Tuning the Surface Oxophilicity of PdAu Alloy Nanoparticles to Favor Electrochemical Reactions: Hydrogen Oxidation and Oxygen Reduction in Anion Exchange Membrane Fuel Cells
Abstract
1. Introduction
2. Results and Discussion
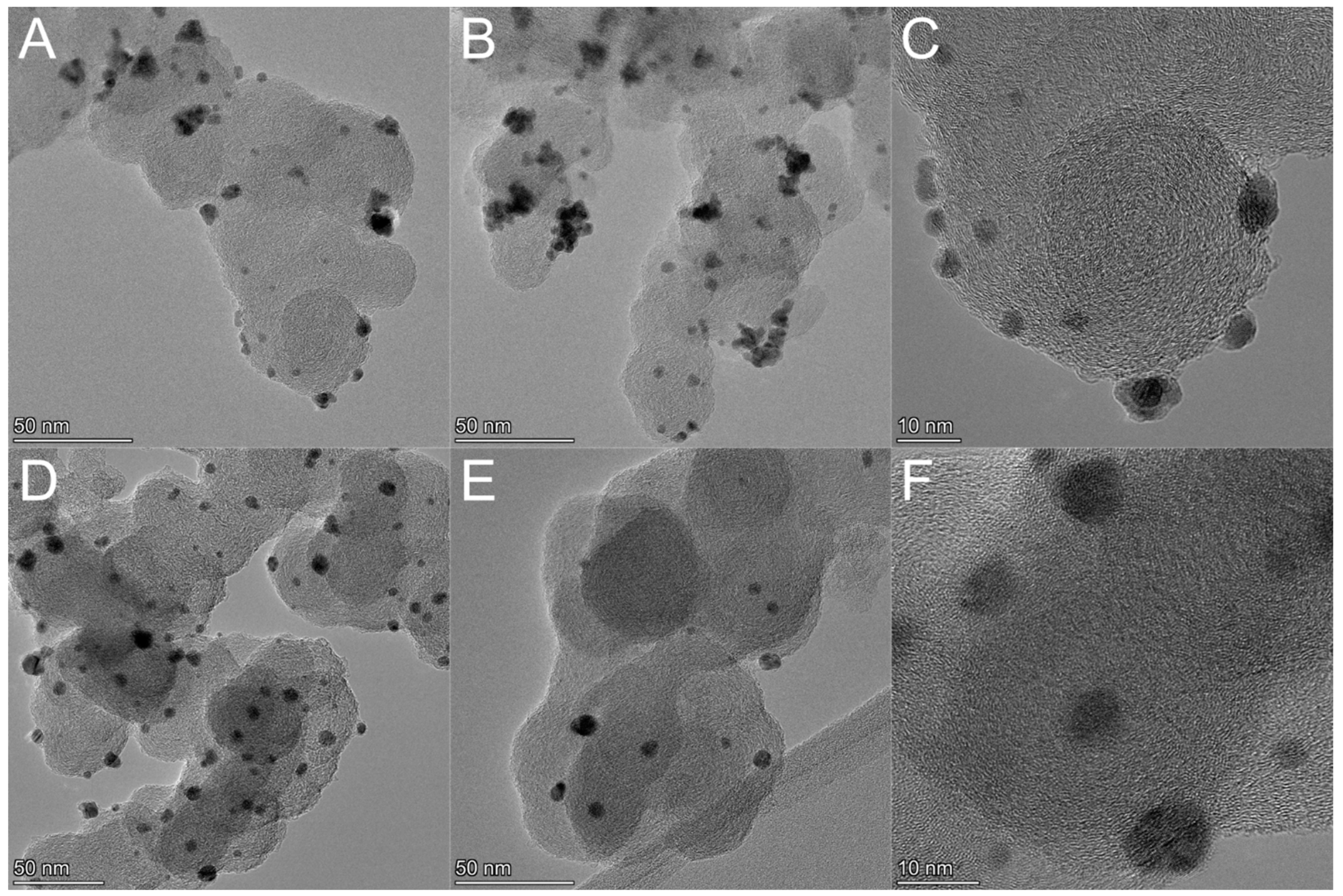
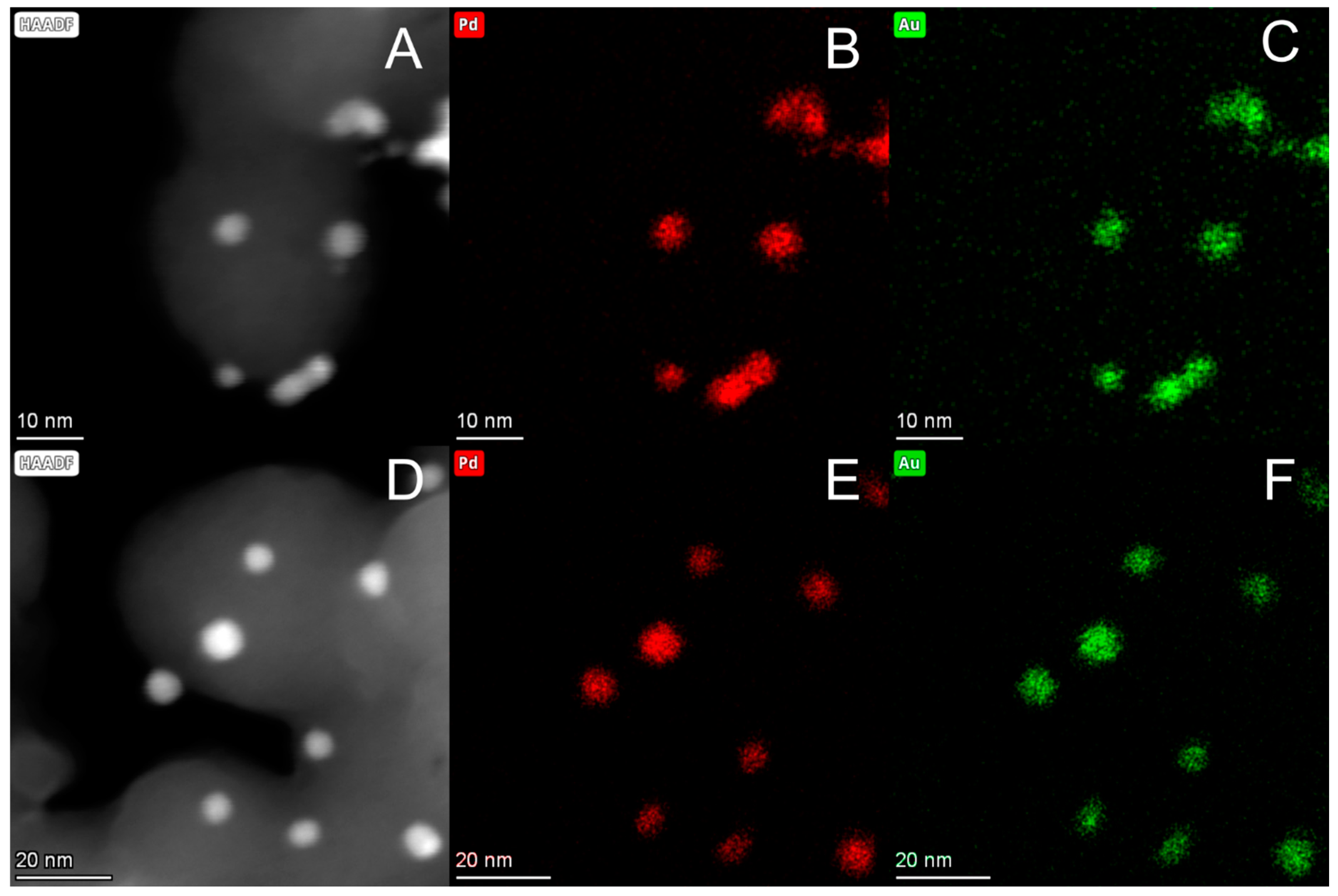
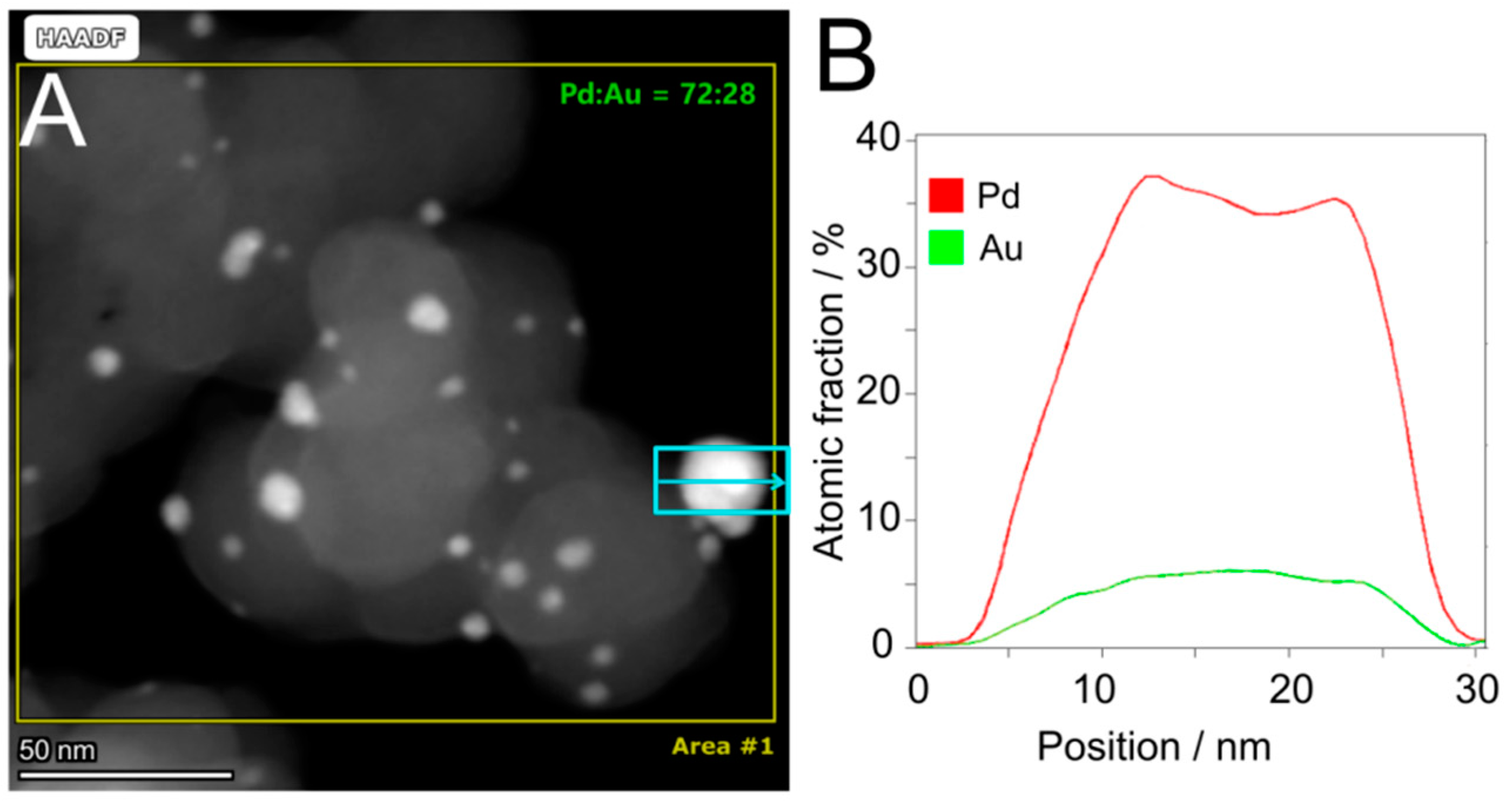
2.1. Synthesis and Chemical–Physical Characterization
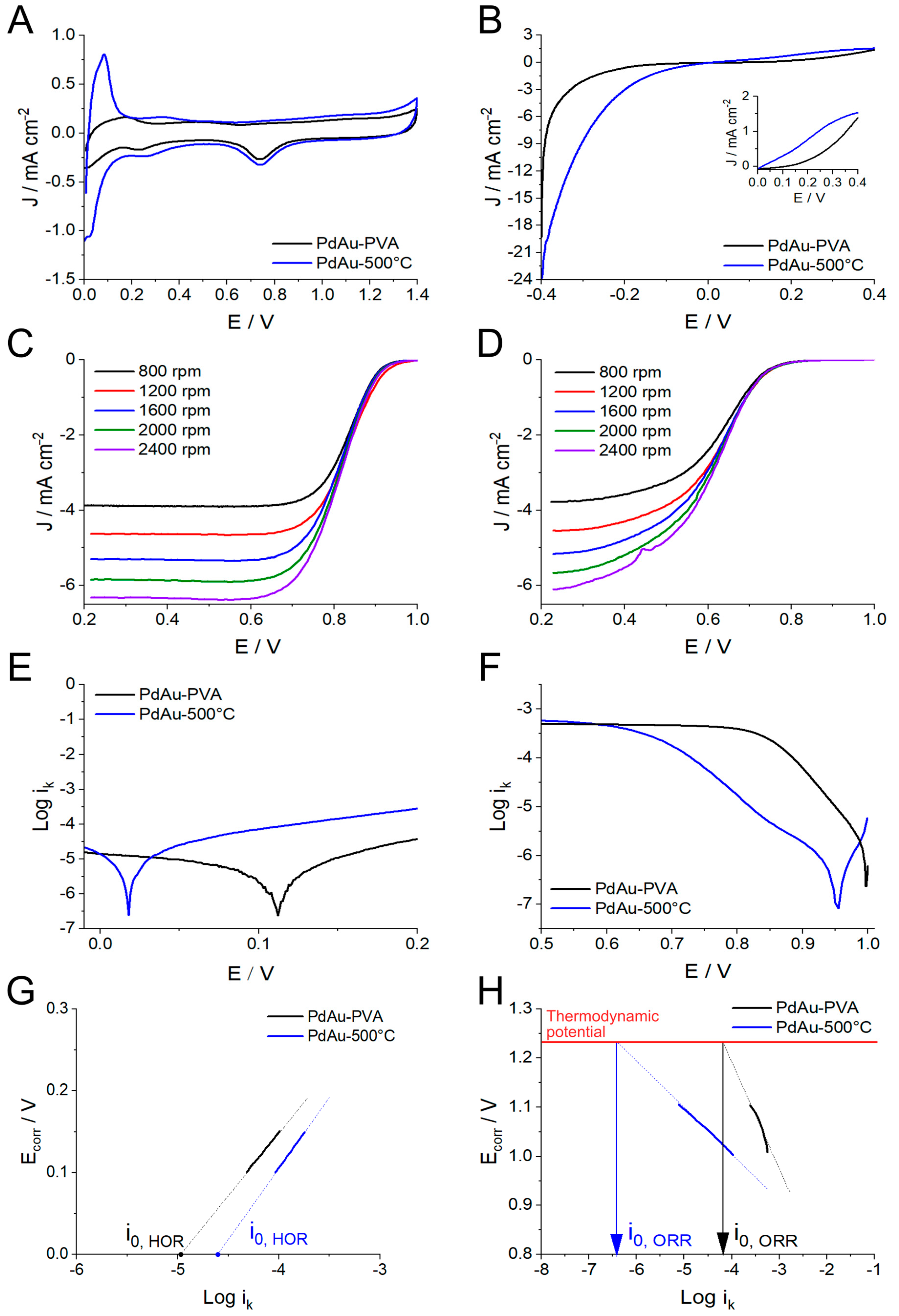
2.2. Electrochemical Testing
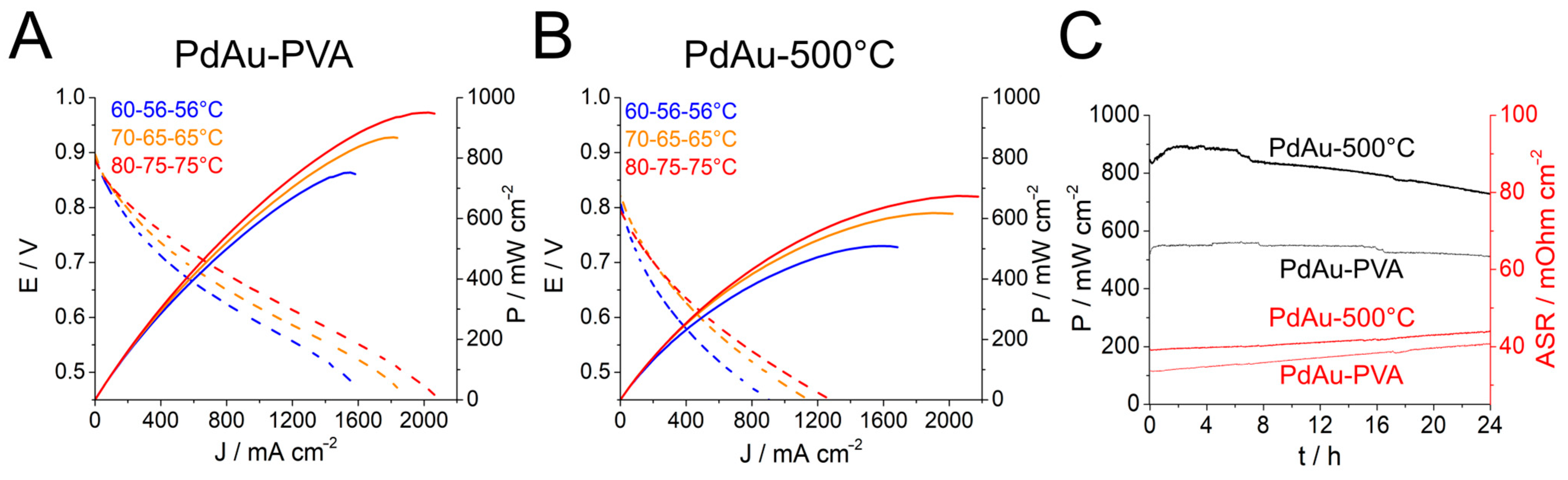
2.3. Discussion on Fuel Cell Testing
3. Experimental
3.1. Synthesis Procedure
3.2. Chemical–Physical Characterization
3.3. Electrochemical Measurements
3.4. Membrane–Electrode–Assembly (MEA) Preparation
3.5. Fuel Cell Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agyekum, E.B.; Odoi-Yorke, F.; Abbey, A.A.; Ayetor, G.K. A Review of the Trends, Evolution, and Future Research Prospects of Hydrogen Fuel Cells—A Focus on Vehicles. Int. J. Hydrogen Energy 2024, 72, 918–939. [Google Scholar]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The Role of Hydrogen and Fuel Cells in the Global Energy System. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar]
- Davydova, E.S.; Zaffran, J.; Dhaka, K.; Toroker, M.C.; Dekel, D.R. Hydrogen Oxidation on Ni-Based Electrocatalysts: The Effect of Metal Doping. Catalysts 2018, 8, 454. [Google Scholar] [CrossRef]
- Bambagioni, V.; Bianchini, C.; Filippi, J.; Lavacchi, A.; Oberhauser, W.; Marchionni, A.; Moneti, S.; Vizza, F.; Psaro, R.; Dal Santo, V.; et al. Single-Site and Nanosized Fe-Co Electrocatalysts for Oxygen Reduction: Synthesis, Characterization and Catalytic Performance. J. Power Sources 2011, 196, 2519–2529. [Google Scholar] [CrossRef]
