Abstract
Formic acid has recently been considered one of the most promising liquid organic hydrogen carriers (LOHCs). Its decomposition to obtain H2 has been fruitfully investigated during recent years using catalysts of a very diverse nature. Most of these catalysts lack stability, so finding stable materials under reaction conditions is highly desirable but challenging. In the present study, catalysts based on Pd nanoparticles supported on C3N4-modified activated carbon derived from biomass residues were developed, characterized, and assessed in the decomposition of formic acid in the liquid phase. These catalysts were prepared using a straightforward method that allowed different nitrogen contents to be achieved in the support and avoided the ex situ reduction in the Pd precursor. The results of the catalytic tests indicated the positive role of incorporating C3N4, leading to catalysts that displayed much better performance than the C3N4-free counterpart. The incorporation of C3N4 resulted in catalysts with small and well-distributed Pd nanoparticles, leaching resistance and modified electronic properties of the Pd species. As a result, promising catalytic activity was observed in the developed materials. Pd/AC_C3N4(19) attained an initial TOF of 2893 h−1, and it preserved most of its catalytic activity for at least six consecutive reaction cycles, which is a remarkable characteristic of the developed catalytic system.
1. Introduction
Formic acid (HCOOH, FA) dehydrogenation has recently shown great promise in the chemical hydrogen storage context, which is due to the properties of that molecule (high hydrogen content, low toxicity and flammability under ambient, biodegradable nature, easy transportation and storage, among others), and also to the fact that such a reaction can proceed under mild conditions upon selection of suitable catalysts [1]. However, dehydrogenation is not the only path for the decomposition of FA, and the side dehydration reaction, which produces H2O and CO, should be avoided to achieve maximum H2 yield and avoid the poisoning of the catalytic active sites by CO molecules. The performance of both homogeneous and heterogeneous catalysts with numerous compositions has been studied in the last two decades [2,3], but the remarkable role of Pd-based catalysts supported on several types of carbon materials becomes obvious while revisiting the recent literature [4,5,6,7,8,9]. In particular, it was found that the performance of carbon-supported catalysts may be significantly enhanced when nitrogen is present in the materials [10,11,12,13]. That strategy has been explored using different carbon materials, including activated carbon (AC), mesoporous carbon, carbon nanofibers, hierarchically porous carbon, carbon xerogels, reduced graphene oxide, etc. [4,5]. The incorporation of nitrogen functional groups in carbon material can be performed by in situ or post-synthesis doping methods, and the experimental strategy differs from study to study [14]. However, achieving significant nitrogen content is sometimes challenging. We have observed that the incorporation of graphitic carbon nitride (g-C3N4) to Multiwalled Carbon Nanotubes (MWCNs) is a promising approach for the preparation of supports with moderate and high nitrogen contents, and the resulting catalysts based on Pd nanoparticles displayed interesting results towards the dehydrogenation of formic acid [15]. As for the Pd active phase, it is usually in the form of nanoparticles, so the atomic efficiency is maximized [16,17,18]. The preparation of those nanoparticles can be carried out by several experimental protocols, but the standard impregnation is the most common synthesis method followed by a reduction in the metal phases, which can be performed using a hydrogen flow at high temperatures or a solid reducing agent such as NaBH4.
In the present study, we extend that synthetic strategy to the preparation of catalysts based on C3N4-modified activated carbon derived from biomass residues. The content of C3N4, and hence the content of N, could be controlled by changing the amount of C3N4 precursor (i.e., dicyandiamide). The developed catalysts were prepared by the standard impregnation of the metal precursor and skipping the subsequent reduction in the metal phase, which significantly simplifies the synthesis of the materials compared with the protocols that are normally used. The derived catalysts displayed interesting performance in the dehydrogenation of formic acid.
2. Results and Discussion
Table 1 lists the results of the support characterization, including the apparent surface area and the nitrogen and C3N4 contents present in the samples, which was determined from the information provided by elemental analysis. As can be seen, supports with N contents ranging from 1.6 to 13.4 wt % were successfully prepared, and those contents are much higher than those previously found in studies devoted to investigation of the effect of the incorporation of nitrogen functional groups in the performance of catalysts towards the decomposition of formic acid [12,19,20].

Table 1.
