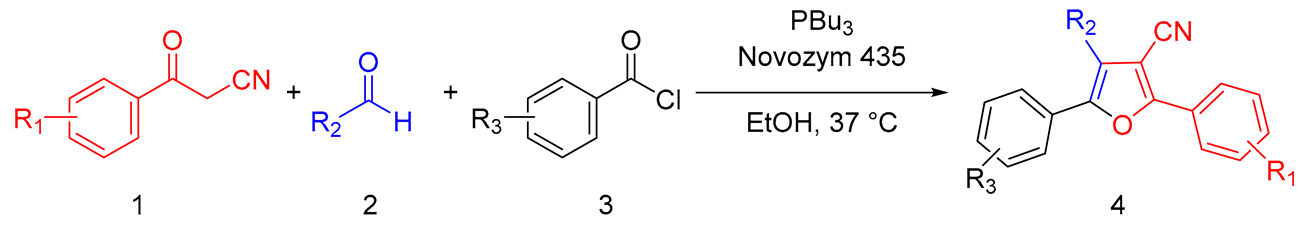
Efficient Synthesis of Tetrasubstituted Furans via Lipase-Catalyzed One-Pot Sequential Multicomponent Reaction
Abstract
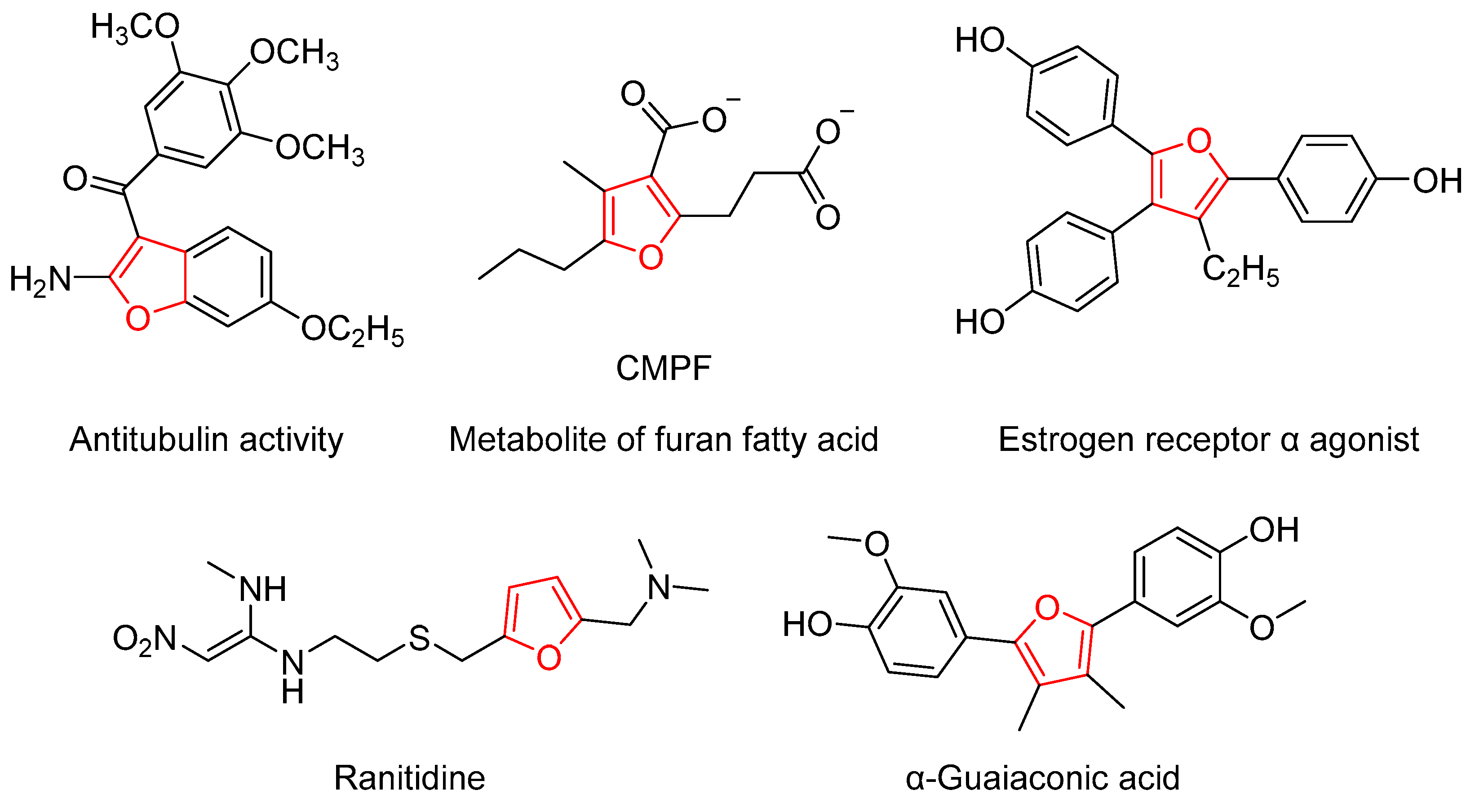
1. Introduction
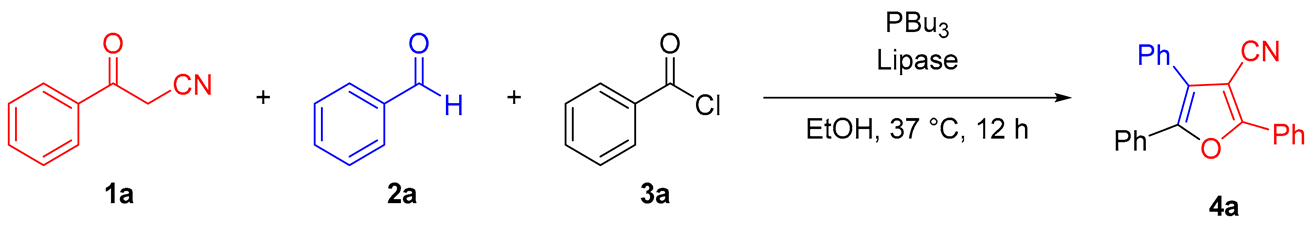
2. Results and Discussion
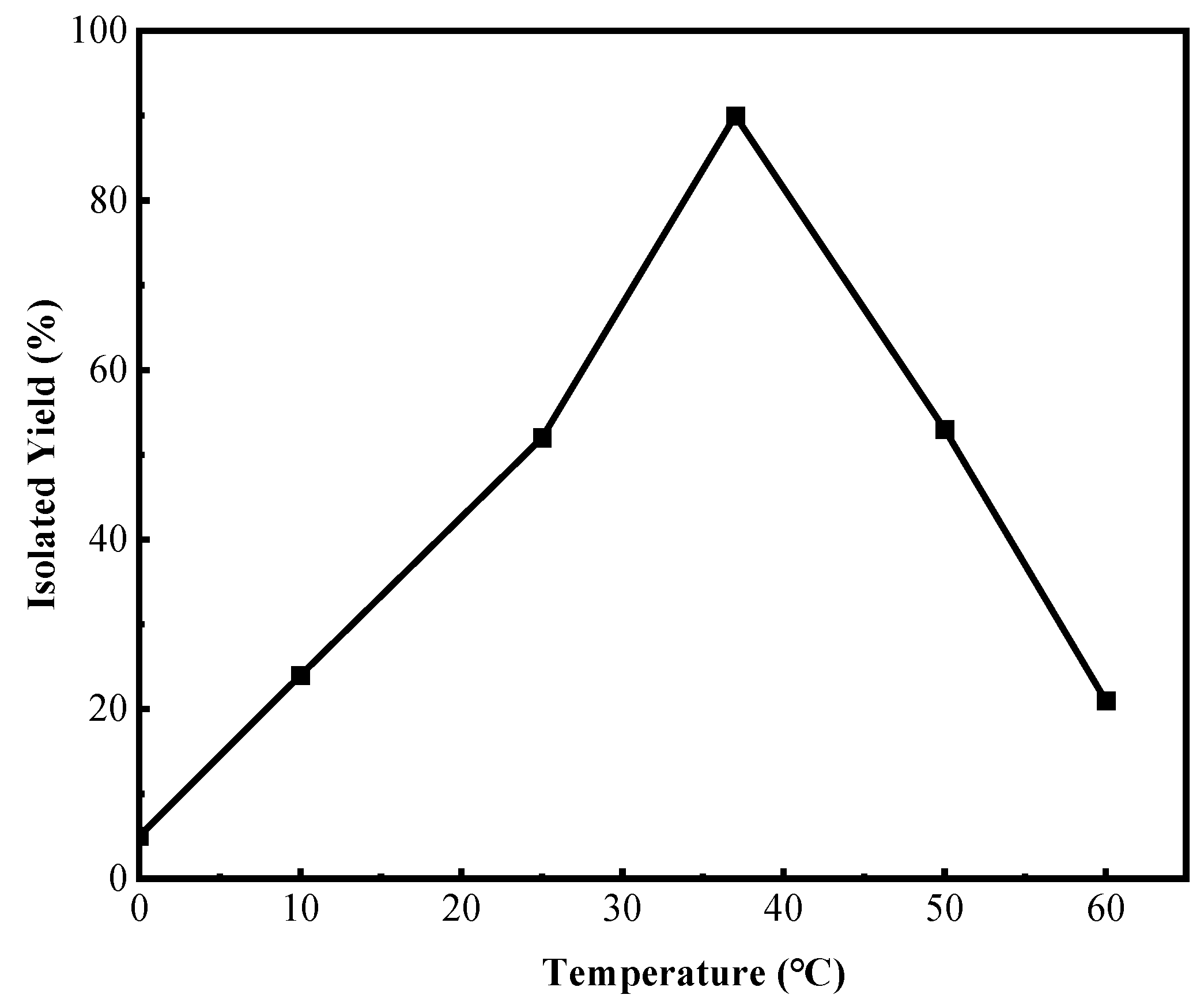
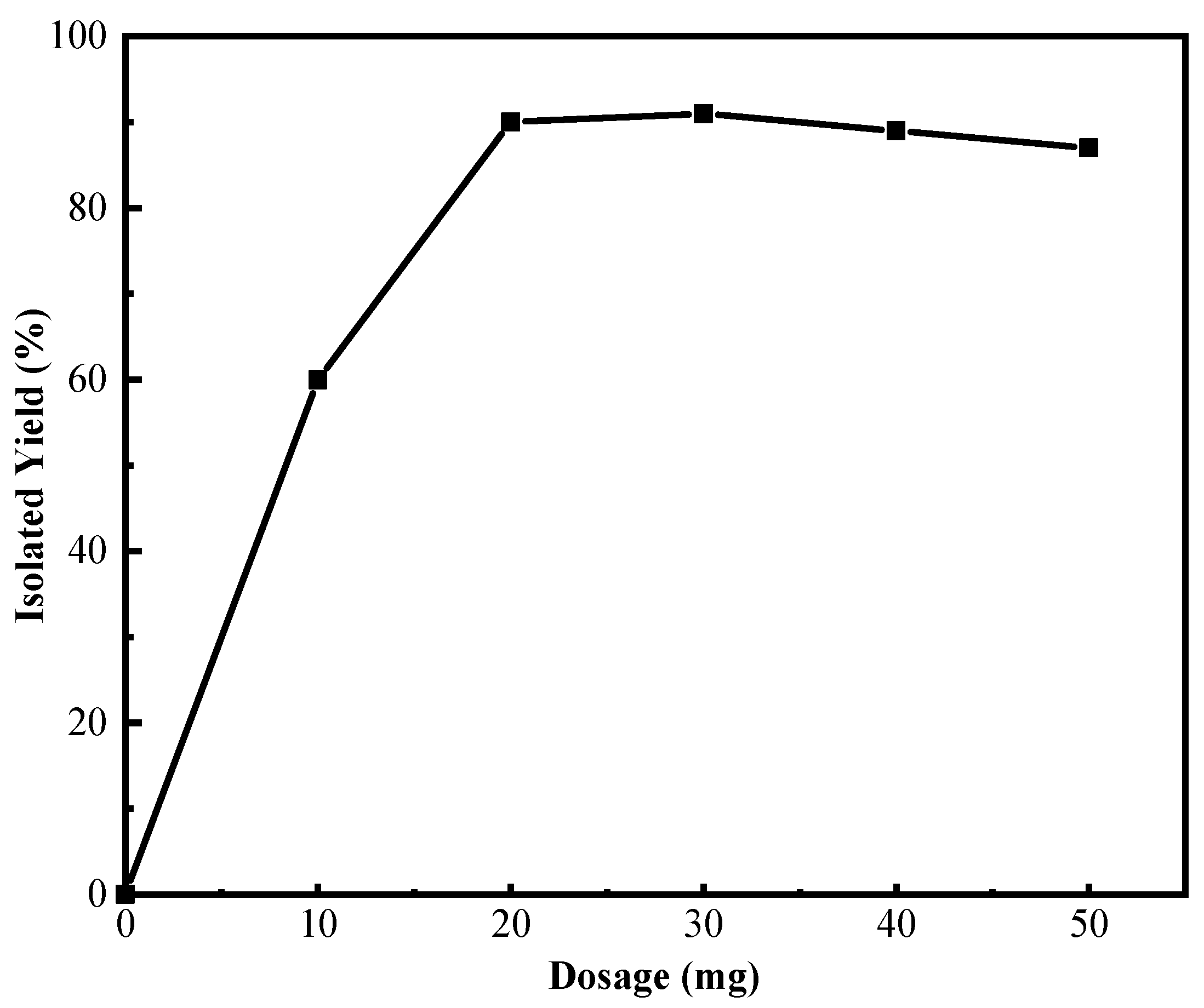
2.1. Optimization of Conditions for the Reaction
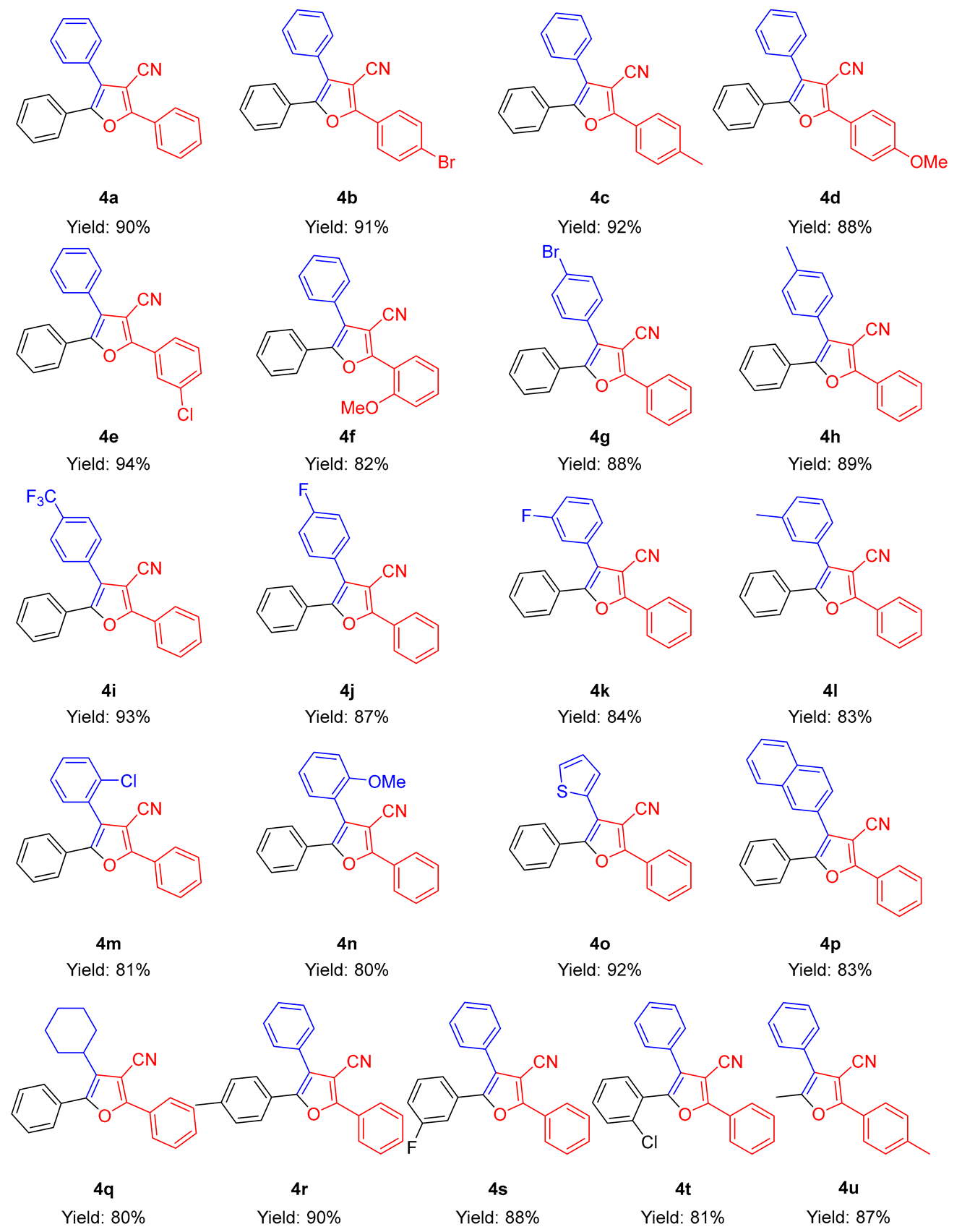
2.2. Substrate Scopes for the Reaction
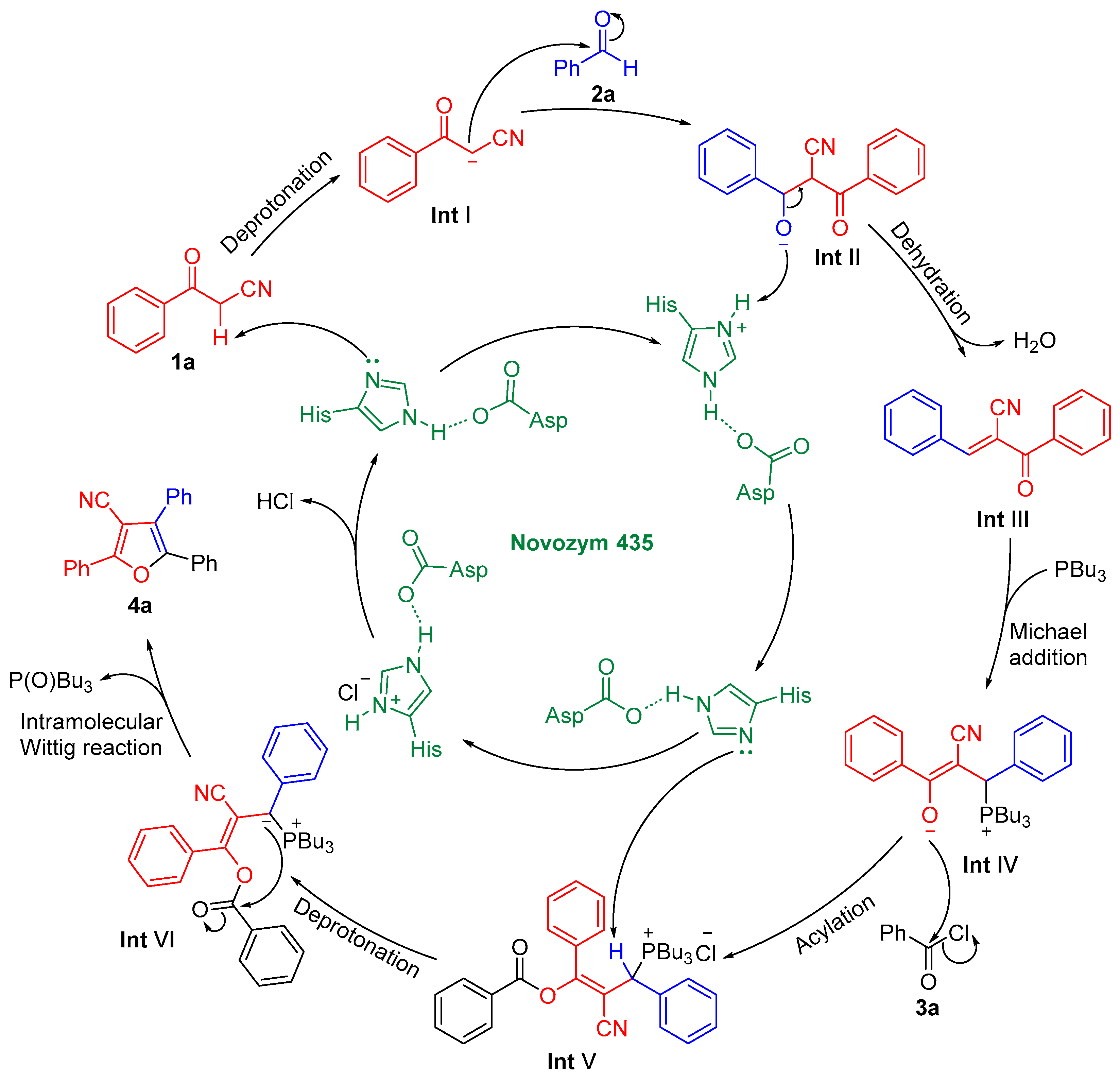
2.3. Control Experiments and Mechanism Study of the Reaction
3. Materials and Methods
3.1. General Information
3.2. Experimental Procedure for Lipase-Catalyzed Synthesis of 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Itoh, T.; Hanefeld, U. Enzyme catalysis in organic synthesis. Green Chem. 2017, 19, 331–332. [Google Scholar] [CrossRef]
- Reisenbauer, J.C.; Sicinski, K.M.; Arnold, F.H. Catalyzing the future: Recent advances in chemical synthesis using enzymes. Curr. Opin. Chem. Biol. 2024, 83, 102536. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, J.; Zhang, S.; Chen, X.; Hao, J.; Wu, Q.; Zhu, D. Discovery and directed evolution of C–C bond formation enzymes for the biosynthesis of β-hydroxy-α-amino acids and derivatives. Crit. Rev. Biotechnol. 2024, 44, 1495–1514. [Google Scholar] [CrossRef]
