Abstract
The Mn-Mn dimer has been found to be catalytically active in various manganese oxides for NO oxidation. However, to date, it remains unclear how the dimer determines catalytic performance. Herein, we employed a combination of DFT theoretical calculations and an experimental approach to investigate the O2 dissociation capability and NO oxidation activity of single Mn sites and Mn-Mn dimer sites with varying bond lengths. Our results indicate that Mn-Mn dimer sites outperform single Mn active sites in both O2 activation and NO oxidation. This enhancement is primarily attributed to the short-range ordered geometry of the Mn-Mn dimers, which suppresses the formation of NO3* intermediates and promotes NO2* desorption. Among the three types of Mn-Mn dimers examined, the Mn-Mn dimer in BaMnO3, with the shortest Mn-Mn bond length, aligns most favorably with O-O, supporting the most efficient O2 activation. Conversely, MnO2, characterized by the longest Mn-Mn bond length, exhibits greater charge transfer and synergistic effects at the local active site, achieving the highest NO catalytic activity. Furthermore, we found that dual-site exposure of Mn-Mn dimers is more effective for catalytic reactions than single-site exposure. This study provides important insights into the structure–activity relationship between the geometric structure of catalytic active sites and the adsorption of intermediates.
1. Introduction
Catalytic reactions encompass a series of intricate steps: reactant molecular adsorption, activation, bond breaking, and reformation [1]. Within these reactions, a crucial determinant of molecular adsorption and activation is the electronic structure matching between active sites and adsorbed molecules [2,3,4]. This matching involves both wavefunction overlap and energy alignment and is widely recognized as pivotal [5,6]. Consequently, numerous descriptors, such as the d-band center [7,8,9,10], eg filling [11,12,13], and p-band center of bulk oxygen [14,15], have been formulated based on the electronic structure to elucidate structure–activity relationships [16]. These descriptors have significantly enriched our understanding of how underlying electronic properties influence catalytic performance.
Meanwhile, the geometric configuration of catalytic sites is equally crucial in determining catalytic activity [17]. It governs the adsorption mode of reactants, which subsequently impacts the activation pathways of adsorbed species [18,19]. Despite its importance, the influence of geometric structure on catalytic behavior remains relatively unexplored. A deeper investigation into how spatial arrangements at active sites affect adsorption and activation could lead to a refined understanding and optimization of catalytic processes.
We propose that unique geometric structures of localized active sites can substantially enhance catalytic activity. Our prior research on Mn-mullite in the context of lithium–sulfur batteries has demonstrated that the remarkable lattice matching between Li-S and Mn-O can efficiently activate polysulfide intermediates, ultimately leading to exceptional SRR catalytic performance [20]. Moreover, Mn-mullite oxides exhibit a distinctive crystal structure featuring alternating pyramidal and octahedral coordination units. This configuration creates active sites where Mn centers are positioned within less than 3 Å of each other, enabling efficient charge transfer and fostering synergistic effects during catalytic reactions. This unexpected behavior results in remarkable enhancement of NO oxidation catalytic activity [21,22,23]. To further explore the structural role of this active site in catalytic activity, Thampy et al. investigated the oxidation active sites in SmMn2O5 and SmMnO3 [24]. Their study revealed that, despite having similar active site densities, SmMn2O5 exhibited significantly higher NO catalytic activity than SmMnO3. This enhanced performance in SmMn2O5 is primarily due to the shorter Mn-Mn distance of 2.85 Å between adjacent Mn4+ ions, compared to a Mn-Mn distance of 3.91 Å between Mn3+ ions in SmMnO3. The short-range ordered Mn-Mn dimer sites in SmMn2O5 enable more efficient charge transfer and cooperative interactions, destabilizing nitrate species and leading to improved catalytic activity.
To further investigate this phenomenon, we systematically studied the catalytic performance of other materials containing Mn-Mn dimer active sites. For a comprehensive comparison, we substituted Sm with Y and selected YMn2O5 mullite and YMnO3 perovskite materials. To further explore the effects of dimer configurations, we included BaMnO3—a face-sharing perovskite with a shorter Mn-Mn bond length—and MnO2, which features a mixed corner- and edge-sharing structure with a longer Mn-Mn bond length. Across various material systems, Mn-Mn bond lengths exhibit significant variability, directly influencing atomic interactions and electron transfer efficiency. Therefore, we need to further investigate the effects of the geometric structure of Mn-Mn dimers on the electronic structure and catalytic activity.
In this study, we showcase the enhanced catalytic performance of Mn-Mn dimer active sites by comparing mullite YMn2O5 (containing Mn-Mn dimers) with perovskite YMnO3 (featuring single Mn sites) using the NO oxidation reaction, which acts as a key process in the nitrogen cycle, as a model reaction. Additionally, we evaluated the catalytic properties of three materials with varying Mn-Mn bond lengths, mullite YMn2O5, perovskite BaMnO3, and manganese dioxide MnO2, assessing their O2 activation and NO oxidation capabilities. Using density functional theory (DFT) calculations in conjunction with advanced characterization techniques, including Raman spectroscopy, O2-programmed temperature desorption (O2-TPD), NO2-programmed temperature desorption (NO2-TPD), X-ray absorption near edge structure (XANES), and extended X-ray absorption fine structure (EXAFS) spectroscopy, we reveal that Mn-Mn bond length plays a critical role in O2 activation and NO oxidation. Specifically, the order of O2 activation is BaMnO3 > YMn2O5 > MnO2, with shorter Mn-Mn dimers exhibiting a higher match with the O-O bond, thereby enhancing O-O activation. Conversely, NO oxidation follows an opposing trend, as longer Mn-Mn bonds inhibit NO3* formation, facilitating charge transfer and cooperative interactions at local sites, ultimately enhancing NO catalytic activity. Additionally, this study explores the impact of dimer exposure modes, revealing that dual-site exposure of Mn-Mn dimers significantly promotes reaction kinetics compared to single-site exposure. These findings provide profound insights into the interplay between the geometric structures of catalytic sites and reaction intermediates, thereby opening up innovative avenues for advancements in catalysis.
2. Results and Discussion
2.1. The Influence of Mn-Mn Bond Length on Geometric and Electronic Structure in Different Manganese Oxides
In catalytic reactions, the electronic structure of active sites is typically the foremost area of focus, given its intimate link to reaction activity. However, the interplay between local geometric configurations and catalytic performance remains crucial and demands deeper exploration. Our investigation into certain manganese oxides has uncovered that when the Mn-Mn distance narrows to below 3 Å, charge interactions between Mn atoms intensify, fostering synergistic reactions. Consequently, these short-range ordered Mn sites are designated as “dimer” sites.
Bader charge analysis (Figure S1) elucidates that Mn atoms within these dimers do not engage in direct interactions. Instead, charge transfer primarily occurs via the intermediary O atom. Furthermore, a reduction in the Mn-Mn distance triggers significant alterations in the dimer’s geometry. Notably, the coordination entity’s stacking mode transitions from edge-sharing to a higher-dimensional face-sharing configuration (Figure 1a), accompanied by a decrease in the Mn-O-Mn bond angle (θ). This, in turn, weakens the Mn-O coupling and impedes charge transfer across the Mn-O-Mn linkage (Figure 1b).