BET surface area and composition of the supports determined by elemental analysis.
Figure 1 presents the N2 adsorption–desorption isotherms of AC, g-C3N4, and AC_C3N4 supports. As can be extracted from the shape of the isotherm of the AC sample, which is a combination of Type I and IV isotherms, the material has micropores and mesopores, as indicated by the sharp N2 uptake at low relative pressures and the slope and the hysteresis loop at higher relative pressures, respectively. C3N4 is a non-porous solid. As for the AC_C3N4 supports, adsorption capacity decreases with increasing C3N4 content and the shape of the isotherm confirms the presence of micropores, while the contribution of mesopores is less pronounced with increasing C3N4 content in the samples.
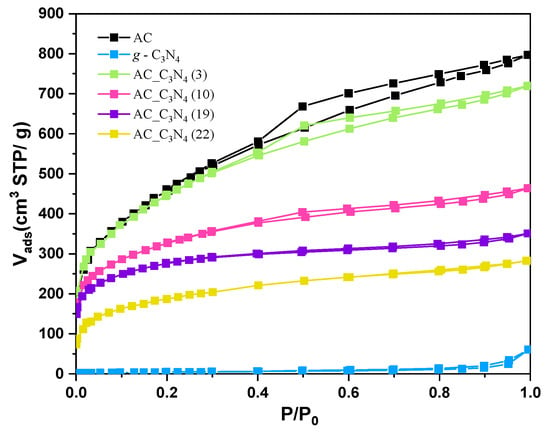
Figure 1.
N2 adsorption–desorption isotherms at −196 °C.
The BET surface area (SBET) obtained from the almond shell (AS)-derived activated carbon was 1675 m2/g, while, as expected, a very low surface area was attained for the pristine g-C3N4. The BET surface area calculated for AC_C3N4-based supports only followed the mixture law for that support with the lowest C3N4 content (AC_C3N4(3)), while much lower values than those expected from the relative proportion of the AC and C3N4 in the materials and their apparent surface area were achieved for the other AC_C3N4 supports. That indicated that the porosity of AC may be partially occupied or blocked by C3N4 even for relatively low contents of C3N4.
As for the catalytic tests, the results of the gas evolution profiles attained in cycle 1 and cycle 2, for the reaction performed at 75 °C, are plotted in Figure 2. As seen in Figure 2a, in the first cycle, none of the samples are active during the first reaction minutes, so an induction time is needed for the samples to catalyze the decomposition of formic acid. After ~3 min of reaction, the catalysts start to become active and the gas evolves. However, all the samples displayed poor activity in the first reaction cycle, which may be related to the absence of Pd0 species in the catalyst. These results suggest that the non-reduced Pd species initially present in the catalysts (notice that no reducing agents were used in the synthesis of the materials) display a moderate activity towards the decomposition of formic acid, and that, as it was previously observed in previous studies [21,22], both Pd0 and Pd2+ are required to attain efficient catalysts for the studied application. Hence, from these results, it seems that, under the experimental conditions used in this study, a first reaction cycle, in which Pd2+ species would be partially reduced to Pd0 with the H2 generated, is needed as a conditioning step for the catalysts to attain optimum working conditions.

Figure 2.
Gas evolution profiles attained in (a) cycle 1 and (b) cycle 2 performed at 75 °C.
As for the results achieved in the second reaction cycle, no induction time was observed and the gas evolved from the beginning of the reaction. As can be seen, Pd/AC and Pd/C3N4 displayed much poorer performance than those samples based on C3N4-modified activated carbon, and only 7.8 and 19.6 mL were achieved after 30 min of reaction for Pd/AC and Pd/C3N4, respectively. Much better activity was observed for Pd/AC_C3N4 catalysts, which is reflected in both the initial reaction rate and the final volume of gas generated after 30 min of reaction. Such an enhancement displayed by AC_C3N4-based catalysts compared to those supported on pristine materials may be related to the combination of the properties of the AC, responsible for a large apparent surface area, and those of C3N4, which provides abundant nitrogen functional groups and, hence, anchorage sites for the metal species and basicity to the final catalysts.
