Abstract
In conventionally used carbon-supported heterogeneous platinum catalysts for hydrogen evolution reaction (HER), low Pt utilization efficiency and poor stability, resulting from weak interactions with the carbon supports, are crucial issues. Here, we report a novel hierarchical structure of TiOx nanoparticles embedded in porous carbon with the in situ growth of highly dispersed Pt on the TiOx surface (Pt-TiOx@C). The as-prepared Pt-TiOx@C electrocatalyst showed excellent catalytic activity during HER with an overpotential of only 10 mV when the current density reached 10 mA cm−2 and the mass activity was 9.24 A mgPt−1 at an overpotential of 30 mV in 0.5 M H2SO4 solution, thus outperforming commercial Pt/C catalysts. Furthermore, it also exhibited highly stable catalytic activity over 10,000 CV cycles of an accelerated degradation test (ADT). This high HER activity and durability could be ascribed to the highly dispersed Pt feature and the strong metal–support interaction (SMSI) between Pt and TiOx. This study also provides a simple and effective method for designing highly active and stable electrocatalysts.
1. Introduction
Hydrogen is regarded as one of the most promising clean energy carriers that could replace fossil fuels, possessing a high gravimetric energy density, good renewability, and zero-pollutant emissions [1,2]. Of current hydrogen production techniques, electrocatalytic water splitting, which involves a hydrogen evolution reaction (HER) and an oxygen evolution reaction (OER), is considered the most low-cost and sustainable strategy [3,4]. The HER, an electrochemical process used to reduce H3O+ (acidic) or H2O (alkaline) to H2, is highly dependent on the electrocatalyst to accelerate the reaction kinetics and increase the energy conversion efficiency of the whole water splitting [5]. Until now, platinum (Pt) has been regarded as the most active electrocatalyst for HER, owing to its nearly zero Gibbs free energy in intermediate adsorptions (ΔGH* = 0.05 eV) [6,7,8]. For example, the platinum nanoparticles supported on carbon nanotubes (Pt/CNT-V_) exhibited an overpotential of 45 mV at a current density of 10 mA cm−2 and a mass activity of 3.28 A mg−1 for the HER in acidic media [9]. However, the scarcity of element Pt, resulting in the high cost of Pt-based electrocatalysts, has seriously hindered its large-scale applications. Thus, it is very important to increase the utilization of Pt and enhance the intrinsic activity of each Pt site [10,11].
Reducing particle size or morphology control are valid approaches for improving the utilization rate and thus reducing the cost of developing Pt-based electrocatalysts [12,13,14,15]. For example, Wei et al. [16] reported a spinel Mn3O4-supported Pt SAC (PtSA-Mn3O4), which showed a low overpotential of 24 mV at a current density of 10 mA cm−2 and a high mass activity of 0.374 mA mgPt−1 at the overpotential of 50 mV. Xie et al. [17] demonstrated in situ-grown platinum nanowires (Pt NW) on ultrathin titanium liquid/gas diffusion layers (LGDLs), which showed a very low cell voltage of 1.643 V with only 15 times lower catalyst loadings than a conventional catalyst in PEMEC tests. Carbon materials are usually employed to load Pt electrocatalysts due to their large specific surface area which can decrease the size of nanoparticles and thus increase the utilization of the element Pt [18,19]. However, the lack of Pt electronic state modification and the weak interaction between carbon and Pt nanoparticles make the HER activity and stability unsatisfactory [20,21,22]. As potential substitutes, transition metal oxides have also been studied as supports, which not only changes the adsorption properties of the molecules on the metal, thus improving its catalytic activity, but also enhances the catalyst stability because of the existence of a strong–metal–support interaction (SMSI) [23,24,25]. For example, Wang et al. [26] found that the single platinum atoms immobilized on monolayer tungsten trioxide nanosheets (Pt-SA/ML-WO3) showed a very small overpotential of 22 mV, resulting in a current density of 10 mA cm−2 during acidic HER. Li et al. [27] reported the Ru-incorporated oxygen vacancy-enriched MoO2 nanosheets (Ru/MoO2−x) with a low overpotential of 29 mV at a current density of 10 mA cm−2 in the base solution. However, the low conductivity of metal oxides and the distribution of Pt on metal oxides support are crucial for this type of HER electrocatalysts [28].
In this study, we report a novel hierarchical structure of TiOx nanoparticles embedded in porous carbon with highly dispersed Pt on its surface (Pt-TiOx@C). The subsize TiOx nanoparticles embedded in porous carbon were prepared by a simple ultrasonic impregnation method followed by high-temperature annealing process in reducing atmosphere (5% H2/95% Ar), which resulted in numerous defects in TiOx. Then the highly dispersed Pt cluster was deposited in situ on the TiOx surface by the spontaneous reaction between Ti3+ and PtCl42+. This Pt-TiOx@C electrocatalyst exhibited an ultra-low overpotential of 10 mV at a current density of 10 mA cm−2 in 0.5 M H2SO4 solution. Moreover, it also showed a very high mass activity of 9.24 A mgPt−1 at an overpotential of only 30 mV, which is 5.9 times that of commercial Pt/C catalysts. In addition, it also exhibited a very stable performance over 10,000 CV cycles of an accelerated degradation test (ADT).
