Study on the Immobilization of a Transaminase Biocatalyst for the Synthesis of Sitagliptin
Abstract
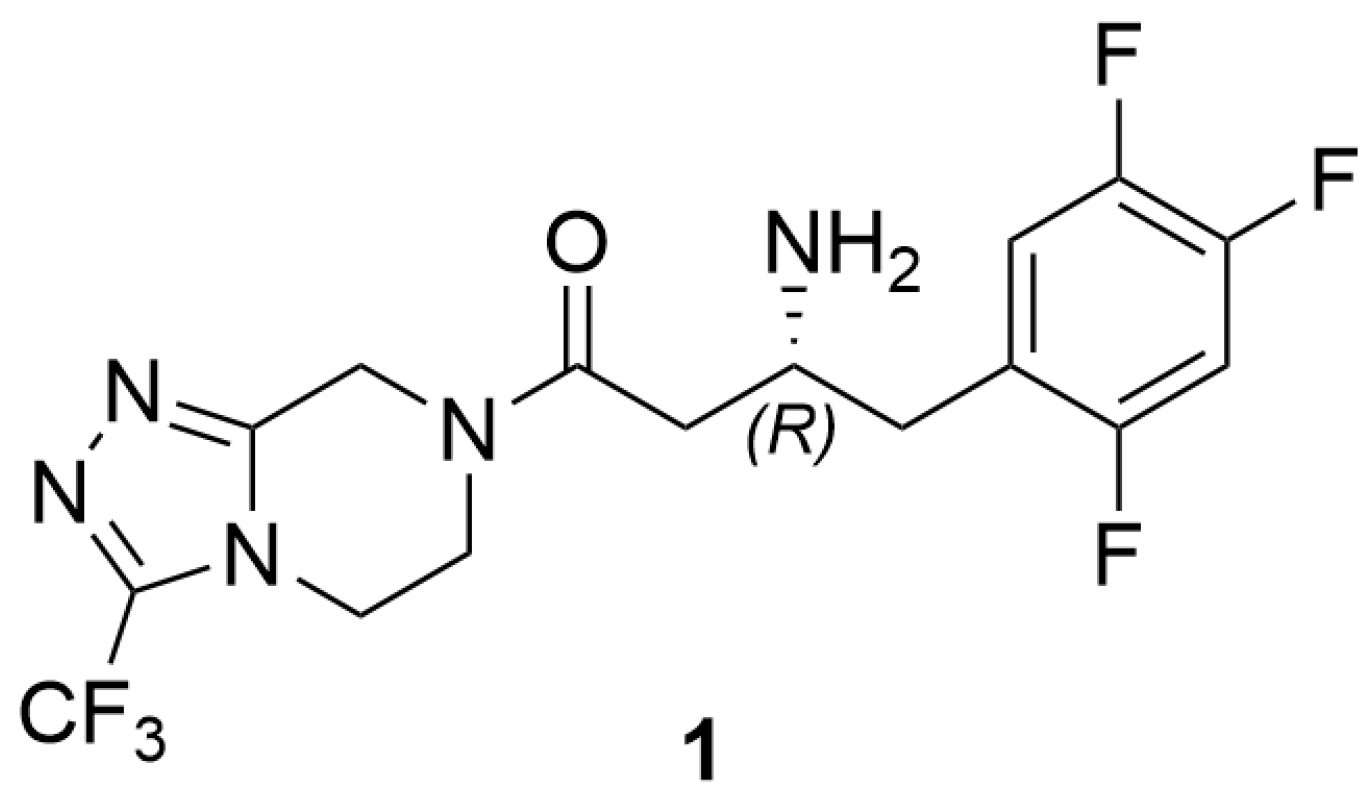
1. Introduction
2. Results and Discussion
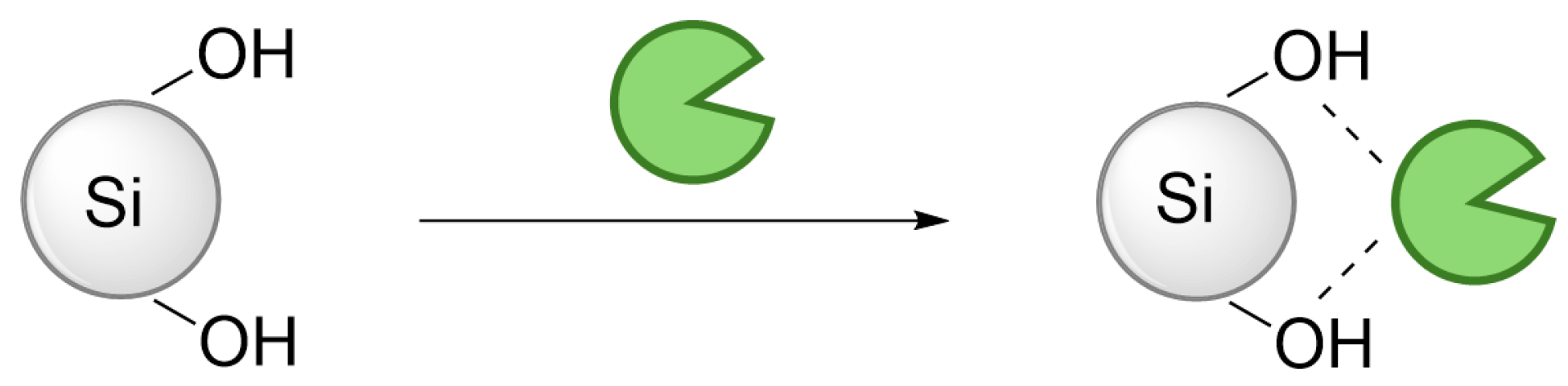
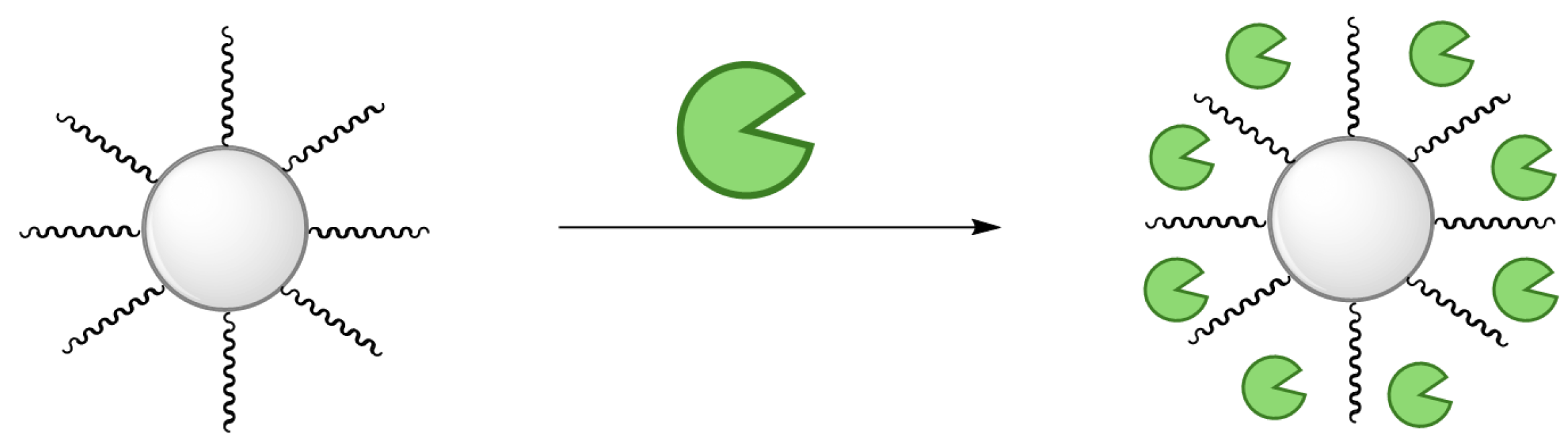
2.1. Immobilization of EMIN041
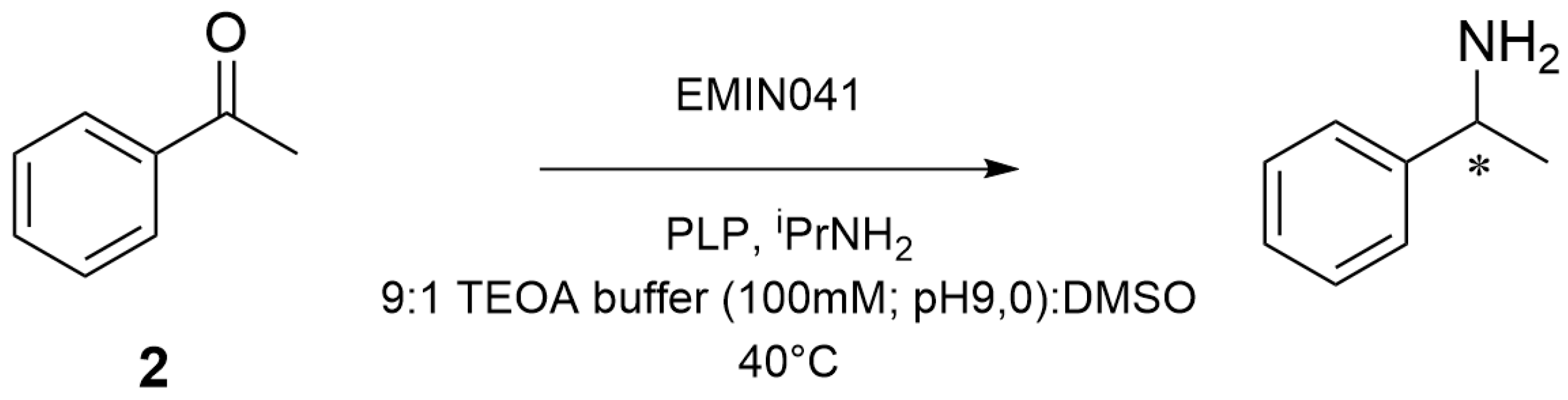
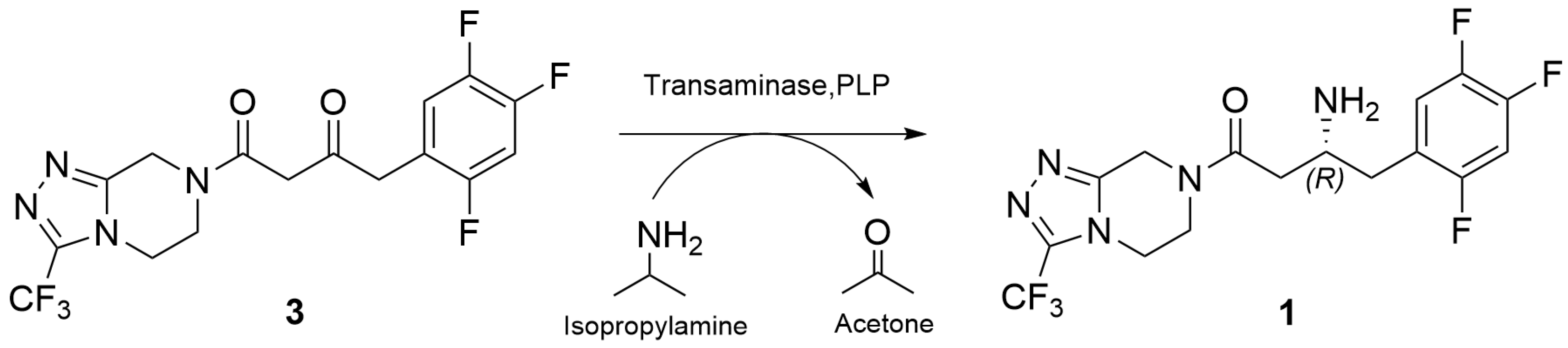
2.2. Enantioselective Transamination of Pro-Sitagliptin Ketone Under Batch Conditions
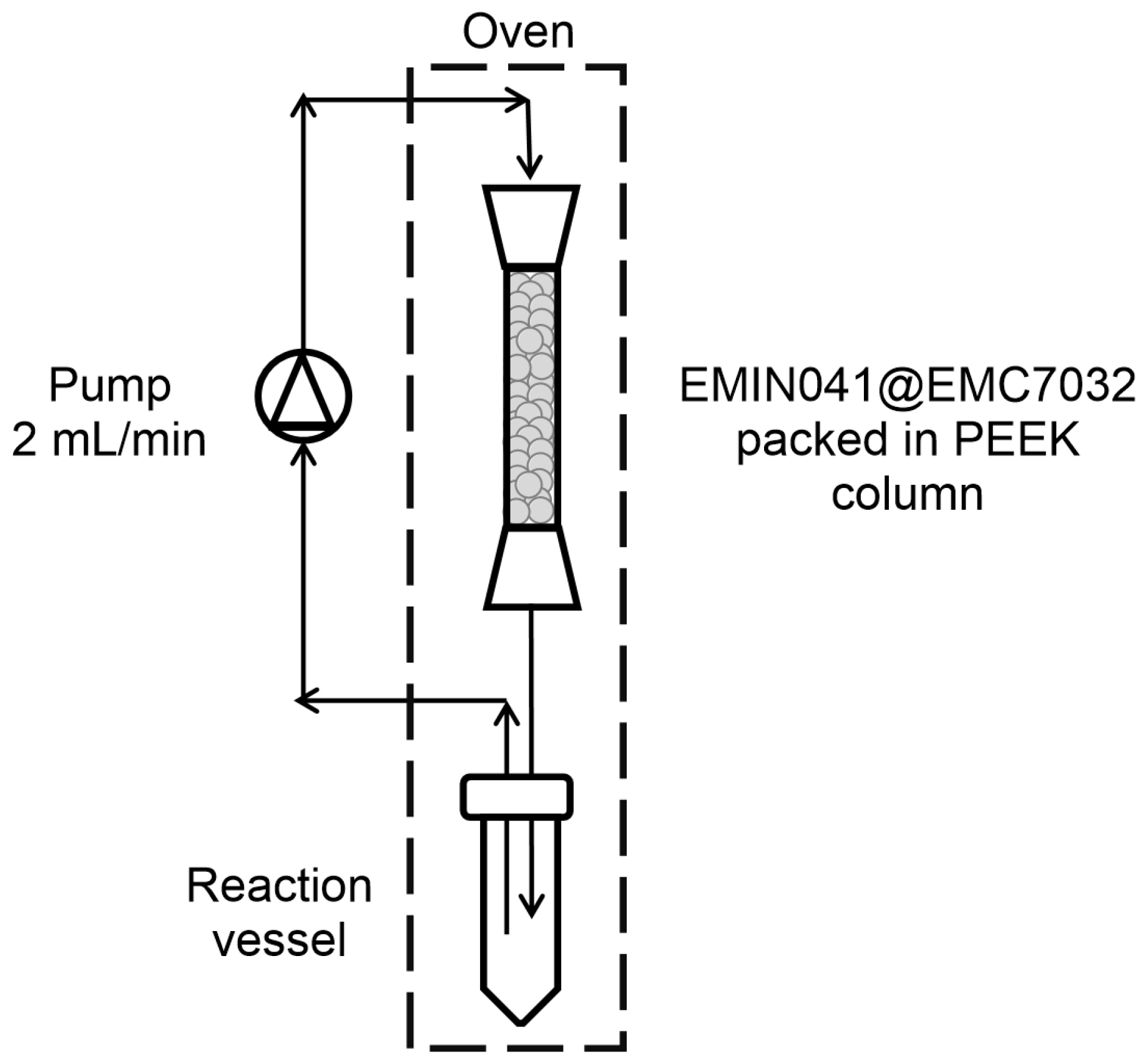
2.3. Enantioselective Transamination of Pro-Sitagliptin Ketone Under Flow Conditions
3. Materials and Methods
3.1. Materials
3.2. HPLC Analysis
3.3. General Transaminase EMIN041 Immobilization Procedure
3.3.1. Immobilization onto Silica Gel
3.3.2. Immobilization onto Octadecyl-Functionalized Resin
3.3.3. Immobilization onto Epoxy-Functionalized Resin
3.3.4. Immobilization onto Amino-Functionalized Resin
3.4. Transamination Reaction with Soluble Transaminase EMIN041
3.5. Transamination Reaction with Immobilized Transaminase EMIN041
3.5.1. Transamination Under Batch Conditions
3.5.2. Transamination Under Flow Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DEA | Diethyl amine |
| DMSO | Dimethyl sulfoxide |
| EtOH | Ethanol |
| HEX | Hexane |
| iPrNH2 | Isopropyl amine |
| MeCN | Acetonitrile |
| PEEK | Polyether ether ketone |
| PLP | Pyridoxal phosphate |
| TEOA | Triethanolamine |
| TFA | Trifluoroacetic acid |
References
