Active Ag-, Fe-, and AC-Modified TiO2 Mesoporous Photocatalysts for Anionic and Cationic Dye Degradation
Abstract
1. Introduction
2. Results
2.1. Structural, Textural, and Morphological Properties of Mesoporous TiO2 Before and After Modification with C, P, Ag, and/or Fe
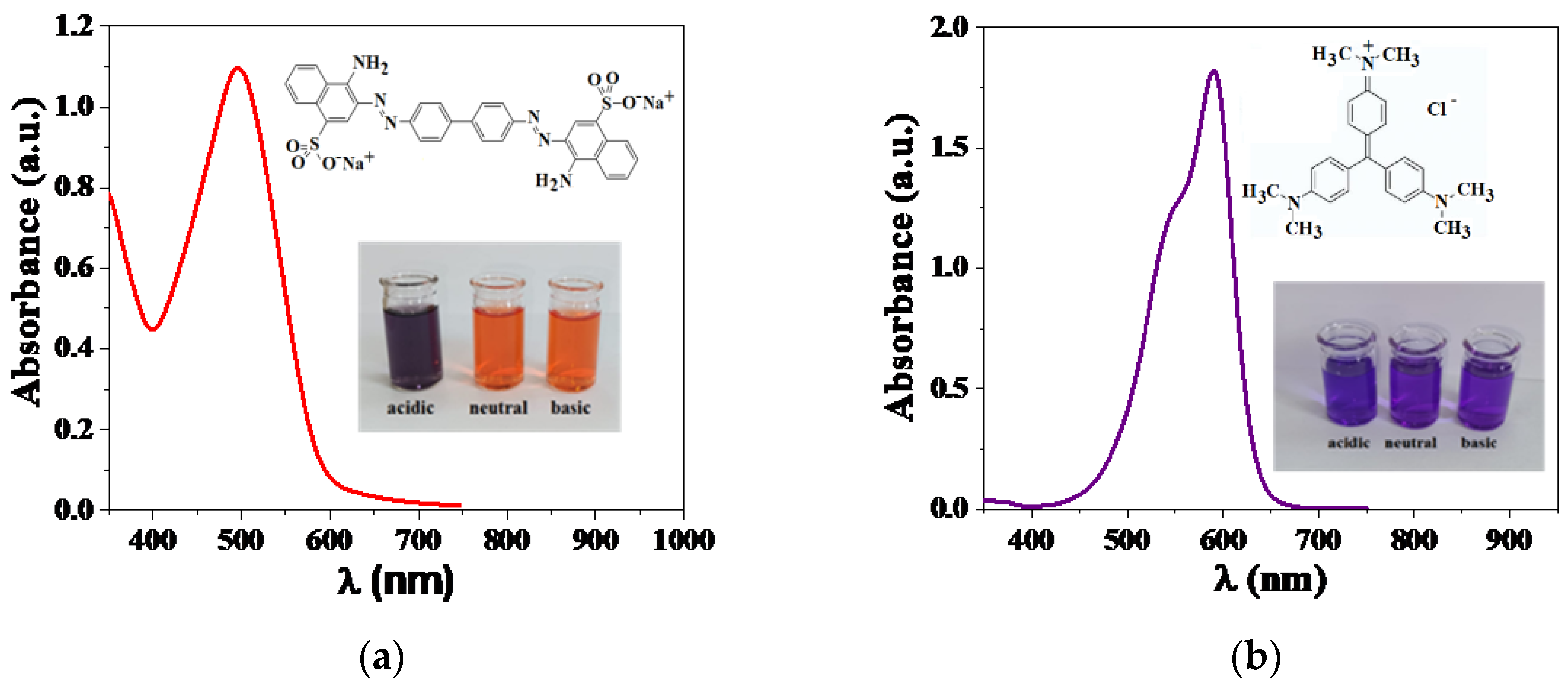
2.2. Surface Chemical Statesand Optical Properties of the Photocatalysts
2.3. Photocatalytic Degradation of Dyes
2.3.1. UV Light Irradiation
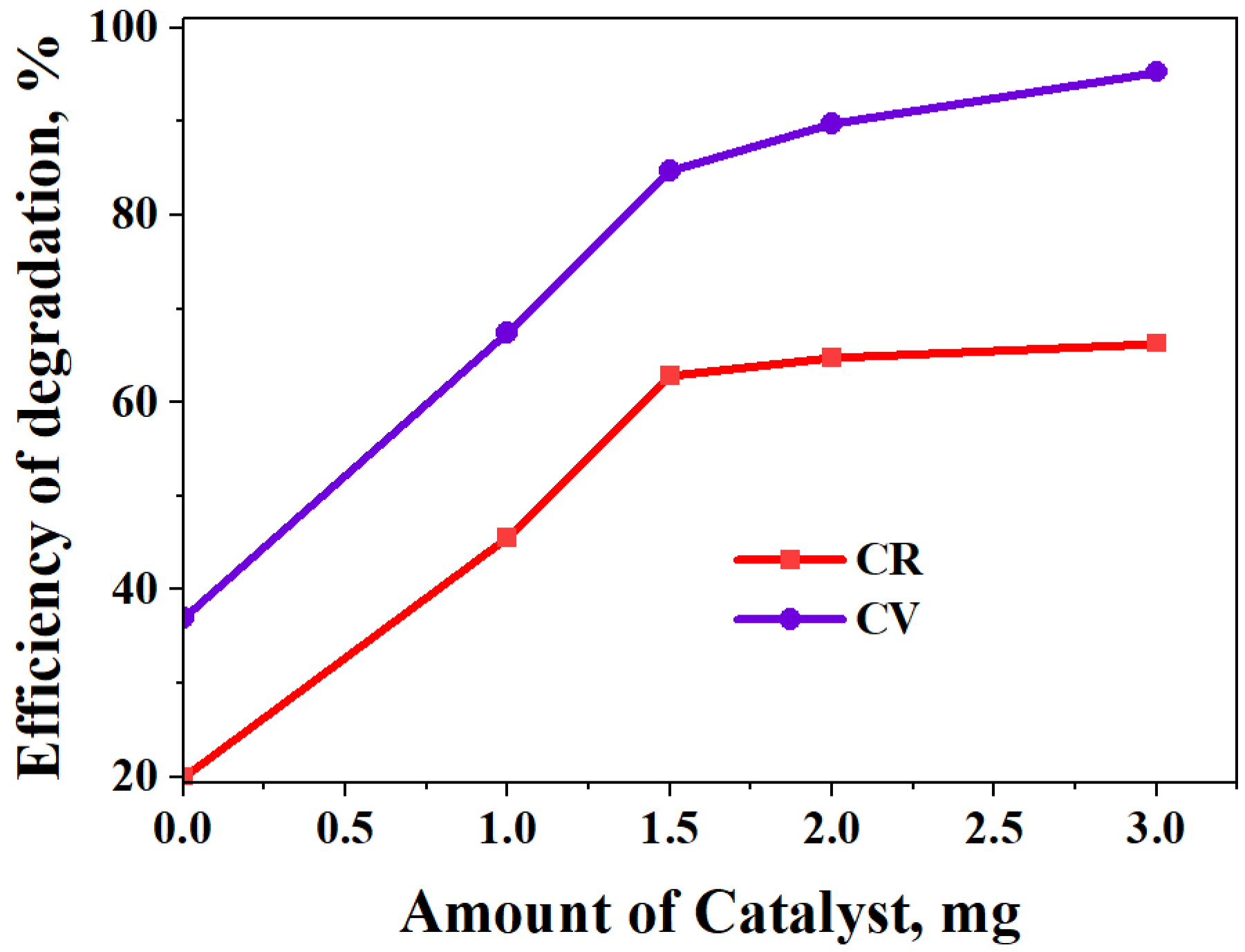
The Effect of the Catalyst Amount
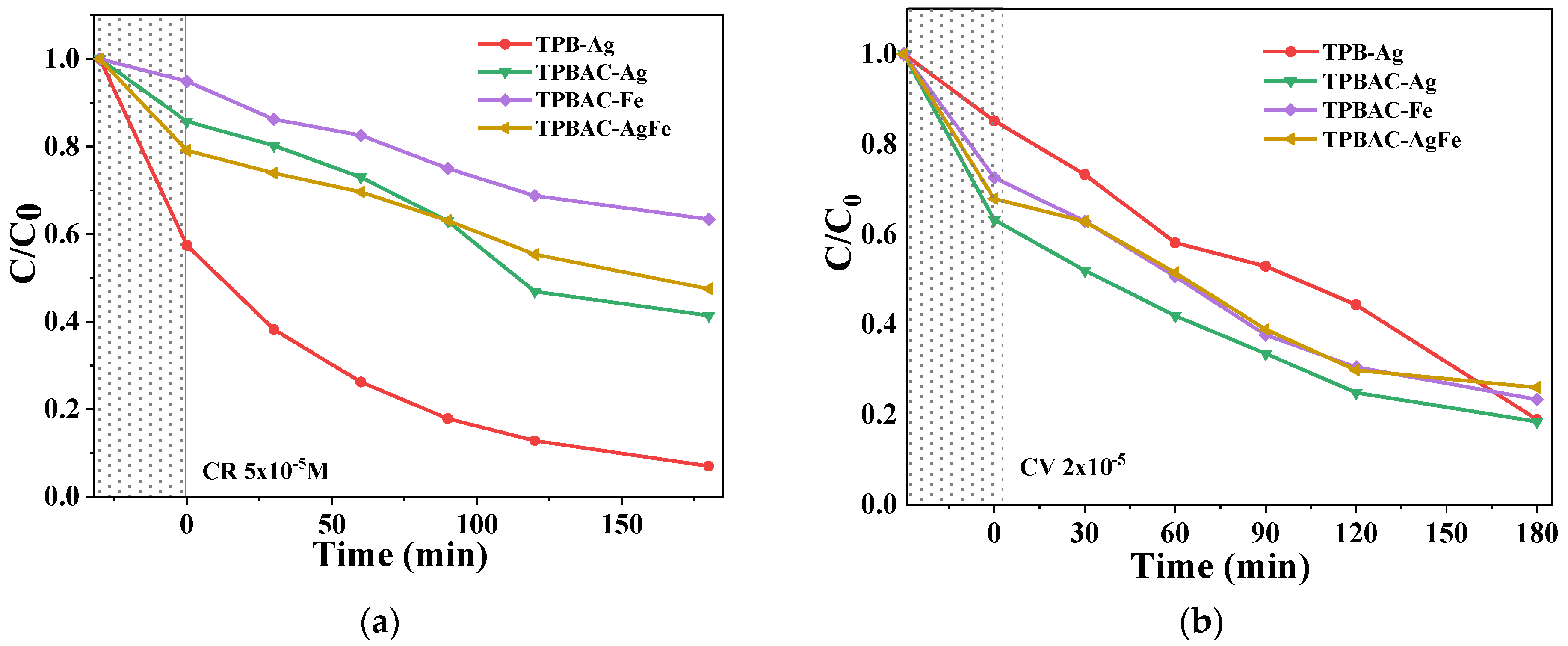
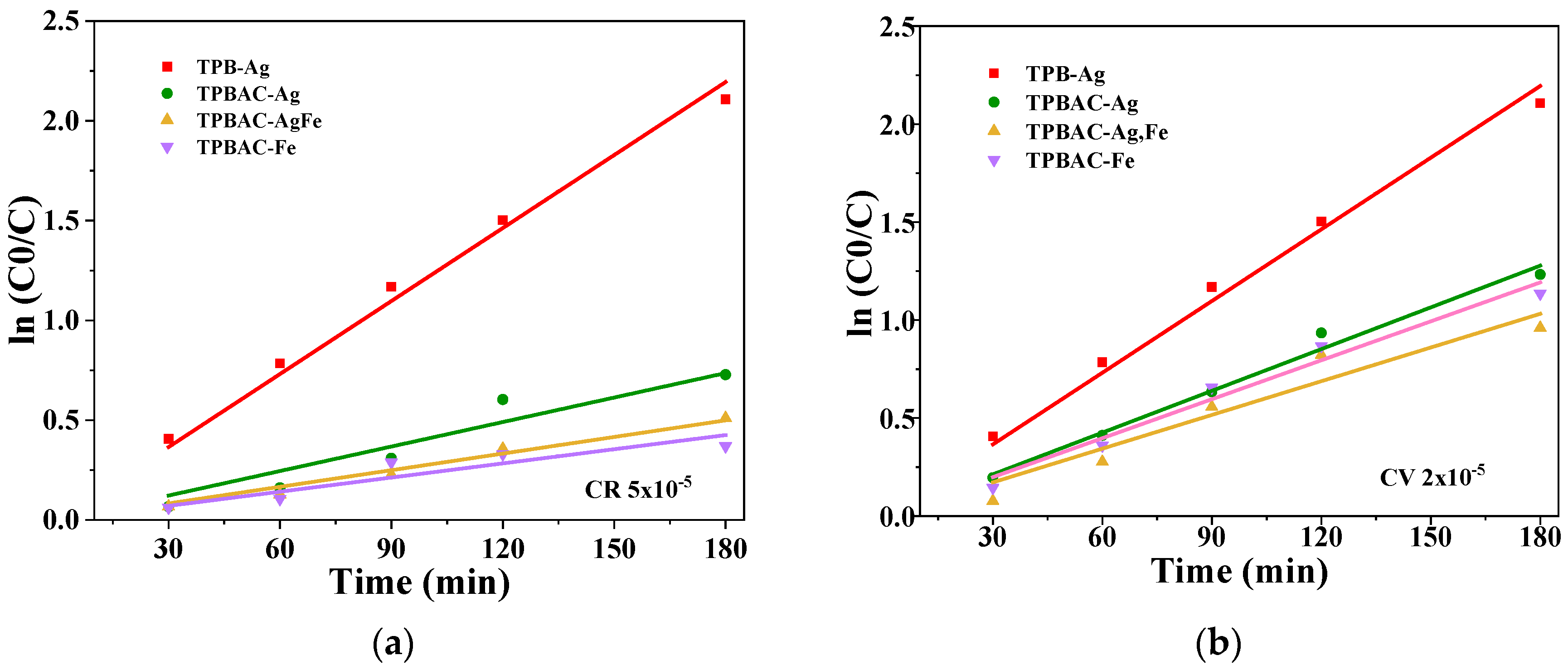
The Effect of the pH and Photodegradation Time
2.3.2. Visible Light Irradiation
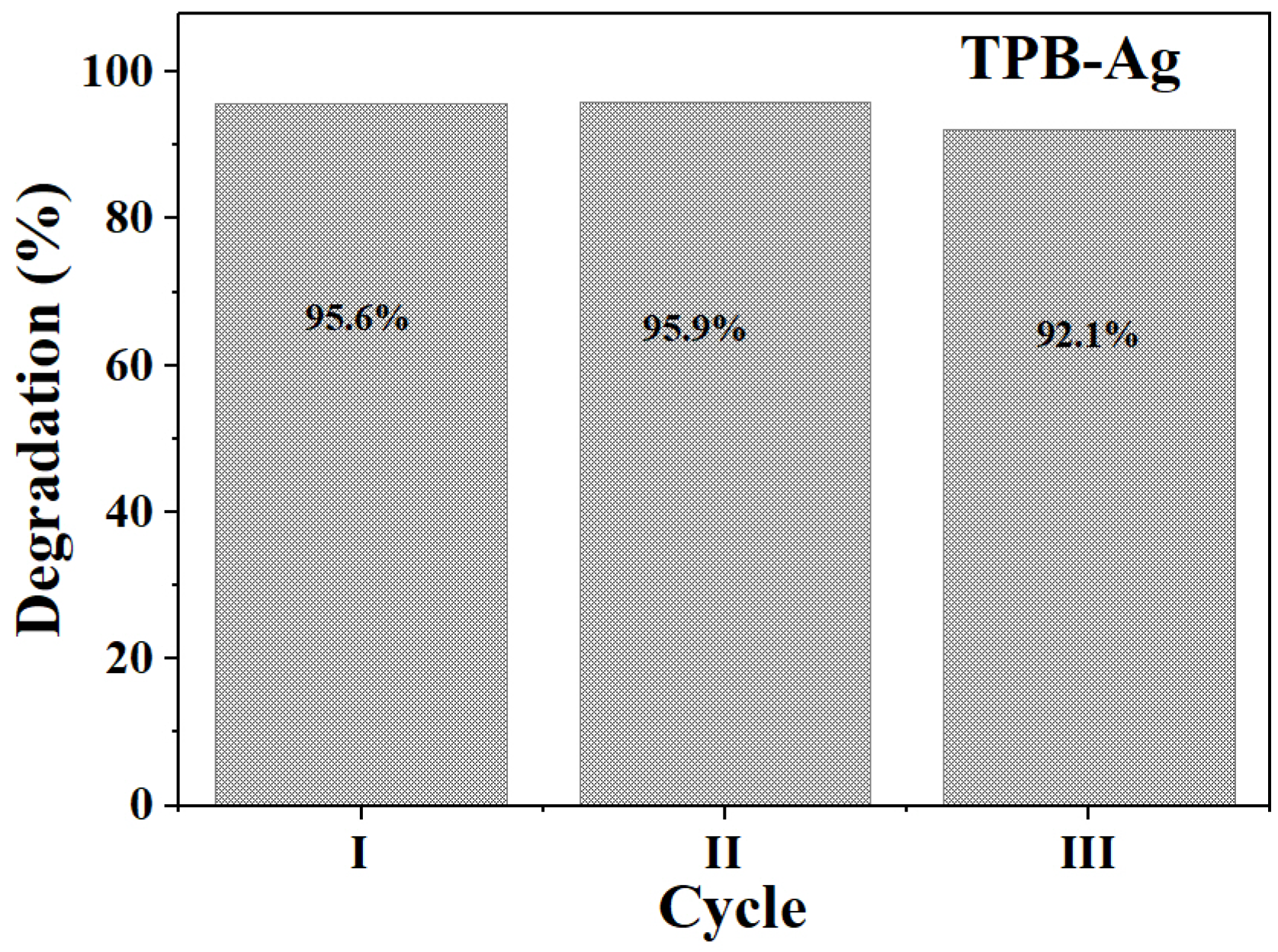
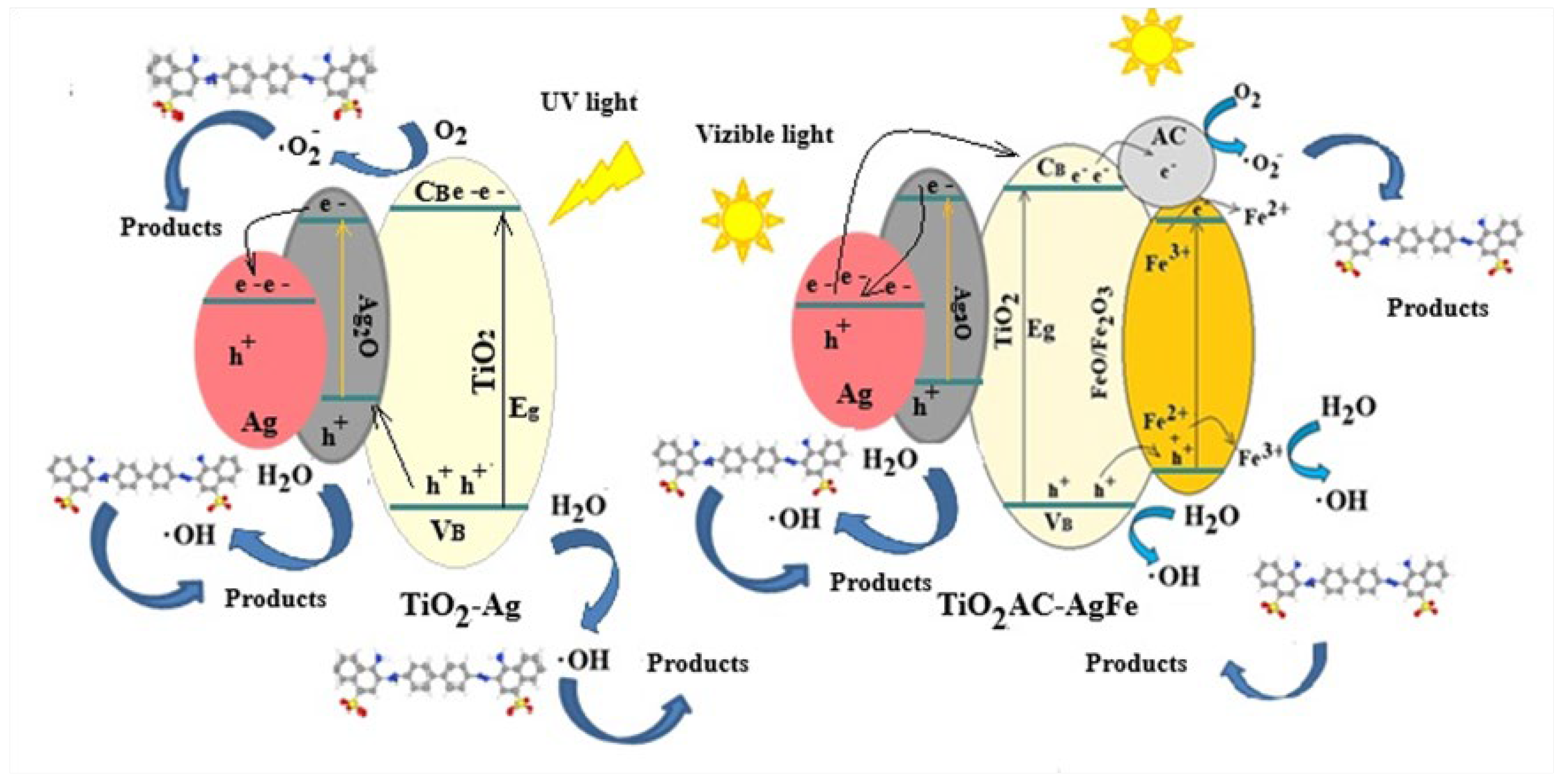
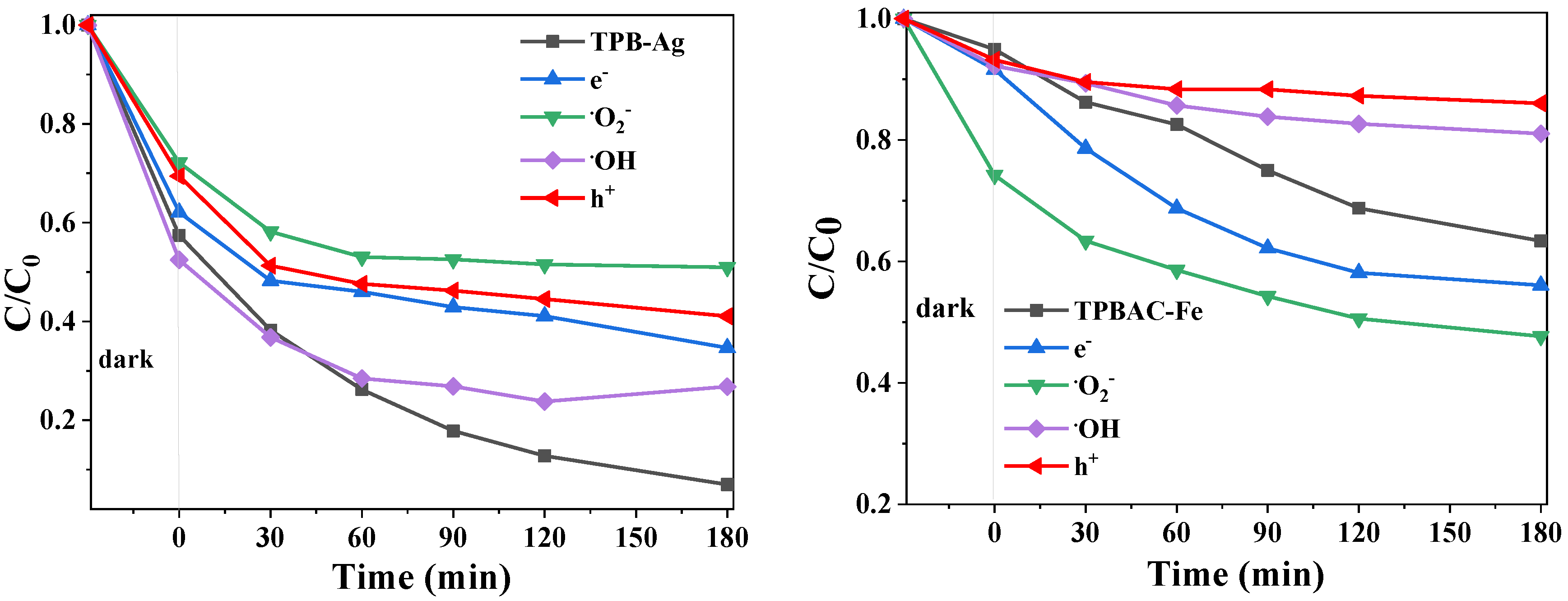
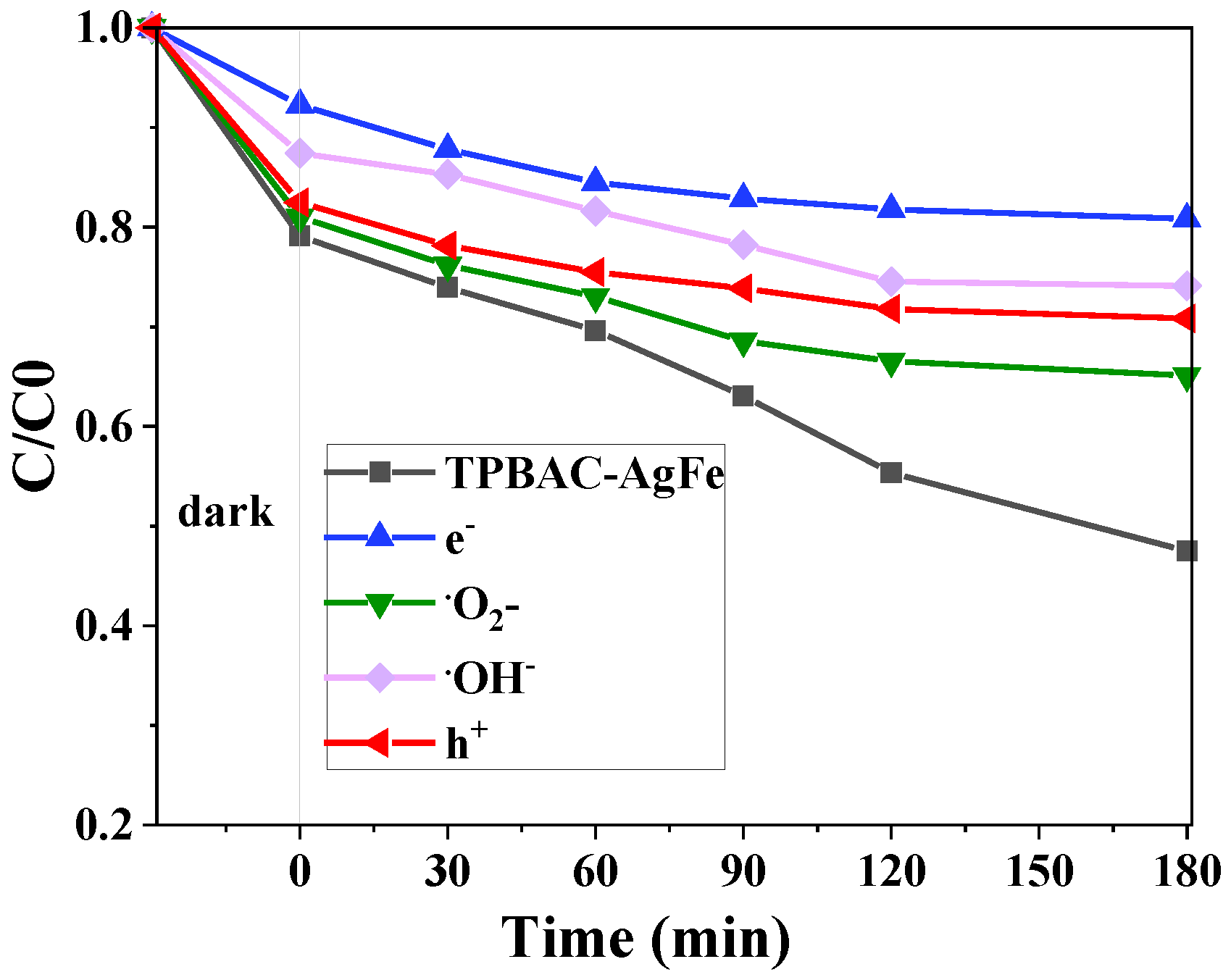
2.3.3. Mechanism of Photocatalytic Reaction
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Characterization Techniques
3.4. Photocatalytic Degradation Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Jiang, J.; Gao, W. Reviewing textile wastewater produced by industries: Characteristics, environmental impacts, and treatment strategies. Water Sci. Technol. 2022, 85, 2076–2096. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, A.P. Recent Advances in the Remediation of Textile-Dye-Containing Wastewater: Prioritizing Human Health and Sustainable Wastewater Treatment. Sustainability 2024, 16, 495. [Google Scholar] [CrossRef]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of textile dyes on health and ecosystem: A review of structure, causes, and potential solutions. Environ. Sci. Pollut. Res. 2023, 30, 9207–9242. [Google Scholar] [CrossRef]
- Zaharia, C.; Suteu, D. Textile organic dyes—Characteristics, polluting effects and separation/elimination procedures from industrial effluents—A critical overview. In Organic Pollutants Ten Years After the Stockholm Convention—Environmental and Analytical Update; Puzyn, T., Mostrag-Szlichtyng, A., Eds.; IntechOpen: Rijeka, Croatia, 2012; Volume 3, pp. 55–86. [Google Scholar]
- Sahoo, C.; Gupta, A.K.; Pal, A. Photocatalytic degradation of Crystal Violet (C.I. Basic Violet 3) on silver ion doped TiO2. Dye. Pigment. 2005, 66, e189–e196. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Irfan, A. Removal of Congo red dye from aqueous medium by its catalytic reduction using sodium borohydride in the presence of various inorganic nano-catalysts: A review. J. Clean. Prod. 2018, 187, e296–e307. [Google Scholar] [CrossRef]
- Negoescu, D.; Atkinson, I.; Gherendi, M.; Culita, D.C.; Baran, A.; Petrescu, S.; Trica, B.; Pelinescu, D.; Ionescu, R.; Bratan, V.; et al. Brij 58–activated carbon assisted synthesis of Ag/Ag2O/TiO2-AC photocatalysts for efficient organic pollutants degradation. J. Alloys Compd. 2023, 931, 167528. [Google Scholar] [CrossRef]
