Amorphous MnO2 Supported on CN@SiO2 for Levofloxacin Degradation via a Non-Radical Pathway by PMS Activation
Abstract
1. Introduction
2. Results
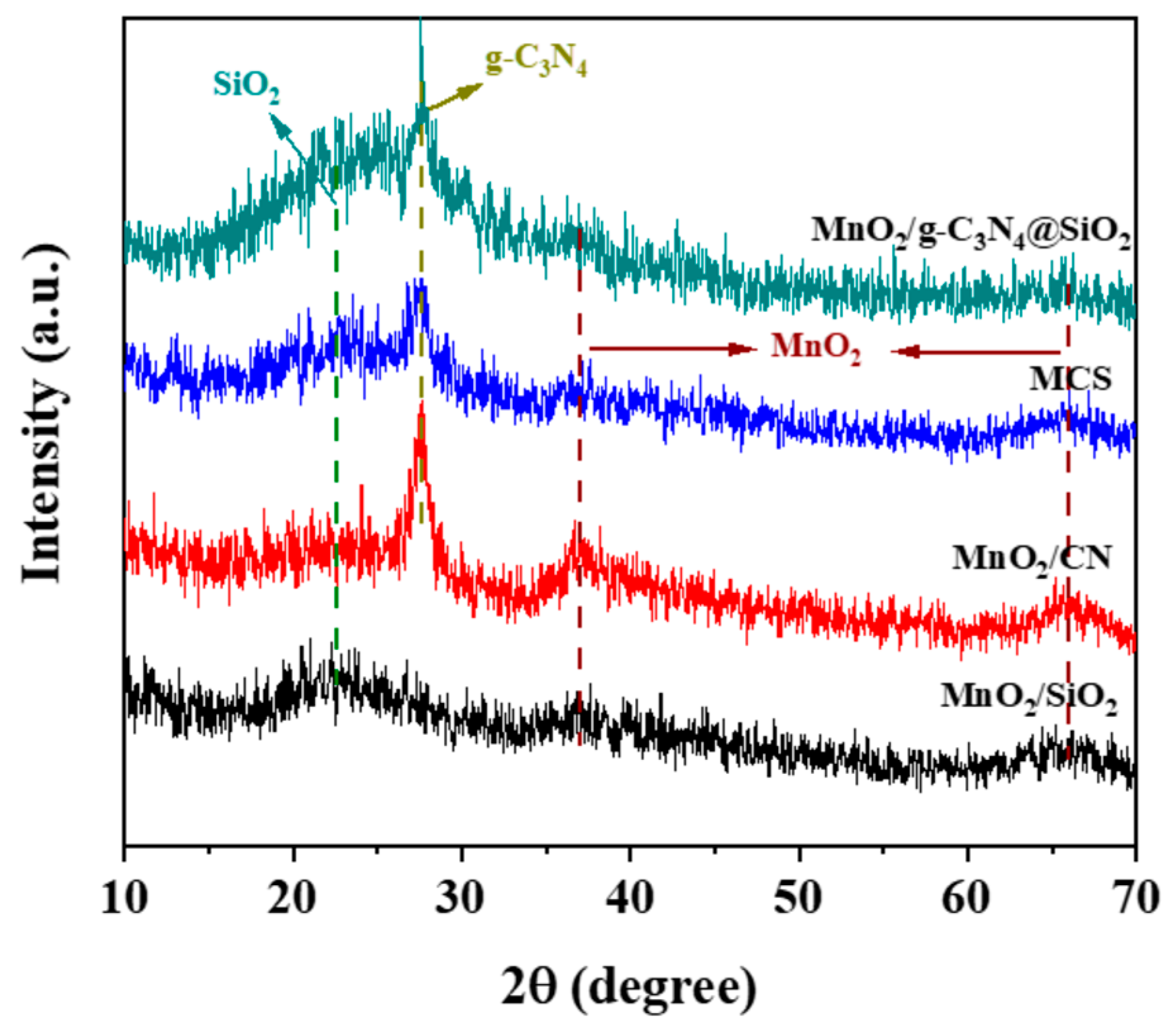
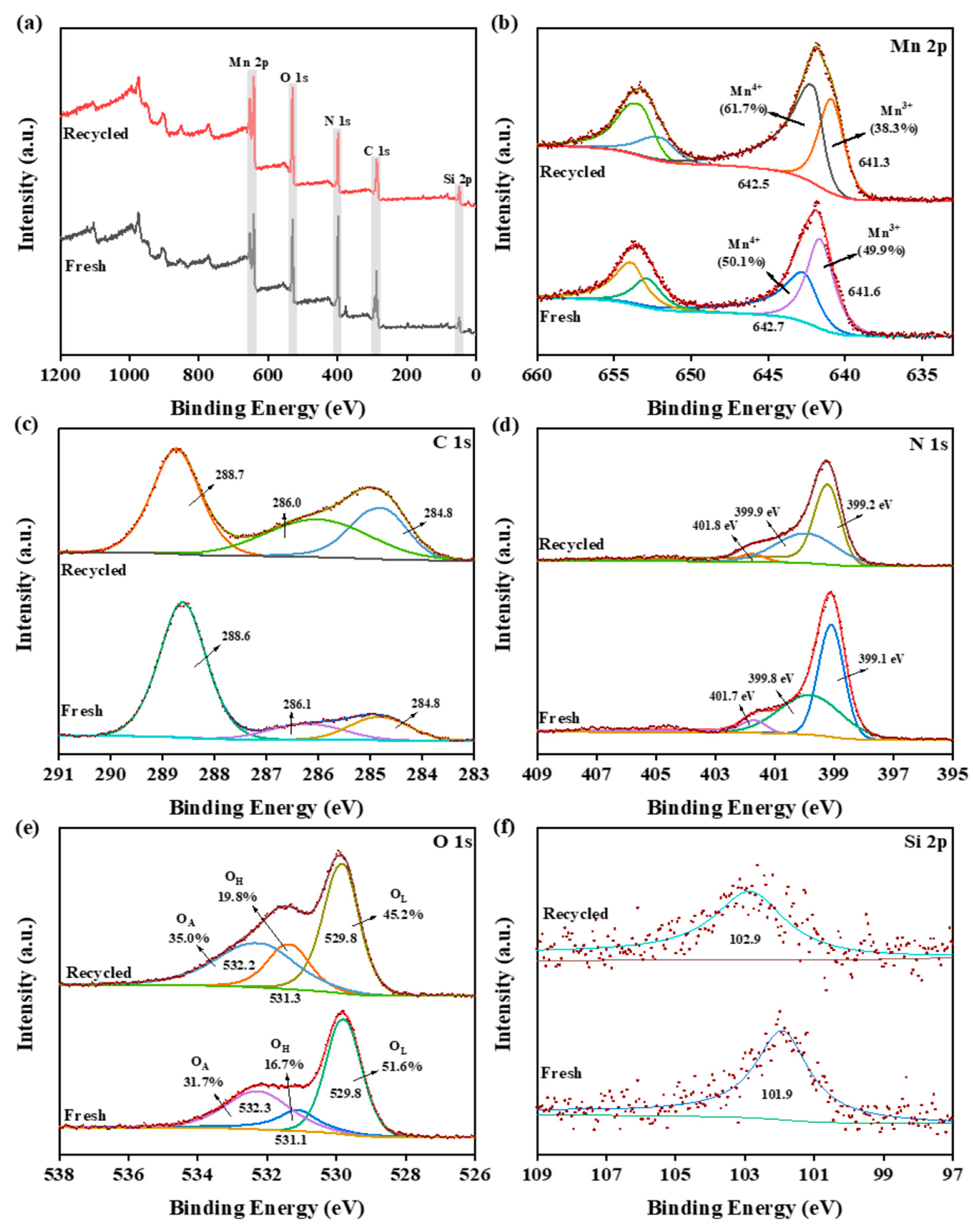
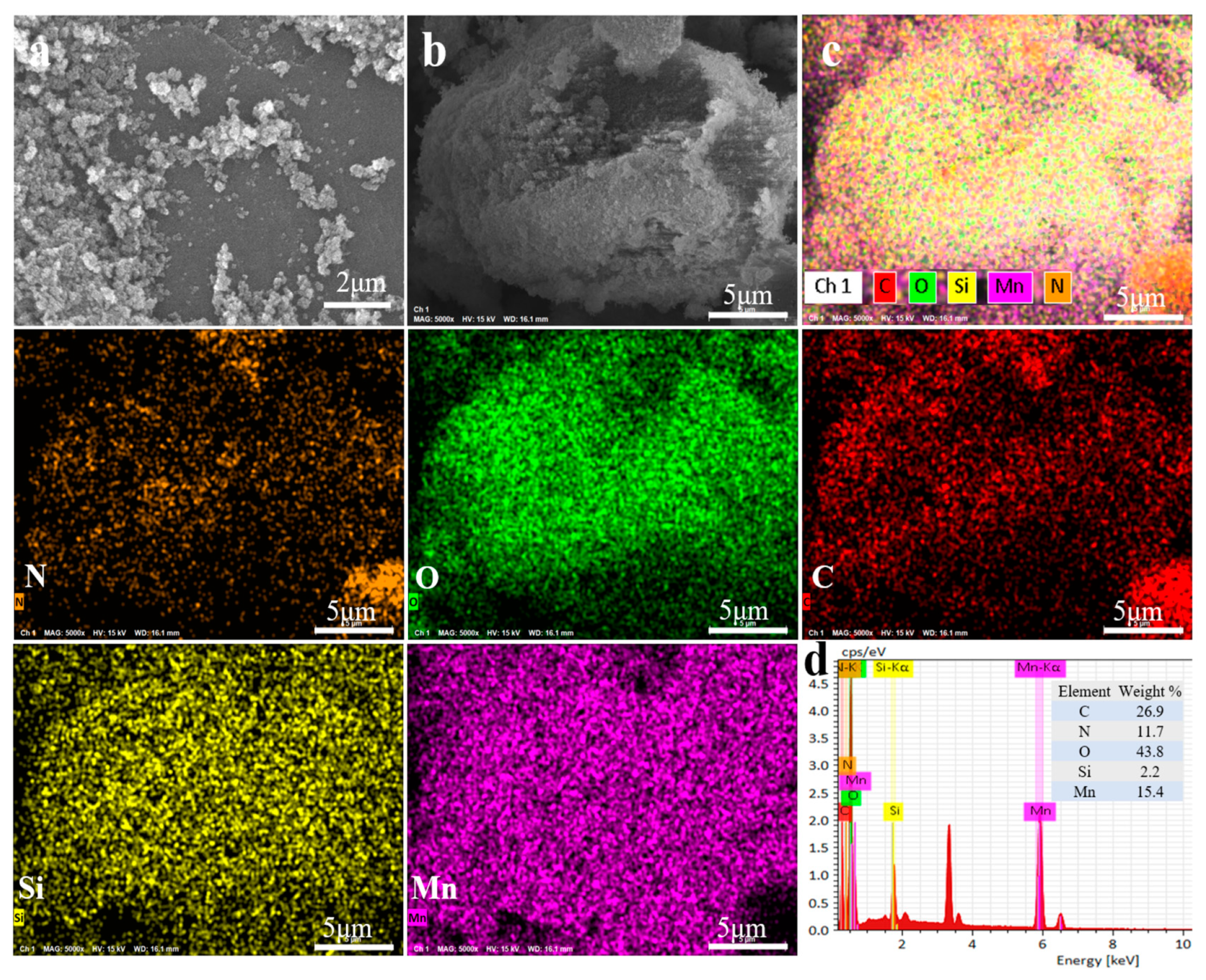
2.1. Characterizations of MnO2/CN@SiO2
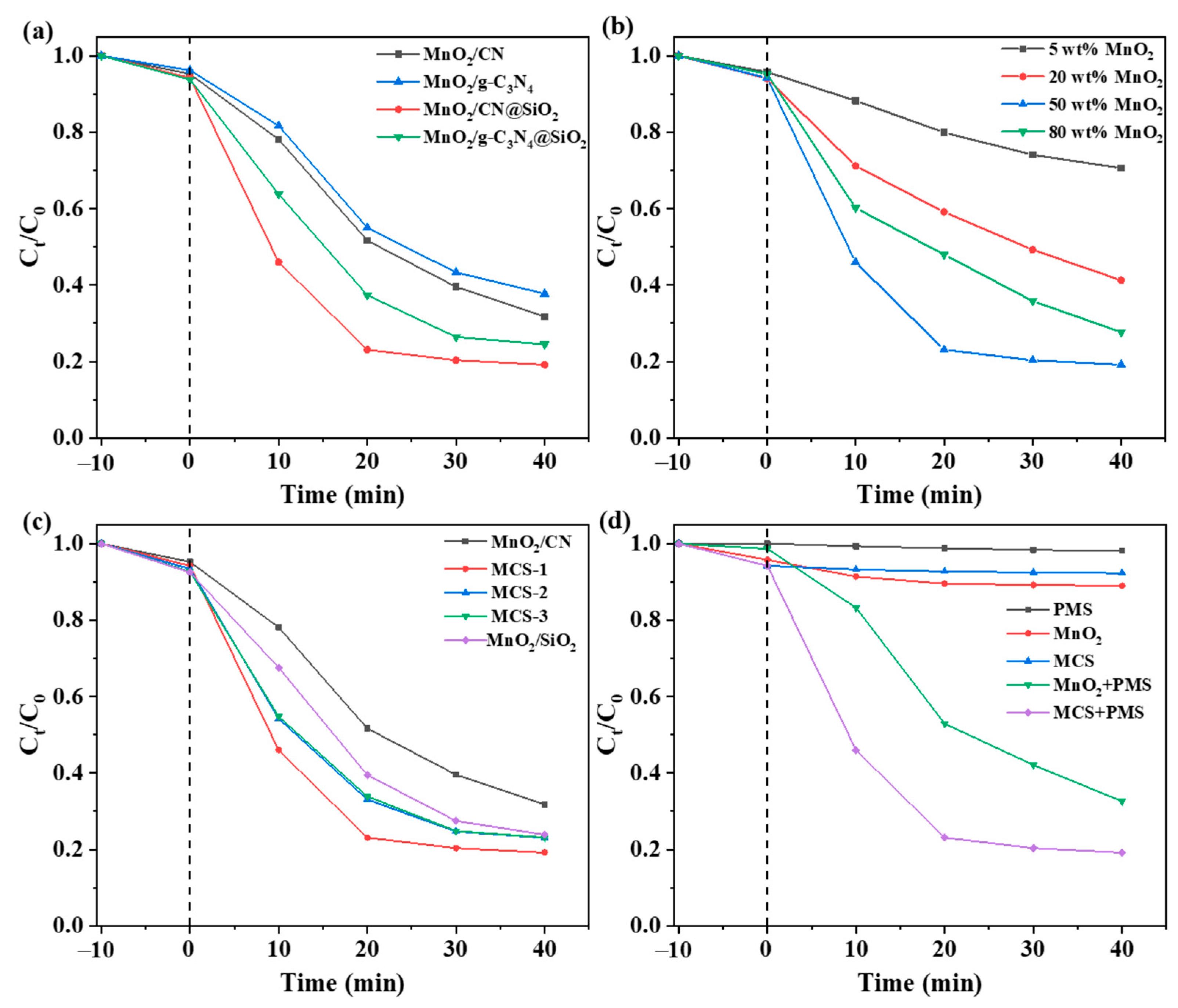
2.2. Effect of Catalyst Composition on LEV Degradation
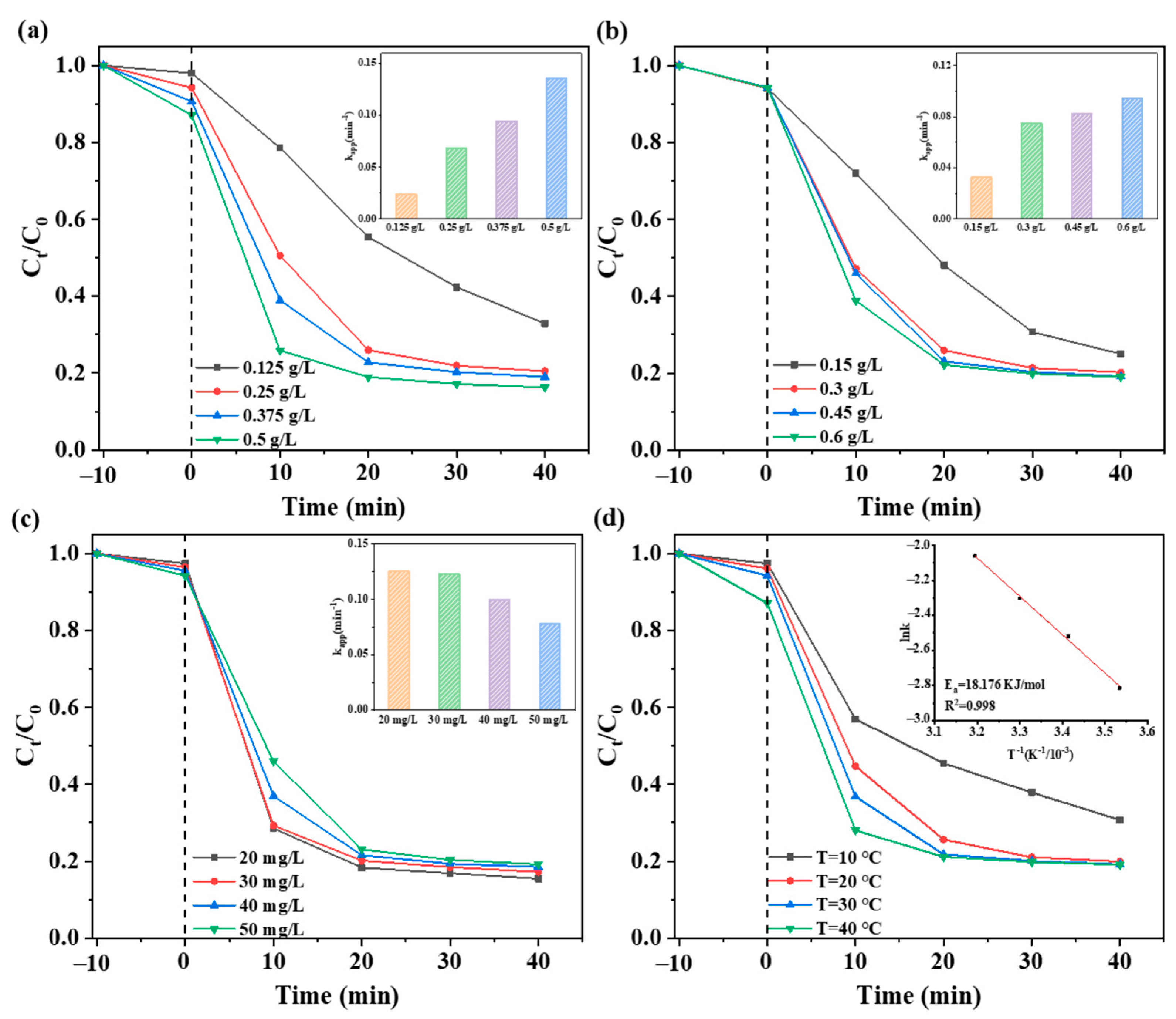
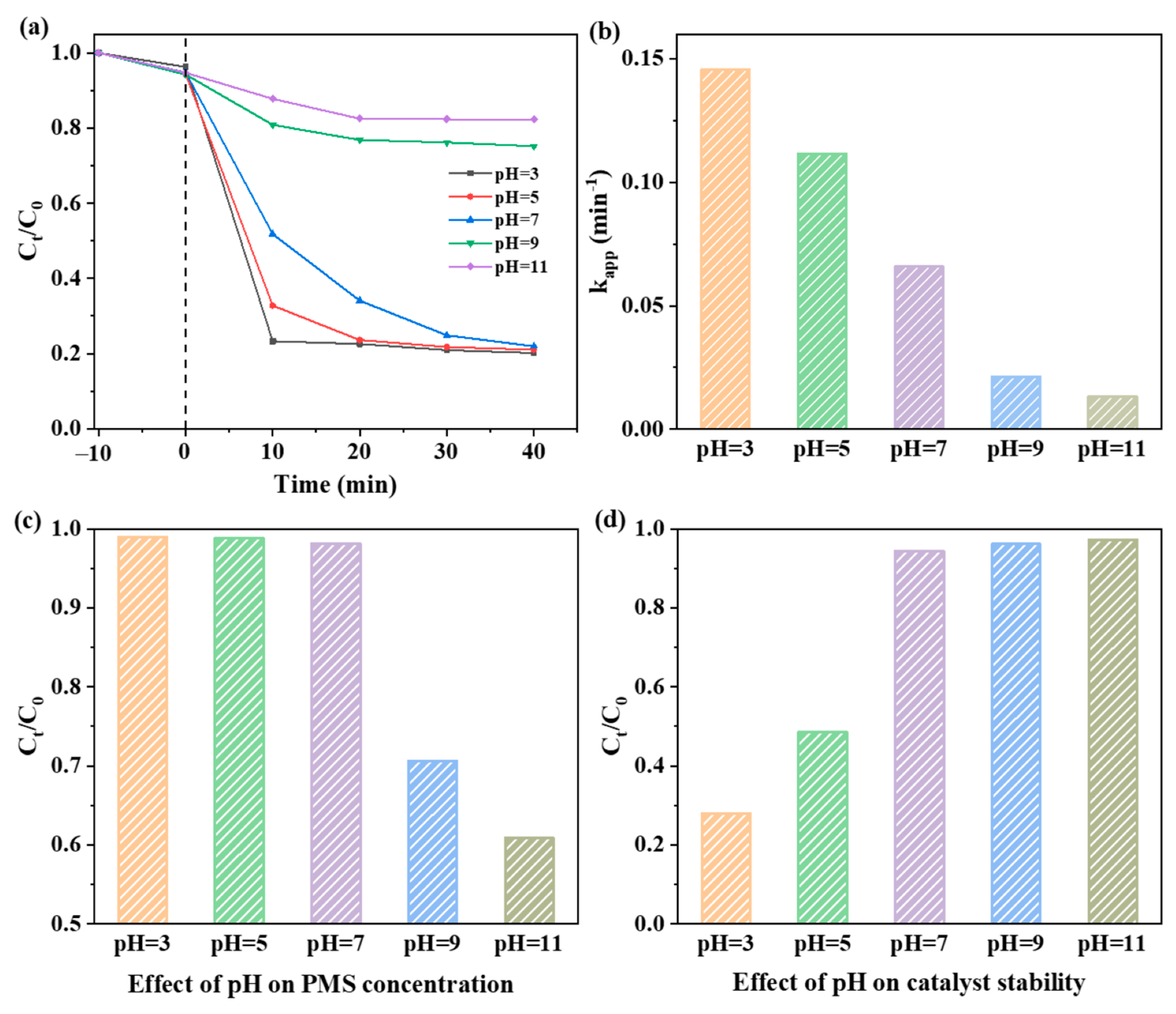
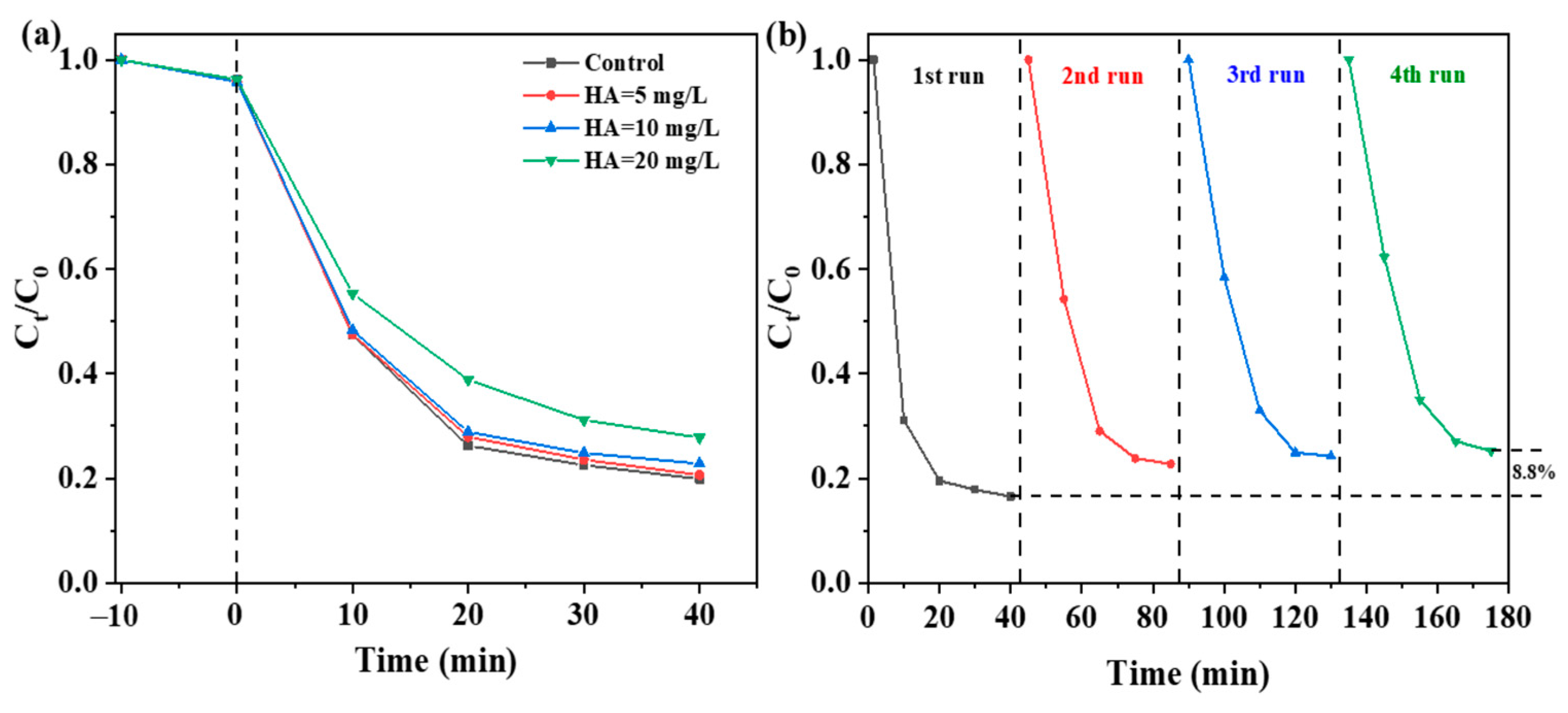
2.3. Effect of Reaction Conditions on LEV Degradation
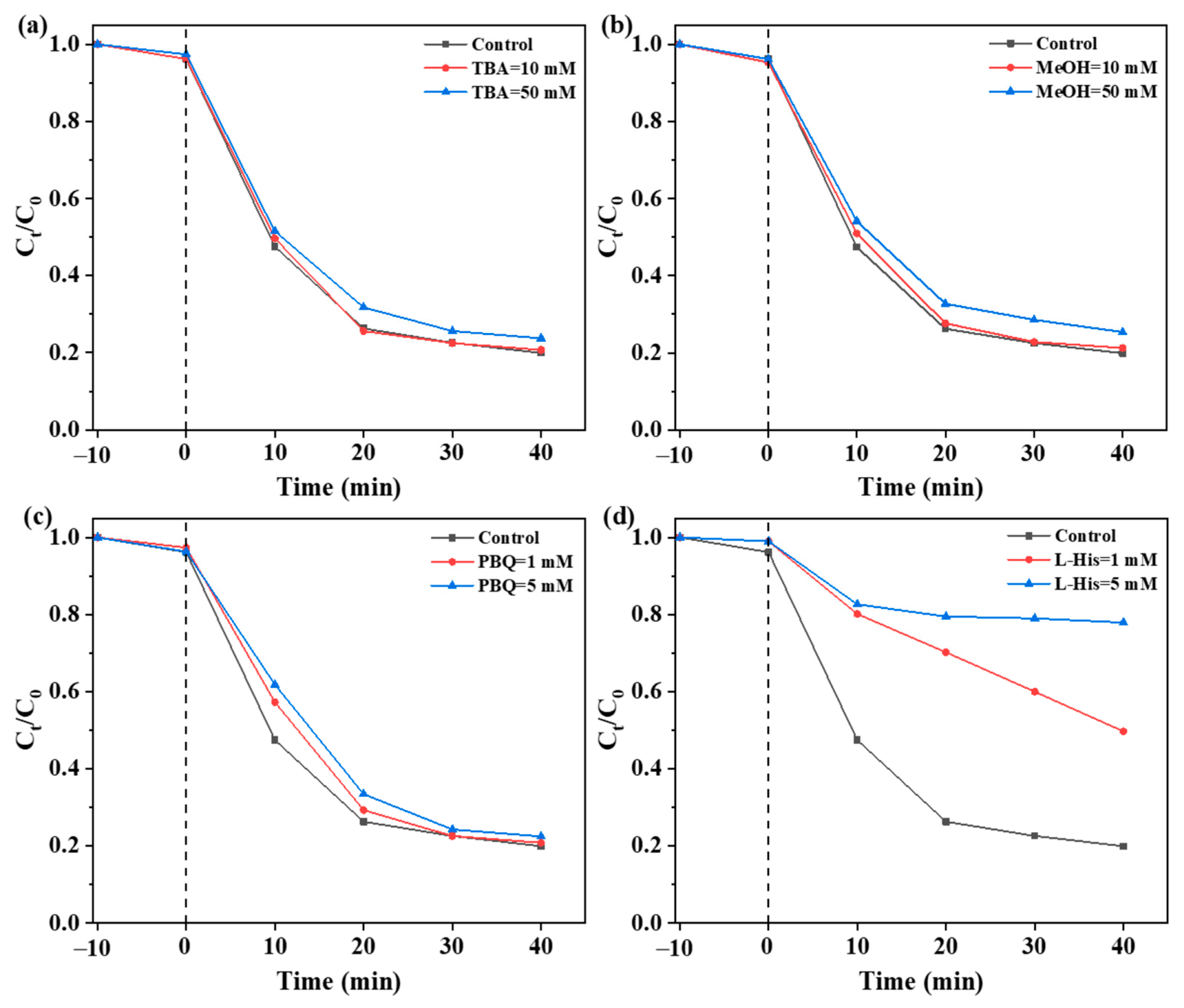
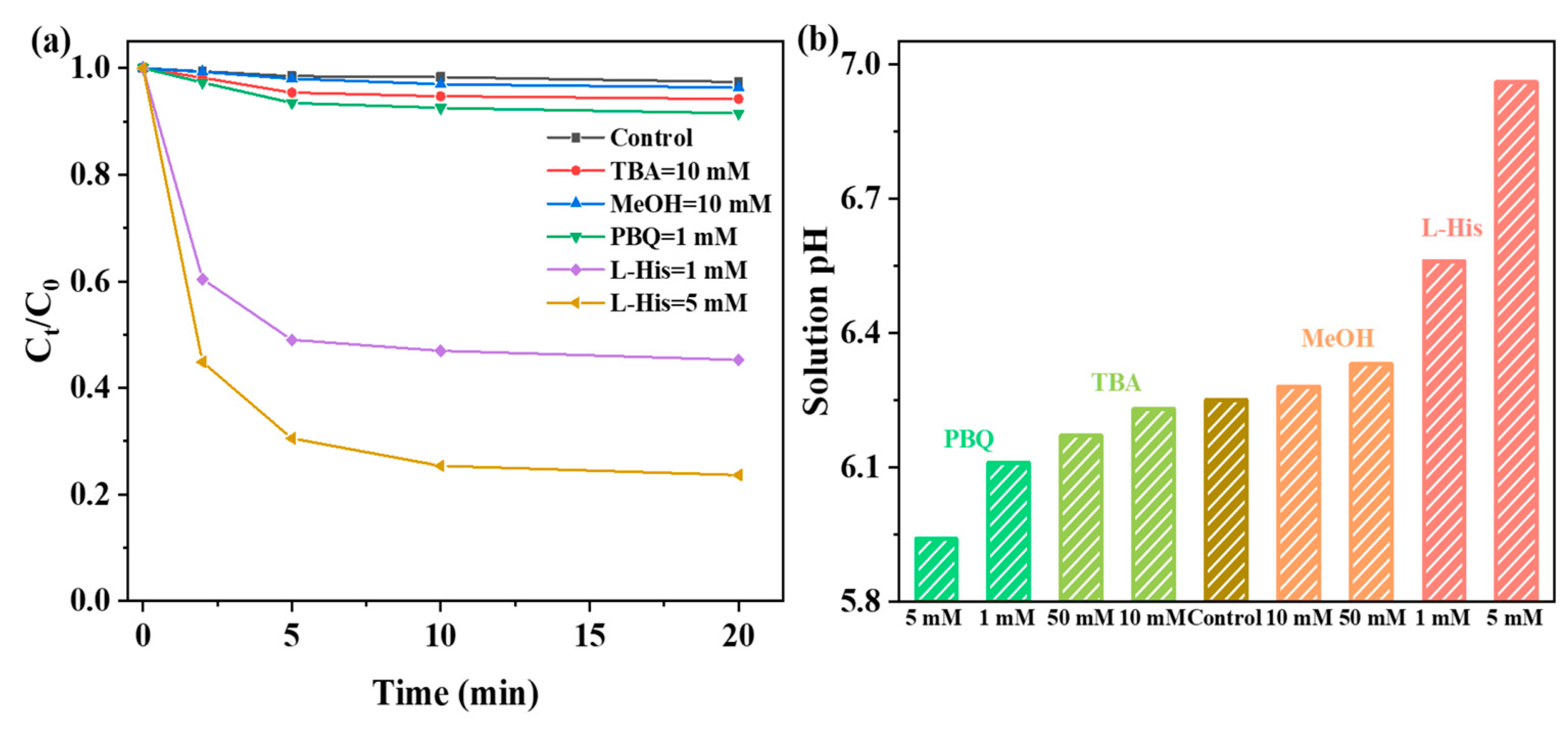
2.4. Identification of Active Species
2.5. Reaction Mechanism
3. Materials and Methods
3.1. Materials
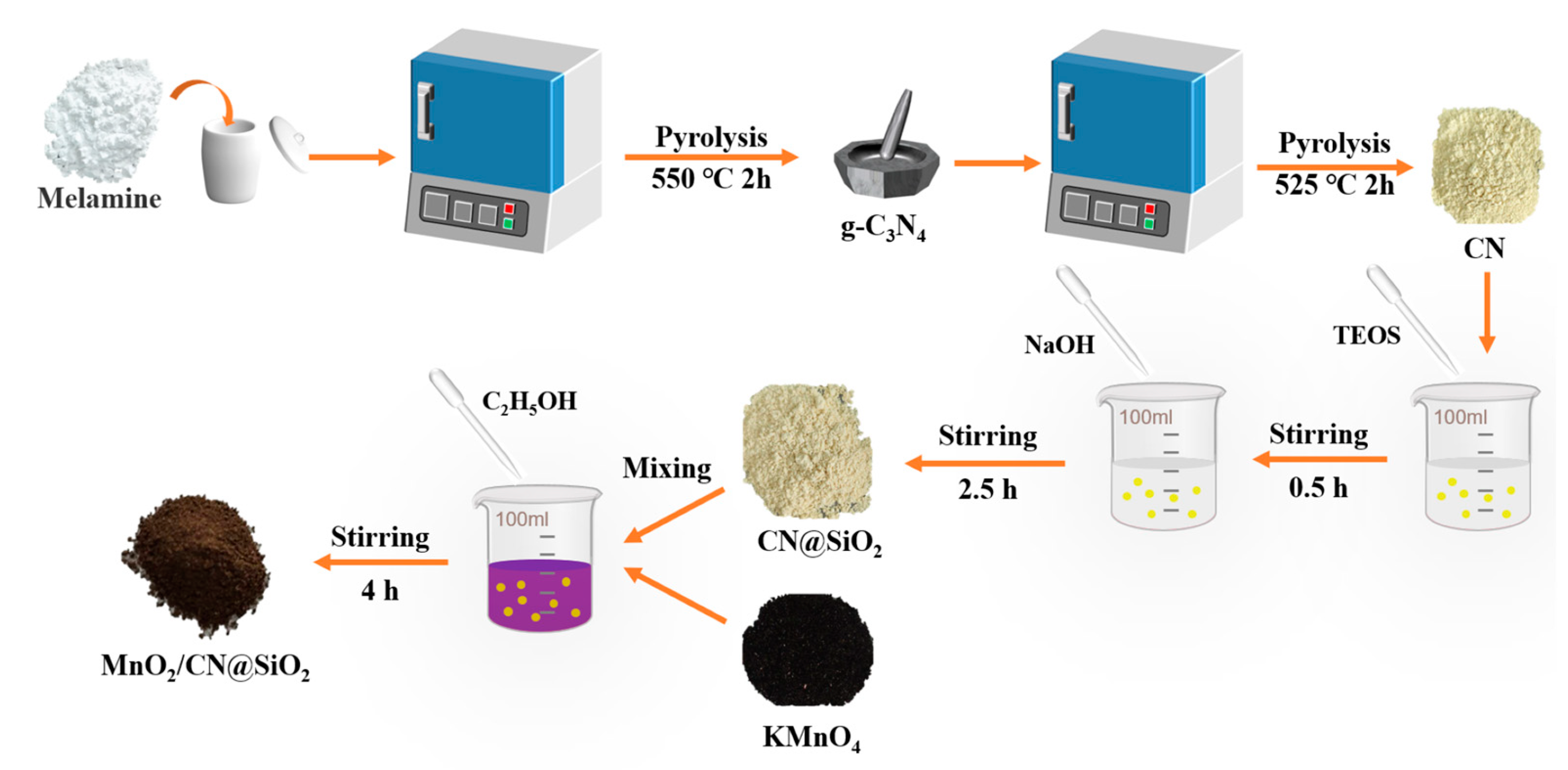
3.2. Preparation of MnO2/CN@SiO2
3.2.1. Synthesis of CN
3.2.2. Synthesis of CN@SiO2
3.2.3. Synthesis of MnO2/CN@SiO2
3.3. Characterizations
3.4. Degradation Tests and Analytical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, K.; Thakur, I.S.; Kaushik, G. Occurrence and distribution of pharmaceutical compounds and their environmental impacts: A review. Bioresour. Technol. Rep. 2021, 16, 100841. [Google Scholar] [CrossRef]
- Gao, F.Z.; Zou, H.Y.; Wu, D.L.; Chen, S.; He, L.Y.; Zhang, M.; Bai, H.; Ying, G.G. Swine farming elevated the proliferation of Acinetobacter with the prevalence of antibiotic resistance genes in the groundwater. Environ. Int. 2020, 136, 105484. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.C.; Ge, M.; Hu, Z.; Guo, C.S. One-pot synthesis of magnetic CuO/Fe2O3/CuFe2O4 nanocomposite to activate persulfate for levofloxacin removal: Investigation of efficiency, mechanism and degradation route. Chem. Eng. J. 2020, 389, 124456. [Google Scholar] [CrossRef]
- Jia, D.; Liu, Q.; Pan, Y.; Xu, S.D.; Li, H.X.; Tang, J.H. The Research Status, Potential Hazards and Toxicological Mechanisms of Fluoroquinolone Antibiotics in the Environment. Antibiotics 2023, 12, 1058. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; McDonald, T.J.; Kim, H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere 2016, 150, 702–714. [Google Scholar] [CrossRef]