The initial TOF attained by all the studied catalysts in the second cycle is shown in Figure 3. As can be seen in that figure, the initial TOF values of Pd/AC_C3N4 catalysts increased with the C3N4 present in the samples, until reaching the best results with the Pd/AC_C3N4(19) sample, which showed an initial TOF value of 2893 h−1, which is higher than those values found in the literature for some other metal-based catalysts (for instance, Au2Pd8/SBA-15-Amine has an initial TOF of 1786 h−1 at 50 °C [23], Pt0.5Pd0.5/NH2-N-rGO yields a TOF value of 511 h−1 at 25 °C [24], Pd@CNFs has a TOF of 75 h−1 at 50 °C [25]). The further addition of C3N4 (sample Pd/AC_C3N4(22)) resulted in a decay in the catalytic activity, probably due to the significant blockage of the porosity of the AC_C3N4(22) support and the C3N4 stacking.
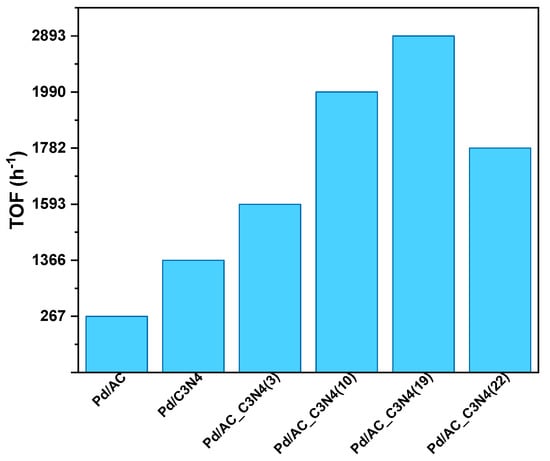
Figure 3.
Initial TOF (h−1) values.
The formation of Pd nanoparticles under reaction conditions was corroborated by recording TEM micrographs of the spent catalysts (used in six consecutive cycles). Representative micrographs of Pd/AC, Pd/C3N4, and Pd/AC_C3N4 catalysts are depicted in Figure 4. All the C3N4-containing samples showed small and well-dispersed nanoparticles, and the average particle size was determined to be 4.2 ± 2.0, 4.8 ± 2.0, 3.8 ± 1.4, 2.3 ± 0.6, and 3.5 ± 1.2 nm for Pd/C3N4, Pd/AC_C3N4(3), Pd/AC_C3N4(10), Pd/AC_C3N4(19), and Pd/AC_C3N4(22), respectively. In contrast, very large aggregates were found in the Pd/AC sample. As for the histograms, the particle size distribution was relatively wide for all the samples except for Pd/AC_C3N4(19), which, aside from the smallest average size of the nanoparticles, also displayed the narrowest size distribution.
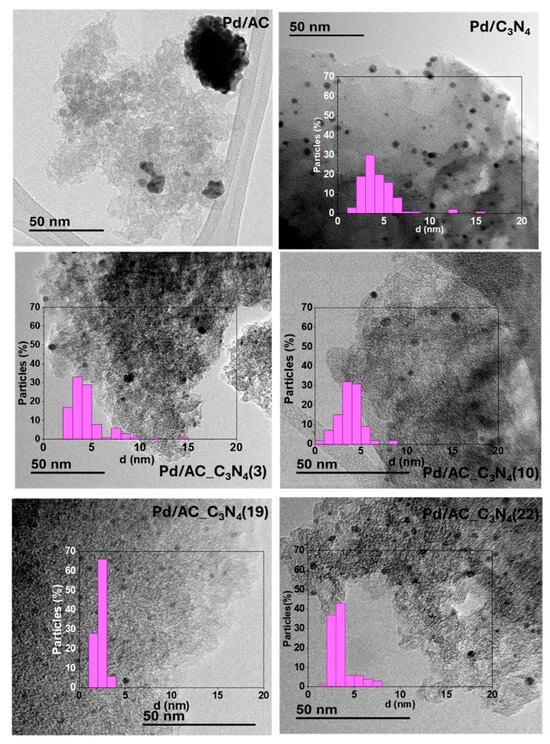
Figure 4.
Representative TEM micrographs and their corresponding histogram with the nanoparticle size distribution.
