Synchronous Removal of Organic Pollutants and Phosphorus from Emergency Wastewater in Chemical Industry Park by Plasma Catalysis System Based on Calcium Peroxide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Various Parameters
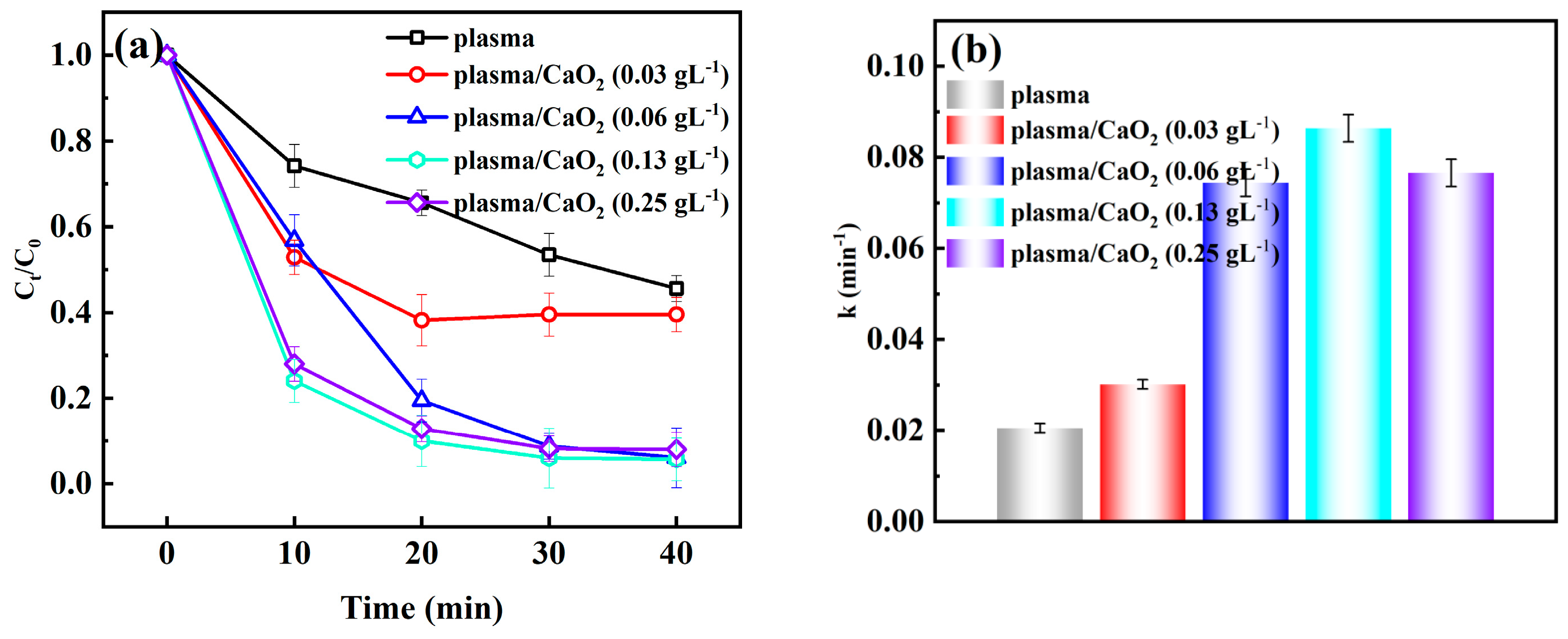
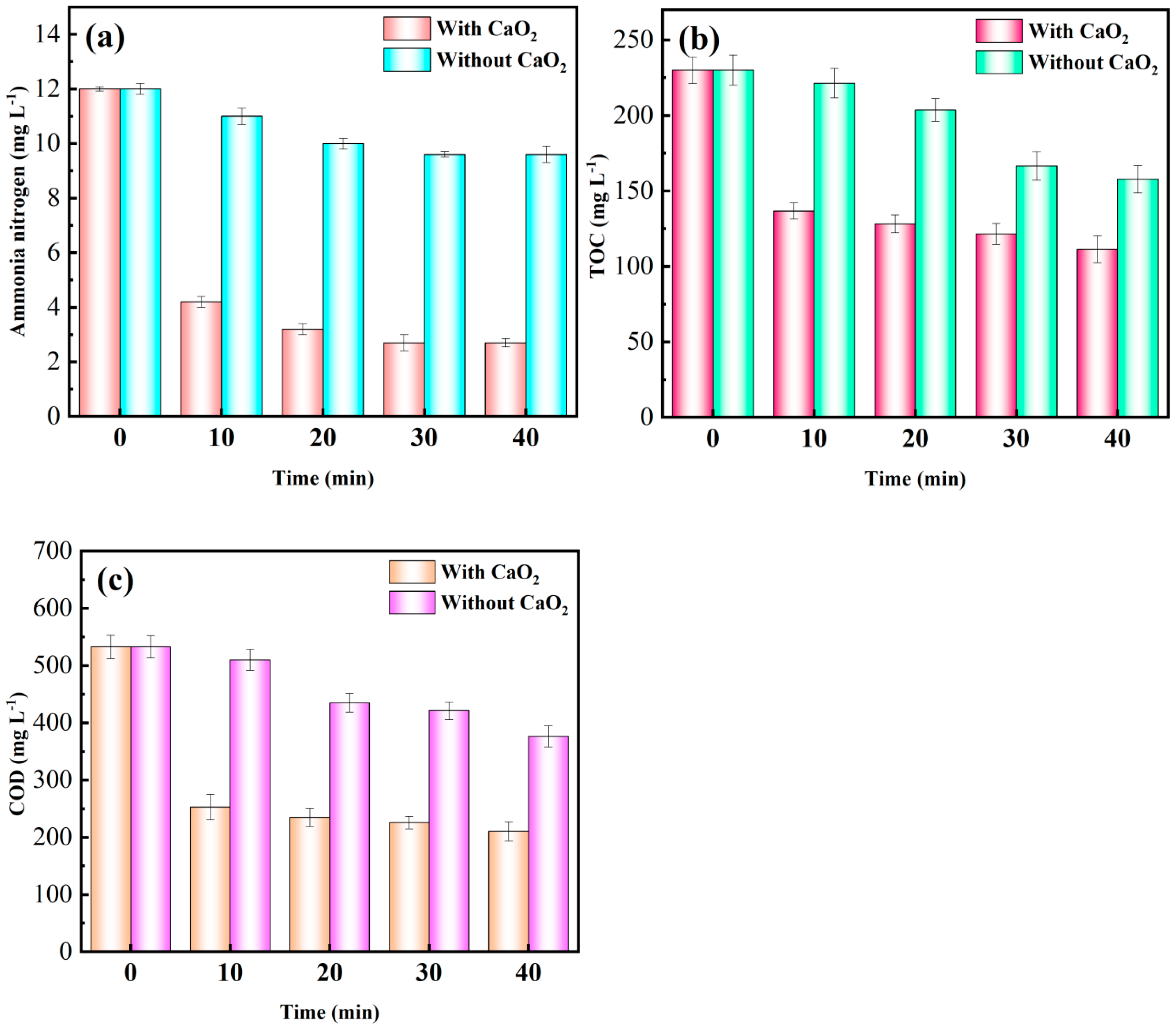
2.1.1. Effect of CaO2 Addition
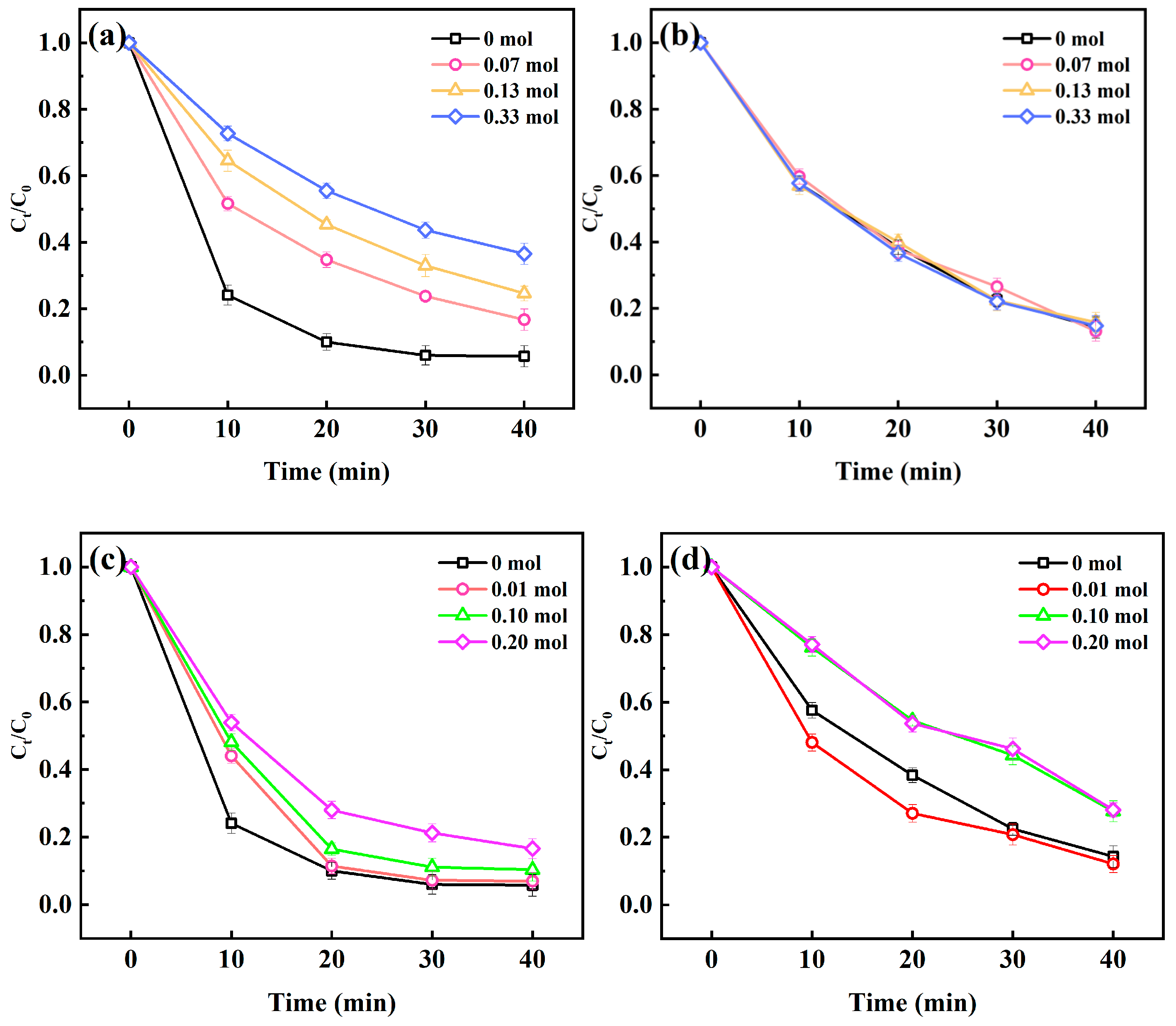
2.1.2. Effect of Initial Concentration
2.1.3. Effect of Discharge Voltage
2.1.4. Effect of Discharge Frequency
2.1.5. Effect of Solution pH
2.2. Effect of Reactive Species
2.3. Detection of Active Substance Production
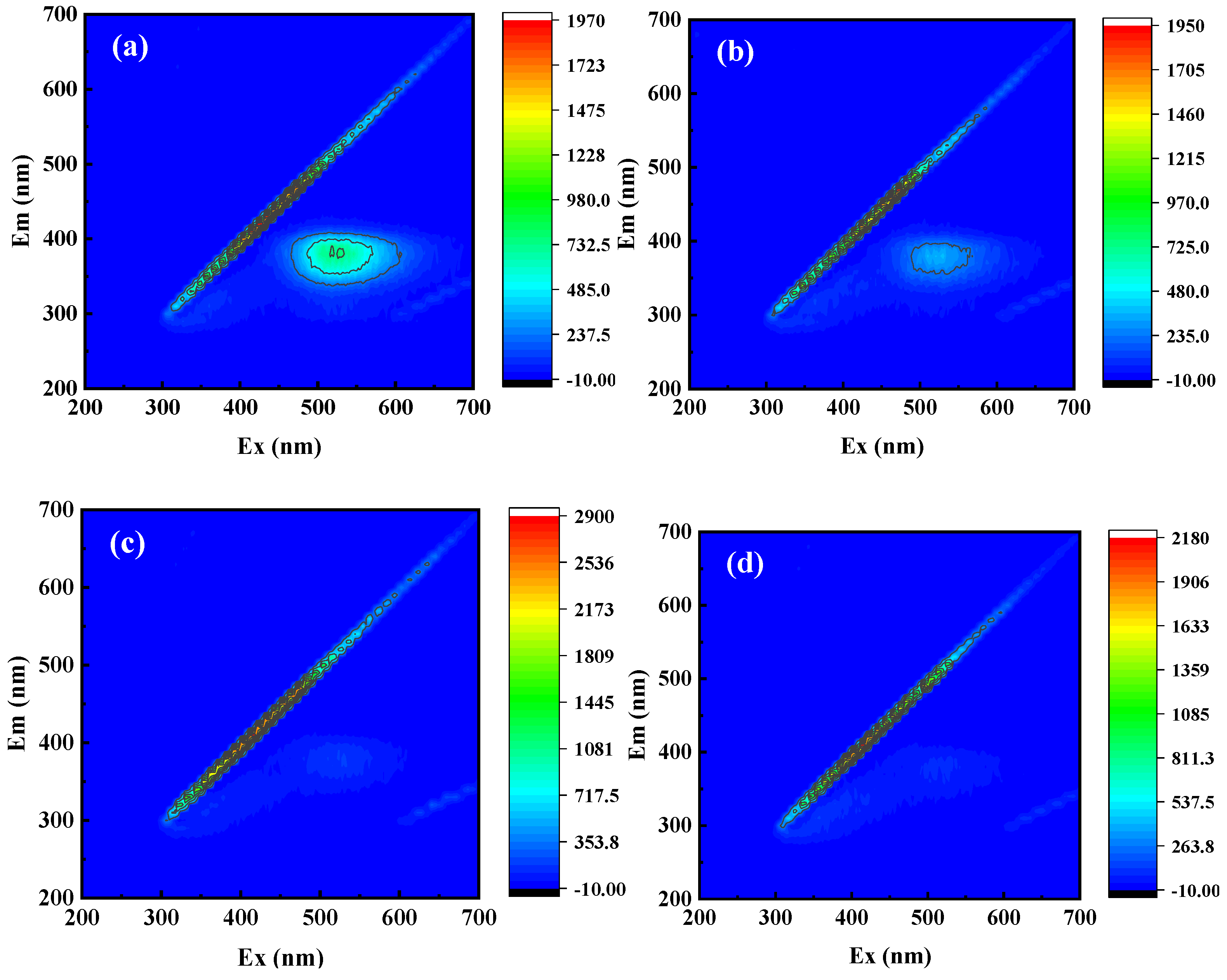
2.4. Analysis of TC Degradation Process
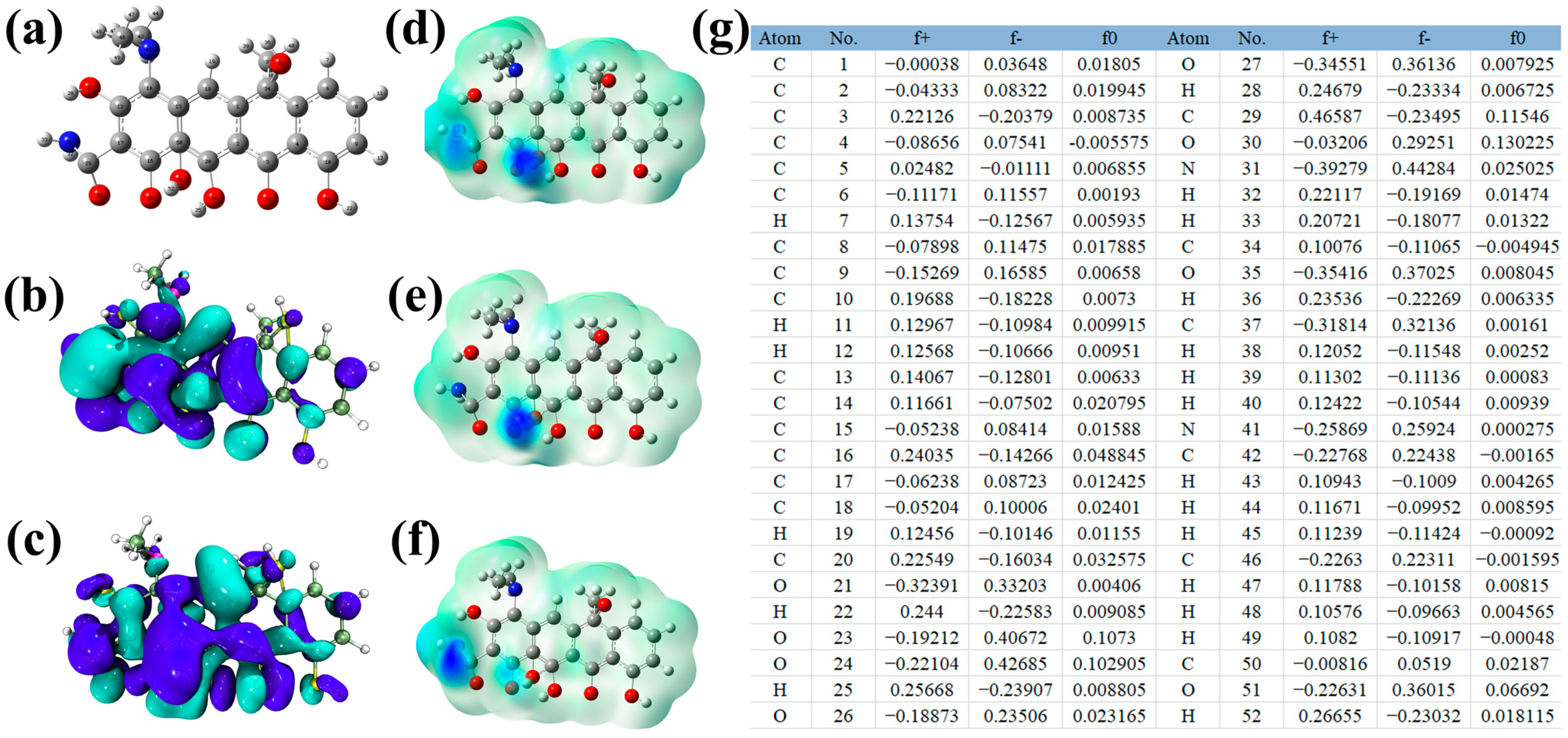
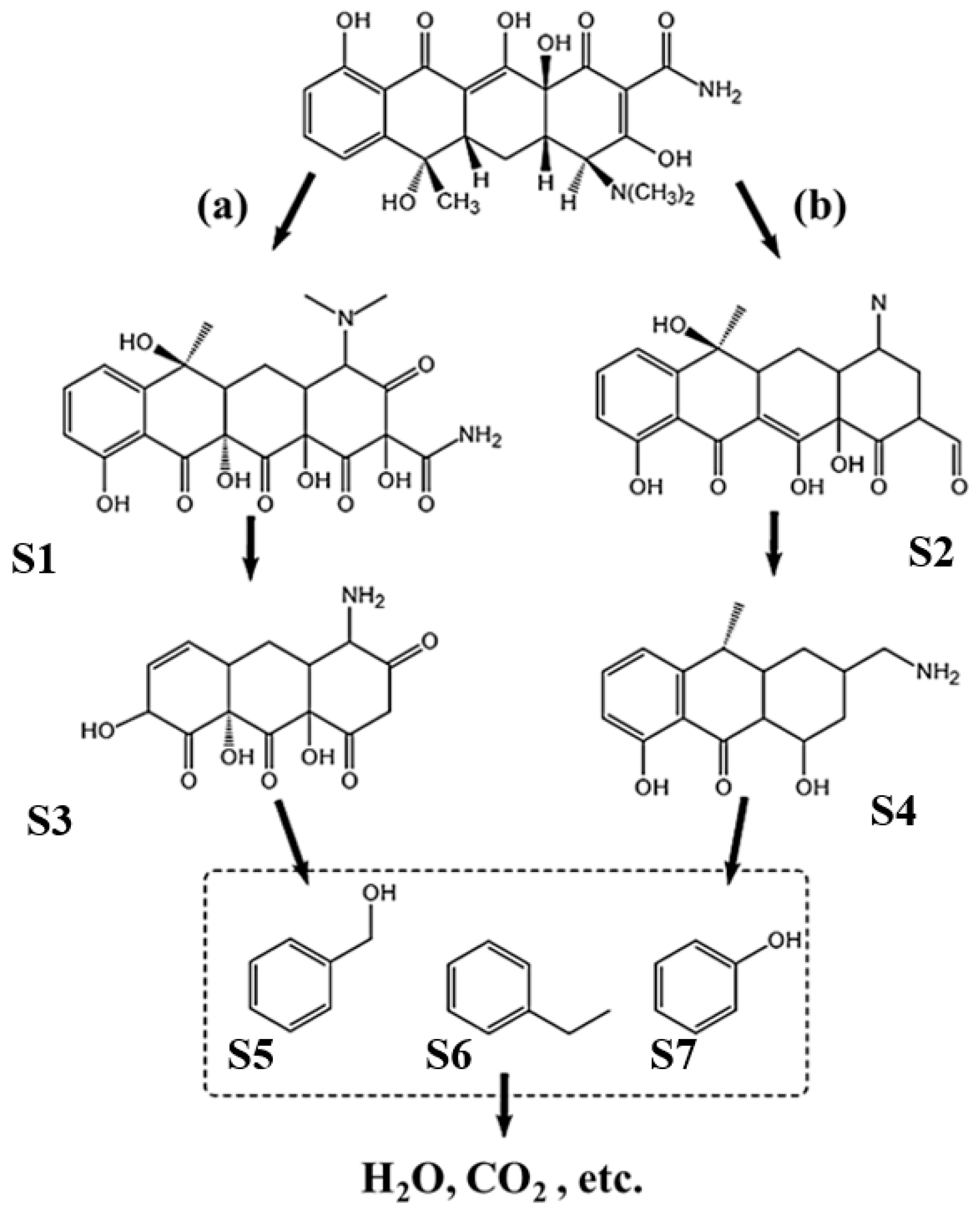
2.5. Analysis of Degradation Products and Pathways
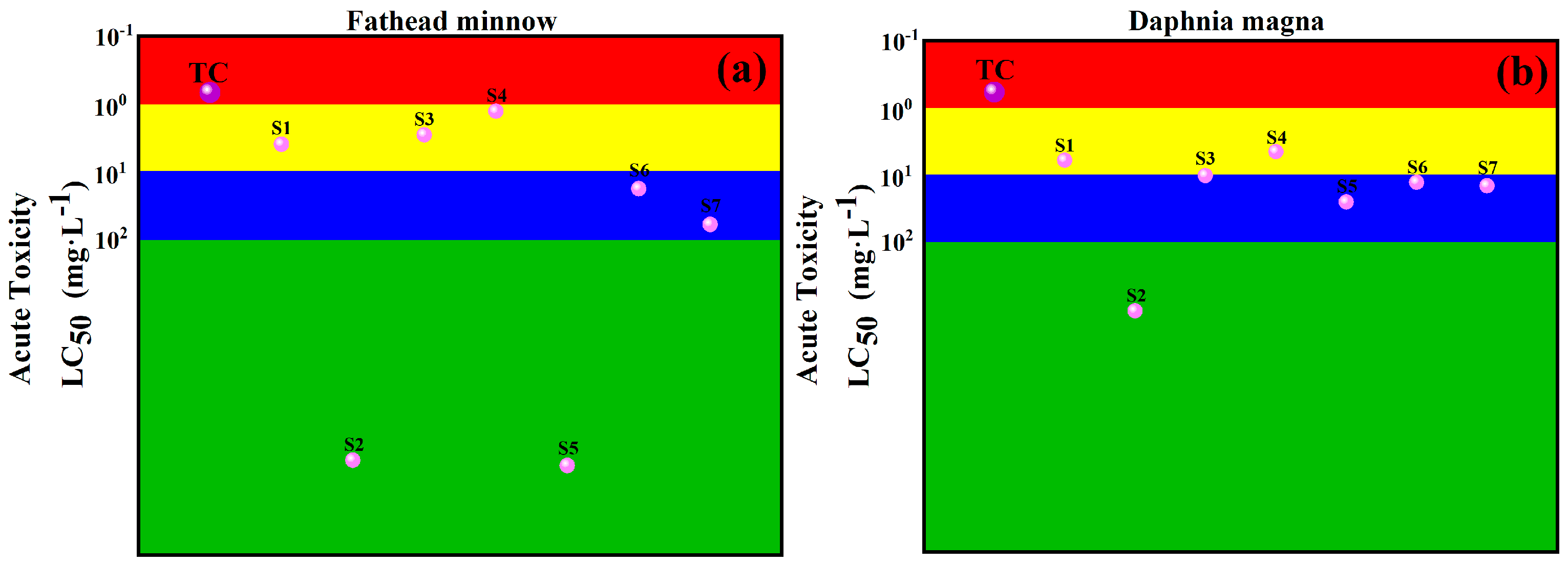
2.6. Toxicity Analysis of Intermediates
3. Experimental
3.1. Chemicals and Experimental Setup
3.2. Experimental Setup
3.3. Calculation and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Escher, B.I.; Stapleton, H.M.; Schymanski, E.L. Tracking complex mixtures of chemicals in our changing environment. Science 2020, 367, 388–392. [Google Scholar] [CrossRef]
- Waheed, A.; Baig, N.; Ullah, N.; Falath, W. Removal of hazardous dyes, toxic metal ions and organic pollutants from wastewater by using porous hyper-cross-linked polymeric materials: A review of recent advances. J. Environ. Manag. 2021, 287, 112360. [Google Scholar] [CrossRef] [PubMed]
