Abstract
The oxidation of imines may give several products, such as oxaziridines, nitrones, amides, and other rearranged compounds. Therefore, its selectivity is a challenge that various methods have to face. The controversial selectivity of the oxidation of imines using urea hydrogen peroxide (UHP) catalyzed by methyltrioxorhenium (MTO) is addressed by varying the solvent, temperature, reaction time, amount of oxidant, and catalyst used. The reactivity and selectivity of the oxidation of imines proved to be particularly sensitive to the type of solvent. The use of methanol furnished the corresponding nitrones as the exclusive products, except for very hindered N-tert-alkyl substituted substrates. Using the ionic liquid [bmim]BF4 as a solvent resulted in a complete switch in reactivity and selectivity. N-methyl substituted imines gave the corresponding amides, while imines with bulkier substituents at nitrogen did not show any reactivity. An exception was the C-phenyl,N-tert-butyl imine—the only substrate that was oxidized to the corresponding oxaziridine, albeit with low conversion. The results reported herein reaffirm the oxidation of imines with UHP/MTO in MeOH as the method of choice for their interconversion to nitrones.
1. Introduction
The oxidation of imines (1) is a tricky reaction that, in principle, may follow concurrent pathways to yield nitrones (2) through oxidation at the nitrogen lone pair, or oxaziridines (3) by oxidation at the π system of the C=N double bond (Scheme 1). As a matter of fact, most of the oxidation methods reported in the literature for imines, including oxidations with peracids [1,2,3,4], oxones [5,6,7], sulfonic peracids [8], UHP/maleic anhydride [9], and H2O2/trichloroacetonitrile [10], as well as several metal catalyzed methods utilizing either H2O2 [11], other peroxides [12], or O2 [13,14], showed remarkable chemoselectivity in favor of oxaziridines (3). Therefore, a reasonable choice of oxidizing reagents and/or catalysts is available for the synthesis of oxaziridines from the corresponding imines. Conversely, only a few methods have been reported to furnish nitrones (2) preferentially, and those suffer from serious limitations in substrate scope, operational conditions, and poor selectivity [2,3,15,16]. Moreover, other methods have been reported to yield amides (4) or products derived from rearrangement (5) as main products, or lead to overoxidation reactions, making the control of selectivity very challenging [1,3,15,17,18,19]. The seemingly trivial N-oxidation of imines to nitrones is, therefore, an elusive transformation. On the other hand, nitrones are very useful building blocks for the synthesis of biologically active nitrogen-containing compounds [20,21,22]. They have been widely employed as either 1,3-dipoles in [3+2] cycloaddition reactions with alkenes [23,24,25,26,27,28,29,30,31], electrophilic acceptors toward organometallic reagents [32,33,34,35,36,37], or other less common reactions [20,30,38]. In addition, nitrones are widely used as spin-trap reagents in biological systems and as therapeutics [39,40,41,42,43,44,45,46,47,48].
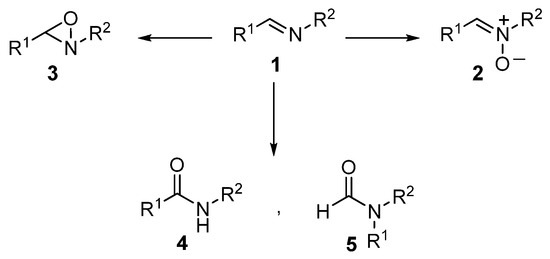
Scheme 1.
Possible products from the oxidation of imines.
More recently, in 2007, we reported on an efficient and broadly applicable method for converting imines into corresponding nitrones [49], that is, via oxidation with urea hydroperoxide (UHP, H2NCONH2.H2O2) [50] in methanol, using catalytic amounts of methyltrioxorhenium (MTO, CH3ReO3, A, Figure 1), which is a well-established catalyst for the oxidation of alkenes [51,52] and other nitrogen-containing substrates [53,54]. It is known that this catalyst reacts with hydrogen peroxide to furnish a monoperoxo and a diperoxo B derivatives, which are considered the active catalytic species for O-transfer to substrates S in the oxidation reactions involving MTO (Figure 1) [51,52]. Successively, we have also reported on the first one-pot direct synthesis of nitrones from aldehydes and primary amines with the same oxidant/catalyst system through the intermediate imines, making the method even more sustainable [55].

Figure 1.
Schematic representation of the general catalytic cycle for the oxidation of substrates S with MTO and H2O2.
However, at approximately the same time, Sain and co-workers published a paper claiming the chemoselective oxidation of imines to oxaziridines utilizing the same UHP/MTO system [56]. We were surprised by the results reported therein, and the question arose as to whether different reaction conditions and/or operational procedures might account for this apparent steering in the course of the reaction. As a matter of fact, during this time lapse, the MTO/UHP method has been utilized by us and others to synthesize nitrones, either from preformed imines [57,58,59,60,61] or through the one-pot procedure from primary amines and aldehydes [62,63,64,65,66]. Conversely, to the best of our knowledge, no example of the preparation of oxaziridines from imines with this combination of reagents has been reported, thus corroborating our findings. Nonetheless, we decided to investigate this reaction in greater detail, in order to uncover possible factors able to drive the reaction to different products and to explain the controversial results. In addition, we extended the diversity of substrates studied to better define the scope of the reaction and disclose potential limitations.
In this paper, we report our findings on this subject, which provide no evidence for the general formation of oxaziridines. If a dichotomy in the chemical behavior of imines in this reaction emerges, it is for yielding amides vs. nitrones.