2. Results and Discussion
2.1. The Synthesis of Pt-TiOx@C Electrocatalyst
The highly dispersed Pt on TiOx nanoparticles embedded in porous carbon were synthesized in two steps. As shown in Figure 1, firstly, TiOx nanoparticles embedded in porous carbon were prepared using the incipient wetness impregnation method followed by an annealing process [29]. Then, the highly dispersed Pt was grown in situ on the surface of TiOx using spontaneous deposition resulting from the reaction between Ti3+ and PtCl42+. Ketjenblack EC-600JD (KB) was selected here as the carbon template due to its abundant surface pores with pore sizes of around 3–5 nm, which are able to confine TiOx nanoparticles (Figure S1). Briefly, 50 mg of KB and 25 mg titanium tetraisopropanolate were dissolved and sonicated in a specific amount of ethanol for 2 h until the liquid was completely absorbed into the carbon. The obtained gel mixture was then dried at 70 °C in a vacuum oven overnight. The dry powder was further calcined at 650 °C for 2 h in 5% H2/95% Ar atmosphere to obtain the TiOx@C sample. Then, this TiOx@C powder was quickly added into 20 mL of 1.25 M K2PtCl4 solution at a temperature of 45 °C and stirred for 1 h. After washing with distilled water and drying, the Pt-TiOx@C catalyst was obtained. As a comparison, the carbon-supported TiOx with Pt in situ growth on its surface (Pt-TiOx/C) was also prepared. The carbon-supported TiOx support was first prepared by the traditional stirring-evaporation method, followed by a similar high-temperature calcination. The Pt deposition was conducted using the same spontaneous process. The Pt weight percent for the two prepared samples were determined by inductively coupled plasma–optical emission spectroscopy (ICP-OES), and the values were 1.43 wt.% and 1.33 wt.% for Pt-TiOx@C and Pt-TiOx/C samples, respectively.
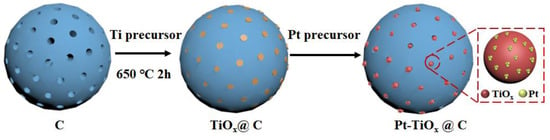
Figure 1.
Schematic illustration of the synthesis of highly dispersed Pt on TiOx embedded in porous carbon.
2.2. The Characterizations of Pt-TiOx@C Electrocatalyst
The phase of TiOx@C and Pt-TiOx@C samples were first analyzed by XRD. Figure S2 shows the XRD patterns of TiOx@C samples, and the diffraction peaks located around 25.2° and 48.0° may be related to the (101) and (200) facets of anatase TiO2 (PDF card no. 21-1272), respectively, while the peak at 54.3° could correspond to the facet of (211) for rutile TiO2 (PDF card no. 21-1276). This indicates that the mixed phases of rutile and anatase TiO2 were obtained after the annealing process, which is in agreement with a previous study [30]. It should be pointed out that no other peaks associated with these two phases of TiO2 were observed, which may be attributed to the embedded feature [29]. Compared to the TiOx@C sample, as shown in Figure 2a, no obvious peaks related to metallic Pt were observed in the XRD pattern of the Pt-TiOx@C sample, and this may indicate the highly dispersed feature of Pt on the TiOx surface. Similarly, no obvious peaks for Pt were found in the XRD pattern of the Pt-TiOx/C sample (Figure S3). The electronic structure of the Pt-TiOx@C sample was investigated by electron paramagnetic resonance (EPR), and as shown in the EPR spectrum (Figure 2b), the intensity of oxygen vacancy at g = 2.007 indicates the existence of oxygen defects [31]. From the TEM and HADDF-STEM images of the Pt-TiOx@C sample (Figure 3), it is easy to see that the nanoparticles of ultrasmall size (about 3–5 nm) were homogeneously dispersed in KB. The calculated d spacing of nanoparticles in the Pt-TiOx@C sample is 0.324 nm, and these are assigned to the (110) plane of rutile TiO2, which is in agreement with the XRD results. As a comparison, large particles were observed in the Pt-TiOx/C sample (Figure S4), and a similar d spacing of 0.324 nm was also observed. This indicates similar phases of TiO2 in the two prepared materials. In addition, no obvious Pt nanoparticles were found in either sample, which reveals the high dispersion of Pt on the TiOx surface [29].
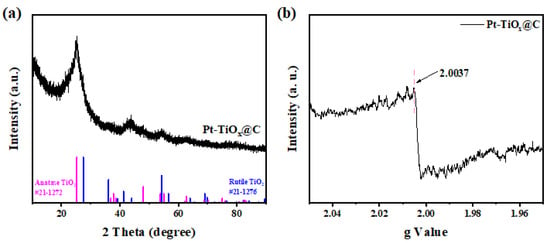
Figure 2.
(a) XRD pattern and (b) electron paramagnetic resonance (EPR) spectrum of the Pt-TiOx@C sample.
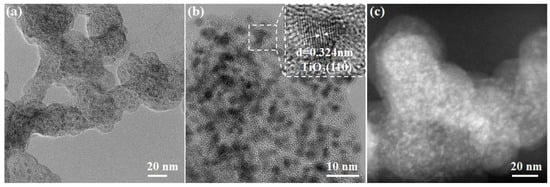
Figure 3.
(a) TEM, (b) HRTEM (insertion shows the d spacing), and (c) HAADF-STEM images of the Pt-TiOx@C sample.
X-ray photoelectron spectroscopy (XPS) was performed to investigate the surface composition and chemical states of the elements presented in the as-prepared Pt-TiOx@C and Pt-TiOx/C samples. Figure 4a and Figure S5a show the survey spectra of Pt-TiOx@C and Pt-TiOx/C samples, respectively, and the characteristic peaks of Pt, Ti, O, and C were observed in both spectra. The Ti 2p high-resolution spectrum for Pt-TiOx@C, as shown in Figure 4b, exhibits two peaks at 459.4 eV and 465.2 eV, corresponding to Ti4+ 2p3/2 and Ti4+ 2p1/2, respectively, while the peaks at 458.3 eV and 463.8 eV are associated with Ti3+ 2p3/2 and Ti3+ 2p1/2 in the sample (Table S1). In addition, two similar pairs of peaks (459.3/465.1 eV and 458.4/463.9 eV) can also be observed in the spectrum of the Pt-TiOx/C sample (Figure S5b and Table S2). The Ti3+ content in Pt-TiOx@C is higher than that in Pt-TiOx/C, and this may be related to the smaller particle size, which leads to easy reduction. As shown in Figure 4c and Figure S5c, for the O 1s spectra, the lattice O 1s peaks of the Pt-TiOx@C and Pt-TiOx/C samples were found to have higher binding energy compared the normal value in metal oxides, and this may have been caused by the strong interaction between Pt and TiOx [32]. The two O 1s (OL and OL′) features for the Pt-TiOx@C and Pt-TiOx/C samples are due to the lattice being O2-bonded with the Ti(IV) and Ti(III) states, respectively, which agrees with the Ti 2p XPS results [33]. As shown in Figure 4d and Figure S5d, the Pt-TiOx@C sample displayed a lower binding energy of Pt0 4f7/2 (70.8 eV) than Pt-TiOx/C (71.4 eV) and a higher portion of Pt0 (80.6%) sample than Pt-TiOx/C (74.9%), indicating a higher reduction degree, which may predict the higher HER activity of the Pt-TiOx@C sample.