- Kamenická, B.; Weidlich, T. A Comparison of Different Reagents Applicable for Destroying Halogenated Anionic Textile Dye Mordant Blue 9 in Polluted Aqueous Streams. Catalysts 2023, 13, 460. [Google Scholar] [CrossRef]
- Hegedus, M.; Lacina, P.; Plotěný, M.; Lev, J.; Kamenická, B.; Weidlich, T. Fast and efficient hydrodehalogenation of chlorinated benzenes in real wastewaters using Raney alloy. J. Water Process Eng. 2020, 38, 101645. [Google Scholar] [CrossRef]
- Jia, Y.Q.; Sun, S.H.; Wang, S.; Yan, X.; Qian, J.S.; Pan, B.C. Phosphorus in water: A review on the speciation analysis and species specific removal strategies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 435–456. [Google Scholar] [CrossRef]
- Li, X.M.; Huang, S.R.; Li, H.; Huang, H.M. The construction of Fe foam-based catalytic filter for the synchronous removal of organic pollutants and phosphorus: Performance, mechanism and applicability. J. Environ. Chem. Eng. 2025, 13, 116112. [Google Scholar] [CrossRef]
- Gottschall, N.; Edwards, M.; Craiovan, E.; Frey, S.K.; Sunohara, M.; Ball, B.; Zoski, E.; Topp, E.; Khan, I.; Clark, I.D.; et al. Amending woodchip bioreactors with water treatment plant residuals to treat nitrogen, phosphorus, and veterinary antibiotic compounds in tile drainage. Ecol. Eng. 2016, 95, 852–864. [Google Scholar] [CrossRef]
- Zhang, H.C.; Zhou, C.; Shangguan, M.H.; Peng, Z.Y.; An, Z.Y. Simultaneous removal of organic pollutants and nutrients from mariculture wastewater using a rotating algal biofilm system. J. Water Process Eng. 2024, 57, 104598. [Google Scholar] [CrossRef]