- Saya, L.; Malik, V.; Gautam, D.; Gambhir, G.; Balendra, W.; Singh, R.; Hooda, S. A comprehensive review on recent advances toward sequestration of levofloxacin antibiotic from wastewater. Sci. Total Environ. 2022, 813, 152529. [Google Scholar] [CrossRef]
- Liu, J.Y.; Li, Z.L.; Wang, M.; Jin, C.Y.; Kang, J.; Tang, Y.W.; Li, S.Y. Eu2O3/Co3O4 nanosheets for levofloxacin removal via peroxymonosulfate activation: Performance, mechanism and degradation pathway. Sep. Purif. Technol. 2021, 274, 118666. [Google Scholar] [CrossRef]
- Yang, W.Q.; Li, J.; Yao, Z.L.; Li, M. A review on the alternatives to antibiotics and the treatment of antibiotic pollution: Current development and future prospects. Sci. Total Environ. 2024, 926, 171757. [Google Scholar] [CrossRef]
- Wang, N.N.; Zheng, T.; Zhang, G.Z.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, H.W.; Zhang, Y.S.; Mahlknecht, J.; Wang, C.Q. A review of metallurgical slags as catalysts in advanced oxidation processes for removal of refractory organic pollutants in wastewater. J. Environ. Manag. 2024, 352, 120051. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, D.; Bai, B.; Ma, Z.Y.; Zong, S.C. Insight into antibiotic removal by advanced oxidation processes (AOPs): Performance, mechanism, degradation pathways, and ecotoxicity assessment. Chem. Eng. J. 2024, 500, 157134. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.L.; Zheng, H.S.; Zheng, Y.J.; Jing, T.; Ma, J.; Nan, J.; Leong, Y.K.; Chang, J.S. Advanced oxidation process based on hydroxyl and sulfate radicals to degrade refractory organic pollutants in landfill leachate. Chemosphere 2022, 297, 134214. [Google Scholar] [CrossRef]
- Usman, M.; Cheema, S.A.; Farooq, M. Heterogeneous Fenton and persulfate oxidation for treatment of landfill leachate: A review supplement. J. Clean. Prod. 2020, 256, 120448. [Google Scholar] [CrossRef]
