Efficient Hydrogenolysis of Lignin into Aromatic Monomers over N-Doped Carbon Supported Co and Dual-Phase MoxC Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
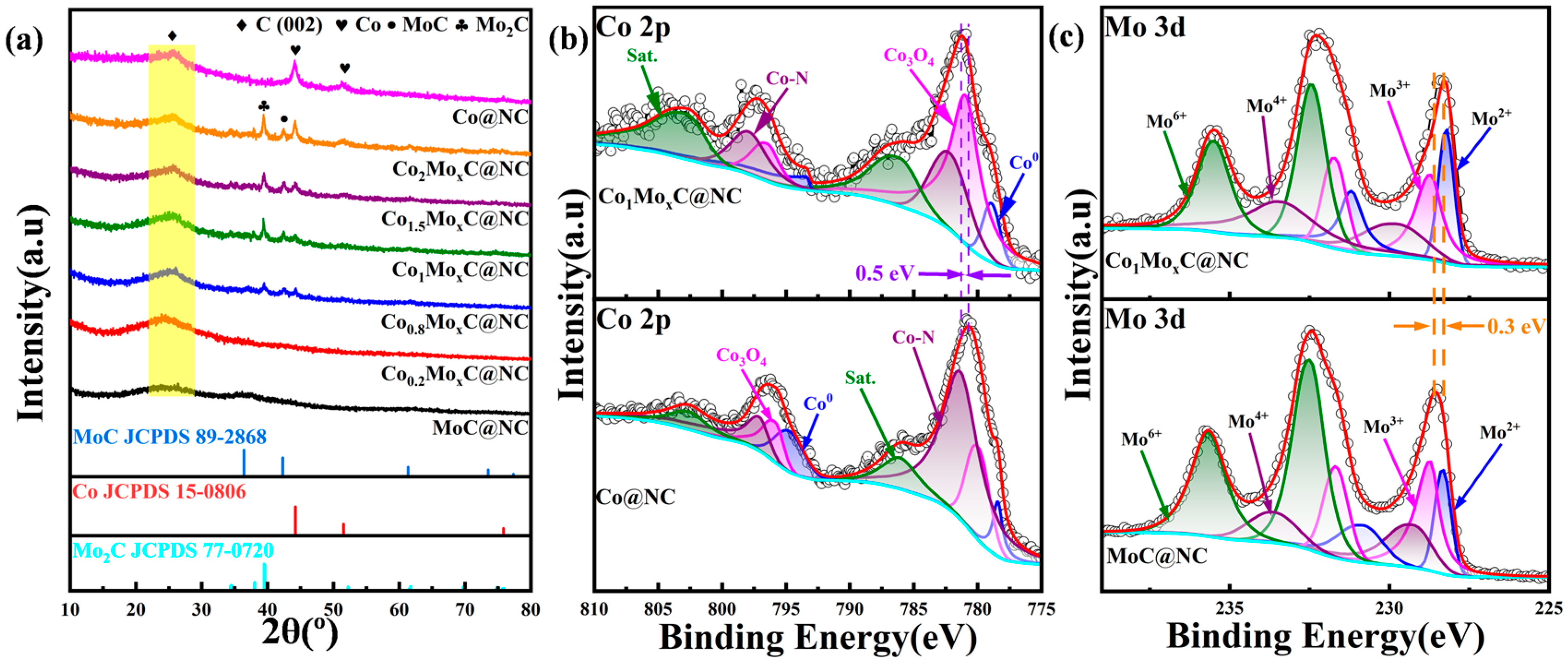
2.1. Structure of CoMoxC Catalysts
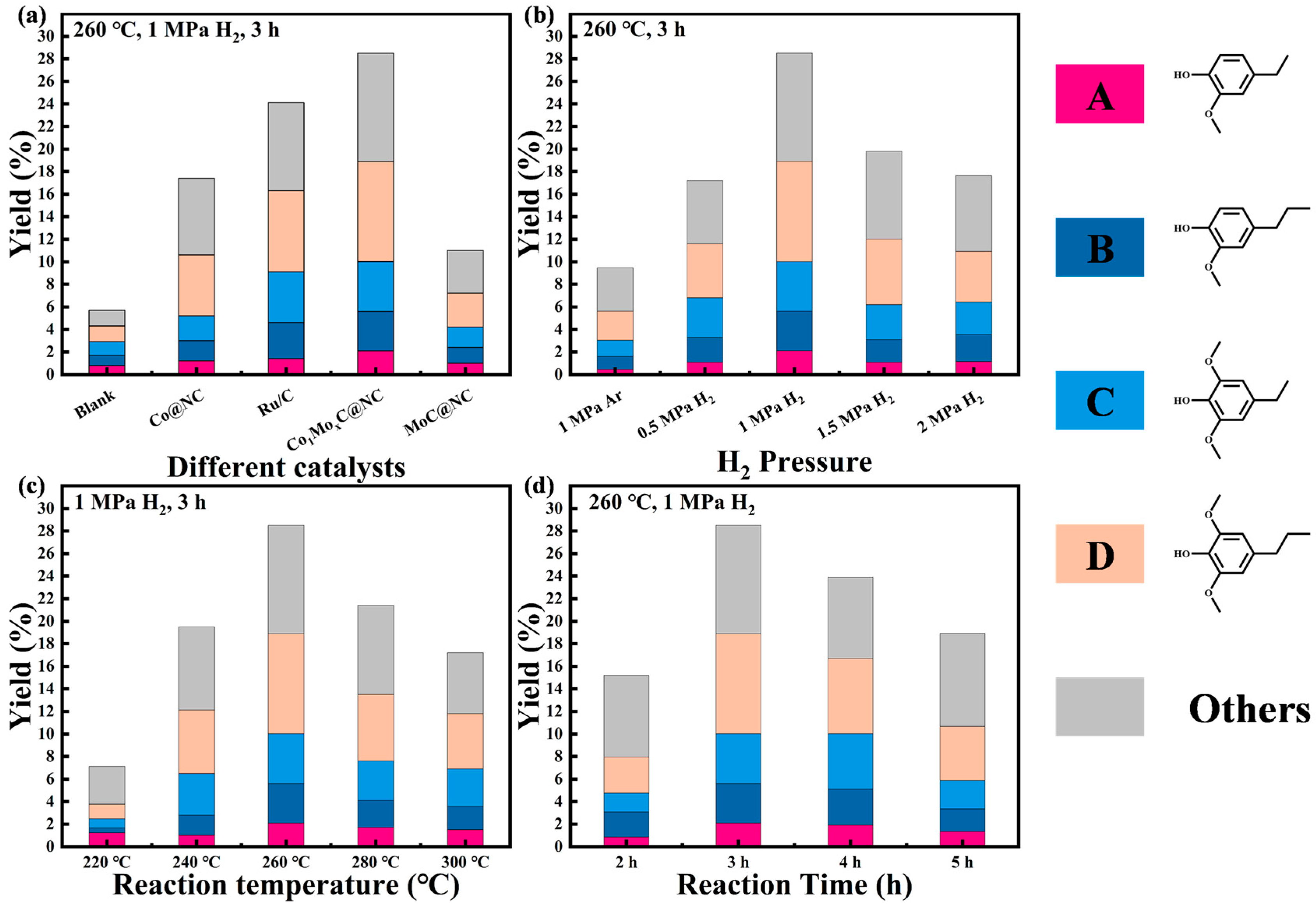
2.2. Catalysts Evaluation for the C–O Bond Cleavage
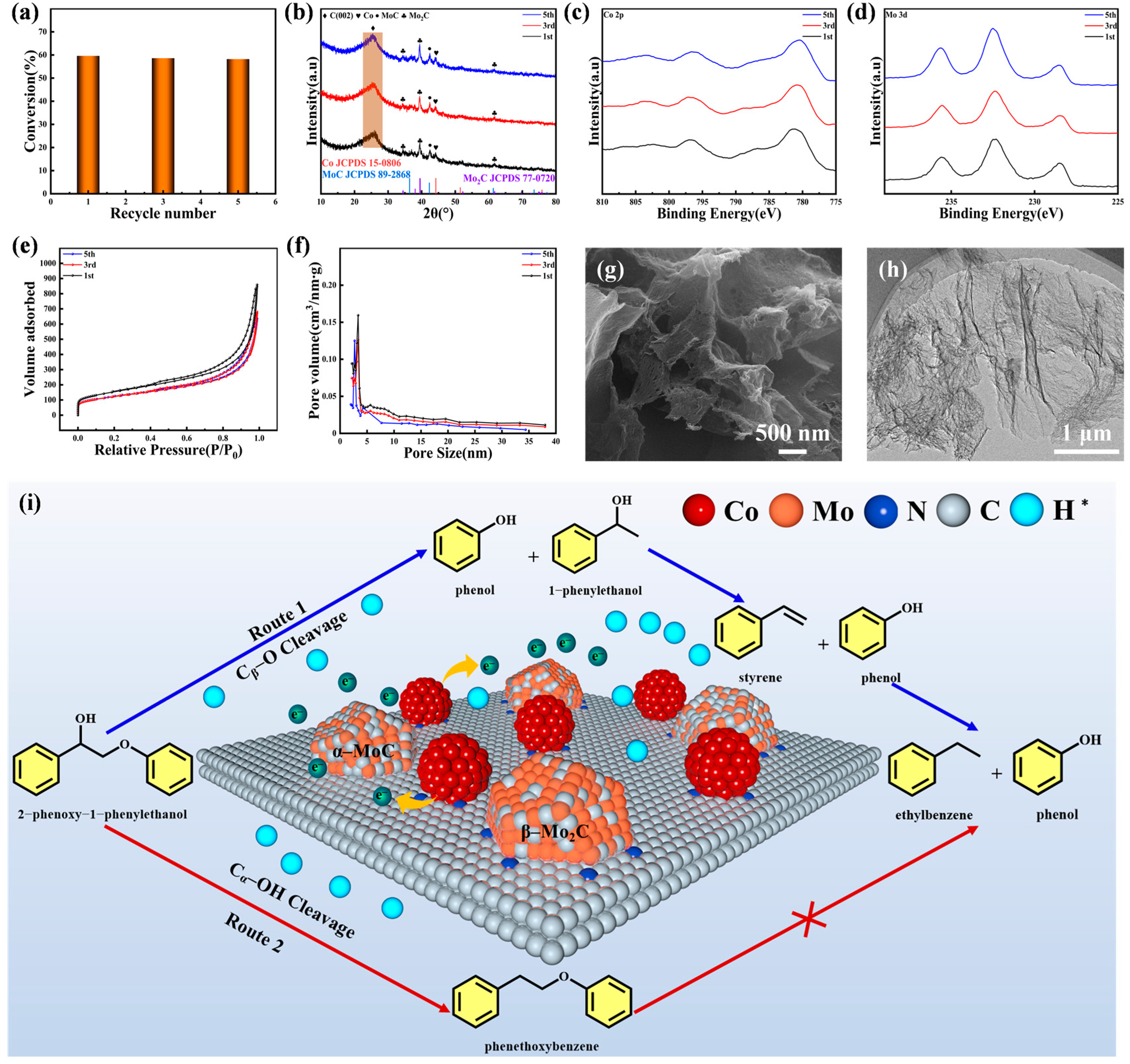
2.3. Reusability of the Catalyst
2.4. Hydrogenolysis of Birch Lignin
3. Material and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalyst Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Wang, X.; Wu, Q.; Pei, W.; Teo, M.J.; Chen, Z.S.; Huang, C. Application of lignin and lignin-based composites in different tissue engineering fields. Int. J. Biol. Macromol. 2022, 222, 994–1006. [Google Scholar] [PubMed]
- Feng, P.; Wang, H.; Huang, P.; Zhong, L.; Gan, S.; Wang, W.; Niu, L. Nitrogen-doped lignin-derived porous carbons for supercapacitors: Effect of nanoporous structure. Chem. Eng. J. 2023, 471, 144817. [Google Scholar]
- Liu, Z.-H.; Li, B.-Z.; Yuan, J.S.; Yuan, Y.-J. Creative biological lignin conversion routes toward lignin valorization. Trends Biotechnol. 2022, 40, 1550–1566. [Google Scholar]
- Cheng, C.; Li, P.; Yu, W.; Shen, D.; Gu, S. Catalytic hydrogenolysis of lignin in ethanol/isopropanol over an activated carbon supported nickel-copper catalyst. Bioresour. Technol. 2021, 319, 124238. [Google Scholar] [PubMed]
