N-Doped Porous Graphene Film Decorated with Palladium Nanoparticles for Enhanced Electrochemical Detection of Hydrogen Peroxide
Abstract
:1. Introduction
2. Results and Discussion
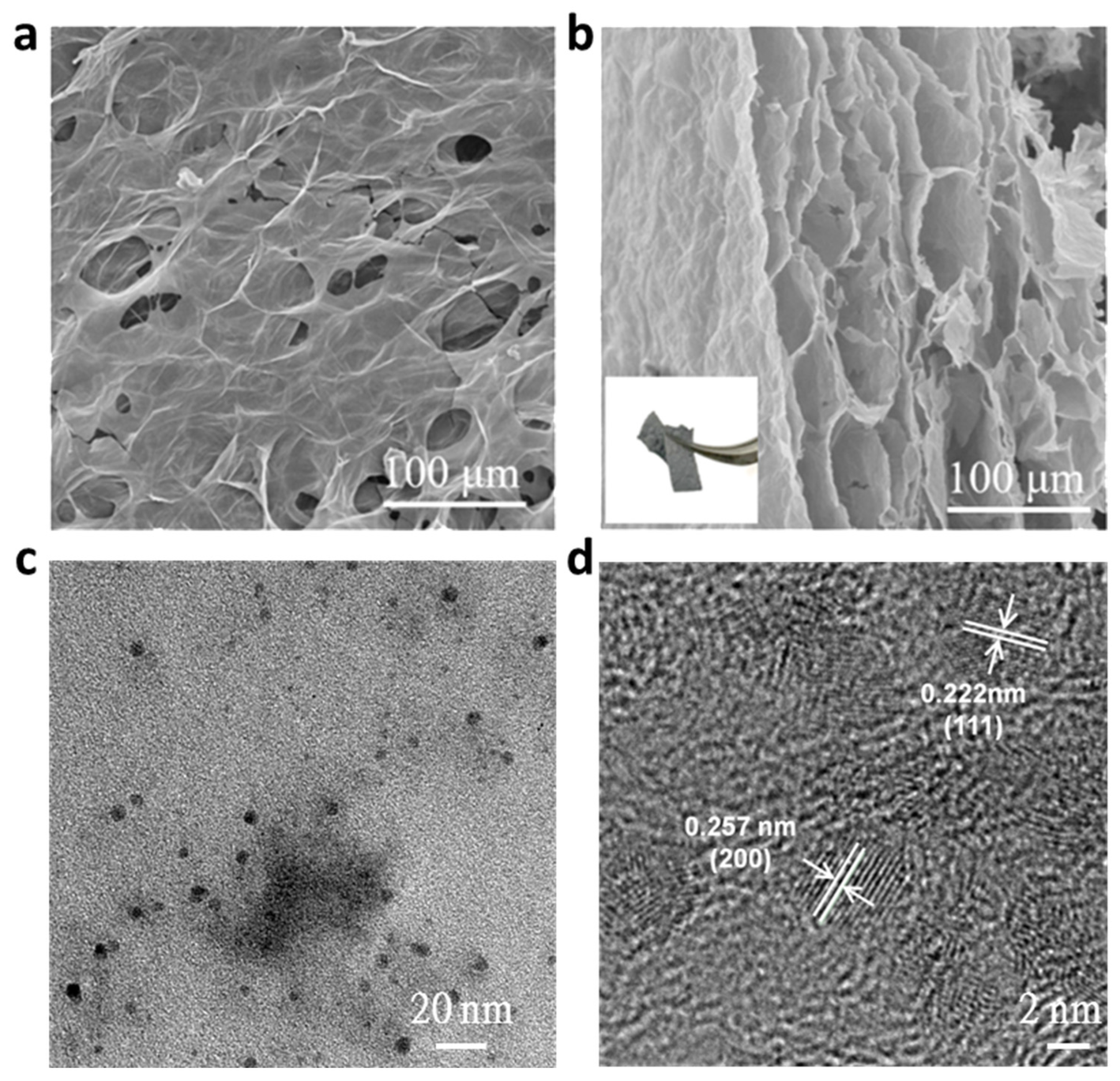
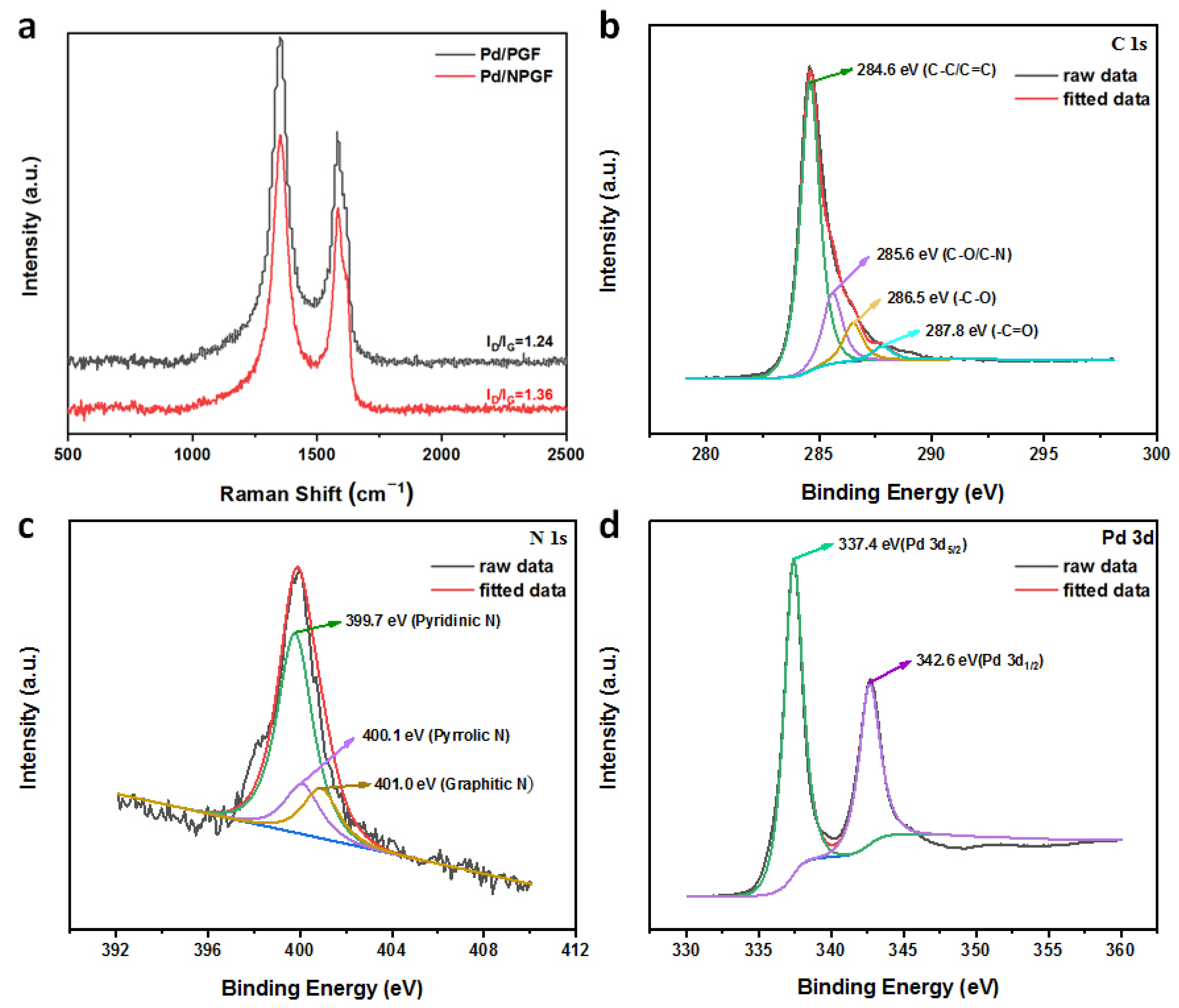
2.1. Morphological and Structural Characterization
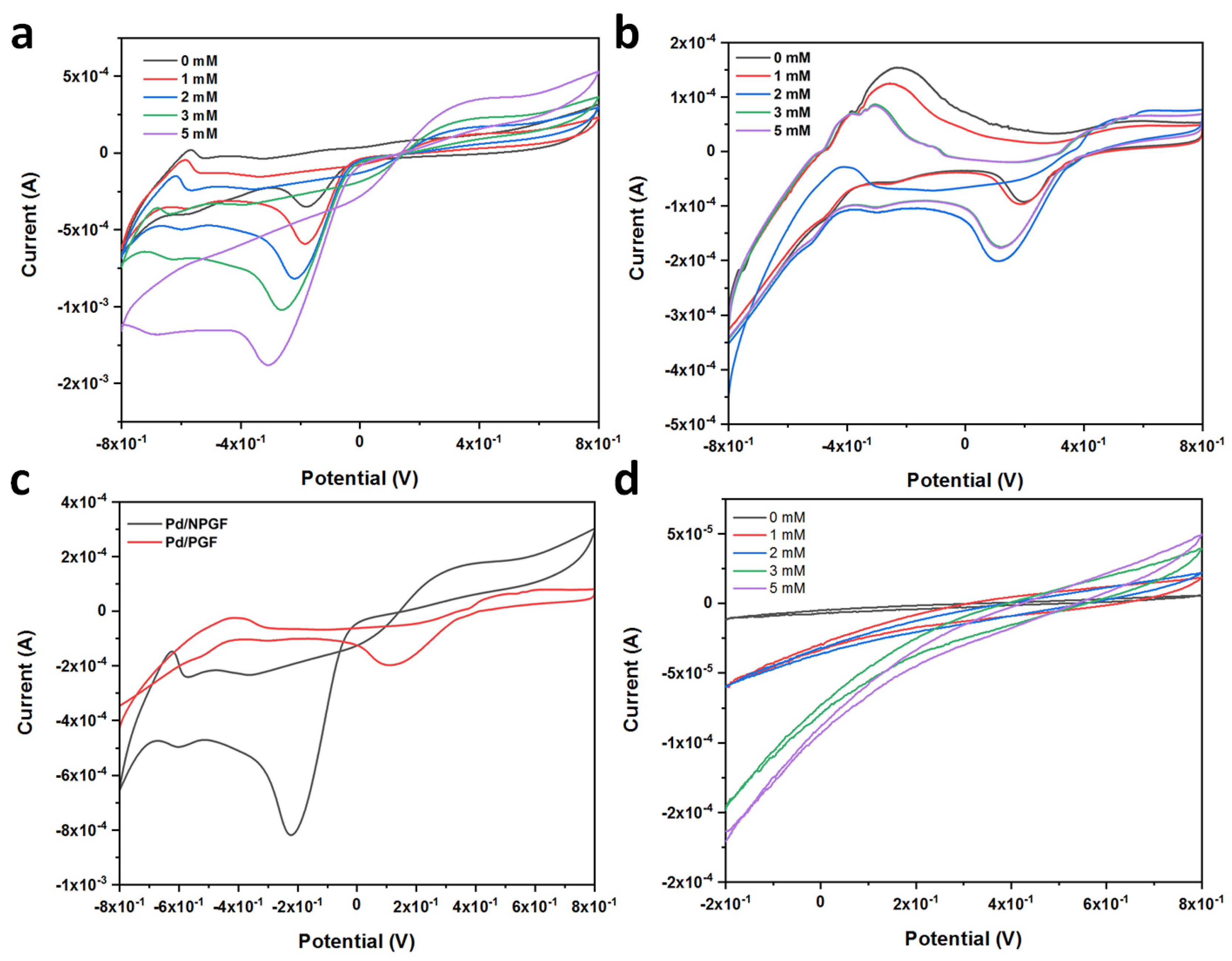
2.2. Electrochemical Behaviors of the Pd/NPGF Modified Electrodes
3. Materials and Methods
3.1. Reagents and Materials
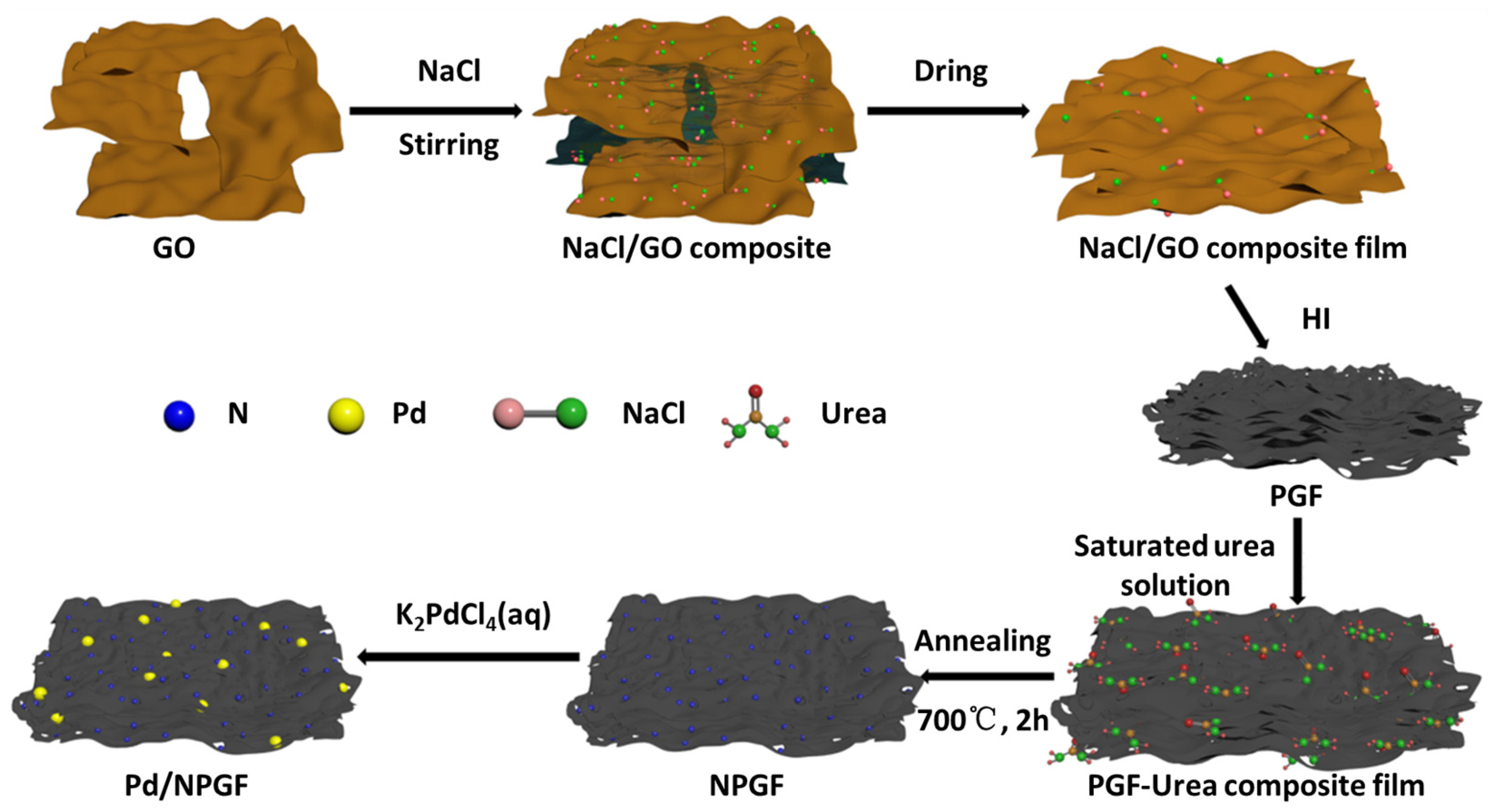
3.2. Preparation of N-Doped Porous Graphene Film (NPGF)
3.3. Preparation of Pd Nanoparticle-Decorated N-Doped Porous Graphene Film (Pd/NPGF)
3.4. Structure and Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [PubMed]
- Li, H.G.; Xia, N. The role of oxidative stress in cardiovascular disease caused by social isolation and loneliness. Redox Biol. 2020, 37, 101585. [Google Scholar]
- Rhee, S.G. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [PubMed]
- Kurowska, E.; Brzózka, A.; Jarosz, M.; Sulka, G.D.; Jaskuła, M. Silver nanowire array sensor for sensitive and rapid detection of H2O2. Electrochim. Acta 2013, 104, 439–447. [Google Scholar]
- Guascito, M.R.; Filippo, E.; Malitesta, C.; Manno, D.; Serra, A.; Turco, A. A new amperometric nanostructured sensor for the analytical determination of hydrogen peroxide. Biosens. Bioelectron. 2008, 24, 1057–1063. [Google Scholar]
- Chen, H.; Zhang, H.; Chi, K.; Zhao, Y. Pyrimidine-containing covalent organic frameworks for efficient photosynthesis of hydrogen peroxide via one-step two electron oxygen reduction process. Nano Res. 2024, 17, 9498–9506. [Google Scholar]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar]
- Salimi, A.; Hallaj, R.; Soltanian, S.; Mamkhezri, H. Nanomolar detection of hydrogen peroxide on glassy carbon electrode modified with electrodeposited cobalt oxide nanoparticles. Anal. Chim. Acta 2007, 594, 24–31. [Google Scholar]
