Evaluation of Vanadium Oxide Nanocatalysts over Graphene for Propylene Production Through Oxidative Propane Dehydrogenations
Abstract
1. Introduction
| Reaction | Description of Reaction |
|---|---|
| The main reaction | |
| Propane absorption on lattice oxygen | |
| Scission of a sigma carbon–hydrogen bond and formation of a propyl intermediate | |
| Reaction of propyl with the surface oxygen and formation of propylene | |
| Formation of water and reduced vanadium site | |
| Reoxidation of the catalyst |
2. Results and Discussion
2.1. FE-SEM
2.2. XRD Analyses
2.3. TGA Analyses
2.4. FTIR Analyses
2.5. TPR Analyses
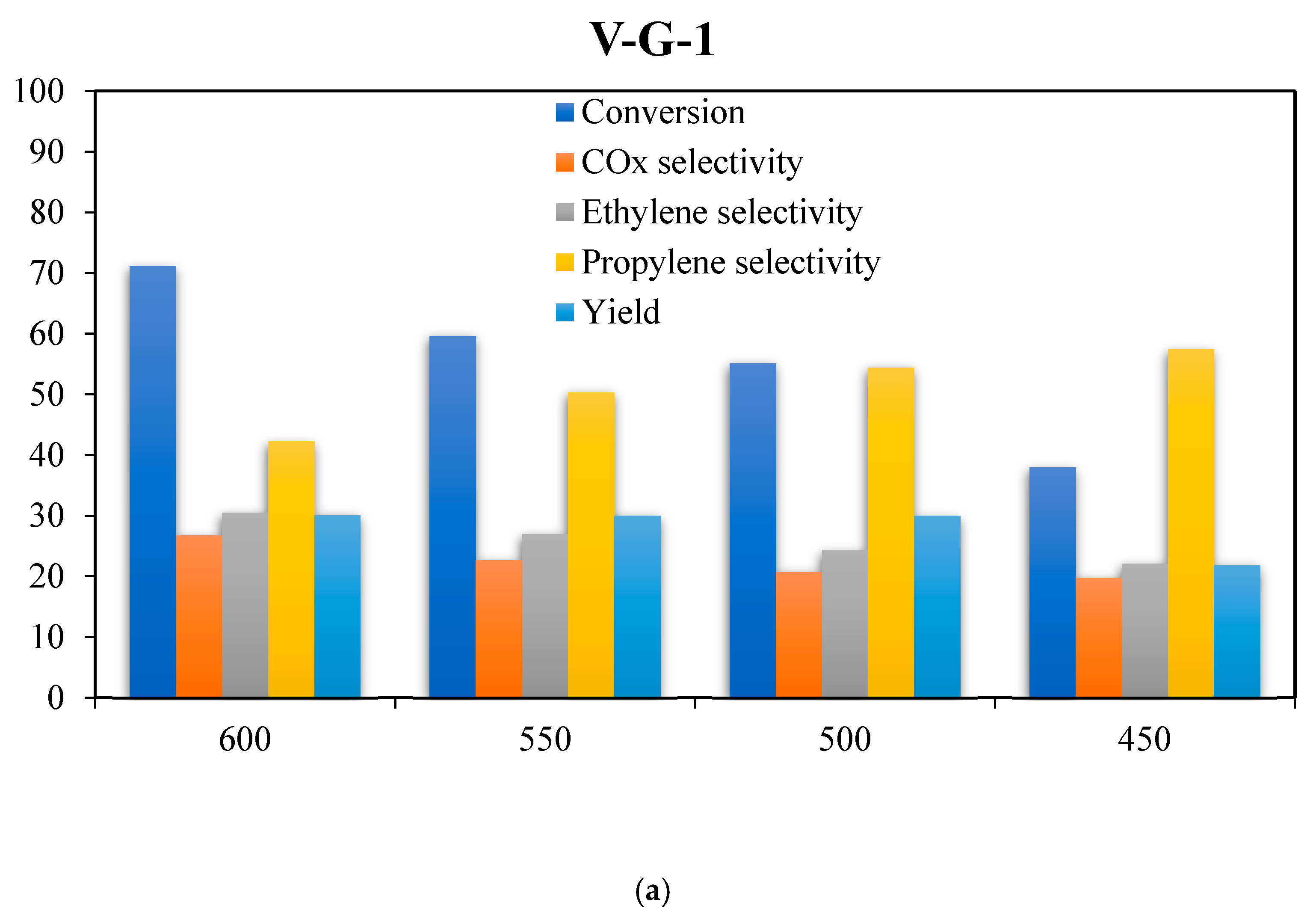
2.6. Catalyst Evaluations
2.7. Comparison of Present Study with Open Literature
3. Methodology
3.1. Materials
3.2. Preparation of Vanadium Oxide/Graphene Nanocatalysts
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fattahi, M.; Kazemeini, M.; Khorasheh, F.; Rashidi, A. An Investigation of the Oxidative Dehydrogenation of Propane Kinetics over a Vanadium–Graphene Catalyst Aiming at Minimizing of the COx Species. Chem. Eng. J. 2014, 250, 14–24. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Zafarnak, S.; Taghvaei, H.; Rahimpour, M.R.; Iulianelli, A. Simultaneous Production of Ethylene and Hydrogen through Carbon-Dioxide-Assisted Conversion of Ethane over Cobalt-Molybdenum Catalysts. J. CO2 Util. 2021, 47, 101499. [Google Scholar] [CrossRef]
- Carter, J.H.; Bere, T.; Pitchers, J.R.; Hewes, D.G.; Vandegehuchte, B.D.; Kiely, C.J.; Taylor, S.H.; Hutchings, G.J. Direct and Oxidative Dehydrogenation of Propane: From Catalyst Design to Industrial Application. Green Chem. 2021, 23, 9747–9799. [Google Scholar] [CrossRef]
- Barghi, B.; Fattahi, M.; Khorasheh, F. Kinetic Modeling of Propane Dehydrogenation over an Industrial Catalyst in the Presence of Oxygenated Compounds. React. Kinet. Mech. Catal. 2012, 107, 141–155. [Google Scholar] [CrossRef]
- Samavati, A.; Fattahi, M.; Khorasheh, F. Modeling of Pt-Sn/γ-Al2O3 Deactivation in Propane Dehydrogenation with Oxygenated Additives. Korean J. Chem. Eng. 2013, 30, 55–61. [Google Scholar] [CrossRef]
- Shirzad, P.; Kantor, I. Toward sustainable propylene: A comparison of current and future production pathways. Renew. Sustain. Energy Transit. 2025, 7, 100099. [Google Scholar] [CrossRef]
- Mitran, G.; Ahmed, R.; Iro, E.; Hajimirzaee, S.; Hodgson, S.; Urdă, A.; Olea, M.; Marcu, I.C. Propane Oxidative Dehydrogenation over VOx/SBA-15 Catalysts. Catal. Today 2018, 306, 260–267. [Google Scholar] [CrossRef]
- Fan, X.; Liu, D.; Zhao, Z.; Li, J.; Liu, J. Influence of Ni/Mo Ratio on the Structure-Performance of Ordered Mesoporous Ni-Mo-O Catalysts for Oxidative Dehydrogenation of Propane. Catal. Today 2020, 339, 67–78. [Google Scholar] [CrossRef]