- Ye, K.; Liu, Y.; Wu, S.; Zhuang, J. A review for lignin valorization: Challenges and perspectives in catalytic hydrogenolysis. Ind. Crops Prod. 2021, 172, 114008. [Google Scholar] [CrossRef]
- Abu-Omar, M.M.; Barta, K.; Beckham, G.T.; Luterbacher, J.S.; Ralph, J.; Rinaldi, R.; Román-Leshkov, Y.; Samec, J.S.M.; Sels, B.F.; Wang, F. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 2021, 14, 262–292. [Google Scholar]
- Cheng, C.; Li, P.; Yu, W.; Shen, D.; Jiang, X.; Gu, S. Nonprecious Metal/Bimetallic Catalytic Hydrogenolysis of Lignin in a Mixed-Solvent System. ACS Sustain. Chem. Eng. 2020, 8, 16217–16228. [Google Scholar]
- Shen, Z.; Shi, C.; Liu, F.; Wang, W.; Ai, M.; Huang, Z.; Zhang, X.; Pan, L.; Zou, J.J. Advances in Heterogeneous Catalysts for Lignin Hydrogenolysis. Adv. Sci. 2024, 11, e2306693. [Google Scholar]
- Guo, T.; Lin, Y.; Pan, D.; Zhang, X.; Zhu, W.; Cai, X.-M.; Huang, G.; Wang, H.; Xu, D.; Kühn, F.E.; et al. Towards bioresource-based aggregation-induced emission luminogens from lignin β–O–4 motifs as renewable resources. Nat. Commun. 2023, 14, 6076. [Google Scholar] [CrossRef]
- Lavoie, J.-M.; Baré, W.; Bilodeau, M. Depolymerization of steam-treated lignin for the production of green chemicals. Bioresour. Technol. 2011, 102, 4917–4920. [Google Scholar]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [PubMed]
- Zhang, W.; Qiu, X.; Wang, C.; Zhong, L.; Fu, F.; Zhu, J.; Zhang, Z.; Qin, Y.; Yang, D.; Xu, C.C. Lignin derived carbon materials: Current status and future trends. Carbon Res. 2022, 1, 14. [Google Scholar]