The leaching resistance of these catalysts was demonstrated by investigating the Pd content of the fresh and spent samples, which was determined by ICP analysis (Table 2). The Pd loss in the used samples is very low when C3N4 is presented in the AC and could be attributed to the properties of the AC_C3N4 support, which combines the porosity provided by the AC that permits an efficient distribution of Pd species, and nitrogen functionalities provided by the C3N4 component that increases the Pd catalyst-interaction.

Table 2.
Pd content (wt %) determined in fresh and used catalysts.
As for the electronic properties of Pd species, they were checked by XPS analysis. Pd spectra are plotted in Figure 5. The relative content of Pd0 and oxidized Pd species is also indicated in Figure 5. The presence of Pd0 is due to the in situ reduction that takes place during the reduction with the H2 that evolved from the decomposition of formic acid. As can be seen, the most significant contribution in Pd/AC is that of Pd0, which indicates that, in the N-free sample, Pd species are very efficiently reduced under reaction conditions. However, different behaviours are observed in the C3N4-containing samples. Pd/C3N4 presents an XPS spectrum in which three different contributions can be distinguished, which are centred at 334.8, 336.5, and 337.9 eV. The first signal (334.8 eV) is related to the presence of reduced Pd species, while the other two are due to electron-deficient species probably in the form of Pd2+ and Pd2+-N, as observed in other studies [15]. As for the Pd/AC_C3N4 catalysts, the signals are shifted towards higher binding energies in Pd/AC_C3N4(19) and Pd/AC_C3N4(22), compared to Pd/AC_C3N4(3) and Pd/AC_C3N4(10), suggesting that the electronic properties of Pd species are dependent on the composition of the support, and that higher N contents may favour the presence of more electron-deficient Pd species, which agrees with the role of nitrogen functional groups in stabilizing Pd2+ species [22,26]. It is important to mention that the presence of such electron-deficient Pd species is important to attain good catalytic performance in the studied application. The role of Pd2+ in catalyzing the decomposition of formic acid has already been claimed in several studies, and it is frequently related to the favoured adsorption of formate ions on the surface of Pd nanoparticles, which is usually described as the first reaction step in the generally accepted mechanism for the decomposition of formic acid [22,27,28].
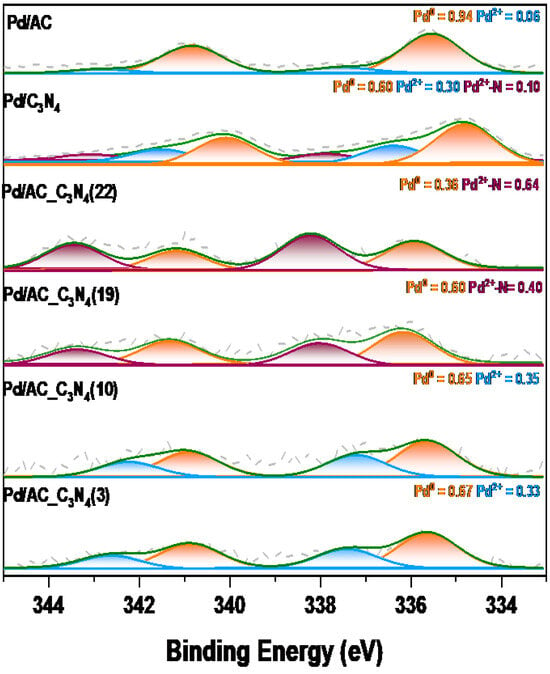
Figure 5.
Pd 3d XPS spectra of the spent catalysts.
The stability of the Pd/AC_C3N4(19) catalyst was assessed by carrying out six consecutive reaction cycles, and the results of the gas generation profiles are plotted in Figure 6. As seen in those profiles, after the first reaction cycle, which, as mentioned before, could be considered a “conditioning” step for the catalysts to reach the operating conditions, the subsequent profiles are quite similar and the catalyst preserved most of its activity after six runs. These results are very promising since the stability of the catalysts is frequently the weak point of the system studied in the present application. Such stability may be related to the properties of the materials, which combine the good development of the porosity and high nitrogen content with the subsequent stabilizing effects of the Pd species that result in small and well-distributed nanoparticles and improved leaching resistance, as well as Pd nanoparticles with modified electronic properties of the Pd species.

