Electrochemical Looping Green Hydrogen Production by Using Water Electrochemically Treated as a Raw Material for the Electrolyzer
Abstract
1. Introduction
2. Results and Discussion
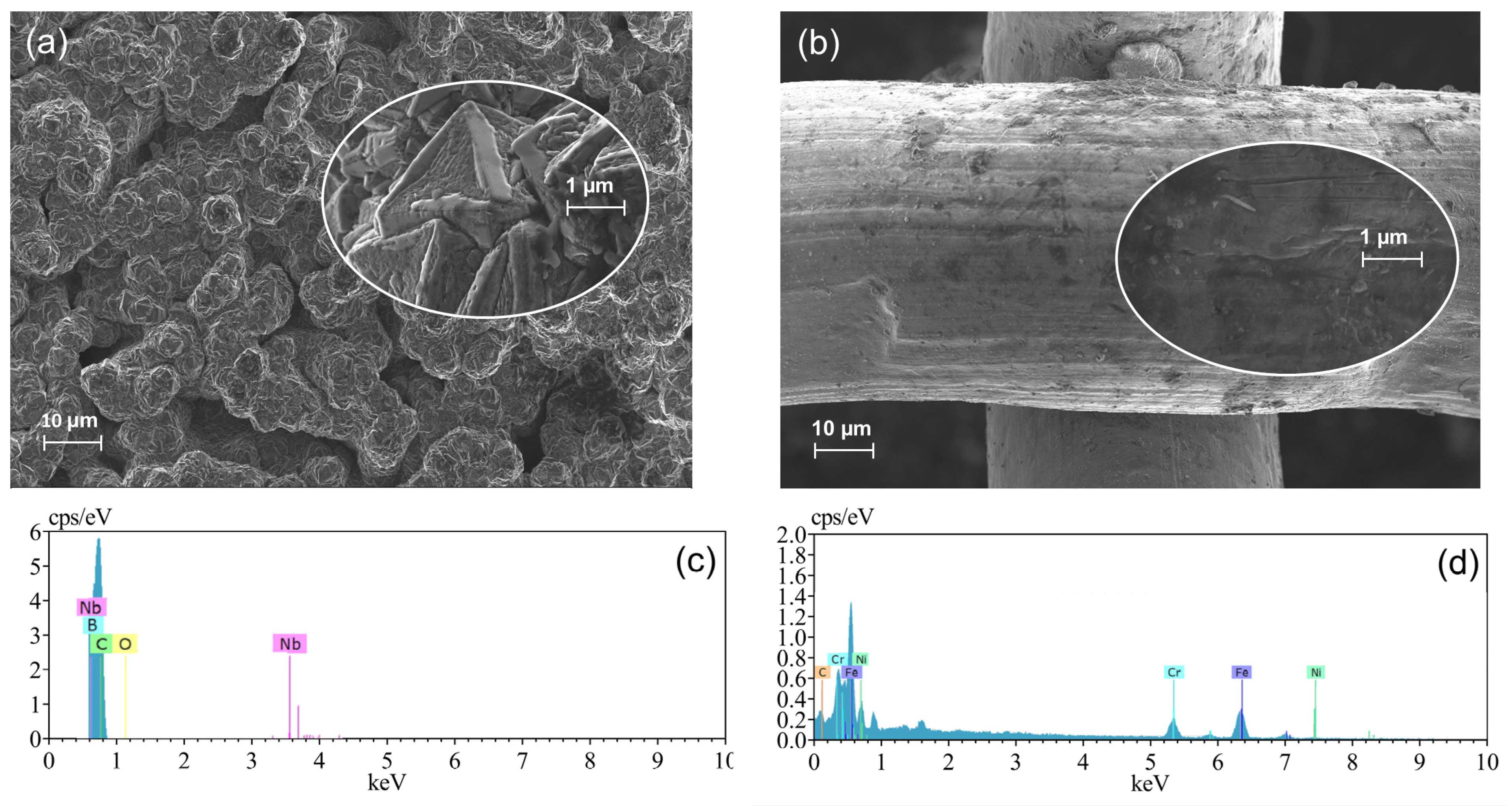
2.1. Electrode Characterizations: BDD as Anode and Ni-Fe-Based SS Mesh as Cathode
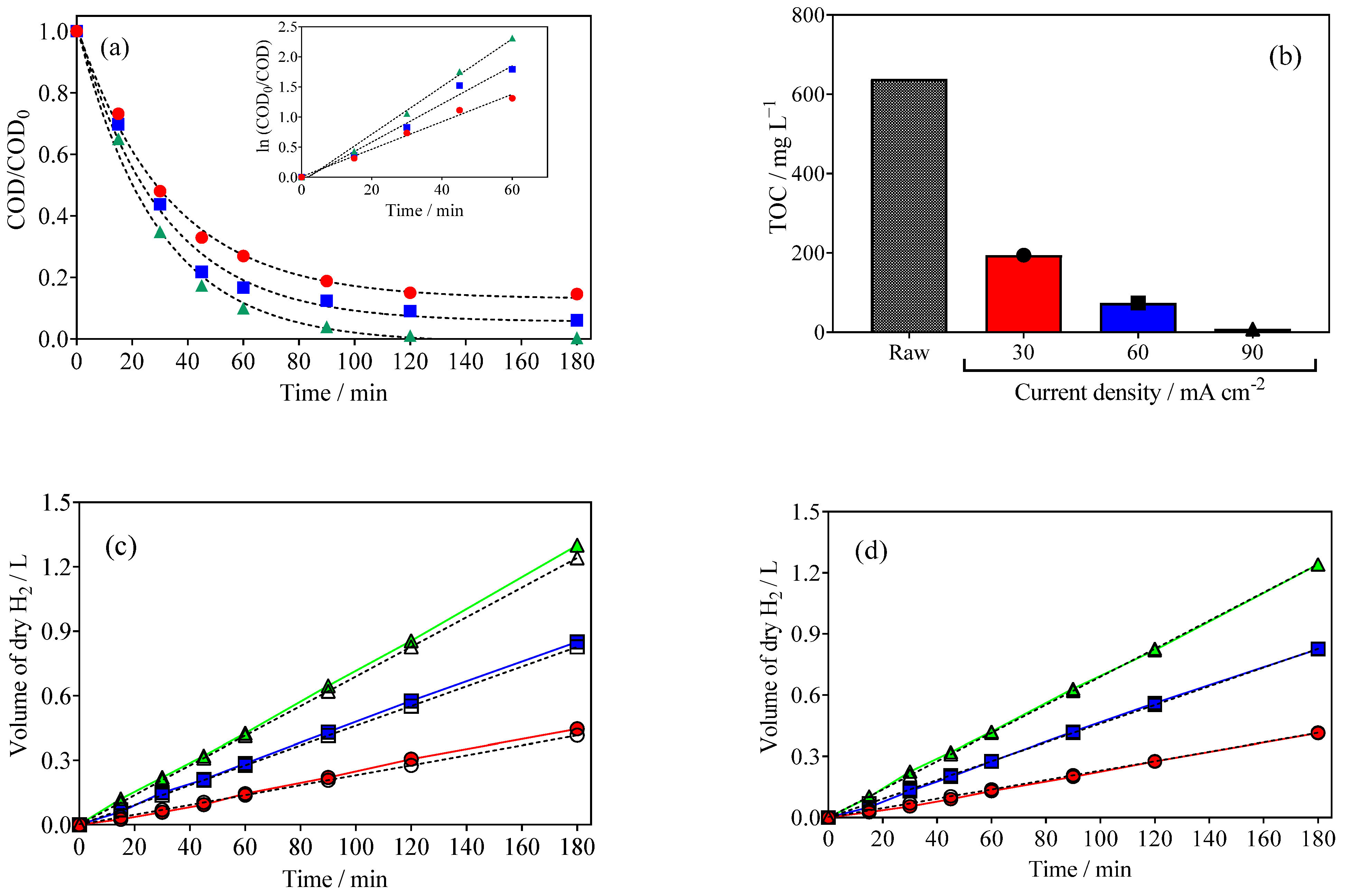
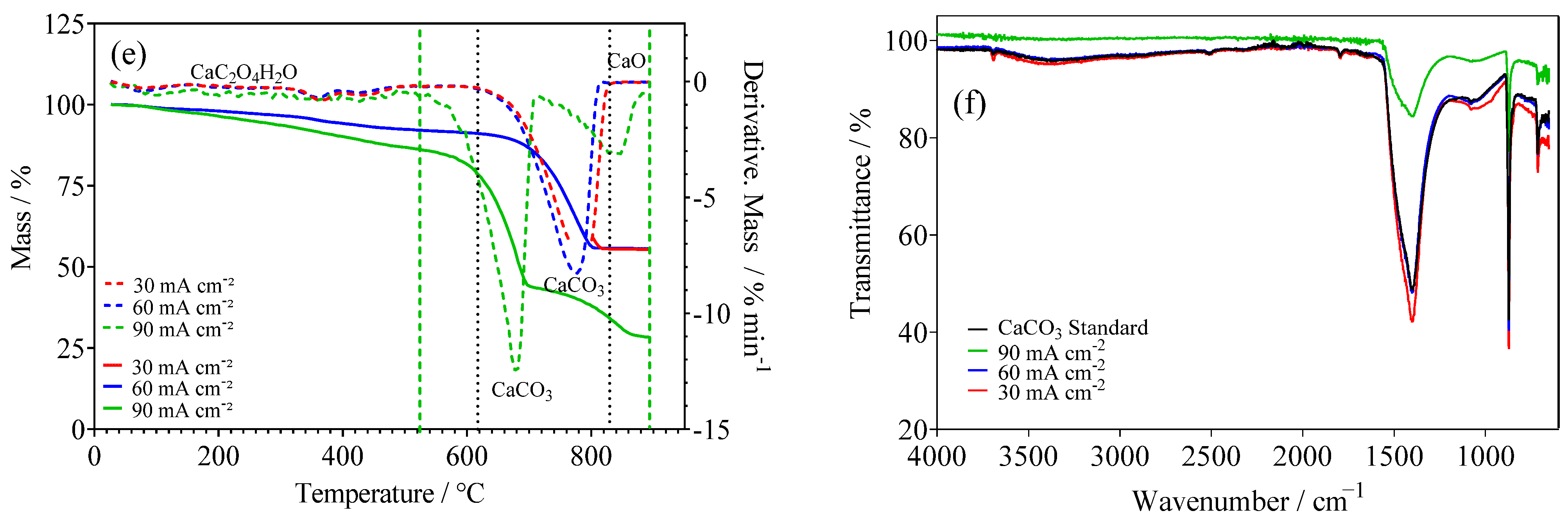
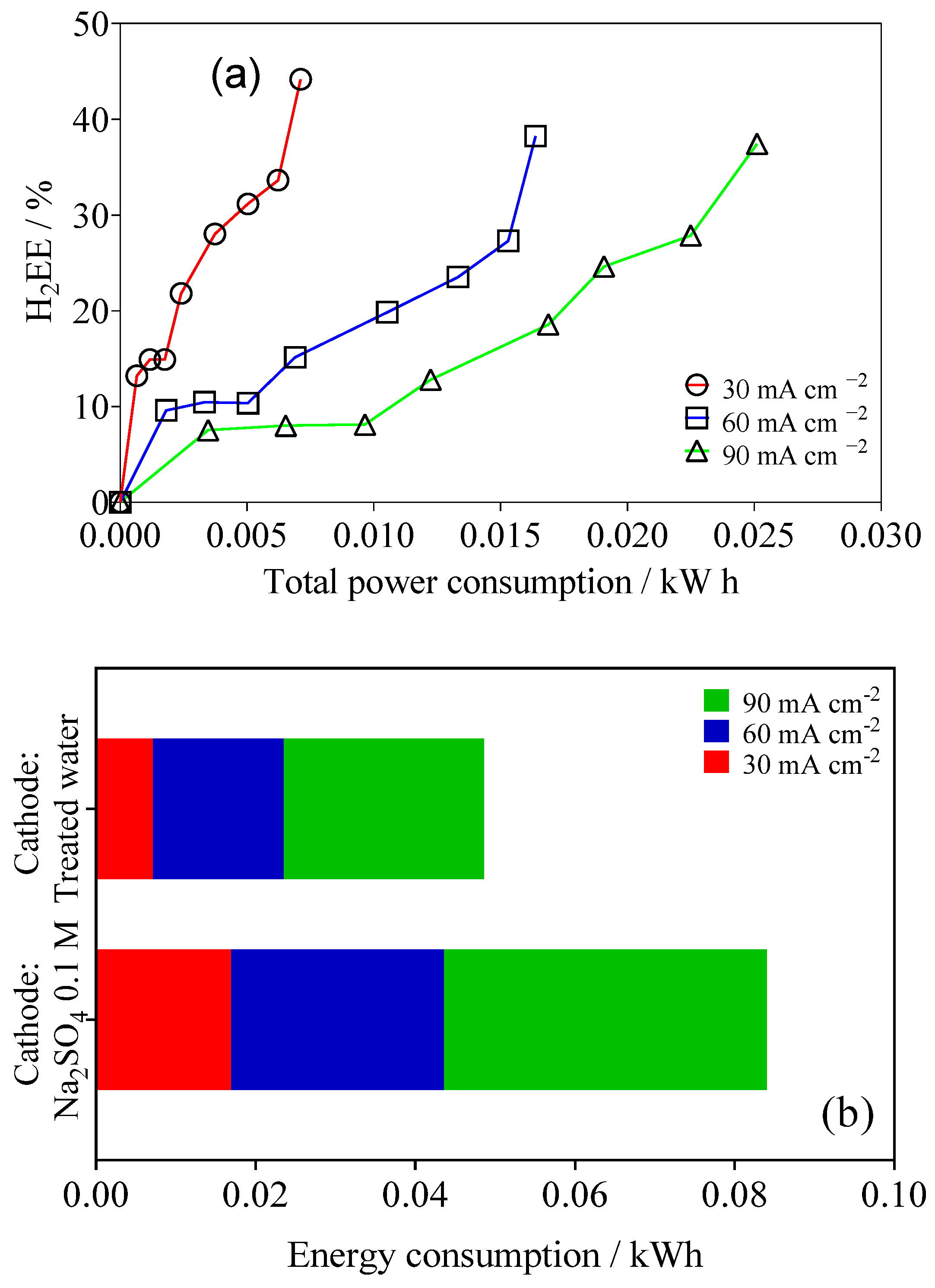
2.2. EO of Wastewater with Simultaneous Green H2 Production