- Bell, E.L.; Finnigan, W.; France, S.P.; Green, A.P.; Hayes, M.A.; Hepworth, L.J.; Lovelock, S.L.; Niikura, H.; Osuna, S.; Romero, E.; et al. Biocatalysis. Nat. Rev. Methods Primers 2021, 1, 46. [Google Scholar]
- Zawodny, W.; Montgomery, S.L. Evolving New Chemistry: Biocatalysis for the Synthesis of Amine-Containing Pharmaceuticals. Catalysts 2022, 12, 595. [Google Scholar] [CrossRef]
- Pandya, S.; Gupte, A. Transaminases for Green Chemistry: Recent Progress and Future Prospects. Microbiol. Biotechnol. Lett. 2023, 51, 333–352. [Google Scholar]
- Kelly, S.A.; Pohle, S.; Wharry, S.; Mix, S.; Allen, C.C.R.; Moody, T.S.; Gilmore, B.F. Application of ω-transaminases in the pharmaceutical industry. Chem. Rev. 2018, 118, 349–367. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Berglund, P. Transaminase biocatalysis: Optimization and application. Green Chem. 2017, 19, 333–360. [Google Scholar] [CrossRef]
- Gomm, A.; O’Reilly, E. Transaminases for chiral amine synthesis. Curr. Opin. Chem. Biol. 2018, 43, 106–112. [Google Scholar] [PubMed]
- Ho, C.-H.; Yi, J.; Wang, X. Biocatalytic Continuous Manufacturing of Diabetes Drug: Plantwide Process Modeling, Optimization, and Environmental and Economic Analysis. ACS Sustain. Chem. Eng. 2019, 7, 1038–1051. [Google Scholar]
- Khanham, W.; Dubey, N.C. Recent advances in immobilized ω-transaminase for chiral amine synthesis. Mater. Today Chem. 2022, 24, 100922. [Google Scholar]
