Designing a Potential Pathway for the Catalytic Synthesis of 1,3-Cyclohexanediamine
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of 1,3-CHDA by One-Pot Reductive Amination of RES
2.2. Synthesis of 1,3-CHDA by Ammonia Pathway from 1,3-CHD
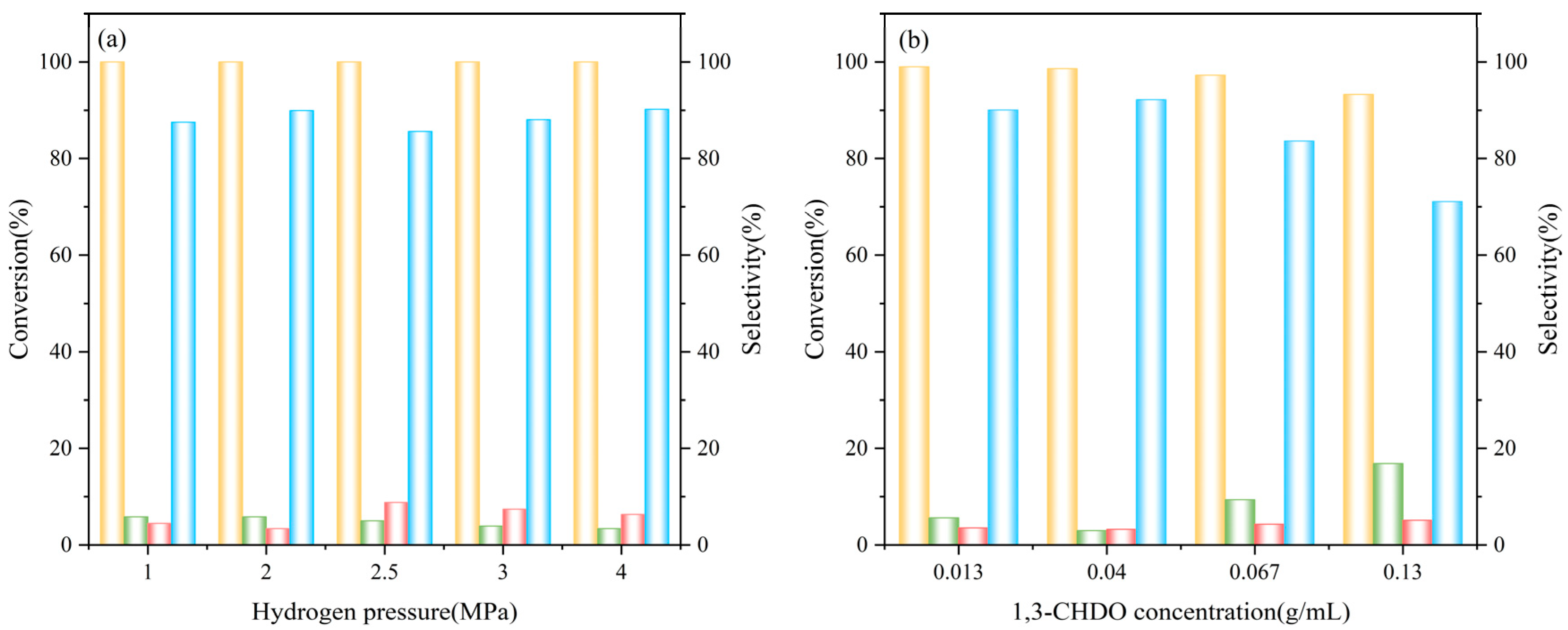
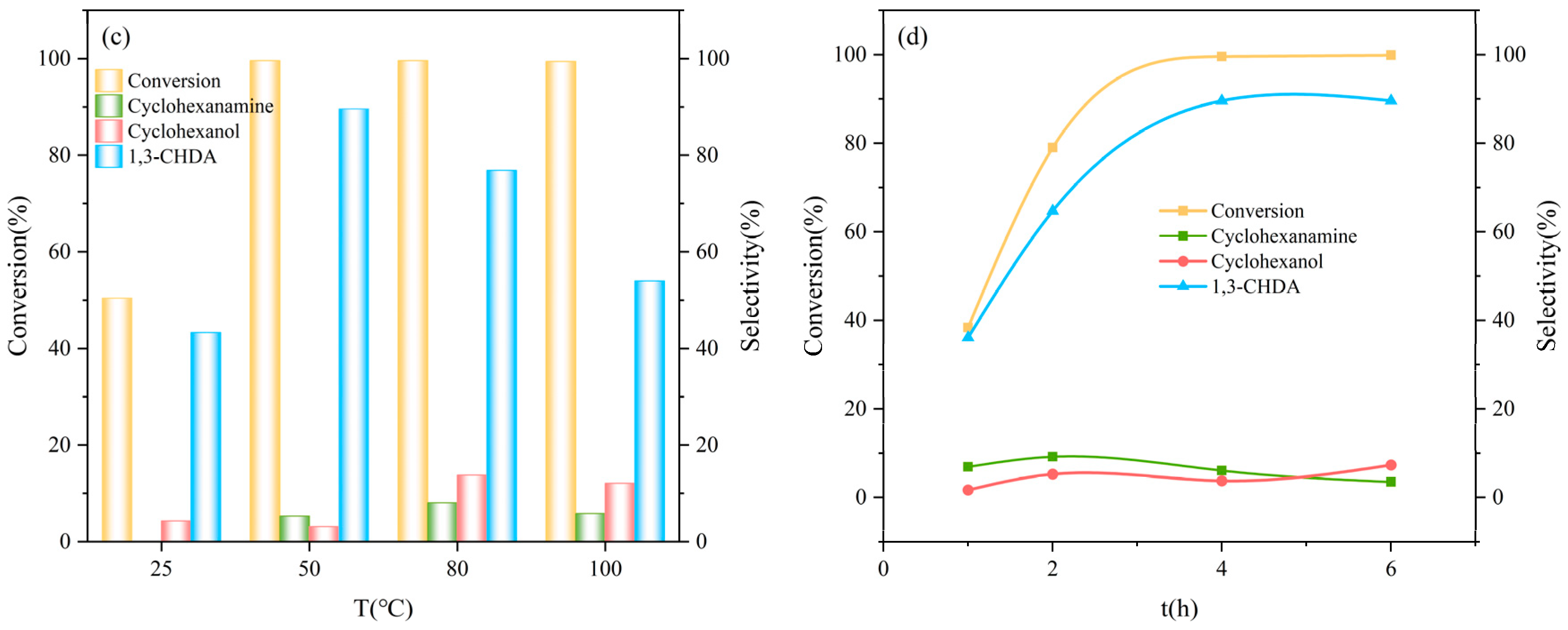
2.3. Synthesis of 1,3-CHDA by Hydroxylamine Hydrochloride Pathway from 1,3-CHD
2.4. Synthesis of 1,3-CHDA by Hydroxylamine Hydrochloride Pathway from RES
3. Experimental Section
3.1. Materials
3.2. Synthesis Section
3.2.1. Synthesis of 1,3-CHD from Hydrogenation of RES
3.2.2. Synthesis of 1,3-CHDA by Reductive Amination of RES (One-Pot Pathway)
3.2.3. Synthesis of 1,3-CHDA by Reductive Amination of 1,3-CHD (Ammonia Pathway)
3.2.4. Synthesis of 1,3-CHDA by Oximation–Hydrogenation of 1,3-CHD (Hydroxylamine Hydrochloride Pathway)
3.3. Purification Procedure
3.4. Analytical Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Park, J.H.; Kim, M.H.; Seo, M.L.; Lee, J.; Jung, J. In situ supramolecular gel formed by cyclohexane diamine with aldehyde derivative. Polymers 2022, 14, 400. [Google Scholar] [CrossRef]
- Jiang, S.; Dong, X.R.; Qiu, Y.T.; Chen, D.; Wu, X.X.; Jiang, S. A new ligand for copper-catalyzed amination of aryl halides to primary(hetero)aryl amines. Tetrahedron Lett. 2020, 61, 151683. [Google Scholar] [CrossRef]
- Wu, J.J.; Darcel, C. Recent developments in manganese, iron and cobalt homogeneous catalyzed synthesis of primary amines via reduction of nitroarenes, nitriles and carboxamides. Adv. Synth. Catal. 2023, 365, 948–964. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhou, K.; Lu, M.; Wang, L.C.; Wei, Z.J.; Li, X.N. Acidic/basic oxides-supported cobalt catalysts for one-pot synthesis of isophorone diamine from hydroamination of isophorone nitrile. Ind. Eng. Chem. Res. 2015, 54, 9124–9132. [Google Scholar] [CrossRef]
- Lee, C.; Kang, H.J.; Hong, S. NiH-catalyzed C-N bond formation: Insights and advancements in hydroamination of unsaturated hydrocarbons. Chem. Sci. 2024, 15, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Reif, P.; Palenicek, P.; Rose, M. Toward renewable amines: Recent advances in the catalytic amination of biomass-derived oxygenates. ACS Catal. 2022, 12, 10400–10440. [Google Scholar] [CrossRef]