- Hammud, H.H.; Traboulsi, H.; Karnati, R.K.; Bakir, E.M. Photodegradation of Congo Red by Modified P25-Titanium Dioxide with Cobalt-Carbon Supported on SiO2 Matrix, DFT Studies of Chemical Reactivity. Catalysts 2022, 12, 248. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium dioxide as the most used photocatalyst for water purification: An overview. Catalysts 2022, 13, 26. [Google Scholar] [CrossRef]
- Negoescu, D.; Culita, D.C.; Atkinson, I.; Bratan, V.; Petrescu, S.; Parvulescu, V. Ti-SBA-15 Mesoporous photocatalysts modified with lanthanides for degradation of dyes in aqueous solution. Rev. Roum. Chim. 2021, 66, 287. [Google Scholar] [CrossRef]
- Predoană, L.; Ciobanu, E.M.; Petcu, G.; Preda, S.; Pandele-Cușu, J.; Anghel, E.M.; Petrescu, S.V.; Culita, D.C.; Baran, A.; Surdu, V.-A.; et al. Photocatalytic Performance of Sn–Doped TiO2 Nanopowders for Photocatalytic Degradation of Methyl Orange Dye. Catalysts 2023, 13, 534. [Google Scholar] [CrossRef]
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.-S.; Jeon, B.-H.; Park, Y.-K. A review on recent advances in the treatment of dye-polluted wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Wu, J.; Zhou, W. Recent progress in defective TiO2 photocatalysts for energy and environmental applications. Renew. Sustain. Energy Rev. 2022, 156, 111980. [Google Scholar] [CrossRef]
- Chauke, N.M.; Mohlala, R.L.; Ngqoloda, S.; Raphulu, M.C. Harnessing visible light: Enhancing TiO2 photocatalysis with photosensitizers for sustainable and efficient environmental solutions. Front. Chem. Eng. 2024, 6, 1356021. [Google Scholar] [CrossRef]
- Niu, B.; Wang, X.; Wu, K.; He, X.; Zhang, R. Mesoporous Titanium Dioxide: Synthesis and Applications in Photocatalysis, Energy and Biology. Materials 2018, 11, 1910. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; Guo, X.; Dai, J.; Xiang, J. Fast Sol–Gel Method to Prepare Mesoporous TiO2 with High Photocatalytic Activity. Nano 2015, 10, 1550001. [Google Scholar] [CrossRef]
- Kachbouri, S.; Elaloui, E.; Moussaoui, Y. The Effect of Surfactant Chain Length and Type on the Photocatalytic Activity of Mesoporous TiO2 Nanoparticles Obtained via Modified Sol-Gel Process. Iran. J. Chem. Chem. Eng. 2019, 93, 17. [Google Scholar]
- Muniz, E.C.; Góes, M.S.; Silva, J.J.; Varela, J.A.; Joanni, E.; Parra, R.; Bueno, P.R. Synthesis and characterization of mesoporous TiO2 nanostructured films prepared by a modified sol–gel method for application in dye solar cells. Ceram. Int. 2011, 37, 1017. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Dell’Edera, M.; Agostiano, A.; Curri, M.L.; Comparelli, R. Scalable Synthesis of Mesoporous TiO2 for Environmental Photocatalytic Applications. Materials 2019, 12, 1853. [Google Scholar] [CrossRef]
- Velázquez-Martínez, S.; Silva-Martínez, S.; Pineda-Arellano, C.A.; Jiménez-González, A.; Salgado-Tránsito, I.; Morales-Pérez, A.A.; Peña-Cruz, M.I. Modified sol-gel/hydrothermal method for the synthesis of microsized TiO2 and iron-doped TiO2, its characterization and solar photocatalytic activity for an azo dye degradation. J. Photochem. Photobiol. A Chem. 2018, 359, 93. [Google Scholar] [CrossRef]