- López Marzo, A.M.; Mayorga-Martinez, C.C.; Pumera, M. 3D-printed graphene direct electron transfer enzyme biosensors. Biosens. Bioelectron. 2020, 151, 111980. [Google Scholar]
- Bai, J.; Jiang, X. A facile one-pot synthesis of copper sulfide-decorated reduced graphene oxide composites for enhanced detecting of H2O2 in biological environments. Anal. Chem. 2013, 85, 8095–8101. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Wu, J.; Li, L.; Ren, F.; Dai, R.; Dai, F.; Dong, S.; Yang, Z. High-loading Bi-MOF nanoparticles anchored on biomass waste-derived carbon for sensitive non-enzymic nitrite sensing. Microchim Acta 2025, 192, 125. [Google Scholar] [CrossRef]
- Tian, F.; Xu, B.; Zhu, L.; Zhu, G. Hydrogen peroxide biosensor with enzyme entrapped within electrodeposited polypyrrole based on mediated sol-gel derived composite carbon electrode. Anal. Chim. Acta 2001, 443, 9–16. [Google Scholar]
- Luo, L.; Li, F.; Zhu, L.; Zhang, Z.; Ding, Y.; Deng, D. Non-enzymatic hydrogen peroxide sensor based on MnO2-ordered mesoporous carbon composite modified electrode. Electrochim. Acta 2012, 77, 179–183. [Google Scholar]
- Xi, J.; Zhang, Y.; Ye, T.; Xiao, J.; Fang, J.; Han, M.; Zhao, A.; Zhang, Y. Self-supported electrochemical sensor based on uniform palladium nanoparticles functionalized porous graphene film for monitoring H2O2 released from living cells. Anal. Bioanal. Chem. 2024, 416, 6995–7006. [Google Scholar]
- Wan, Y.; Wang, Y.; Wu, J.; Zhang, D. Graphene oxide sheet-mediated silver enhancement for application to electrochemical biosensors. Anal. Chem. 2011, 83, 648–653. [Google Scholar]
- Yao, Y.; Ping, J. Recent advances in graphene-based freestanding paper-like materials for sensing applications. TrAC Trends Anal. Chem. 2018, 105, 75–88. [Google Scholar]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance. Angew. Chem. Int. Ed. 2012, 51, 11496–11500. [Google Scholar]
