Co-Doped Ni@Ni(OH)2 Core–Shell Catalysts for Dual-Function Water and Urea Oxidation
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
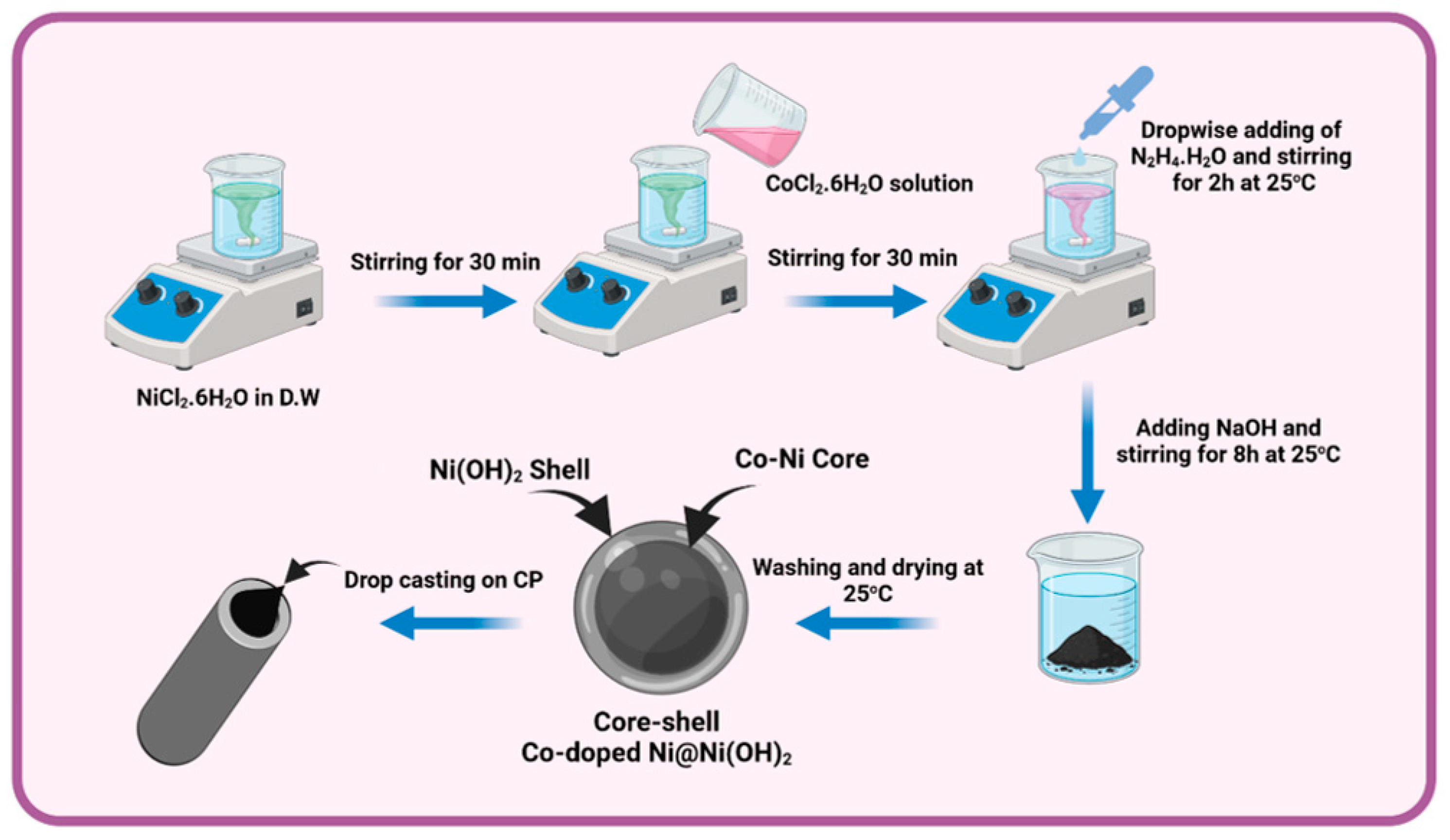
2.2. Chemical Deposition of Co-Doped Ni@Ni(OH)2 Core-Shell Catalysts
2.3. Characterization
3. Results and Discussion
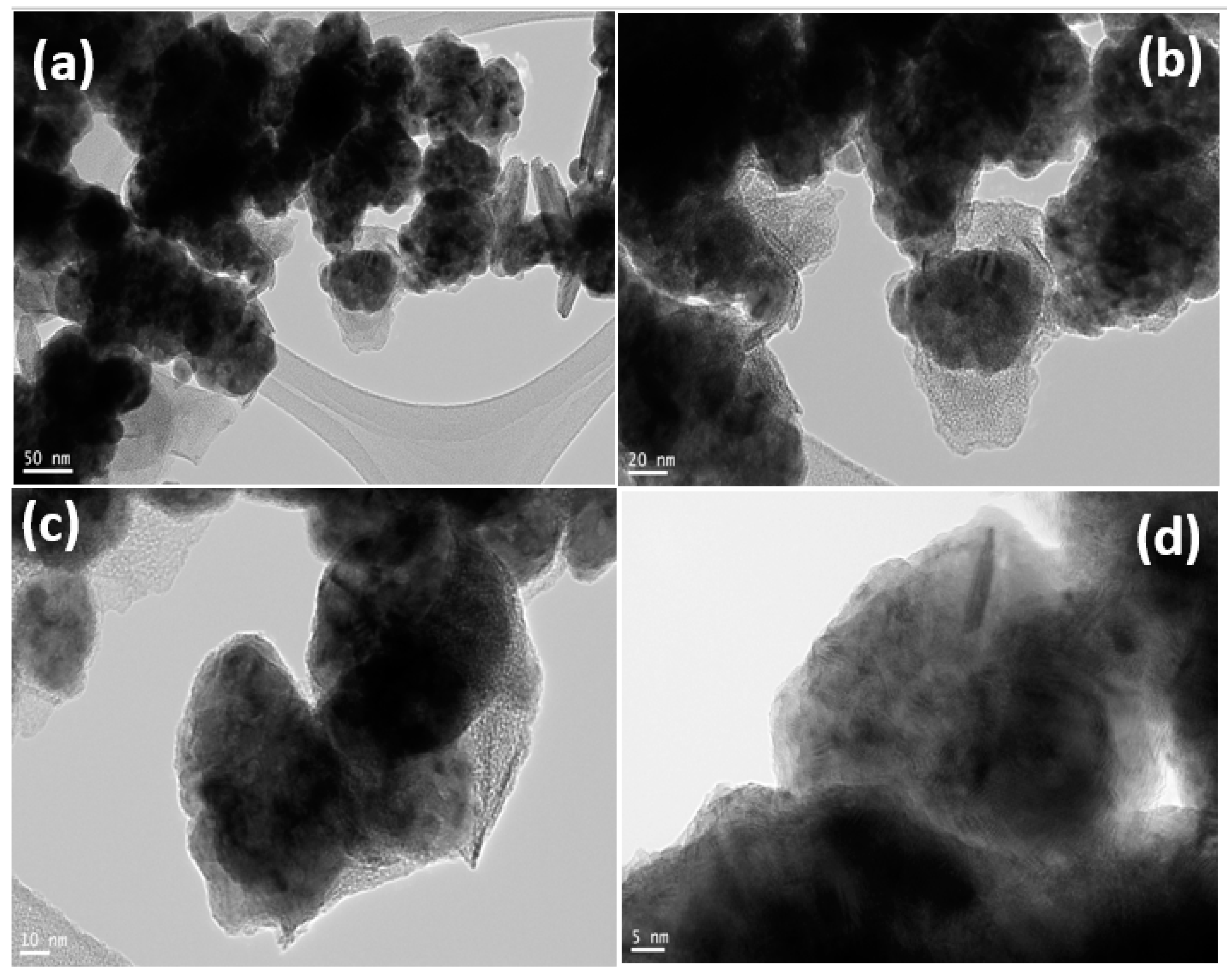
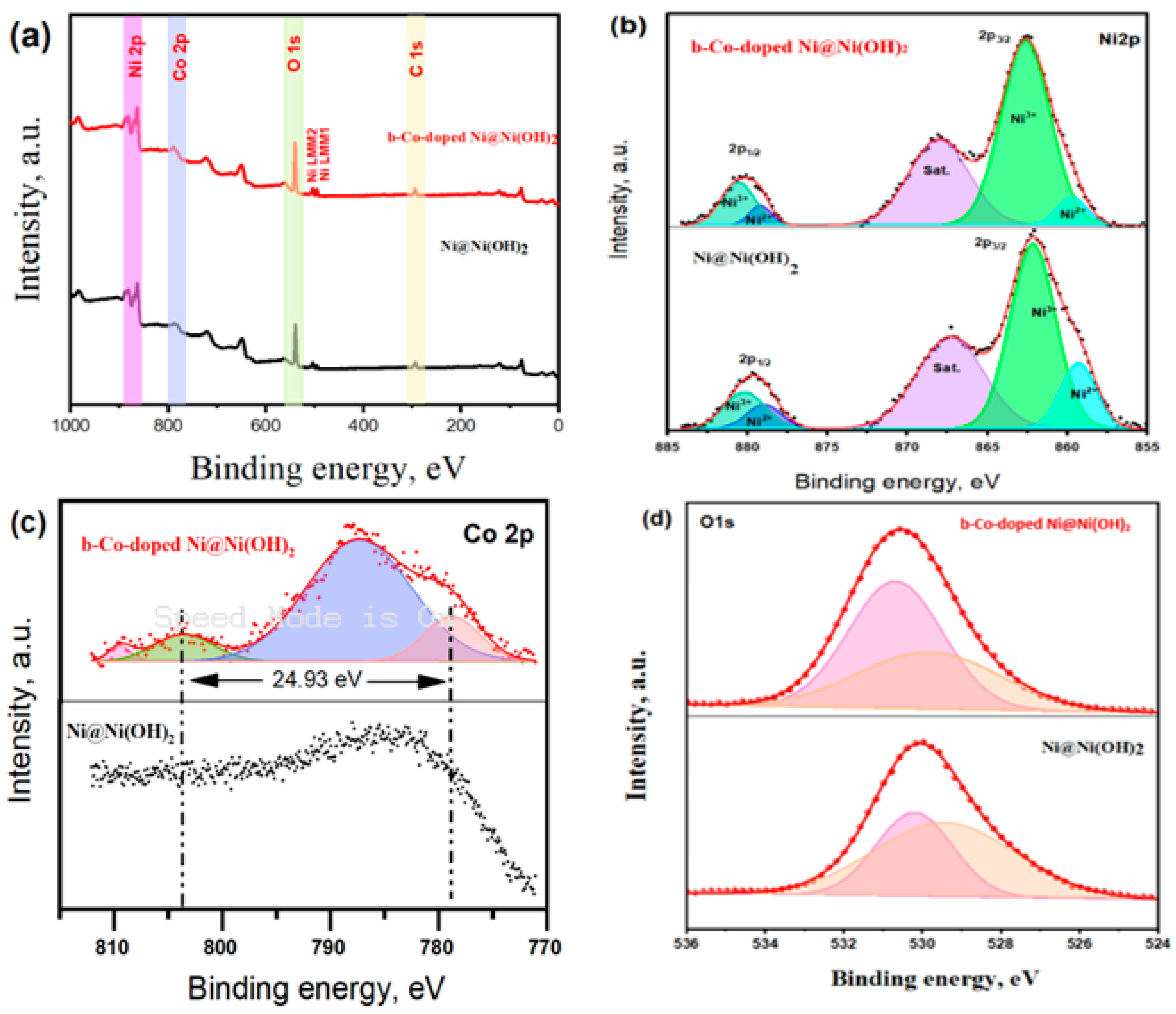
3.1. Catalysts Characterization
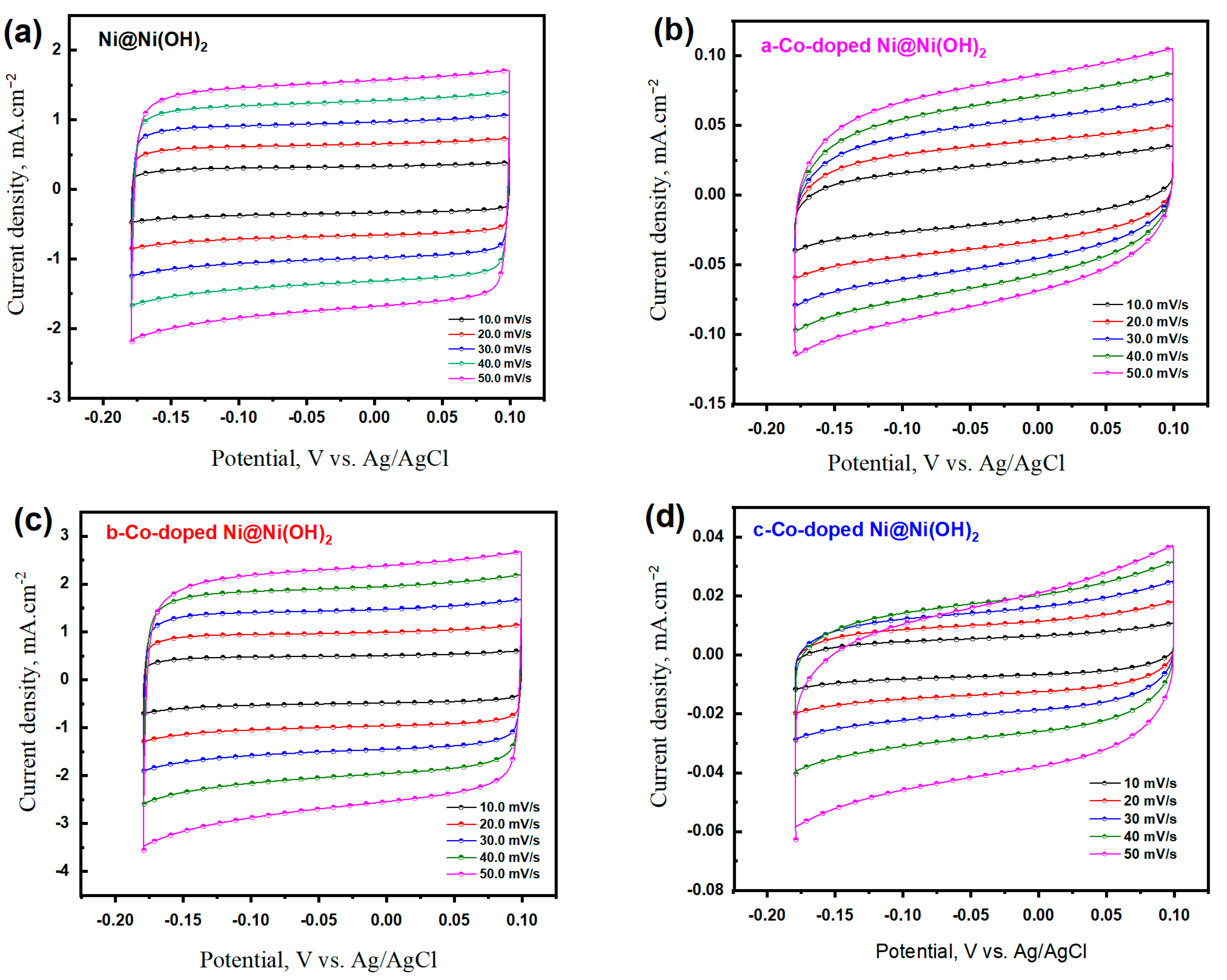
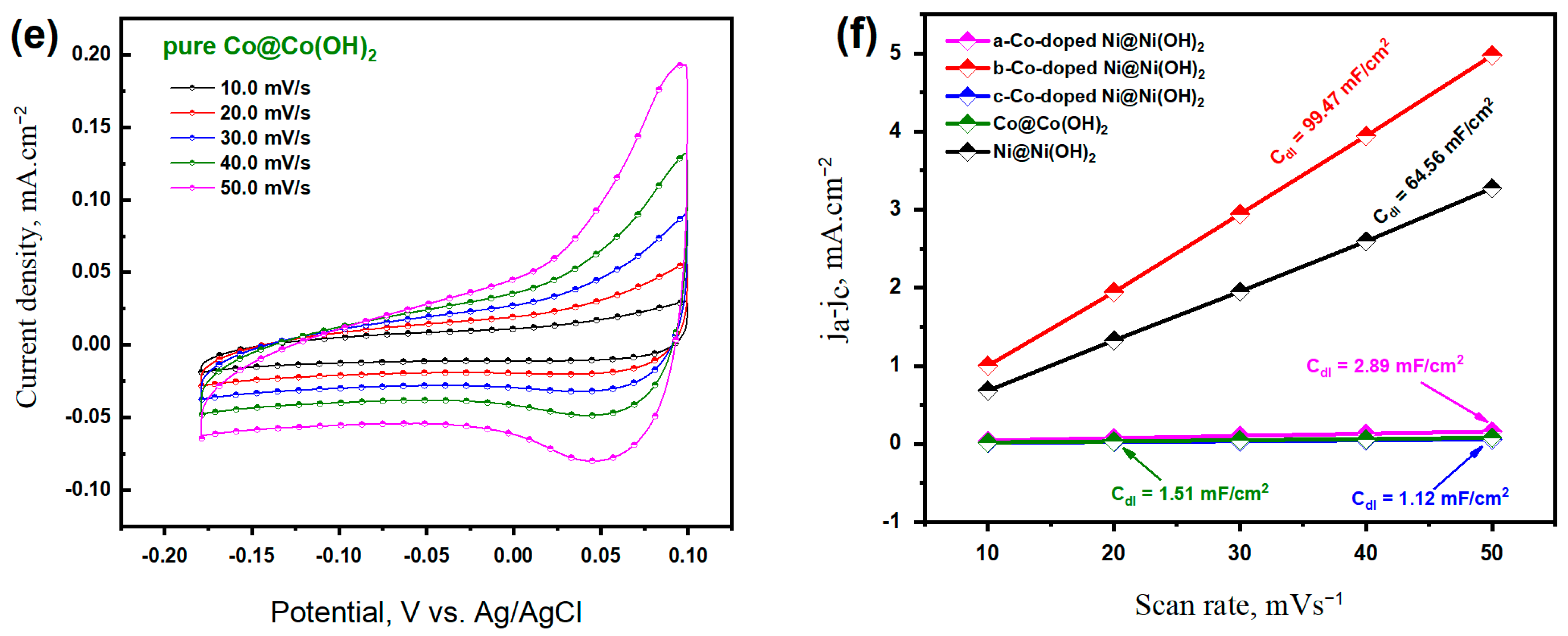
3.2. Electrochemical Properties of Co-Doped Ni@Ni(OH)2 Catalysts
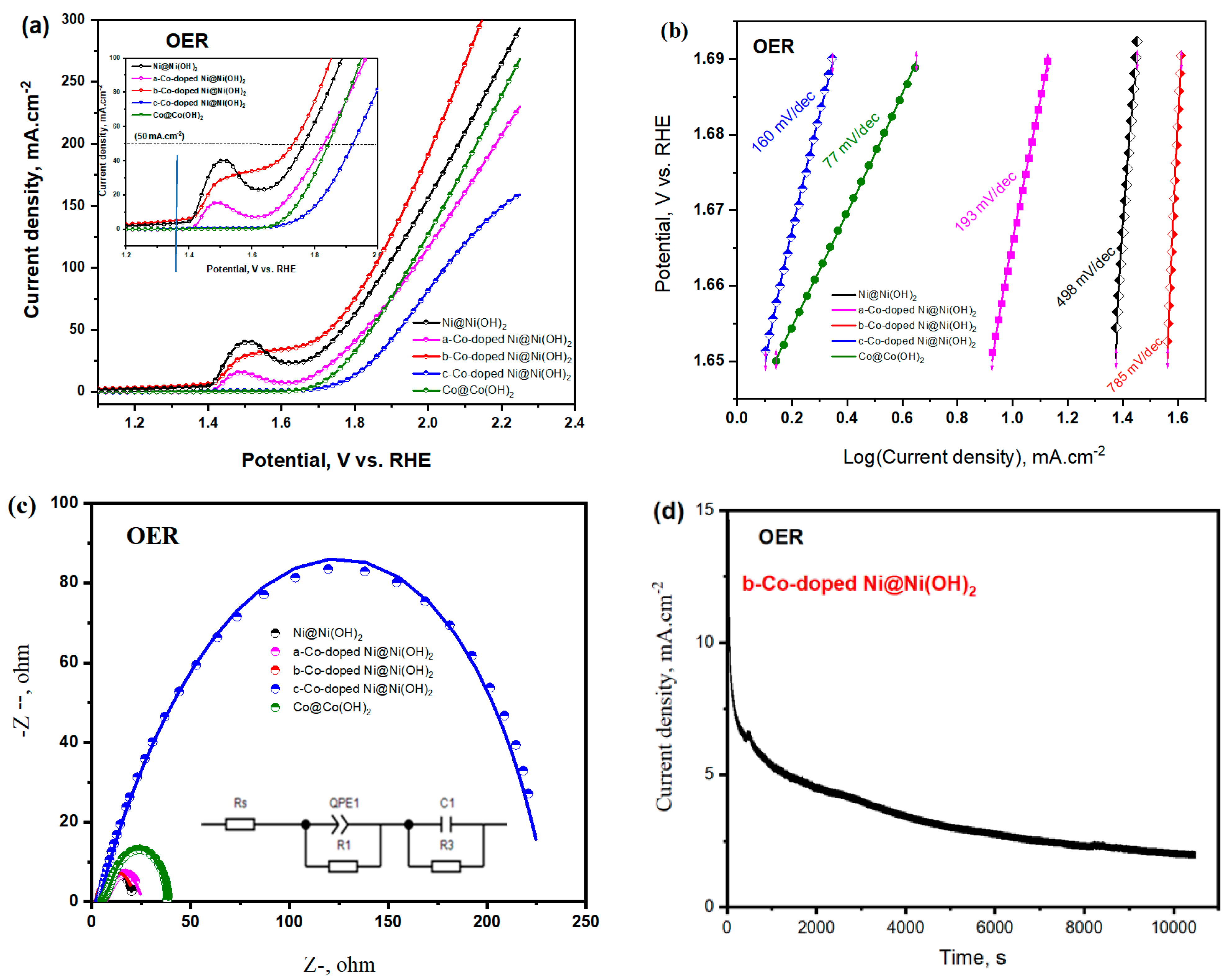
3.2.1. OER Characteristics
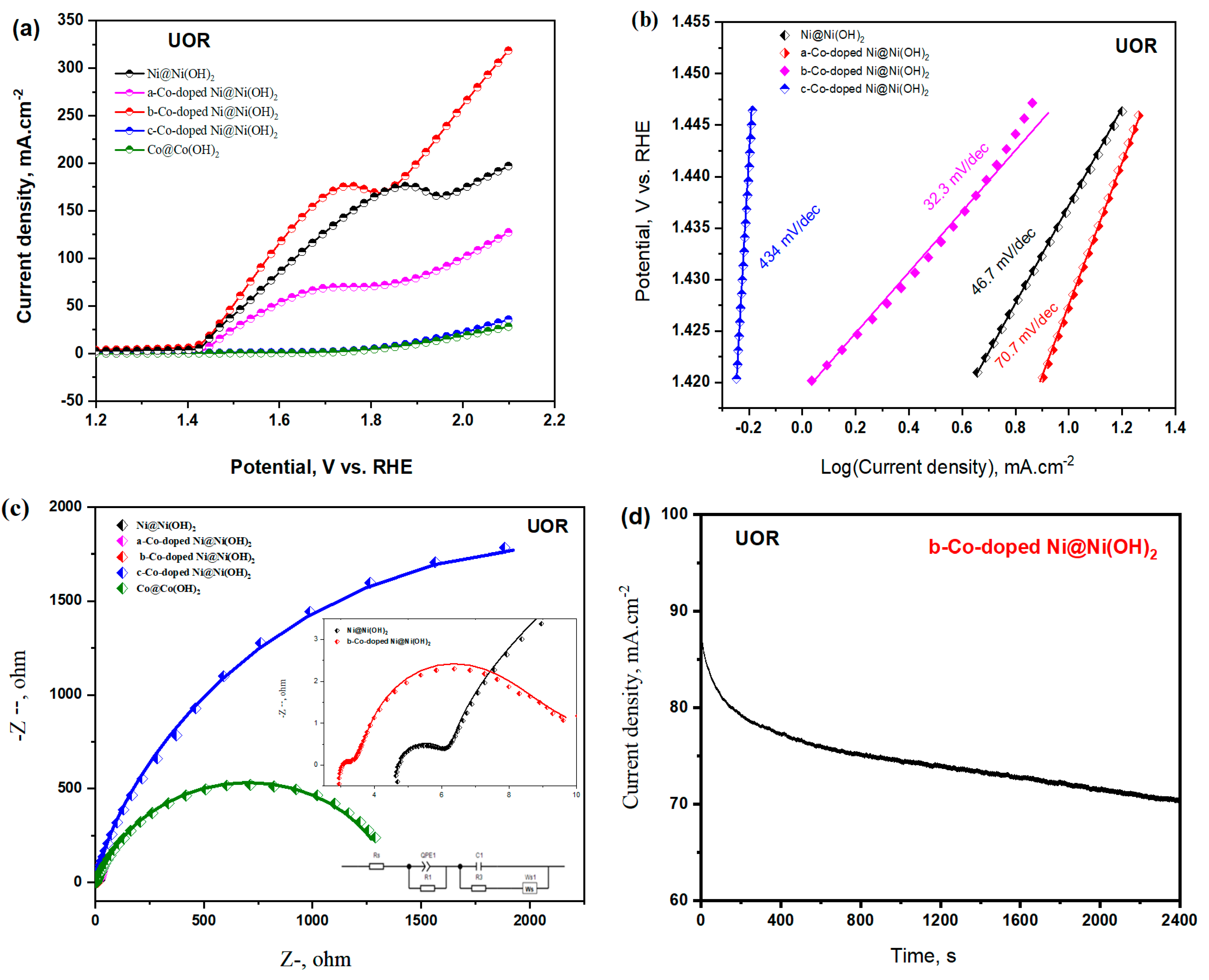
3.2.2. UOR Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Syirat, B.; Jern, P.; Mohamed, H.; Chyuan, H.; Fattah, I.M.R.M.R.; Rahman, S.M.A.M.A.; Nghiem, L.D.; Mahlia, T.M.I.; Zainal, B.S.; Ker, P.J.; et al. Recent Advancement and Assessment of Green Hydrogen Production Technologies. Renew. Sustain. Energy Rev. 2024, 189, 113941. [Google Scholar]
- Agyekum, E.B.; Nutakor, C.; Agwa, A.M.; Kamel, S.; Kame, S. A Critical Review of Renewable Hydrogen Production Methods: Factors Affecting Their Scale-Up and Its Role in Future Energy Generation. Membranes 2022, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, J.; Wu, N.; Xie, S.; Meng, C.; Zheng, Y.; Wang, X.; Zhao, Y. Review and Outlook on the International Renewable Energy Development. Energy Built Environ. 2022, 3, 139–157. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Liang, J.; Yin, P.; Tian, Y. Orthorhombic α-NiOOH Nanosheet Arrays: Phase Conversion and Efficient Bifunctional Electrocatalysts for Full Water Splitting. ACS Sustain. Chem. Eng. 2017, 5, 3808–3818. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A Review of Recent Developments in Hydrogen Production via Biogas Dry Reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, L.; Liu, J. Recent Advances in Cobalt-Based Electrocatalysts for Hydrogen and Oxygen Evolution Reactions. J. Alloys Compd. 2020, 19, 1–68. [Google Scholar] [CrossRef]
- Zhu, B.; Liang, Z.; Zou, R. Designing Advanced Catalysts for Energy Conversion Based on Urea Oxidation Reaction, Reveiw. Small 2020, 16, 1–19. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Chavan, H.S.; Jo, Y.; Im, H.; Kim, H. Nonprecious Bimetallic NiFe-Layered Hydroxide Nanosheets as a Catalyst for Highly Efficient Electrochemical Water Splitting. Int. J. Energy Res. 2021, 45, 16963–16972. [Google Scholar] [CrossRef]