- Chen, S.Y.; Habib, Z.; Wang, Z.W.; Zhao, P.; Song, W.L.; Wang, X.H. Integrating anaerobic acidification with two-stage forward osmosis concentration for simultaneously recovering organic matter, nitrogen and phosphorus from municipal wastewater. Water Res. 2023, 245, 120595. [Google Scholar] [CrossRef]
- Wang, Y.S.; Song, J.L.; Zhu, R.T.; Peng, M.B.; Long, J.; Bao, T. Degradation of pharmaceutical contaminants in wastewater by non-thermal plasma technology: A comprehensive Review. J. Environ. Chem. Eng. 2025, 13, 116150. [Google Scholar] [CrossRef]
- Guo, H.; Pan, S.; Hu, Z.X.; Wang, Y.W.; Jiang, W.X.; Yang, Y.X.; Wang, T.C.; Han, J.G.; Wu, Y.F.; Wang, T.C. Persulfate activated by non-thermal plasma for organic pollutants degradation: A review. Chem. Eng. J. 2023, 470, 144094. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.W.; Liao, L.N.; Li, Z.; Pan, S.J.; Puyang, C.D.; Su, Y.Y.; Zhang, Y.; Wang, T.C.; Ren, J.Y.; et al. Review on remediation of organic-contaminated soil by discharge plasma: Plasma types, impact factors, plasma-assisted catalysis, and indexes for remediation. Chem. Eng. J. 2022, 436, 135239. [Google Scholar] [CrossRef]
- Zhang, A.; Zhou, Y.Q.; Li, Y.M.; Liu, Y.B.; Li, X.; Xue, G.; Miruka, A.C.; Zheng, M.; Liu, Y.N. Motivation of reactive oxygen and nitrogen species by a novel non-thermal plasma coupled with calcium peroxide system for synergistic removal of sulfamethoxazole in waste activated sludge. Water Res. 2022, 212, 118128. [Google Scholar] [CrossRef]