2. Results
A careful comparison of the substrates and reaction conditions employed by us [49] vs. those used by Sain and co-workers [56] allowed us to identify some clues to investigate the possible divergent behavior in the oxidation of imines with UHP catalyzed by MTO. The optimal reaction conditions selected by Sain consisted of the use of an ionic liquid (butylmethylimidazolium tetrafluoroborate, [bmim]BF4) as the reaction medium at a temperature of 50 °C, while we carried out the oxidations at room temperature in methanol, which was superior to other options [49]. They also used much higher imine concentrations (ca. 6.7 M vs. 0.5 M) and a lower amount of oxidant (UHP, 2 equiv. vs. 3 equiv.) and catalyst (MTO, 1 mol% vs. 2 mol%) than those employed in our study. Thus, the first option involved a different type of solvent and conditions; however, in the preliminary screening of solvents, Sain and co-workers also performed a reaction in methanol, while still reporting the selective formation of oxaziridine. Another possible reason for the different results reported can be ascribed to the different temperatures utilized. Furthermore, we observed that the substrates used successfully by Sain consisted of C-aryl,N-alkyl imines. Most of them bore bulky secondary or tertiary substituents at nitrogen, while we observed that steric encumbrance at nitrogen gave more sluggish reactions and lower product yields. The different nature of the substrates used could be another reason for the divergent behavior, albeit the same work also reported an example of an N-methyl imine that still yielded the corresponding oxaziridine [56].
Based on the above observations, we conducted a full series of experiments in order to firmly clarify this matter. We first carried out the oxidation reactions on C-phenyl,N-alkyl imines 1a–e, which are representative of a large range of imines possessing increasingly bulkier substituents at nitrogen (Me, nBu, iPr, cHex, tBu). The results are reported in Scheme 2 and Table 1.
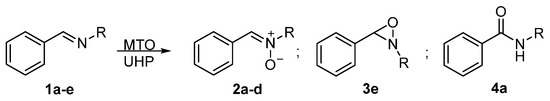
Scheme 2.
Products detected in the oxidation of C-phenyl,N-alkyl imines 1a–e with MTO/UHP.

Table 1.
Results in the oxidation of C-phenyl,N-alkyl imines 1a–e with MTO/UHP.
The oxidation of N-phenyl,C-alkyl imines 1a–d under the optimized conditions used in our original communication [49] (0.5 M in MeOH, 2–4 mol% MTO, 3 equiv. UHP) confirmed the completely selective formation of the corresponding nitrones 2a–d (Table 1, entries 1, 10, 14, 18). The structural assignment was unequivocally based on the nitrone C-H signal, which resonates at a low field in the 1H NMR spectrum (δ = 7.2–9.2 ppm), and by comparison with literature data (see Supplementary Materials). It is worth noting that only imine 1b has been previously employed in this oxidation method [49], all other substrates are reported here for the first time. In this respect, we now report that in the presence of bulky secondary alkyl groups, such as the isopropyl and cyclohexyl in 1c and 1d, respectively, the reaction was sluggish (entries 14 and 18); it was found to be more practical to increase the amount of MTO (4 mol%, entries 15 and 19) in order to reach the complete conversion of the starting material, which required longer reaction times. It should be emphasized that all the reactions were completely selective for the formation of the nitrones 2, as well as very clean, with no trace of oxaziridines 3, amides 4, or other products detected in the crude reaction mixtures by 1H NMR. When the products were subjected to isolation and purification (see Table 1 and Supplementary Materials), the nitrones 2 were collected in high yields. Increasing the bulkiness of the N-substituent by placing a tert-butyl in imine 1e resulted in inhibiting any reaction, with the starting imine being recovered unaltered (entry 25). It is then apparent that N-oxidation of the imines is highly sensitive to the steric hindrance of the substituent at nitrogen.
The first variations tested to mimic the operational conditions employed by Sain regarded the temperature and the imine concentration, as well as the amount of catalyst/oxidant. Raising the temperature to 50 °C did not affect the selectivity but, with the exception of imine 1a (entry 2), was detrimental to the reaction rate, resulting in lower conversions of imines 1b–d to the corresponding nitrones 2b–d (entries 11, 16, and 20). This unexpected effect might be ascribed to the inactivation or decomposition of the MTO-derived effective rhenium peroxo catalytic species. Increasing the imine 1a concentration in MeOH to 6 M and reducing the amount of oxidant to 2 equiv. and the catalyst to 1 mol% resulted in a shortage in selectivity, producing 54% of amide 4a and 46% of nitrone 2a (entry 3). Given the lack of selectivity, these conditions were not replicated with the other imines. The formation of amide 4a under these conditions may be attributed to direct oxidation by hydrogen peroxide without the intervention of the catalyst, which competes at higher concentrations and lower amounts of catalyst. This assumption was proved by subjecting imine 1a to oxidation in MeOH with UHP in the absence of MTO, which only yielded amide 4a, especially under high concentration (53% conversion after 20 h at 20 °C).