- Available online: https://www.januvia.com/ (accessed on 27 February 2025).
- Savile, C.K.; Janey, J.M.; Mundorff, E.C.; Moore, J.C.; Tam, S.; Jarvis, W.R.; Colbeck, J.C.; Krebber, A.; Fleitz, F.J.; Brands, J.; et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 2010, 329, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, J.; Ding, X.; Wang, P. Efficient enantioselective biocatalytic production of a chiral intermediate of sitagliptin by a newly Filamentous fungus isolate. Appl. Biochem. Biotech. 2016, 180, 695–706. [Google Scholar]
- Truppo, M.D.; Rozzell, J.D.; Turner, N.J. Efficient production of enantiomerically pure chiral amines at concentrations of 50 g/L using transaminases. Org. Process Res. Dev. 2010, 14, 234–237. [Google Scholar]
- Truppo, M.D.; Strotman, H.; Hughes, G. Development of an Immobilized Transaminase Capable of Operating in Organic Solvent. ChemCatChem 2012, 4, 1071–1074. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, H.; Liu, N.; Wang, X.; Cheng, F.; Liu, Z.; Zheng, Y. A novel self-sufficient biocatalyst based on transaminase and pyridoxal 5′-T phosphate covalent co-immobilization and its application in continuous biosynthesis of sitagliptin. Enz. Microb. Technol. 2019, 130, 109362. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhou, J.; Liu, T.; Zhao, W.; Zhang, X.; Men, D. Self-Assembled Enzymatic Nanowires with a “Dry and Wet” Interface Improve the Catalytic Performance of Januvia Transaminase in Organic Solvent. ACS Catal. 2022, 12, 372–382. [Google Scholar]