- Chang, L.; Xue, X.J.; Deng, Q.C.; Xie, X.Y.; Zhang, X.D.; Cheng, C.; Chai, H.X.; Huang, Y. Modulating the electronic structure of Co center via MgO@C co-doping for PMS activation to remove levofloxacin. Sep. Purif. Technol. 2023, 321, 124151. [Google Scholar] [CrossRef]
- Bai, Y.B.; Han, C.; Li, Z.M.; Zhang, H.M.; Deng, Q.Y.; Tong, H.N.; Zhang, C.L. Efficient degradation of levofloxacin using peroxymonosulfate activated by a novel magnetic catalyst derived from waste walnut shell biomass. Colloids Surf. A Physicochem. Eng. Asp. 2024, 699, 134668. [Google Scholar] [CrossRef]
- Li, J.J.; Liang, Y.Q.; Jin, P.L.; Zhao, B.; Zhang, Z.H.; He, X.J.; Tan, Z.L.; Wang, L.; Cheng, X.W. Heterogeneous Metal-Activated Persulfate and Electrochemically Activated Persulfate: A Review. Catalysts 2022, 12, 1024. [Google Scholar] [CrossRef]
- Deng, Q.C.; Zhang, X.D.; Chang, L.; Chai, H.X.; Huang, Y.M. The MOF/LDH derived heterostructured Co3O4/MnCo2O4 composite for enhanced degradation of levofloxacin by peroxymonosulfate activation. Sep. Purif. Technol. 2022, 294, 121182. [Google Scholar] [CrossRef]
- Zheng, J.L.; Lin, Q.T.; Liu, Y.X.; Fan, X.D.; Xu, K.H.; Ma, Y.J.; He, J.; Fu, H.Y. Peroxymonosulfate activation by Mg-introduced Fe-N carbon nanotubes to accelerate sulfamethoxazole degradation: Singlet oxygen-dominated nonradical pathway. Chem. Eng. J. 2023, 452, 139233. [Google Scholar] [CrossRef]
- Zheng, X.X.; Niu, X.J.; Zhang, D.Q.; Lv, M.Y.; Ye, X.Y.; Ma, J.L.; Lin, Z.; Fu, M.L. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Huang, J.Z.; Dai, Y.F.; Singewald, K.; Liu, C.C.; Saxena, S.; Zhang, H.C. Effects of MnO2 of different structures on activation of peroxymonosulfate for bisphenol A degradation under acidic conditions. Chem. Eng. J. 2019, 370, 906–915. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Okamoto, N.L.; Ichitsubo, T. Thermal stability of MnO2 polymorphs. J. Solid State Chem. 2022, 305, 122683. [Google Scholar] [CrossRef]
- Boyom-Tatchemo, F.W.; Devred, F.; Ndiffo-Yemeli, G.; Laminsi, S.; Gaigneaux, E.M. Plasma-induced redox reactions synthesis of nanosized α-, γ- and δ-MnO2 catalysts for dye degradation. Appl. Catal. B Environ. 2020, 260, 118159. [Google Scholar] [CrossRef]