- Aziz, T.; Haque, M.A.; Saha, S.; Mondal, B.; Jain, S.; Dutta, A. A Review of Nanostructured Transition Metal Phosphide-Driven Electrocatalytic Oxygen Evolution Reaction. Energy Fuels 2023, 37, 18291–18309. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Z.; Zheng, H.; Cao, R. Recent Progress on Defect-Rich Transition Metal Oxides and Their Energy-Related Applications. Chem. Asian J. 2020, 15, 3717–3736. [Google Scholar] [CrossRef]
- Omar ben Gubaer, S.; Shaddad, M.N.; Arunachalam, P.; Amer, M.S.; Aladeemy, S.A.; Al-Mayouf, A.M. Enhanced Electrocatalytic Oxygen Redox Reactions of Iron Oxide Nanorod Films by Combining Oxygen Vacancy Formation and Cobalt Doping. RSC Adv. 2023, 13, 33242–33254. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; Arunachalam, P.; Al-Mayouf, A.M.; Sudha, P.N.; Rekha, A.; Vidhya, A.; Hemapriya, J.; Latha, S.; Prasad, P.S.; Pavithra, S.; et al. Engineered CoS/Ni3S2 Heterointerface Catalysts Grown Directly on Carbon Paper as an Efficient Electrocatalyst for Urea Oxidation. Catalysts 2024, 14, 570. [Google Scholar] [CrossRef]
- Amer, M.S.; Arunachalam, P.; Alsalman, A.M.; Al-Mayouf, A.M.; Almutairi, Z.A.; Aladeemy, S.A.; Hezam, M. Facile Synthesis of Amorphous Nickel Iron Borate Grown on Carbon Paper as Stable Electrode Materials for Promoted Electrocatalytic Urea Oxidation. Catal. Today 2022, 397, 197–205. [Google Scholar] [CrossRef]
- Al-Sulmi, W.A.; Ghanem, M.A.; Aladeemy, S.A.; Al-Mayouf, A.M.; Alotaibi, N.H. Chemical Deposition from a Liquid Crystal Template: A Highly Active Mesoporous Nickel Phosphate Electrocatalyst for Hydrogen Green Production via Urea. Arab. J. Chem. 2023, 16, 105266. [Google Scholar] [CrossRef]
- Boggs, B.K.; King, R.L.; Botte, G.G. Urea Electrolysis: Direct Hydrogen Production from Urine. Chem. Commun. 2009, 32, 4859–4861. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Direct Evidence of the Mechanism for the Electro-Oxidation of Urea on Ni(OH)2 Catalyst in Alkaline Medium. Electrochim. Acta 2013, 108, 660–665. [Google Scholar] [CrossRef]