- Liu, Y.; Li, M.; Zhou, B.; Xuan, X.; Li, H. Flexible B, N co-doped graphene electrodes for electrochemical detection of serotonin in bodily fluids. Electrochim. Acta 2023, 457, 142494. [Google Scholar]
- Xi, J.B.; Zhang, Y.; Wang, Q.J.; Xiao, J.; Chi, K.; Duan, X.M.; Chen, J.; Tang, C.Y.; Sun, Y.M.; Xiao, F.; et al. Multi-element doping design of high-efficient carbocatalyst for electrochemical sensing of cancer cells. Sens. Actuators B Chem. 2018, 273, 108–117. [Google Scholar]
- Kaushal, S.; Kaur, M.; Kaur, N.; Kumari, V.; Singh, P.P. Heteroatom-doped graphene as sensing materials: A mini review. RSC Adv. 2020, 10, 28608–28629. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Jia, A.; Song, J.; Cao, S.; Wang, N.; Liu, X. Metal-support interactions in heterogeneous catalytic hydrogen production of formic acid. Chem. Eng. J. 2023, 474, 145612. [Google Scholar]
- Huang, J.; Li, X.; Xie, R.-H.; Tan, X.; Xi, J.; Tian, F.; Liu, P.; Willum Hansen, T.; Bai, Z.-W. Defect anchoring of atomically dispersed Pd on nitrogen-doped holey carbon nanotube for catalytic hydrogenation of nitroarenes. Appl. Surf. Sci. 2023, 615, 156344. [Google Scholar]
- Zhao, J.; Zhang, A.; Li, Y.; Hu, H.; Xi, J. Pd nanoparticles decorated N-doped holey graphene assembled on aluminum silicate fibers agglomerate for catalytic continuous-flow reduction of nitroarenes. Chem. Eng. Sci. 2024, 286, 119656. [Google Scholar]
- Chen, X.; Wu, G.; Chen, J.; Chen, X.; Xie, Z.; Wang, X. Synthesis of “clean” and well-dispersive Pd nanoparticles with excellent electrocatalytic property on graphene oxide. J. Am. Chem. Soc. 2011, 133, 3693–3695. [Google Scholar] [CrossRef]
- Meku, E.; Du, C.; Wang, Y.; Du, L.; Sun, Y.; Kong, F.; Yin, G. Concentration gradient Pd-Ir-Ni/C electrocatalyst with enhanced activity and methanol tolerance for oxygen reduction reaction in acidic medium. Electrochim. Acta 2016, 192, 177–187. [Google Scholar]
