Abstract
The global reliance on fossil fuels, particularly natural gas, underscores the urgency of developing sustainable methods for methane (CH4) and carbon dioxide (CO2) conversion. Methane, which constitutes 95% of natural gas, is a critical feedstock and fuel source. However, its high bond dissociation energy and volatility pose challenges for large-scale utilization and transport. Current research emphasizes the catalytic and plasma-assisted conversion of CH4 and CO2 into value-added products such as methanol, higher hydrocarbons, and organic oxygenates. Advancements in these technologies aim to overcome obstacles such as high operating temperatures, coking, and low product selectivity while addressing methane’s environmental impact, as leakage during extraction and distribution significantly contributes to global warming. Plasma-assisted conversion has emerged as a promising approach, leveraging electron impact processes to generate reactive species that facilitate CH4 and CO2 transformation at near-room temperatures. The integration of catalysts within plasma environments enhances reaction pathways, product yields, and selectivity by modifying plasma properties and surface interactions. This review comprehensively discusses the various methods investigated for CH4 conversion and energy efficiency. We attempt to highlight the recent progress in plasma-assisted catalytic processes for CH4 and CO2 valorization, with a focus on the mechanisms of product formation, catalyst modifications, and their impact on plasma discharge characteristics. The insights gained could pave the way for scalable, energy-efficient solutions to produce sustainable fuels and chemicals, thereby contributing to global efforts in carbon cycle fixation and climate change mitigation.
1. Introduction
Recent reports indicate that approximately 80% of the global energy demand is met by fossil fuels such as oil, natural gas, and coal. Natural gas has witnessed a significant increase in consumption over the past few decades due to its extensive availability and relatively lower emissions compared to oil and coal. Estimates suggest that natural gas reserves, encompassing both conventional and unconventional sources, amount to approximately 6330 trillion m3 [1].
The methane cycle diagram illustrates the various sources and sinks of CH4 in the environment. It includes processes such as fossil fuel combustion, emissions from landfills, CH4 release from wetlands, plant decomposition, methane release due to permafrost melting, and digestion by termites and animals, among others. These processes contribute to the natural flux of CH4 in the atmosphere, impacting its concentration and ultimately influencing climate change and global warming, and are illustrated in Figure 1 [2].
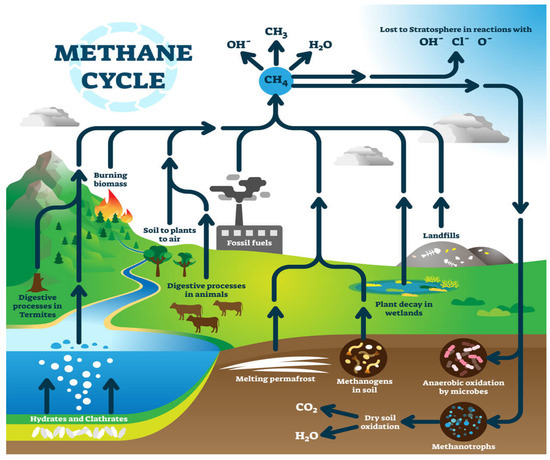
Figure 1.
Methane cycle diagram involving fossil fuel combustion, landfills, wetland plant deterioration, permafrost melting, termite and animal digestion, and so on [2].
According to studies, using natural gas reduces CO2 emissions by 25–45% when compared to burning coal or oil. Crucially, using natural gas results in significantly lower emissions of genotoxic substances, aromatic hydrocarbons, and different oxides (NOx, Sox, and CO). Methane (CH4) makes up most of the natural gas (approximately 95%) and is often acquired as a byproduct of petroleum processing [3]. Shale gas stands out as a major unconventional source of natural gas, where methane is trapped within the micropores of sedimentary rocks, namely, shales. Hydraulic fracturing combined with horizontal drilling techniques is employed to extract the gas from shale reserves. The challenges, potential opportunities, and associated environmental concerns related to the hydraulic recovery of CH4 from shale gas have been extensively discussed [4]. Another potentially abundant source of CH4 is natural gas hydrates [5].
CH4 serves dual roles as a feedstock chemical and a fuel source across various sectors including residential, electric power generation, industrial processes, and transportation. However, its liquefaction is crucial for facilitating long-distance transportation from remote natural gas reserves to market centers. Yet, the energy-intensive process of compressing CH4 into a liquid state poses economic challenges for commercial viability [6]. Transporting CH4 in its gaseous form is hindered by its extremely low boiling point (−161.6 °C at 1 atm) and high flammability, making it challenging and limiting its applications, especially from remote sources [7]. Consequently, research emphasis has shifted towards controlled oxidation of CH4 to produce liquid fuels such as methanol (CH3OH) or converting it into easily liquefiable hydrocarbons. Advancements in this technology would enable the efficient storage, transportation, and utilization of CH4 on a larger scale [8,9].
The demand for CH3OH production is rising due to its application in fuel cells for energy generation. However, activating CH4 poses significant challenges due to the covalent nature of its C–H bonds [10]. Only strong superacid, as demonstrated by Olah et al. [11,12], can induce hydride ion abstraction to generate methyl carbocation. Overcoming these challenges is imperative for advancing the cost-effective conversion of CH4 into liquid fuels, thereby expanding its utilization and impact across various sectors.
The release of CH4 into the atmosphere poses a significant environmental challenge due to its high volatility. Approximately 8% of CH4 escapes during various stages of shale gas extraction, transportation, storage, and distribution. This leakage can be mitigated by converting methane into less volatile or liquid forms [13]. The escalating emissions of both CH4 and CO2 have drawn increased scrutiny in recent decades due to the widespread reliance on fossil fuels and the extensive exploitation of natural gas resources.
To address the urgent issue of global warming and associated climate changes, it is imperative to focus on the transformation of CH4 and CO2 into more useful feedstocks. Ongoing research in the catalytic conversion of CO2 and CH4 plays a pivotal role in fostering sustainable development and carbon cycle fixation [14,15,16]. Figure 2 shows the various methods of the CH4 conversion process, including photochemical, electrochemical, biological, catalytic, and plasma-assisted techniques. The large-scale production of valuable products, such as higher hydrocarbons, hydrogen (H2), synthesis gas (CO + H2), alkanes, CH3OH, carboxylic acids, alkenes, and aromatics, was accomplished through the effective use of thermal and/or catalytic pyrolysis, oxidative coupling, biological techniques, and plasma activation [17,18,19,20,21]. Notably, C-5+ hydrocarbons and liquid organic oxygenates are the most desired products. Additionally compared to CH4, the C-2 to C-4 hydrocarbons are readily compressible and hold promise for more cost-effective transportation.
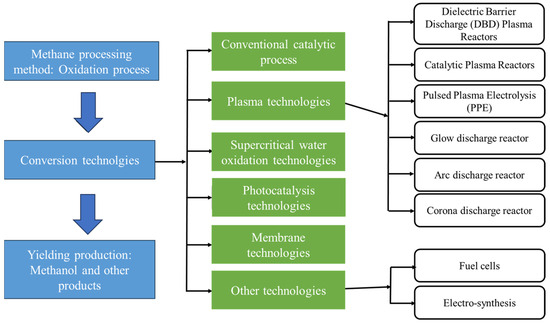
Figure 2.
Various methods of methane-to-energy conversion.
The most promising avenue for significant advancement in CH4 catalytic conversion technology lies in the identification of a pathway leading to the formation of oxygenates such as CH3OH and formaldehyde (HCHO) [19,20,22]. Current theories propose that H2, released as a byproduct during the dehydrogenation of CH4, combines with oxygen (O2) on the catalyst surface, giving rise to a surface peroxide species. This peroxide species is believed to play a crucial role in activating CH4 and facilitating its conversion into oxygenated compounds [23]. Some studies have investigated the oxidation of CH4 using oxygen over molybdenum-based catalysts, demonstrating low CH4 conversions (<10%) with a notable selectivity (50%) toward HCHO. Additionally, research involving zeolite-based catalysts has explored their capability to convert CH4 into CH3OH. Notably, Cu-exchanged zeolites have been found to effectively convert CH4 to CH3OH at temperatures ranging from 130 to 200 °C, utilizing molecular oxygen as the oxidant [21,22,23].
As previously mentioned, the catalytic conversion of CH4 and CO2 poses significant challenges, including the need for high operating temperatures, issues such as coking and sintering, and low conversion rates and limited product selectivity. A significant challenge in the thermal activation of CH4 for liquid product formation is that many of these products begin to decompose at the high temperatures necessary for effective CH4 conversion. This issue complicates the ability to achieve both high conversion rates and selectivity simultaneously, ultimately restricting single-pass product yields.