Then, we investigated the MTO/UHP oxidation of imines 1a–e in the ionic liquid medium, namely in [bmim]BF4 as reported [56]. Using 2 equiv. UHP and 1 mol% MTO with imine 1a (6 M in [bmim]BF4) at 50 °C required reaction times as long as 18 h to allow the oxidation to reach completion (Table 1, entries 4 and 5), exclusively furnishing amide 4a. The same result was obtained by lowering the reaction temperature to 20 °C and increasing the oxidant and catalyst to 3 equiv. and 2 mol%, respectively, under a more diluted 0.5 M concentration of imine (entry 7). Thus, variation of the solvent from MeOH to [bmim]BF4 effectively resulted in a switching of the selectivity of the reaction, yielding, in our hands, amide 4a rather than oxaziridine 3a. We speculated that the amide might be derived from a rearrangement of the oxaziridine or the nitrone, formed as primary oxidation products, which are reactions known under different conditions [2,4,67]. This hypothesis was ruled out since both oxaziridine 3a, synthesized according to the literature [68], as well as nitrone 2a, failed to give the amide when heated under the same reaction conditions for long time periods. Moreover, upon carrying out the reaction for just 1 h, neither nitrone 2a nor oxaziridine 3a were detected, but only amide 4a (39% conversion, entry 6). Replacing UHP with a 35% aqueous H2O2 solution at 20 °C slowed down the oxidation, which reached a 57% conversion after 20 h, once again giving amide 4a as the exclusive product (entry 8). Finally, [bmim]PF6, as the ionic liquid solvent, showed the same efficiency as [bmim]BF4 but resulted in lower selectivity, yielding 79% of amide 4a accompanied by 21% of nitrone 2a (entry 9), thus demonstrating that the nature of the liquid ionic anion has a role in the outcome of the reaction. Some representative 1H NMR spectra of crude reaction mixtures (experiments in entries 2, 3, 4, 6, and 9) are reported in the Supplementary Materials in order to exemplify the conversions and product ratios calculated. In contrast to the behavior of imine 1a, when N-alkyl benzaldimines 1b–d were reacted in [bmim]BF4 under the optimal conditions (6 M concentration of imine, 2 equiv. UHP, 1 mol% MTO, 50 °C or rt), no reaction occurred and the starting materials were recovered unaltered (entries 12, 17, 21, and 22). N-tert-butyl benzaldimine 1e showed instead a partial conversion to the corresponding oxaziridine 3e, as assessed by the typical signal at δ = 4.7 ppm in the 1H NMR spectrum for C-H at the three-membered ring, albeit with a low 11% conversion after 20 h (entry 27). Notably, this was the only case where the formation of an oxaziridine was detected. Finally, the treatment of imines 1b and 1d in [bmim]PF6 (0.5 M concentration of imine, 3 equiv. UHP, 2 mol% MTO, 50 °C or rt) resulted in the partial but selective conversion to the corresponding nitrones 2b and 2d (80% after 4 h and 33% after 18 h, respectively, entries 13 and 23). Therefore, a generally higher propensity to yield nitrones upon switching from the tetrafluoroborate to the hexafluorophosphate salt of butylmethylimidazolium was demonstrated.
The results collected in Table 1 did not evidence any reaction condition that was able to steer the selectivity of the oxidation of imines toward the formation of oxaziridines. Rather, upon replacing the methanolic solvent with the ionic liquid [bmim]BF4, a switch from the obtainment of nitrones to amides was observed, which was limited to the N-methyl-substituted imine 1a. We deemed this observation unprecedented and worthy to be investigated in greater detail, in order to establish if this is a general behavior for N-methyl aromatic aldimines. To this aim, we subjected the C-aryl,N-methyl imines 1f–k to the UHP/MTO oxidation either in MeOH or [bmim]BF4, and the results are reported in Scheme 3 and Table 2.
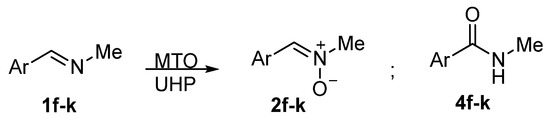
Scheme 3.
Products detected in the oxidation of C-aryl,N-methyl imines 1f–k with MTO/UHP.

Table 2.
Results of the oxidation of C-aryl,N-methyl imines 1f–k with MTO/UHP.
The standard procedure for the oxidation in MeOH (0.5 M concentration of imine, 3 equiv. UHP, 2 mol% MTO, 20 °C) proved to be satisfying for the selective conversion of imines 1f–k to the corresponding nitrones 2f–k as expected (Table 2, entries 1, 5, 7, 9, 11, and 13). The structural assignment was unequivocally based on the signals of N-H as a broad singlet and N-methyl as a doublet (δ = 2.8–3.1 ppm) in the 1H NMR spectrum, and by comparing with the literature data (see Supplementary Materials). Only in the case of p-nitrobenzaldimine 1j did the reaction not go to complete conversion (82% after 20 h, entry 11), showing that N-oxidation is slightly affected by electronic factors with electron-withdrawing groups slowing down the process. Moreover, in this case, and in the case of C-2-naphthyl substituted imine 1k, small amounts (ca. 6%) of amides 4j and 4k, respectively, were detected alongside the nitrones 2j and 2k (entries 11 and 13).
The best procedure for the oxidation of 1a in the ionic liquid [bmim]BF4 (6 M concentration of imine, 2 equiv. UHP, 1 mol% MTO, 50 °C) was employed with p-methoxybenzaldimine 1f, yielding the corresponding N-methyl amide 4f exclusively, thus confirming the selectivity observed for imine 1a, but with a moderate 45% conversion after 4 h (entry 2). Increasing the amount of MTO to 2 mol% and the reaction time to 48 h gave almost the same conversion (46%, entry 3), while carrying out the reaction at room temperature in a more dilute solution was deleterious even upon increasing the catalyst and oxidant amounts (entry 4). The conditions of entry 3 were then used in the oxidation of the other C-aryl,N-methyl imines 1g–k. In all cases, the reactions were completely selective, producing only the corresponding N-methyl amides 4g–k (entries 6, 8, 10, 12, and 14). However, none of the reactions went to completion, stopping at a 23–27% conversion for the more electron-rich aromatic rings (entries 8, 10, and 14) and a poorer 9–10% conversion when the aromatic ring brought the electron-withdrawing bromo and nitro substituents (entries 6 and 12).
3. Discussion and Conclusions
In this work, we addressed the controversial issue of selectivity in the oxidation of imines using hydrogen peroxide (utilized in the more easily manageable form of its complex with urea, UHP) as a sustainable oxidant in the presence of catalytic amounts of methyltrioxorhenium.
The results collected for the oxidation of a series of N-alkyl,C-aryl imines 1a–k (Table 1 and Table 2) have enabled us to definitely establish that when MeOH is used as the solvent, the oxidation gives the corresponding nitrones 2a–k with complete (or nearly complete) selectivity, except for the very hindered imine 1e substituted at nitrogen with the bulky tert-butyl group that failed to give any reaction. Therefore, these conditions represent a unique methodology for the conversion of imines into the corresponding nitrones, with a generality that is unparalleled by any other method and should be regarded as the method of choice for this functional group interconversion. It is worth stressing that this method, in contrast to other available oxidation methods from different substrates (amines [53], hydroxylamines [54]), yields nitrones with no regioselectivity issue, since the C=N double bond is already fixed in the starting imine. The only limitation for the application of this method found so far concerns imines unusually hindered at the nitrogen atom.