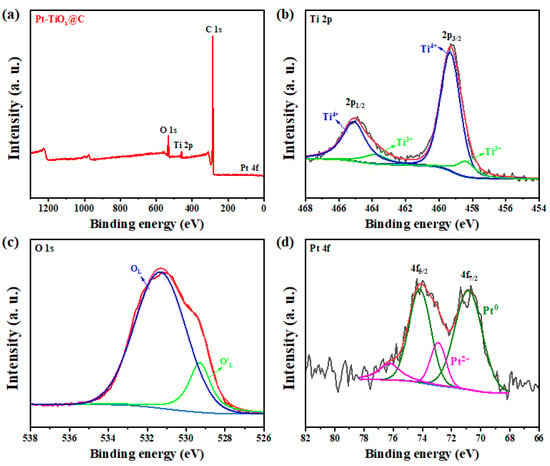
Figure 4.
(a) XPS survey, (b) Ti 2p, (c) O 1s, and (d) Pt 4f spectra of Pt-TiOx@C sample. The red lines in b-d are the fitting curves.
2.3. HER Performance of the Pt-TiOx@C Electrocatalyst
To investigate the electrocatalytic performance of the Pt-TiOx@C sample towards HER, the electrochemical characterizations were performed in a typical three-electrode configuration with 0.5 M H2SO4 as an electrolyte. As comparisons, the as-prepared Pt-TiOx/C sample and commercial Pt/C catalyst were also examined. The linear sweeping voltammetry (LSV) technique was first employed with a scan rate of 2 mV s−1, and the HER polarization curves are shown in Figure 5a and Figure S6. In addition, the HER performance TiOx@C and bare glassy carbon electrode (GCE) were also studied, and their activities were very poor (Figure S7). Obviously, the Pt-TiOx@C sample exhibited the most positive curve, indicating the best HER electrocatalytic performance among the studied three catalysts. Moreover, the overpotentials (η10 and η50) of these samples when the current densities reached 10 mA cm−2 and 50 mA cm−2, respectively, are shown as Figure 5b. The η10 of the Pt-TiOx@C sample was only 10 mV, which is about 9 mV and 26 mV lower than those of the Pt-TiOx/C and commercial Pt/C catalysts, respectively. In addition, when the current density reached 50 mA cm−2, this value (η50) only increased to 26 mV, which is significantly lower than those of 63 mV and 115 mV for Pt-TiOx/C and commercial Pt/C catalysts, respectively. Normalized to the Pt loading, the calculated mass activities were compared at overpotentials of 20 mV and 30 mV and are shown in Figure 3c. For the Pt-TiOx@C electrocatalyst, its mass activity was 4.56 A mgPt−1 at an overpotential of 20 mV, and this increased to 9.24 A mgPt−1 at an overpotential of 30 mV, which is about 3.5 times and 5.92 times those of Pt-TiOx/C and commercial Pt/C catalysts, respectively. Moreover, the Pt-TiOx@C electrocatalyst also exhibited the almost highest activity values among metal oxide-containing, supported Pt-based catalysts (Table S3) and non-Pt-based catalysts (Table S4) previously reported, and this further confirms the good HER activity of the Pt-TiOx@C electrocatalyst. For the HER path in acidic solutions, two paths have been proposed, namely the Volmer–Heyrovsky or the Volmer–Tafel paths [32]. Generally, the rate-limiting step of the HER can be revealed by the Tafel slope, an inherent property of electrocatalysts [34]. As shown in Figure 5d, the Tafel slope of Pt-TiOx@C is significantly lower than those of Pt-TiOx/C and commercial Pt/C catalysts, and this demonstrates the HER kinetic superiority over the Pt-TiOx@C catalyst [35]. Furthermore, since the Tafel slope of Pt-TiOx@C was 17.9 mV dec−1, this indicates that the HER process for the Pt-TiOx@C follows the Volmer–Tafel path, in which the Tafel reaction is the rate-determining step [36]. To further reveal the electrocatalytic kinetics, the electrochemical impedance measurement was implemented. Figure S8 shows the Nyquist plots of Pt-TiOx@C and Pt-TiOx/C at an overpotential of 10 mV. In these plots, the semicircle diameter at high frequencies typically reflects the charge transfer resistance (Rct) at the electrode–electrolyte interface, with a smaller radius indicating the more efficient electron transfer and faster reaction kinetics. The impedance of Pt-TiOx@C was lower than that of Pt-TiOx/C, revealing the superior HER activity of the former sample [37,38]. This excellent HER catalytic activity of Pt-TiOx@C may be associated with the dispersed Pt feature which increases the exposure of Pt sites [10] and the SMSI between Pt and TiOx, resulting in the enhanced HER catalytic ability of each Pt site [23].
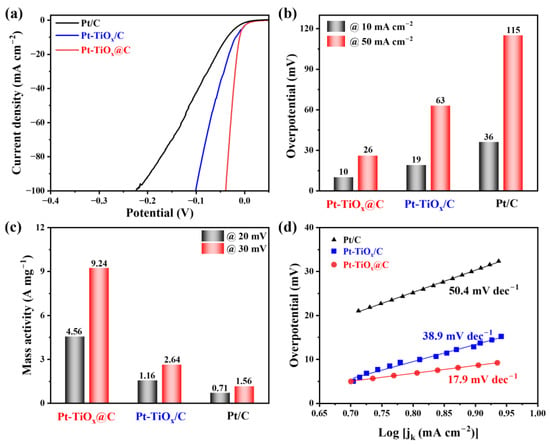
Figure 5.