- Wang, L.; Ao, C.; Zhai, Y.; Feng, B.; Duan, J.; Qian, S.; Zhao, W.; Zhang, L.; Liu, F. Highly Active and Stable Co3O4 Catalyst for the Low-Temperature Oxidative Dehydrogenation of Propane. Inorg. Chem. Commun. 2020, 112, 107725. [Google Scholar] [CrossRef]
- Fattahi, M.; Kazemeini, M.; Khorasheh, F.; Rashidi, A. Kinetic Modeling of Oxidative Dehydrogenation of Propane (ODHP) over a Vanadium–Graphene Catalyst: Application of the DOE and ANN Methodologies. J. Ind. Eng. Chem. 2014, 20, 2236–2247. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Xu, A.; Ma, F.; Chen, F.; Lu, W. Research on the Nanocrystal FeVxOy Catalysts for New Reaction from Propane to Propylene and CO. Appl. Surf. Sci. 2014, 320, 552–557. [Google Scholar] [CrossRef]
- Miranda, G.P.; Ferreira Neto, V.J.M.; Young, A.F.; Silveira, E.B.; Pries de Oliveira, P.G.; Mendes, F.M.T. Oxidative Dehydrogenation of Propane: Developing Catalysts Containing VOX, V-P-O and V-Mg-O Species Supported on MCM-41 and Activated Carbon. Catal. Today 2020, 348, 148–156. [Google Scholar] [CrossRef]
- Xiong, G.; Sang, J. Oxidative Dehydrogenation of Propane over Nanodiamond Modified by Molybdenum Oxide. J. Mol. Catal. A Chem. 2014, 392, 315–320. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Z.; Luo, M.; Zhao, Z.; Wang, J.; Xie, Z.; Guo, L. Vanadium-Containing Dendritic Mesoporous Silica Nanoparticles: Multifunctional Catalysts for the Oxidative and Non-Oxidative Dehydrogenation of Propane to Propylene. Microporous Mesoporous Mater. 2019, 282, 133–145. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, S.; Zou, Y.; Wang, Y.M.; Zhou, X.; Shen, Y.; Zheng, X. Highly Efficient V–Sb–O/SiO2 Catalyst with Sb Atom-Isolated VOx Species for Oxidative Dehydrogenation of Propane to Propene. Catal. Commun. 2014, 45, 158–161. [Google Scholar] [CrossRef]
- Rostom, S.; de Lasa, H.I. Propane Oxidative Dehydrogenation Using Consecutive Feed Injections and Fluidizable VOx/γAl2O3 and VOx/ZrO2–γAl2O3 Catalysts. Ind. Eng. Chem. Res. 2017, 56, 13109–13124. [Google Scholar] [CrossRef]
- Burri, A.; Hasib, M.A.; Mo, Y.-H.; Reddy, B.M.; Park, S.-E. An Efficient Cr-TUD-1 Catalyst for Oxidative Dehydrogenation of Propane to Propylene with CO2 as Soft Oxidant. Catal. Lett. 2018, 148, 576–585. [Google Scholar] [CrossRef]
- Wu, G.; Hao, Y.; Zhang, N.; Guan, N.; Li, L.; Grünert, W. Oxidative Dehydrogenation of Propane with Nitrous Oxide over Fe–O–Al Species Occluded in ZSM-5: Reaction and Deactivation Mechanisms. Microporous Mesoporous Mater. 2014, 198, 82–91. [Google Scholar] [CrossRef]
- Wu, G.; Hei, F.; Zhang, N.; Guan, N.; Li, L.; Grünert, W. Oxidative Dehydrogenation of Propane with Nitrous Oxide over Fe-ZSM-5 Prepared by Grafting: Characterization and Performance. Appl. Catal. A Gen. 2013, 468, 230–239. [Google Scholar] [CrossRef]