- Zheng, G.; Altman, E.I. The oxidation of Pd(111). Surf. Sci. 2000, 462, 151–168. [Google Scholar]
- Zhang, J.; Jia, K.; Huang, Y.; Liu, X.; Xu, Q.; Wang, W.; Zhang, R.; Liu, B.; Zheng, L.; Chen, H.; et al. Intrinsic wettability in pristine graphene. Adv. Mater. 2022, 34, 2103620. [Google Scholar]
- Zhao, A.; She, J.; Xiao, C.; Xi, J.; Xu, Y.; Manoj, D.; Sun, Y.; Xiao, F. Green and controllable synthesis of multi-heteroatoms co-doped graphene fiber as flexible and biocompatible microelectrode for in situ electrochemical detection of biological samples. Sens. Actuators B Chem. 2021, 335, 129683. [Google Scholar]
- Hu, C.; Dai, L. Doping of carbon materials for metal-free electrocatalysis. Adv. Mater. 2019, 31, 1804672. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Han, L.; Wang, X.; Li, W.; Guo, H.; Wei, H. Nanozyme sensor arrays based on heteroatom-doped graphene for detecting pesticides. Anal. Chem. 2020, 92, 7444–7452. [Google Scholar] [PubMed]
- Hu, H.; Liu, P.; Cao, S.; You, L.; Zhang, N.; Xi, J.; Guo, S.; Zhou, K. Single metal atoms anchored on N-doped holey graphene as efficient dual-active-component catalysts for nitroarene reduction. Adv. Funct. Mater. 2024, 34, 2307162. [Google Scholar]
- Ma, X.; Wang, J.; Zhu, Z.; Wang, N.; Wang, C.; Nie, G. A two-pronged strategy to boost the capacitive deionization performance of nitrogen-doped porous carbon nanofiber membranes. Desalination 2025, 594, 118293. [Google Scholar]
- Du, S.; Cao, S.; Chen, W.; Xi, J. Fibrous catalyst based on atomic Pd and N-doped holey graphene functionalized cotton fiber for continuous-flow reaction. Int. J. Biol. Macromol. 2024, 280, 136049. [Google Scholar]
- Wang, M.; Liang, L.; Liu, X.; Sun, Q.; Guo, M.; Bai, S.; Xu, Y. Selective semi-hydrogenation of alkynes on palladium-selenium nanocrystals. J. Catal. 2023, 418, 247–255. [Google Scholar]