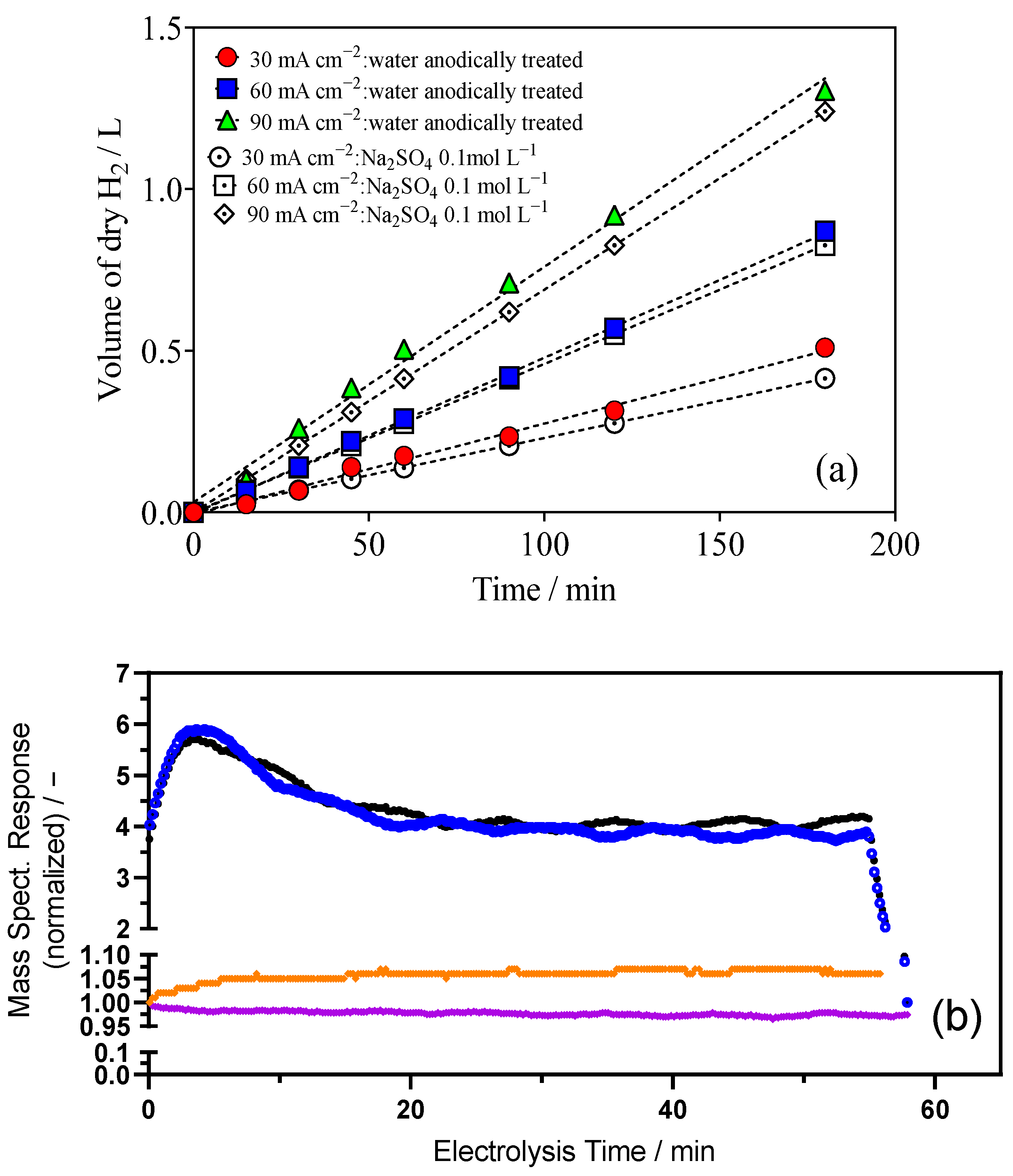
2.3. Reuse of Treated Water in Cathodic Process for H2 Production
- (i)
- The increase in the volume of H2 gas (reported in Figure 3a by applying 90 mA cm−2), as a function of time, is explained as the increase of N2 gas,
- (ii)
- The lower production of N2 is due to the electro conversion of NO3− to NO2−, and after to N2, and
- (iii)
- The preference of Ni-Fe mesh cathode to adsorb H2 is higher than NO3− [42].
3. Materials and Methods
3.1. Experimental Setup
- Firstly, the electrochemical treatment of the 350 mL of raw water (by adding 0.1 mol L−1 Na2SO4 as the supporting electrolyte) was carried out for 180 min using a power supply (Minipa, model MLP-3305, Minipa do Brasil Ltd.a, São Paulo, Brazil) under galvanostatic conditions (30, 60, and 90 mA cm−2), connected to the solar photovoltaic (PV)-battery system (as reported in SM, Figures S3 and S4). The effluent was recirculated through the anode compartment using a peristaltic pump at a constant rate of 125 mL min−1 while a volume of 40 mL of supporting electrolyte solution (0.1 mol L−1 Na2SO4) was used in the cathodic compartment, without flow conditions. The water treatment was reserved for a second stage.
- The treated water in the preceding stage (without additional electrolyte), which was analyzed, was used in the cathodic compartment under similar experimental conditions in (1) to examine the impact of water quality on H2 generation (Table 2).