- Balogun, M.L.; Khan, W.U.; Shaikh, M.N.; Adamu, S.; Arjah, A.S.; Al-Bogami, S.A.; Al-Ghamdi, S.; Hossain, M.M. Redox MoO3/CaMnO3 Catalysts for Chemical-Looping Oxidative Dehydrogenation of Propane: A Greener Approach of Propylene Production. Appl. Catal. A Gen. 2023, 655, 119087. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, Z.; Zhu, L.; Lu, W.; He, L.; Wang, D. Oxidative Dehydrogenation of Propane on Boron Phosphate: A Computational Mechanistic Study. J. Phys. Chem. C 2023, 127, 12942–12952. [Google Scholar] [CrossRef]
- Wang, X.; Pei, C.; Zhao, Z.J.; Chen, S.; Li, X.; Sun, J.; Song, H.; Sun, G.; Wang, W.; Chang, X.; et al. Coupling Acid Catalysis and Selective Oxidation over MoO3-Fe2O3 for Chemical Looping Oxidative Dehydrogenation of Propane. Nat. Commun. 2023, 14, 2039. [Google Scholar] [CrossRef] [PubMed]
- Monguen, C.K.F.; Daniel, S.; Tian, Z.Y. Controlled Synthesis of Cu-Cr Double-Layer Thin Films for Oxidative Dehydrogenation of Propane at Low Temperature. Chem. Eng. J. 2023, 474, 145769. [Google Scholar] [CrossRef]
- Schumacher, L.; Pfeiffer, J.; Shen, J.; Gutmann, T.; Breitzke, H.; Buntkowsky, G.; Hofmann, K.; Hess, C. Collaborative Mechanistic Effects between Vanadia and Titania during the Oxidative Dehydrogenation of Propane Investigated by Operando and Transient Spectroscopies. ACS Catal. 2023, 13, 8139–8160. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Reja, D.; Koné-Guira, S.; Miche, A.; Costentin, G.; Thomas, C. Cobalt on Dealuminated-Siβ as a Catalyst for the Oxidative Dehydrogenation of Propane. Appl. Catal. A Gen. 2023, 657, 119119. [Google Scholar] [CrossRef]
- Qiu, B.; Lu, W.D.; Gao, X.Q.; Sheng, J.; Ji, M.; Wang, D.; Lu, A.H. Boosting the Propylene Selectivity over Embryonic Borosilicate Zeolite Catalyst for Oxidative Dehydrogenation of Propane. J. Catal. 2023, 417, 14–21. [Google Scholar] [CrossRef]
- Cao, L.; Yan, P.; Wen, S.; Bao, W.; Jiang, Y.; Zhang, Q.; Yu, N.; Zhang, Y.; Cao, K.; Dai, P.; et al. Antiexfoliating H-BN⊃In2O3 Catalyst for Oxidative Dehydrogenation of Propane in a High-Temperature and Water-Rich Environment. J. Am. Chem. Soc. 2023, 145, 6184–6193. [Google Scholar] [CrossRef]
- Fu, Z.; Li, D.Z.; Zhou, L.D.; Li, Y.M.; Guo, J.W.; Li, Y.Q.; Liu, H.M.; Zhang, Q.J. A Mini Review on Oxidative Dehydrogenation of Propane over Boron Nitride Catalysts. Pet. Sci. 2023, 20, 2488–2498. [Google Scholar] [CrossRef]
- Grant, J.T.; Carrero, C.A.; Love, A.M.; Verel, R.; Hermans, I. Enhanced Two-Dimensional Dispersion of Group V Metal Oxides on Silica. ACS Catal. 2015, 5, 5787–5793. [Google Scholar] [CrossRef]
- Gao, X.; Liu, M.; Huang, Y.; Xu, W.; Zhou, X.; Yao, S. Dimensional Understanding of Boron-Based Catalysts for Oxidative Propane Dehydrogenation: Structure and Mechanism. ACS Catal. 2023, 13, 9667–9687. [Google Scholar] [CrossRef]