- Zhou, Z.G.; Du, H.M.; Dai, Z.H.; Mu, Y.; Tong, L.L.; Xing, Q.J.; Liu, S.S.; Ao, Z.M.; Zou, J.P. Degradation of organic pollutants by peroxymonosulfate activated by MnO2 with different crystalline structures: Catalytic performances and mechanisms. Chem. Eng. J. 2019, 374, 170–180. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.S.; Yu, J.; Huang, Z.; Yu, J.; Deng, S.W.; Jiang, Y.Y.; Zhu, W.W. Insights into the potential applications of permanganate/peroxymonosulfate systems: Enhancement via amorphous MnO2, effects of water matrices, and optimization using response surface methodology. RSC Adv. 2024, 14, 4116–4128. [Google Scholar] [CrossRef]
- Edathil, A.A.; Kannan, P.; Haija, M.A.; Banat, F. Sulfide remediation from wastewater using hydrothermally synthesized δ-MnO2/porous graphitic carbon as adsorbent. Environ. Res. 2021, 196, 110429. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, H.; Liu, Z.L.; Su, Y.G.; Du, C.F. Oxygen vacancies and interfacial engineering of MnO/Mn3O4 heterojunction for peroxymonosulfate activation to promote Bisphenol A removal. Appl. Surf. Sci. 2023, 628, 157302. [Google Scholar] [CrossRef]
- Wang, L.H.; Jiang, J.; Pang, S.Y.; Zhou, Y.; Li, J.; Sun, S.F.; Gao, Y.; Jiang, C.C. Oxidation of bisphenol A by nonradical activation of peroxymonosulfate in the presence of amorphous manganese dioxide. Chem. Eng. J. 2018, 352, 1004–1013. [Google Scholar] [CrossRef]
- Oseghe, E.O.; Akpotu, S.O.; Mombeshora, E.T.; Oladipo, A.O.; Ombaka, L.M.; Maria, B.B.; Idris, A.O.; Mamba, G.; Ndlwana, L.; Ayanda, O.S.; et al. Multi-dimensional applications of graphitic carbon nitride nanomaterials–A review. J. Mol. Liq. 2021, 344, 117820. [Google Scholar] [CrossRef]
- Ganesan, S.; Kokulnathan, T.; Sumathi, S.; Palaniappan, A. Efficient photocatalytic degradation of textile dye pollutants using thermally exfoliated graphitic carbon nitride (TE–g–C3N4). Sci. Rep. 2024, 14, 2284. [Google Scholar] [CrossRef]