Figure 6.
Gas evolution profile achieved with Pd/AC_C3N4(19) catalyst.
3. Materials and Methods
The activated carbon used for the preparation of the support was synthesized from almond shells using H₃PO₄-assisted Hydrothermal Carbonization (HTC) under the experimental conditions used in our previous study [29]. g-C₃N₄ was synthesized from dicyandiamide following the protocol reported elsewhere [30,31]. AC_C₃N₄ supports with different compositions were prepared using a straightforward method. For this, 0.8 g of AC was dispersed in 20 mL of water using an ultrasonic bath for 30 min. Subsequently, an aqueous solution of dicyandiamide was added to the AC dispersion, and the mixture was stirred for 1 h at room temperature. The resulting product was then heated to 100 °C and continuously stirred until complete solvent removal. The dicyandiamide concentration was varied to obtain supports with different compositions. After water evaporation, the solid was gently ground to ensure a homogeneous blend. The material was then heat-treated at 520 °C for 4 h (with a heating rate of 5 °C/min) under a nitrogen atmosphere to convert the precursor into C₃N₄. Finally, the composite supports were ground and collected. They were designated as AC_C₃N₄(x), where “x” represents the C₃N₄ wt % calculated from the nitrogen content determined by elemental analysis.
3.1. Synthesis of Pd/AC_C3N4 Catalysts
Pd/AC_C3N4 catalysts with a nominal Pd loading of 1 wt % were synthesized using standard impregnation with Pd(OAc)2 as the metal precursor. For that, 1 g of AC_C3N4 (x) was dispersed in 40 mL of acetone, they were mixed with a solution of Pd(OAc)2, and the mixture was stirred for 2 h. After that, it was filtered, washed, and dried overnight. The same experimental conditions were used for the preparation of the reference samples (Pd/AC and Pd/C3N4).
3.2. Characterization of the Materials
The surface area of the supports was calculated from nitrogen adsorption–desorption isotherms performed at −196 °C using the BET equation (SBET). The samples were previously outgassed at 250 °C for 4 h to remove any possible adsorbed impurity. The nitrogen content was determined by elemental analysis using a microanalyzer Thermo Finnigan Flash 1112 (Thermo Fisher Scientific, Dreieich, Germany). The Pd loading present in the catalysts was determined by Inductively Coupled Plasma–Optical Emission Spectroscopy (ICP-OES) with a Perkin-Elmer Optima 4300 system (PerkinElmer, Waltham, MA, USA). The morphology of the samples was observed by Transmission Electron Microscopy (TEM). The average size of the nanoparticles was determined from representative micrographs registered by TEM after measuring the nanoparticles with ImageJ (version 1.54i) software. X-ray photoelectron spectroscopy (XPS) analysis was carried out in a VG-Microtech Multilab 3000 spectrometer (VG Scientific, Sussex, UK) equipped with a semispherical electron analyzer and a Mg Kα (hν = 1253.6 eV) 300 W X-ray source. The binding energies referred to the C 1s line at 284.6 eV. The different Pd species and their relative contents were calculated from the deconvoluted spectra. Avantage (version Avantage5.9929) software was used for the analysis of XPS spectra. The deconvolution of the spectra was carried out by using Gaussian functions with 20% of the Lorentzian component. The FWHM of the peaks was kept at 1.6 eV.
3.3. Catalytic Tests
The developed catalysts were assessed in the decomposition of formic acid in the liquid phase. For that, the gas evolution (i.e., H2 and CO2) was followed during 30 min of reaction at 75 °C using a burette system. The catalytic tests were performed with 0.15 g of sample and an aqueous solution of formic acid and sodium formate with a molar ratio of 9 to 1 (final concentration of 1 M). The performance of Pd/AC and Pd/C3N4 was also checked for comparison. Initial TOF values (h−1) were calculated with the following equation:
The produced H2 (mole) was the mole of H2 generated after 2 min of reaction, and Pd atoms were determined from the Pd content obtained by ICP-OES analysis in the fresh catalysts.