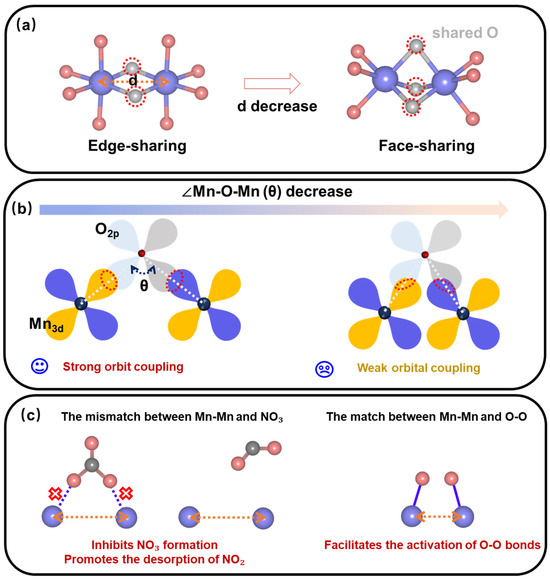
Figure 1.
(a) Stacking modes of Mn-Mn dimer coordination entities with varying bond lengths. (b) Electron transfer capabilities of Mn-Mn dimers with different bond lengths. (c) The ability of Mn-Mn dimers with varying bond lengths to facilitate (hinder) NO3 formation, NO2 desorption, and O2 dissociation.
To delve into the ramifications of these structural transformations, we examined Mn-Mn dimer sites with varying bond lengths in MnO2, YMn2O5, and BaMnO3, utilizing NO oxidation as a model reaction to assess the influence of Mn-Mn bond lengths on both geometric and electronic structure. Our results underscore the pivotal role of Mn-Mn bond length: longer Mn-Mn bonds hinder nitrate (NO3*) formation and favor monodentate NO2 desorption, whereas shorter Mn-Mn bonds facilitate O-O bond cleavage, furnishing reactive oxygen species for subsequent reactions (Figure 1c). The detailed reaction mechanisms will be elaborated in the subsequent sections.
2.2. Enhanced NO Oxidation Activity of Mn-Mn Dimers in Mullite YMn2O5 Compared to Single Mn Sites in Perovskite YMnO3
To explore the structural role of Mn-Mn dimers in catalytic activity, we selected mullite YMn2O5 with a Mn-Mn distance of 2.81 Å and perovskite YMnO3 with a Mn-Mn distance of 3.84 Å as model systems to evaluate their NO oxidation activity (Figure 2a). In the simulation calculations, we ensured that the active site density and oxidation states remained consistent, choosing the 010 surface of YMn2O5 and the 100 surface of YMnO3 for analysis. As shown in Figure 2a and Figure S2, the desorption of NO2 emerged as the rate-limiting step in the overall reaction process, with desorption energies of 0.44 eV for Mn-Mn dimer sites in YMn2O5 and 1.87 eV for single Mn sites in YMnO3. This indicates that Mn-Mn dimer active sites significantly facilitate NO2 desorption relative to single Mn sites. Consequently, YMn2O5, with its Mn-Mn dimer sites, displays superior NO catalytic oxidation activity compared to YMnO3. Furthermore, NO catalytic oxidation on the YMn2O5 (121) surface, which contains both dimer and single Mn sites, provides additional evidence for the advantages of Mn-Mn dimer sites (Figures S3 and S4).
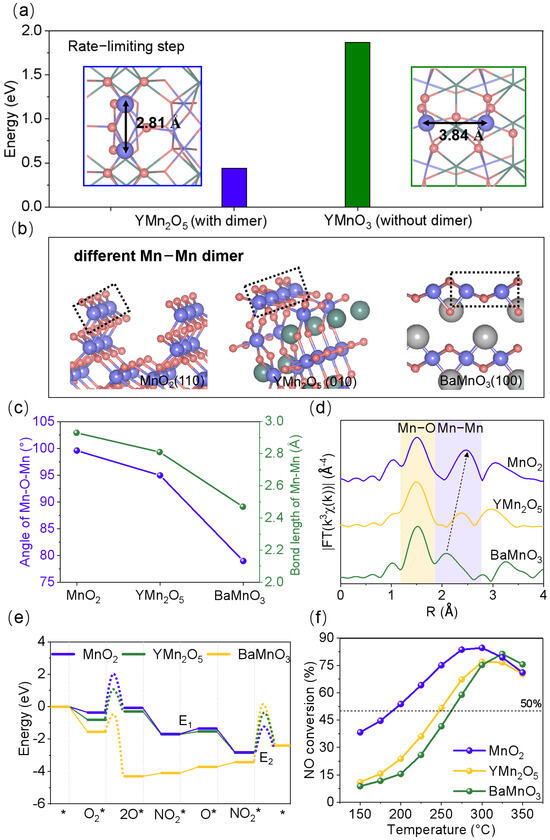
Figure 2.
(a) The thermodynamic energy barrier of NO catalytic oxidation of YMn2O5 (010) and YMnO3 (100). The blue and pink represent Mn and O atoms, respectively. (b) Geometric configurations of MnO2 (110), YMn2O5 (010), and BaMnO3 (100) surfaces. Green, light gray, blue, and pink represent Y, Ba, Mn, and O atoms, respectively. (c) The bond length of Mn-Mn and the angle of Mn-O-Mn for MnO2 (110), YMn2O5 (010), and BaMnO3 (100). (d) The k3-weighted Fourier transform spectra of EXAFS curves of MnO2, YMn2O5, and BaMnO3. (e) The NO catalytic oxidation energy diagram of MnO2 (110), YMn2O5 (010), and BaMnO3 (100). The symbol * represents clean catalyst surface. (f) NO oxidation activity of MnO2, YMn2O5, and BaMnO3.
2.3. Geometric Structure Characterization and NO Oxidation Activity of Mn-Based Oxides with Mn-Mn Dimers of Varying Bond Lengths
Due to their unique geometric and electronic structures, Mn-Mn dimers exhibit exceptional catalytic activity in NO catalytic oxidation. We also observed that different manganese oxides possess Mn-Mn dimer sites with varying bond lengths, suggesting that the distance between these dimers may be a critical factor in facilitating charge transfer and cooperative interactions among manganese atoms. To investigate the impact of Mn-Mn dimer active sites with different bond lengths on catalytic reactions, perovskite BaMnO3, manganese dioxide MnO2, and mullite YMn2O5, all containing Mn-Mn dimer active sites, were experimentally synthesized and compared (Figure S5). The crystal structures of the three materials were confirmed based on their XRD patterns (Figure S6), corresponding to MnO2 (JCPDS 44-0141) [25], YMn2O5 (JCPDS 34-0067) [26], and BaMnO3 (JCPDS 26-0168) [27]. The morphology and microstructure of the three materials were characterized using SEM, TEM, and HRTEM, showing irregularly aggregated nanostructures and well-defined lattice fringes (Figures S7–S9). EDS elemental mapping (Figures S10–S12) demonstrated that the elements are uniformly distributed within MnO2, YMn2O5, and BaMnO3. Notably, the edge-sharing MnO2 features the longest Mn-Mn bond lengths and the largest Mn-O-Mn bond angles, followed by YMn2O5, while the face-sharing Mn-Mn dimer in BaMnO3 exhibits the shortest bond lengths and smallest bond angles (Figure 2b,c and Figure S13 and Tables S2 and S3). The electronic structure and Mn-O coordination were further characterized using XANES and EXAFS. The normalized Mn K-edge XANES spectra [28,29,30], along with the magnified view (Figure S14), revealed that the average oxidation state of Mn increases in the order of YMn2O5 < BaMnO3 < MnO2, consistent with the Mn 2p XPS results [31,32] (Figures S15 and S16 and Table S4). The k3-weighted Fourier transform spectra of EXAFS (FT-EXAFS) curves (Figure 2d) show Mn-O peaks in the range of approximately 1.2–1.8 Å and Mn-Mn peaks in the range of approximately 1.8–2.8 Å. The average Mn-Mn bond lengths for the three materials followed the order: MnO2 > YMn2O5 > BaMnO3. This indicated that MnO2 possesses superior electron transfer capabilities relative to YMn2O5 and BaMnO3, thereby enhancing charge transfer during the reaction process and improving the adsorption behavior of intermediates.