- Liu, H.; Sun, S.; Li, D.; Lei, Y. Catalyst Development for O2-Assisted Oxidative Dehydrogenation of Propane to Propylene. Chem. Commun. 2024, 60, 7535–7554. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-T.; Ke, M.; Zhang, J.; Peng, Y.-L.; Chen, G. Advancements of MOFs in the Field of Propane Oxidative Dehydrogenation for Propylene Production. Molecules 2024, 29, 1212. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Su, Y.; Duan, X.; Xie, K. High-Density Lewis Acid Sites in Porous Single-Crystalline Monoliths to Enhance Propane Dehydrogenation at Reduced Temperatures. Angew. Chem. Int. Ed. 2021, 60, 9311–9315. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yu, Q.; Roghair, I.; Wang, K.; van Sint Annaland, M. Chemical Looping Oxidative Dehydrogenation of Propane: A Comparative Study of Ga-Based, Mo-Based, V-Based Oxygen Carriers. Chem. Eng. Process.-Process Intensif. 2020, 157, 108137. [Google Scholar] [CrossRef]
- Chen, K.; Xie, S.; Iglesia, E.; Bell, A.T. Structure and Properties of Zirconia-Supported Molybdenum Oxide Catalysts for Oxidative Dehydrogenation of Propane. J. Catal. 2000, 189, 421–430. [Google Scholar] [CrossRef]
- Liu, Y.M.; Cao, Y.; Yi, N.; Feng, W.L.; Dai, W.L.; Yan, S.R.; He, H.Y.; Fan, K.N. Vanadium Oxide Supported on Mesoporous SBA-15 as Highly Selective Catalysts in the Oxidative Dehydrogenation of Propane. J. Catal. 2004, 224, 417–428. [Google Scholar] [CrossRef]
- Chen, K.; Xie, S.; Bell, A.T.; Iglesia, E. Structure and Properties of Oxidative Dehydrogenation Catalysts Based on MoO3/Al2O3. J. Catal. 2001, 198, 232–242. [Google Scholar] [CrossRef]
- Darvishi, A.; Davand, R.; Khorasheh, F.; Fattahi, M. Modeling-Based Optimization of a Fixed-Bed Industrial Reactor for Oxidative Dehydrogenation of Propane. Chin. J. Chem. Eng. 2016, 24, 612–622. [Google Scholar] [CrossRef]
- Kotanjac, Ž.S.; van Sint Annaland, M.; Kuipers, J.A.M. A Packed Bed Membrane Reactor for the Oxidative Dehydrogenation of Propane on a Ga2O3/MoO3 Based Catalyst. Chem. Eng. Sci. 2010, 65, 441–445. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Z.; Bollini, P.; Balakotaiah, V. Scale-up Analysis of the Oxidative Dehydrogenation of Ethane over MoVTeNbOx Catalysts in an Autothermal Reactor. Chem. Eng. Sci. 2023, 273, 118649. [Google Scholar] [CrossRef]
- Chen, J.; Bollini, P.; Balakotaiah, V. Oxidative Dehydrogenation of Ethane over Mixed Metal Oxide Catalysts: Autothermal or Cooled Tubular Reactor Design? AIChE J. 2021, 67, e17168. [Google Scholar] [CrossRef]