Recent advancements in plasma-assisted conversion techniques present promising solutions to many of these challenges. As a result, there has been growing interest and extensive research in the plasma-assisted valorization of CH4 and CO2 [24,25,26]. Within a plasma environment, processes such as electron impact excitation, dissociation, and ionization produce a diverse array of reactive species. These reactive species subsequently engage in recombination reactions, facilitating the formation of value-added neutral molecules.
Controlling product formation within a plasma environment involves adjusting the density of generated species, achievable through regulating parameters such as the gas flow rate, discharge power, and feed gas composition. Introducing appropriate catalysts into the discharge zone often enhances product yield and selectivity. Catalysts play multiple roles in plasma environments, facilitating efficient adsorption of reactive species on their surfaces and thus altering reaction pathways. Furthermore, catalysts within the discharge zone can significantly impact plasma properties, inducing effects such as surface discharge [27]. These effects vary depending on the physical and chemical properties of the catalyst material.
This review extensively explores the interaction between catalysts and plasma properties. It focuses on the latest advancements in plasma-assisted processes, both catalytic and non-catalytic, for the activation and conversion of CH4 and CO2 into valuable chemicals. Special attention is given to the mechanisms of product formation, particularly the synthesis of higher hydrocarbons and organic oxygenates. Moreover, summary tables are presented to highlight the complexity of methane conversion processes and underscore the importance of catalyst design and reaction engineering in controlling product selectivity and yield. These tables provide a comprehensive overview of the various pathways, catalysts, and reaction conditions that influence the formation of desired products from methane conversion. Additionally, it delves into important surface processes and the role of plasma in modifying various catalyst materials, both physically and chemically.
2. Methane-to-Energy Conversion
Converting CH4 to energy is an important part of sustainability since it tackles environmental problems related to CH4 emissions, which is a powerful greenhouse gas. In the upcoming sections, we will explore various sustainable technologies developed for CH4 conversion, discussing their efficiency as well as their merits and demerits (Figure 3).
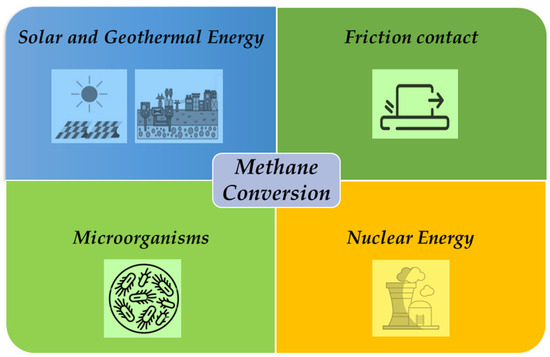
Figure 3.
Different energies developed for CH4 conversion.
2.1. Sustainable Technologies Developed for CH4 Conversion
2.1.1. Solar-Energy-Derived Methane Conversion
Solar-energy-driven CH4 conversion is a potential green technique for reducing the environmental effect of CH4 emissions and decreasing dependency on fossil fuels. Solar energy can transform CH4 into other useful products via numerous chemical processes [28]. There are several pathways for solar-energy-derived CH4 conversion such as solar–thermal-energy-derived CH4 conversion [29,30], solar–photovoltaic-derived CH4 conversion [31,32], and photocatalytic CH4 convention [33,34].
Dong, Chunyang et al. [35] have demonstrated a revolutionary photocatalytic reaction route that allows for the direct production of CH3COOH from CH4, CO, and H2O over Pt/polyoxometalate/TiO2 at room temperature. Under ideal circumstances, they produced a phenomenal yield of 5.7 mmol·L−1 CH3COOH within 60 h, with exceptional selectivity surpassing 90%. Using isotopic labeling and in situ spectroscopy, the researchers discovered that CH4 is activated by ·OH radicals and then reacts with surface-bound CO molecules on Pt single atom sites to form acetyl groups. Further oxidation resulted in the production of CH3COOH. Despite impressive advances in catalyst development and the investigation of new reaction routes for photocatalytic CH4 conversion, the problem of selectively activating and functionalizing CH4 into useful molecules remains tough.
Ma et al. [36] created a unique heat pipe tubular reactor including a heat transfer cavity and a reaction cavity. This system offered a constant hybrid energy source by combining concentrated solar energy with photovoltaic power. Stability tests over 24 h found that the system maintained about 90% CH4 conversion and 72% H2 selectivity. This emphasizes the importance of combining thermal and photovoltaic systems to completely utilize solar energy, rather than depending simply on one method. Such integration may also be applied to processes such as CH4 breakdown and other high-energy-demand endothermic reactions.
2.1.2. Microbe-Driven CH4 Conversion
Microbe-driven methane conversion uses microorganisms such as bacteria or archaea to convert CH4 into various chemicals. This process is often carried out by bacteria that contain enzymes capable of metabolizing CH4. Microbial CH4 conversion occurs naturally in a variety of habitats, including wetlands, rice paddies, and the digestive tracts of some animals. Biological conversion has emerged as an innovative and sustainable approach to carbon sequestration and the conversion of CH4 into valuable compounds such as liquid fuels, bioplastics, and chemicals, in contrast to thermochemical processes. Bacteria, including different types of bacteria such as ammonia-oxidizing bacteria, can activate the stable C–H bond in CH4 molecules under normal circumstances. This activation is most likely due to catalytic mechanisms assisted by particular enzymes. This biological technique functions in milder conditions than standard thermochemical procedures, providing an ecologically benign and energy-efficient option for exploiting methane’s potential in a variety of industrial applications [37,38,39].
In the study by Patel et al. [40], several support materials, such as chitosan modified with glutaraldehyde (GLA), 3-aminopropyltriethoxysilane (APTES), polyethyleneimine, and APTES followed by GLA (APTES-GLA), were investigated to improve methanol synthesis from biogas. Among these materials, chitosan-APTES-GLA showed the greatest improvement in immobilization yield and relative efficiency of Methylomicrobium album, reaching 56.4% and 97.7%, respectively. Notably, the maximal cell loading of M. album was 236 mg dry cell mass per g-support, representing a remarkable 7.7-fold increase over chitosan alone. The immobilized M. album produced significantly more methanol, approximately 23.9 times that of free cells.
2.1.3. Nuclear-Driven Methane Conversion
For the conversion of CH4 (methane), nuclear energy is an attractive and sustainable source of thermal energy. This is due to processes such as steam reforming and methane breakdown, which are characterized by endothermic reactions that produce H2. Nuclear energy provides an alternative to the traditional Steam Methane Reforming (SMR) method, which produces significant CO2 emissions from the burning of CH4. In this case, CH4 not only acts as a reactant to produce H2, but it also helps the process by releasing heat during combustion.
By using nuclear heat in these processes, as opposed to traditional combustion techniques, greenhouse gas emissions and other toxic pollutants are reduced. Furthermore, nuclear-driven systems that convert thermal energy directly to H2 show a far faster rate of H2 generation than indirect conversions that include thermal–electrical–H2 energy pathways. This highlights the environmental benefits and efficiency improvements linked to nuclear H2 generation via steam CH4 reforming, thus establishing it as a very advantageous choice in the context of sustainable energy supplies [41,42].
2.1.4. Tribochemical-Driven Methane Conversion
Tribochemical-driven methane conversion is the process by which methane (CH4) is converted into various compounds using chemical reactions sparked by the mechanical forces produced during frictional contact. This method does not require high temperatures or outside energy sources to drive chemical reactions since it uses mechanical energy, which is normally produced by rubbing or friction. “Tribology”, the study of friction, and “chemical” are combined to form the phrase “tribochemical”.
Tribochemical reactions involving sliding surfaces and additives such as lubricants, ambient air, and some tribomaterials are frequently triggered at sliding interfaces by high contact pressures [43], frictional heating [44], and shear stresses [45]. At the contact interface, this causes a protective boundary layer or tribofilm to form. For instance, atmospheric pressures of CH4 are used during the production of carbon-based compounds such as graphene [46], polycrystalline diamond [47], or diamond-like carbon [48]. It has been shown that the methyl group (–CH3) with a diamond structure plays a critical role in the deposition of diamond films. Methyl groups with sp3 hybrid orbitals engage with the surface of the substrate to produce C–C covalent bonds, and they nucleate on the surface of the matrix to form the nucleus of a diamond crystal. Active methyl groups progressively replace H atoms in the crystal nucleus by continuous impact with high-energy particles, promoting the connection and creation of a diamond film. Consequently, it is more advantageous to use carbon sources such CH4, acetone, methanol, etc., that have a diamond-like structure for diamond film deposition [49].