- Ma, L.; Li, J.; Chen, D.; He, Y.; Chen, B.; Xia, J.; Li, J.; Tang, H.; Wang, L.; Li, Z. Green Synthesis of Chiral Beta Amino Acids Using Biological Enzymes and Immobilized Aminotransferase Used. CN118063351, 28 June 2024. [Google Scholar]
- Liu, Z.; Wang, X.; Liu, N.; Zhang, X.; Zheng, Y. Method for Co-Immobilizing Aminotransferase by Liposome Embedding-Metal Framework and Co-Immobilized Aminotransferase. CN118028285, 28 March 2024. [Google Scholar]
- Dong, Z.; Han, R.; Lyu, X.; Wu, M. Transaminase, Immobilized Transaminase and Application Thereof. CN117946992, 2 February 2024. [Google Scholar]
- Chen, B.; Zhong, Z.; Yan, J.; Qu, Y.; Ma, L. High Chiral Purity Sitagliptin and Method for Preparing Same by Using Immobilized Transaminase. CN117467733, 12 March 2024. [Google Scholar]
- Liu, Z.; Huang, M.; Zhang, X.; Zheng, Y.; Liu, N. Method for Covalently Immobilizing Transaminase by Using Aminated Carbon Nano Tube. CN117286130, 10 January 2023. [Google Scholar]
- Rao, J.; Wang, Z.; Yue, Y.; Shen, Y.; Lu, F.; Lin, L.; Ke, S. Preparation Method of Sitagliptin Phosphate Monohydrate. CN116694697, 25 April 2023. [Google Scholar]
- Jiao, Q.; Huang, Y.; Tian, Z.; Wang, S.; Cheng, H.Z.; Sun, C. Transaminase, Immobilized Transaminase and Use for Preparing Sitagliptin. WO2022257686, 15 December 2022. [Google Scholar]
- Hong, H.; Gage, J.; Zhang, N.; Rojas, V.W.; Cui, Y.; Zhao, J.; Zhang, C. Immobilized Enzyme, Preparation Method and Application Thereof. CN114606221, 2 September 2022. [Google Scholar]