3.2. Characterization Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alamiery, A. Advancements in Materials for Hydrogen Production: A Review of Cutting-Edge Technologies. ChemPhysMater 2023. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An Overview of Water Electrolysis Technologies for Green Hydrogen Production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y.; Djire, A. Recent Development in Electrocatalysts for Hydrogen Production through Water Electrolysis. Int. J. Hydrogen Energy 2021, 46, 32284–32317. [Google Scholar] [CrossRef]
- Santos, J.E.L.; Da Silva, D.R.; Martínez-Huitle, C.A.; Dos Santos, E.V.; Quiroz, M.A. Cathodic Hydrogen Production by Simultaneous Oxidation of Methyl Red and 2,4-Dichlorophenoxyacetate Aqueous Solutions Using Pb/PbO2, Ti/Sb-Doped SnO2 and Si/BDD Anodes. Part 1: Electrochemical Oxidation. RSC Adv. 2020, 10, 37695–37706. [Google Scholar] [CrossRef]
- Santos, J.E.L.; Da Silva, D.R.; Martínez-Huitle, C.A.; Dos Santos, E.V.; Quiroz, M.A. Cathodic Hydrogen Production by Simultaneous Oxidation of Methyl Red and 2,4-Dichlorophenoxyacetate in Aqueous Solutions Using PbO2, Sb-Doped SnO2 and Si/BDD Anodes. Part 2: Hydrogen Production. RSC Adv. 2020, 10, 37947–37955. [Google Scholar] [CrossRef]
- Barreto, J.P.d.P.; Santos, J.E.L.; Cardozo, J.C.; de Santana Souza, D.F.; Cavalcanti, L.N.; Gondim, A.D.; Martínez-Huitle, C.A.; dos Santos, E.V. Energy-Saving Electrochemical Green Hydrogen Production Coupled with Persulfate or Hydrogen Peroxide Valorization at Boron-Doped Diamond Electrodes. J. Environ. Chem. Eng. 2024, 12, 114837. [Google Scholar] [CrossRef]
- da Silva, L.M.G.; Costa, L.G.A.; Santos, J.E.L.; de A. Costa, E.C.T.; de Morais Araújo, A.M.; Gondim, A.D.; Cavalcanti, L.N.; Quiroz, M.A.; dos Santos, E.V.; Martínez-Huitle, C.A. Electro-Refinery in Organics to Produce Energy Carriers: Co-Generation of Green Hydrogen and Carboxylic Acids by Glycerol Electrooxidation Using Dimensionally Stable Anode. Catalysts 2025, 15, 333. [Google Scholar] [CrossRef]
- Campos da Paixão, I.; Cardozo, J.C.; Sales Monteiro, M.K.; Gondim, A.D.; Cavalcanti, L.N.; Fabiano de Santana Souza, D.; Martínez-Huitle, C.A.; Vieira dos Santos, E. A Sustainable Solar-Driven Electrochemical Process for Reforming Lignocellulosic Biomass Effluent into High Value-Added Products: Green Hydrogen, Carboxylic and Vanillic Acids. RSC Adv. 2023, 13, 35755–35765. [Google Scholar] [CrossRef]
- Oliveira, H.L.; Barros, T.M.; Santos, J.E.L.; Gondim, A.D.; Quiroz, M.A.; Martínez-Huitle, C.A.; dos Santos, E.V. Electrochemical Oxidation of a Real Effluent Using Selective Cathodic and Anodic Strategies to Simultaneously Produce High Value-Added Compounds: Green Hydrogen and Carboxylic Acids. Electrochem. Commun. 2023, 154, 107553. [Google Scholar] [CrossRef]
- Oliveira, H.L.; Santos, J.E.L.; Gondim, A.D.; Cavalcanti, L.N.; Correia de Carvalho, F.; Castro, S.S.L.; Martínez-Huitle, C.A.; dos Santos, E.V. Replacing Oxygen Evolution Reaction in Water Splitting Process by Electrochemical Energy-Efficient Production of High-Added Value Chemicals with Co-Generation of Green Hydrogen. Electrochim. Acta 2024, 499, 144692. [Google Scholar] [CrossRef]
- dos Santos, E.V.; Cardozo, J.C.; Feijoó Zambrano, T.N.; Quiroz Alfaro, M.A.; Martínez-Huitle, C.A. Towards Win-Win Electrochemical Alternative for Depolluting Water and Producing Green H2: Understanding and Unrevealing the Participation of Sacrificial Organic Compound on H2 Production. Int. J. Hydrogen Energy 2024, 110, 598–608. [Google Scholar] [CrossRef]
- de Araujo, D.M.; Barbosa Segundo, I.D.; Cardozo, J.C.; Santos, J.E.L.; Nascimento, J.H.O.; Gondim, A.D.; dos Santos, E.V.; Martínez-Huitle, C.A. Produced Water Electrolysis with Simultaneous Green H2 Generation: From Wastewater to the Future of the Energetic Industry. Fuel 2024, 373, 132369. [Google Scholar] [CrossRef]