- Wang, D.; Botte, G.G. In Situ X-Ray Diffraction Study of Urea Electrolysis on Nickel Catalysts. ECS Electrochem. Lett. 2014, 3, 29–32. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Alharthi, A.I.; Aladeemy, S.A.; Arunachalam, P. Engineering Oxygen Vacancy on Nickel-Doped Iron Oxide Nanorods as Efficient Bifunctional Electrocatalysts for Oxygen Evolution and Urea Oxidation Reaction. J. Taiwan Inst. Chem. Eng. 2024, 105928. [Google Scholar] [CrossRef]
- Cheng, Y.; Liao, F.; Dong, H.; Wei, H.; Geng, H.; Shao, M. Engineering CoN/Ni(OH)2 Heterostructures with Improved Intrinsic Interfacial Charge Transfer toward Simultaneous Hydrogen Generation and Urea-Rich Wastewater Purification. J. Power Sources 2020, 480, 229151. [Google Scholar] [CrossRef]
- Kim, J.; Monllor-Satoca, D.; Choi, W.; Environ, E.; Kim, J. Simultaneous Production of Hydrogen with the Degradation of Organic Pollutants Using TiO 2 Photocatalyst Modified with Dual Surface Components†. Energy Environ. Sci. 2012, 5, 7647–7656. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, J.; Li, J.; Wu, Q. Urea Electrooxidation: Current Development and Understanding of Ni-Based Catalysts. ChemElectroChem 2020, 7, 3211–3228. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Khalaf, M.M.; Mohamed, I.M.A. Advances in Phosphorus-Based Catalysts for Urea Electrooxidation: A Pathway to Sustainable Waste to Energy Conversion Through Electrocatalysis. Catalysts 2024, 14, 1–30. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Pan, T.; Zhu, Y.; Pang, H. Ni-Based Electrocatalysts for Urea Oxidation Reaction: Mechanism, Catalyst Design Strategies and Future Perspectives. Sci. China Mater. 2025, 68, 317–340. [Google Scholar] [CrossRef]
- Almutairi, E.M.; Ghanem, M.A.; Al-Warthan, A.; Shaik, M.R.; Adil, S.F.; Almutairi, A.M. Chemical Deposition and Exfoliation from Liquid Crystal Template: Nickel/Nickel (II) Hydroxide Nanoflakes Electrocatalyst for a Non-Enzymatic Glucose Oxidation Reaction. Arab. J. Chem. 2022, 15, 103467. [Google Scholar] [CrossRef]
- Saepudin, E.; Yuliani, T.; Musyarofah, N.R.R.; Nasution, M.A.F.; Gunlazuardi, J.; Einaga, Y.; Ivandini, T.A. Nickel Hydroxide Nanoparticles for Application in Immunochromatographic Strip Tests of Melamine. Sens. Mater. 2021, 33, 1027–1036. [Google Scholar] [CrossRef]
- Su, Y.Z.; Xiao, K.; Li, N.; Liu, Z.Q.; Qiao, S.Z. Amorphous Ni(OH)2@Three-Dimensional Ni Core-Shell Nanostructures for High Capacitance Pseudocapacitors and Asymmetric Supercapacitors. J. Mater. Chem. A 2014, 2, 13845–13853. [Google Scholar] [CrossRef]