- Li, T.; Lin, H.; Ouyang, X.; Qiu, X.; Wan, Z.; Ruan, T. Impact of nitrogen species and content on the catalytic activity to C–O bond cleavage of lignin over N-doped carbon supported Ru-based catalyst. Fuel 2020, 278, 118324. [Google Scholar]
- Guo, H.; Lu, X.; Yang, Y.; Wei, J.; Wu, L.; Tan, L.; Tang, Y.; Gu, X. Harvesting alkyl phenols from lignin monomers via selective hydrodeoxygenation under ambient pressure on Pd/α-MoC catalysts. Mol. Catal. 2023, 540, 113041. [Google Scholar]
- Gao, Z.; Li, C.; Fan, G.; Yang, L.; Li, F. Nitrogen-doped carbon-decorated copper catalyst for highly efficient transfer hydrogenolysis of 5-hydroxymethylfurfural to convertibly produce 2,5-dimethylfuran or 2,5-dimethyltetrahydrofuran. Appl. Catal. B Environ. 2018, 226, 523–533. [Google Scholar]
- Guo, H.; Zhao, J.; Chen, Y.; Lu, X.; Yang, Y.; Ding, C.; Wu, L.; Tan, L.; Long, J.; Yang, G.; et al. Mechanistic Insights into Hydrodeoxygenation of Lignin Derivatives over Ni Single Atoms Supported on Mo2C. ACS Catal. 2024, 14, 703–717. [Google Scholar]
- Wu, W.; Liu, H.; Wu, H.; Zheng, B.; Han, S.; Zhang, K.; Mei, X.; Xu, C.; He, M.; Han, B. Selective Hydrogenolysis of Lignin Model Compounds to Aromatics over a Cobalt Nanoparticle Catalyst. ACS Sustain. Chem. Eng. 2021, 9, 11862–11871. [Google Scholar]
- Wang, Z.; Chen, X.; Xie, X.; Yang, S.; Sun, L.; Li, T.; Chen, L.; Hua, D. Synthesis of aromatic monomers via hydrogenolysis of lignin over nickel catalyst supported on nitrogen-doped carbon nanotubes. Fuel Process. Technol. 2023, 248, 107810. [Google Scholar]