- Yang, H.H.; Wang, L.G.; Xu, S.; Cao, Y.; He, P.; Chen, J.Q.; Zheng, Z.; Li, H.Q. Green and selective hydrogenation of aromatic diamines over the nanosheet Ru/g-C3N4-H2 catalyst prepared by ultrasonic assisted impregnation-deposition method. Green Energy Environ. 2022, 7, 1361–1376. [Google Scholar] [CrossRef]
- Tomkins, P.; Müller, T.E. Enhanced selectivity in the hydrogenation of anilines to cyclo-aliphatic primary amines over lithium-modified Ru/CNT catalysts. ChemCatChem 2018, 10, 1438–1445. [Google Scholar] [CrossRef]
- Saha, B.; De, S.; Dutta, S. Recent advancements of replacing existing aniline production process with environmentally friendly one-pot process: An overview. Crit. Rev. Environ. Sci. Technol. 2011, 43, 84–120. [Google Scholar] [CrossRef]
- Li, X.F.; Wang, Z.; Mao, S.J.; Chen, Y.Q.; Tang, M.H.; Li, H.R.; Wang, Y. Insight into the role of additives in catalytic synthesis of cyclohexylamine from nitrobenzene. Chin. J. Chem. 2018, 36, 1191–1196. [Google Scholar] [CrossRef]
- Rathod, V.D.; Paganelli, S.; Piccolo, O. Selective reduction of halo-nitro aromatic compounds using [Rh]/DHTANa as catalyst in an aqueous bi-phase system. Catal. Commun. 2016, 84, 52–55. [Google Scholar] [CrossRef]
- Wei, Z.J.; Pan, R.F.; Hou, Y.X.; Yang, Y.; Liu, Y.X. Graphene-supported Pd catalyst for highly selective hydrogenation of resorcinol to 1, 3-cyclohexanedione through giant pi-conjugate interactions. Sci. Rep. 2015, 5, 15664. [Google Scholar] [CrossRef]
- Hou, Y.X.; Xu, L.B.; Wei, Z.J.; Liu, Y.X.; Li, X.H.; Deng, S.G. Reaction process and kinetics of the selective hydrogenation of resorcinol into 1,3-cyclohexanedione. J. Taiwan Ins. Chem. Eng. 2014, 45, 1428–1434. [Google Scholar] [CrossRef]
- Wei, Z.; Jin, J.; Liu, Y.; Cheng, Y. A Method for Preparing 3-Amino Cyclohexanone by Reducing and Amidating 1,3-Cyclohexanedione. CN114085159A, 25 February 2022. [Google Scholar]