- Fazlinezhad, A.; Fattahi, M.; Tavakoli-Chaleshtori, R.; Rezaveisi, H. Sensitivity Analysis and Multi-Objective Optimization of Oxidative Dehydrogenation of Propane in a Fixed-Bed Reactor over Vanadium/Graphene for Propylene Production. Chem. Eng. Technol. 2022, 45, 309–318. [Google Scholar] [CrossRef]
- Cai, R.; Brody, L.; Tian, Y.; Neal, L.; Bose, A.; Li, F. Numerical Modeling of Chemical Looping Oxidative Dehydrogenation of Ethane in Parallel Packed Beds. Chem. Eng. J. 2023, 469, 143930. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, Y.; Deng, H.; Wang, S.; Dong, H. Multiscale Modeling and Simulation on Oxidative Dehydrogenation of Ethane to Ethylene. Chem. Eng. Res. Des. 2023, 195, 235–246. [Google Scholar] [CrossRef]
- Reyes-Antonio, C.A.; Cordero, M.E.; Pérez-Pastenes, H.; Uribe, S.; Al-Dahhan, M. Analysis of the Effect of Hydrodynamics over the Activity and Selectivity of the Oxidative Dehydrogenation of Propane Process in a Packed Bed Reactor through CFD Techniques. Fuel 2020, 280, 118510. [Google Scholar] [CrossRef]
- Khraibet, S.A.; Mazloom, G.; Banisharif, F. Comparative Study of Different Two-Phase Models for the Propane Oxidative Dehydrogenation in a Bubbling Fluidized Bed Containing the VOx/γ-Al2O3 Catalyst. Ind. Eng. Chem. Res. 2021, 60, 9729–9738. [Google Scholar] [CrossRef]
- Rostom, S.; de Lasa, H. Downer Fluidized Bed Reactor Modeling for Catalytic Propane Oxidative Dehydrogenation with High Propylene Selectivity. Chem. Eng. Process.-Process Intensif. 2019, 137, 87–99. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Du, Y.; Liu, H.; Yang, C. Numerical Investigations of the Oxidative Dehydrogenation of Propane in a Spouted Bed Reactor. Energy Fuels 2020, 34, 10858–10871. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Yao, Y.; Liu, Q.; Lu, F.; Wang, X. Embedding Isolated Fe Species in Titania Increases Olefins for Oxidative Propane Dehydrogenation. AIChE J. 2023, 69, e18088. [Google Scholar] [CrossRef]
- Lu, F.; Wang, J.; Chai, S.; Wang, Y.; Yao, Y.; Wang, X. Asymmetric Coupled Binuclear-Site Catalysts for Low-Temperature Selective Acetylene Semi-Hydrogenation. Angew. Chem. Int. Ed. 2025, 64, e202414719. [Google Scholar] [CrossRef]
- Chen, K.; Bell, A.T.; Iglesia, E. Kinetics and Mechanism of Oxidative Dehydrogenation of Propane on Vanadium, Molybdenum, and Tungsten Oxides. J. Phys. Chem. B 2000, 104, 1292–1299. [Google Scholar] [CrossRef]
- Minić, D.M.; Blagojević, V.A. Hydrothermal Synthesis and Controlled Growth of Vanadium Oxide Nanocrystals. CrystEngComm 2013, 15, 6617–6624. [Google Scholar] [CrossRef]
- Hu, P.; Hu, P.; Vu, T.D.; Li, M.; Wang, S.; Ke, Y.; Zeng, X.; Mai, L.; Long, Y. Vanadium Oxide: Phase Diagrams, Structures, Synthesis, and Applications. Chem. Rev. 2023, 123, 4353–4415. [Google Scholar] [CrossRef] [PubMed]