A strong dependence of the outcome of the oxidation reaction on the solvent used was highlighted. Indeed, the use of the ionic liquid solvent [bmim]BF4 completely inhibited the formation of nitrones. However, the reported formation of oxaziridines [56] was observed only in the case of N-tert-butyl imine 1e and the reaction proceeded sluggishly, giving a very low conversion after 20 h. For N-alkyl imines 1b–d with primary and secondary alkyl groups at nitrogen, no reaction was observed. Only N-methyl benzaldimine 1a gave an interesting switch of selectivity toward the formation of the corresponding N-methyl benzamide 4a. In principle, this might constitute a novel oxidative method for the preparation of N-methyl secondary amides. This behavior proved to be general for a series of C-aryl,N-methyl imines 1f–k (Table 2), but the reactivity was too low—especially with electron-poor aryl moieties—for the method to be practical. As a matter of fact, the choice of the solvent was the most critical parameter in the outcome of the MTO/UHP oxidation of imines. At this point, the reason for the observed switch in selectivity cannot be rationalized and any suggestion would be merely speculative.
Further studies are required and would require broadening the nature of ionic liquids, given the dependence of the reaction on its counterion and structure, in order to speed up this transformation and more precisely define the effect and role of the solvent. A critical dependence of both reactivity and selectivity in the oxidation of imines on the imine substituents and the solvent used has been commonly observed with other oxidation methods [2,69].
4. Materials and Methods
Commercial reagents were used as received. All reactions were carried out under magnetic stirring and monitored by TLC on 0.25 mm silica gel plates (Merck F254, Darmstadt, Germany). Column chromatography was carried out on silica gel (230–400 mesh, Merck, Darmstadt, Germany). Yields refer to spectroscopically and analytically pure compounds unless otherwise stated. 1H NMR spectra were recorded on a Varian Gemini-200 instrument at 25 °C. Chemical shifts are reported relative to CDCl3 (1H: 7.26 ppm). Integrals are in accordance with assignments; coupling constants are given in Hz. Splitting patterns are described by using the following abbreviations: br, broad; s, singlet; d, doublet; t, triplet; q, quartet; quint, quintet; m, multiplet.
General procedure for the oxidation of imines with UHP/MTO in MeOH (Method A):
To a stirred solution of the imine 1a–k (1 mmol) in MeOH (2 mL), UHP (282 mg, 3 mmol) and MTO (5 mg, 0.02 mmol) were added sequentially. The resulting yellow solution was stirred at room temperature and monitored by TLC (for reaction time, see Table 1 and Table 2). After the removal of the solvent under reduced pressure, the reaction mixture was added with CH2Cl2, and the undissolved urea was filtered off. Removal of the solvent yielded the crude product, which was purified by flash column chromatography on silica gel, if necessary.
General procedure for the oxidation of imines with UHP/MTO in [bmim]BF4 (Method B):
To a stirred solution of the imine 1a–k (3 mmol) in [bmim]BF4 (0.5 mL), UHP (564 mg, 6 mmol) and MTO (7.5 mg, 0.03 mmol) were added sequentially. The resulting yellow solution was stirred at 50 °C (for reaction time, see Table 1 and Table 2) and monitored via 1H NMR. The reaction mixture was extracted with AcOEt and the organic layer was washed with water (2 times). Finally, the organic layer was separated and dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure to yield the crude product.
General procedure for the oxidation of imines with UHP/MTO in [bmim]PF6 (Method C):
To a stirred solution of the imine 1a, 1b, or 1d (1 mmol) in [bmim]PF6 (2 mL), UHP (282 mg, 3 mmol) and MTO (5 mg, 0.02 mmol) were added sequentially. The resulting yellow solution was stirred at room temperature or 50 °C (for the reaction time, see Table 1) and monitored via 1H NMR. The reaction mixture was extracted with AcOEt and the organic layer was washed with water (2 times). Finally, the organic layer was separated and dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure to yield the crude product.
All the obtained products are known compounds reported in the literature, namely, nitrones 2a–d,f–k [70,71,72,73,74,75,76,77], oxaziridine 3e [78], and amides 4a,f–k [79,80,81,82]. Their 1H NMR spectral data are reported in the Supplementary Materials and are in agreement with the literature.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15040344/s1. General procedures for the synthesis of imines 1b–k; procedure for the oxidation of imine 1a with MTO/H2O2 in [bmim]BF4; procedure for the Oxidation of Imines with with UHP/MTO in MeOH (Method A); procedure for the Oxidation of Imines with with UHP/MTO in [bmim]BF4 (Method B); procedure for the Oxidation of Imines with with UHP/MTO in [bmim]BF6 (Method C); 1H NMR spectral data of nitrones 2a–d,f–k, oxaziridine 3e and amides 4a–d,f–k; representative 1H NMR spectra of crude reaction mixtures for the determination of conversions and product ratios reported in the Tables. Refs. [70,71,72,73,74,75,76,77,78,79,80,81,82] are mentioned in the Supplementary Materials.