- Chen, H.M.; Fan, Y.Y.; Xu, H.Y.; Cui, D.F.; Xue, C.Y.; Zhang, W.D. A facile and green microwave hydrothermal method for fabricating g-C3N4 nanosheets with improved hydrogen evolution performance. J. Alloys Compd. 2021, 863, 158448. [Google Scholar] [CrossRef]
- Khan, A.; Zhang, K.K.; Taraqqi-A-Kamal, A.; Wang, X.G.; Chen, Y.; Zhang, Y.R. Degradation of antibiotics in aqueous media using manganese nanocatalyst-activated peroxymonosulfate. J. Colloid Interface Sci. 2021, 599, 805–818. [Google Scholar] [CrossRef]
- Sodipo, B.K.; Aziz, A.A. Recent advances in synthesis and surface modification of superparamagnetic iron oxide nanoparticles with silica. J. Magn. Magn. Mater. 2016, 416, 275–291. [Google Scholar] [CrossRef]
- Ma, M.Y.; Li, H.W.; Xiong, Y.Z.; Dong, F.P. Rational design, synthesis, and application of silica/graphene-based nanocomposite: A review. Mater. Des. 2021, 198, 109367. [Google Scholar] [CrossRef]
- Du, R.; Zhu, H.Y.; Zhao, H.Y.; Lu, H.; Dong, C.; Liu, M.T.; Yang, F.; Yang, J.; Pan, J. Coupling ultrafine plasmonic Co3O4 with thin-layer carbon over SiO2 nanosphere for dual-functional PMS activation and solar interfacial water evaporation. J. Alloys Compd. 2023, 940, 168816. [Google Scholar] [CrossRef]
- Yuan, D.S.; Li, Z.Y.; Chen, X.R.; Ding, J.; Wan, H.; Guan, G.F. Homodispersed B–CN/P–CN S-scheme homojunction for enhanced visible-light-driven hydrogen evolution. Green Energy Environ. 2022, 7, 1119–1127. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Zhang, P.P.; Hu, K.S.; Duan, X.G.; Ren, Y.X.; Sun, H.Q.; Wang, S.B. Sustainable redox processes induced by peroxymonosulfate and metal doping on amorphous manganese dioxide for nonradical degradation of water contaminants. Appl. Catal. B Environ. 2021, 286, 119903. [Google Scholar] [CrossRef]
- Arkhipova, E.A.; Ivanov, A.S.; Maslakov, K.I.; Novotortsev, R.Y.; Savilov, S.V.; Xia, H.; Desyatov, A.V.; Aldoshin, S.M. Facile hydrothermal synthesis of α-MnO2 and δ-MnO2 for pseudocapacitor applications. Ionics 2022, 28, 3501–3509. [Google Scholar] [CrossRef]