- Nippatlapalli, N.; Ganta, A. Recent progress on application of nonthermal plasma for the degradation of aqueous emerging contaminants: A review on mechanism, reactor strategies, integrated systems and future perspective. Process Saf. Environ. Prot. 2024, 187, 1454–1470. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, W.; Dong, N.; Chen, P.; Ge, L.; Tan, F.; Wang, X.; Qiao, X.; Wong, P.K. A dual-oxidant advanced oxidation process system containing CaO2 and peroxymonosulfate for organic pollutant degradation: High adaptability and synergistic effect. Sep. Purif. Technol. 2023, 308, 122909. [Google Scholar] [CrossRef]
- Xu, Q.X.; Huang, Q.S.; Wei, W.; Sun, J.; Dai, X.H.; Ni, B.J. Improving the treatment of waste activated sludge using calcium peroxide. Water Res. 2020, 187, 116440. [Google Scholar] [CrossRef]
- Wang, H.F.; Zhao, Y.S.; Li, T.Y.; Chen, Z.; Wang, Y.N.; Qin, C.Y. Properties of calcium peroxide for release of hydrogen peroxide and oxygen: A kinetics study. Chem. Eng. J. 2016, 303, 450–457. [Google Scholar] [CrossRef]
- Lapointe, M.C.; Martel, R.; Cassidy, D.P. RDX degradation by chemical oxidation using calcium peroxide in bench scale sludge systems. Environ. Res. 2020, 188, 109836. [Google Scholar] [CrossRef]
- Wu, L.; Wei, Q.; Zhang, Y.; Fan, Y.; Li, M.; Rong, L.; Xiao, X.; Huang, X.; Zou, X. Effects of antibiotics on enhanced biological phosphorus removal and its mechanisms. Sci. Total Environ. 2021, 774, 145571. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Meropoulis, S.; Hatzisymeon, M.; Lada, Z.G.; Rassias, G. Degradation of antibiotic enrofloxacin in water by gas-liquid nsp-DBD plasma: Parametric analysis, effect of H2O2 and CaO2 additives and exploration of degradation mechanisms. Chem. Eng. J. 2020, 398, 125622. [Google Scholar] [CrossRef]
- Jiang, N.; He, J.; Zhang, A.; Zhou, Y.; Zheng, M.; Xu, X.; Miruka, A.C.; Li, X.; Liu, Y.; Xue, G. Synergistic improvement of short-chain fatty acid production from waste activated sludge via anaerobic fermentation by combined plasma-calcium peroxide process. Bioresour. Technol. 2022, 361, 127754. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, Y.; Yang, Y.; Xu, F.; Yang, C.; Guo, H.; Hou, J.; Han, J. Simultaneous removal of organic matters and phosphorus by a novel dielectric barrier discharge coupled with CaO2 system: Feasible and mechanism. Sep. Puriϧcation Technol. 2025, 354, 129519. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, X.; Xue, Y. Application of calcium peroxide in water and soil treatment: A review. J. Hazard. Mater. 2017, 337, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Qian, Y.; Chen, J.; Zhang, Y.; Zhou, X. A critical review of solid peroxides in environmental remediation and water purification: From properties to field applications. Chem. Eng. J. 2023, 465, 142424. [Google Scholar] [CrossRef]
- Sanito, R.C.; You, S.J.; Wang, Y.F. Degradation of contaminants in plasma technology: An overview. J. Hazard. Mater. 2022, 424, 127390. [Google Scholar] [CrossRef]