Author Contributions
Conceptualization, A.G. and F.C.; methodology, A.G. and M.B.; formal analysis, A.G., C.M. and M.B.; data curation, C.M. and M.B.; writing—original draft preparation, A.G.; writing—review and editing, all authors; supervision, A.G.; funding acquisition, A.G. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Financial support provided by the MUR–Dipartimenti di Eccellenza 2023–2027 (DICUS 2.0) to the Department of Chemistry “Ugo Schiff” of the University of Florence is acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ogata, Y.; Sawaki, Y. Peracid Oxidation of Imines. Kinetics of Oxazirane Formation from Benzylidene-tert-butylamines and Perbenzoic Acid. J. Am. Chem. Soc. 1973, 95, 4687–4692. [Google Scholar] [CrossRef]
- Christensen, D.; Jørgensen, K.A. Oxidation of Imines to Nitrones by the Permanganate Ion. J. Org. Chem. 1989, 54, 126–131. [Google Scholar] [CrossRef]
- Boyd, D.R.; Coulter, P.B.; McGuckin, M.R.; Sharma, N.D.; Jennings, W.B.; Wilson, V.E. Imines and derivatives. Part 24. Nitrone synthesis by imine oxidation using either a peroxyacid or dimethyldioxirane. J. Chem. Soc. Perkin Trans. 1 1990, 1990, 301–306. [Google Scholar] [CrossRef]
- Kitagawa, O.; Vander Velde, D.; Dutta, D.; Morton, M.; Takusagawa, F.; Aubé, J. Structural Analysis of β-Tum Mimics Containing a Substituted 6-Aminocaproic Acid Linker. J. Am. Chem. Soc. 1995, 117, 5169–5178. [Google Scholar] [CrossRef]
- Davis, F.A.; Chattopadhyay, S.; Towson, J.C.; Lal, S.; Reddy, T. Chemistry of Oxaziridines. 9. Synthesis of 2-Sulfonyl- and 2-Sulfamyloxaziridines Using Potassium Peroxymonosulfate (Oxone). J. Org. Chem. 1988, 53, 2087–2089. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Pyne, S.G. A rapid and efficient synthesis of oxaziridines and diaryl nitrones using Oxone. J. Chem. Res. (S) 1992, 24, 388. [Google Scholar] [CrossRef]
- Mohajer, D.; Iranpoor, N.; Rezaeifard, A. Simple and highly efficient synthesis of oxaziridines by tetrabutylammonium Oxone®. Tetrahedron Lett. 2004, 45, 631–634. [Google Scholar] [CrossRef]
- Kluge, R.; Schulz, M.; Liebsch, S. Sulfonic Peracids—III. Heteroatom Oxidation and Chemoselectivity. Tetrahedron 1996, 52, 5773–5782. [Google Scholar] [CrossRef]
- Damavandi, J.A.; Karami, B.; Zolfigol, M.A. Selective Oxidation of N-Alkyl Imines to Oxaziridines using UHP/Maleic Anhydride System. Synlett 2002, 6, 933–934. [Google Scholar] [CrossRef]
- Kraïem, J.; Ben Othman, R.; Ben Hassine, B. Synthesis of oxaziridines by oxidation of imines with the trichloroacetonitrile–hydrogen peroxide system. Comptes Rendus Chim. 2004, 7, 1119–1126. [Google Scholar] [CrossRef]
- Shailaja, M.; Manjula, A.; Rao, B.V. An inexpensive and selective oxygenation of N-alkyl imines to oxaziridines. Synlett 2005, 7, 1176–1178. [Google Scholar] [CrossRef]
- Singhal, S.; Jain, S.L.; Prasad, V.V.D.N.; Sain, B. An Environmentally Friendly Oxidation System for the Selective Oxygenation of Aldimines to Oxaziridines with Anhydrous TBHP and Alumina-SupportedMoO3 as a Recyclable Heterogeneous Catalyst. Eur. J. Org. Chem. 2007, 2007, 2051–2054. [Google Scholar] [CrossRef]
- Martiny, L.; Jørgensen, K.A. Oxidation of imines to oxaziridines catalysed by transition metal complexes using molecular oxygen as the terminal oxidant. J. Chem. Soc. Perkin Trans. 1 1995, 1995, 699–704. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Miller, M.J. Oxidation of Primary Amines to Oxaziridines Using Molecular Oxygen (O2) as the Ultimate Oxidant. J. Org. Chem. 2001, 66, 8282–8285. [Google Scholar] [CrossRef]
- Larsen, J.; Jørgensen, K.A.; Christensen, D. Duality of the permanganate ion in the oxidation of imines. Oxidation of imines to amides. J. Chem. Soc. Perkin Trans. 1 1991, 1991, 1187–1190. [Google Scholar] [CrossRef]
- Busqué, F.; de March, P.; Figueredo, M.; Font, J.; Gallagher, T.; Milán, S. Efficient synthesis of (S)-3,4-dihydro-2-pivaloyloxymethyl-2H-pyrrole 1-oxide. Tetrahedron Asymmetry 2002, 13, 437–445. [Google Scholar] [CrossRef]
- Nongkunsarn, P.; Ramsden, C.A. Oxidative Rearrangement of Imines to Formamides using Sodium Perborate. Tetrahedron 1997, 53, 3805–3830. [Google Scholar] [CrossRef]
- An, G.-I.; Kim, M.; Kim, J.Y.; Rhee, H. Oxidation of aldimines to amides by m-CPBA and BF3·OEt2. Tetrahedron Lett. 2003, 44, 2183–2186. [Google Scholar] [CrossRef]
- Llopis, N.; Gisbert, P.; Baeza, A. Direct Synthesis of N,N-Disubstituted Formamides by Oxidation of Imines Using an HFIP/UHP System. J. Org. Chem. 2020, 85, 11072–11079. [Google Scholar] [CrossRef]