2.1.5. Geothermal-Driven Methane Conversion
Geothermal energy, a clean and sustainable resource with a widespread distribution and significant reserves, is still in its early phases of development. The concept of utilizing geothermal heat to convert CH4 was introduced, with an improved geothermal system (EGS) reservoir. This technique used two wells, one of which injected water for chemical reactions and heat transmission, resulting in smaller, more cost-effective wells [50]. Geothermal energy may also be transformed into electricity using thermoelectric generators. Li et al. [51] report on successful field testing that produced 500 W of electrical energy from a geothermal–thermoelectric generator with a temperature differential of 152 °C. The study of thermoelectric materials in geothermal energy conversion is predicted to progress quickly, with the possibility of using the produced electric power for industrial production and CH4 conversion in the future [52].
2.2. Energy Efficiency
Table 1 compares the energy efficiency of various processes employed in energy production using sustainable technology. Researchers have been exploring methane (CH4) conversion utilizing renewable energy sources, specifically solar and nuclear energy. Early efforts used heat or electricity-based techniques to produce hydrogen (H2), with considerable CO and CO2 byproducts and 11.4% energy efficiency [53]. Photocatalytic technology has also evolved, which uses semiconductor materials to convert CH4 into different C1/C2 oxygenates using daylight, and about 14.1% energy efficiency was reached [54]. Despite development, difficulties remain, including kinetic constraints and the generation of byproducts.

Table 1.
Different energy-driven CH4 conversion technologies.
Microbial-driven methane conversion has an amazing 80% energy efficiency [55], outperforming other approaches. The direct use of sustainable energy is essential to boosting efficiency, exhibiting the potential of previously untapped sources such as wind energy and ocean waves. Furthermore, unused energy, such as leftover heat and electrochemical neutralization energy, offers chances for driving or helping CH4 conversion. Further research into these driving forces is required for sustained progress in sustainable methane conversion [56,57].
Although there are not many papers on sustainable energy-driven methane (CH4) conversion, it is clear that developing effective, well-controlled systems in moderate climates is still a difficult task. It is possible to partially alleviate the energy crisis and solve global environmental issues by incorporating alternative energy sources into existing CH4 technology. Thankfully, continued research has advanced fundamental knowledge, reactor design, catalyst production, characterization methods, and theoretical explorations. However, converting CH4 just using renewable energy sources will not cut it in the long run. Research on more efficient catalysts is now underway, as is the development of sustainable energy-driven reactors and the seamless integration of these systems with current CH4 converters to improve overall conversion performance and energy utilization efficiency. To make significant advancements in the sector moving forward, researchers should investigate a variety of pathways in sustainable energy-driven CH4 conversion [58,59].
3. Plasma Technology for CH4-to-Energy Conversion
Non-thermal plasma (NTP) can be generated through a straightforward method involving the placement of two electrodes in a gas medium and the application of an electrical potential difference. This potential can be in the form of direct current (DC), alternating current (AC), or pulsed electrical signals. Alternatively, induction coils or microwaves can also be employed to provide the necessary electrical energy. Depending on the chosen gas and the method of plasma generation, electrons in NTP can achieve an average temperature ranging from 10,000 to 100,000 K (1–10 eV), despite the gas temperature remaining as low as room temperature [60,61].
Elevated electron temperatures are pivotal in the distinctive chemistry of NTP, enabling the activation and dissociation of stable atoms and molecules, thus facilitating diverse reactions. NTP encompasses several primary categories as depicted in Figure 4 [62].
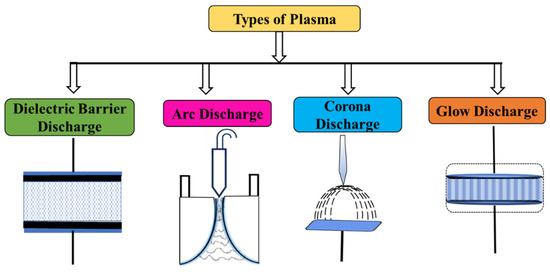
Figure 4.
Various types of plasma reactors.
For chemical conversion processes, operating at pressures close to atmospheric levels is generally preferred. This preference stems from the considerable expense and technical challenges associated with low-pressure systems, along with their limited favorability for kinetic reactions. Typically, high pressures are utilized in industrial catalytic processes where thermodynamic constraints permit, thereby accelerating reaction rates. Although plasma can operate at higher pressures, it is generally less preferred due to the escalating voltage requirements for sustaining discharge. Addressing voltage limitations may involve reducing the discharge gap, but this approach could render the gap too narrow for accommodating potential catalyst integration within the discharge zone. Consequently, it is unsurprising that most of the research in CH4 activation chemistry is conducted using atmospheric pressure plasmas [61].
3.1. CH4 Conversion to Energy or Oxygenated Products
Extensive research on greenhouse gas conversion using plasma has primarily focused on the valorization of CH4. This is achieved through plasma-assisted non-oxidative coupling, which facilitates the formation of higher hydrocarbons, and oxidative coupling, which leads to the production of organic oxygenates. This section provides an overview of plasma-based CH4 valorization processes, highlighting key influencing factors. A summary of the findings from previous studies on CH4 valorization across different plasma reactors is presented in Table 2.

Table 2.
CH4 valorization in various non-catalytic plasma reactors.
3.2. Non-Oxidative CH4 Conversion
NTP-assisted non-oxidative CH4 conversion is widely investigated owing to its simple methodology. This process primarily involves two mechanisms: (i) electron collision activation and (ii) thermal activation. Both mechanisms strip hydrogen from CH4, creating active species to produce stable products through further reactions. Predominant products include C2H6 and C2H2, along with hydrogen. While higher hydrocarbons such as C-5+ are detected, their yields are typically low. Importantly, an undesired outcome of this process is the formation of pure carbon or coke, which can hinder energy efficiency. Despite this, if CH4 needs to be utilized due to transportation challenges, even the production of C2H6, C2H4, and C2H2 can be considered valorization, albeit safety concerns arise, especially with C2H2. These gases offer advantages such as cheaper transportation due to greater compressibility and potential transformation into liquids or other valuable products. A few studies even reported on the synthesis of high- graphene nano-flakes and sheets as high-value carbon products. The study reveals that manipulating the pressure can successfully regulate the properties of graphene nanoflakes, although it comes with considerations regarding energy usage. Notably, a significantly low energy consumption of approximately 0.13 kWh/g was attained under 200 kPa, indicating promising opportunities for refining NTP methods to enhance the mass production of graphene nanoflakes on a large scale [79].
3.3. Partial Oxidation of CH4
Partial oxidation of CH4 by NTP is a promising avenue for producing oxygenates, initiated by electron collision with CH4 and O2 molecules. CH3 and O are key intermediate species produced by plasma. Interestingly, controlling the complete oxidation of CH4 to H2O and CO2 is crucial to maximize the oxygenate yield [80,81,82,83,84,85,86]. Achieving high selectivity, such as gliding arcs near 100% syngas selectivity at an O2/CH4 ratio of 0.6, is vital [81].
Thermal plasma is not favored; instead, DBD plasma is commonly employed. It is essential to manage CH3OH decomposition and selectivity, especially as the residence time affects conversion and selectivity. Optimizing the residence time is critical for maximizing the oxygenate yield while considering specific energy input adjustments, typically controlled via the total gas flow rate at constant power.
Another crucial aspect to consider is the breakdown of CH3OH in plasma and the shifting preference towards complete combustion products as the duration of residence increases, a phenomenon extensively discussed in a review by Indarto et al. [82]. It is worth noting that determining the optimal residence time is essential to maximize the yield of CH3OH or other oxygenates, as there exists a trade-off between CH4 conversion and the selectivity towards organic oxygenates concerning the specific energy input (SEI). The SEI can be fine-tuned by regulating the flow rate of gas under equivalent power conditions, as demonstrated in previous studies [87].
3.4. CH4 Conversion to Syngas
Traditional syngas production methods utilize catalytic reformers, which are associated with high economic investment costs and several drawbacks [83,84]. These drawbacks include the need to activate catalytic sites at high temperatures and low flow rates of inlet gas [85]. To address these issues, alternative technologies such as plasma (both thermal and non-thermal) are gaining traction [86]. Luche et al., investigated the behavior of CH4–air mixtures injected into an NTP reactor at atmospheric pressure and ambient temperature. The research demonstrated successful syngas (H2 and CO) production across a wide range of fuel flow rates and CH4 concentrations in air. Notably, the lowest energy cost for H2 production is approximately 45 kWh/kg (H2) at the highest mass flow rate. The numerical simulations further validate that the NTP reactor achieves optimal electrical power utilization with high mass flow rates and low inlet CH4 concentrations in air [87].