4. Conclusions
A set of catalysts based on Pd nanoparticles loaded on C3N4-modified activated carbon derived from biomass residues were prepared using a simple method and skipping the ex situ reduction in the Pd precursor prior to the reaction. Catalysts with high nitrogen contents were achieved by using different amounts of C3N4 precursor in the synthesis. The developed materials displayed a good development of porosity, and also interesting properties of the Pd nanoparticles (i.e., small size, leaching resistance, and modified electronic properties), which were dependent on the composition of the support. The beneficial role of the AC_C3N4 support was demonstrated by the comparison of the catalytic activity of Pd/AC_C3N4 samples with both Pd/AC and Pd/C3N4 reference materials, and it may be attributed to the combination of both a large surface area provided by the AC and the effect of nitrogen functionalities coming from C3N4. As a result, very interesting catalytic performance was attained, especially by Pd/AC_C3N4(19), which had an initial TOF of 2893 h−1 and preserved catalytic activity for at least six consecutive reaction cycles.
Author Contributions
Conceptualization, M.N.-G. and D.C.-A.; Methodology, M.N.-G. and M.B.-V.; Software, M.B.-V.; Validation, M.B.-V., M.N.-G. and D.C.-A.; Formal Analysis, M.B.-V., M.N.-G. and D.C.-A.; Investigation, M.B.-V., M.N.-G. and D.C.-A.; Resources, M.N.-G. and D.C.-A.; Data Curation, M.B.-V. and M.N.-G.; Writing—Original Draft Preparation, M.B.-V. and M.N.-G.; Writing—Review and Editing, M.N.-G. and D.C.-A.; Visualization, M.N.-G. and D.C.-A.; Supervision, M.N.-G. and D.C.-A.; Project Administration, M.N.-G. and D.C.-A.; Funding Acquisition, M.N.-G. and D.C.-A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the PID2021-123079OB-I00 project funded by MICIU/AEI/10.13039/501100011033 and ERDF, EU. M. Bernal-Vela acknowledges the PRE2022-104824 grant funded by MICIU/AEI/10.13039/501100011033 and by “ESF+”. M. Navlani-García acknowledges the Ramón y Cajal contract (RYC2021-034199-I) funded by MCIN/AEI/10.13039/501100011033 and the European Union «NextGenerationEU»/PRTR.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Navlani-García, M.; Salinas-Torres, D.; Cazorla-Amorós, D. Hydrogen Production from Formic Acid Attained by Bimetallic Heterogeneous PdAg Catalytic Systems. Energies 2019, 12, 4027. [Google Scholar] [CrossRef]
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chem. Rev. 2018, 118, 372–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; He, P.; Wu, J.; Chen, N.; Pan, C.; Shi, E.; Jia, H.; Hu, T.; He, K.; Cai, Q.; et al. Reviews on Homogeneous and Heterogeneous Catalysts for Dehydrogenation and Recycling of Formic Acid: Progress and Perspectives. Energy Fuels 2023, 37, 17075–17093. [Google Scholar] [CrossRef]
- Navlani-García, M.; Mori, K.; Kuwahara, Y.; Yamashita, H. Recent Strategies Targeting Efficient Hydrogen Production from Chemical Hydrogen Storage Materials over Carbon-Supported Catalysts. NPG Asia Mater. 2018, 10, 277–292. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Navlani-García, M.; Mori, K.; Kuwahara, Y.; Cazorla-Amorós, D.; Yamashita, H. Recent Advances in Catalytic Hydrogen Generation from Formic Acid Using Carbon-Based Catalysts. In Advanced Nanomaterials and Their Applications in Renewable Energy, 2nd ed.; Louise-Liu, J., Yan, T., Bashir, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 273–301. [Google Scholar] [CrossRef]