- Islam, M.T.; Roni, M.N.P.; Ali, M.Y.; Islam, M.R.; Hossan, M.S.; Rahman, M.H.; Zahid, A.A.S.M.; Alam, M.N.E.; Hanif, M.A.; Akhtar, M.S. Selectivity of Sol-Gel and Hydrothermal TiO2 Nanoparticles towards Photocatalytic Degradation of Cationic and Anionic Dyes. Molecules 2023, 28, 6834. [Google Scholar] [CrossRef]
- Cano-Casanova, L.; Amorós-Pérez, A.; Ángeles Lillo-Ródenas, M.; del Carmen Román-Martínez, M. Effect of the Preparation Method (Sol-Gel or Hydrothermal) and Conditions on the TiO2 Properties and Activity for Propene Oxidation. Materials 2018, 11, 2227. [Google Scholar] [CrossRef]
- Kao, L.H.; Hsu, T.; Lu, H. Sol–gel synthesis and morphological control of nanocrystalline TiO2 via urea treatment. J. Colloid Interface Sci. 2007, 316, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhou, J.; Liu, Y.; Yang, Y.; Zheng, S.; Wang, Q. Constructing Ag2O nanoparticle modified TiO2 nanotube arrays for enhanced photocatalytic performances. J. Alloys Compd. 2020, 849, 56493. [Google Scholar] [CrossRef]
- Patil, S.B.; Basavarajappa, P.S.; Ganganagappa, N.; Jyothi, M.S.; Raghu, A.V.; Reddy, K.R. Recent advances in non-metals-doped TiO2 nanostructured photocatalysts for visible-light driven hydrogen production, CO2 reduction and air purification. Int. J. Hydrogen Energy 2019, 44, 13022. [Google Scholar] [CrossRef]
- Yalçin, Y.; Kiliç, M.; Cinar, Z. The Role of Non-Metal Doping in TiO2 Photocatalysis. J. Adv. Oxid. Technol. 2010, 13, 281. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Jheng, H.-K.; Siang-En Syu, S.-E. Effect of non-metal doping on the photocatalytic activity of titanium dioxide on the photodegradation of aqueous bisphenol A. Environ. Technol. 2021, 42, 1603. [Google Scholar] [CrossRef]
- Sultana, M.; Mondal, A.; Islam, S.; Khatun, M.A.; Rahaman, M.H.; Chakraborty, A.K.; Rahman, M.S.; Rahman, M.M.; Nur, A.S.M. Strategic development of metal doped TiO2 photocatalysts for enhanced dye degradation activity under UV–Vis irradiation: A review. Curr. Res. Green Sustain. 2023, 7, 100383. [Google Scholar] [CrossRef]
- Negoescu, D.; Bratan, V.; Gherendi, M.; Atkinson, I.; Culita, D.C.; Neacsu, A.; Baran, A.; Petrescu, S.; Parvulescu, V. Iron Promoted TiO2-Activated Carbon Nanocomposites for Photocatalytic Degradation of Congo Red in Water. Catalysts 2024, 14, 844. [Google Scholar] [CrossRef]
- Peñas-Garzón, M.; Gómez-Avilés, A.; Belver, C.; Rodriguez, J.J.; Bedia, J. Degradation pathways of emerging contaminants using TiO2-activated carbon heterostructures in aqueous solution under simulated solar light. J. Chem. Eng. 2020, 392, 124867. [Google Scholar] [CrossRef]
- Ren, W.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B Environ. 2007, 69, 138. [Google Scholar] [CrossRef]