- Bezborodov, A.M.; Zagustina, N.A. Lipases in catalytic reactions of organic chemistry. Appl. Biochem. Microbiol. 2014, 50, 313–337. [Google Scholar] [CrossRef]
- Khanra, M.; Ravichandiran, V.; Swain, S.P. Lipase Enzymes for Sustainable Synthesis of Pharmaceuticals and Chiral Organic Building Blocks. Adv. Sustainable Syst. 2025, 9, 2400495. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, G.; Nian, B.; Hu, Y. Study on the Mechanism of Synergistic Enhancement of Lipase Efficiency in Vitamin E Succinate Synthesis through Immobilization and Solvent Design Strategies. Acta Chim. Sinica 2025, 83, 119–125. [Google Scholar] [CrossRef]
- Sarno, M.; Iuliano, M.; Polichetti, M.; Ciambelli, P. High activity and selectivity immobilized lipase on Fe3O4 nanoparticles for banana flavour synthesis. Process Biochem. 2017, 56, 98–108. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Abellanas-Perez, P.; de Andrades, D.; Tavano, O.; Mendes, A.A.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. A comprehensive review of lipase-catalyzed acidolysis as a method for producing structured glycerides. Int. J. Biol. Macromol. 2025, 309, 142878. [Google Scholar] [CrossRef]