- Miller, H.A.; Pagliaro, M.V.; Bellini, M.; Bartoli, F.; Wang, L.; Salam, I.; Varcoe, J.R.; Vizza, F. Integration of a Pd-CeO2/C Anode with Pt and Pt-Free Cathode Catalysts in High Power Density Anion Exchange Membrane Fuel Cells. ACS Appl. Energy Mater. 2020, 3, 10209–10214. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhong, Y.; Ran, R.; Shao, Z. Ruddlesden–Popper Perovskites in Electrocatalysis. Mater. Horiz. 2020, 7, 2519–2565. [Google Scholar] [CrossRef]
- Arges, C.G.; Ramani, V.; Pintauro, P.N. The Chalkboard: Anion Exchange Membrane Fuel Cells. Electrochem. Soc. Interface 2010, 19, 31–35. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion Exchange Membranes for Alkaline Fuel Cells: A Review. J. Memb. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of Cell Performance in Anion Exchange Membrane Fuel Cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.; Zelenay, P.; Kim, Y.S. Anion Exchange Membrane Fuel Cells: Current Status and Remaining Challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low Cost Hydrogen Production by Anion Exchange Membrane Electrolysis: A Review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Li, G.; Huang, B.; Pan, J.; Liu, Q.; Han, J.; Xiao, L.; Lu, J.; Zhuang, L. Pt-Ru Catalyzed Hydrogen Oxidation in Alkaline Media: Oxophilic Effect or Electronic Effect? Energy Environ. Sci. 2015, 8, 177–181. [Google Scholar] [CrossRef]
- Marković, N.M.; Schmidt, T.J.; Stamenković, V.; Ross, P.N. Oxygen Reduction Reaction on Pt and Pt Bimetallic Surfaces: A Selective Review. Fuel Cells 2001, 1, 105–116. [Google Scholar]