- Li, J.; Cai, T.; Feng, Y.; Liu, X.; Wang, N.; Sun, Q. Subnanometric bimetallic Pt-Pd clusters in zeolites for efficient hydrogen production and selective tandem hydrogenation of nitroarenes. Sci China Chem 2024, 67, 2911–2917. [Google Scholar] [CrossRef]
- Hu, J.; Li, F.; Wang, K.; Han, D.; Zhang, Q.; Yuan, J.; Niu, L. One-step synthesis of graphene-AuNPs by HMTA and the electrocatalytical application for O2 and H2O2. Talanta 2012, 93, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhou, S.; Zhang, X.; Zeng, T.; Zhang, W.; Li, H.; Liu, X.; Zhao, P. One pot synthesis of nitrogen-doped hollow carbon spheres with improved electrocatalytic properties for sensitive H2O2 sensing in human serum. Sens. Actuators B Chem. 2018, 270, 530–537. [Google Scholar]
- Yao, H.; Zhang, W.-T.; Yan, T.-Y.; Li, X.-Q.; Wang, X.-F. Electrochemical sensor for detection of hydrogen peroxide based on Cu-doped ZIF-8 material modified with chitosan and cytochrome c. Int. J. Electrochem. Sci. 2022, 17, 220654. [Google Scholar]
- Mathivanan, D.; Shalini Devi, K.S.; Sathiyan, G.; Tyagi, A.; da Silva, V.A.O.P.; Janegitz, B.C.; Prakash, J.; Gupta, R.K. Novel polypyrrole-graphene oxide-gold nanocomposite for high performance hydrogen peroxide sensing application. Sens. Actuators A Phys. 2021, 328, 112769. [Google Scholar]
- Li, H.; Zhao, H.; He, H.; Shi, L.; Cai, X.; Lan, M. Pt-Pd bimetallic nanocoral modified carbon fiber microelectrode as a sensitive hydrogen peroxide sensor for cellular detection. Sens. Actuators B Chem. 2018, 260, 174–182. [Google Scholar] [CrossRef]
- Chen, D.; Zhuang, X.; Zhai, J.; Zheng, Y.; Lu, H.; Chen, L. Preparation of highly sensitive Pt nanoparticles-carbon quantum dots/ionic liquid functionalized graphene oxide nanocomposites and application for H2O2 detection. Sens. Actuators B Chem. 2018, 255, 1500–1506. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, J.; Sheng, Q. One-step synthesis of Au@Pt-Graphene nanocomposites and their electrochemical properties. J. Nanosci. Nanotechnol. 2019, 19, 5546–5553. [Google Scholar] [CrossRef]