HER performance of Pt-TiOx@C electrocatalyst. (a) HER polarization curves; (b) overpotentials at current densities of 10 mA cm−2 and 50 mA cm−2; (c) mass activities at overpotentials of 20 mV and 30 mV; (d) Tafel slopes.
The durability of the Pt-TiOx@C catalyst was further evaluated via accelerated degradation tests (ADTs) by applying 10,000 continuous cyclic voltammetry cycles between −0.03 V and 0.05 V (vs. RHE) at a scan rate of 50 mV s−1, and the LSV curve was recorded again after ADTs using the same procedure. As shown in Figure 6a, the HER polarization curve of Pt-TiOx@C after the ADT was almost the same as that for the fresh catalyst and was even slightly more positive under a high current density. This indicates the excellent durability of the Pt-TiOx@C electrocatalyst during HER in acidic conditions. The mass activities for the catalyst before and after the ADT were further calculated at overpotentials of 20 mV and 30 mV and are shown in Figure 6b. At an overpotential of 20 mV, the initial mass activity was 4.56 A mgPt−1; after ADT, it was 4.53 A mgPt−1, which was a loss of only 0.07%. Interestingly, after the ADT, the mass activity increased slightly from 9.24 A mgPt−1 to 10.55 A mgPt−1 at an overpotential of 30 mV. The presented excellent stability could be attributed to the highly dispersed feature and the SMSI between TiOx support and Pt, which resulted in the inhibition of particle migration and agglomeration during the cycling test [39]. In addition, the reconstruction of Pt on the TiOx surface may be the reason for the slight increase in activity in the high current density range [40].
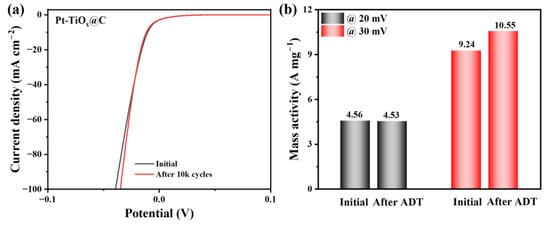
Figure 6.
(a) The HER polarization curves of Pt-TiOx@C at the initial stage and after 10,000 cycles of the ADT; (b) the mass activities comparison at the initial stage and after 10,000 cycles at overpotentials of 20 mV and 30 mV.
3. Experimental Section
3.1. Chemicals
Titanium tetraisopropanolate (C12H28O4Ti), potassium hexachloroplatinate (K2PtCl4), and sulfuric acid (H2SO4, AR, 95–98%) were purchased from Sinopharm Chemical Reagent Co., Ltd (Beijing, China). Anhydrous ethanol was purchased from Shanghai Titan Technology Co., Ltd (Shanghai, China). Ketjenblack EC-600JD was purchased from AkzoNobel (Tianjin, China). Nafion solution (5 wt.%) was purchased from Ion Power (Tyrone, PA, USA). Commercial Pt/C (20 wt.%) catalysts were purchased from Johnson Matthey (London, UK). All chemicals were used as received.
3.2. Synthesis of Pt-TiOx@C Catalyst
Briefly, 25 mg of titanium tetraisopropanolate was dissolved in 1.6 mL of ethanol, which formed a homogeneous solution, and 50 mg of KB was immersed into the solution, then ultrasonicated for 2 h. The slurry was dried in the vacuum oven (Shanghai Yiheng, China) at 70 °C for 12 h. The dried powder was then transferred to tube furnace (Hefei Kejing, China) and annealed at 650 °C for 2 h under a 5% H2/95% Ar atmosphere to obtain the precursor. Then, the precursor was quickly added into 20 mL of 1.25 M K2PtCl4 solution at a temperature of 45 °C and stirred for 1 h. After washing with distilled water and drying, the Pt-TiOx@C catalyst was obtained.
3.3. Synthesis of Pt-TiOx/C Catalyst
Briefly, 50 mg KB was dispersed in 20 mL of ethanol, forming a homogeneous solution by sonication, and 25 mg of titanium tetraisopropanolate in 2 mL of ethanol was added into above solution, followed by stirring for 2 h. Then, the solvent was evaporated at 80 °C to obtain a wet slurry. The wet slurry was further dried in a vacuum oven at 70 °C for 12 h. The following annealing and the spontaneous Pt deposition were similar processes to those utilized for the Pt-TiOx@C catalyst. Finally, the Pt-TiOx/C catalyst was obtained.
3.4. Material Characterization
X-ray diffraction (XRD) patterns were measured by a Bruker D8 Advanced (Berlin, Germany) with Cu Kα radiation (λ = 1.5418 Å) at a scanning rate of 5° min−1. X-ray photoelectron spectroscopy (XPS) was conducted on a Thermo Fisher ESXCALAB Xi+ (Waltham, MA, USA) with an Al Kα X-ray source. The morphology of the catalyst was characterized by transmission electron microscopy (TEM) on a Talos F200X G2 microscope (Thermo Fisher, Waltham, MA, USA) at accelerating voltages of 200 kV. Pt content in catalyst was determined by inductively coupled plasma atomic emission spectroscopy (710-ES, ICP-OES, Varian, Palo Alto, CA, USA).
3.5. Electrochemical Measurements
The electrochemical tests were performed in a standard three-electrode system using a CHI 760E electrochemical workstation (CHI Instruments, Shanghai, China). A graphite rod was used as the counter electrode and Ag/AgCl was utilized as a reference electrode. An electrolytic bridge was used between the reference electrode and electrolyte to minimize the leakage of chlorides. The working electrode was prepared as follows: a certain amount of the as-prepared catalyst was dispersed in 950 μL of water/ethanol (v/v ratio of 5:95) and 50 μL of 5 wt.% Nafion solution, followed by sonication for 30 min to obtain a uniform ink. Subsequently, 20 μL of the catalyst ink was dropped onto the surface of a glassy carbon electrode (d = 5 mm; the geometric electrode area was 0.196 cm2) with a Pt metal loading of 7 µg cm−2 and dried at room temperature. The HER polarization measurement was carried out using linear sweep voltammetry (LSV) technology in N2-saturated 0.5 M H2SO4 solutions at a scan rate of 2 mV s−1 with an electrode rotation rate of 2500 rpm. All LSV curves were iR-compensated. Electrochemical impedance spectroscopy (EIS) measurements were carried out at an overpotential of 10 mV (vs. RHE) within a frequency range of 100 kHz to 0.1 Hz with an AC amplitude of 5 mV. As a comparison, the HER performance of the commercial Pt/C (Johnson Matthey, 20 wt.% Pt) was also tested using the same Pt loading.