- Miller, H.A.; Vizza, F. Electrocatalysts and Mechanisms of Hydrogen Oxidation in Alkaline Media for Anion Exchange Membrane Fuel Cells. In Anion Exchange Membrane Fuel Cells: Principles, Materials and Systems; An, L., Zhao, T.S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 79–103. ISBN 978-3-319-71371-7. [Google Scholar]
- Miller, H.A.; Vizza, F.; Marelli, M.; Zadick, A.; Dubau, L.; Chatenet, M.; Geiger, S.; Cherevko, S.; Doan, H.; Pavlicek, R.K.; et al. Highly Active Nanostructured Palladium-Ceria Electrocatalysts for the Hydrogen Oxidation Reaction in Alkaline Medium. Nano Energy 2017, 33, 293–305. [Google Scholar] [CrossRef]
- Bellini, M.; Pagliaro, M.V.; Lenarda, A.; Fornasiero, P.; Marelli, M.; Evangelisti, C.; Innocenti, M.; Jia, Q.; Mukerjee, S.; Jankovic, J.; et al. Palladium-Ceria Catalysts with Enhanced Alkaline Hydrogen Oxidation Activity for Anion Exchange Membrane Fuel Cells. ACS Appl. Energy Mater. 2019, 2, 4999–5008. [Google Scholar] [CrossRef]
- Lenarda, A.; Bellini, M.; Marchionni, A.; Miller, H.A.; Montini, T.; Melchionna, M.; Vizza, F.; Prato, M.; Fornasiero, P. Nanostructured Carbon Supported Pd-Ceria as Anode Catalysts for Anion Exchange Membrane Fuel Cells Fed with Polyalcohols. Inorganica Chim. Acta 2018, 470, 213–220. [Google Scholar] [CrossRef]
- Pagliaro, M.V.; Miller, H.A.; Evangelisti, C.; Bellini, M.; Tuci, G.; Pham-Huu, C.; Giambastiani, G.; Marelli, M.; Vizza, F. Synergy between Nickel Nanoparticles and N-Enriched Carbon Nanotubes Enhances Alkaline Hydrogen Oxidation and Evolution Activity. ACS Appl. Nano Mater. 2021, 4, 3586–3596. [Google Scholar] [CrossRef]