- Yan, L.G.; Xu, Y.Y.; Yu, H.Q.; Xin, X.D.; Wei, Q.; Du, B. Adsorption of phosphate from aqueous solution by hydroxy-aluminum, hydroxy-iron and hydroxy-iron-aluminum pillared bentonites. J. Hazard. Mater. 2010, 179, 244–250. [Google Scholar] [CrossRef]
- Ma, M.; Duan, W.; Huang, X.; Zeng, D.; Hu, L.; Gui, W.; Zhu, G.; Jiang, J. Application of calcium peroxide in promoting resource recovery from municipal sludge: A review. Chemosphere 2024, 354, 141704. [Google Scholar] [CrossRef]
- Butxon, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar]
- Wang, X.W.; Wang, Y.D.; Zhu, Z.Y.; Jin, L.; Li, J.H.; Liu, X. Research progress of pathways of the production of singlet oxygen in water treatment. Ind. Water Treat. 2023, 43, 1–15. [Google Scholar]
- Schweikl, H.; Godula, M.; Petzel, C.; Bolay, C.; Hiller, K.A.; Buchalla, W. Critical role of superoxide anions and hydroxyl radicals in HEMA-induced apoptosis. Dent. Mater. 2016, 33, 110–118. [Google Scholar] [CrossRef]
- Wang, S.H.; Zhou, L.L.; Li, Z.J.; Han, J.L.; Liu, R.J.; Yun, J. Research progress on degradation of organic pollutants in water by catalytic ozonation. Chin. J. Process Eng. 2022, 22, 586–600. [Google Scholar]
- Chou, Y.F.; Yang, Z.Y.; Song, W.F.; Chen, S.Q.; Ma, S.N.; Bai, X.Y. Preparation of Fe3O4-CeOx/AC magnetic catalyst and degradation of tetracycline hydrochloride by catalytic ozonation. Acta Sci. Circumstantiae 2022, 42, 146–155. [Google Scholar]
- Xu, Z.L.; Qi, W.X.; Gu, P.C.; Guo, W.Z.; Zhao, L. Kinetics of Degradation of Unsymmetrical Dimethylhydrazine in Water by Hydrogen Peroxide Enhanced Ultraviolet Photocatalytic Ozone. Chem. Eng. Des. Commun. 2019, 45, 146. [Google Scholar]