- Yang, Q.; Li, N.; Li, Q.; Chen, S.; Wang, H.-L.; Yang, H. Amperometric sarcosine biosensor based on hollow magnetic Pt-Fe3O4@C nanospheres. Anal. Chim. Acta 2019, 1078, 161–167. [Google Scholar] [CrossRef] [PubMed]
- He, F.-G.; Yin, J.-Y.; Sharma, G.; KUmar, A.; Stadler, F.J.; Du, B. Facile fabrication of hierarchical rGO/PANI@PtNi nanocomposite via microwave-assisted treatment for non-enzymatic detection of hydrogen peroxide. Nanomaterials 2019, 9, 1109. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Sun, F.; Yang, N. 3D heteroatom-doped graphene-wrapped flexible carbon fiber microsensor for real-time hydrogen peroxide detection in live cancer cells. Appl. Surf. Sci. 2023, 611, 155655. [Google Scholar] [CrossRef]
- Qi, C.; Luo, Y.; Dong, Y. Synergistic effects of Fe-Se dual single-atom sites for boosting electrochemical nonenzymatic H2O2 sensing. Appl. Surf. Sci. 2023, 637, 157900. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Liu, Y.; Cao, X.; Zhang, F.; Xia, J.; Wang, Z. MOF-derived porous carbon nanozyme-based flexible electrochemical sensing system for in situ and real-time monitoring of H2O2 released from cells. Talanta 2024, 266, 125132. [Google Scholar] [CrossRef]
- Lee, G.-G.; Hong, H.-G. Catalytically synthesized prussian blue by a one-step copolymerization of polydopamine-polypyrrole for electrochemical sensing of hydrogen peroxide. Electrochim. Acta 2023, 465, 142949. [Google Scholar] [CrossRef]
- Nasir, A.; Khalid, S.; Mazare, A.; Yasin, T. Non-enzymatic hydrogen peroxide detection on a novel nanohybrid composite of chitosan and grafted graphene oxide. Mater. Res. Bull. 2023, 167, 112427. [Google Scholar] [CrossRef]
- Li, G.; Zheng, Y.; Hu, G.; Chen, B.; Gu, Y.; Yang, J.; Yang, H.; Hu, F.; Li, C.; Guo, C. Boosting photo-electro-fenton process via atomically dispersed iron sites on graphdiyne for invitro hydrogen peroxide detection. Small 2023, 19, 2301540. [Google Scholar]