- Chen, H.; Bong, Y.; Wang, J.; Cai, B.; Shang, C.; Bocola, M. Engineered Transaminase Polypeptides and Uses Thereof. WO2019128894A1, 4 July 2019. [Google Scholar]
- Armani, D.; Piccolo, O.; Petri, A. Immobilization of Alcohol Dehydrogenases on Silica-Based Supports and Their Application in Enantioselective Ketone Reductions. Catalysts 2024, 14, 148. [Google Scholar] [CrossRef]
- Armani, D.; Piccolo, O.; Petri, A. Biocatalytic Asymmetric Synthesis of (S)-1[3,5-bis (trifluoromethyl) phenyl] ethanol by an Immobilized KRED in Batch and Flow Conditions. ChemCatChem 2023, 15, e202300809. [Google Scholar]
- Sheldon, R.A.; Basso, A.; Brady, D. New frontiers in enzyme immobilization: Robust biocatalysts for a circular bio-based economy. Chem. Soc. Rev. 2021, 50, 5850–5862. [Google Scholar] [PubMed]
- Arango, H.M.; van den Biggelaar, L.; Soumillion, P.; Luis, P.; Leyssens, T.; Paradisi, F.; Debecker, D.P. Continuous flow-mode synthesis of (chiral) amines with transaminase: A strategic biocatalytic approach to essential building blocks. React. Chem. Eng. 2023, 8, 1505–1544. [Google Scholar]
- Petri, A.; Colonna, V.; Piccolo, O. Asymmetric Synthesis of a High Added Value Chiral Amine Using Immobilized ω-Transaminases. Beilstein J. Org. Chem. 2019, 15, 60–66. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochemistry 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]