- Sanij, F.D.; Balakrishnan, P.; Leung, P.; Shah, A.; Su, H.; Xu, Q. Advanced Pd-Based Nanomaterials for Electro-Catalytic Oxygen Reduction in Fuel Cells: A Review. Int. J. Hydrogen Energy 2021, 46, 14596–14627. [Google Scholar]
- Fortunato, G.V.; Pizzutilo, E.; Cardoso, E.S.F.; Lanza, M.R.V.; Katsounaros, I.; Freakley, S.J.; Mayrhofer, K.J.J.; Maia, G.; Ledendecker, M. The Oxygen Reduction Reaction on Palladium with Low Metal Loadings: The Effects of Chlorides on the Stability and Activity towards Hydrogen Peroxide. J. Catal. 2020, 389, 400–408. [Google Scholar]
- Xu, C.; Chen, Q.; Ding, R.; Huang, S.; Zhang, Y.; Fan, G. Sustainable Solid-State Synthesis of Uniformly Distributed PdAg Alloy Nanoparticles for Electrocatalytic Hydrogen Oxidation and Evolution. Chin. J. Catal. 2021, 42, 251–258. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z. Grain Boundary Engineering: An Emerging Pathway toward Efficient Electrocatalysis. InfoMat 2024, 6, e12608. [Google Scholar] [CrossRef]
- Hu, Q.; Zhan, W.; Guo, Y.; Luo, L.; Zhang, R.; Chen, D.; Zhou, X. Heat Treatment Bimetallic PdAu Nanocatalyst for Oxygen Reduction Reaction. J. Energy Chem. 2020, 40, 217–223. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, P.; Guo, S.; Zhang, X.; Shen, X.; Lu, G.; Su, D.; Huang, X. Ordered PdCu-Based Nanoparticles as Bifunctional Oxygen-Reduction and Ethanol-Oxidation Electrocatalysts. Angew. Chem. 2016, 128, 9176–9181. [Google Scholar] [CrossRef]
- Kang, Y.S.; Choi, K.H.; Ahn, D.; Lee, M.J.; Baik, J.; Chung, D.Y.; Kim, M.J.; Lee, S.Y.; Kim, M.; Shin, H.; et al. Effect of Post Heat-Treatment of Composition-Controlled PdFe Nanoparticles for Oxygen Reduction Reaction. J. Power Sources 2016, 303, 234–242. [Google Scholar] [CrossRef]