Furthermore, to investigate their catalytic activity, we performed DFT calculations on the catalytic oxidation of NO using the Mn-Mn dimer double-exposed surfaces of MnO2 (110), YMn2O5(010), and BaMnO3 (100) (Figure 2b and Figure S17). The results indicated that the order of catalytic activity is MnO2 > YMn2O5 > BaMnO3. For both YMn2O5 and BaMnO3, the rate-limiting steps occur during the desorption of NO2, whereas for MnO2, the rate-limiting step occurs during the dissociation of O2. The corresponding energy barriers for these rate-limiting steps were found to be 1.01 eV, 1.59 eV, and 0.98 eV, respectively (Figure 2e and Table S7). The specific adsorption configuration is shown in Figures S18–S20. The catalytic oxidation performance of MnO2, YMn2O5, and BaMnO3 for 500 ppm NO was tested experimentally (Figure 2f). Notably, MnO2 exhibited the highest NO conversion rate at 300 °C (84.7%), significantly outperforming YMn2O5 (77.0%) and BaMnO3 (75.2%). The T50 of the MnO2 sample, defined as the temperature at which the NO conversion exceeds 50%, was 190 °C, approximately 57 °C and 72 °C lower than that of the YMn2O5 and BaMnO3 samples, respectively, which also demonstrated higher NO oxidation activity than the commercial Pt/Al2O3 catalyst [21]. The catalytic activity order of the three materials aligned with the theoretical results.
2.4. Dynamic Regulation of Adsorbed Intermediates by Different Bond Lengths in Mn-Mn Dimers
Raman spectroscopy and DFT-based theoretical calculations were employed to investigate the dynamic regulation of Mn-Mn dimers during the reaction process. The lattice vibration modes of the three materials were characterized using Raman spectroscopy. As shown in Figure 3a, the Raman band at ~571 cm−1 in MnO2 corresponds to the ν3 mode, attributed to Mn-O stretching vibrations along the [MnO6] plane [33,34]. In YMn2O5, the Raman band at ~625 cm−1 represents the Ag(11) mode, associated with the bending vibrations of the Mn4+-O-Mn4+ structure [35,36]. The Raman band at ~654 cm−1 in BaMnO3 corresponds to the A1g(2) mode, related to the relative displacement of Mn-O vibrations perpendicular to the c-axis [37,38]. The order of the Mn-O vibration peaks, MnO2 > YMn2O5 > BaMnO3, indicated that the Mn-O bond strength follows the same order. This suggested that Mn-O coupling in MnO2 is stronger, favoring more efficient charge transfer. Analysis of the projected density of states (PDOS) and d-band center of the Mn-Mn dimers further supports this trend (Figure 3b), with the d-band center order being MnO2 > YMn2O5 > BaMnO3, suggesting that MnO2 dimer sites have enhanced regulatory capabilities.
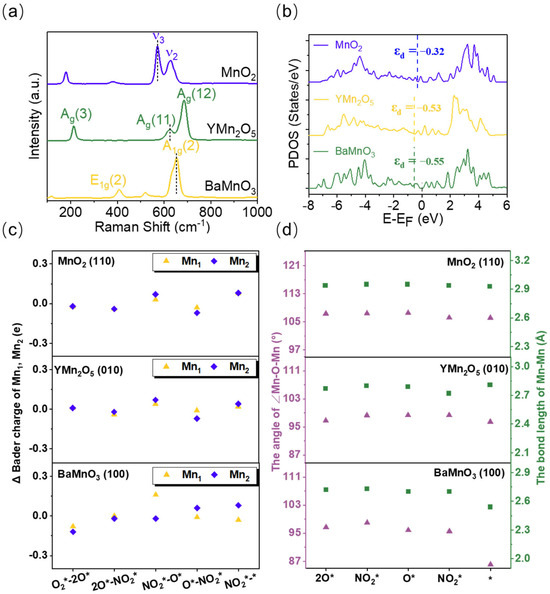
Figure 3.
(a) Raman spectrum of MnO2, YMn2O5, and BaMnO3. (b) The PDOS of the Mn-Mn dimer of MnO2 (110), YMn2O5 (010), and BaMnO3 (100). (c) The variation in the Bader charge of the Mn-Mn site of MnO2 (110), YMn2O5 (010), and BaMnO3 (100). (d) The bond length of Mn-Mn and the angle of Mn-O-Mn of MnO2 (110), YMn2O5 (010), and BaMnO3 (100) during the reaction process. The symbol * represents clean catalyst surface.
Additionally, we examined the variations in Mn-Mn bond lengths, bond angles, and charge during the reaction (Figure 3c,d). Although NO adsorption and NO2 desorption interact directly with only one Mn site in the Mn-Mn dimer, significant charge transfer was observed at both Mn sites, and the Mn-Mn dimer underwent substantial geometric changes throughout the reaction. This finding indicates that the Mn-Mn dimer can regulate local charge transfer through geometric adjustments, thereby tuning the adsorption strength of reaction intermediates and enhancing catalytic activity. This may be a key reason for the superior performance of Mn-Mn dimer sites over single Mn sites.
Moreover, among the three materials, compared to the shorter Mn-Mn dimers in BaMnO3, YMn2O5, and MnO2 possess longer Mn-Mn bond lengths and larger Mn-O-Mn bond angles. During NO adsorption, Mn sites lose electrons, while charge returns to Mn sites upon NO2 desorption, showing superior regulatory capabilities compared to BaMnO3. Furthermore, MnO2 exhibits smaller changes in bond length and angle throughout the reaction, indicating greater structural stability.