4. Conclusions
In summary, the highly dispersed Pt on TiOx nanoparticles embedded in porous carbon were prepared using a facile method combining the incipient wetness impregnation method and the annealing process, followed by spontaneous deposition. This hierarchical structure of Pt-TiOx@C electrocatalysts shows excellent HER catalytic activity with an overpotential of only 10 mV at a current density of 10 mA cm−2 and a mass activity of 9.24 A mgPt−1 at the overpotential of 30 mV in 0.5 M H2SO4 solution, which is about 5.92 times that of the commercial Pt/C catalyst. In addition, this method even results in a slightly higher mass activity after the ADT. The high Pt utilization rate was a result of the dispersed Pt feature, and the SMSI between Pt and TiOx may be the reason for the excellent catalytic activity and durability during the HER of the Pt-TiOx@C electrocatalyst.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15050487/s1. Figure S1: (a) N2 adsorption–desorption isotherm curve and (b) pore–size distribution curve of Ketjenblack EC 600JD; Figure S2: XRD pattern of TiOx@C sample; Figure S3: XRD pattern of Pt-TiOx/C sample; Figure S4: (a) TEM, (b) HRTEM (insertion shows the d spacing), and (c) HAADF-STEM images of Pt-TiOx/C sample; Figure S5: (a) XPS survey, (b) Ti 2p, (c) O 1s, and (d) Pt 4f spectra of Pt-TiOx/C sample; Figure S6: HER polarization curve of Pt-TiOx@C catalyst in 0.5M H2SO4 solution at a scan rate of 2 mV s−1; Figure S7: HER polarization curves of TiOx@C and bare glassy carbon electrode (GCE) in 0.5 M H2SO4 solution at a scan rate of 2 mV s−1; Figure S8: Nyquist plots of Pt-TiOx@C and Pt-TiOx/C at an overpotential of 10 mV; Table S1: Binding energies and atomic ratios of Ti species in the as-prepared Pt-TiOx@C and Pt-TiOx/C samples; Table S2: Binding energies and atomic ratios of O species in the as-prepared Pt-TiOx@C and Pt-TiOx/C samples; Table S3: Comparison of the overpotentials at 10 mA cm−2 and mass activity for various Pt supported on metal oxides-based HER catalysts under acidic conditions; Table S4: Comparison of overpotentials at 10 mA cm−2 and mass activity for various non-Pt based HER catalysts under acidic conditions. References [6,31,32,39,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63] are citied in the Supplementary Materials.
Author Contributions
Investigation, data curation, writing—original draft, Z.W.; investigation, data curation, X.C.; investigation, data curation, P.D.; data curation, J.L.; data curation, Z.C.; data curation, C.Y.; methodology, project administration, funding acquisition, supervision, writing—review and editing, Z.M.; supervision, writing—review and editing, G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (22208214).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zeng, L.; Zhao, Z.; Huang, Q.; Zhou, C.; Chen, W.; Wang, K.; Li, M.; Lin, F.; Luo, H.; Gu, Y.; et al. Single-atom Cr-N4 sites with high oxophilicity interfaced with Pt atomic clusters for practical alkaline hydrogen evolution catalysis. J. Am. Chem. Soc. 2023, 145, 21432–21441. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Küspert, S.; Balaghi, S.E.; Hussein, H.E.M.; Ortlieb, N.; Knäbbeler-Buß, M.; Hügenell, P.; Pollitt, S.; Hug, N.; Melke, J.; et al. Ultrahigh mass activity Pt entities consisting of Pt single atoms, clusters, and nanoparticles for improved hydrogen evolution reaction. Small 2023, 19, 2205885. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhu, Y.; Chen, Y.; Wen, X.; Long, M.; Wu, X.; Hu, Z.; Guan, D.; Wang, X.; Zhou, C.; et al. Hydrogen spillover in complex oxide multifunctional sites improves acidic hydrogen evolution electrocatalysis. Nat. Commun. 2022, 13, 1189. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Gu, W.; Zhang, B.; Zhao, Y.; Zhang, A.; Xiao, W.; Wei, S.; Pang, H. Research progress of non-noble metal-based self-supporting electrode for hydrogen evolution reaction at high current density. Adv. Funct. Mater. 2025, 2423760. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Chen, K.; Deng, S.; Lu, Y.; Gong, M.; Hu, Y.; Zhao, T.; Shen, T.; Wang, D. Molybdenum-doped titanium dioxide supported low-Pt electrocatalyst for highly efficient and stable hydrogen evolution reaction. Chin. Chem. Lett. 2021, 32, 765–769. [Google Scholar] [CrossRef]
- Yue, C.; Feng, C.; Sun, G.; Liu, N.; Hao, H.; Bao, W.; Zhang, X.; Sun, F.; Zhang, C.; Bi, J.; et al. Hierarchically stabilized Pt single-atom catalysts induced by an atomic substitution strategy for an efficient hydrogen evolution reaction. Energy Environ. Sci. 2024, 17, 5227–5240. [Google Scholar] [CrossRef]
- Feng, Y.; Xie, Y.; Yu, Y.; Chen, Y.; Liu, Q.; Bao, H.; Luo, F.; Pan, S.; Yang, Z. Electronic metal-support interaction induces hydrogen spillover and platinum utilization in hydrogen evolution reaction. Angew. Chem. Int. Edit. 2025, 64, e202413417. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Y.; Sun, H.; Yu, N.; Ni, G. Effect of bias in argon plasma on electronic structure of electrocatalyst Pt/CNT for hydrogen evolution reaction. Catal. Lett. 2025, 155, 110. [Google Scholar] [CrossRef]