- Bonnefont, A.; Simonov, A.N.; Pronkin, S.N.; Gerasimov, E.Y.; Pyrjaev, P.A.; Parmon, V.N.; Savinova, E.R. Hydrogen Electrooxidation on PdAu Supported Nanoparticles: An Experimental RDE and Kinetic Modeling Study. Catal. Today 2013, 202, 70–78. [Google Scholar] [CrossRef]
- Edström, H.; Schaefer, A.; Jacobse, L.; von Allmen, K.; Hagman, B.; Carlsson, P.; Gustafson, J. Alloying and oxidation of PdAu thin films. Thin Solid Films 2024, 790, 140212. [Google Scholar] [CrossRef]
- Ma, C.; Du, Y.; Feng, J.; Cao, X.; Yang, J.; Li, D. Fabrication of Supported PdAu Nanoflower Catalyst for Partial Hydrogenation of Acetylene. J. Catal. 2014, 317, 263–271. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A. Precise Calculation of Crystallite Size of Nanomaterials: A Review. J. Alloys Compd. 2023, 968, 171914. [Google Scholar]
- Prieur, D.; Bonani, W.; Popa, K.; Walter, O.; Kriegsman, K.W.; Engelhard, M.H.; Guo, X.; Eloirdi, R.; Gouder, T.; Beck, A.; et al. Size Dependence of Lattice Parameter and Electronic Structure in CeO2 Nanoparticles. Inorg. Chem. 2020, 59, 5760–5767. [Google Scholar] [CrossRef]
- Casey, É.; Holmes, J.D.; Collins, G. PdAu Nanosheets for Visible-Light-Driven Suzuki Cross-Coupling Reactions. ACS Appl. Nano Mater. 2022, 5, 16196–16206. [Google Scholar] [CrossRef]
- Castello, C.; Peruzzolo, T.; Bellini, M.; Pagliaro, M.V.; Bartoli, F.; Berretti, E.; Poggini, L.; Pitzalis, E.; Evangelisti, C.; Miller, H.A. Direct Formate Anion Exchange Membrane Fuel Cells with a PdAu Bimetallic Nanoparticle Anode Electrocatalyst Obtained by Metal Vapor Synthesis. Energy Adv. 2024, 3, 2520–2529. [Google Scholar] [CrossRef]