- Bhardwaj, K.K.; Gupta, R. Synthesis of Chirally Pure Enantiomers by Lipase. J. Oleo Sci. 2017, 66, 1073–1084. [Google Scholar] [CrossRef]
- Melani, N.B.; Tambourgi, T.E.; Silveira, E. Lipases: From Production to Applications. Sep. Purif. Rev. 2020, 49, 143–158. [Google Scholar] [CrossRef]
- Ranganathan, S.; Gärtner, T.; Wiemann, L.O.; Sieber, V. A one pot reaction cascade of in situ hydrogen peroxide production and lipase mediated in situ production of peracids for the epoxidation of monoterpenes. J. Mol. Catal. B Enzym. 2015, 114, 72–76. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, C.; Chunyu, W.; Liu, Z.; Fengxi, L.; Weian, Z.; Peng, C.; Wang, L. A mild and efficient Dakin reaction mediated by lipase. Green Chem. Lett. Rev. 2017, 10, 269–273. [Google Scholar] [CrossRef]
- Li, W.; Liu, D.; Geng, X.; Li, Z.; Gao, R. Real-time regulation of catalysis by remote-controlled enzyme-conjugated gold nanorod composites for aldol reaction-based applications. Catal. Sci. Technol. 2019, 9, 2221–2230. [Google Scholar] [CrossRef]
- Guan, Z.; Fu, J.-P.; He, Y.-H. Biocatalytic promiscuity: Lipase-catalyzed asymmetric aldol reaction of heterocyclic ketones with aldehydes. Tetrahedron Lett. 2012, 53, 4959–4961. [Google Scholar] [CrossRef]