3.5. Plasma Coupling of CH4 and CO2
While most investigations into the simultaneous conversion of CH4 and CO2 have focused on dry reforming processes, some studies have explored the conversion of these gases into liquid chemicals [88,89]. Dry reforming processes are typically conducted in warmer plasmas such as a spark or gliding arcs, whereas research targeting liquid products has predominantly utilized dielectric barrier discharge (DBD) systems [90,91]. Catalysts were strategically positioned around the high-voltage electrode within the central discharge zone of the reactor to capitalize on the synergistic effects of the catalyst and plasma. With an input power of 140 W from DBD plasma and no additional heating, impressive conversion rates of 80.3% for CO2 and 86.4% for CH4 are achieved. Furthermore, the catalyst proves advantageous in producing higher-value C2H4 products under plasma promotion. These findings hold significant implications for optimizing plasma-promoted dry reforming reactions to yield high-value products and are shown in Figure 5 [92].
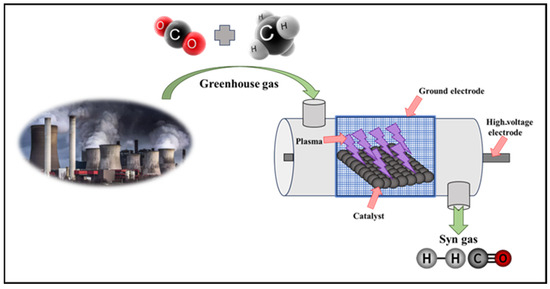
Figure 5.
Plasma coupling of CH4 and CO2 of DBD reactor.
Martini et al. [93] reported the formation of liquid products such as CH3OH and C2H5OH, along with formic, acetic, propanoic, and butanoic acids. The gas products consisted primarily of syngas (89%), with smaller amounts of C-2 (8.5%) and C-3 hydrocarbons (1.5%). The authors suggested that the plasma-induced dissociation of the CH4/CO2 mixture generates the reactive species CH3, H, CO, and O, which subsequently contribute to product formation (Table 3). Additionally, potential mechanisms were proposed through density functional theory (DFT) calculations. For instance, acetic acid could be produced through successive additions of CO and OH to methyl radicals, or via the addition of COOH. It is hypothesized that COOH primarily forms through the recombination of CO and OH radicals.

Table 3.
List of catalysts combined with plasma-assisted DRM.
4. Plasma-Catalysis for Energy Production
NTP technology diverges from conventional thermal catalytic methods by prioritizing the conversion of electrical energy into reactive species and electrons. While this presents a promising alternative for direct CH4 coupling, the gas-phase non-catalytic plasma reaction typically operates through a free radical mechanism, making product selectivity challenging to control [104,105,106]. To address this limitation and enhance methane conversion, significant research has focused on integrating NTP with heterogeneous catalyst systems. Various plasma types, such as dielectric barrier discharge, corona, glow discharge, and gliding arc, have been explored. Studies indicate that manipulating electron energy through reactor design and catalyst selection significantly impacts product selectivity, particularly in DBD and corona discharge setups [107].
The fusion of NTP with catalytic systems opens a promising avenue for directly upgrading CH4 into higher-value products, operating at significantly lower temperatures compared to conventional thermal-catalytic methods [108]. Over the past two decades, extensive experimental investigations have delved into plasma-activated CH4 conversion, both with and without oxidants. These studies, involving a diverse range of catalysts and plasma discharge configurations, consistently showcase the process’s potential [109,110]. However, a common challenge lies in the limited understanding of the mechanistic intricacies underlying the observed performance outcomes. The interplay between plasma and catalyst in plasma-catalysis systems introduces high complexity, emphasizing the need for an increased focus on modeling efforts to elucidate some of the intricate interactions at play [111,112].
Recent research has been directed towards modeling the influence of vibrationally excited species in elucidating the synergistic relationship observed between the plasma and catalyst phases in various chemical processes. For instance, Engelmann et al. [113] conducted a study on the nonoxidative coupling of CH4 over different transition metals utilizing a surface microkinetic model. Their findings indicated a notable enhancement in the turnover frequency of vibrationally excited CH4, particularly with low-activity metals characterized by weak binding. Similarly, Loenders et al. [114] employed a similar methodology to investigate the partial oxidation of CH4 on the Pt surface.
These investigations underscore the necessity for kinetic models that encompass the diverse array of species originating from the plasma phase and the elucidation of their interactions with the catalyst. However, most existing studies have primarily focused on initial rates within a static gas phase framework, where populations of vibrationally excited states were predetermined based on a fixed vibrational temperature distribution. Dynamic studies integrating plasma-kinetic models with catalytic surface models have been limited to the production of ammonia [115,116]. Furthermore, recent research in thermal catalysis by the Dauenhauer group has demonstrated the potential exploitation of dynamical effects to achieve performance surpassing that of steady-state conditions [117].
Combining catalysts with plasma reactors enhances CH4 conversion with improved selectivity for desired products compared to plasma alone. This synergy modifies the surface chemistry, activating reactants at mild conditions and facilitating selective reactions on catalyst surfaces. Plasma generates reactive species and alters catalyst properties, promoting catalyst activation and reducing the activation barrier. Catalysts enhance electric fields, change plasma discharge behavior, and increase adsorption rates, leading to higher product selectivity, catalyst stability, and energy efficiency while reducing carbon formation in DRM [118]. Catalyst placement depends on reactor geometry and temperature, with high-thermal-stability catalysts being suitable for simple plasma reactor geometries.
Noble metal catalysts, notably Pt, coupled with plasma reactors show promise for the thermal DRM reaction. Wang et al. utilized Ag, Pt, Pd, Ir, and Re fixed on CZSM5 and UZSM5 catalysts combined with DBD reactors for biogas reforming, achieving significant liquid product yields (60%) with low carbon deposition (5.1%) [100]. Another Pt catalyst, PtUiO-67, exhibited enhanced CH4 conversion and H2 yield in a DBD reactor, attributed to increased CH4 dissociation and dehydrogenation reactions [20]. In situ DRIFTS studies provided direct evidence for surface reactions under plasma conditions.
Metal oxides such as zeolite [102], La2O3/Al2O3 [119], γ-Al2O3 [120], and CaO [121] show limited activity in the thermally driven dry reforming of methane (DRM). However, when combined with plasma, significant enhancements in conversion and selectivity are observed. For instance, Nguyen et al. demonstrated a 55.9% increase in CO2 conversion and a 67.7% increase in CH4 conversion using a zeolite-packed dielectric barrier discharge (DBD) plasma reactor [102]. Zeolite also improved CO selectivity by 45.3% by enhancing CO2 decomposition. Zeolite A was found to suppress carbon deposition and enhance C2-C4 selectivity [102]. La2O3/Al2O3 in a DBD reactor increased CH4 conversion three-fold due to surface oxygen species aiding in CH4 activation. La2O3/γ-Al2O3 combined with pulse corona plasma produced C2 hydrocarbons with notable yields [121]. γ-Al2O3 facilitated CH4 adsorption and activation under plasma, leading to the formation of various hydrocarbon products [122]. Additionally, decreasing the CaO grain size in DBD reactors increased CH4 and CO2 conversions by 14% and 22%, respectively, attributed to enhanced gas-phase reactions favoring ethane formation [122].
Photocatalytic DRM typically suffers from low reaction rates and syngas yield due to inefficient solar energy utilization. However, packing photocatalysts into plasma reactors can induce a synergistic effect called SEPC, improving energy efficiency (EE), selectivity, and stability. Chung et al. demonstrated this by packing perovskite photocatalysts into a spark discharge reactor, resulting in a 42% increase in syngas production efficiency compared to plasma alone [101]. The plasma reduces particle size, extends the lifetime of electron-hole pairs, and increases the specific surface area, leading to the faster diffusion of electron-hole pairs and enhanced reduction and oxidation rates. Moreover, the packing of photocatalyst increases the plasma current density, facilitating the dissociation of CO2 and CH4. Additionally, Liu’s group showed that DBD plasma can alter the surface structure of catalysts, inhibiting certain reactions and improving carbon resistance in DRM reactions [122].
5. Conclusions
The integration of non-thermal plasma (NTP) with heterogeneous catalytic systems represents a transformative approach for CH4 conversion, offering significant advantages over conventional thermal catalytic methods. NTP prioritizes the generation of energetic electrons rather than heating gases, enabling reactions at lower temperatures. However, challenges in product selectivity due to the gas-phase non-catalytic plasma reaction’s free radical mechanism have spurred extensive research into combining NTP with catalysts. Studies have demonstrated that this synergy enhances CH4 conversion and selectivity, leveraging plasma-generated reactive species and catalyst-induced modifications to the surface chemistry. Advanced reactor designs, coupled with active catalysts, have shown significant potential, enabling selective product formation, increased energy efficiency, and reduced carbon deposition in CH4 reforming processes.