- Miller, H.A.; Lavacchi, A.; Vizza, F.; Marelli, M.; Di Benedetto, F.; D’Acapito, F.; Paska, Y.; Page, M.; Dekel, D.R. A Pd/C-CeO2Anode Catalyst for High-Performance Platinum-Free Anion Exchange Membrane Fuel Cells. Angew. Chem. Int. Ed. 2016, 55, 6004–6007. [Google Scholar] [CrossRef]
- Mukundan, V.; Yin, J.; Hu, S.; Noborikawa, J. Pd-Based Bimetallic and Trimetallic Catalyst for Direct Formic Acid Fuel Cells. ECS Trans. 2014, 64, 1113. [Google Scholar]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel Slopes from a Microkinetic Analysis of Aqueous Electrocatalysis for Energy Conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanendran, N.; Ravichandran, S.; Xu, Q.; Maiyalagan, T.; Su, H. A Quick Guide to the Assessment of Key Electrochemical Performance Indicators for the Oxygen Reduction Reaction: A Comprehensive Review. Int. J. Hydrogen Energy 2022, 47, 7113–7138. [Google Scholar] [CrossRef]
- Adachi, Y.; Nagai, T.; Kuroda, Y.; Ohgi, Y.; Matsuzawa, K.; Mitsushima, S.; Ota, K.; Ishihara, A. Factors Affecting ORR Activity of Nb-Added TiO x Catalyst Using Carbon Support for PEFC. ECS Trans. 2020, 98, 555–563. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Li, F.; Chan, S.H.; Tu, Z. Recent Development of Anion Exchange Membrane Fuel Cells and Performance Optimization Strategies: A Review. Chem. Rec. 2024, 24, e202300067. [Google Scholar] [CrossRef]