- Cai, J.-F.; Guan, Z.; He, Y.-H. The lipase-catalyzed asymmetric C–C Michael addition. J. Mol. Catal. B: Enzym. 2011, 68, 240–244. [Google Scholar] [CrossRef]
- Li, K.; He, T.; Li, C.; Feng, X.-W.; Wang, N.; Yu, X.-Q. Lipase-catalysed direct Mannich reaction in water: Utilization of biocatalytic promiscuity for C–C bond formation in a “one-pot” synthesis. Green Chem. 2009, 11, 777–779. [Google Scholar] [CrossRef]
- Mortensen, D.S.; Rodriguez, A.L.; Carlson, K.E.; Sun, J.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A. Synthesis and Biological Evaluation of a Novel Series of Furans: Ligands Selective for Estrogen Receptor α. J. Med. Chem. 2001, 44, 3838–3848. [Google Scholar] [CrossRef]
- Oliva, P.; Romagnoli, R.; Manfredini, S.; Brancale, A.; Ferla, S.; Hamel, E.; Ronca, R.; Maccarinelli, F.; Giacomini, A.; Rruga, F.; et al. Design, synthesis, in vitro and in vivo biological evaluation of 2-amino-3-aroylbenzo[b]furan derivatives as highly potent tubulin polymerization inhibitors. Eur. J. Med. Chem. 2020, 200, 112448. [Google Scholar] [CrossRef]
- Kotaka, Y.; Nakajima, M.; Nemoto, T. Collective Synthesis of Furanocoumarin Natural Products Through a Radical-mediated Construction of Furanocoumarin Skeletons. Asian J. Org. Chem. 2024, 13, e202400277. [Google Scholar] [CrossRef]