- Murahashi, S.-I.; Imada, Y. Synthesis and Transformations of Nitrones for Organic Synthesis. Chem. Rev. 2019, 119, 4684–4716. [Google Scholar] [CrossRef]
- Breuer, E. Nitrones and nitronic acid derivatives: Their structure and their roles in synthesis. In The Chemistry of Amino, Nitroso and Nitro Compounds and Their Derivatives—Part 1; Patai, S., Ed.; Wiley Interscience: New York, NY, USA, 1982; pp. 460–564. [Google Scholar]
- Grigor’ev, I.A. Nitrones: Novel Strategies in Synthesis. In Nitrile Oxides, Nitrones, and Nitronates in Organic Synthesis; Feuer, H., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 129–434. [Google Scholar]
- Tufariello, J.J. Nitrones. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; John Wiley & Sons: New York, NY, USA, 1984; Volume 2, pp. 83–168. [Google Scholar]
- Frederickson, M. Optically active isoxazolidines via asymmetric cycloaddition reactions of nitrones with alkenes: Applications in organic synthesis. Tetrahedron 1997, 53, 403–425. [Google Scholar] [CrossRef]
- Gothelf, K.V.; Jørgensen, K.A. Asymmetric 1,3-Dipolar Cycloaddition Reactions. Chem. Rev. 1998, 98, 863–909. [Google Scholar] [CrossRef] [PubMed]
- Goti, A.; Cicchi, S.; Cordero, F.M.; Fedi, V.; Brandi, A. A Straightforward Route to Enantiopure Pyrrolizidines and Indolizidines by Cycloaddition to Pyrroline N-Oxides Derived from the Chiral Pool. Molecules 1999, 4, 1–12. [Google Scholar] [CrossRef]
- Jones, R.C.F.; Martin, J.N. Nitrones. In Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; The Chemistry of Heterocyclic Compounds; Padwa, A., Pearson, W.H., Eds.; John Wiley & Sons: New York, NY, USA, 2002; Volume 59, pp. 1–81. [Google Scholar] [CrossRef]
- Brandi, A.; Cardona, F.; Cicchi, S.; Cordero, F.M.; Goti, A. [3 + 2] Dipolar Cycloadditions of Cyclic Nitrones with Alkenes. Org. React. 2017, 94, 1–529. [Google Scholar] [CrossRef]
- Koumbis, A.E.; Gallos, J.K. 1,3-Dipolar Cycloadditions in the Synthesis of Carbohydrate Mimics. Part 2: Nitrones and Oximes. Curr. Org. Chem. 2003, 7, 585–628. [Google Scholar] [CrossRef]
- Bilodeau, D.A.; Margison, K.D.; Serhan, M.; Pezacki, J.P. Bioorthogonal Reactions Utilizing Nitrones as Versatile Dipoles in Cycloaddition Reactions. Chem. Rev. 2021, 121, 6699–6717. [Google Scholar] [CrossRef]
- Brandi, A.; Cardona, F.; Cicchi, S.; Cordero, F.M.; Goti, A. Stereocontrolled Cyclic Nitrone Cycloaddition Strategy for the Synthesis of Pyrrolizidine and Indolizidine Alkaloids. Chem. Eur. J. 2009, 15, 7808–7821. [Google Scholar] [CrossRef]
- Bloch, R. Additions of Organometallic Reagents to C = N Bonds: Reactivity and Selectivity. Chem. Rev. 1998, 98, 1407–1438. [Google Scholar] [CrossRef]
- Enders, D.; Reinhold, U. Asymmetric synthesis of amines by nucleophilic 1,2-addition of organometallic reagents to the CN-double bond. Tetrahedron Asymmetry 1997, 8, 1895–1946. [Google Scholar] [CrossRef]
- Lombardo, M.; Trombini, C. Nucleophilic Additions to Nitrones. Synthesis 2000, 2000, 759–774. [Google Scholar] [CrossRef]
- Merino, P.; Franco, S.; Merchán, F.L.; Tejero, T. Nucleophilic Additions to Chiral Nitrones: New Approaches to Nitrogenated Compounds. Synlett 2000, 2000, 442–454. [Google Scholar] [CrossRef]
- Lombardo, M.; Trombini, C. The Reaction of Nitrones with Organometallic Compounds: Scope, Limitations and Synthetic Applications. Curr. Org. Chem. 2002, 6, 695–713. [Google Scholar] [CrossRef]
- Merino, P. New developments in nucleophilic additions to nitrones. Comptes Rendus Chim. 2005, 8, 775–788. [Google Scholar] [CrossRef]
- Cardona, F.; Goti, A. The Discovery of Novel Metal-Induced Reactions of Nitrones: Not Only Electrophiles and Reagents for [3+2]Cycloadditions. Angew. Chem. Int. Ed. 2005, 44, 7832–7835. [Google Scholar] [CrossRef]
- Janzen, E.G. A critical review of spin trapping in biological systems. In Free Radicals in Biology; Pryor, W.A., Ed.; Academic Press: New York, NY, USA, 1980; pp. 115–154. [Google Scholar]
- Janzen, E.G.; Haire, D.L. Two decades of spin-trapping. In Advances in Free Radical Chemistry; Tanner, D.D., Ed.; JAI Press: Greenwich, CT, USA, 1990; Volume 1, pp. 253–295. [Google Scholar]
- Frejaville, C.; Karoui, H.; Tuccio, B.; Le Moigne, F.; Culcasi, M.; Pietri, S.; Lauricella, R.; Tordo, P. 5-(Diethoxyphosphoryl)-5-methyl-l-pyrroline N-Oxide: A New Efficient Phosphorylated Nitrone for the in Vitro and in Vivo Spin Trapping of Oxygen-Centered Radicals. J. Med. Chem. 1995, 38, 258–265. [Google Scholar] [CrossRef]
- Fevig, T.L.; Bowen, S.M.; Janowick, D.A.; Jones, B.K.; Munson, H.R.; Ohlweiler, D.F.; Thomas, C.E. Design, Synthesis, and in Vitro Evaluation of Cyclic Nitrones as Free Radical Traps for the Treatment of Stroke. J. Med. Chem. 1996, 39, 4988–4996. [Google Scholar] [CrossRef]