Despite these advancements, the complex interplay between plasma and catalysts remains a critical research focus. Investigations into plasma–catalyst systems have highlighted the importance of developing kinetic models to better understand the dynamic interactions at play, including the influence of vibrationally excited species and plasma-generated radicals. Experimental and modeling studies suggest that optimizing the plasma discharge configurations, catalyst compositions, and placement can further enhance performance. Additionally, incorporating photocatalysts within plasma reactors has revealed a promising avenue for improving energy efficiency and reaction stability. By addressing the current limitations in mechanistic understanding and reactor design, this emerging field holds the potential to revolutionize CH4 conversion technologies, enabling the sustainable production of high-value chemicals and fuels.
Author Contributions
Conceptualization, S.L. and A.A.A.; methodology, S.L. and A.A.A.; writing—original draft preparation, S.M., H.M. and N.B.; writing—review and editing, S.L., L.K. and A.A.A.; supervision, S.L. and A.A.A.; project administration, S.L. and A.A.A.; funding acquisition, A.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2503).
Data Availability Statement
All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Iaquaniello, G.; Salladini, A.; Palo, E.; Centi, G. Catalytic partial oxidation coupled with membrane purification to improve resource and energy efficiency in syngas production. ChemSusChem 2015, 8, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K. How Methanogenic Archaea Contribute to Climate Change; American Society for Microbiology: Washington, DC, USA, 2022. [Google Scholar]
- Hayhoe, K.; Kheshgi, H.S.; Jain, A.K.; Wuebbles, D.J. Substitution of natural gas for coal: Climatic effects of utility sector emissions. Clim. Change 2002, 54, 107–139. [Google Scholar] [CrossRef]
- Kargbo, D.M.; Wilhelm, R.G.; Campbell, D.J. Natural gas plays in the Marcellus shale: Challenges and potential opportunities. Environ. Sci. Technol. 2010, 44, 5679–5684. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-G.; Li, X.-S. Research progress on methane production from natural gas hydrates. RSC Adv. 2015, 5, 54672–54699. [Google Scholar] [CrossRef]
- Ceri, H.; Conrad, S.; Hermans, I. Oxidative methane upgrading. ChemSusChem 2012, 5, 1668–1686. [Google Scholar]
- Webb, J.R.; Tamara, B.; Brent, G.T. Catalytic oxy-functionalization of methane and other hydrocarbons: Fundamental advancements and new strategies. ChemSusChem 2011, 4, 37–49. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Wan Daud, W.M.A.; Abbas, H.F. Production of greenhouse gas free hydrogen by thermocatalytic decomposition of methane–A review. Renew. Sustain. Energy Rev. 2015, 44, 221–256. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Peppel, T.; Seeburg, D.; Kondratenko, V.A.; Kalevaru, N.; Martin, A.; Wohlrab, S. Methane conversion into different hydrocarbons or oxygenates: Current status and future perspectives in catalyst development and reactor operation. Catal. Sci. Technol. 2017, 7, 366–381. [Google Scholar] [CrossRef]
- Mierczynski, P.; Vasilev, K.; Mierczynska, A.; Maniukiewicz, W.; Ciesielski, R.; Rogowski, J.; Szynkowska, I.M.; Trifonov, A.Y.; Dubkov, S.V.; Gromov, D.G.; et al. The effect of gold on modern bimetallic Au–Cu/MWCNT catalysts for the oxy-steam reforming of methanol. Catal. Sci. Technol. 2016, 6, 4168–4183. [Google Scholar] [CrossRef]
- Olah, G.A.; Richard, H.S. Chemistry in super acids. I. Hydrogen exchange and polycondensation of methane and alkanes in FSO3H-SbF5 (“magic acid”) solution. Protonation of alkanes and the intermediacy of CH5+ and related hydrocarbon ions. The high chemical reactivity of “paraffins” in ionic solution reactions. J. Am. Chem. Soc. 1968, 90, 2726–2727. [Google Scholar]
- Olah, G.A.; Gilles, K.; Schlosberg, R.H. Super acids. III. Protonation of alkanes and intermediacy of alkanonium ions, pentacoordinated carbon cations of CH5+ type. Hydrogen exchange, protolytic cleavage, hydrogen abstraction; polycondensation of methane, ethane, 2, 2-dimethylpropane and 2, 2, 3, 3-tetramethylbutane in FSO3H-SbF5. J. Am. Chem. Soc. 1969, 91, 3261–3268. [Google Scholar]
- Howarth, R.W.; Santoro, R.; Ingraffea, A. Methane and the greenhouse-gas footprint of natural gas from shale formations: A letter. Clim. Change 2011, 106, 679–690. [Google Scholar] [CrossRef]
- Yu, B.; Liang-Nian, H. Upgrading carbon dioxide by incorporation into heterocycles. ChemSusChem 2015, 8, 52–62. [Google Scholar] [CrossRef]
- Guo, X.; Fang, G.; Li, G.; Ma, H.; Fan, H.; Yu, L.; Ma, C.; Wu, X.; Deng, D.; Wei, M.; et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 2014, 344, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, T.V.; Erhan, A.; Wayne, G.D. Nonoxidative activation of methane. Catal. Rev. 2003, 45, 151–203. [Google Scholar] [CrossRef]
- Holmen, A. Direct conversion of methane to fuels and chemicals. Catal. Today 2009, 142, 2–8. [Google Scholar] [CrossRef]
- Alvarez-Galvan, M.C.; Mota, N.; Ojeda, M.; Rojas, S.; Navarro, R.M.; Fierro, J.L. Direct methane conversion routes to chemicals and fuels. Catal. Today 2011, 171, 15–23. [Google Scholar] [CrossRef]
- Annapragada, A.V.; Erdogan, G. Fe P0 catalysts for methane utilization—Catalyst development and identification. J. Catal. 1990, 123, 130–146. [Google Scholar] [CrossRef]
- Horn, R.; Robert, S. Methane activation by heterogeneous catalysis. Catal. Lett. 2015, 145, 23–39. [Google Scholar] [CrossRef]
- Da Silva, M.J. Synthesis of methanol from methane: Challenges and advances on the multi-step (syngas) and one-step routes (DMTM). Fuel Process. Technol. 2016, 145, 42–61. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Matus, E.V.; Kerzhentsev, M.A.; Tsikoza, L.T.; Ismagilov, I.Z.; Dosumov, K.D.; Mustafin, A.G. Methane conversion to valuable chemicals over nanostructured Mo/ZSM-5 catalysts. Pet. Chem. 2011, 51, 174–186. [Google Scholar] [CrossRef]
- Specchia, S. Fuel processing activities at European level: A panoramic overview. Int. J. Hydrogen Energy 2014, 39, 17953–17968. [Google Scholar] [CrossRef]
- Baig, S.; Sajjadi, B. Non-thermal plasma enhanced catalytic conversion of methane into value added chemicals and fuels. J. Energy Chem. 2024, 97, 265–301. [Google Scholar] [CrossRef]
- Qin, W.; Wu, H.; Chen, Q.; Sun, J.; Liu, N.; Liu, B.; Zhang, M. From electric field catalysis to plasma catalysis: A combined experimental study and kinetic modeling to understand the synergistic effects in methane dry reforming. Chem. Eng. J. 2025, 508, 161015. [Google Scholar] [CrossRef]
- Gharahshiran, V.S.; Zheng, Y. Synergistic plasma and platinum catalysts interactions in CO2 reforming of propane at room temperature: The role of supports. Chem. Eng. J. 2024, 499, 156492. [Google Scholar] [CrossRef]
- Kim, J.; Abbott, M.S.; Go, D.B.; Hicks, J.C. Enhancing C–H bond activation of methane via temperature-controlled, catalyst–plasma interactions. ACS Energy Lett. 2016, 1, 94–99. [Google Scholar] [CrossRef]