- Cimino, G.; De Stefano, S.; Minale, L. Further linear furanoterpenes from marine sponges. Tetrahedron 1972, 28, 5983–5991. [Google Scholar] [CrossRef]
- Kratochvil, J.F.; Burris, R.H.; Seikel, M.K.; Harkin, J.M. Isolation and characterization of α-guaiaconic acid and the nature of guaiacum blue. Phytochemistry 1971, 10, 2529–2531. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, P.; Jin, H.; Yi, J.; Xie, X.; Yang, M.; Gao, T.; Yang, L.; Hu, C.; Zhang, X.; et al. Circulating 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid (CMPF) levels are associated with hyperglycemia and β cell dysfunction in a Chinese population. Sci. Rep. 2017, 7, 3114. [Google Scholar] [CrossRef]
- Helman, C.A.; Tim, L.O. Pharmacology and Clinical Efficacy of Ranitidine, a New H2-Receptor Antagonist. Pharmacotherapy 1983, 3, 185–191. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, K.; Li, H.; Liu, K.; Li, J.; Chu, Y.; Prithiviraj, B.; Yue, B.; Zhang, X. Antibacterial and anti-virulence effects of furazolidone on Trueperella pyogenes and Pseudomonas aeruginosa. BMC Vet. Res. 2022, 18, 114. [Google Scholar] [CrossRef]