2.5. The Influence of Mn-Mn Bond Length on O2 Activation Efficiency in NO Oxidation Catalysis
Oxygen activation is a crucial step in the NO oxidation reaction. Through O2-TPD experiments and theoretical calculations, we investigated the O2 dissociation capabilities at different Mn-Mn dimer sites. As shown in Figure 4a, BaMnO3, with the shortest Mn-Mn bond length, exhibits the lowest O2 dissociation barrier, followed by YMn2O5, with MnO2 having the highest such barrier. Additionally, the O2-TPD spectra (Figure 4b) showed that MnO2 has the lowest onset temperature at around 100 °C, indicating that O2 desorption occurs more readily in MnO2, followed by YMn2O5. In the range of ~150–300 °C, MnO2 still exhibited the lowest desorption temperature, indicating that its surface active oxygen species are more reactive [39]. In contrast, BaMnO3 showed a significant lattice oxygen desorption peak only above 800 °C, suggesting stronger binding to O2 [40]. This indicated that the binding strength of O2 among the three materials follows the order: MnO2 < YMn2O5 < BaMnO3. Consequently, weaker O2 binding leads to more difficult cleavage of the O=O bond, which is consistent with the computational results. The coupling strength of Mn-O and O-O was assessed using the integration of crystal orbital Hamilton population (ICOHP), and were performed for bonding states below the Fermi level (Figure 4c,d and Tables S5 and S6). For the three materials, the order of ICOHP for Mn-O is BaMnO3 > YMn2O5 > MnO2; that for O-O is totally reversed. In other words, BaMnO3, with the shortest Mn-Mn bond length among the examined materials, exhibits the strongest O2 adsorption, making it highly favorable for O=O bond activation. This is reflected in the longest O=O bond length (1.35 Å) and the highest charge accumulation on O2 (0.49 e) (Figure 4e). As the Mn-Mn bond length increases, the strength of O2 adsorption decreases, and the activation capacity diminishes correspondingly, as confirmed by both the Δ Bader charge and -ICOHP values of O2.
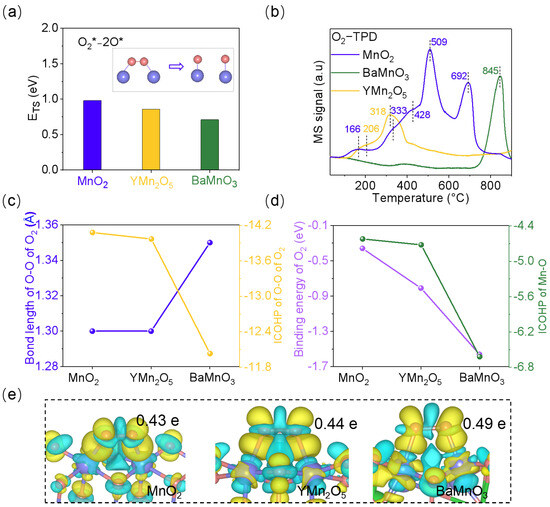
Figure 4.
(a) The dissociation energy barrier of O2 on the Mn-Mn dimer of MnO2 (110), YMn2O5 (010), and BaMnO3 (100). The symbol * represents clean catalyst surface. (b) O2-TPD profiles of MnO2, YMn2O5, and BaMnO3. (c) The bond length and ICOHP of O-O on MnO2 (110), YMn2O5 (010), and BaMnO3 (100). (d) The binding energy of O2 and ICOHP of Mn-O on MnO2 (110), YMn2O5 (010), and BaMnO3 (100). (e) The Bader charge and differential charge of O2 on MnO2 (110), YMn2O5 (010), and BaMnO3 (100). Isosurface = 0.002 eV/Å3. Yellow indicates charge accumulation and blue indicates charge depletion. The blue and pink represent Mn and O atoms, respectively.
From a geometric standpoint, BaMnO3 also demonstrates the closest match between the Mn-Mn dimer and the O=O bond lengths. Upon O2 relaxation, the Mn-Mn bond length in BaMnO3 contracts by only 0.02 Å, compared to 0.08 Å and 0.09 Å in YMn2O5 and MnO2, respectively (Figure S21). We therefore suggest that the matching degree between the Mn-Mn bond length and the O=O bond length is a key factor in determining the efficiency of O2 activation. When the Mn-Mn distance aligns closely with the O=O bond length, resonance interactions between Mn-Mn and O=O are maximized, facilitating easier activation of the O2 molecule.
2.6. The Role of Mn-Mn Bond Length in Mechanisms of NO Oxidation Catalysis
In addition to O2 dissociation, NO adsorption and subsequent NO2 desorption are critical steps in the NO oxidation reaction. Using YMn2O5 as an example, NO can adsorb at Mn-Mn dimer sites in two configurations: monodentate NO2* and bidentate NO3*. These configurations lead to two distinct reaction mechanisms, i.e., Mechanism I, involving NO2* [41], and Mechanism II, involving NO3* [26] (Figure S22). A comparison of the two mechanisms reveals that the rate-limiting step for Mechanism II (NO₃*-involved) is the transition from NO3* to ONO2*, while for Mechanism I (NO2*-involved), it is the second desorption of NO2*(Figure 2e and Figure S23). Mechanism II’s rate-limiting energy barrier is significantly higher than that of Mechanism I (Figure 5a–c and Table S7), explaining why dimer sites demonstrate far superior catalytic performance compared to single Mn sites. On single Mn sites, NO3* formation is unavoidable, which makes subsequent NO2* desorption challenging and results in reduced catalytic efficiency.

Figure 5.
(a) The dissociation energy barrier of NO3* to ONO2* on the Mn-Mn dimer of MnO2 (110), YMn2O5 (010), and BaMnO3 (100). The symbol * represents clean catalyst surface. (b) The two adsorption energy of NO on the Mn-Mn dimer of MnO2 (110), YMn2O5 (010), and BaMnO3 (100). (c) The second desorption energy and CINEB barrier of NO2 on the Mn-Mn dimer of MnO2 (110), YMn2O5(010), and BaMnO3 (100). The blue, grey and pink represent Mn, N and O atoms, respectively. (d) NO2-TPD profiles of MnO2, YMn2O5, and BaMnO3.
Furthermore, DFT calculations and NO2-TPD experiments were used to investigate the origin of the superior NO oxidation activity with Mn-Mn dimers with longer bond lengths. As shown in Figure 5b,c, the NO adsorption strength follows the order MnO2 > YMn2O5 > BaMnO3. Strong NO adsorption weakens the Mn-O bond, thereby facilitating NO2 desorption (Figure S24 and Table S8). Consequently, MnO2, with the longest Mn-Mn bond length, exhibits the lowest NO2 desorption energy barrier. This is primarily attributed to its longer Mn-Mn distance and larger Mn-O-Mn bond angle, where the extended Mn-Mn distance avoids strong NO3* formation and the larger bond angle enhances charge transfer capacity, which more effectively regulates the critical intermediate NO2*, leading to superior catalytic activity. Conversely, as the Mn-Mn distance shortens, BaMnO3, with its face-sharing structure, has the shortest Mn-Mn bond and smallest Mn-O-Mn bond angle, resulting in weaker charge transfer and reduced regulation capability. Its too-weak NO adsorption hinders NO2* desorption, resulting in lower NO oxidation activity. NO2-TPD curves (Figure 5d) showed that MnO2 has the lowest onset temperature of around 70 °C, indicating that NO2 desorbs more easily from MnO2. As the temperature increased, YMn2O5 began to desorb NO2, while BaMnO3 exhibited the highest NO2 desorption temperature, suggesting that NO2 desorption from BaMnO3 is more difficult. These results further confirmed the theoretical predictions, with the desorption energy following the order: MnO2 < YMn2O5 < BaMnO3.
2.7. Catalytic Advantage of Dual-Site Mn-Mn Dimer Exposure over Single-Site Exposure in NO Oxidation
As discussed earlier, simultaneous exposure of Mn-Mn dimer dual-sites enhances catalytic activity. In practical scenarios, Mn-Mn dimers may not be simultaneously exposed. Considering other crystal facets, we further investigated the structure of singly exposed Mn-Mn dimers. DFT calculations for NO oxidation were performed on singly exposed Mn-Mn dimer surfaces of MnO2 (001), YMn2O5 (121), and BaMnO3 (001) (Figure S17). Consistent with previous findings, singly exposed Mn-Mn dimers exhibited superior catalytic activity for NO oxidation compared to isolated Mn sites (Figure S25). Although only one Mn atom in the dimer directly engages in the reaction, the unique geometry of the Mn-Mn dimer allows the second Mn atom to dynamically influence and regulate the entire process, with significant adjustments observed in the geometric and electronic structures of both Mn atoms (Figures S26 and S27).