- Wang, X.; Xue, X.; Liu, X.; Xing, X.; Li, Q.; Yang, J. Synergistic effect of single-electron-trapped oxygen vacancies and carbon species on the visible light photocatalytic activity of carbon-modified TiO2. Mater. Chem. Phys. 2015, 153, 117. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Diwvyaanak Kutiang, F.; Lim, Y.C.; Goh, P.S. Recent progress of Ag/TiO2 photocatalyst for wastewater treatment: Doping, co-doping, and green materials functionalization. Appl. Mater. Today 2022, 27, 101500. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; el kacem Qaiss, A.; Rachid Bouhfid, R. Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638. [Google Scholar] [CrossRef]
- Gogoi, D.; Namdeo, A.; Golder, A.K.; Peela, N.R. Ag-doped TiO2 photocatalysts with effective charge transfer for highly efficient hydrogen production through water splitting. Int. J. Hydrogen Energy 2020, 45, 2729. [Google Scholar] [CrossRef]
- Kumar, M.K.; Bhavani, K.; Naresh, G.; Srinivas, B.; Venugopal, A. Plasmonic resonance nature of Ag-Cu/TiO2 photocatalyst under solar and artificial light: Synthesis, characterization and evaluation of H2O splitting activity. Appl. Catal. B Environ. 2016, 199, 282. [Google Scholar]
- Leong, K.H.; Gan, B.L.; Ibrahim, S.; Saravanan, P. Synthesis of surface plasmon resonance (SPR) triggered Ag/TiO2 photocatalyst for degradation of endocrine disturbing compounds. Appl. Surf. Sci. 2014, 319, 128. [Google Scholar] [CrossRef]
- Wu, F.; Hu, X.; Fan, J.; Liu, E.; Sun, T.; Kang, L.; Hou, W.; Zhu, C.; Liu, H. Photocatalytic Activity of Ag/TiO2 Nanotube Arrays Enhanced by Surface Plasmon Resonance and Application in Hydrogen Evolution by Water Splitting. Plasmon 2013, 8, 501. [Google Scholar] [CrossRef]
- Handoko, T.; Moustakas, N.G.; Peppel, T.; Springer, A.; Oropeza, F.E.; Huda, A.; Bustan, M.D.; Yudono, B.; Gulo, F.; Strunk, J. Characterization and effect of Ag(0) vs. Ag(I) species and their localized plasmon resonance on photochemically inactive TiO2. Catalysts 2019, 9, 323. [Google Scholar] [CrossRef]
- Awazu, K.; Fujimaki, M.; Rockstuhl, C.; Tominaga, J.; Murakami, H.; Ohki, Y.; Yoshida, N.; Watanabe, T.A. Plasmonic Photocatalyst Consisting of Silver Nanoparticles Embedded in Titanium Dioxide. J. Am. Chem. Soc. 2008, 130, 1676. [Google Scholar] [CrossRef] [PubMed]
- Chena, C.; Leic, X.F.; Xue, M.Z. A simple method to synthesise Ag-doped TiO2 photocatalysts with different Ag0:Ag2O atomic ratios for enhancing visible-light photocatalytic activity. J. Chem. Res. 2017, 41, 475. [Google Scholar] [CrossRef]
- Han, G.; Kim, J.Y.; Kim, K.-J.; Lee, H.; Kim, Y.-M. Controlling surface oxygen vacancies in Fe-doped TiO2 anatase nanoparticles for superior photocatalytic activities. Appl. Surf. Sci. 2020, 507, 144916. [Google Scholar] [CrossRef]
- Adormaa, B.B.; Darkwah, W.K.; Ao, Y. Oxygen vacancies of the TiO2 nano-based composite photocatalysts in visible light responsive photocatalysis. RSC Adv. 2018, 8, 33551. [Google Scholar] [CrossRef]