- Koussi-Daoud, S.; Planchat, A.; Renaud, A.; Pellegrin, Y.; Odobel, F.; Pauporté, T. Solvent-Templated Electrodeposition of Mesoporous Nickel Oxide Layers for Solar Cell Applications. ChemElectroChem 2017, 4, 2618–2625. [Google Scholar] [CrossRef]
- Zhu, X.; Dou, X.; Dai, J.; An, X.; Guo, Y.; Zhang, L.; Tao, S.; Zhao, J.; Chu, W.; Zeng, X.C.; et al. Metallic Nickel Hydroxide Nanosheets Give Superior Electrocatalytic Oxidation of Urea for Fuel Cells. Angew. Chem. Int. Ed. 2016, 55, 12465–12469. [Google Scholar] [CrossRef]
- Li, B.; Ai, M.; Xu, Z. Mesoporous β-Ni(OH)2: Synthesis and Enhanced Electrochemical Performance. Chem. Commun. 2010, 46, 6267–6269. [Google Scholar] [CrossRef]
- Prathap, M.U.A.; Satpati, B.; Srivastava, R. Facile Preparation of β-Ni(OH)2-NiCo2O4 Hybrid Nanostructure and Its Application in the Electro-Catalytic Oxidation of Methanol. Electrochim. Acta 2014, 130, 368–380. [Google Scholar] [CrossRef]
- Xie, J.; Gao, L.; Cao, S.; Liu, W.; Lei, F.; Hao, P.; Xia, X.; Tang, B. Copper-Incorporated Hierarchical Wire-on-Sheet α-Ni(OH)2 Nanoarrays as Robust Trifunctional Catalysts for Synergistic Hydrogen Generation and Urea Oxidation. J. Mater. Chem. A 2019, 7, 13577–13584. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Ghaemi, M.; Sabour, B.; Dalvand, S. Electrochemical Preparation of α-Ni(OH)2 Ultrafine Nanoparticles for High-Performance Supercapacitors. J. Solid State Electrochem. 2014, 18, 1569–1584. [Google Scholar] [CrossRef]
- Lin, C.; Gao, Z.; Zhang, F.; Yang, J.; Liu, B.; Jin, J. In Situ Growth of Single-Layered α-Ni(OH)2 Nanosheets on a Carbon Cloth for Highly Efficient Electrocatalytic Oxidation of Urea. J. Mater. Chem. A 2018, 6, 13867–13873. [Google Scholar] [CrossRef]
- Eom, S.; Jung, J.; Kim, D.H. One-Pot Synthesis of Nanostructured Ni@Ni(OH)2 and Co-Doped Ni@Ni(OH)2 via Chemical Reduction Method for Supercapacitor Applications. Materials 2023, 16, 380. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, S.; Li, Y.; Wang, J.; Du, Y.; Li, Z.; Han, X.; Sun, J.; Xu, P. A Crystalline-Amorphous Ni-Ni(OH)2 Core-Shell Catalyst for the Alkaline Hydrogen Evolution Reaction. J. Mater. Chem. A 2020, 8, 23323–23329. [Google Scholar] [CrossRef]