The order of NO oxidation activity is MnO2 > YMn2O5 > BaMnO3 (Figure 6a and Figure S28), while the order for O2 dissociation energy is BaMnO3 > YMn2O5 > MnO2 (Figure 6b and Figure S29 and Tables S9–S11). The specific adsorption configuration is shown in Figures S30–S32. These results confirm that the bond-length effect of Mn-Mn dimers remains stable, irrespective of the exposure mode. Notably, in terms of both NO oxidation activity and O2 dissociation capability, dual-site exposure of Mn-Mn dimers consistently outperforms single-site exposure, underscoring the advantage of dual-site exposure configurations. This is likely due to the stronger lattice constraints on singly exposed Mn-Mn dimer sites, leading to more significant geometric distortions of the Mn-Mn dimers, which indicates a weakened regulatory effect. Moreover, the singly exposed Mn-Mn dimers suppress the dual-site synergistic interaction, resulting in poorer NO oxidation performance.
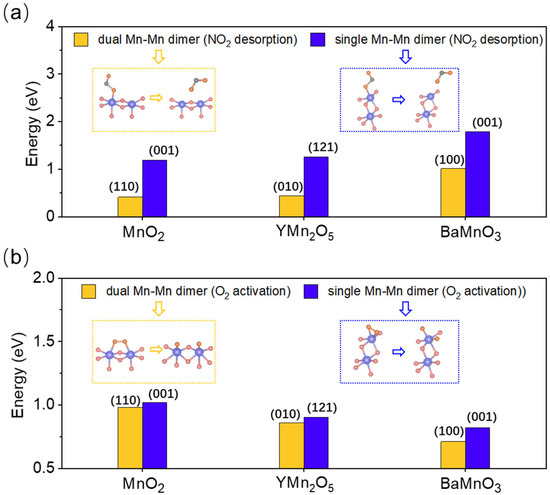
Figure 6.
(a) The second desorption energy of NO2 on single-exposed (blue) and double-exposed (yellow) dimer sites for MnO2, YMn2O5, and BaMnO3. The blue, pink, gray, and orange represent Mn, O, N, and adsorbed O atoms, respectively. (b) The activation energy of O2 on single-exposed (blue) and double-exposed (yellow) dimer sites for MnO2, YMn2O5, and BaMnO3.
3. Materials and Methods
3.1. Catalyst Synthesis
MnO2 was synthesized using the hydrothermal method [42,43]. A solution of 0.525 g MnSO4·H2O (AR, GENERAL-REAGENT®, Shanghai, China) and 1.25 g KMnO4 (AR, DAMAO, Tianjin, China) was prepared in 60 mL deionized water. After stirring, 2 mL of 68% nitric acid was added, and the mixture was stirred at room temperature until a homogeneous solution formed. The solution was then transferred to a 100 mL Teflon-lined stainless-steel autoclave and heated at 100 °C for 36 h. The resulting product was filtered, washed, and dried at 60 °C to yield MnO2.
BaMnO3 and YMn2O5 were synthesized using the sol–gel method [37]. For BaMnO3, 2 mmol of Ba(CH3COO)2 (AR, MERYER, Shanghai, China), 2 mmol of Mn(CH3COO)2·4H2O (AR, MERYER, Shanghai, China), and 1 g of PVP-K90 (Coolaber, Beijing, China) were dissolved in a mixture of deionized water and absolute ethanol (1:1 volume ratio, total volume of 20 mL). After magnetic stirring for 12 h, a yellow gel formed. The gel was collected and dried in an oven at 100 °C for 8 h and then thoroughly ground to obtain the precursor. The precursor was calcined in a muffle furnace at a heating rate of 2 °C/min up to 900 °C and held for 5 h to produce BaMnO3. For YMn2O5, 2 mmol of Y(NO3)3·6H2O (AR, Xiya, Shandong, China), 4 mmol of Mn(CH3COO)2·4H2O, and 1 g of PVP-K90 were used, followed by calcination at 800 °C for 5 h to obtain YMn2O5.
3.2. Characterization
The crystal structure, morphology, and elemental distribution of the catalysts were characterized using powder X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), and energy-dispersive X-ray spectroscopy (EDS). Surface composition, bond strength, chemical states, and electronic structure were analyzed through X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, X-ray absorption near-edge structure (XANES), and extended X-ray absorption fine structure (EXAFS). Additionally, temperature-programmed desorption (TPD) was employed to investigate the desorption behavior of gases, providing insights into the reaction mechanisms.
XRD patterns were recorded on a Rigaku MiniFlex600 (Rigaku Corporation, Tokyo, Japan) Benchtop X-ray diffractometer with Cu Kα radiation (40 kV, 40 mA). The morphological observations of the samples were obtained through SEM (Zeiss Sigma 500, Carl Zeiss AG, Oberkochen, Germany). The local microstructures were further characterized using TEM and HRTEM (JEM-2800, JEOL Ltd., Musashino, Japan). The elemental composition and distribution were recorded using an EDS detector. XPS with a Thermo Escalab 250Xi (Thermo Fisher Scientific, Waltham, MA, USA) spectrometer equipped with an Al Ka X-ray source was used to detect the surface elemental composition of the samples. The vibration of chemical bonds on the surface of the samples was characterized using Raman spectroscopy (HORIBA LabRAM HR Evolution, 532 nm, HORIBA Scientific, Kyoto, Japan). The XANES and EXFAS spectra of Mn K-edge (E0 = 6539 eV) for catalysts were recorded at the BL11B beamline of the Shanghai Synchrotron Radiation Facility (SSRF). TPD was performed on an ALTAMIRA AMI-300 (Altamira Instruments, Pittsburgh, PA, USA) micro-reactor followed by a ThermoStar mass spectrum. More details about TPD are listed in Table S1.
3.3. Catalytic Activity Measurements
The catalytic activity for NO oxidation under lean conditions was investigated in a fixed-bed reactor. A total of 200 mg of catalyst was placed in the center of a quartz tube with an inner diameter of 8 mm for in situ analysis. The reactant gas mixture consisted of 500 ppm NO, 10% O2, and balanced N2, with a total flow rate of 200 mL/min, corresponding to a weight hourly space velocity (WHSV) of 6000 mL gcat−1 h−1. The catalyst was heated in a quartz fixed-bed reactor from 150 °C to 350 °C at a rate of 2 °C/min. During this process, data points were recorded at 50 °C intervals, with each temperature point held for ~15 min to allow the reaction to reach equilibrium. The composition of the gas at varying temperatures was analyzed using an Antaris™ IGS infrared spectrometer (Thermo Fisher Scientific). The conversion of NO to NO2 was defined as follows:
3.4. Computational Methods
All DFT calculations in this study were performed using the Vienna Ab-initio Simulation Package (VASP 5.4.4). To account for electron exchange and correlation effects, the generalized gradient approximation (GGA) with the Perdew–Burke–Ernzerhof (PBE) functional was applied [44,45,46]. Projector-augmented wave (PAW) potentials were used to describe electron–ion interactions, with valence electron configurations of 4s24p65s24d1 for Y, 5s25p66s2 for Ba, 3d54s2 for Mn, and 2s22p4 for O. A plane-wave energy cutoff of 400 eV was chosen to ensure convergence, with the energy convergence criterion set to 10−5 eV between electronic steps. Structural optimizations proceeded until the maximum Hellmann–Feynman force on each atom fell below 0.02 eV/Å. To avoid periodic boundary effects, a vacuum layer of 15 Å was introduced in the slab model.