- Galkina, O.L.; Vinogradov, V.V.; Agafonov, A.V.; Vinogradov, A.V. Surfactant-assisted sol-gel synthesis of TiO2 with uniform particle size distribution. Int. J. Inorg. Chem. 2011, 2011, 108087. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Søgaard, E.G. Sol–gel reactions of titanium alkoxides and water: Influence of pH and alkoxy group on cluster formation and properties of the resulting products. J. Solgel. Sci. Technol. 2010, 53, 485. [Google Scholar] [CrossRef]
- Pandele-Cusu, J.; Petrescu, S.; Preda, S.; Petcu, G.; Ciobanu, M.; Predoana, L. Comparative study of the TiO2 nanopowders prepared from different precursors and chemical methods for heterogeneous photocatalysis application. J. Therm. Anal. Calorim. 2022, 147, 13111. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Jean Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051. [Google Scholar] [CrossRef]
- Ismail, M.A.; Hedhili, M.N.; Anjum, D.H.; Singaravelu, V.; Chung, S.H. Synthesis and Characterization of Iron-Doped TiO2 Nanoparticles Using Ferrocene from Flame Spray Pyrolysis. Catalysts 2021, 11, 438. [Google Scholar] [CrossRef]
- Tabakova, T.; Nikolova, D.; Ivanov, I.; Anghel, E.M.; Karashanova, D.; Karakirova, Y.; Venezia, A.M.; Vakros, J.; Crişan, M.; Tenchev, K.; et al. Study of ceria-doped Au/TiO2 catalysts for boosting hydrogen production by water-gas shift reaction. Int. J. Hydrogen Energy 2024, 70, 389. [Google Scholar] [CrossRef]
- Cui, Y.; Ma, Q.; Deng, X.; Meng, Q.; Cheng, X.; Xie, M.; Li, X.; Cheng, Q.; Liu, H. Fabrication of Ag-Ag2O/reduced TiO2 nanophotocatalyst and its enhanced visible light driven photocatalytic performance for degradation of diclofenac solution. Appl. Catal. B Environ. 2017, 206, 136. [Google Scholar] [CrossRef]
- Indudhar, P.V.; Anusha, B.S.; Pruthvija, M.; Savitha, S.; Ravindra, S.; Nagaveni, M.; Poojitha, P.S.; Swathi, N. Bamboo and coconut shell based activated carbon: A Raman spectroscopic study. Mater. Chem. Phys. 2024, 318, 129240. [Google Scholar]
- Vasquez, C.G.; Andrea Peche-Herrero, M.; Maestre, D.; Aleman, B.; Ramırez-Castellanos, J.; Cremades, A.; Gonzalez-Calbet, J.M.; Piqueras, J. Influence of Fe and Al doping on the stabilization of the anatase phase in TiO2 nanoparticles. J. Mater. Chem. C 2014, 2, 10377. [Google Scholar] [CrossRef]
- Yu, C.C.; Chiang, K.Y.; Okuno, M.; Seki, T.; Ohto, T.; Yu, X.; Korepanov, V.; Hamaguchi, H.; Nagata, Y. Vibrational couplings and energy transfer pathways of water’s bending mode. Nat. Commun. 2020, 11, 5977. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Fu, F.; Niu, L.; Jin, M.; Wang, X. Effect of support on hydrogen generation over iron oxides in the chemical looping process. RSC Adv. 2021, 11, 37552. [Google Scholar] [CrossRef] [PubMed]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564. [Google Scholar] [CrossRef]
- Xue, J.; Bao, J. Interfacial charge transfer of heterojunction photocatalysts: Characterization and calculation. Surf. Interfaces 2010, 25, 101265. [Google Scholar] [CrossRef]