- Biancolli, A.L.G.; Herranz, D.; Wang, L.; Stehlíková, G.; Bance-Soualhi, R.; Ponce-González, J.; Ocón, P.; Ticianelli, E.A.; Whelligan, D.K.; Varcoe, J.R.; et al. ETFE-Based Anion-Exchange Membrane Ionomer Powders for Alkaline Membrane Fuel Cells: A First Performance Comparison of Head-Group Chemistry. J. Mater. Chem. A Mater. 2018, 6, 24330–24341. [Google Scholar] [CrossRef]
- Wang, L.; Peng, X.; Mustain, W.E.; Varcoe, J.R. Radiation-Grafted Anion-Exchange Membranes: The Switch from Low- to High-Density Polyethylene Leads to Remarkably Enhanced Fuel Cell Performance. Energy Environ. Sci. 2019, 12, 1575–1579. [Google Scholar] [CrossRef]

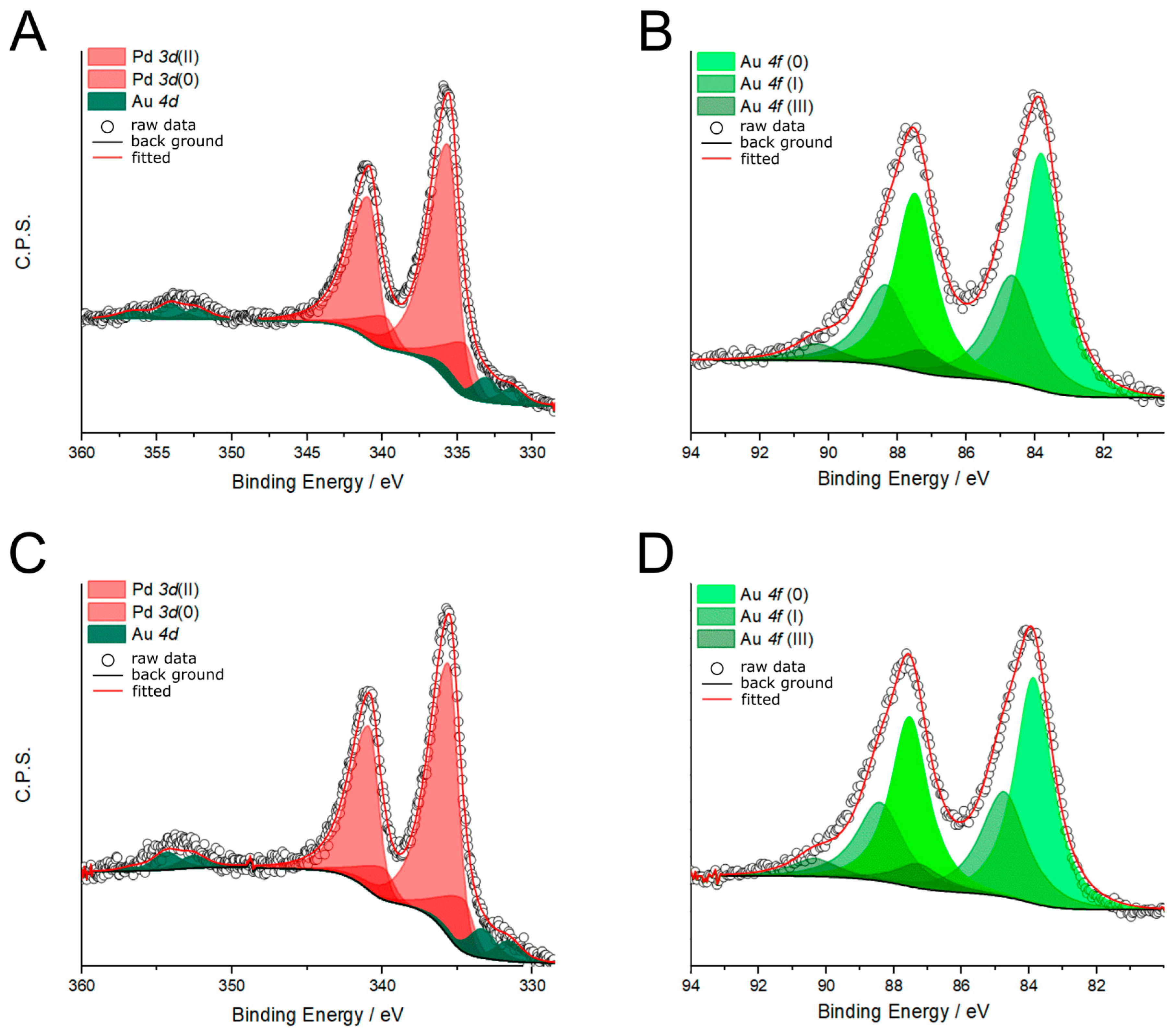

| Catalyst | %Pd | %Au | Pd/Au | %Pd(0) | %Pd(II) | %Au(0) | %Au(I) | %Au(III) |
|---|---|---|---|---|---|---|---|---|
| PdAu-PVA | 73.2 | 26.8 | 2.7 | 21.3 | 78.7 | 60.5 | 32.6 | 6.9 |
| PdAu-500 °C | 72.7 | 22.3 | 2.7 | 19.2 | 80.8 | 58.6 | 33.6 | 7.8 |
| Catalyst | JHOR@0.2V mA cm−2 | |JHER|@0.2V mA cm−2 | I0 A g−1 | EASA m2 g−1 | JHOR,EASA @0.2V A m−2 | JHER,EASA @0.2V A m−2 |
|---|---|---|---|---|---|---|
| PdAu-PVA | 0.181 | 0.600 | 1.5 | 12.8 | 0.52 | 1.74 |
| PdAu-500 °C | 0.772 | 3.058 | 4.8 | 15.3 | 1.94 | 7.69 |
| Catalyst | |Jl|@1600RPM mA cm−2 | Vonset V | E@1/2Jl V | Koutecky–Levich Analysis (mA cm−2)−1 vs. ω−1/2 | I0 A g−1 | |
|---|---|---|---|---|---|---|
| Jk | ne- | |||||
| PdAu-PVA | 5.28 | 0.91 | 0.82 | 23.8–50.0 [700–500 mV] | 3.9–4.0 | 13.1 |
| PdAu-500 °C | 5.17 | 0.74 | 0.62 | 7.8–19.6 [600–400 mV] | 4.0 | 0.8 |
| MEA | OCV mV | Anode | Cathode | Pmax mW cm−2 | J@Pmax mA cm−2 | Operating Conditions Cell–Anode–Cathode °C H2-O2 Flow Rate L min−1 |
|---|---|---|---|---|---|---|
| 1 | 891 897 886 | PdAu-500 °C | Pt | 757 871 952 | 1532 1791 2005 | 60-56-56 0.5-0.5 70-65-65 0.5-0.5 80-75-75 0.5-0.5 |