- Feng, G.; Zhao, Y.; Ma, C.; Chen, W.; Li, T.; Zhao, X.; Li, G.; Dong, X.; Song, Y.; Wei, W.; et al. Solar driven efficient direct conversion of methane to multicarbon oxygenates. J. Mater. Chem. A 2022, 10, 7856–7868. [Google Scholar] [CrossRef]
- Abuseada, M.; Fisher, T.S. Continuous solar-thermal methane pyrolysis for hydrogen and graphite production by roll-to-roll processing. Appl. Energy 2023, 352, 121872. [Google Scholar] [CrossRef]
- Agrafiotis, C.; von Storch, H.; Roeb, M.; Sattler, C. Solar thermal reforming of methane feedstocks for hydrogen and syngas production—A review. Renew. Sustain. Energy Rev. 2014, 29, 656–682. [Google Scholar] [CrossRef]
- Lee, J.; Yang, J.; Moon, J.H. Solar cell-powered electrochemical methane-to-methanol conversion with CuO/CeO2 catalysts. ACS Energy Lett. 2021, 6, 893–899. [Google Scholar] [CrossRef]
- Liang, H.; Wang, F.; Yang, L.; Cheng, Z.; Shuai, Y.; Tan, H. Progress in full spectrum solar energy utilization by spectral beam splitting hybrid PV/T system. Renew. Sustain. Energy Rev. 2021, 141, 110785. [Google Scholar] [CrossRef]
- Hameed, A.; Ismail, I.M.; Aslam, M.; Gondal, M.A. Photocatalytic conversion of methane into methanol: Performance of silver impregnated WO3. Appl. Catal. A Gen. 2014, 470, 327–335. [Google Scholar] [CrossRef]
- Li, A.; Ming, T.; Xiong, H.; Wu, Y.; Shi, T.; Li, W.; de Richter, R.; Chen, Y.; Tang, X.; Yuan, Y. A high-performance solar chimney in building integrated with photocatalytic technology for atmospheric methane removal. Solar Energy 2023, 260, 126–136. [Google Scholar] [CrossRef]
- Dong, C.; Marinova, M.; Tayeb, K.B.; Safonova, O.V.; Zhou, Y.; Hu, D.; Chernyak, S.; Corda, M.; Zaffran, J.; Khodakov, A.Y.; et al. Direct photocatalytic synthesis of acetic acid from methane and CO at ambient temperature using water as oxidant. J. Am. Chem. Soc. 2023, 145, 1185–1193. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, B.; Li, L.; Yu, K.; Zhang, Q.; Lv, Z.; Tang, D. A high temperature tubular reactor with hybrid concentrated solar and electric heat supply for steam methane reforming. Chem. Eng. J. 2022, 428, 132073. [Google Scholar] [CrossRef]
- Hwang, I.Y.; Nguyen, A.D.; Nguyen, T.T.; Nguyen, L.T.; Lee, O.K.; Lee, E.Y. Biological conversion of methane to chemicals and fuels: Technical challenges and issues. Appl. Microbiol. Biotechnol. 2018, 102, 3071–3080. [Google Scholar] [CrossRef]
- Sahoo, K.K.; Goswami, G.; Das, D. Biotransformation of methane and carbon dioxide into high-value products by methanotrophs: Current state of art and future prospects. Front. Microbiol. 2021, 12, 636486. [Google Scholar] [CrossRef]
- Guo, S.; Nguyen, D.T.; Chau, T.H.; Fei, Q.; Lee, E.Y. Systems Metabolic Engineering of Methanotrophic Bacteria for Biological Conversion of Methane to Value-Added Compounds. In One-Carbon Feedstocks for Sustainable Bioproduction; Springer International Publishing: Cham, Switzerland, 2022; pp. 91–126. [Google Scholar]
- Patel, S.K.; Gupta, R.K.; Kondaveeti, S.; Otari, S.V.; Kumar, A.; Kalia, V.C.; Lee, J.K. Conversion of biogas to methanol by methanotrophs immobilized on chemically modified chitosan. Bioresour. Technol. 2020, 315, 123791. [Google Scholar] [CrossRef]
- Orhan, M.F.; Dincer, I.; Rosen, M.A.; Kanoglu, M. Integrated hydrogen production options based on renewable and nuclear energy sources. Renew. Sustain. Energy Rev. 2012, 16, 6059–6082. [Google Scholar] [CrossRef]
- Kugler, K.; Niessen, H.F.; Roth-Kamat, M.; Bocker, D.; Ruter, B.; Theis, K.A. Transport of nuclear heat by means of chemical energy. Nucl. Eng. Des. 1975, 34, 65. [Google Scholar] [CrossRef]
- Nakagawa, S.; Tachibana, Y.; Takamatsu, K.; Ueta, S.; Hanawa, S. Performance test of HTTR. Nucl. Eng. Des. 2004, 233, 291–300. [Google Scholar] [CrossRef]
- Shirani, A.; Gu, J.; Wei, B.; Lee, J.; Aouadi, S.M.; Berman, D. Tribologically enhanced self-healing of niobium oxide surfaces. Surf. Coat. Technol. 2019, 364, 273–278. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Gu, J.; Berman, D. Self-healing ceramic coatings that operate in extreme environments: A review. J. Vac. Sci. Technol. A 2020, 38, 050802. [Google Scholar] [CrossRef]
- Ramirez, G.; Eryilmaz, O.L.; Fatti, G.; Righi, M.C.; Wen, J.; Erdemir, A. Tribochemical conversion of methane to graphene and other carbon nanostructures: Implications for friction and wear. ACS Appl. Nano Mater. 2020, 3, 8060–8067. [Google Scholar] [CrossRef]
- Chen, L.; Wu, J.; Lu, Z.; Shang, L.; Zhang, G.; Xue, Q. Probing the lubrication of shear-induced self-assembled layer on amorphous carbon films in methane atmosphere. Tribol. Lett. 2022, 70, 18. [Google Scholar] [CrossRef]
- Palau, J.; Assadi, A.A.; Penya-roja, J.M.; Bouzaza, A.; Wolbert, D.; Martínez-Soria, V. Isovaleraldehyde degradation using UV photocatalytic and dielectric barrier discharge reactors, and their combinations. J. Photochem. Photobiol. A Chem. 2015, 299, 110–117. [Google Scholar] [CrossRef]
- Kobashi, K.; Nishimura, K.; Kawate, Y.; Horiuchi, T. Synthesis of diamonds by use of microwave plasma chemical-vapor deposition: Morphology and growth of diamond films. Phys. Rev. B 1988, 38, 4067. [Google Scholar] [CrossRef] [PubMed]
- Jody, B.J.; Doctor, R.D.; Petchsingto, T.; Snyder, S.W. Concept for production of chemicals and power using geothermal energy. Appl. Therm. Eng. 2013, 58, 564–569. [Google Scholar] [CrossRef]
- Li, K.; Garrison, G.; Zhu, Y.; Moore, M.; Liu, C.; Hepper, J.; Bandt, L.; Horne, R.; Petty, S. Thermoelectric power generator: Field test at Bottle Rock geothermal power plant. J. Power Sources 2021, 485, 229266. [Google Scholar] [CrossRef]
- Assareh, E.; Alirahmi, S.M.; Ahmadi, P. A Sustainable model for the integration of solar and geothermal energy boosted with thermoelectric generators (TEGs) for electricity, cooling and desalination purpose. Geothermics 2021, 92, 102042. [Google Scholar]
- Gu, R.; Ding, J.; Wang, Y.; Yuan, Q.; Wang, W.; Lu, J. Heat transfer and storage performance of steam methane reforming in tubular reactor with focused solar simulator. Appl. Energy 2019, 233, 789–801. [Google Scholar] [CrossRef]
- Luo, L.; Gong, Z.; Xu, Y.; Ma, J.; Liu, H.; Xing, J.; Tang, J. Binary Au–Cu reaction sites decorated ZnO for selective methane oxidation to C1 oxygenates with nearly 100% selectivity at room temperature. J. Am. Chem. Soc. 2021, 144, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Conrado, R.J.; Gonzalez, R. Envisioning the bioconversion of methane to liquid fuels. Science 2014, 343, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Weng, G.M. Sustainable Energy Resources for Driving Methane Conversion. Adv. Energy Mater. 2023, 13, 2301734. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Jansen, I.; Christus, J.S. Renewable energy based catalytic CH4 conversion to fuels. Catal. Sci. Technol. 2014, 4, 2397–2411. [Google Scholar] [CrossRef]
- Wang, B.; Albarracín-Suazo, S.; Pagán-Torres, Y.; Nikolla, E. Advances in methane conversion processes. Catal. Today 2017, 285, 147–158. [Google Scholar] [CrossRef]