4. Conclusions
Integrated with the theoretical calculations and experimental techniques, we first conducted a systematic comparison of the superior NO oxidation performance arising from the unique geometric and electronic structural effects of the Mn-Mn dimer active site. Specifically, a Mn-Mn dimer with a longer bond length suppresses the formation of NO3*, thereby altering the NO oxidation mechanism, whereas a Mn-Mn dimer with a shorter bond length is more favorable for activating the O-O bond. Since the rate-determining step in NO oxidation is often the desorption of NO2, a larger Mn-O-Mn bond angle facilitates charge transfer and promotes the desorption of NO2*. Consequently, MnO2 with a longer Mn-Mn bond length exhibits the best NO oxidation activity. Furthermore, our study explored the impact of dimer exposure modes, revealing that dual-exposed Mn-Mn dimers significantly accelerate reaction kinetics compared to single-site exposure. This research offers a crucial understanding of how the geometric arrangement of catalytic active sites influences the adsorption behavior of intermediates, establishing a significant structure–activity correlation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15040307/s1. Figure S1: (a) Charge transfer between Mn3+ dimers; (b) Charge transfer between Mn4+ dimers. Green, blue, and pink represent Y, Mn, and O atoms, respectively. Yellow indicates charge accumulation, and cyan indicates charge depletion. Figure S2: (a) The surface structure of YMn2O5 and YMnO3. The blue and pink represent Mn and O atoms, respectively. (b) The NO catalytic oxidation energy diagram of YMn2O5 (010) and YMnO3 (100). Figure S3: (a) Geometric structure of the YMn2O5 (121) surface, where cyan, blue, and pink represent Y, Mn, and O atoms, respectively. (b) Catalytic reaction pathway for NO oxidation on the YMn2O5 (121) surface. Figure S4: (a) O2 adsorption configuration on the YMn2O5 (121) surface, with cyan, blue, pink, and orange representing Y, Mn, O, and adsorbed O atoms, respectively. (b) Energy barrier for O2 activation on the YMn2O5 (121) surface. Figure S5: The crystal structure of MnO2, YMn2O5, and BaMnO3. The cyan, green, blue, and pink represent Y, Ba, Mn, and O atoms, respectively. Figure S6: XRD patterns of MnO2, YMn2O5, and BaMnO3. Figure S7: SEM images of (a,b) MnO2, (c,d) YMn2O5, and (e,f) BaMnO3. Figure S8: TEM images of (a) MnO2, (b) YMn2O5, and (c) BaMnO3. Figure S9: HRTEM images of (a) MnO2, (b) YMn2O5, and (c) BaMnO3. Figure S10: HAADF-STEM image and corresponding EDS elemental mapping of MnO2. Figure S11: HAADF-STEM image and corresponding EDS elemental mapping of YMn2O5. Figure S12: HAADF-STEM image and corresponding EDS elemental mapping of BaMnO3. Figure S13: The bond length of Mn-Mn and the angle of Mn-O-Mn for MnO2 (110), YMn2O5(121), and BaMnO3 (100). Figure S14: Mn K-edge XANES spectra of MnO2, YMn2O5, and BaMnO3. Figure S15: XPS survey spectrum of MnO2, YMn2O5, and BaMnO3. Figure S16: Mn 2p3/2 XPS spectra of MnO2, YMn2O5, and BaMnO3. Figure S17: Geometric configurations of single-exposed and double-exposed dimer sites for MnO2, YMn2O5, and BaMnO3. Cyan, light gray, blue, and pink represent Y, Ba, Mn, and O atoms, respectively. Figure S18: The adsorption configuration on MnO2 (110). The green, dark gray, blue, and pink represent absorbed O, N, Mn, and O atoms, respectively. Figure S19: The adsorption configuration on YMn2O5 (010). The green, dark gray, blue, cyan, and pink represent absorbed O, N, Mn, Y, and O atoms, respectively. Figure S20: The adsorption configuration on BaMnO3 (100). The green, dark gray, blue, light gray, and pink represent absorbed O, N, Mn, Ba, and O atoms, respectively. Figure S21: Geometric configurations for O2 activation at dimer sites on MnO2 (110), YMn2O5(010), and BaMnO3 (100). Blue, pink, and orange represent Mn, O, and adsorbed O atoms, respectively. Figure S22: Two reaction mechanisms for NO oxidation. Blue, pink, gray, and orange represent Mn, O, N, and adsorbed O atoms, respectively. Figure S23: The NO catalytic oxidation energy diagram of MnO2 (110), YMn2O5(010), and BaMnO3 (100) under Mechanism II. Figure S24: Structure of the second NO adsorption on the Mn-Mn dimer dual active site of MnO2 (110), YMn2O5(010), and BaMnO3 (100). Figure S25: The NO catalytic oxidation energy diagram of MnO2 (001), YMn2O5(121), and BaMnO3 (001). Figure S26: The bond length of Mn-Mn and the angle of Mn-O-Mn of MnO2 (001), YMn2O5(121), and BaMnO3 (001) during the reaction process. Figure S27: The variation in the Bader charge of the Mn-Mn site of MnO2 (001), YMn2O5(121), and BaMnO3 (001) during the reaction process. Figure S28: (a) The two adsorption energies of NO on the Mn-Mn dimer of MnO2 (001), YMn2O5(121), and BaMnO3 (001). (b) The two desorption energy of NO2 on the Mn-Mn dimer of MnO2 (001), YMn2O5(121), and BaMnO3 (001). The blue, pink, and orange represent Mn, O, and adsorbed O atoms, respectively. Figure S29: (a) The Bader charge and differential charge of O2 on MnO2 (001), YMn2O5 (121), and BaMnO3 (001). Isosurface = 0.002 eV/Å3. Yellow indicates charge accumulation and cyan indicates charge depletion. The blue and pink represent Mn and O atoms, respectively. (b) The dissociation energy barrier of O2 on the Mn-Mn dimer of MnO2 (001), YMn2O5(121), and BaMnO3 (001). Figure S30: The adsorption configuration on MnO2 (001). The green, dark gray, blue, and pink represent absorbed O, N, Mn, and O atoms, respectively. Figure S31: The adsorption configuration on YMn2O5 (121). The green, dark gray, blue, cyan, and pink represent absorbed O, N, Mn, Y, and O atoms, respectively. Figure S32: The adsorption configuration on BaMnO3 (001). The green, dark gray, blue, light gray, and pink represent absorbed O, N, Mn, Ba, and O atoms, respectively. Table S1: Summary of experimental conditions of TPD 50 mg samples for each test. Table S2: The bond length of Mn-Mn and the angle of Mn-O-Mn for MnO2 (001), YMn2O5(121), and BaMnO3 (001). Table S3: The bond length of Mn-Mn and the angle of Mn-O-Mn for MnO2 (110), YMn2O5(010), and BaMnO3 (100). Table S4: Surface element compositions of MnO2, YMn2O5 and BaMnO3. Table S5: The bond length of O-O and the ICOHP of O-O for MnO2 (110), YMn2O5(010), and BaMnO3 (100). Table S6: The binding energy of O2 and the ICOHP of Mn-O for MnO2 (110), YMn2O5(010), and BaMnO3 (100). Table S7: The dissociation energy barrier of NO3 to ONO2 on the Mn-Mn dimer of MnO2 (110), YMn2O5(010), and BaMnO3 (100). Table S8: The bond length of N-O and Mn-O when NO is adsorbed on MnO2 (110), YMn2O5(010), and BaMnO3 (100). Table S9: The bond length of O-O and the ICOHP of O-O for MnO2 (001), YMn2O5(121), and BaMnO3 (001). Table S10: The binding energy of O2 and the ICOHP of Mn-O for MnO2 (001), YMn2O5(121), and BaMnO3 (001). Table S11: The Bader charge of O2 for MnO2 (001), YMn2O5 (121), and BaMnO3 (001).