- Ma, Z.; Cano, Z.P.; Yu, A.; Chen, Z.; Jiang, G.; Fu, X.; Yang, L.; Wu, T.; Bai, Z.; Lu, J. Enhancing oxygen reduction activity of Pt-based electrocatalysts: From theoretical mechanisms to practical methods. Angew. Chem. Int. Edit. 2020, 59, 18334–18348. [Google Scholar] [CrossRef]
- Jauhar, A.M.; Ma, Z.; Xiao, M.; Jiang, G.; Sy, S.; Li, S.; Yu, A.; Chen, Z. Space-confined catalyst design toward ultrafine Pt nanoparticles with enhanced oxygen reduction activity and durability. J. Power Sources 2020, 473, 228607. [Google Scholar] [CrossRef]
- Yu, F.; Gao, Y.; Lang, Z.; Ma, Y.; Yin, L.; Du, J.; Tan, H.; Wang, Y.; Li, Y. Electrocatalytic performance of ultrasmall Mo2C affected by different transition metal dopants in hydrogen evolution reaction. Nanoscale 2018, 10, 6080–6087. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shang, H.; Wang, C.; Du, Y. Ultrafine Pt-based nanowires for advanced catalysis. Adv. Funct. Mater. 2020, 30, 2000793. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, G.; Cui, X.; Chen, B.; Zhu, Y.; Gong, Y.; Saleem, F.; Xi, S.; Du, Y.; Borgna, A.; et al. Crystal phase and architecture engineering of lotus-thalamus-shaped Pt-Ni anisotropic superstructures for highly efficient electrochemical hydrogen evolution. Adv. Mater. 2018, 30, 1801741. [Google Scholar] [CrossRef]
- Hao, R.; Feng, Q.-L.; Wang, X.-J.; Zhang, Y.-C.; Li, K.-S. Morphology-controlled growth of large-area PtSe2 films for enhanced hydrogen evolution reaction. Rare Met. 2022, 41, 1314–1322. [Google Scholar] [CrossRef]
- Wei, J.; Xiao, K.; Chen, Y.; Guo, X.-P.; Huang, B.; Liu, Z.-Q. In situ precise anchoring of Pt single atoms in spinel Mn3O4 for a highly efficient hydrogen evolution reaction. Energy Environ. Sci. 2022, 15, 4592–4600. [Google Scholar] [CrossRef]
- Xie, Z.; Yu, S.; Yang, G.; Li, K.; Ding, L.; Wang, W.; Cullen, D.A.; Meyer, H.M.; Retterer, S.T.; Wu, Z.; et al. Ultrathin platinum nanowire based electrodes for high-efficiency hydrogen generation in practical electrolyzer cells. Chem. Eng. J. 2021, 410, 128333. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Zhang, J.; Ding, J.; Cheng, Y.; Wang, T.; Li, J.; Hu, F.; Yang, H.B.; Liu, B. Recent advances in carbon-supported noble-metal electrocatalysts for hydrogen evolution reaction: Syntheses, structures, and properties. Adv. Energy Mater. 2022, 12, 2200928. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Chen, S.; Yan, H.; Wang, C.; Wu, C.; Haleem, Y.A.; Duan, S.; Lu, J.; Ge, B.; et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy 2019, 4, 512–518. [Google Scholar] [CrossRef]
- Yu, B.; Liu, Q.; Nichols, F.; Mayford, K.; Pan, D.; Kuo, H.-L.; Lu, J.Q.; Bridges, F.; Chen, S. Platinum-anchored iron oxide nanostructures for efficient hydrogen evolution reaction in acidic media. J. Phys. Chem. C 2023, 127, 3996–4005. [Google Scholar] [CrossRef]
- Cheng, X.; Li, Y.; Zheng, L.; Yan, Y.; Zhang, Y.; Chen, G.; Sun, S.; Zhang, J. Highly active, stable oxidized platinum clusters as electrocatalysts for the hydrogen evolution reaction. Energy Environ. Sci. 2017, 10, 2450–2458. [Google Scholar] [CrossRef]
- Liu, J.; Guo, P.; Liu, D.; Yan, X.; Tu, X.; Pan, H.; Wu, R. Activating TiO2 through the phase transition-mediated hydrogen spillover to outperform Pt for electrocatalytic pH-universal hydrogen evolution. Small 2024, 20, 2400783. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Han, X.; Ma, Z.; Zhang, B.; Zheng, X.; Liu, Y.; Gao, M.; Zhao, G.; Lin, Y.; Pan, H.; et al. Unraveling the unique strong metal-support interaction in titanium dioxide supported platinum clusters for the hydrogen evolution reaction. Angew. Chem. Int. Edit. 2024, 63, e202406728. [Google Scholar] [CrossRef]
- Zhang, W.; Du, M.; Xi, W.; Zhang, H.; Liu, S.F.; Yan, J. Platinum species on oxygen vacancy-rich titania for efficient basic electrocatalytic hydrogen evolution. Langmuir 2023, 39, 12715–12724. [Google Scholar] [CrossRef]
- Zeng, H.; Ji, Y.; Wen, J.; Li, X.; Zheng, T.; Jiang, Q.; Xia, C. Pt nanocluster-catalyzed hydrogen evolution reaction: Recent advances and future outlook. Chin. Chem. Lett. 2025, 36, 109686. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Du, N.; Hou, W. Single platinum atoms immobilized on monolayer tungsten trioxide nanosheets as an efficient electrocatalyst for hydrogen evolution reaction. Adv. Funct. Mater. 2021, 31, 2009770. [Google Scholar] [CrossRef]
- Li, C.; Jang, H.; Kim, M.G.; Hou, L.; Liu, X.; Cho, J. Ru-incorporated oxygen-vacancy-enriched MoO2 electrocatalysts for hydrogen evolution reaction. Appl. Catal. B Environ. Energy 2022, 307, 121204. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, Q.; Zhong, Y.; Tahini, H.A.; Shao, Z.; Wang, H. Metal oxide-based materials as an emerging family of hydrogen evolution electrocatalysts. Energy Environ. Sci. 2020, 13, 3361–3392. [Google Scholar] [CrossRef]