- Wȩgrzyniak, A.; Jarczewski, S.; Wȩgrzynowicz, A.; Michorczyk, B.; Kuśtrowski, P.; Michorczyk, P. Catalytic Behavior of Chromium Oxide Supported on Nanocasting-Prepared Mesoporous Alumina in Dehydrogenation of Propane. Nanomaterials 2017, 7, 249. [Google Scholar] [CrossRef]
- Chen, D.; Yi, R.; Chen, S.; Xu, T.; Gordin, M.L.; Lv, D.; Wang, D. Solvothermal Synthesis of V2O5/Graphene Nanocomposites for High Performance Lithium Ion Batteries. Mater. Sci. Eng. B 2014, 185, 7–12. [Google Scholar] [CrossRef]
- Boukhoubza, I.; Achehboune, M.; El Khouja, O.; Mohamed, M.A.; Mindroc, M.; Derkaoui, I.; Enculescu, M.; Matei, E. Enhanced Photocatalytic Performance of V2O5 NRs/RGO Nanocomposites for Rhodamine-B Decolorization under Solar Irradiation: Experimental and Theoretical Study. J. Phys. Chem. Solids 2025, 201, 112654. [Google Scholar] [CrossRef]
- Cao, Z.; Wei, B. V2O5/Single-Walled Carbon Nanotube Hybrid Mesoporous Films as Cathodes with High-Rate Capacities for Rechargeable Lithium Ion Batteries. Nano Energy 2013, 2, 481–490. [Google Scholar] [CrossRef]
- Shan, Y.L.; Sun, H.L.; Zhao, S.L.; Li, K.X.; Xia, K.H.; Ding, J.W.; Yu, W.L. Enhancing Propane Direct Dehydrogenation Performances through Temperature Induced VOx Dispersion and Alumina Support Phase Transformation. Chem. Eng. J. 2022, 450, 137969. [Google Scholar] [CrossRef]
- Yam, K.M.; Guo, N.; Jiang, Z.; Li, S.; Zhang, C. Graphene-Based Heterogeneous Catalysis: Role of Graphene. Catalysts 2020, 10, 53. [Google Scholar] [CrossRef]
- Habeeb, A.I.; Al-Asadi, A.S. Fabrication of Hybrid Graphene Nanosheets/Vanadium(V) Oxide Nanoparticles Composite Electrodes for Supercapacitor Application. Solid. State Commun. 2023, 360, 115024. [Google Scholar] [CrossRef]
- Mikhaylov, A.A.; Medvedev, A.G.; Grishanov, D.A.; Sladkevich, S.; Gun, J.; Prikhodchenko, P.V.; Xu, Z.J.; Nagasubramanian, A.; Srinivasan, M.; Lev, O. Vanadium Oxide Thin Film Formation on Graphene Oxide by Microexplosive Decomposition of Ammonium Peroxovanadate and Its Application as a Sodium Ion Battery Anode. Langmuir 2018, 34, 2741–2747. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, Z.-F.; Liu, Y.; Zhang, H.; Ren, Y.; Sun, C.-J.; Lu, W.; Zhou, Y.; Stanciu, L.; Stach, E.A.; et al. Graphene-Modified Nanostructured Vanadium Pentoxide Hybrids with Extraordinary Electrochemical Performance for Li-Ion Batteries. Nat. Commun. 2015, 6, 6127. [Google Scholar] [CrossRef] [PubMed]
- Carrero, C.A.; Schloegl, R.; Wachs, I.E.; Schomaecker, R. Critical Literature Review of the Kinetics for the Oxidative Dehydrogenation of Propane over Well-Defined Supported Vanadium Oxide Catalysts. ACS Catal. 2014, 4, 3357–3380. [Google Scholar] [CrossRef]
- Kube, P.; Frank, B.; Schlögl, R.; Trunschke, A. Isotope Studies in Oxidation of Propane over Vanadium Oxide. ChemCatChem 2017, 9, 3446–3455. [Google Scholar] [CrossRef]