- Ghanem, M.A.; Al-Mayouf, A.M.; Singh, J.P.; Arunachalam, P. Concurrent Deposition and Exfoliation of Nickel Hydroxide Nanoflakes Using Liquid Crystal Template and Their Activity for Urea Electrooxidation in Alkaline Medium. Electrocatalysis 2017, 8, 16–26. [Google Scholar] [CrossRef]
- Bamuqaddam, A.M.; Aladeemy, S.A.; Ghanem, M.A.; Al-mayouf, A.M.; Alotaibi, N.H.; Marken, F. Foam Synthesis of Nickel/Nickel (II) Hydroxide Nanoflakes Using Double Templates of Surfactant Liquid Crystal and Hydrogen Bubbles: A High-Performance Catalyst for Methanol Electrooxidation in Alkaline Solution. Nanomaterials 2022, 12, 879. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; Al-Mayouf, A.M.; Shaddad, M.N.; Amer, M.S.; Almutairi, N.K.; Ghanem, M.A.; Alotaibi, N.H.; Arunachalam, P. Electrooxidation of Urea in Alkaline Solution Using Nickel Hydroxide Activated Carbon Paper Electrodeposited from DMSO Solution. Catalysts 2021, 11, 102. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; Al-Mayouf, A.; Amer, M.; Alotaibi, N.; Weller, M.T.; Ghanem, M.A. Structure and Electrochemical Activity of Nickel Aluminium Fl Uoride Nanosheets during Urea Electro-Oxidation in an Alkaline Solution. RSC Adv. 2021, 11, 3190–3201. [Google Scholar] [CrossRef]
- Pashazadeh, A.; Habibi, B. A Nickel Ion-Incorporating Zinc-Mesoporous Metal Organic Framework Thin Film Nanocomposite Modified Glassy Carbon Electrode for Electrocatalytic Oxidation of Methanol in Alkaline Media. New J. Chem. 2021, 45, 2597–2608. [Google Scholar] [CrossRef]
- Zhu, W.; Ren, M.; Hu, N.; Zhang, W.; Luo, Z.; Wang, R.; Wang, J.; Huang, L.; Suo, Y.; Wang, J. Traditional NiCo2S4 Phase with Porous Nanosheets Array Topology on Carbon Cloth: A Flexible, Versatile and Fabulous Electrocatalyst for Overall Water and Urea Electrolysis. ACS Sustain. Chem. Eng. 2018, 6, 5011–5020. [Google Scholar] [CrossRef]
- Liu, N.; Guan, J. Core–Shell Co3O4@FeOx Catalysts for Efficient Oxygen Evolution Reaction. Mater. Today Energy 2021, 21, 100715. [Google Scholar]
- Lu, B.; Cao, D.; Wang, P.; Wang, G.; Gao, Y. Oxygen Evolution Reaction on Ni-Substituted Co3O4 Nanowire Array Electrodes. Int. J. Hydrogen Energy 2011, 36, 72–78. [Google Scholar] [CrossRef]