Author Contributions
Conceptualization, H.L. and R.W.; methodology, H.L. and W.W. (Wanying Wang); software, H.L.; validation, R.W., A.D. and X.W.; formal analysis, H.L., A.D., W.W. (Wanying Wang) and J.X.; investigation, H.L., R.W. and S.Z.; resources, H.L. and W.W. (Wanying Wang); data curation, H.L., W.W. (Wanying Wang) and C.Z.; writing—original draft preparation, H.L. and R.W.; writing—review and editing, H.L., R.W. and C.Z.; visualization, H.L., R.W. and C.Z.; supervision, C.Z. and W.W. (Weichao Wang); project administration, C.Z.; funding acquisition, W.W. (Wen Wang), R.H., G.C., C.Z. and W.W. (Weichao Wang). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development program (2022YFA1504000), the National Natural Science Foundation of China (22302101), the Fundamental Research Funds for the Central Universities (63185015), Shenzhen Science and Technology Program (JCYJ20210324121002007, JCYJ20230807151503007), the China Postdoctoral Science Foundation (2022M721699), Guangdong Basic and Applied Basic Research Foundation (2024A1515010347), and Yunnan Provincial Science and Technology Project at Southwest United Graduate School (No. 202402AO370001).
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials; further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Anqi Dong is employed by the company China Automotive Technology & Research Center Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhou, X.; Min, Y.; Zhao, C.; Chen, C.; Ke, M.-K.; Xu, S.-L.; Chen, J.-J.; Wu, Y.; Yu, H.-Q. Constructing sulfur and oxygen super-coordinated main-group electrocatalysts for selective and cumulative H2O2 production. Nat. Commun. 2024, 15, 193. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wang, Z.; Wu, H.; Xiao, M.; Liu, C.; Xing, W. High-density Ir single sites from rapid ligand transformation for efficient water electrolysis. Chin. J. Catal. 2024, 66, 223–232. [Google Scholar] [CrossRef]
- Li, W.; Hong, J.; Shang, J.; Yamashita, H.; Wei, C.; Hu, Y. In situ construction of CuBi-MOF derived heterojunctions with electron-rich effects enhances localized CO2 enrichment integrated with Si photocathodes for CO2 reduction. Appl. Catal. B Environ. 2025, 365, 124890. [Google Scholar] [CrossRef]
- Chang, J.; Shi, Y.; Wu, H.; Yu, J.; Jing, W.; Wang, S.; Waterhouse, G.I.N.; Tang, Z.; Lu, S. Oxygen radical coupling on short-range ordered Ru atom arrays enables exceptional activity and stability for acidic water oxidation. J. Am. Chem. Soc. 2024, 146, 12958–12968. [Google Scholar] [CrossRef]
- Fukui, K. Role of frontier orbitals in chemical reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef]
- Fukui, K.; Inagaki, S. Orbital interaction rationale for the role of catalysts. J. Am. Chem. Soc. 1975, 97, 4445–4452. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Rossmeisl, J.; Christensen, C.H. Towards the computational design of solid catalysts. Nat. Chem. 2009, 1, 37–46. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Vojvodic, A.; Nørskov, J.K.; Abild-Pedersen, F. Electronic structure effects in transition metal surface chemistry. Top. Catal. 2014, 57, 25–32. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, I.; Chorkendorff, J.; Nørskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Yoneyama, H.; Tamura, H. Catalytic activity for electrochemical reduction of oxygen of lanthanum nickel oxide and related oxides. J. Electroanal. Chem. Interfacial Electrochem. 1977, 79, 319–326. [Google Scholar] [CrossRef]
- Wei, C.; Feng, Z.; Scherer, G.G.; Barber, J.; Shao-Horn, Y.; Xu, Z.J. Cations in octahedral sites: A descriptor for oxygen electrocatalysis on transition-metal spinels. Adv. Mater. 2017, 29, 1606800. [Google Scholar] [CrossRef] [PubMed]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef]
- Grimaud, A.; May, K.J.; Carlton, C.E.; Lee, Y.-L.; Risch, M.; Hong, W.T.; Zhou, J.; Shao-Horn, Y. Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution. Nat. Commun. 2013, 4, 2439. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Kleis, J.; Rossmeisl, J.; Shao-Horn, Y.; Morgan, D. Prediction of solid oxide fuel cell cathode activity with first-principles descriptors. Energy Environ. Sci. 2011, 4, 3966–3970. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Chen, H.; Du, X.; Zhang, B.; Hong, Z.; Sun, S.; Wang, W. Progress and challenges toward the rational design of oxygen electrocatalysts based on a descriptor approach. Adv. Sci. 2020, 7, 1901614. [Google Scholar] [CrossRef]
- Li, R.; Hu, A.; Zhao, C.; Zhou, B.; He, M.; Fan, Y.; Chen, J.; Yan, Z.; Pan, Y.; Long, J. Tailoring mixed geometrical configurations in amorphous catalysts to activate oxygen electrode reactions of lithium-oxygen batteries. Chem. Eng. J. 2023, 452, 139162. [Google Scholar]
- Chen, S.; Guan, J. Structural regulation strategies of nitrogen reduction electrocatalysts. Chin. J. Catal. 2024, 66, 20–52. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Zheng, W.; Sun, Y.; Xie, Y.; Ma, K.; Zhang, Z.; Liao, Q.; Tian, Z.; Kang, Z.; et al. Identifying and interpreting geometric configuration-dependent activity of spinel catalysts for water reduction. J. Am. Chem. Soc. 2022, 144, 19163–19172. [Google Scholar] [CrossRef]
- Wang, L.; Hua, W.; Wan, X.; Feng, Z.; Hu, Z.; Li, H.; Niu, J.; Wang, L.; Wang, A.; Liu, J.; et al. Design rules of a sulfur redox electrocatalyst for lithium-sulfur batteries. Adv. Mater. 2022, 34, 2110279. [Google Scholar] [CrossRef]
- Wang, W.; McCool, G.; Kapur, N.; Yuan, G.; Shan, B.; Nguyen, M.; Graham, U.M.; Davis, B.H.; Jacobs, G.; Cho, K.; et al. Mixed-phase oxide catalyst based on Mn-mullite (Sm, Gd)Mn2O5 for NO oxidation in diesel exhaust. Science 2012, 337, 832–835. [Google Scholar]