- Câmara Cardozo, J.; da Silva, D.R.; Martínez-Huitle, C.A.; Quiroz, M.A.; Dos Santos, E.V. Photovoltaic Electrochemically Driven Degradation of Calcon Dye with Simultaneous Green Hydrogen Production. Materials 2022, 15, 7445. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Zhang, P.S.; Jiang, Y.X.; Sun, L.; Sun, X.H. Wastewater Treatment by Anodic Oxidation in Electrochemical Advanced Oxidation Process: Advance in Mechanism, Direct and Indirect Oxidation Detection Methods. Chemosphere 2023, 311, 136993. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, X.; Kamali, M.; Zhang, X.; Feijoo, S.; Al-Salem, S.M.; Dewil, R.; Appels, L. Biochar in Hydroxyl Radical-Based Electrochemical Advanced Oxidation Processes (EAOPs)—Mechanisms and Prospects. Chem. Eng. J. 2023, 467, 143291. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; Moratalla, Á.; Sáez, C.; Dos Santos, E.V.; Rodrigo, M.A. Production of Chlorine Dioxide Using Hydrogen Peroxide and Chlorates. Catalysts 2021, 11, 1478. [Google Scholar] [CrossRef]
- Jlassi, K.; Oturan, M.A.; Fauzi Ismail, A.; Chehimi, M.M. Clean Water: Next Generation Technologies; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Ahmed, M.; Mavukkandy, M.O.; Giwa, A.; Elektorowicz, M.; Katsou, E.; Khelifi, O.; Naddeo, V.; Hasan, S.W. Recent Developments in Hazardous Pollutants Removal from Wastewater and Water Reuse within a Circular Economy. Npj Clean Water 2022, 5, 1–25. [Google Scholar] [CrossRef]
- He, Y.; Huang, W.; Chen, R.; Zhang, W.; Lin, H.; Li, H. Anodic Oxidation of Aspirin on PbO2, BDD and Porous Ti/BDD Electrodes: Mechanism, Kinetics and Utilization Rate. Sep. Purif. Technol. 2015, 156, 124–131. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A. Nature, Mechanisms and Reactivity of Electrogenerated Reactive Species at Thin-Film Boron-Doped Diamond (BDD) Electrodes During Electrochemical Wastewater Treatment. ChemElectroChem 2019, 6, 2379–2392. [Google Scholar] [CrossRef]
- Oliveira, K.S.G.C.; dos Santos, E.V.; Loor-Urgilés, L.D.; Shabanloo, A.; Martínez-Huitle, C.A. The World Impact of Boron Doped Diamond Electrodes and Low-Cost Strategies for Novel Production Systems for Sustainable Wastewater Treatment. Curr. Opin. Electrochem. 2025, 50, 101648. [Google Scholar] [CrossRef]
- Mbaye, M.; Diaw, P.A.; Mbaye, O.M.A.; Oturan, N.; Gaye Seye, M.D.; Trellu, C.; Coly, A.; Tine, A.; Aaron, J.J.; Oturan, M.A. Rapid Removal of Fungicide Thiram in Aqueous Medium by Electro-Fenton Process with Pt and BDD Anodes. Sep. Purif. Technol. 2022, 281, 119837. [Google Scholar] [CrossRef]
- Ridruejo, C.; Salazar, C.; Cabot, P.L.; Centellas, F.; Brillas, E.; Sirés, I. Electrochemical Oxidation of Anesthetic Tetracaine in Aqueous Medium. Influence of the Anode and Matrix Composition. Chem. Eng. J. 2017, 326, 811–819. [Google Scholar] [CrossRef]
- Clematis, D.; Panizza, M. Application of Boron-Doped Diamond Electrodes for Electrochemical Oxidation of Real Wastewaters. Curr. Opin. Electrochem. 2021, 30, 100844. [Google Scholar] [CrossRef]
- Castillo-Cabrera, G.X.; Pliego-Cerdán, C.I.; Méndez, E.; Espinoza-Montero, P.J. Step-by-Step Guide for Electrochemical Generation of Highly Oxidizing Reactive Species on BDD for Beginners. Front. Chem. 2023, 11, 1298630. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sirés, I.; Scialdone, O. A Critical Review on Latest Innovations and Future Challenges of Electrochemical Technology for the Abatement of Organics in Water. Appl. Catal. B 2023, 328, 122430. [Google Scholar] [CrossRef]
- Skolotneva, E.; Kislyi, A.; Klevtsova, A.; Clematis, D.; Mareev, S.; Panizza, M. Mathematical Modeling of the Anodic Oxidation of Organic Pollutants: A Review. Environ. Chem. Lett. 2024, 22, 1521–1561. [Google Scholar] [CrossRef]
- Araújo, K.C.; dos Santos, E.V.; Nidheesh, P.V.; Martínez-Huitle, C.A. Fundamentals and Advances on the Mechanisms of Electrochemical Generation of Persulfate and Sulfate Radicals in Aqueous Medium. Curr. Opin. Chem. Eng. 2022, 38, 100870. [Google Scholar] [CrossRef]