- Zhang, L.; Mohamed, H.H.; Dillert, R.; Bahnemann, D. Kinetics and mechanisms of charge transfer processes in photocatalytic systems: A review. J. Photochem. Photobio. C Photochem. Rev. 2012, 13, 263–276. [Google Scholar] [CrossRef]
- State, R.-N.; Morosan, M.-A.; Cretu, L.; Straca, A.-I.; Vasile, A.; Bratan, V.; Culita, D.; Atkinson, I.; Balint, I.; Papa, F. The Effect of the Metal Oxide as the Support for Silver Nanoparticles on the Catalytic Activity for Ammonia Ozonation. Catalysts 2025, 15, 104. [Google Scholar] [CrossRef]
- Loan, T.T.; Long, N.N. Effect of Co2+ doping on Raman spectra and the phase transformation of TiO2: Co2+ nanowires. J. Phys. Chem. Solids 2019, 124, 336–342. [Google Scholar] [CrossRef]
- Ghamsari, M.S.; Gaeeni, M.R.; Han, W.; Park, H.H. Highly stable colloidal TiO2 nanocrystals with strong violet-blue emission. J. Lumin. 2016, 178, 89. [Google Scholar] [CrossRef]
- Rej, S.; Hejazi, S.H.; Badura, Z.; Zoppellaro, G.; Kalytchuk, S.; Kment, S.; Fornasiero, P.; Naldoni, A. Light-induced defect formation and Pt single atoms synergistically boost photocatalytic H2 production in 2D TiO2-bronze nanosheets. ACS Sustain. Chem. Eng. 2022, 10, 17286. [Google Scholar] [CrossRef]
- Csillag, K.; Emri, T.; Rangel, D.E.; Pócsi, I. pH-dependent effect of Congo Red on the growth of Aspergillus nidulans and Aspergillus niger. Fungal Biol. 2023, 127, 1180. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Harrache, Z.; Trari, M. Removal of gentian violet in aqueous solution by activated carbon equilibrium, kinetics, and thermodynamic study. Adsorpt. Sci. Technol. 2019, 37, 566. [Google Scholar] [CrossRef]
- Boulahbel, H.; Benamira, M.M.; Bouremmad, F.; Ahmia, N.; Kiamouche, S.; Lahmar, H.; Souici, A.; Trari, M. Enhanced photodegradation of Congo red dye under sunlight irradiation by p-n NiFe2O4/TiO2 heterostructure. Inorg. Chem. Commun. 2023, 154, 110921. [Google Scholar] [CrossRef]
- Wahab, H.S.; Hussain, A.A. Photocatalytic oxidation of phenol red onto nanocrystalline TiO2 particles. J. Nanostruct. Chem. 2016, 6, 261. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.S.W.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Z.; Tian, F.; Ye, B.C.; Tong, Y. Synthesis of N and La co-doped TiO2/AC photocatalyst by microwave irradiation for the photocatalytic degradation of naphthalene. J. Alloys Compd. 2016, 676, 489. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Ibrahim, S.S.; Shahien, M.G. Photocatalytic degradation and photo-Fenton oxidation of Congo red dye pollutants in water using natural chromite-response surface optimization. Appl. Water Sci. 2017, 7, 4743. [Google Scholar] [CrossRef]
- Elaziouti, A.; Ahmed, B. ZnO-Assisted Photocatalytic Degradation of Congo Red and Benzopurpurine 4B in Aqueous Solution. J. Chem. Eng. Process Technol. 2011, 2, 1. [Google Scholar]
- Fowsiya, J.; Madhumitha, G.; Al-Dhabi, N.A.; Arasu, M.V. Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 162, 395. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Said, A.; Ali, F.; Raziq, F.; Ali, Z.; Bilal, M.; Reinert, L.; Begum, T.; Iqbal, H.M.N. Photocatalytic Degradation of Congo Red Dye from Aqueous Environment Using Cobalt Ferrite Nanostructures: Development, Characterization, and Photocatalytic Performance. Water Air Soil Pollut. 2020, 231, 50. [Google Scholar] [CrossRef]