- Yang, W.; Su, Z.A.; Xu, Z.; Yang, W.; Peng, Y.; Li, J. Comparative study of α-, β-, γ- and δ-MnO2 on toluene oxidation: Oxygen vacancies and reaction intermediates. Appl. Catal. B Environ. 2020, 260, 118150. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Nguyen, T.B.; Tran, L.H.; Nguyen, T.G.; Fatimah, I.; Kuncoro, E.P.; Doong, R.A. Z-scheme S, B co-doped g-C3N4 nanotube@MnO2 heterojunction with visible-light-responsive for enhanced photodegradation of diclofenac by peroxymonosulfate activation. Chem. Eng. J. 2023, 452, 139249. [Google Scholar] [CrossRef]
- Jing, L.Q.; Xie, M.; Xu, Y.G.; Tong, C.; Du, X.; Zhao, H.; Zhong, N.; Li, H.M.; Hu, J.G. Advanced oxidation via the synergy of C-defective/C–O band modified ultrathin porous g-C3N4 and PMS for efficient photothermal degradation of bisphenol pollutants and lignin derivatives. Green Energy Environ. 2024, 9, 1159–1170. [Google Scholar] [CrossRef]
- Li, W.C.; Wen, X.Y.; Wang, X.J.; Li, J.; Ren, E.B.; Shi, Z.F.; Liu, C.M.; Mo, D.Q.; Mo, S.P. Oriented growth of δ-MnO2 nanosheets over core-shell Mn2O3@δ-MnO2 catalysts: An interface-engineered effects for enhanced low-temperature methanol oxidation. Mol. Catal. 2021, 514, 11847. [Google Scholar] [CrossRef]
- Liu, M.T.; Ma, S.L.; Zhao, H.Y.; Lu, H.; Yang, J.; Tang, S.; Gao, S.Y.; Yang, F. Compositing ultrafine CoFe2O4 spinel with porous silica as catalyst for photothermal PMS activation and interfacial water evaporation. J. Alloys Compd. 2023, 949, 169901. [Google Scholar] [CrossRef]
- Duan, X.G.; Indrawirawan, S.; Kang, J.; Tian, W.J.; Zhang, H.Y.; Sun, H.Q.; Wang, S.B. Temperature-dependent evolution of hydroxyl radicals from peroxymonosulfate activation over nitrogen-modified carbon nanotubes. Sustain. Mater. Technol. 2018, 18, e00082. [Google Scholar] [CrossRef]
- Fan, J.H.; Yao, Z.L.; Wu, D.L.; Liu, X.; Ma, L.M. Pilot-scale study on an advanced Fe-Cu process for refractory wastewater pretreatment. J. Hazard. Mater. 2023, 457, 131756. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Gu, C.Y.; Tang, B.L.; Zhou, Y.H.; Gan, M.; Zhu, J.Y. Expeditious degradation of SMX by high-valent cobalt-oxo species derived from cobalt-doped C3N5-activated peroxymonosulfate with the assistance of visible light. Sep. Purif. Technol. 2022, 301, 122009. [Google Scholar] [CrossRef]