- George, A.; Shen, B.; Craven, M.; Wang, Y.; Kang, D.; Wu, C.; Tu, X. A Review of Non-Thermal Plasma Technology: A novel solution for CO2 conversion and utilization. Renew. Sustain. Energy Rev. 2021, 135, 109702. [Google Scholar] [CrossRef]
- Puliyalil, H.; Jurković, D.L.; Dasireddy, V.D.; Likozar, B. A review of plasma-assisted catalytic conversion of gaseous carbon dioxide and methane into value-added platform chemicals and fuels. RSC Adv. 2018, 8, 27481–27508. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, C.; Bai, H.; Zhang, S.; Ostrikov, K.K.; Shao, T. Energy pooling mechanism for catalyst-free methane activation in nanosecond pulsed non-thermal plasmas. Chem. Eng. J. 2020, 396, 125185. [Google Scholar] [CrossRef]
- Khalifeh, O.; Mosallanejad, A.; Taghvaei, H.; Rahimpour, M.R.; Shariati, A. Decomposition of methane to hydrogen using nanosecond pulsed plasma reactor with different active volumes.; voltages and frequencies. Appl. Energy 2016, 169, 585–596. [Google Scholar] [CrossRef]
- Lotfalipour, R.; Ghorbanzadeh, A.M.; Mahdian, A. Methane conversion by repetitive nanosecond pulsed plasma. J. Phys. D Appl. Phys. 2014, 47, 365201. [Google Scholar] [CrossRef]
- Mei, D.; Duan, G.; Fu, J.; Liu, S.; Zhou, R.; Zhou, R.; Fang, Z.; Cullen, P.J.; Ostrikov, K.K. CO2 reforming of CH4 in single and double dielectric barrier discharge reactors: Comparison of discharge characteristics and product distribution. J. CO2 Util. 2021, 53, 101703. [Google Scholar] [CrossRef]
- Yap, D.; Tatibouët, J.M.; Batiot-Dupeyrat, C. Carbon dioxide dissociation to carbon monoxide by non-thermal plasma. J. CO2 Util. 2015, 12, 54–61. [Google Scholar] [CrossRef]
- Alawi, N.M.; Barifcani, A.; Abid, H.R. Optimisation of CH4 and CO2 conversion and selectivity of H2 and CO for the dry reforming of methane by a microwave plasma technique using a B ox–B ehnken design. Asia-Pac. J. Chem. Eng. 2018, 13, e2254. [Google Scholar] [CrossRef]
- Scapinello, M.; Martini, L.M.; Dilecce, G.; Tosi, P. Conversion of CH4/CO2 by a nanosecond repetitively pulsed discharge. J. Phys. D Appl. Phys. 2016, 49, 075602. [Google Scholar] [CrossRef]
- Slaets, J.; Aghaei, M.; Ceulemans, S.; Van Alphen, S.; Bogaerts, A. CO2 and CH4 conversion in “real gas mixtures in a gliding arc plasmatron: How do N2 and O2 affect the performance? Green Chem. 2020, 22, 1366–1377. [Google Scholar] [CrossRef]
- Wang, X.; Ning-Shi-Ya, N.; Qian, T.C.; Zhou, S. Plasma-Assisted Dry Reforming of Methane over the Ti–Ni Catalysts. Ind. Eng. Chem. Res. 2025, 64, 2626–2635. [Google Scholar] [CrossRef]
- Rutberg, P.G.; Nakonechny, G.V.; Pavlov, A.V.; Popov, S.D.; Serba, E.O.; Surov, A.V. AC plasma torch with a H2O/CO2/CH4 mix as the working gas for methane reforming. J. Phys. D Appl. Phys. 2015, 48, 245204. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Nasonova, A.; Nah, I.W.; Kim, K.S. Analysis on CO2 reforming of CH4 by corona discharge process for various process variables. J. Ind. Eng. Chem. 2015, 32, 58–62. [Google Scholar] [CrossRef]
- Chun, S.M.; Hong, Y.C.; Choi, D.H. Reforming of methane to syngas in a microwave plasma torch at atmospheric pressure. J. CO2 Util. 2017, 19, 221–229. [Google Scholar] [CrossRef]
- Montesano, C.; Faedda, M.; Martini, L.M.; Dilecce, G.; Tosi, P. CH4 reforming with CO2 in a nanosecond pulsed discharge. The importance of the pulse sequence. J. CO2 Util. 2021, 49, 101556. [Google Scholar] [CrossRef]
- Wanten, B.; Maerivoet, S.; Vantomme, C.; Slaets, J.; Trenchev, G.; Bogaerts, A. Dry reforming of methane in an atmospheric pressure glow discharge: Confining the plasma to expand the performance. J. CO2 Util. 2022, 56, 101869. [Google Scholar] [CrossRef]
- Zhu, B.; Li, X.S.; Liu, J.L.; Zhu, X.; Zhu, A.M. Kinetics study on carbon dioxide reforming of methane in kilohertz spark-discharge plasma. Chem. Eng. J. 2015, 264, 445–452. [Google Scholar] [CrossRef]
- Wu, A.; Yan, J.; Zhang, H.; Zhang, M.; Du, C.; Li, X. Study of the dry methane reforming process using a rotating gliding arc reactor. Int. J. Hydrogen Energy 2014, 39, 17656–17670. [Google Scholar] [CrossRef]
- Iwarere, S.A.; Rohani, V.J.; Ramjugernath, D.; Fulcheri, L. Dry reforming of methane in a tip–tip arc discharge reactor at very high pressure. Int. J. Hydrogen Energy 2015, 40, 3388–3401. [Google Scholar] [CrossRef]
- Mohanta, A.; Lanfant, B.; Asfaha, M.; Leparoux, M. Methane dissociation process in inductively coupled Ar/H2/CH4 plasma for graphene nano-flakes production. Appl. Phys. Lett. 2017, 110, 093109. [Google Scholar] [CrossRef]
- Wu, A.; Li, X.; Yan, J.; Yang, J.; Du, C.; Zhu, F.; Qian, J. Co-generation of hydrogen and carbon aerosol from coalbed methane surrogate using rotating gliding arc plasma. Appl. Energy 2017, 195, 67–79. [Google Scholar] [CrossRef]
- Lee, D.H.; Song, Y.H.; Kim, K.T.; Lee, J.O. Comparative study of methane activation process by different plasma sources. Plasma Chem. Plasma Process. 2013, 33, 647–661. [Google Scholar] [CrossRef]
- Indarto, A. A review of direct methane conversion to methanol by dielectric barrier discharge. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 1038–1043. [Google Scholar] [CrossRef]
- Deluga, G.A.; Salge, J.R.; Schmidt, L.D.; Verykios, X.E. Renewable hydrogen from ethanol by autothermal reforming. Science 2004, 303, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Paulmier, T.; Fulcheri, L. Use of non-thermal plasma for hydrocarbon reforming. Chem. Eng. J. 2005, 106, 59–71. [Google Scholar] [CrossRef]
- Indarto, A.; Choi, J.W.; Lee, H.; Song, H.K. Kinetic modeling of plasma methane conversion using gliding arc. J. Nat. Gas. Chem. 2005, 14, 13–21. [Google Scholar]
- Petitpas, G.; Rollier, J.D.; Darmon, A.; Gonzalez-Aguilar, J.; Metkemeijer, R.; Fulcheri, L. A comparative study of non-thermal plasma assisted reforming technologies. Int. J. Hydrogen Energy 2007, 32, 2848–2867. [Google Scholar] [CrossRef]
- Luche, J.; Aubry, O.; Khacef, A.; Cormier, J.M. Syngas production from methane oxidation using a non-thermal plasma: Experiments and kinetic modeling. Chem. Eng. J. 2009, 149, 35–41. [Google Scholar] [CrossRef]
- Gao, B.; Cao, G.; Feng, Y.; Jiao, Y.; Li, C.; Zhao, J.; Fang, Y. CO2 Reforming of Biomass Gasification Tar over Ni-Fe-Based Catalysts in a DBD Plasma Reactor. Molecules 2025, 30, 1032. [Google Scholar] [CrossRef]
- Jiao, H.; Wang, G.-C. AComprehensive Theoretical Study of the Mechanism for Dry Reforming of Methane on a Ni4/ZrO2(101) Catalyst Under External Electric Fields: The Role of Interface and Oxygen Vacancy. ACS Catal. 2025, 15, 3846–3859. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Lemasle, M.; Wolbert, D. Removal of trimethylamine and isovaleric acid from gas streams in a continuous flow surface discharge plasma reactor. Chem. Eng. Res. Des. 2015, 93, 640–651. [Google Scholar] [CrossRef]
- Cleiren, E.; Heijkers, S.; Ramakers, M.; Bogaerts, A. Dry reforming of methane in a gliding arc plasmatron: Towards a better understanding of the plasma chemistry. ChemSusChem 2017, 10, 4025–4036. [Google Scholar] [CrossRef]