- Hitkari, G.; Chowdhary, P.; Kumar, V.; Singh, S.; Motghare, A. Potential of Copper-Zinc Oxide nanocomposite for photocatalytic degradation of congo red dye. Clean. Chem. Eng. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Turcu, E.; Coromelci, C.G.; Harabagiu, V.; Ignat, M. Enhancing the Photocatalytic Activity of TiO2 for the Degradation of Congo Red Dye by Adjusting the Ultrasonication Regime Applied in Its Synthesis Procedure. Catalysts 2023, 13, 345. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Priyadharsini, G.M.A.; Kaviyarasu, K.; Jothi, A.I.; Simiyon, G.G. Synthesis and characterization of TiO2 doped cobalt ferrite nanoparticles via microwave method: Investigation of photocatalytic performance of congo red degradation dye. Surf. Interfaces 2021, 25, 101296. [Google Scholar] [CrossRef]
- Saeed, M.; Albalawi, K.; Khan, I.; Akram, N.; Abd El-Rahim, I.H.; Alhag, S.K.; Ahmed, A.E. Synthesis of pn NiO-ZnO heterojunction for photodegradation of crystal violet dye. Alex. Eng. J. 2023, 65, 561. [Google Scholar] [CrossRef]
- Habib, M.A.; Muslim, M.; Shahadat, M.T.; Islam, M.N.; Ismail, I.M.I.; Islam, T.S.A.; Mahmood, A.J. Photocatalytic decolorization of crystal violet in aqueous nano-ZnO suspension under visible light irradiation. J. Nanostruct. Chem. 2013, 3, 70. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, Q.; Wang, X.; Kawazoe, N.; Yang, Y. Nonmetal-Metal-Semiconductor-Promoted P/Ag/Ag2O/Ag3PO4/TiO2 Photocatalyst with Superior Photocatalytic Activity and Stability. J. Mater. Chem. 2015, 3, 17858. [Google Scholar] [CrossRef]
- Wang, C.; Zhan, Z.; Liu, H.; Li, Y.; Wu, J.; Sun, P.; Shen, G. Single-atom iron cocatalyst for highly enhancing TiO2 photocatalytic degradation of antibiotics and antibiotic-resistant genes. Chem. Eng. J. 2024, 482, 148906. [Google Scholar] [CrossRef]
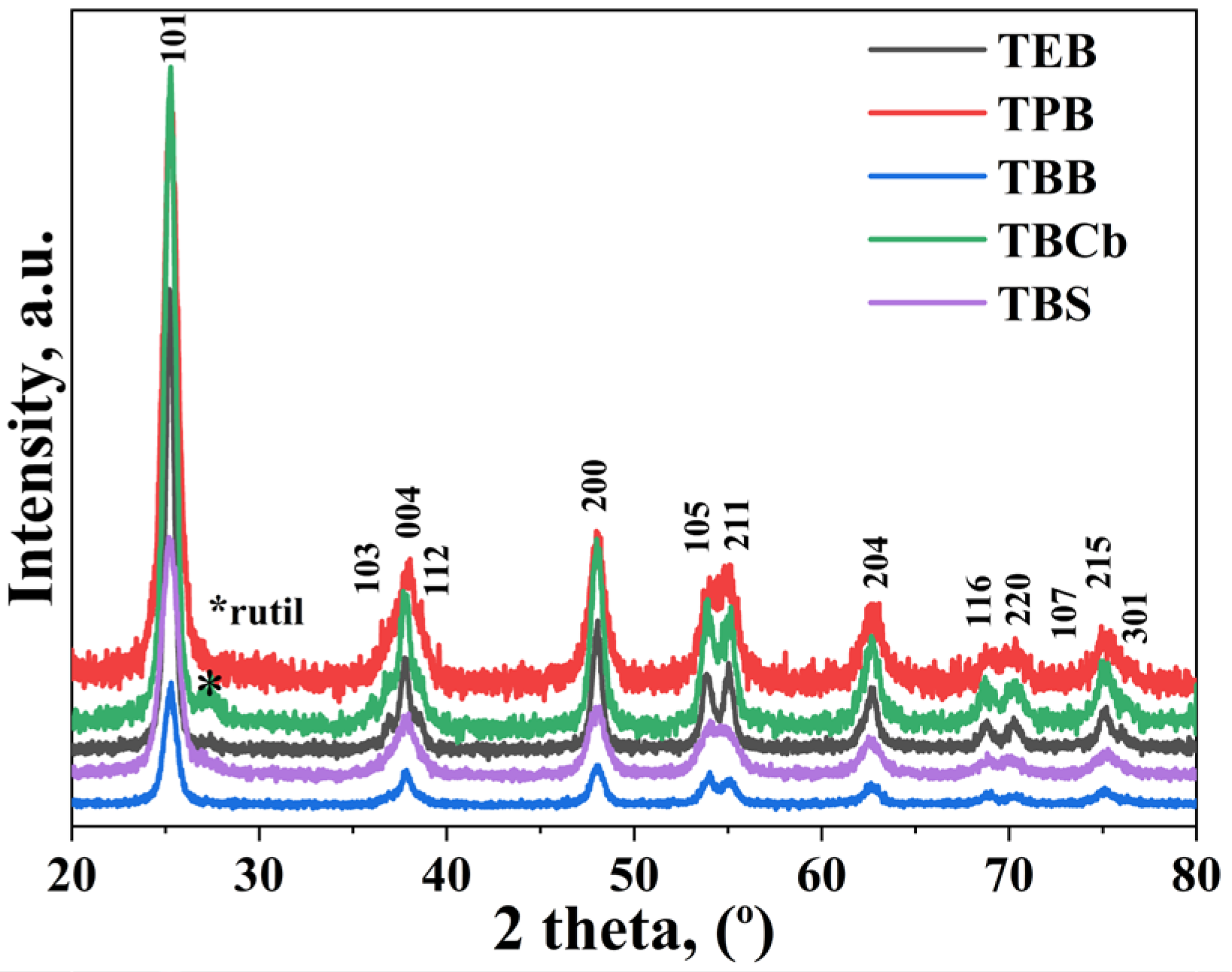


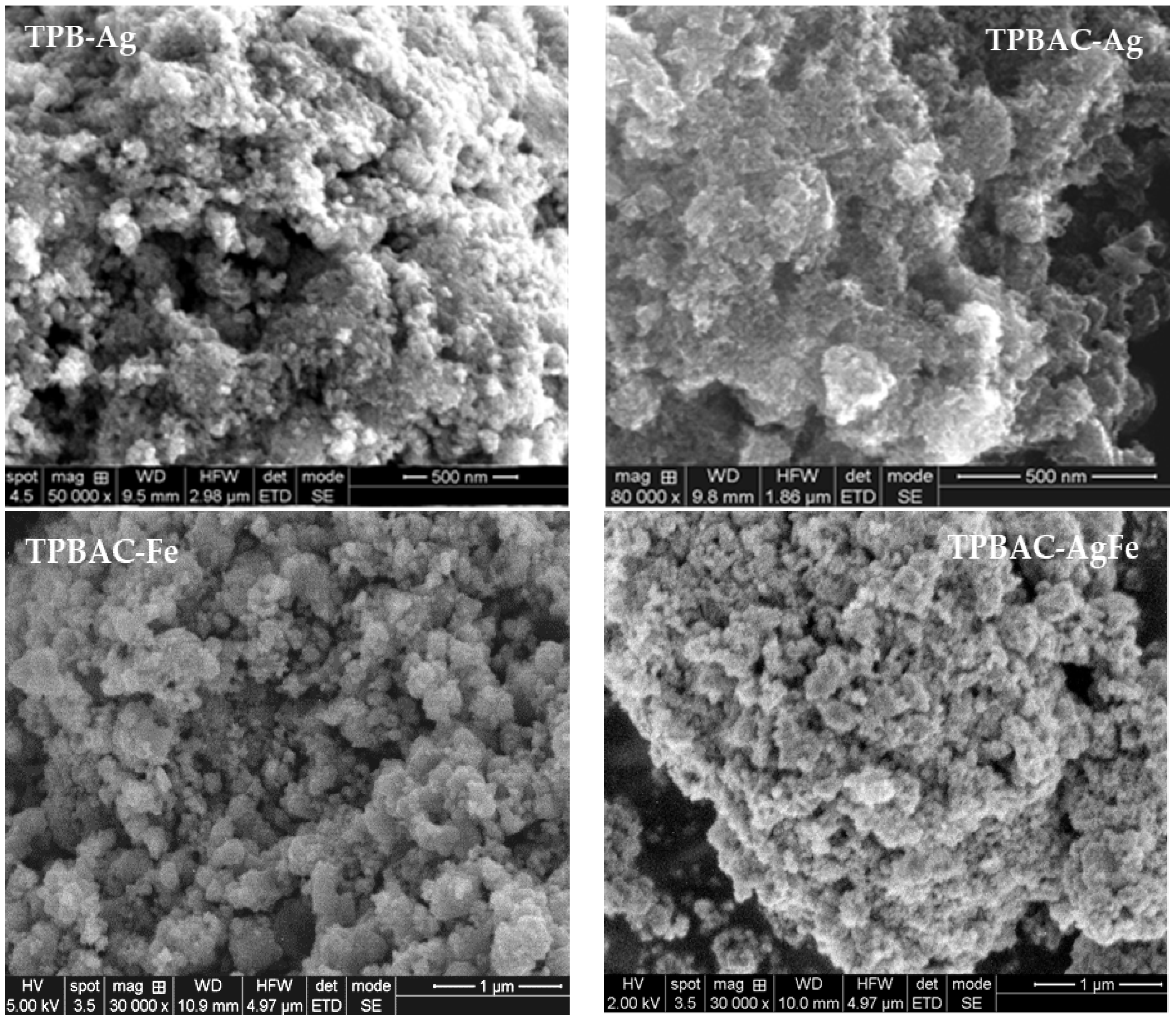
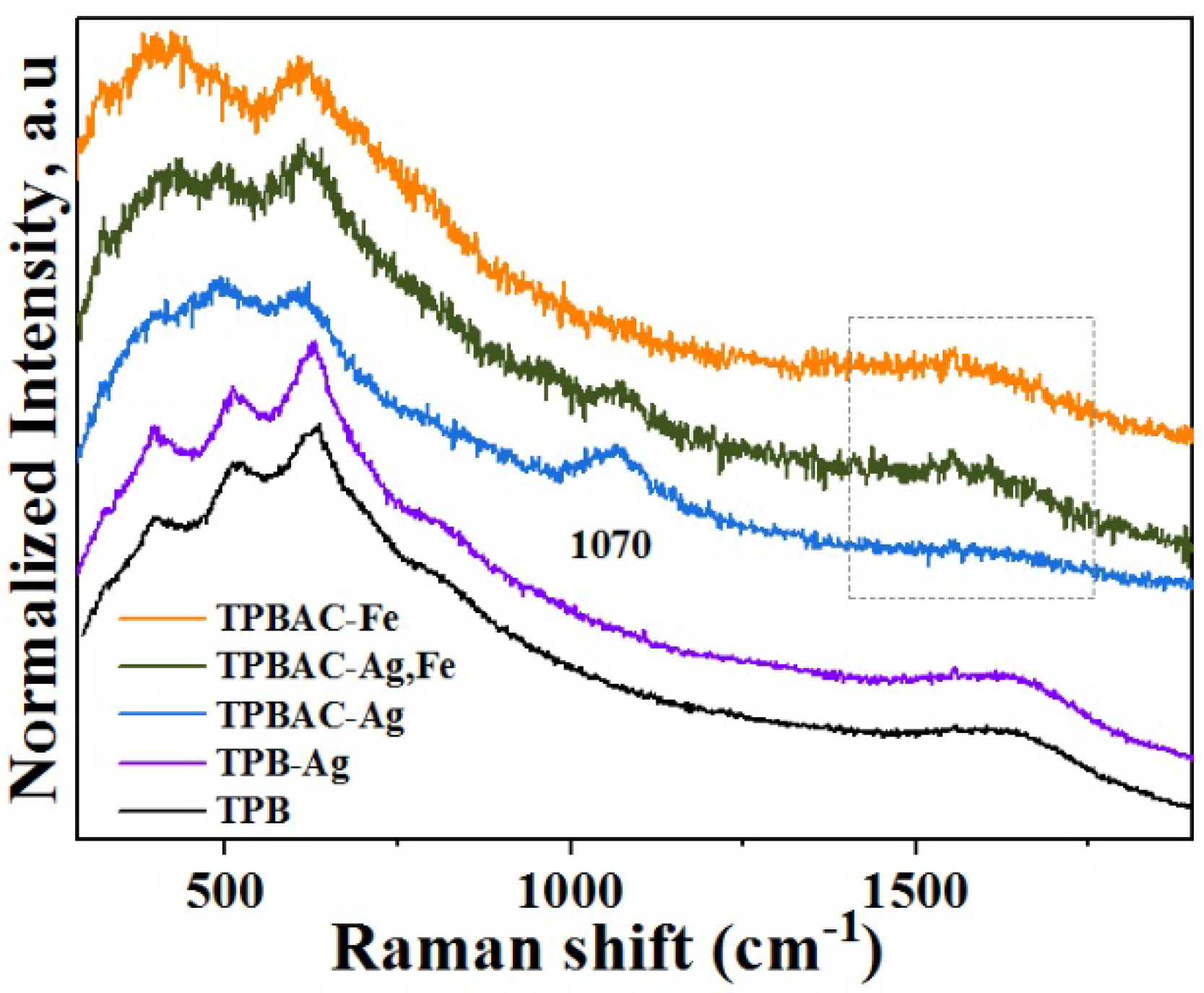



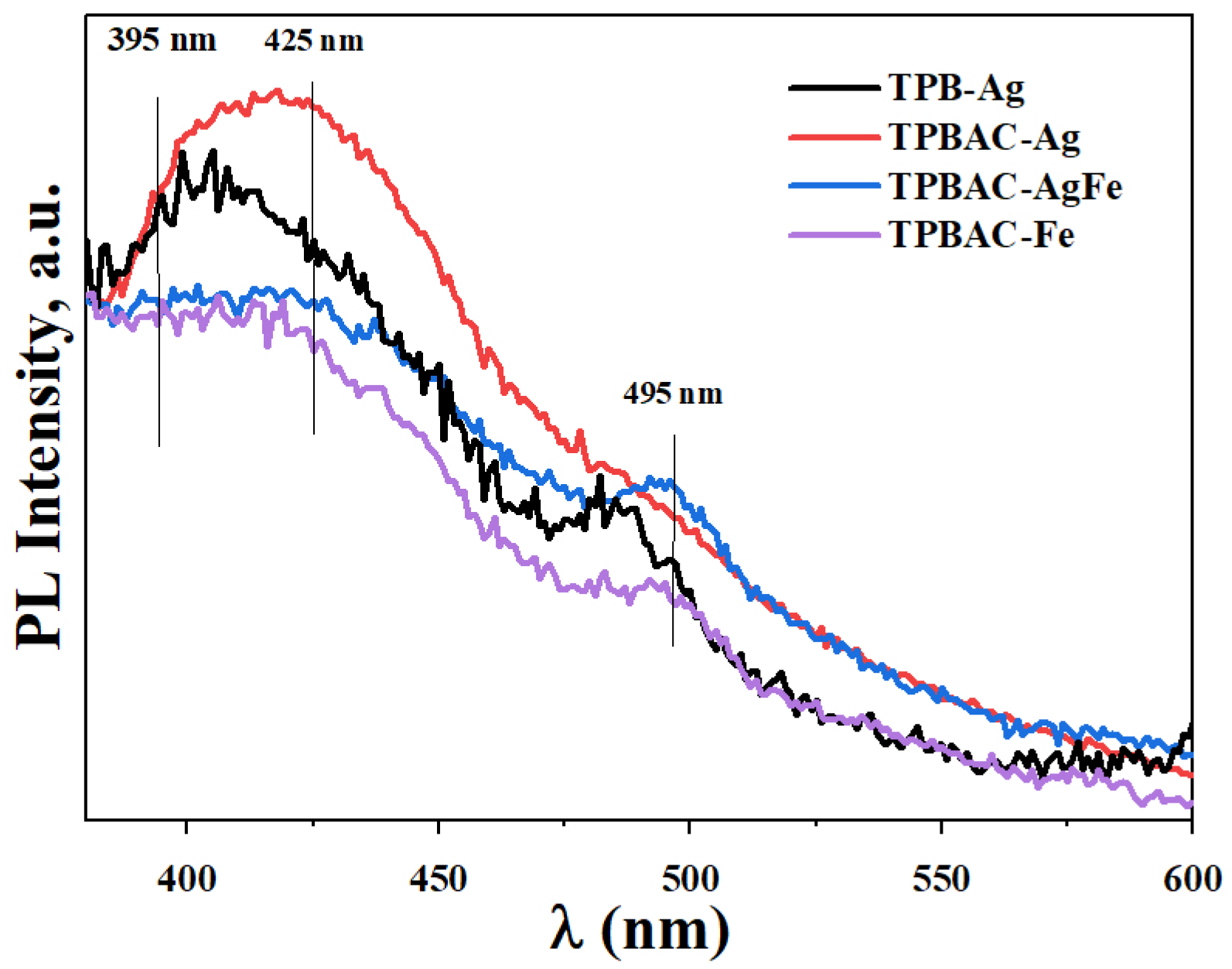



| Sample | Precursor/Surfactant | a = b | c | DA 1 (nm) | SBET 2 (m2/g) | Vp 3 (nm3) | Dp 4 (nm) |
|---|---|---|---|---|---|---|---|
| TEB | TET/Brij58 | 3.79 | 9.39 | 12.2 | 84.4 | 0.18 | 5.9 |
| TPB | TTIP/Brij58 | 3.76 | 9.37 | 8.4 | 90.2 | 0.20 | 6.4 |
| TBB | TBT/Brij58 | 3.78 | 9.44 | 9.2 | 105.6 | 0.23 | 6.1 |
| TBCb | TBT/CTAB | 3.78 | 9.44 | 8.9 | 74.2 | 0.24 | 9.8 |
| TBS | TBT/SDS | 3.78 | 9.42 | 8.2 | 25.0 | 0.08 | 9.8 |
| Sample | P (%) | C (%) | Ag (%) | Fe (%) | DA 1 (nm) | SBET 2 (m2/g) | Vp 3 (nm3) | Dp 4 (nm) | Eg 5 (eV) |
|---|---|---|---|---|---|---|---|---|---|
| TPB-Ag | - | - | 1.1 | - | 10.0 | 81.8 | 0.20 | 6.9 | 3.08 |
| TPBAC | 1.3 | 2.4 | - | - | 9.4 | 185.6 | 0.34 | 9 | 3.36 |