- Martin, C.; Quintanilla, A.; Vega, G.; Casas, J.A. Formic Acid-to-Hydrogen on Pd/AC Catalysts: Kinetic Study with Catalytic Deactivation. Appl. Catal. B 2022, 317, 121802. [Google Scholar] [CrossRef]
- Ortega-Murcia, A.; Navlani-García, M.; Morallón, E.; Cazorla-Amorós, D. MWCNT-Supported PVP-Capped Pd Nanoparticles as Efficient Catalysts for the Dehydrogenation of Formic Acid. Front. Chem. 2020, 8, 359. [Google Scholar] [CrossRef]
- Navlani-García, M.; Mori, K.; Nozaki, A.; Kuwahara, Y.; Yamashita, H. Screening of Carbon-Supported PdAg Nanoparticles in the Hydrogen Production from Formic Acid. Ind. Eng. Chem. Res. 2016, 55, 7612–7620. [Google Scholar] [CrossRef]
- Navlani-García, M.; Mori, K.; Nozaki, A.; Kuwahara, Y.; Yamashita, H. Investigation of Size Sensitivity in the Hydrogen Production from Formic Acid over Carbon-Supported Pd Nanoparticles. Chem. Sel. 2016, 1, 1879–1886. [Google Scholar] [CrossRef]
- Poldorn, P.; Wongnongwa, Y.; Zhang, R.-Q.; Nutanong, S.; Tao, L.; Rungrotmongkol, T.; Jungsuttiwong, S. Mechanistic Insights into Hydrogen Production from Formic Acid Catalyzed by Pd@N-Doped Graphene: The Role of the Nitrogen Dopant. Int. J. Hydrogen Energy 2023, 48, 16341–16357. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.H. Hydrogen Production from Formic Acid Dehydrogenation over a Pd Supported on N-Doped Mesoporous Carbon Catalyst: A Role of Nitrogen Dopant. Appl. Catal. A Gen. 2020, 608, 117887. [Google Scholar] [CrossRef]
- Bi, Q.-Y.; Lin, J.-D.; Liu, Y.-M.; He, H.-Y.; Huang, F.-Q.; Cao, Y. Dehydrogenation of Formic Acid at Room Temperature: Boosting Palladium Nanoparticle Efficiency by Coupling with Pyridinic-Nitrogen-Doped Carbon. Angew. Chem. Inter. Ed. 2016, 55, 11849–11853. [Google Scholar] [CrossRef]
- Navlani-García, M.; Salinas-Torres, D.; Mori, K.; Léonard, A.F.; Kuwahara, Y.; Job, N.; Yamashita, H. Insights on Palladium Decorated Nitrogen-Doped Carbon Xerogels for the Hydrogen Production from Formic Acid. Catal. Today 2019, 324, 90–96. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Navlani-García, M.; Mori, K.; Kuwahara, Y.; Yamashita, H. Nitrogen-Doped Carbon Materials as a Promising Platform toward the Efficient Catalysis for Hydrogen Generation. Appl. Catal. Gen. A 2019, 571, 25–41. [Google Scholar] [CrossRef]
- Navlani-García, M.; Salinas-Torres, D.; Vázquez-Álvarez, F.D.; Cazorla-Amorós, D. Formic Acid Dehydrogenation Attained by Pd Nanoparticles-Based Catalysts Supported on MWCNT-C3N4 Composites. Catal. Today 2022, 397–399, 428–435. [Google Scholar] [CrossRef]
- Jeon, H.; Chung, Y.-M. Hydrogen Production from Formic Acid Dehydrogenation over Pd/C Catalysts: Effect of Metal and Support Properties on the Catalytic Performance. Appl. Catal. B 2017, 210, 212–222. [Google Scholar] [CrossRef]
- Chen, Z.; Stein, C.A.M.; Qu, R.; Rockstroh, N.; Bartling, S.; Weiß, J.; Kubis, C.; Junge, K.; Junge, H.; Beller, M. Designing a Robust Palladium Catalyst for Formic Acid Dehydrogenation. ACS Catal. 2023, 13, 4835–4841. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Q. Metal-Nanoparticle-Catalyzed Hydrogen Generation from Formic Acid. Acc. Chem. Res. 2017, 50, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhao, Y.; Liu, J.; Chen, G.; Yan, B.; Cheng, Z.; Qi, J.; Wang, L. Pd Nanoparticles on Functionalized Carbon Nanotubes for Enhanced Formic Acid Hydrogen Production under Ambient Conditions. Surfaces Interfaces 2024, 51, 104833. [Google Scholar] [CrossRef]