- Zheng, Y.; Thampy, S.; Ashburn, N.; Dillon, S.; Wang, L.; Jangjou, Y.; Tan, K.; Kong, F.; Nie, Y.; Kim, M.J.; et al. Stable and active oxidation catalysis by cooperative lattice oxygen redox on SmMn2O5 mullite surface. J. Am. Chem. Soc. 2019, 141, 10722–10728. [Google Scholar] [PubMed]
- Ma, S.-J.; Wang, X.-W.; Chen, T.; Yuan, Z.-H. Effect of surface morphology on catalytic activity for NO oxidation of SmMn2O5 nanocrystals. Chem. Eng. J. 2018, 354, 191–196. [Google Scholar]
- Thampy, S.; Zheng, Y.; Dillon, S.; Liu, C.; Jangjou, Y.; Lee, Y.-J.; Epling, W.S.; Xiong, K.; Chabal, Y.J.; Cho, K.; et al. Superior catalytic performance of Mn-Mullite over Mn-Perovskite for NO oxidation. Catal. Today 2018, 310, 195–201. [Google Scholar]
- Yang, W.; Su, Z.A.; Xu, Z.; Yang, W.; Peng, Y.; Li, J. Comparative study of α-, β-, γ- and δ-MnO2 on toluene oxidation: Oxygen vacancies and reaction intermediates. Appl. Catal. B Environ. 2020, 260, 118150. [Google Scholar]
- Zhang, T.; Li, H.; Yang, Z.; Cao, F.; Li, L.; Chen, H.; Liu, H.; Xiong, K.; Wu, J.; Hong, Z.; et al. Electrospun YMn2O5 nanofibers: A highly catalytic activity for NO oxidation. Appl. Catal. B Environ. 2019, 247, 133–141. [Google Scholar]
- Ao, R.; Ma, L.; Guo, Z.; Yang, J.; Mu, L.; Yang, J.; Wei, Y. NO oxidation performance and kinetics analysis of BaMO3 (M=Mn, Co) perovskite catalysts. Environ. Sci. Pollut. Res. 2021, 28, 6929–6940. [Google Scholar]
- Stueben, B.L.; Cantrelle, B.; Sneddon, J.; Beck, J.N. Manganese K-edge XANES studies of Mn speciation in Lac des Allemands as a function of depth. Microchem. J. 2004, 76, 113–120. [Google Scholar]
- Celorrio, V.; Calvillo, L.; Granozzi, G.; Russell, A.E.; Fermin, D.J. AMnO3 (A = Sr, La, Ca, Y) perovskite oxides as oxygen reduction electrocatalysts. Top. Catal. 2018, 61, 154–161. [Google Scholar]
- Chaboy, J.; Prieto, C.; Hernando, M.; Parras, M.; González-Calbet, J. Ab initio X-ray absorption study of the manganese K-edge XANES spectra in Mn- and Zn-related hexagonal perovskites. Phys. Rev. B 2006, 74, 174433. [Google Scholar]
- Kim, K.; Jeong, J.; Azad, A.K.; Jin, S.B.; Kim, J.H. X-ray photoelectron spectroscopic study of direct reforming catalysts Ln0.5Sr0.5Ti0.5Mn0.5O3±d (Ln = La, Nd, and Sm) for high temperature-operating solid oxide fuel cell. Appl. Surf. Sci. 2016, 365, 38–46. [Google Scholar]
- Zheng, Y.; Liu, Q.; Shan, C.; Su, Y.; Fu, K.; Lu, S.; Han, R.; Song, C.; Ji, N.; Ma, D. Defective ultrafine MnOx nanoparticles confined within a carbon matrix for low-temperature oxidation of volatile organic compounds. Environ. Sci. Technol. 2021, 55, 5403–5411. [Google Scholar] [PubMed]
- Xiong, T.; Yu, Z.G.; Wu, H.; Du, Y.; Xie, Q.; Chen, J.; Zhang, Y.-W.; Pennycook, S.J.; Lee, W.S.V.; Xue, J. Defect engineering of oxygen-deficient manganese oxide to achieve high-performing aqueous zinc ion battery. Adv. Energy Mater. 2019, 9, 1803815. [Google Scholar]
- Chen, X.; Li, Y.; Fu, W.; Tian, S.; Yang, Y.; Yang, K.; Xu, X.; Zhang, X. Oriented generation of singlet oxygen in H2O2 activation for water decontamination: Regulation of oxygen vacancies over α-MnO2 nanocatalysts. Environ. Sci. Nano 2023, 10, 1428–1440. [Google Scholar] [CrossRef]
- Litvinchuk, A.P. Lattice dynamics and spin-phonon interactions in multiferroic RMn2O5: Shell model calculations. J. Magn. Magn. Mater. 2009, 321, 2373–2377. [Google Scholar]
- Zhao, C.; Zhu, A.; Gao, S.; Wang, L.; Wan, X.; Wang, A.; Wang, W.-H.; Xue, T.; Yang, S.; Sun, D.; et al. Phonon resonance catalysis in NO oxidation on Mn-based mullite. ACS Catal. 2022, 12, 12113–12122. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, X.; Yu, M.; Wang, A.; Wang, L.; Xue, L.; Liu, J.; Yang, Z.; Wang, W. Cooperative catalysis toward oxygen reduction reaction under dual coordination environments on intrinsic AMnO3-type perovskites via regulating stacking configurations of coordination units. Adv. Mater. 2020, 32, 2006145. [Google Scholar]
- Sacchetti, A.; Baldini, M.; Postorino, P.; Martin, C.; Maignan, A. Raman spectroscopy on cubic and hexagonal SrMnO3. J. Raman Spectrosc. 2006, 37, 591–596. [Google Scholar]
- Joly, J.P.; Méhier, C.; Béré, K.E.; Abon, M. TPD study of labile oxygen on a (VO)2P2O7 catalyst active in n-butane partial oxidation. Appl. Catal. A Gen. 1998, 169, 55–63. [Google Scholar]
- Li, B.; Yang, Q.; Peng, Y.; Chen, J.; Deng, L.; Wang, D.; Hong, X.; Li, J. Enhanced low-temperature activity of LaMnO3 for toluene oxidation: The effect of treatment with an acidic KMnO4. Chem. Eng. J. 2019, 366, 92–99. [Google Scholar]
- Yang, J.; Zhang, J.; Liu, X.; Duan, X.; Wen, Y.; Chen, R.; Shan, B. Origin of the superior activity of surface doped SmMn2O5 mullites for NO oxidation: A first-principles based microkinetic study. J. Catal. 2018, 359, 122–129. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Cai, G.; Wang, Y.; Gao, Z.; Hao, R.; Bao, X.; Zhao, C.; Wang, W. Spatial geometric effect driven by the different [MnO6] octahedra entity stacking configurations to facilitate the catalytic decomposition of H2O2 in wastewater. Appl. Surf. Sci. 2024, 669, 160589. [Google Scholar] [CrossRef]
- Yang, R.; Guo, Z.; Cai, L.; Zhu, R.; Fan, Y.; Zhang, Y.; Han, P.; Zhang, W.; Zhu, X.; Zhao, Q.; et al. Investigation into the phase-activity relationship of MnO2 nanomaterials toward ozone-assisted catalytic oxidation of toluene. Small 2021, 17, 2103052. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. 1996, 6, 15–50. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).