- Chen, X.; Chen, X.; Qi, J.; Liang, C. Self-assembly synthesis of lamellar molybdenum carbides with controllable phases for hydrodeoxygenation of diphenyl ether. Mol. Catal. 2020, 492, 110972. [Google Scholar]
- Li, C.; Li, H.; Wang, Y.; Tang, Z.; Shi, J.; Chen, M. Efficient liquefaction of Kraft lignin over N-doped carbon supported size-controlled MoC nanoparticles in supercritical ethanol. Fuel 2023, 333, 126360. [Google Scholar]
- Wu, K.; Yang, C.; Zhu, Y.; Wang, J.; Wang, X.; Liu, C.; Liu, Y.; Lu, H.; Liang, B.; Li, Y. Synthesis-Controlled α- and β-Molybdenum Carbide for Base-Promoted Transfer Hydrogenation of Lignin to Aromatic Monomers in Ethanol. Ind. Eng. Chem. Res. 2019, 58, 20270–20281. [Google Scholar] [CrossRef]
- Feng, P.; Wang, H.; Gan, S.; Liao, B.; Niu, L. Novel Lignin-Functionalized Waterborne Epoxy Composite Coatings with Excellent Anti-Aging, UV Resistance, and Interfacial Anti-Corrosion Performance. Small 2024, 20, 2312085. [Google Scholar]
- Li, M.; Wang, H.; Zhu, W.; Li, W.; Wang, C.; Lu, X. RuNi Nanoparticles Embedded in N-Doped Carbon Nanofibers as a Robust Bifunctional Catalyst for Efficient Overall Water Splitting. Adv. Sci. 2020, 7, 1901833. [Google Scholar]
- Yan, K.; Wang, D.; Li, H. Atom Doping Engineering of Metal/Carbon Catalysts for Biomass Hydrodeoxygenation. ACS Sustain. Chem. Eng. 2021, 9, 16531–16555. [Google Scholar]