- Chen, Y.S.; Zhang, X.S.; Chang, S.; Dai, Y.C.; Yuan, W.K. Study on the degradation mechanism of organic compounds in the aqueous solution by pulsed high-voltage discharge plasma. Acta Sci. Circumstantiae 2005, 25, 113–116. [Google Scholar]
- Wang, Y.W.; Huang, J.W.; Guo, H.; Puyang, C.D.; Han, J.G.; Li, Y.; Ruan, Y.X. Mechanism and process of sulfamethoxazole decomposition with persulfate activated by pulse dielectric barrier discharge plasma. Sep. Purif. Technol. 2022, 287, 120540. [Google Scholar] [CrossRef]
- Li, Q.B.; Wei, Y.; Cui, H.X.; Feng, H.; Lang, J.Y. Quantitative Analysis of TOC in Water Quality Based on UV-Vis Spectroscopy. Spectrosc. Spectr. Anal. 2022, 42, 376–380. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Toxicity Estimation Software Tool (T.E.S.T.), Version 4.1.; National Center for Computational Toxicology, Office of Research and Development, EPA: Washington DC, USA, 2012.
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.0.16; Semichem Inc.: Shawnee Mission, KS, USA, 2016.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Wang, H.J.; Guo, H.; Wu, Q.S.; Zhou, G.S.; Yi, C.W. Effect of activated carbon addition on H2O2 formation and dye decoloration in a pulsed discharge plasma system. Vacuum 2016, 128, 99–101. [Google Scholar] [CrossRef]
- Bander, H.; Hoigné, J. Determination of ozone in water by the indigo method. Ozone Sci. Eng. 1982, 4, 449–456. [Google Scholar]