- Ma, Z.; Li, S.; Wu, L.; Song, L.; Jiang, G.; Liang, Z.; Su, D.; Zhu, Y.; Adzic, R.R.; Wang, J.X.; et al. NbOx nano-nail with a Pt head embedded in carbon as a highly active and durable oxygen reduction catalyst. Nano Energy 2020, 69, 104455. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, X.; She, Z.; Pope, M.A.; Li, Y. Ultrasmall TiOx nanoparticles rich in oxygen vacancies synthesized through a simple strategy for ultrahigh-rate lithium-ion batteries. ChemElectroChem 2020, 7, 4124–4130. [Google Scholar] [CrossRef]
- Wei, Z.-W.; Wang, H.-J.; Zhang, C.; Xu, K.; Lu, X.-L.; Lu, T.-B. Reversed charge transfer and enhanced hydrogen spillover in platinum nanoclusters anchored on titanium oxide with rich oxygen vacancies boost hydrogen evolution reaction. Angew. Chem. Int. Edit. 2021, 60, 16622–16627. [Google Scholar] [CrossRef] [PubMed]
- Naik, K.M.; Higuchi, E.; Inoue, H. Pt nanoparticle-decorated two-dimensional oxygen-deficient TiO2 nanosheets as an efficient and stable electrocatalyst for the hydrogen evolution reaction. Nanoscale 2020, 12, 11055–11062. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Leung, K.T.; Pradhan, D. Nitrogen doped reduced graphene oxide based Pt-TiO2 nanocomposites for enhanced hydrogen evolution. J. Phys. Chem. C 2015, 119, 19117–19125. [Google Scholar] [CrossRef]
- van der Heijden, O.; Park, S.; Vos, R.E.; Eggebeen, J.J.J.; Koper, M.T.M. Tafel slope plot as a tool to analyze electrocatalytic reactions. ACS Energy Lett. 2024, 9, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Deng, J.; Xu, Y.; Tao, W.; Wang, X.; Lin, Z.; Zhang, Q.; Gu, L.; Zhong, W. Pollen-like self-supported FeIr alloy for improved hydrogen evolution reaction in acid electrolyte. J. Energy Chem. 2022, 66, 560–565. [Google Scholar] [CrossRef]
- Yang, J.; Ning, G.; Yu, L.; Wang, Y.; Luan, C.; Fan, A.; Zhang, X.; Liu, Y.; Dong, Y.; Dai, X. Morphology controllable synthesis of PtNi concave nanocubes enclosed by high-index facets supported on porous graphene for enhanced hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 17790–17796. [Google Scholar] [CrossRef]
- Wang, J.; Zang, W.; Liu, X.; Sun, J.; Xi, S.; Liu, W.; Kou, Z.; Shen, L.; Wang, J. Switch Volmer-Heyrovsky to Volmer-Tafel pathway for efficient acidic electrocatalytic hydrogen evolution by correlating Pt single atoms with clusters. Small 2024, 20, 2309427. [Google Scholar] [CrossRef]
- Yuan, H.; Li, J.; Tang, Z.; Wang, Y.; Wu, T.; Huang, M.; Zhao, L.; Zhao, Z.; Liu, H.; Xu, C.; et al. Enhanced interfacial stability of Pt/TiO2/Ti via Pt-O bonding for efficient acidic electrolyzer. Chem. Eng. J. 2024, 492, 152339. [Google Scholar] [CrossRef]
- Cho, S.; Yim, G.; Koh, J.; Jang, H.; Park, J.T. One-pot synthesis of Pt@TiO2 core-shell nanoparticles for stable hydrogen evolution reaction in acidic and alkaline media. Mater. Today Chem. 2023, 32, 101644. [Google Scholar] [CrossRef]
- Luo, X.; Tan, X.; Ji, P.; Chen, L.; Yu, J.; Mu, S. Surface reconstruction-derived heterostructures for electrochemical water splitting. EnergyChem 2023, 5, 100091. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhao, Y.; Wang, Y.; Zhang, Z.; Wu, T.; Qin, W.; Liu, S.; Jia, B.; Wu, H.; et al. Ultrahigh Pt-mass-activity hydrogen evolution catalyst electrodeposited from bulk Pt. Adv. Funct. Mater. 2022, 32, 2112207. [Google Scholar] [CrossRef]
- Zhu, Y.a.; Luo, Y.; Yao, J.; Dai, W.; Zhong, X.; Lu, T.; Pan, Y. Atomically dispersed Pt-O coordination boosts highly active and durable acidic hydrogen evolution reaction. Chem. Eng. J. 2022, 440, 135957. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, B.; Cheng, B.; Macyk, W.; Kuang, P.; Yu, J. Hollow carbon sphere-supported Pt/CoOx hybrid with excellent hydrogen evolution activity and stability in acidic environment. Appl. Catal. B Environ. 2022, 314, 121503. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, P.; Li, Q.; Xiao, W.; Li, Z.; Xu, G.; Liu, F.; Jia, B.; Ma, T.; Feng, S.; et al. Microwave synthesis of Pt clusters on black TiO2 with abundant oxygen vacancies for efficient acidic electrocatalytic hydrogen evolution. Angew. Chem. Int. Edit. 2023, 62, e202300406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, M.; An, J.; Shi, H.; Dai, L.; Jiao, S. Ultra-stable Ti vacancies-Pt atomic clusters structure on titanium oxycarbide supports for high current density hydrogen evolution reaction. Small 2024, 20, 2309823. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, L.; Wang, J.; Cheng, L.; Chen, R.; Ni, H. Binary electrocatalyst composed of Mo2C nanocrystals with ultra-low Pt loadings anchored in TiO2 nanotube arrays for hydrogen evolution reaction. Appl. Surf. Sci. 2020, 509, 144679. [Google Scholar] [CrossRef]