- Wei, Z.; Ma, Z.; Cheng, Y.; Xu, G.; Huang, L.; Jin, Z. A Method for Preparing 1,3-Cyclohexanediamine by Reducing and Amidating 1,3-Cyclohexanedione. CN118666688A, 7 June 2024. [Google Scholar]
- Zheng, B.X.; Xu, J.; Song, J.L.; Wu, H.H.; Mei, X.L.; Zhang, K.L.; Han, W.Y.; Wu, W.; He, M.; Han, B. Nanoparticles and single atoms of cobalt synergistically enabled low-temperature reductive amination of carbonyl compounds. Chem. Sci. 2022, 13, 9047–9055. [Google Scholar] [CrossRef] [PubMed]
- Merling, G. Ueber Dihydroresorcin. Justus Liebigs Ann. Der Chemie 1894, 278, 20–57. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Kon, K.; Onodera, W.; Yamazaki, H.; Kondo, J.N. Heterogeneous Ni catalyst for direct synthesis of primary amines from alcohols and ammonia. ACS Catal. 2012, 3, 112–117. [Google Scholar] [CrossRef]
- Cuypers, T.; Morias, T.; Windels, S.; Marquez, C.; Van Goethem, C.; Vankelecom, I.; De Vos, D.E. Ni-Catalyzed reductive amination of phenols with ammonia or amines into cyclohexylamines. Green Chem. 2020, 22, 1884–1893. [Google Scholar] [CrossRef]
- Wei, Z.J.; Cheng, Y.R.; Huang, H.; Ma, Z.H.; Zhou, K.; Liu, Y.X. Reductive amination of 5-hydroxymethylfurfural to 2,5-bis(aminomethyl)furan over alumina-supported Ni-based catalytic systems. ChemSusChem 2022, 15, e202200233. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, H.; Shu, H.; Xiao, S.; Guo, D.; Liu, Y.; Wei, Z.; Li, X. A comprehensive study on the reductive amination of 5-hydroxymethylfurfural into 2,5-bisaminomethylfuran over Raney Ni through DFT calculations. ChemCatChem 2019, 11, 2649–2656. [Google Scholar] [CrossRef]
- Ming, J.X.; Ping, S.Y.; Ning, C.Z.; Zhang, X.; Wei, Z.; Ping, S.W. Differentiation between enamines and tautomerizable imines in the oxidation reaction with TEMPO. Nat. Commun. 2018, 9, 5002. [Google Scholar]
- McGonagle, F.I.; MacMillan, D.S.; Murray, J.; Sneddon, H.F.; Jamieson, C.; Watson, A.J.B. Development of a solvent selection guide for aldehyde-based direct reductive amination processes. Green Chem. 2013, 15, 21159. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Guieu, S.; White, N.G.; Lelj, F.; MacLachlan, M.J. The rich tautomeric behavior of campestarenes. Chem. Eur. J. 2016, 22, 17657–17672. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, Y.Z.; Yan, N. A remarkable solvent effect on reductive amination of ketones. Mol. Catal. 2018, 454, 87–93. [Google Scholar] [CrossRef]