- Hou, Y.; Tu, L.; Qin, S.; Yu, Z.; Yan, Y.; Xu, Y.; Song, H.; Lin, H.; Chen, Y.; Wang, S. Dye Wastewater Treatment and Hydrogen Production in Microbial Electrolysis Cells Using MoS2-Graphene Oxide Cathode: Effects of Dye Concentration, Co-Substrate and Buffer Solution. Process Biochem. 2021, 102, 51–58. [Google Scholar] [CrossRef]
- Gómez, M.J.; Benavente-Llorente, V.; Hainer, A.; Lacconi, G.I.; Scaiano, J.C.; Franceschini, E.A.; Lanterna, A.E. Evaluation of Different Ni-Semiconductor Composites as Electrodes for Enhanced Hydrogen Evolution Reaction. Sustain. Energy Fuels 2020, 4, 3963–3970. [Google Scholar] [CrossRef]
- Buccheri, B.; Ganci, F.; Patella, B.; Aiello, G.; Mandin, P.; Inguanta, R. Ni-Fe Alloy Nanostructured Electrodes for Water Splitting in Alkaline Electrolyser. Electrochim. Acta 2021, 388, 138588. [Google Scholar] [CrossRef]
- Kim, J.S.; Han, J.W.; Lee, B.J. Alloying Non-Precious Metals into Ni-Based Electrocatalysts for Enhanced Hydrogen Oxidation Reaction in Alkaline Media: A Computational Study. Appl. Surf. Sci. 2021, 554, 149627. [Google Scholar] [CrossRef]
- Meshram, A.A.; Aashish Moses, K.; Baral, S.S.; Sontakke, S.M. Hydrogen Production from Water Splitting of Real-Time Industry Effluent Using Novel Photocatalyst. Adv. Powder Technol. 2022, 33, 103488. [Google Scholar] [CrossRef]
- Tak, S.S.; Shetye, O.; Muley, O.; Jaiswal, H.; Malik, S.N. Emerging Technologies for Hydrogen Production from Wastewater. Int. J. Hydrogen Energy 2022, 47, 37282–37301. [Google Scholar] [CrossRef]
- Klidi, N.; Clematis, D.; Carpanese, M.P.; Gadri, A.; Ammar, S.; Panizza, M. Electrochemical Oxidation of Crystal Violet Using a BDD Anode with a Solid Polymer Electrolyte. Sep. Purif. Technol. 2019, 208, 178–183. [Google Scholar] [CrossRef]
- Fresenius, W.; Quentin, K.E.; Schneider, W. Others Water Analysis; a Practical Guide to Physico-Chemical, Chemical and Microbiological Water Examination and Quality Assurance; Springer: Berlin/Heidelberg, Germany, 1988; ISBN 9788578110796. [Google Scholar]
- Guenot, B.; Cretin, M.; Lamy, C. Clean Hydrogen Generation from the Electrocatalytic Oxidation of Methanol inside a Proton Exchange Membrane Electrolysis Cell (PEMEC): Effect of Methanol Concentration and Working Temperature. J. Appl. Electrochem. 2015, 45, 973–981. [Google Scholar] [CrossRef]
- Ferro, S.; Martínez-Huitle, C.A.; De Battisti, A. Electroxidation of Oxalic Acid at Different Electrode Materials. J. Appl. Electrochem. 2010, 40, 1779–1787. [Google Scholar] [CrossRef]
- Valor, A.; Reguera, E.; Torres-García, E.; Mendoza, S.; Sanchez-Sinencio, F. Thermal Decomposition of the Calcium Salts of Several Carboxylic Acids. Thermochim. Acta 2002, 389, 133–139. [Google Scholar] [CrossRef]
- Karunadasa, K.S.P.; Manoratne, C.H.; Pitawala, H.M.T.G.A.; Rajapakse, R.M.G. Thermal Decomposition of Calcium Carbonate (Calcite Polymorph) as Examined by in-Situ High-Temperature X-Ray Powder Diffraction. J. Phys. Chem. Solids 2019, 134, 21–28. [Google Scholar] [CrossRef]
- Wada, N.; Horiuchi, N.; Nakamura, M.; Nozaki, K.; Nagai, A.; Yamashita, K. Controlled Crystallization of Calcium Carbonate via Cooperation of Polyaspartic Acid and Polylysine under Double-Diffusion Conditions in Agar Hydrogels. ACS Omega 2018, 3, 16681–16692. [Google Scholar] [CrossRef]
- Chen, D.; Yin, D.; Zhang, S.; Yip, S.P.; Ho, J.C. Nitrate Electroreduction: Recent Development in Mechanistic Understanding and Electrocatalyst Design. Mater. Today Energy 2024, 44, 101610. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, D.; Kim, K.; Hoffmann, M.R. Electrochemical Production of Hydrogen Coupled with the Oxidation of Arsenite. Environ. Sci. Technol. 2014, 48, 2059–2066. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Guo, Z.; Zhang, W.; Li, H.; Huang, W. Recent Developments and Advances in Boron-Doped Diamond Electrodes for Electrochemical Oxidation of Organic Pollutants. Sep. Purif. Technol. 2019, 212, 802–821. [Google Scholar] [CrossRef]