- Liu, W.; Cao, T.; Dai, X.; Bai, Y.; Lu, X.; Li, F.; Qi, W. Nitrogen-Doped Graphene Monolith Catalysts for Oxidative Dehydrogenation of Propane. Front. Chem. 2021, 9, 759936. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Grant, J.T.; Venegas, J.M.; McDermott, W.P.; Hermans, I. Aerobic Oxidations of Light Alkanes over Solid Metal Oxide Catalysts. Chem. Rev. 2018, 118, 2769–2815. [Google Scholar] [CrossRef]
- Sheng, J.; Yan, B.; Lu, W.-D.; Qiu, B.; Gao, X.-Q.; Wang, D.; Lu, A.-H. Oxidative Dehydrogenation of Light Alkanes to Olefins on Metal-Free Catalysts. Chem. Soc. Rev. 2021, 50, 1438–1468. [Google Scholar] [CrossRef]
- Rostom, S.; de Lasa, H. Propane Oxidative Dehydrogenation on Vanadium-Based Catalysts under Oxygen-Free Atmospheres. Catalysts 2020, 10, 418. [Google Scholar] [CrossRef]
- Corma, A.; López-Nieto, J.M.; Paredes, N.; Pérez, M.; Shen, Y.; Cao, H.; Suib, S.L. Oxidative Dehydrogenation of Propane over Supported-Vanadium Oxide Catalysts. Stud. Surf. Sci. Catal. 1992, 72, 213–220. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Ovsitser, O.; Radnik, J.; Schneider, M.; Kraehnert, R.; Dingerdissen, U. Influence of Reaction Conditions on Catalyst Composition and Selective/Non-Selective Reaction Pathways of the ODP Reaction over V2O3, VO2 and V2O5 with O2 and N2O. Appl. Catal. A Gen. 2007, 319, 98–110. [Google Scholar] [CrossRef]
- Balderas-Tapia, L.; Hernández-Pérez, I.; Schacht, P.; Córdova, I.R.; Aguilar-Ríos, G.G. Influence of Reducibility of Vanadium–Magnesium Mixed Oxides on the Oxidative Dehydrogenation of Propane. Catal. Today 2005, 107–108, 371–376. [Google Scholar] [CrossRef]
- Clark, P.D.; Dowling, N.I.; Long, X.; Li, Y. Oxidative Dehydrogenation of Propane to Propene in the Presence of H2S at Short Contact Times. J. Sulfur. Chem. 2004, 25, 381–387. [Google Scholar] [CrossRef]
- Sugiyama, S.; Iizuka, Y.; Nitta, E.; Hayashi, H.; Moffat, J.B. Role of Tetrachloromethane as a Gas-Phase Additive in the Oxidative Dehydrogenation of Propane over Cerium Oxide. J. Catal. 2000, 189, 233–237. [Google Scholar] [CrossRef]
- Kazerooni, H. Investigation of Oxidative Propane Dehydrogenation Using Metal Oxide Nanocatalysts Based on Magnesium Oxide; College of Engineering, University of Tehran: Tehran, Iran, 2011. [Google Scholar]
- Fattahi, M.; Kazemeini, M.; Khorasheh, F.; Rashidi, A.M. Morphological Investigations of Nanostructured V2O5 over Graphene Used for the ODHP Reaction: From Synthesis to Physiochemical Evaluations. Catal. Sci. Technol. 2015, 5, 910–924. [Google Scholar] [CrossRef]
- Rashidi, A.; Mahmudian, L.; Dehghani, H. Producing Graphene and Nanoporous Graphene. US10221069B2, 5 March 2019. [Google Scholar]

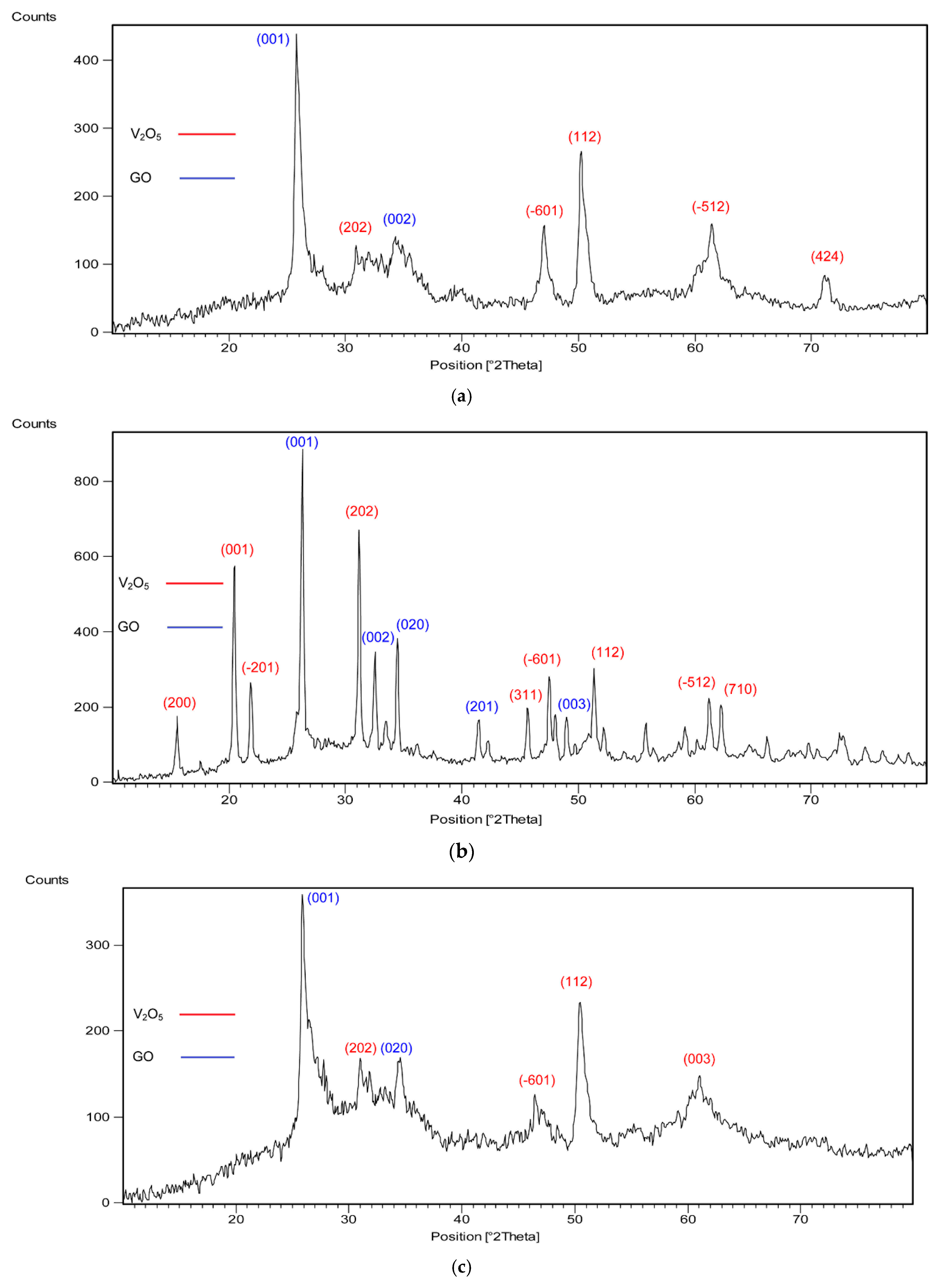
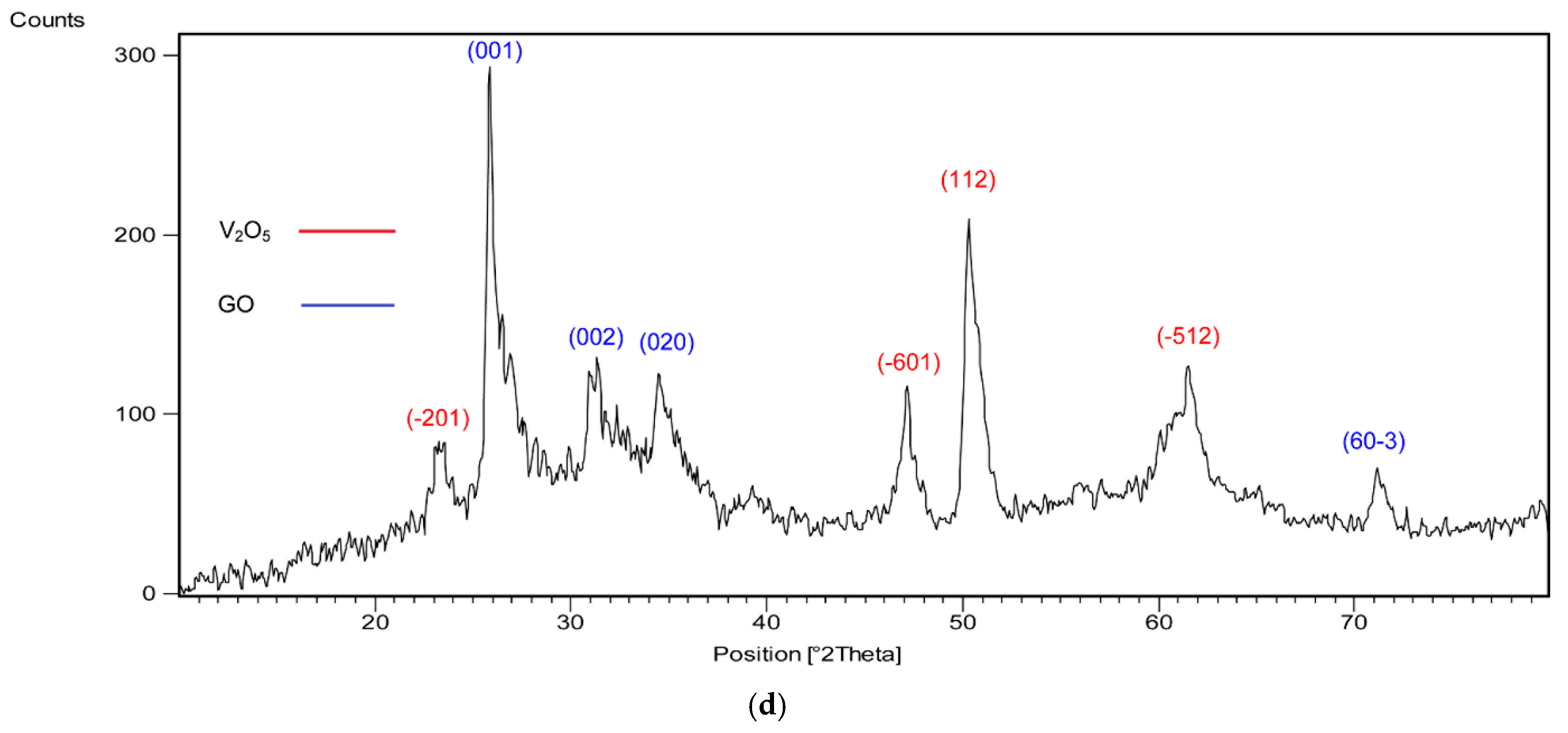