| TPBAC-Ag | 1 | 2.1 | 1.3 | - | 6.1 | 99.9 | 0.29 | 11.0 | 3.34 |
| TPBAC-AgFe | 0.8 | 1.9 | 1.2 | 1.2 | 5.7 | 95.2 | 0.31 | 12.0 | 3.13 |
| TPBAC-Fe [31] | 1 | 1.9 | - | 0.8 | 5.1 | 101.4 | 0.33 | 11.96 | 3.29 |
| Sample | CR | CV | ||||
|---|---|---|---|---|---|---|
| k1·10−3 (min−1) | t1/2 (min) | R2 | k1·10−3 (min−1) | t1/2 (min) | R2 | |
| TPB-Ag | 12.2 | 56.8 | 0.9890 | 12.3 | 56.3 | 0.9890 |
| TPBAC-Ag | 4.1 | 169.1 | 0.9910 | 7.1 | 97.6 | 0.9863 |
| TPBAC-AgFe | 2.8 | 247.5 | 0.9766 | 5.7 | 121.6 | 0.9633 |
| TPBAC-Fe [31] | 2.1 | 328.4 | 0.9910 | 6.6 | 105.0 | 0.9733 |
| Photocatalyst | Light Irradiation | * Q (mg/L) | * C mg/L) | * T (min) | * E % | Ref. |
|---|---|---|---|---|---|---|
| Congo Red | ||||||
| ZnO | UV | 500 | 20 | 60 | 95.02 | [71] |
| ZnO | UV | 1 | 697 | 135 | 97 | [72] |
| CoFe2O4 | UV | 10 | 10 | 90 | 91 | [73] |
| CuO composites | Xe lamp | 65 | 50 | 50 | 77 | [74] |
| ZnO | Xe lamp | 50 | 50 | 50 | 25 | [74] |
| TiO2 | UV | 50 | 50 | 90 | 98.28 | [75] |
| TiO2-CoFe2O4 | Vis | 80 | 10 | 120 | 85 | [76] |
| TPB-Ag | UV | 187.5 | 14 | 180 | 69.6 | This study |
| TPB-Ag | Vis | 500 | 14 | 180 | 93.0 | This study |
| TPBAC-Ag | Vis | 500 | 14 | 180 | 58.6 | This study |
| TPBAC-AgFe | Vis | 500 | 14 | 180 | 52.5 | This study |
| Crystal Violet | ||||||
| AgTiO2 | UV | 1000 | 0.02 | 105 | 97 | [5] |
| AgTiO2 | Solar simulator | 1000 | 0.02 | 600 | 88 | [5] |
| 10NiO-ZnO | UV | 100 | 100 | 180 | 100 | [77] |
| ZnO | Vis | 2500 | 14 | 60 | 70.57 | [78] |
| ZnO | Vis | 5000 | 7 | 60 | 86.79 | [78] |
| TPB-Ag | UV | 187.5 | 14 | 180 | 90.2 | This study |
| TPB-Ag | Vis | 500 | 8.2 | 180 | 95.6 | This study |
| TPBAC-Ag | Vis | 500 | 8.2 | 180 | 81.6 | This study |
| TPBAC-AgFe | Vis | 500 | 8.2 | 180 | 74.0 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negoescu, D.; Atkinson, I.; Gherendi, M.; Culita, D.C.; Baran, A.; Petrescu, S.; Bratan, V.; Parvulescu, V. Active Ag-, Fe-, and AC-Modified TiO2 Mesoporous Photocatalysts for Anionic and Cationic Dye Degradation. Catalysts 2025, 15, 479. https://doi.org/10.3390/catal15050479
Negoescu D, Atkinson I, Gherendi M, Culita DC, Baran A, Petrescu S, Bratan V, Parvulescu V. Active Ag-, Fe-, and AC-Modified TiO2 Mesoporous Photocatalysts for Anionic and Cationic Dye Degradation. Catalysts. 2025; 15(5):479. https://doi.org/10.3390/catal15050479
Chicago/Turabian StyleNegoescu, Daniela, Irina Atkinson, Mihaela Gherendi, Daniela C. Culita, Adriana Baran, Simona Petrescu, Veronica Bratan, and Viorica Parvulescu. 2025. "Active Ag-, Fe-, and AC-Modified TiO2 Mesoporous Photocatalysts for Anionic and Cationic Dye Degradation" Catalysts 15, no. 5: 479. https://doi.org/10.3390/catal15050479
APA StyleNegoescu, D., Atkinson, I., Gherendi, M., Culita, D. C., Baran, A., Petrescu, S., Bratan, V., & Parvulescu, V. (2025). Active Ag-, Fe-, and AC-Modified TiO2 Mesoporous Photocatalysts for Anionic and Cationic Dye Degradation. Catalysts, 15(5), 479. https://doi.org/10.3390/catal15050479