- Ponnappa, S.P.; Macleod, J.; Umer, M.; Soda, N.; Pannu, A.S.; Shiddiky, M.J.A.; Ayoko, G.A.; O’Mullane, A.P.; Sonar, P. Electropolymerized Porous Polymer Films on Flexible Indium Tin Oxide Using Trifunctional Furan Substituted Benzene Conjugated Monomer for Biosensing. ACS Appl. Polym. Mater. 2020, 2, 351–359. [Google Scholar] [CrossRef]
- Wailzer, B.; Kocker, J.; Wolschann, P.; Buchbauer, G. Structural Features for Furan-Derived Fruity and Meaty Aroma Impressions. Nat. Prod. Commun. 2016, 11, 1475–1479. [Google Scholar] [CrossRef]
- Adair, L.; Egan, B.A.; Pearson, C.M.; Lopez-Gonzalez, R.; Kuchar, M.; Mendoza-Mendoza, A.; Prunet, J.; Marquez, R. Isobenzofurans as Synthetic Intermediates: Synthesis and Biological Activity of 8-epi-(–)-Ajudazol B. Eur. J. Org. Chem. 2020, 2020, 6661–6672. [Google Scholar] [CrossRef]
- Song, B.; Hu, K.; Qin, A.; Tang, B.Z. Oxygen as a Crucial Comonomer in Alkyne-Based Polymerization toward Functional Poly(tetrasubstituted furan)s. Macromolecules 2018, 51, 7013–7018. [Google Scholar] [CrossRef]
- Dvorko, M.Y.; Shabalin, D.A.; Ushakov, I.A.; Schmidt, E.Y.; Trofimov, B.A. Superbase-Catalyzed Steric-Hindrance-Induced Dimerization of Alkynones to Highly Functionalized Furans. Eur. J. Org. Chem. 2023, 26, e202201464. [Google Scholar] [CrossRef]
- Almaraz-Ortiz, W.E.; Ramos Orea, A.; Casadiego-Díaz, O.; Reyes-Salgado, A.; Mejía-Galindo, A.; Torres-Ochoa, R.O. Divergent copper-catalyzed syntheses of 3-carboxylpyrroles and 3-cyanofurans from O-acetyl oximes and β-ketoesters/nitriles. RSC Adv. 2022, 12, 26673–26679. [Google Scholar] [CrossRef]