- Chen, L.; van Muyden, A.P.; Cui, X.; Laurenczy, G.; Dyson, P.J. Selective hydrogenation of lignin-derived compounds under mild conditions. Green Chem. 2020, 22, 3069–3073. [Google Scholar] [CrossRef]
- Kölmel, D.K.; Kool, E.T. Oximes and hydrazones in bioconjugation: Mechanism and catalysis. Chem. Rev. 2017, 117, 10358–10376. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.M.; Zhao, Y.L.; Sun, Q.; Ouyang, X.H.; Li, J.H. Recent advances in N-O bond cleavage of oximes and hydroxylamines to construct N-heterocycle. Molecules 2023, 28, 1775. [Google Scholar] [CrossRef]
- Mas-Roselló, J.; Smejkal, T.; Cramer, N. Iridium-catalyzed acid-assisted asymmetric hydrogenation of oximes to hydroxylamines. Science 2020, 368, 1098–1102. [Google Scholar] [CrossRef]
- Hu, H.Y.; Cai, X.T.; Xu, Z.Y.; Yan, X.Y.; Zhao, S.X. Beckmann rearrangement of ketoxime catalyzed by N-methyl-imidazolium hydrosulfate. Molecules 2018, 23, 1764. [Google Scholar] [CrossRef]
- Li, J.T.; Li, X.L.; Li, T.S. Synthesis of oximes under ultrasound irradiation. Ultrason. Sonochem. 2006, 13, 200–202. [Google Scholar] [CrossRef]
- Ahmad, G.; Rasool, N.; Rizwan, K.; Altaf, A.A.; Rashid, U.; Hussein, M.Z.; Mahmood, T.; Ayub, K. Role of pyridine nitrogen in palladium-catalyzed imine hydrolysis: A case study of (E)-1-(3-bromothiophen-2-yl)-N-(4-methylpyridin-2-yl)methanimine. Molecules 2019, 24, 2609. [Google Scholar] [CrossRef]


| Entry | Substrate | Reaction Conditions | Product (Yield) | |||
|---|---|---|---|---|---|---|
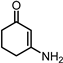
| 1 | 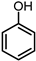 | 0.5 MPa NH3, 2 MPa H2 |  | |||
| 91.3 | ||||||
| 2 | 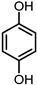 | 0.5 MPa NH3, 2 MPa H2 |  | |||
| 58.5% | ||||||
| 3 | 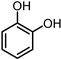 | 0.5 MPa NH3, 2 MPa H2 |  |  | ||