- Lin, S.S.; Li, P.R.; Jiang, H.B.; Diao, J.F.; Xu, Z.N.; Guo, G.C. Plasma promotes dry reforming reaction of CH4 and CO2 at room temperature with highly dispersed NiO/γ-Al2O3 catalyst. Catalysts 2021, 11, 1433. [Google Scholar] [CrossRef]
- Martini, L.M.; Dilecce, G.; Guella, G.; Maranzana, A.; Tonachini, G.; Tosi, P. Oxidation of CH4 by CO2 in a dielectric barrier discharge. Chem. Phys. Lett. 2014, 593, 55–60. [Google Scholar] [CrossRef]
- Liu, J.L.; Li, Z.; Liu, J.H.; Li, K.; Lian, H.Y.; Li, X.S.; Zhu, X.; Zhu, A.M. Warm-plasma catalytic reduction of CO2 with CH4. Catal. Today 2019, 330, 54–60. [Google Scholar] [CrossRef]
- Xin, Z.; Li, X.; Zheng, Z.; Hu, Y.; Cao, R.; Sun, A.; Zhao, F.; Yu, W. Study on the kinetics and energy transfer during ignition of methane excited by NRP-SDBD non-equilibrium plasma. J. Energy Inst. 2025, 118, 101929. [Google Scholar] [CrossRef]
- Hrycak, B.; Czylkowski, D.; Jasiński, M.; Dors, M.; Mizeraczyk, J. Hydrogen production via synthetic biogas reforming in atmospheric-pressure microwave (915 MHz) plasma at high gas-flow output. Plasma Chem. Plasma Process. 2019, 39, 695–711. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Zhang, S.; Sun, H.; Li, J.; Shao, T. Nanosecond pulsed plasma assisted dry reforming of CH4: The effect of plasma operating parameters. Appl. Energy 2019, 243, 132–144. [Google Scholar] [CrossRef]
- Vakili, R.; Gholami, R.; Stere, C.E.; Chansai, S.; Chen, H.; Holmes, S.M.; Jiao, Y.; Hardacre, C.; Fan, X. Plasma-assisted catalytic dry reforming of methane (DRM) over metal-organic frameworks (MOFs)-based catalysts. Appl. Catal. B Environ. 2020, 260, 118195. [Google Scholar] [CrossRef]
- Chung, W.C.; Tsao, I.Y.; Chang, M.B. Novel plasma photocatalysis process for syngas generation via dry reforming of methane. Energy Convers. Manag. 2018, 164, 417–428. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Kim, K.S. Combination of plasmas and catalytic reactions for CO2 reforming of CH4 by dielectric barrier discharge process. Catal. Today 2015, 256, 88–95. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A. Cold plasma dielectric barrier discharge reactor for dry reforming of methane over Ni/ɤ-Al2O3-MgO nanocomposite. Fuel Process. Technol. 2018, 178, 166–179. [Google Scholar] [CrossRef]
- Tu, X.; Whitehead, J.C. Plasma-catalytic dry reforming of methane in an atmospheric dielectric barrier discharge: Understanding the synergistic effect at low temperature. Appl. Catal. B Environ. 2012, 125, 439–448. [Google Scholar] [CrossRef]
- Mei, D.; Ashford, B.; He, Y.L.; Tu, X. Plasma-catalytic reforming of biogas over supported Ni catalysts in a dielectric barrier discharge reactor: Effect of catalyst supports. Plasma Process. Polym. 2017, 14, 1600076. [Google Scholar] [CrossRef]
- Wang, C.; Jie, X.; Qiu, Y.; Zhao, Y.; Al-Megren, H.A.; Alshihri, S.; Edwards, P.P.; Xiao, T. The importance of inner cavity space within Ni@ SiO2 nanocapsule catalysts for excellent coking resistance in the high-space-velocity dry reforming of methane. Appl. Catal. B Environ. 2019, 259, 118019. [Google Scholar] [CrossRef]
- Li, Z.; Das, S.; Hongmanorom, P.; Dewangan, N.; Wai, M.H.; Kawi, S. Silica-based micro-and mesoporous catalysts for dry reforming of methane. Catal. Sci. Technol. 2018, 8, 2763–2778. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, W.; Li, S.; Wu, G.; Ma, X.; Wang, X.; Gong, J. Sintering-resistant Ni-based reforming catalysts obtained via the nanoconfinement effect. Chem. Commun. 2013, 49, 9383–9385. [Google Scholar] [CrossRef]
- Ashok, J.; Kawi, S. Nickel–iron alloy supported over iron–alumina catalysts for steam reforming of biomass tar model compound. ACS Catal. 2014, 4, 289–301. [Google Scholar] [CrossRef]
- Banos, R.; Manzano-Agugliaro, F.; Montoya, F.G.; Gil, C.; Alcayde, A.; Gómez, J. Optimization methods applied to renewable and sustainable energy: A review. Renew. Sustain. Energy Rev. 2011, 15, 1753–1766. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Hamid, M.Y. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Dębek, R.; Radlik, M.; Motak, M.; Galvez, M.E.; Turek, W.; Da Costa, P.; Grzybek, T. Ni-containing Ce-promoted hydrotalcite derived materials as catalysts for methane reforming with carbon dioxide at low temperature–on the effect of basicity. Catal. Today 2015, 257, 59–65. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Influence of Ce/Zr molar ratio on catalytic performance of hydrotalcite-derived catalysts at low temperature CO2 methane reforming. Int. J. Hydrogen Energy 2017, 42, 23556–23567. [Google Scholar] [CrossRef]
- Li, Z.; Mo, L.; Kathiraser, Y.; Kawi, S. Yolk–satellite–shell structured Ni–yolk@ Ni@ SiO2 nanocomposite: Superb catalyst toward methane CO2 reforming reaction. ACS Catal. 2014, 4, 1526–1536. [Google Scholar] [CrossRef]
- Li, Z.; Yasotha, K.; Sibudjing, K. Facile synthesis of high surface area yolk–shell Ni@ Ni embedded SiO2 via Ni phyllosilicate with enhanced performance for CO2 reforming of CH4. ChemCatChem 2015, 7, 160–168. [Google Scholar] [CrossRef]
- Li, Z.; Kathiraser, Y.; Ashok, J.; Oemar, U.; Kawi, S. Simultaneous tuning porosity and basicity of nickel@ nickel–magnesium phyllosilicate core–shell catalysts for CO2 reforming of CH4. Langmuir 2014, 30, 14694–14705. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sibudjing, K. Multi-Ni@ Ni phyllosilicate hollow sphere for CO2 reforming of CH4: Influence of Ni precursors on structure, sintering, and carbon resistance. Catal. Sci. Technol. 2018, 8, 1915–1922. [Google Scholar] [CrossRef]
- Li, Z.; Kawi, S. Facile Synthesis of Multi-Ni-Core@ Ni Phyllosilicate@ CeO2 Shell Hollow Spheres with High Oxygen Vacancy Concentration for Dry Reforming of CH4. ChemCatChem 2018, 10, 2994–3001. [Google Scholar] [CrossRef]
- Kobayashi, T.; Furuya, T.; Fujitsuka, H.; Tago, T. Synthesis of Birdcage-type zeolite encapsulating ultrafine Pt nanoparticles and its application in dry reforming of methane. Chem. Eng. J. 2019, 377, 120203. [Google Scholar] [CrossRef]
- Neyts, E.C.; Bogaerts, A. Understanding plasma catalysis through modelling and simulation—A review. J. Phys. D Appl. Phys. 2014, 47, 224010. [Google Scholar] [CrossRef]
- Yap, D.; Tatibouët, J.-M.; Batiot-Dupeyrat, C. Catalyst assisted by non-thermal plasma in dry reforming of methane at low temperature. Catal. Today 2018, 299, 263–271. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, B.; Zhu, A.; Gong, W.; Liu, C. The simultaneous activation of methane and carbon dioxide to C2 hydrocarbons under pulse corona plasma over La2O3/γ-Al2O3 catalyst. Catal. Today 2002, 72, 223–227. [Google Scholar] [CrossRef]
- Bouchoul, N.; Fourré, E.; Tatibouët, J.M.; Batiot-Dupeyrat, C. Plasma-catalytic dry reforming of CH4 over calcium oxide: Catalyst structural and textural modifications. Plasma Chem. Plasma Process. 2019, 39, 713–727. [Google Scholar] [CrossRef]
- Hu, X.; Jia, X.; Zhang, X.; Liu, Y.; Liu, C.J. Improvement in the activity of Ni/ZrO2 by cold plasma decomposition for dry reforming of methane. Catal. Commun. 2019, 128, 105720. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).