- Chourasiya, M.; Kumar, A.; Vikram, V.N.; Tadigoppula, N. Ru(ii)-catalyzed synthesis of poly-substituted furans via intermolecular oxidative annulation reaction of ethyl 3-oxo-3-phenylpropanoates with aryl alkynes/heteroaryl alkynes. Chem. Commun. 2023, 59, 9650–9653. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.-T.; Syu, S.-E.; Jhang, Y.-W.; Lin, W. Preparation of Tetrasubstituted Furans via Intramolecular Wittig Reactions with Phosphorus Ylides as Intermediates. Org. Lett. 2010, 12, 3066–3069. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- van der Kamp, M.W.; Prentice, E.J.; Kraakman, K.L.; Connolly, M.; Mulholland, A.J.; Arcus, V.L. Dynamical origins of heat capacity changes in enzyme-catalysed reactions. Nat. Commun. 2018, 9, 1177. [Google Scholar] [CrossRef]

 | ||
|---|---|---|
| Entry | Lipase a | Yield (%) b |
| 1 | - | N.D. c |
| 2 | CSL | 29 |
| 3 | PSL | 33 |
| 4 | MML | 42 |
| 5 | PPL | 70 |
| 6 | CALB | 82 |
| 7 | Novozym 435 | 90 |
| 8 | Novozym 435 d | N.D. c |
| 9 | Novozym 435 e | N.D. c |
| 10 | BSA | N.D. c |
 | ||
|---|---|---|
| Entry | Solvent | Yield (%) a |
| 1 | n-Hexane | Trace |