- Cheng, D.; Wu, H.; Feng, C.; Ding, Y.; Mei, H. Bifunctional photoelectrochemical sensor based on Bi/Bi2S3/BiVO4 for detecting hexavalent chromium and hydrogen peroxide. Sens. Actuators B Chem. 2022, 353, 131108. [Google Scholar] [CrossRef]
- Du, Y.; Jia, M.; Zhang, X.; Ma, M.; Zhang, X.; Li, C.; Wang, S.; Zhang, J.; Li, D.; He, W.; et al. Graphene oxide covered WO3 nanosheet arrays as photoelectrode for photoelectrochemical detection of trace H2O2. ACS Appl. Nano Mater. 2024, 7, 7896–7905. [Google Scholar]
- Murugan, P.; Sundramoorthy, A.K.; Nagarajan, R.D.; Atchudan, R.; Shanmugam, R.; Ganapathy, D.; Arya, S.; Alothman, A.A.; Ouladsmane, M.; Murthy, H.C.A. Electrochemical detection of H2O2 on graphene nanoribbons/cobalt oxide nanorods-modified electrode. J. Nanomater. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Ju, J.; Chen, W. In situ growth of surfactant-free gold nanoparticles on nitrogen-doped graphene quantum dots for electrochemical detection of hydrogen peroxide in biological environments. Anal. Chem. 2015, 87, 1903–1910. [Google Scholar]
- Xu, F.; Sun, Y.; Zhang, Y.; Shi, Y.; Wen, Z.; Li, Z. Graphene-Pt nanocomposite for nonenzymatic detection of hydrogen peroxide with enhanced sensitivity. Electrochem. Commun. 2011, 13, 1131–1134. [Google Scholar]
- Bhaduri, S.N.; Ghosh, D.; Chatterjee, S.; Biswas, R.; Bhaumik, A.; Biswas, P. Fe(iii)-incorporated porphyrin-based conjugated organic polymer as a peroxidase mimic for the sensitive determination of glucose and H2O2. J. Mater. Chem. B 2023, 11, 8956–8965. [Google Scholar]

| Electrode Material | Linear Range (mM) | Sensitivity (μA·mM−1·cm−2) | LOD (μM) | Reference |
|---|---|---|---|---|
| N-HCS/GCE | 0.05–29.5, 29.5–47.5 | - | 0.00002 | [38] |
| Cu-doped ZIF-8/Chitosan | 0.01–5.2 | - | 3.7 | [39] |