| 63.2% | <1.0% | |||||
| 4 | 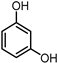 | 0.5 MPa NH3, 2 MPa H2 | 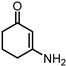 |  | ||
| 38.0% | <1.0% | |||||
| 5 | 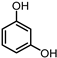 | 0.63 g NH3OH∙HCl, 0.40 g NaOH, 2 MPa H2 | 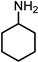 | 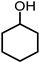 |  | |
| 23.5% | 15.0% | <1.0% | ||||
| Entry | Catalyst | Conv. (%) | Yield (%) | |||
|---|---|---|---|---|---|---|
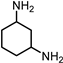 | 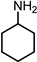 | 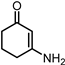 | Others | |||
| (I) | (II) | (III) | ||||
| 1 | Pd/C | 100 | - | 25.1 | 68.3 | 6.60 |
| 2 | Pt/C | 100 | 0.63 | 2.11 | 91.5 | 5.76 |
| 3 | Ru/C | 100 | - | - | 96.4 | 3.60 |
| 4 | Rh/C | 100 | - | 1.90 | 87.0 | 11.1 |
| 5 | Raney Ni | 100 | 37.5 | 26.8 | 20.1 | 15.6 |
| 6 | Raney Ni a | 97.5 | - | 0.10 | 94.6 | 2.80 |
| 7 | Raney Ni b | 80.5 | 26.9 | 37.6 | - | 16.0 |
| Entry | Solvent | Conv. (%) | Yield (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
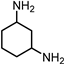 | 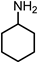 |  |  | 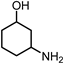 | 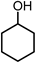 | Others | |||
| (I) | (II) | (III) | (IV) | (V) | (VI) | ||||
| 1 | H2O | 100 | 0.51 | - | 79.5 | 18.8 | 0.32 | 0.12 | 0.75 |
| 2 | Methanol | 100 | 5.00 | - | 68.0 | 27.0 | - | - | - |
| 3 | 1-Butanol | 100 | 14.7 | 17.5 | 36.3 | 20.4 | 8.67 | 1.31 | 1.12 |
| 4 | Isopropanol | 100 | 12.9 | 27.7 | 16.7 | 13.1 | 25.1 | 4.50 | - |
| 5 | Toluene | 100 | 34.4 | 30.6 | 13.0 | 5.84 | 4.16 | 6.87 | 5.13 |
| 6 | Cyclohexane | 100 | 34.3 | 13.4 | - | 2.20 | 31.2 | 4.50 | 14.4 |
| 7 | 1,4-Dioxane | 100 | 37.5 | 26.8 | 20.1 | 1.05 | 10.4 | 4.02 | 0.13 |
| 8 | 1,4-Dioxane a | 100 | 53.1 | 30.6 | 0.46 | 2.04 | 8.41 | 1.39 | 4.00 |
| Entry | Solvent | Base | n (NH3OH·HCl: 1,3-CHD) | n (Base: NH3OH·HCl) | pH Value | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | Methanol | TEA | 2.50 | 1.20 | - | 85.5 |
| 2 | Methanol–H2O a | TEA | 2.50 | 1.20 | - | 54.2 |
| 3 | H2O | TEA | 2.50 | 1.20 | 5.00 | 88.3 |