- Morozov, D.A.; Kirilyuk, I.A.; Komarov, D.A.; Goti, A.; Bagryanskaya, I.Y.; Kuratieva, N.V.; Grigor’ev, I.A. Synthesis of a Chiral C2-Symmetric Sterically Hindered Pyrrolidine Nitroxide Radical via Combined Iterative Nucleophilic Additions and Intramolecular 1,3-Dipolar Cycloadditions to Cyclic Nitrones. J. Org. Chem. 2012, 77, 10688–10698. [Google Scholar] [CrossRef]
- Floyd, R.A.; Kopke, R.D.; Choi, C.-H.; Foster, S.B.; Doblas, S.; Towner, R.A. Nitrones as therapeutics. Free Radic. Biol. Med. 2008, 45, 1361–1374. [Google Scholar] [CrossRef]
- Villamena, F.A.; Das, A.; Nash, K.M. Potential implication in the chemical properties and bioactivity of nitrone spin traps for therapeutics. Future Med. Chem. 2012, 4, 1171–1207. [Google Scholar] [CrossRef]
- Rosselin, M.; Poeggeler, B.; Durand, G. Nitrone Derivatives as Therapeutics: From Chemical Modification to Specific-targeting. Curr. Top. Med. Chem. 2017, 17, 2006–2022. [Google Scholar] [CrossRef]
- Floyd, R.A. Nitrones as therapeutics in age-related diseases. Aging Cell 2006, 5, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Marco-Contelles, J. Recent Advances on Nitrones Design for Stroke Treatment. J. Med. Chem. 2020, 63, 13413–13427. [Google Scholar] [CrossRef] [PubMed]
- Soldaini, G.; Cardona, F.; Goti, A. Catalytic Oxidation of Imines Based on Methyltrioxorhenium/Urea Hydrogen Peroxide: A Mild and Easy Chemo- and Regioselective Entry to Nitrones. Org. Lett. 2007, 9, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Heaney, H. Novel Organic Peroxygen Reagents for Use in Organic Synthesis. Top. Curr. Chem. 1993, 164, 1–19. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Kühn, F.E. Organorhenium Oxides. Acc. Chem. Res. 1997, 30, 169–180. [Google Scholar] [CrossRef]
- Espenson, J.H. Atom-transfer reactions catalyzed by methyltrioxorhenium(VII)—Mechanisms and applications. Chem. Commun. 1999, 1999, 479–488. [Google Scholar] [CrossRef]
- Nannelli, L.; Goti, A. Synthesis of Nitrones by Methyltrioxorhenium Catalyzed Direct Oxidation of Secondary Amines. Tetrahedron Lett. 1996, 37, 6025–6028. [Google Scholar] [CrossRef]
- Saladino, R.; Neri, V.; Cardona, F.; Goti, A. Oxidation of N,N-Disubstituted Hydroxylamines to Nitrones with Hydrogen Peroxide Catalyzed by Polymer-Supported Methylrhenium Trioxide Systems. Adv. Synth. Catal. 2004, 346, 639–647. [Google Scholar] [CrossRef]
- Cardona, F.; Bonanni, M.; Soldaini, G.; Goti, A. One-Pot Synthesis of Nitrones from Primary Amines and Aldehydes Catalyzed by Methyltrioxorhenium. ChemSusChem 2008, 1, 327–332. [Google Scholar] [CrossRef]
- Jain, S.L.; Singhal, S.; Sain, B. [Bmim]BF4-immobilized rhenium-catalyzed highly efficient oxygenation of aldimines to oxaziridines using solid peroxides as oxidants. J. Organomet. Chem. 2007, 692, 2930–2935. [Google Scholar] [CrossRef]
- Kamath, V.P.; Xue, J.; Juarez-Brambila, J.J.; Morris, C.B.; Ganorkar, R.; Morris, P.E., Jr. Synthesis of analogs of forodesine HCl, a human purine nucleoside phosphorylase inhibitor—Part I. Bioorg. Med. Chem. Lett. 2009, 19, 2624–2626. [Google Scholar] [CrossRef] [PubMed]
- Diez-Martinez, A.; Gultekin, Z.; Delso, I.; Tejero, T.; Merino, P. Synthesis of N-(Benzyloxyethyl)- and N-(Alkoxycarbonylmethyl)nitrones. Synthesis 2010, 4, 678–688. [Google Scholar] [CrossRef]
- Davis, F.A.; Theddu, N.; Edupuganti, R. Asymmetric Total Synthesis of (S)-(+)-Cocaine and the First Synthesis of Cocaine C-1 Analogs from N-Sulfinyl β-Amino Ester Ketals. Org. Lett. 2010, 12, 4118–4121. [Google Scholar] [CrossRef]
- Dong, C.; Dickie, D.A.; Maio, W.A.; Manz, T.A. Synthesis and Characterization of N,N′-Bismesityl Phenanthrene-9,10-diimine and Imine−Nitrone. ACS Omega 2018, 3, 16858–16865. [Google Scholar] [CrossRef]
- Biyani, S.A.; Lytle, C.; Hyun, S.-H.; McGuire, M.A.; Pendyala, R.; Thompson, D.H. Development of a Continuous Flow Synthesis of Lorazepam. Org. Process Res. Dev. 2022, 26, 2715–2727. [Google Scholar] [CrossRef]
- Najjar, R.; Safa, K.D. Methyltrioxorhenium Catalyzed Synthesis of Dinitrones from Primary Diamines and Non-Enolizable Aldehydes. Lett. Org. Chem. 2011, 8, 495–499. [Google Scholar] [CrossRef]
- Merino, P.; Greco, G.; Tejero, T.; Hurtado-Guerrero, R.; Matute, R.; Chiacchio, U.; Corsaro, A.; Pistarà, V.; Romeo, R. Stereoselective 1,3-dipolar cycloadditions of nitrones derived from amino acids. Asymmetric synthesis of N-(alkoxycarbonylmethyl)-3-hydroxypyrrolidin-2-ones. Tetrahedron 2013, 69, 9381–9390. [Google Scholar] [CrossRef]