| PPy-GO-AuNPs/GCE | 2.5–25 | 41.35 | 5 | [40] |
| Pt-Pd/CFME | 0.005–3.92 | 11.6 | 0.42 | [41] |
| PtNPs-CDs/IL-GO/GCE | 0.001–0.9 | 40.3 | 0.1 | [42] |
| Au@Ptgraphene | 0.5–22.3 | - | 0.2 | [43] |
| Pt-Fe3O4@C | 0.0005–0.06 | 48.8 | 0.43 | [44] |
| rGO/PANI@Pt | 0.1–0.126 | - | 1.1 | [45] |
| CF@3D-NBG | 0.0005–4.25 | 233 | 0.1 | [46] |
| Fe1Se1/NC | 0.02–13 | 1508.6 | 11.5 | [47] |
| PCNSs | 0.001–20 | 41.2 | 0.76 | [48] |
| PB-PDA-PPY/GCE | 0.005–11.6 | 112 | 3.64 | [49] |
| CS/GOP | 0.0005–0.2 | 0.77 | 17.3 | [50] |
| Fe-GDY | 0.0001–48.160 | - | 0.033 | [51] |
| Bi-BSV | 0.001–130 | - | 0.089 | [52] |
| GO/WO3 | 0.1 to 2.0 | 8.14 | 100 | [53] |
| GNR/Co3O4 | 0.01–0.200 | 5100 | 1.27 | [54] |
| Au NPs−N-GQDs | 0.00025–13.327 | 186.22 | 0.12 | [55] |
| Graphene-Pt | 0.002–0.71 | - | 0.5 | [56] |
| Fe-DMP-POR | 0.005–2 | 947.67 | 3.16 | [57] |
| Pd/NPGF | 0.005–36.3 | 176.7 | 2.3 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zheng, S.; Xiao, J.; Xi, J. N-Doped Porous Graphene Film Decorated with Palladium Nanoparticles for Enhanced Electrochemical Detection of Hydrogen Peroxide. Catalysts 2025, 15, 298. https://doi.org/10.3390/catal15040298
Zhang Y, Zheng S, Xiao J, Xi J. N-Doped Porous Graphene Film Decorated with Palladium Nanoparticles for Enhanced Electrochemical Detection of Hydrogen Peroxide. Catalysts. 2025; 15(4):298. https://doi.org/10.3390/catal15040298
Chicago/Turabian StyleZhang, Yue, Shi Zheng, Jian Xiao, and Jiangbo Xi. 2025. "N-Doped Porous Graphene Film Decorated with Palladium Nanoparticles for Enhanced Electrochemical Detection of Hydrogen Peroxide" Catalysts 15, no. 4: 298. https://doi.org/10.3390/catal15040298
APA StyleZhang, Y., Zheng, S., Xiao, J., & Xi, J. (2025). N-Doped Porous Graphene Film Decorated with Palladium Nanoparticles for Enhanced Electrochemical Detection of Hydrogen Peroxide. Catalysts, 15(4), 298. https://doi.org/10.3390/catal15040298