- Qiao, L.L.; Zhang, X.Y.; Li, T.L.; Wang, H.T. Interfacial photothermal-enhanced FeCo@BC nanocomposites activating peroxymonosulfate for efficient tetracycline degradation. Sep. Purif. Technol. 2025, 354, 129391. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, J.H.; Xiao, D.X.; Guo, Y.G.; Fang, C.L.; Lou, X.Y.; Wang, Z.H.; Liu, J.S. Transformations of chloro and nitro groups during the peroxymonosulfate-based oxidation of 4-chloro-2-nitrophenol. Chemosphere 2015, 134, 446–451. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, Q.; Wang, Z.Q.; Hua, S.; Ding, D.H.; Cai, T.M.; Zhang, R.H. Pyrrolic N-rich biochar without exogenous nitrogen doping as a functional material for bisphenol A removal: Performance and mechanism. Appl. Catal. B Environ. 2021, 291, 120093. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, L.L.; Zhang, X.Y.; Liu, Z.L.; Li, T.L.; Wang, H.T. Green synthesis of FeCu@biochar nanocomposites through a mechanochemical method for enhanced tetracycline degradation via peroxymonosulfate activation. Sep. Purif. Technol. 2024, 328, 125077. [Google Scholar] [CrossRef]
- Dong, J.R.; Li, P.; Ji, X.Y.; Kang, Y.; Yuan, X.; Tang, J.C.; Shen, B.X.; Dong, H.J.; Lyu, H.H. Electrons of d-orbital (Mn) and p-orbital (N) enhance the photocatalytic degradation of antibiotics by biochar while maintaining biocompatibility: A combined chemical and biological analysis. J. Hazard. Mater. 2023, 451, 131083. [Google Scholar] [CrossRef]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Wang, L.L.; Lan, X.; Peng, W.Y.; Wang, Z.H. Uncertainty and misinterpretation over identification, quantification and transformation of reactive species generated in catalytic oxidation processes: A review. J. Hazard. Mater. 2021, 408, 124436. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.K.; Xie, T.P.; Han, G.K.; Zhu, Q.X.; Wang, Y.J.; Peng, Y.; Liu, S.L.; Yao, Z.P. SiO2 mediated templating synthesis of γ-Fe2O3/MnO2 as peroxymonosulfate activator for enhanced phenol degradation dominated by singlet oxygen. Appl. Surf. Sci. 2021, 560, 149984. [Google Scholar] [CrossRef]