- Al-Sharif, M.S.; Arunachalam, P.; Abiti, T.; Amer, M.S.; Al-Shalwi, M.; Ghanem, M.A. Mesoporous Cobalt Phosphate Electrocatalyst Prepared Using Liquid Crystal Template for Methanol Oxidation Reaction in Alkaline Solution. Arab. J. Chem. 2020, 13, 2873–2882. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, J.; Chen, W.; Wu, Y.; Li, S.; Du, X.; Zhang, M. Nickel-Cobalt Derived Nanowires/Nanosheets as Electrocatalyst for Efficient H2 Generation via Urea Oxidation Reaction. J. Alloys Compd. 2021, 891, 161790. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Shi, Y.; Wang, J.; Zheng, Y.; Pan, J.; Li, C.; Cao, J. Realizing Highly-Effcient Urea Oxidation via Decreasing the Energy Barrier of Deprotonation over Regulated Electronic Structure of Co Doped Ni(OH)2. Appl. Surf. Sci. 2023, 640, 158391. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, S.; Zhou, S.; Li, J.; Qu, K.; Cai, W. Mutual Promotion Effect of Ni and Mo2C Encapsulated in N-Doped Porous Carbon on Bifunctional Overall Urea Oxidation Catalysis. Catalysts 2022, 405, 606–613. [Google Scholar] [CrossRef]
- Zhang, J.W.; Lv, X.W.; Ren, T.Z.; Wang, Z.; Bandosz, T.J.; Yuan, Z.Y. Engineering Heterostructured Ni@Ni(OH)2 Core-Shell Nanomaterials for Synergistically Enhanced Water Electrolysis. Green Energy Environ. 2022, 7, 1024–1032. [Google Scholar] [CrossRef]
- Wang, X.; Xu, R.; Wang, R.; Wang, H.; Brett, D.J.L.; Pollet, B.G.; Ji, S. Nano-Sized Co/Co(OH)2 Core-Shell Structure Synthesized in Molten Salt as Electrode Materials for Supercapacitors. Ionics 2017, 23, 725–730. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Botte, G.G. Nickel and Cobalt Bimetallic Hydroxide Catalysts for Urea Electro-Oxidation. Electrochim. Acta 2012, 61, 25–30. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Understanding the Electro-Catalytic Oxidation Mechanism of Urea on Nickel Electrodes in Alkaline Medium. Electrochim. Acta 2012, 81, 292–300. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; Arunachalam, P.; Amer, M.S.; Al-Mayouf, A.M. Electrochemically Embedded Heterostructured Ni/NiS Anchored onto Carbon Paper Aas Bifunctional Electrlcatalysts for Urea Oxidation and Hydrogen Reaction. RSC Adv. 2025, 15, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Banerjee, J.; Chakraborty, P.; Banerjee, A.; Chanda, S.; Ray, K.; Acharya, K.; Sarkar, J. Green Synthesis of Copper/Copper Oxide Nanoparticles and Their Applications: A Review. Green Chem. Lett. Rev. 2022, 15, 187–215. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; AlRijraji, T.R.; Amer, M.S.; Arunachalam, P.; Al-Mayouf, A.M. Electrooxidation of Ethylene Glycol Coupled with Hydrogen Production on Porous NiO/Ni@NF Nanosheet Electrocatalysts. Catal. Sci. Technol. 2025, 15, 2571–2583. [Google Scholar] [CrossRef]
- Seo, B.; Woo, J.; Kim, E.; Cheong, S.H.; Lee, D.K.; Lee, H. Insight toward the role of Fe in layered Ni(OH)2 for electrochemical oxidations of water and 5-Hydroxymethylfurfural. Catal. Commun. 2022, 170, 106501. [Google Scholar] [CrossRef]
- Luan, C.; Liu, G.; Liu, Y.; Yu, L.; Wang, Y.; Xiao, Y.; Qiao, H.; Dai, X.; Zhang, X. Structure Effects of 2D Materials on α-Nickel Hydroxide for Oxygen Evolution Reaction. ACS Nano 2018, 12, 3875–3885. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, H.; He, H.; Xu, X.; Jin, Y. A high-performance binary Ni-Co hydroxide-based water oxidation electrode with three-dimensional coaxial nanotube array structure. Adv. Funct. Mater. 2014, 24, 4698–4705. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Xiao, M.; Liu, C.; Hou, S.; Ge, J.; Xing, W. Regulating the pore structure and oxygen vacancies of cobaltosic oxide hollow dodecahedra for an enhanced oxygen evolution reaction. NPG Asia Mater. 2020, 12, 73. [Google Scholar] [CrossRef]
- Iqbal, W.; Hameed, A.; Tariq, I.; Shah, S.S.A.; Nadeem, M.A. Cobalt hydroxide supported nickel nanoparticles for an efficient electrocatalytic urea oxidation reaction. Electrochim. Acta 2023, 467, 143055. [Google Scholar] [CrossRef]
- Toufani, M.; Besic, H.; Tong, W.; Farràs, P. Exploring the role of different morphologies of β–Ni(OH)2 for electrocatalytic urea oxidation and the effects of electrochemically active surface area. Results Chem. 2023, 6, 101031. [Google Scholar] [CrossRef]
- Wu, T.H.; Hou, B.W. Superior catalytic activity of α-Ni(OH)2 for urea electrolysis. Catal. Sci. Technol. 2021, 11, 4292–4300. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, Y.; Ma, Z.; Qiu, Y. Oxygen Vacancy-rich Ni/NiO@NC Nanosheets with Schottky Heterointerface for Efficient Urea Oxidation Reaction. ChemSusChem 2020, 13, 5004–5014. [Google Scholar] [CrossRef] [PubMed]


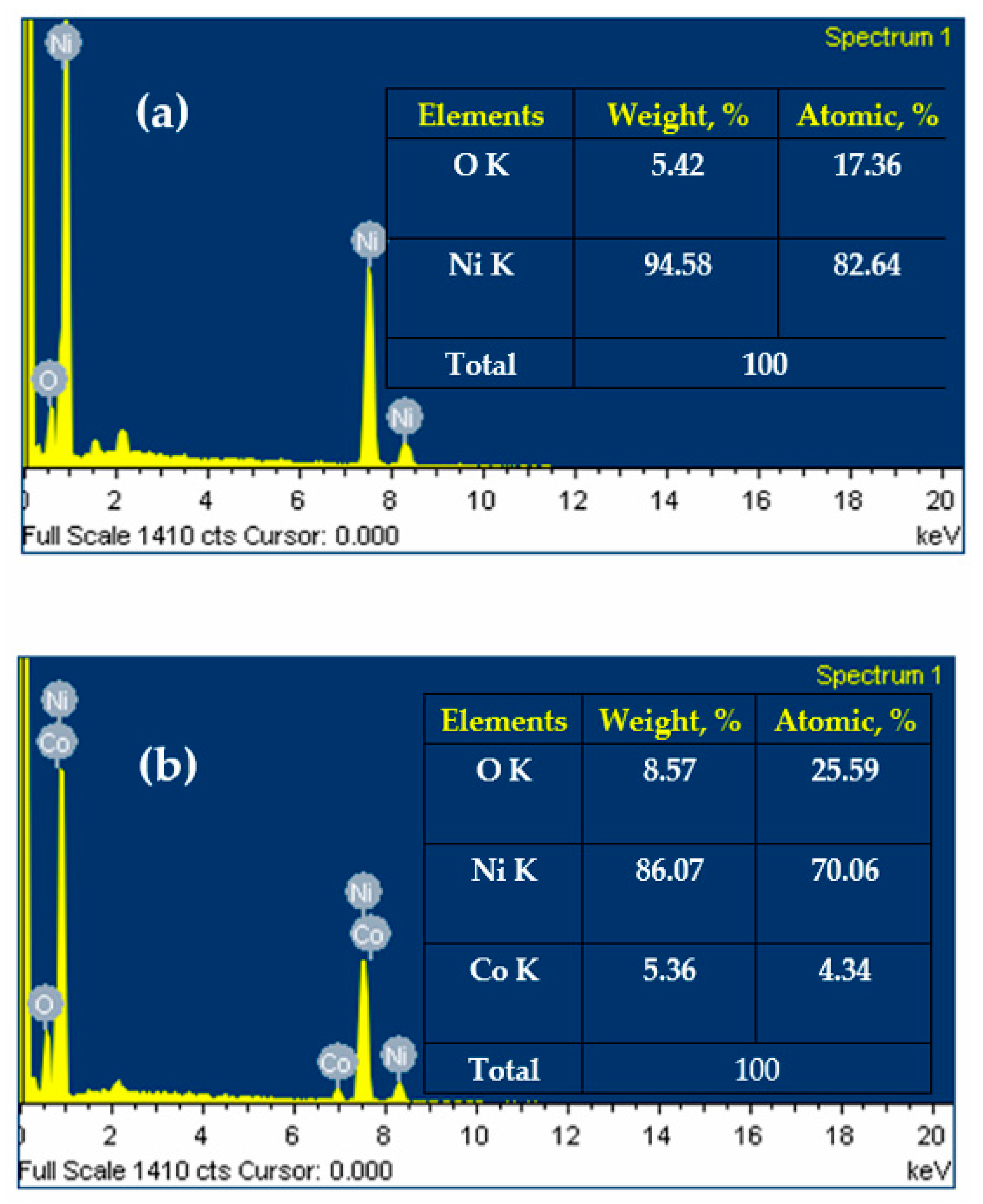
| Materials, Elements | Pure Ni@Ni(OH)2 (Atomic, %) | b-Co-doped Ni@Ni(OH)2 (Atomic, %) |