- Brosler, P.; Girão, A.V.; Silva, R.F.; Tedim, J.; Oliveira, F.J. In-House vs. Commercial Boron-Doped Diamond Electrodes for Electrochemical Degradation of Water Pollutants: A Critical Review. Front. Mater. 2023, 10, 1020649. [Google Scholar] [CrossRef]
- Muddemann, T.; Neuber, R.; Haupt, D.; Graßl, T.; Issa, M.; Bienen, F.; Enstrup, M.; Möller, J.; Matthée, T.; Sievers, M.; et al. Improving the Treatment Efficiency and Lowering the Operating Costs of Electrochemical Advanced Oxidation Processes. Processes 2021, 9, 1482. [Google Scholar] [CrossRef]
- Barbosa Segundo, I.D.; Cardozo, J.C.; Castro, P.S.; Gondim, A.D.; dos Santos, E.V.; Martínez-Huitle, C.A. Cost-Effective Smartphone-Based Method for Low Range Chemical Oxygen Demand Analysis. MethodsX 2023, 11, 102300. [Google Scholar] [CrossRef]
- de Castro, C.M.; Olivi, P.; de Freitas Araújo, K.C.; Barbosa Segundo, I.D.; dos Santos, E.V.; Martínez-Huitle, C.A. Environmental Application of a Cost-Effective Smartphone-Based Method for COD Analysis: Applicability in the Electrochemical Treatment of Real Wastewater. Sci. Total Environ. 2023, 855, 158816. [Google Scholar] [CrossRef]
| Parameters | Contaminated Water | After Treatment | ||
|---|---|---|---|---|
| 30 mA cm−2 | 60 mA cm−2 | 90 mA cm−2 | ||
| Conductivity (μS cm−2) | 2009 | 31.28 | 26.51 | 21.88 |
| pH | 7.77 | 1.73 | 1.94 | 1.56 |
| UT Turbidity | 16.58 | 11.75 | 11.53 | 11.85 |
| Nitrate (mg/L) | 4.69 | 8.84 | 13.22 | 17.88 |
| Total Nitrogen (chemiluminescence) (mg L−1) | 49.86 | 1.34 | 2.13 | 2.72 |
| Calcium (mg L−1) | 27.09 | 16.01 | 6.56 | 2.56 |
| Sodium (mg L−1) | 301.42 | 2486 | 3105 | 3287 |
| Acetic acid (mg L−1) | ND | 1.25 | 1.98 | 0.46 |
| Formic acid (mg L−1) | ND | 2.05 | 3.74 | 0.82 |
| Malonic acid (mg L−1) | ND | ND | 1.51 | 0.85 |
| Oxalic acid (mg L−1) | ND | 1.1 | 0.7 | 0.5 |
| Entry | Anodic Compartment | Cathodic Compartment | j, mA cm−2 | Flow Rate r(H2), mmol min−1 |
|---|---|---|---|---|
| 1 | Wastewater 0.1 mol L−1 Na2SO4 | 0.1 mol L−1 Na2SO4 | 30 | 0.22 |
| 2 | Wastewater 0.1 mol L−1 Na2SO4 | 0.1 mol L−1 Na2SO4 | 60 | 0.31 |
| 3 | Wastewater 0.1 mol L−1 Na2SO4 | 0.1 mol L−1 Na2SO4 | 90 | 0.45 |
| 4 | Wastewater 0.1 mol L−1 Na2SO4 | water treated | 30 | 0.28 |
| 5 | Wastewater 0.1 mol L−1 Na2SO4 | water treated | 60 | 0.44 |
| 6 | Wastewater 0.1 mol L−1 Na2SO4 | water treated | 90 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, M.K.S.; Cardozo, J.C.; Araújo, A.M.d.M.; Gondim, A.D.; Feijoó, T.N.; Loor-Urgilés, L.D.; Martínez-Huitle, C.A.; Quiroz, M.A.; dos Santos, E.V. Electrochemical Looping Green Hydrogen Production by Using Water Electrochemically Treated as a Raw Material for the Electrolyzer. Catalysts 2025, 15, 447. https://doi.org/10.3390/catal15050447
Monteiro MKS, Cardozo JC, Araújo AMdM, Gondim AD, Feijoó TN, Loor-Urgilés LD, Martínez-Huitle CA, Quiroz MA, dos Santos EV. Electrochemical Looping Green Hydrogen Production by Using Water Electrochemically Treated as a Raw Material for the Electrolyzer. Catalysts. 2025; 15(5):447. https://doi.org/10.3390/catal15050447
Chicago/Turabian StyleMonteiro, Mayra K. Sales, Jussara C. Cardozo, Aruzza M. de Morais Araújo, Amanda D. Gondim, Tabata N. Feijoó, Luis D. Loor-Urgilés, Carlos A. Martínez-Huitle, Marco A. Quiroz, and Elisama V. dos Santos. 2025. "Electrochemical Looping Green Hydrogen Production by Using Water Electrochemically Treated as a Raw Material for the Electrolyzer" Catalysts 15, no. 5: 447. https://doi.org/10.3390/catal15050447
APA StyleMonteiro, M. K. S., Cardozo, J. C., Araújo, A. M. d. M., Gondim, A. D., Feijoó, T. N., Loor-Urgilés, L. D., Martínez-Huitle, C. A., Quiroz, M. A., & dos Santos, E. V. (2025). Electrochemical Looping Green Hydrogen Production by Using Water Electrochemically Treated as a Raw Material for the Electrolyzer. Catalysts, 15(5), 447. https://doi.org/10.3390/catal15050447