- Wang, Y.C.; Tong, Y.P.; Chen, D.Z.; Zhou, T.L.; Zhang, Q.Z.; Zou, J.P. Activation of peroxymonosulfate by g-C3N4/ε-MnO2 microspheres for nonradical pathway degradation of organic pollutants in water: Catalytic mechanism and degradation path. Chem. Eng. J. 2023, 459, 141643. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Zhao, J.H.; Yang, C. Efficient removal of ciprofloxacin by peroxymonosulfate/Mn3O4-MnO2 catalytic oxidation system. Chem. Eng. J. 2017, 327, 481–489. [Google Scholar] [CrossRef]
- Yuan, R.X.; Jiang, Z.Q.; Wang, Z.H.; Gao, S.M.; Liu, Z.J.; Li, M.L.; Boczkaj, G. Hierarchical MnO2 nanoflowers blooming on 3D nickel foam: A novel micro-macro catalyst for peroxymonosulfate activation. J. Colloid Interface Sci. 2020, 571, 142–154. [Google Scholar] [CrossRef]
- Wang, Z.M.; Wang, Z.H.; Li, W.; Lan, Y.P.; Chen, C. Performance comparison and mechanism investigation of Co3O4-modified different crystallographic MnO2 (α, β, γ, and δ) as an activator of peroxymonosulfate (PMS) for sulfisoxazole degradation. Chem. Eng. J. 2022, 427, 130888. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, L.; Wang, X.; Li, J.; Xu, D. Amorphous MnO2 Supported on CN@SiO2 for Levofloxacin Degradation via a Non-Radical Pathway by PMS Activation. Catalysts 2025, 15, 419. https://doi.org/10.3390/catal15050419
Xia L, Wang X, Li J, Xu D. Amorphous MnO2 Supported on CN@SiO2 for Levofloxacin Degradation via a Non-Radical Pathway by PMS Activation. Catalysts. 2025; 15(5):419. https://doi.org/10.3390/catal15050419
Chicago/Turabian StyleXia, Longfei, Xilin Wang, Jiahui Li, and Dongyan Xu. 2025. "Amorphous MnO2 Supported on CN@SiO2 for Levofloxacin Degradation via a Non-Radical Pathway by PMS Activation" Catalysts 15, no. 5: 419. https://doi.org/10.3390/catal15050419
APA StyleXia, L., Wang, X., Li, J., & Xu, D. (2025). Amorphous MnO2 Supported on CN@SiO2 for Levofloxacin Degradation via a Non-Radical Pathway by PMS Activation. Catalysts, 15(5), 419. https://doi.org/10.3390/catal15050419