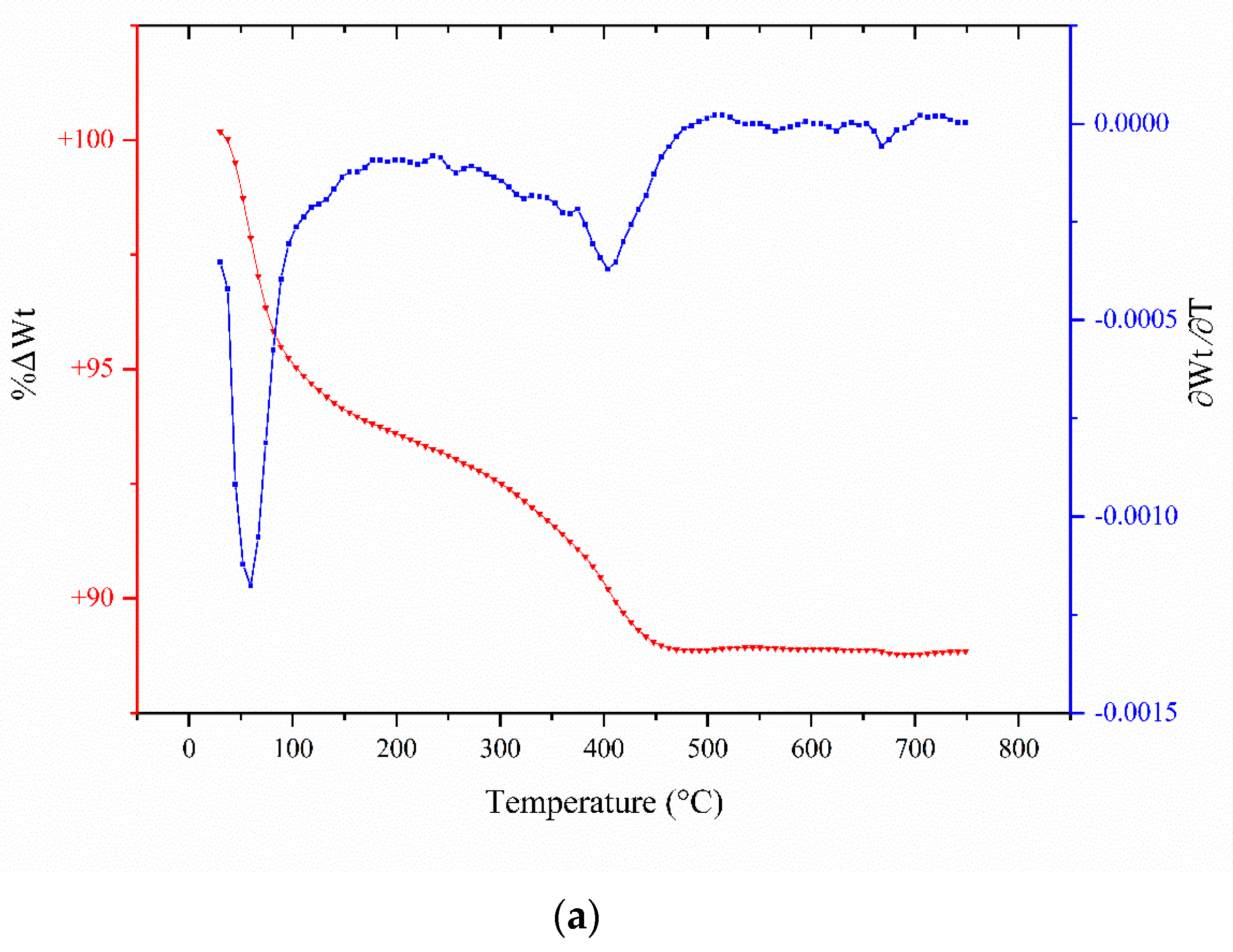
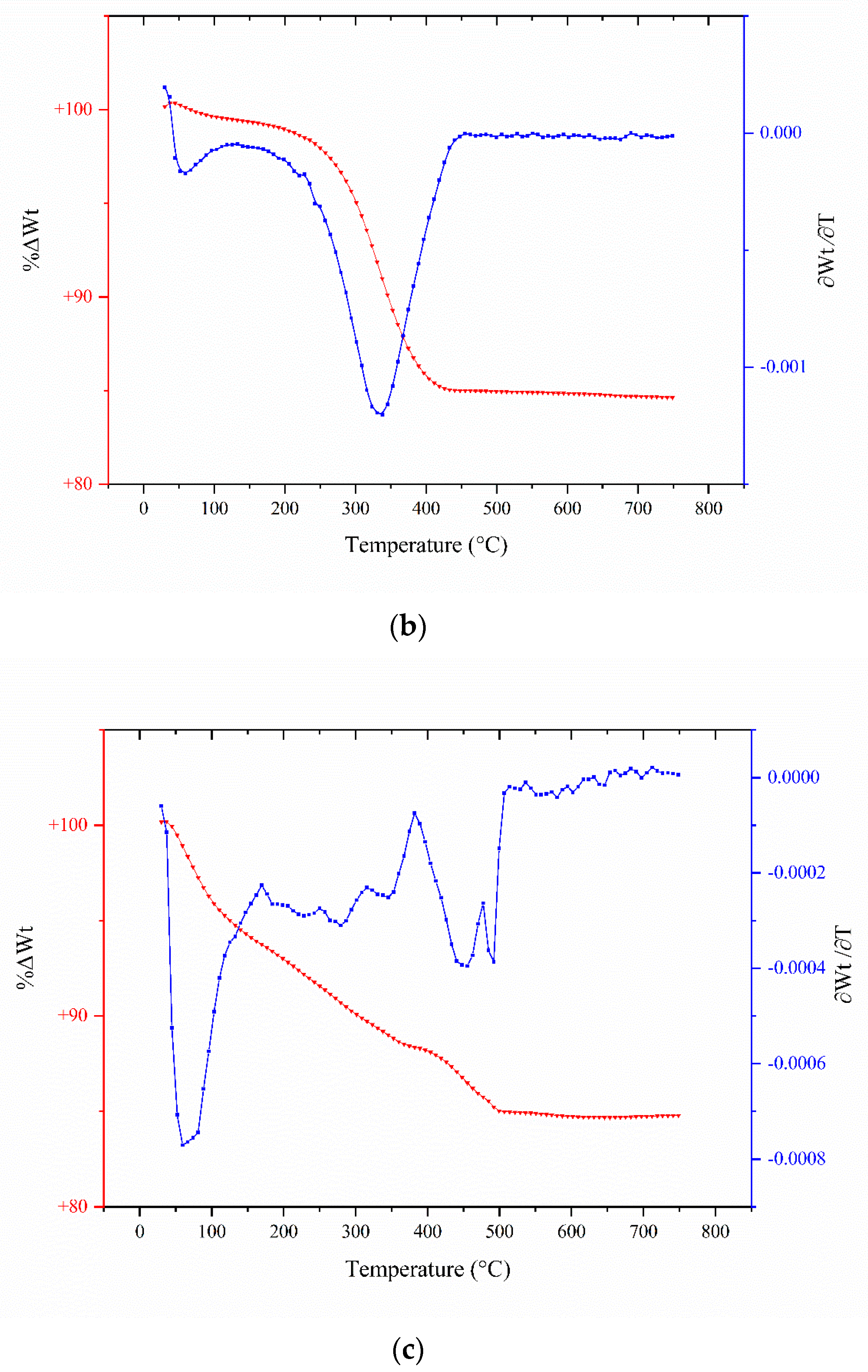
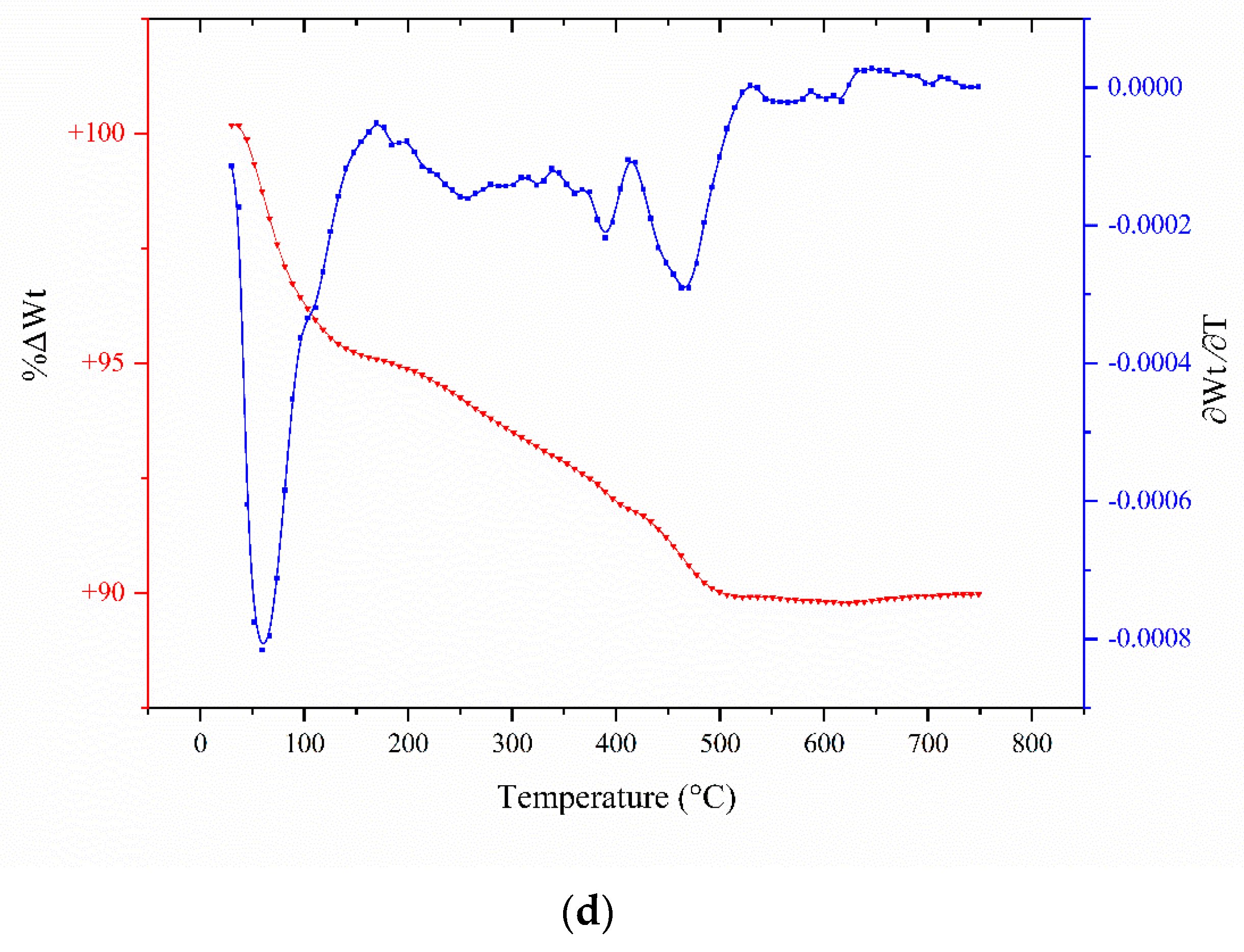


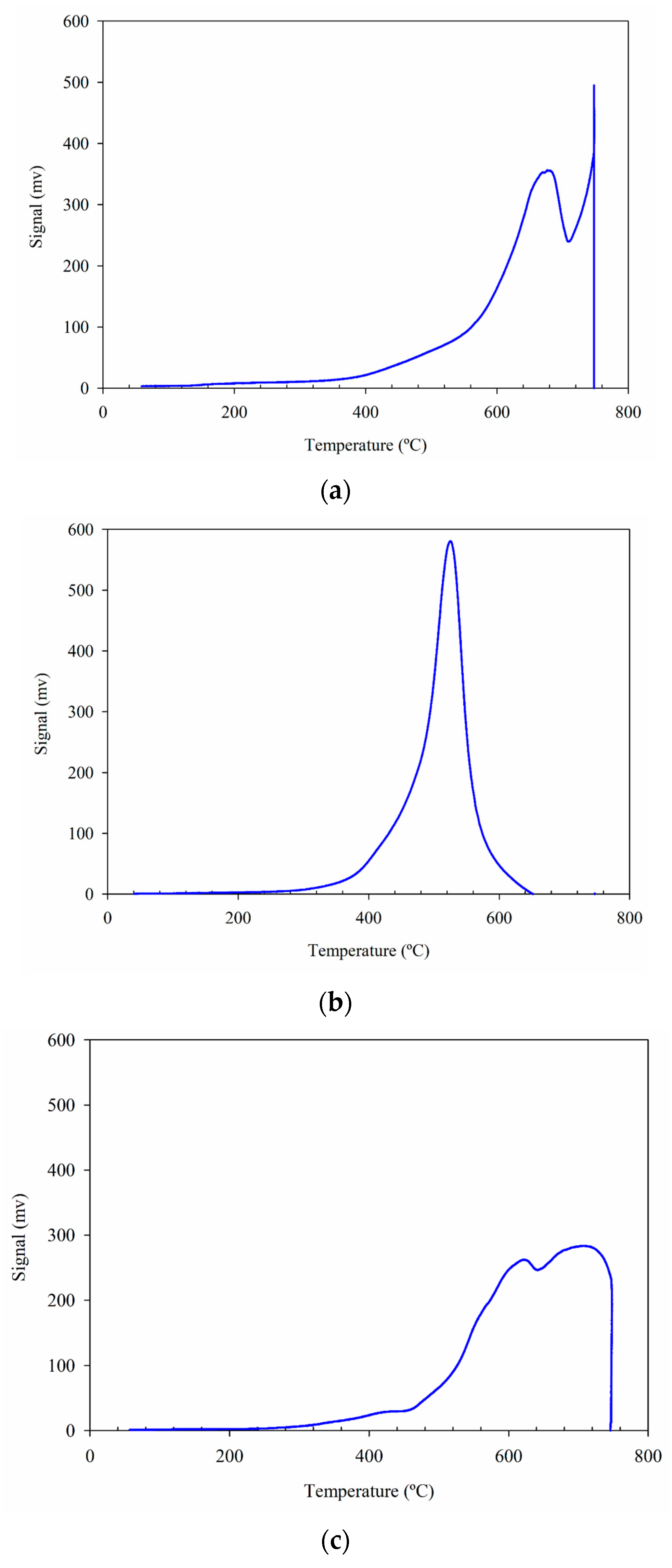
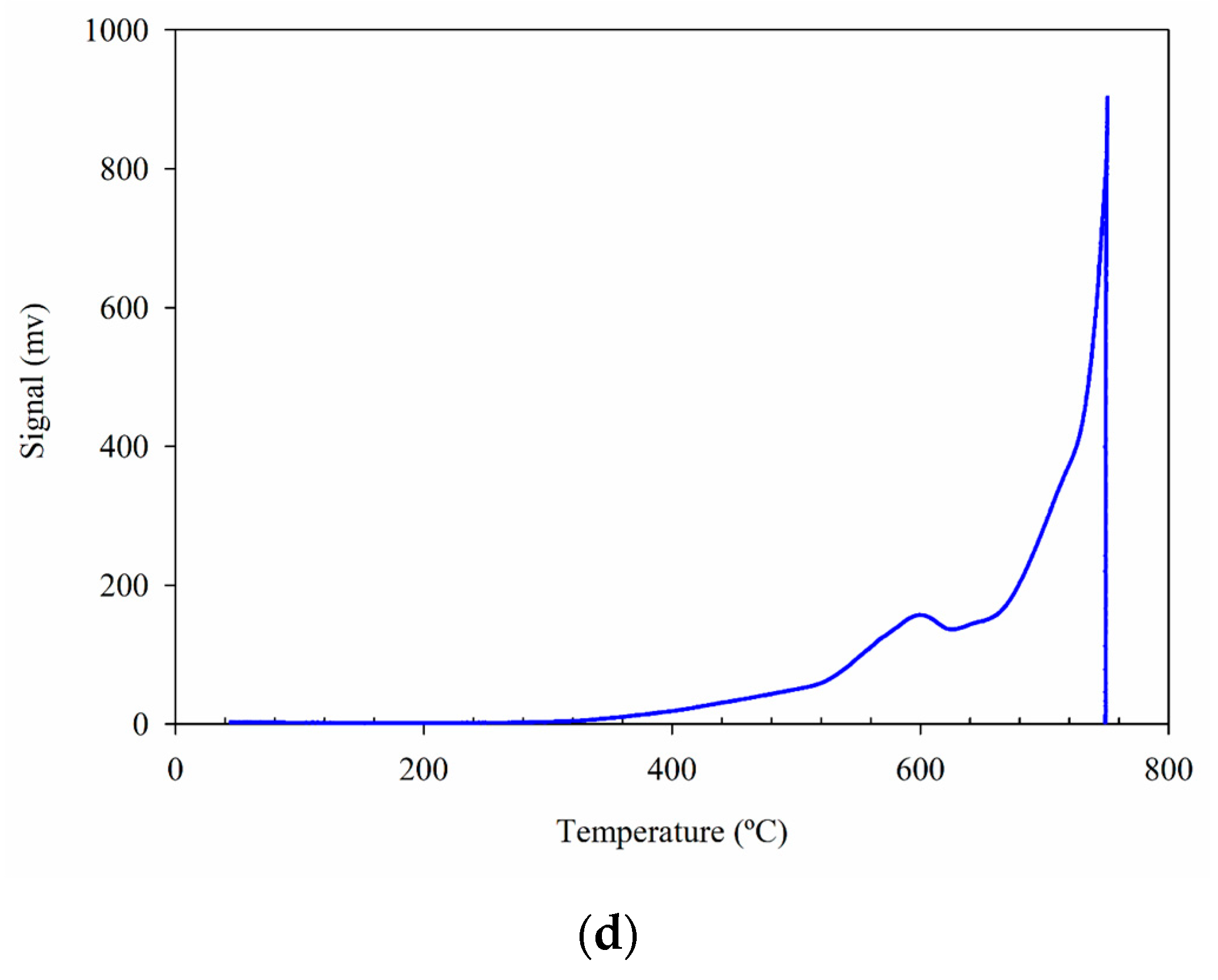
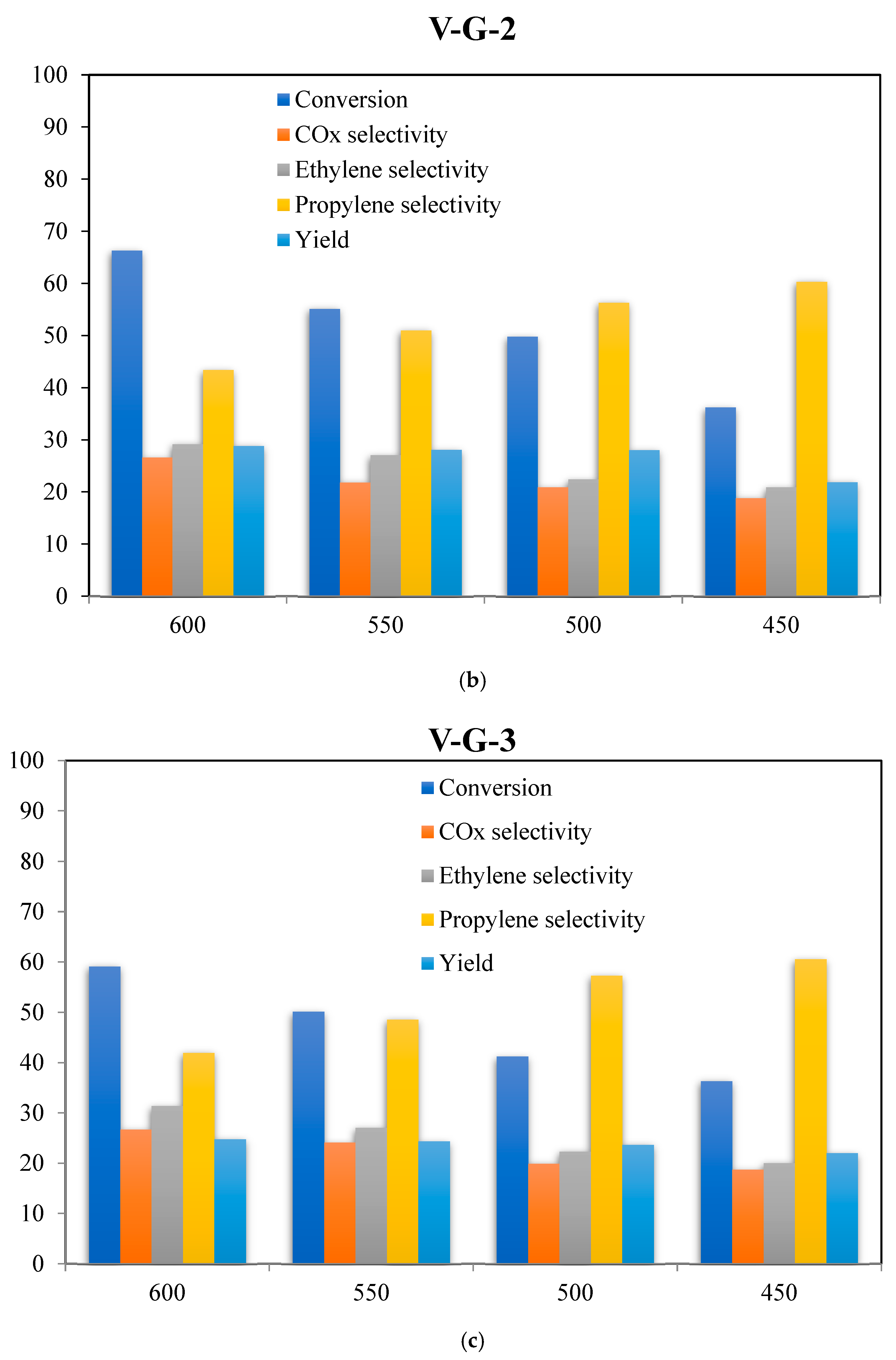
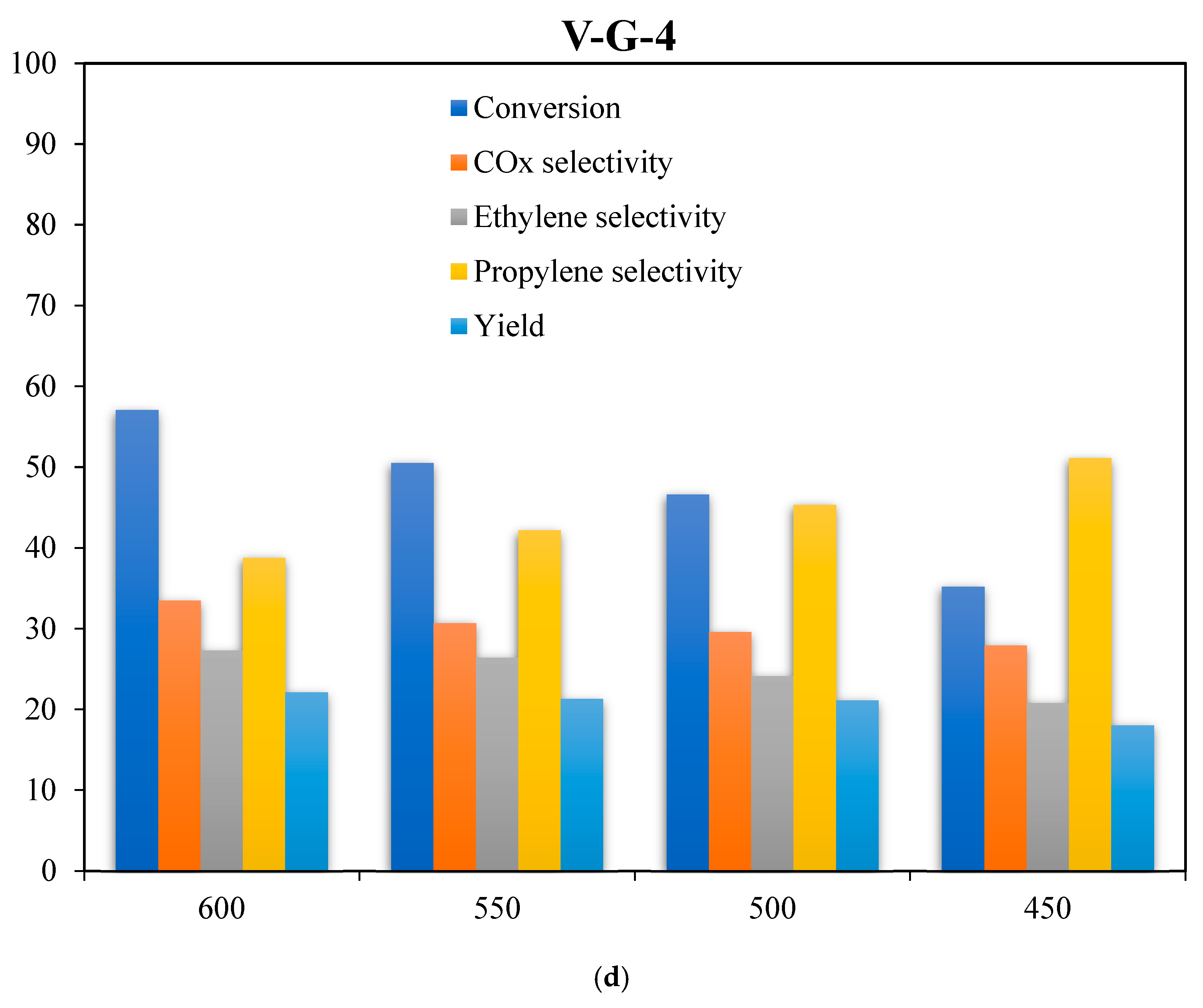
| Catalyst/Oxidizing Agent | T (°C) | Propane Conversion (%) | Selectivity (%) | C3H6 Yield (%) | Reference | ||
|---|---|---|---|---|---|---|---|
| COx | C3H6 | C2H4 | |||||
| V2O5/O2 | 500 | 6.73 | - | 25.26 | 73.70 | 1.69 | [71] |
| V2O5/N2O | 500 | 4.30 | - | 72.25 | 27.21 | 3.11 | [71] |
| V2O5/Air | 460 | 3.50 | - | 15.3 | 89.7 | 0.54 | [72] |
| Al2O3/(O2 and H2S) | 700 | 24.2 | 13.9 | 71.3 | 5.7 | 17.2 | [73] |
| Al2O3/O2 | 700 | 26.8 | 34.8 | 44.5 | 3.1 | 11.9 | [73] |
| V-Al2O3/(O2 and H2S) | 700 | 53.7 | 25.1 | 56.5 | 1.0 | 30.4 | [73] |
| V-Al2O3/O2 | 700 | 22.8 | 29.9 | 25.0 | 22.3 | 5.7 | [73] |
| CeO2 | 450 | 7.25 | 0.0 | 18.14 | 82.21 | 1.32 | [74] |
| V2O5-CNT/Air | 500 | 17.21 | 3.45 | 51.02 | 47.24 | 8.78 | [75] |
| V2O5-Benzylamine/Air | 500 | 73 | 34.5 | 47.59 | 10.8 | 34.74 | [75] |
| V2O5-Dodecyl Amine/Air | 500 | 50.44 | 17.44 | 41.03 | 41.28 | 20.69 | [76] |
| V2O5-Aniline-Graphene/Air | 500 | 50.65 | 21.49 | 51.86 | 25.75 | 26.27 | [76] |
| V-G-1 | 600 | 71.20 | 26.72 | 42.25 | 30.50 | 30.08 | Present study |
| V-G-1 | 500 | 55.10 | 20.68 | 54.38 | 24.32 | 30.10 | Present study |
| Sample | V2O5/Graphene Molar Ratio | Hydrothermal Conditions | |
|---|---|---|---|
| Time (h) | Temperature (°C) | ||
| V-G-1 | 1/1 | 48 | 200 |
| V-G-2 | 24 | ||
| V-G-3 | 10 | ||
| V-G-4 | 2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi, R.; Fazlinezhad, A.; Fallah Shojaei, A.; Rashidi, A.; Fattahi, M. Evaluation of Vanadium Oxide Nanocatalysts over Graphene for Propylene Production Through Oxidative Propane Dehydrogenations. Catalysts 2025, 15, 409. https://doi.org/10.3390/catal15050409
Mousavi R, Fazlinezhad A, Fallah Shojaei A, Rashidi A, Fattahi M. Evaluation of Vanadium Oxide Nanocatalysts over Graphene for Propylene Production Through Oxidative Propane Dehydrogenations. Catalysts. 2025; 15(5):409. https://doi.org/10.3390/catal15050409
Chicago/Turabian StyleMousavi, Robabeh, Armin Fazlinezhad, Abdollah Fallah Shojaei, Alimorad Rashidi, and Moslem Fattahi. 2025. "Evaluation of Vanadium Oxide Nanocatalysts over Graphene for Propylene Production Through Oxidative Propane Dehydrogenations" Catalysts 15, no. 5: 409. https://doi.org/10.3390/catal15050409
APA StyleMousavi, R., Fazlinezhad, A., Fallah Shojaei, A., Rashidi, A., & Fattahi, M. (2025). Evaluation of Vanadium Oxide Nanocatalysts over Graphene for Propylene Production Through Oxidative Propane Dehydrogenations. Catalysts, 15(5), 409. https://doi.org/10.3390/catal15050409