| 2 | Toluene | 10 |
| 3 | Dichloromethane | 35 |
| 4 | Tetrahydrofuran | 40 |
| 5 | Ethyl acetate | 48 |
| 6 | Acetonitrile | 57 |
| 7 | Dimethyl sulfoxide | 70 |
| 8 | Ethanol | 90 |
| 9 | Water | N.D. b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Tang, Y.; Xu, M.; Wang, D.; Wang, Z.; Gao, Y.; Wang, L. Efficient Synthesis of Tetrasubstituted Furans via Lipase-Catalyzed One-Pot Sequential Multicomponent Reaction. Catalysts 2025, 15, 482. https://doi.org/10.3390/catal15050482
Zeng Y, Tang Y, Xu M, Wang D, Wang Z, Gao Y, Wang L. Efficient Synthesis of Tetrasubstituted Furans via Lipase-Catalyzed One-Pot Sequential Multicomponent Reaction. Catalysts. 2025; 15(5):482. https://doi.org/10.3390/catal15050482
Chicago/Turabian StyleZeng, Yongqi, Yong Tang, Minglu Xu, Dantong Wang, Zhi Wang, Yin Gao, and Lei Wang. 2025. "Efficient Synthesis of Tetrasubstituted Furans via Lipase-Catalyzed One-Pot Sequential Multicomponent Reaction" Catalysts 15, no. 5: 482. https://doi.org/10.3390/catal15050482
APA StyleZeng, Y., Tang, Y., Xu, M., Wang, D., Wang, Z., Gao, Y., & Wang, L. (2025). Efficient Synthesis of Tetrasubstituted Furans via Lipase-Catalyzed One-Pot Sequential Multicomponent Reaction. Catalysts, 15(5), 482. https://doi.org/10.3390/catal15050482