|---|---|---|
| Ni | 18.71 | 15.22 |
| O | 65.88 | 66.41 |
| Co | - | 3.86 |
| C | 15.41 | 14.51 |
| Total | 100 | |
| Anodic Materials | EIS Parameters: Rs + Q1/R1 + C2/R3 | ||||
|---|---|---|---|---|---|
| Rs, ohm | Q1, F | R1, ohm | C2, F | R3, ohm | |
| Ni@Ni(OH)2 | 2.67 | 4.44 × 10−11 | 10.05 | 0.0205 | 13.33 |
| a-Co-doped Ni@Ni(OH)2 | 4.49 | 1.5 6 × 10−4 | 11.50 | 0.0082 | 10.32 |
| b-Co-doped Ni@Ni(OH)2 | 3.46 | 0.0124 | 7.23 | 0.0475 | 9.77 |
| c-Co-doped Ni@Ni(OH)2 | 3.75 | 4.39 × 10−5 | 132.91 | 0.0009 | 95 |
| Co@Co(OH)2 | 4.95 | 1.65 × 10−5 | 0.467 | 0.0005 | 22.21 |
| Anodic Materials | EIS Parameters: Rs + Q1/R1 + C2/R3+Ws | |||||
|---|---|---|---|---|---|---|
| Rs, ohm | Q1, F | R1, ohm | C2, F | R3, ohm | Ws | |
| Ni@Ni(OH)2 | 4.6948 | 8.6547 × 10−5 | 1.5918 | 0.00695 | 1.052 | 16.398 |
| a-Co-doped Ni@Ni(OH)2 | 4.7738 | 0.00023 | 24.528 | 0.01051 | 53.57 | 5.372 |
| b-Co-doped Ni@Ni(OH)2 | 3.0142 | 0.00284 | 1.0938 | 0.01695 | 3.414 | 10.146 |
| c-Co-doped Ni@Ni(OH)2 | 3.5389 | 0.00012 | 69.242 | 0.00027 | 423.5 | 2.7949 |
| Co@Co(OH)2 | 4.9638 | 4.5011 × 10−5 | 63.822 | 0.00014 | 450.8 | 0.5679 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aladeemy, S.A.; Shaddad, M.N.; Qahtan, T.F.; Alharthi, A.I.; Shalabi, K.; Al-Mayouf, A.M.; Arunachalam, P. Co-Doped Ni@Ni(OH)2 Core–Shell Catalysts for Dual-Function Water and Urea Oxidation. Catalysts 2025, 15, 474. https://doi.org/10.3390/catal15050474
Aladeemy SA, Shaddad MN, Qahtan TF, Alharthi AI, Shalabi K, Al-Mayouf AM, Arunachalam P. Co-Doped Ni@Ni(OH)2 Core–Shell Catalysts for Dual-Function Water and Urea Oxidation. Catalysts. 2025; 15(5):474. https://doi.org/10.3390/catal15050474
Chicago/Turabian StyleAladeemy, Saba A., Maged N. Shaddad, Talal F. Qahtan, Abdulrahman I. Alharthi, Kamal Shalabi, Abdullah M. Al-Mayouf, and Prabhakarn Arunachalam. 2025. "Co-Doped Ni@Ni(OH)2 Core–Shell Catalysts for Dual-Function Water and Urea Oxidation" Catalysts 15, no. 5: 474. https://doi.org/10.3390/catal15050474
APA StyleAladeemy, S. A., Shaddad, M. N., Qahtan, T. F., Alharthi, A. I., Shalabi, K., Al-Mayouf, A. M., & Arunachalam, P. (2025). Co-Doped Ni@Ni(OH)2 Core–Shell Catalysts for Dual-Function Water and Urea Oxidation. Catalysts, 15(5), 474. https://doi.org/10.3390/catal15050474