| 2 | 794 820 806 | PdAu-PVA | Pt | 510 619 674 | 1579 1902 2092 | 60-56-56 0.5-0.5 70-65-65 0.5-0.5 80-75-75 0.5-0.5 |
| 3 | 920 923 | PtRu | PdAu-PVA | 1091 890 | 1802 1680 | 60-55-55 0.5-0.5 80-75-75 0.5-1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagliaro, M.V.; Poggini, L.; Bellini, M.; Fei, L.; Peruzzolo, T.; Miller, H.A. Tuning the Surface Oxophilicity of PdAu Alloy Nanoparticles to Favor Electrochemical Reactions: Hydrogen Oxidation and Oxygen Reduction in Anion Exchange Membrane Fuel Cells. Catalysts 2025, 15, 306. https://doi.org/10.3390/catal15040306
Pagliaro MV, Poggini L, Bellini M, Fei L, Peruzzolo T, Miller HA. Tuning the Surface Oxophilicity of PdAu Alloy Nanoparticles to Favor Electrochemical Reactions: Hydrogen Oxidation and Oxygen Reduction in Anion Exchange Membrane Fuel Cells. Catalysts. 2025; 15(4):306. https://doi.org/10.3390/catal15040306
Chicago/Turabian StylePagliaro, Maria V., Lorenzo Poggini, Marco Bellini, Lorenzo Fei, Tailor Peruzzolo, and Hamish A. Miller. 2025. "Tuning the Surface Oxophilicity of PdAu Alloy Nanoparticles to Favor Electrochemical Reactions: Hydrogen Oxidation and Oxygen Reduction in Anion Exchange Membrane Fuel Cells" Catalysts 15, no. 4: 306. https://doi.org/10.3390/catal15040306
APA StylePagliaro, M. V., Poggini, L., Bellini, M., Fei, L., Peruzzolo, T., & Miller, H. A. (2025). Tuning the Surface Oxophilicity of PdAu Alloy Nanoparticles to Favor Electrochemical Reactions: Hydrogen Oxidation and Oxygen Reduction in Anion Exchange Membrane Fuel Cells. Catalysts, 15(4), 306. https://doi.org/10.3390/catal15040306