- Xue, F.; Lu, H.; He, L.; Li, W.; Zhang, D.; Liu, X.-Y.; Qin, Y. Formal Total Syntheses of (−)- and (+)-Actinophyllic Acid. J. Org. Chem. 2018, 83, 754–764. [Google Scholar] [CrossRef]
- Clemente, F.; Matassini, C.; Giachetti, S.; Goti, A.; Morrone, A.; Martínez-Bailén, M.; Orta, S.; Merino, P.; Cardona, F. Piperidine Azasugars Bearing Lipophilic Chains: Stereoselective Synthesis and Biological Activity as Inhibitors of Glucocerebrosidase (GCase). J. Org. Chem. 2021, 86, 12745–12761. [Google Scholar] [CrossRef]
- Singh, B.; Jain, S.L.; Rana, B.S.; Khatri, P.K.; Sinha, A.K.; Sain, B. Silica-Immobilized Highly Dispersed Oxo–Rhenium and its Catalytic Activity for the Direct Synthesis of Nitrones. ChemCatChem 2010, 2, 1260–1264. [Google Scholar] [CrossRef]
- Leung, C.H.; Voutchkova, A.M.; Crabtree, R.H.; Balcells, D.; Eisenstein, O. Atom economic synthesis of amides via transition metal catalyzed rearrangement of oxaziridines. Green Chem. 2007, 9, 976–979. [Google Scholar] [CrossRef]
- Pews, R.G. A Novel Synthesis of 3-Phenyloxaziridines. J. Org. Chem. 1967, 32, 1628. [Google Scholar] [CrossRef]
- Karami, B.; Montazerozohori, M.; Moghadam, M.; Farahi, M. Iron and manganese (III)—Porphyrins as new applicable catalysts for selective oxidation of imines with urea–hydrogen peroxide. J. Chem. Res. 2007, 2007, 275–277. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, K.; Chen, G. Synthesis and Application of Phenyl Nitrone Derivatives as Acidic and Microbial Corrosion Inhibitors. J. Chem. 2015, 2015, 201259. [Google Scholar] [CrossRef]
- Colonna, S.; Pironti, V.; Carrea, G.; Pasta, P.; Zambianchi, F. Oxidation of secondary amines by molecular oxygen and cyclohexanone monooxygenase. Tetrahedron 2004, 60, 569–575. [Google Scholar] [CrossRef]
- Ballard, N.; Aguirre, M.; Simula, A.; Agirre, A.; Leiza, J.R.; Asua, J.M.; van Es, S. New Class of Alkoxyamines for Efficient Controlled Homopolymerization of Methacrylates. ACS Macro Lett. 2016, 5, 1019–1022. [Google Scholar] [CrossRef]
- Chan, K.S.; Yeung, M.L.; Chan, W.-k.; Wang, R.-J.; Mak, T.C.W. Chromium and Tungsten Pentacarbonyl Groups as Reactivity and Selectivity Auxiliaries in [3 + 2] Cycloaddition of Alkynyl Fischer Carbene Complexes with N-Alkyl Nitrones. J. Org. Chem. 1995, 60, 1741–1747. [Google Scholar] [CrossRef]
- Dias, A.G.; Santos, C.E.V.; Cyrino, F.Z.G.A.; Bouskela, E.; Costa, P.R.R. N-tert-Butyl and N-methyl nitrones derived from aromatic aldehydes inhibit macromolecular permeability increase induced by ischemia/reperfusion in hamsters. Bioorg. Med. Chem. 2009, 17, 3995–3998. [Google Scholar] [CrossRef]
- Poulsen, P.H.; Vergura, S.; Monleón, A.; Jørgensen, D.K.B.; Jørgensen, K.A. Controlling Asymmetric Remote and Cascade 1,3-Dipolar Cycloaddition Reactions by Organocatalysis. J. Am. Chem. Soc. 2016, 138, 6412–6415. [Google Scholar] [CrossRef]
- Hammami, R.; Maldivi, P.; Philouze, C.; Carret, S.; Darses, B.; Touil, S.; Poisson, J.-F. Synthesis of 4-Phosphinylpyrrolidin-3-ones via [3+2] Cycloaddition of Nitrones with Phosphinylallenes. Adv. Synth. Catal. 2023, 365, 1385–1390. [Google Scholar] [CrossRef]
- Li, T.-Z.; Liu, S.-J.; Sun, Y.-W.; Deng, S.; Tan, W.; Jiao, Y.; Zhang, Y.-C.; Shi, F. Regio- and Enantioselective (3+3) Cycloaddition of Nitrones with 2-Indolylmethanols Enabled by Cooperative Organocatalysis. Angew. Chem. Int. Ed. 2021, 60, 2355–2363. [Google Scholar] [CrossRef] [PubMed]
- Boudou, C.; Berges, M.; Sagnes, C.; Sopkova-De Oliveira Santos, J.; Perrio, S.; Metzner, P. trans-(±)-2-tert-Butyl-3-phenyloxaziridine: A Unique Reagent for the Oxidation of Thiolates into Sulfenates. J. Org. Chem. 2007, 72, 5403–5406. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Ju, J.; Choe, J.; Song, K.H.; Lee, S. The Scope and Limitation of Nickel-Catalyzed Aminocarbonylation of Aryl Bromides from Formamide Derivatives. J. Org. Chem. 2009, 74, 6358–6361. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, X.; Zhang, Y.; Chen, C.; Chen, W. Copper-Catalyzed N-Methylation of Amides and O-Methylation of Carboxylic Acids by Using Peroxides as the Methylating Reagents. Org. Lett. 2013, 15, 3326–3329. [Google Scholar] [CrossRef]
- Scott, G.D. Method Using Lifespan-Altering Compounds for Altering the Lifespan of Eukaryotic Organisms, and Screening for such Compounds. US20090163545 A1, 25 June 2009. [Google Scholar]
- Wen, X.; Chen, W.; Chen, J. Nickel-catalyzed aminocarbonylation of aryl halides with carbamoylsilanes: Efficient synthesis of secondary (primary) aromatic amides. Appl. Organomet. Chem. 2019, 33, e5174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).