| Entry | TA | Conversion (%) 1 |
|---|---|---|
| 1 | EMIN041 | 33.6 |
| 2 | EMIN041@SiO2 | 59.4 |
| 3 | EMIN041@ECR8806 | 58.1 |
| 4 | EMIN041@ECR8215 | 38.9 |
| 5 | EMIN041@EMC7032 | 37.5 |
| 6 | EMIN041@ECR8309 | 26.1 |
| 7 | EMIN041@ECR8409 | 22.7 |
| Entry | TA | Conversion (%) 1 | ee (%) 1 |
|---|---|---|---|
| 1 | EMIN041 | 99.0 | >99 |
| 2 | EMIN041@SiO2 | 35.5 | >99 |
| 3 | EMIN041@ECR8806 | 5 | - |
| 4 | EMIN041@ECR8215 | 98 | >99 |
| 5 | EMIN041@EMC7032 | 100 | >99 |
| 6 | EMIN041@ECR8309 | 21.3 | >99 |
| 7 | EMIN041@ECR8409 | 34.5 | >99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosati, C.; Piccolo, O.; Petri, A. Study on the Immobilization of a Transaminase Biocatalyst for the Synthesis of Sitagliptin. Catalysts 2025, 15, 326. https://doi.org/10.3390/catal15040326
Rosati C, Piccolo O, Petri A. Study on the Immobilization of a Transaminase Biocatalyst for the Synthesis of Sitagliptin. Catalysts. 2025; 15(4):326. https://doi.org/10.3390/catal15040326
Chicago/Turabian StyleRosati, Chiara, Oreste Piccolo, and Antonella Petri. 2025. "Study on the Immobilization of a Transaminase Biocatalyst for the Synthesis of Sitagliptin" Catalysts 15, no. 4: 326. https://doi.org/10.3390/catal15040326
APA StyleRosati, C., Piccolo, O., & Petri, A. (2025). Study on the Immobilization of a Transaminase Biocatalyst for the Synthesis of Sitagliptin. Catalysts, 15(4), 326. https://doi.org/10.3390/catal15040326