- Guo, H.; Li, D.S.; Li, Z.; Lin, S.Y.; Wang, Y.W.; Pan, S.J.; Han, J.G. Promoted elimination of antibiotic sulfamethoxazole in water using sodium percarbonate activated by ozone: Mechanism, degradation pathway and toxicity assessment. Sep. Purif. Technol. 2021, 266, 118543–118545. [Google Scholar] [CrossRef]
- Kang, J.Y. Study of improved method for determination of orthophosphate in water. Electr. Power Technol. Environ. Prot. 2020, 36, 27–30. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.; Qian, C.; Wen, J.; Wang, T. Synchronous Removal of Organic Pollutants and Phosphorus from Emergency Wastewater in Chemical Industry Park by Plasma Catalysis System Based on Calcium Peroxide. Catalysts 2025, 15, 486. https://doi.org/10.3390/catal15050486
Li A, Qian C, Wen J, Wang T. Synchronous Removal of Organic Pollutants and Phosphorus from Emergency Wastewater in Chemical Industry Park by Plasma Catalysis System Based on Calcium Peroxide. Catalysts. 2025; 15(5):486. https://doi.org/10.3390/catal15050486
Chicago/Turabian StyleLi, Aihua, Chengjiang Qian, Jinfeng Wen, and Tiecheng Wang. 2025. "Synchronous Removal of Organic Pollutants and Phosphorus from Emergency Wastewater in Chemical Industry Park by Plasma Catalysis System Based on Calcium Peroxide" Catalysts 15, no. 5: 486. https://doi.org/10.3390/catal15050486
APA StyleLi, A., Qian, C., Wen, J., & Wang, T. (2025). Synchronous Removal of Organic Pollutants and Phosphorus from Emergency Wastewater in Chemical Industry Park by Plasma Catalysis System Based on Calcium Peroxide. Catalysts, 15(5), 486. https://doi.org/10.3390/catal15050486