- Yan, J.; Xi, Z.; Cong, L.; Lv, K.; Xin, R.; Cao, B.; Liu, B.; He, J.; Zhang, J. Synergy of platinum single atoms and platinum atomic clusters on sulfur-doped titanium nitride nanotubes for enhanced hydrogen evolution reaction. Small 2022, 18, 2205603. [Google Scholar] [CrossRef]
- Wang, S.; Sang, L.; Jiao, Z.; Zhang, F.; Wang, K.; Xu, B.; Zhang, P.; Liu, B.; Wang, Y.; Li, Y.; et al. Pt nanoparticles supported on in-situ growth titanium dioxide nanowire arrays with oxygen vacancies for hydrogen evolution reaction. Appl. Surf. Sci. 2025, 687, 162257. [Google Scholar] [CrossRef]
- Samanta, R.; Mishra, R.; Barman, S. Interface-engineered porous Pt–PdO nanostructures for highly efficient hydrogen evolution and oxidation reactions in base and acid. ACS Sustain. Chem. Eng. 2022, 10, 3704–3715. [Google Scholar] [CrossRef]
- Pham, H.Q.; Huynh, T.T.; Huynh, Q. Mixed-oxide-containing composite-supported MoPt with ultralow Pt content for accelerating hydrogen evolution performance. Chem. Comm. 2023, 59, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Roy, S.B.; Moon, S.; Yoo, S.; Choi, H.; Parale, V.G.; Kim, Y.; Lee, J.; Jun, S.C.; Kang, K.; et al. Highly dispersed Pt clusters on F-doped tin(IV) oxide aerogel matrix: An ultra-robust hybrid catalyst for enhanced hydrogen evolution. ACS Nano 2022, 16, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Q.; Shao, Y.; Wang, R.; Cheng, M.; Hu, J.; Wei, T.; Liu, B.; Jiang, H.; Qi, L.; et al. Single-atom nickel encapsulated in nanosheet-coiled rGO-CTAB-MoS2 nanoflowers for high-efficiency and long-term hydrogen evolution in acidic medium. Adv. Funct. Mater. 2025, 2425826. [Google Scholar] [CrossRef]
- Su, Y.; Wu, H.; Wang, S.; Hu, Z.; Li, J.; Chang, J.; Yin, G.; Lu, S. Enhanced overall water splitting by CQDs-coupled RuO2-IrO2 heterojunction in acidic media. J. Energy Chem. 2025, 106, 331–339. [Google Scholar] [CrossRef]
- Jian, C.; Yuan, J.; Wang, T.; Zeng, L.; Liu, W. Construction of Ru single-atom and cluster/np-Mo synergistic active sites with hydrogen spillover for efficient hydrogen evolution reaction. Chem. Eng. J. 2025, 510, 161683. [Google Scholar] [CrossRef]
- Kim, T.; Jung, H.; Choi, H.; Lee, W.; Patil, U.M.; Parale, V.G.; Kim, Y.; Kim, J.; Kim, S.-H.; Park, H.-H. Partially oxidized inter-doped RuNi alloy aerogel for the hydrogen evolution reaction in both alkaline and acidic media. Mater. Horiz. 2024, 11, 4123–4132. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Liu, Y.; Li, J.; Feng, K.; Zhong, J.; Huang, H.; Shao, M.; Fan, Z.; Liao, F.; Liu, Y.; et al. Enhanced acidic hydrogen evolution reaction kinetics via nitrogen-doped iridium nanosheet with optimized hydrogen adsorption energy. Chem. Eng. J. 2024, 495, 153214. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, P.F.; Pan, L.F.; Wang, H.F.; Yang, Z.Z.; Zheng, L.R.; Hu, P.; Zhao, H.J.; Gu, L.; Yang, H.G. Local atomic structure modulations activate metal oxide as electrocatalyst for hydrogen evolution in acidic water. Nat. Comm. 2015, 6, 8064. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, C.; Xiao, L.; Han, C.; Zhao, X.; Yin, P.; Dong, C.; Liu, H.; Du, X.; Yang, J. Construction of Co–Se–W at interfaces of phase-mixed cobalt selenide via spontaneous phase transition for platinum-like hydrogen evolution activity and long-term durability in alkaline and acidic media. Adv. Mater. 2024, 36, 2401880. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, R.; Xiang, H.; Wu, J.; Zhong, W.; Li, W.; Zhang, Q.; Yang, N.; Li, X. Ni-activated transition metal carbides for efficient hydrogen evolution in acidic and alkaline solutions. Adv. Energ. Mater. 2020, 10, 2002260. [Google Scholar] [CrossRef]
- Wang, Z.; Chi, K.; Yang, S.; Xiao, J.; Xiao, F.; Zhao, X.; Wang, S. Optimizing the electronic structure of atomically dispersed Ru sites with CoP for highly efficient hydrogen evolution in both alkaline and acidic media. Small 2023, 19, 2301403. [Google Scholar] [CrossRef]
- Wang, L.; He, W.; Yin, D.; Zhang, H.; Liu, D.; Yang, Y.; Yu, W.; Dong, X. CoN/MoC embedded in nitrogen-doped multi-channel carbon nanofibers as an efficient acidic and alkaline hydrogen evolution reaction electrocatalysts. Renew. Sust. Energ. Rev. 2023, 181, 113354. [Google Scholar] [CrossRef]
- Qian, Y.; Sun, Y.; Zhang, F.; Luo, X.; Li, K.; Shen, L.; Shi, H.; Kang, D.J.; Pang, H. In situ construction of layered transition metal phosphides/sulfides heterostructures for efficient hydrogen evolution in acidic and alkaline media. Chem. Eng. J. 2024, 490, 151693. [Google Scholar] [CrossRef]
- Jung, K.; Pratama, D.S.A.; Haryanto, A.; Jang, J.I.; Kim, H.M.; Kim, J.C.; Lee, C.W.; Kim, D.W. Iridium-cluster-implanted ruthenium phosphide electrocatalyst for hydrogen evolution reaction. Adv. Fiber Mater. 2024, 6, 158–169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).