| 4 | H2O | Pyridine | 2.50 | 1.20 | 4.50 | 88.4 |
| 5 | H2O | Na2CO3 | 2.50 | 1.20 | 7.00 | 71.9 |
| 6 | H2O | NaOH | 2.50 | 1.20 | 7.67 | 64.5 |
| 7 | H2O | NaOH | 2.50 | 1.30 | 10.93 | 26.9 |
| 8 | H2O | NaOH | 2.50 | 1.10 | 6.90 | 68.4 |
| 9 | H2O | NaOH | 2.50 | 1.05 | 6.60 | 73.3 |
| 10 | H2O | NaOH | 2.25 | 1.05 | 7.07 | 78.1 |
| 11 | H2O | NaOH | 2.20 | 1.05 | 6.58 | 92.1 |
| 12 | H2O | NaOH | 2.10 | 1.05 | 6.27 | 91.1 |
| 13 | H2O b | NaOH | 2.20 | 1.05 | 6.58 | 97.5 |
| Entry | Solvent | Cat. Dosage (g) | Conv. (%) | Yield (%) | |||
|---|---|---|---|---|---|---|---|
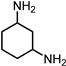 |  |  | Others | ||||
| 1 | Methanol | 0.06 | 100 | 60.0 | 2.10 | 8.30 | 29.6 |
| 2 | Methanol | 0.1 | 100 | 79.0 | 7.15 | 4.92 | 8.93 |
| 3 | Methanol | 0.3 | 100 | 87.4 | 6.06 | 4.34 | 2.20 |
| 4 | N-Methyl-pyrrolidone | 0.3 | 100 | 52.6 | 47.4 | - | - |
| 5 | H2O | 0.3 | 84.1 | - | 44.2 | 39.9 | - |
| 6 | 1-Butanol | 0.3 | 100 | 92.7 | 3.52 | 3.36 | 0.42 |
| 7 | Isopropanol | 0.3 | 100 | 92.3 | 5.10 | 0.27 | 2.33 |
| 8 | 1,4-dioxiane | 0.3 | 98.7 | 92.8 | 1.68 | 0.74 | 3.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Ma, Z.; Cheng, Y.; Xu, G.; Huang, L.; Zhou, T.; Wei, Z.; Liu, Y. Designing a Potential Pathway for the Catalytic Synthesis of 1,3-Cyclohexanediamine. Catalysts 2025, 15, 446. https://doi.org/10.3390/catal15050446
Sun D, Ma Z, Cheng Y, Xu G, Huang L, Zhou T, Wei Z, Liu Y. Designing a Potential Pathway for the Catalytic Synthesis of 1,3-Cyclohexanediamine. Catalysts. 2025; 15(5):446. https://doi.org/10.3390/catal15050446
Chicago/Turabian StyleSun, Danna, Zhihe Ma, Yuran Cheng, Gengxin Xu, Le Huang, Tingyu Zhou, Zuojun Wei, and Yingxin Liu. 2025. "Designing a Potential Pathway for the Catalytic Synthesis of 1,3-Cyclohexanediamine" Catalysts 15, no. 5: 446. https://doi.org/10.3390/catal15050446
APA StyleSun, D., Ma, Z., Cheng, Y., Xu, G., Huang, L., Zhou, T., Wei, Z., & Liu, Y. (2025). Designing a Potential Pathway for the Catalytic Synthesis of 1,3-Cyclohexanediamine. Catalysts, 15(5), 446. https://doi.org/10.3390/catal15050446