- Deng, M.; Yang, A.; Ma, J.; Yang, C.; Cao, T.; Yang, S.; Yao, M.; Liu, F.; Wang, X.; Cao, J. Enhanced Catalytic Performance of N-Doped Carbon Sphere-Supported Pd Nanoparticles by Secondary Nitrogen Source Regulation for Formic Acid Dehydrogenation. ACS Appl. Mater. Interfaces 2022, 14, 18550–18560. [Google Scholar] [CrossRef]
- Lv, Q.; Meng, Q.; Liu, W.; Sun, N.; Jiang, K.; Ma, L.; Peng, Z.; Cai, W.; Liu, C.; Ge, J.; et al. Pd–PdO Interface as Active Site for HCOOH Selective Dehydrogenation at Ambient Condition. J. Phys. Chem. C 2018, 122, 2081–2088. [Google Scholar] [CrossRef]
- Chaparro-Garnica, J.; Navlani-García, M.; Salinas-Torres, D.; Morallón, E.; Cazorla-Amorós, D. Highly Stable N-Doped Carbon-Supported Pd-Based Catalysts Prepared from Biomass Waste for H2 Production from Formic Acid. ACS Sustain. Chem. Eng. 2020, 8, 15030–15043. [Google Scholar] [CrossRef]
- Xu, L.; Yao, F.; Luo, J.; Wan, C.; Ye, M.; Cui, P.; An, Y. Facile Synthesis of Amine-Functionalized SBA-15-Supported Bimetallic Au-Pd Nanoparticles as an Efficient Catalyst for Hydrogen Generation from Formic Acid. RSC Adv. 2017, 7, 4746–4752. [Google Scholar] [CrossRef]
- Li, S.-J.; Zhou, Y.-T.; Kang, X.; Liu, D.-X.; Gu, L.; Zhang, Q.-H.; Yan, J.-M.; Jiang, Q. A Simple and Effective Principle for a Rational Design of Heterogeneous Catalysts for Dehydrogenation of Formic Acid. Adv. Mater. 2019, 31, 1806781. [Google Scholar] [CrossRef]
- Abdullah, M.; Aziz, I.; Noshear Arshad, S.; Zaheer, M. Development of Functionalized Carbon Nanofibers with Integrated Palladium Nanoparticles for Catalytic Hydrogen Generation. Results Chem. 2022, 4, 100554. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Zacharska, M.; Shlyakhova, E.V.; Chuvilin, A.L.; Guo, Y.; Beloshapkin, S.; Okotrub, A.V.; Bulusheva, L.G. Single Isolated Pd2+ Cations Supported on N-Doped Carbon as Active Sites for Hydrogen Production from Formic Acid Decomposition. ACS Catal. 2016, 6, 681–691. [Google Scholar] [CrossRef]
- Mori, K.; Dojo, M.; Yamashita, H. Pd and Pd–Ag Nanoparticles within a Macroreticular Basic Resin: An Efficient Catalyst for Hydrogen Production from Formic Acid Decomposition. ACS Catal. 2013, 3, 1114–1119. [Google Scholar] [CrossRef]
- Riquelme-García, P.; Chaparro-Garnica, J.; Navlani-García, M.; Cazorla-Amorós, D. Exploring the Effects Behind the Outstanding Catalytic Performance of PdAg Catalysts Supported on Almond Shell-Derived Activated Carbon Towards the Dehydrogenation of Formic Acid. ChemCatChem 2024, 16, e202400160. [Google Scholar] [CrossRef]
- Chaparro-Garnica, J.; Navlani-García, M.; Salinas-Torres, D.; Berenguer-Murcia, Á.; Morallón, E.; Cazorla-Amorós, D. Efficient Production of Hydrogen from a Valuable CO2-Derived Molecule: Formic Acid Dehydrogenation Boosted by Biomass Waste-Derived Catalysts. Fuel 2022, 320, 123900. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, M.; Navlani-García, M.; Kuwahara, Y.; Mori, K.; Yamashita, H. Palladium Nanoparticles Supported on Titanium-Doped Graphitic Carbon Nitride for Formic Acid Dehydrogenation. Chem. Asian J. 2017, 12, 860–867. [Google Scholar] [CrossRef]
- Navlani-García, M.; Verma, P.; Kuwahara, Y.; Kamegawa, T.; Mori, K.; Yamashita, H. Visible-Light-Enhanced Catalytic Activity of Ru Nanoparticles over Carbon Modified g-C3N4. J. Photochem. Photobiol. A Chem. 2018, 358, 327–333. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).