- Zhang, H.; Jin, H.; Yang, Y.; Sun, F.; Liu, Y.; Du, X.; Zhang, S.; Song, F.; Wang, J.; Wang, Y.; et al. Understanding the synergetic interaction within α-MoC/β-Mo2C heterostructured electrocatalyst. J. Energy Chem. 2019, 35, 66–70. [Google Scholar]
- Ji, N.; Ri, P.; Diao, X.; Rong, Y.; Kim, C. Supported transition metal (Mo, W) carbide and nitride catalysts for lignin hydrodeoxygenation: Interplay of supports, structure, and catalysis. Catal. Sci. Technol. 2023, 13, 2618–2637. [Google Scholar]
- Zhu, Y.; Ma, Y.; Sun, Y.; Wang, L.; Ding, J.; Zhong, Y.; Zhang, J.; Wang, L.; Li, Y. In-situ construction of N-doped hollow carbon polyhedral cage anchored Co–Ni dual binding sites as nanoreactor for efficient real lignin oil hydrodeoxygenation. Renew. Energy 2023, 217, 119222. [Google Scholar]
- Wang, Z.; Chen, X.; Sun, Y.; Hua, D.; Yang, S.; Sun, L.; Li, T.; Chen, L. Co-pyrolysis induced strong metal-support interaction in N-doped carbon supported Ni catalyst for the hydrogenolysis of lignin. Chem. Eng. J. 2023, 473, 145182. [Google Scholar]
- Li, T.; Lin, H.; Ouyang, X.; Qiu, X.; Wan, Z. In Situ Preparation of Ru@N-Doped Carbon Catalyst for the Hydrogenolysis of Lignin To Produce Aromatic Monomers. ACS Catal. 2019, 9, 5828–5836. [Google Scholar]
- Li, J.; Li, Z.; Dong, J.; Fang, R.; Chi, Y.; Hu, C. Hexaniobate as a Recyclable Solid Base Catalyst to Activate C–H Bonds in Lignin Linkage Boosting the Production of Aromatic Monomers. ACS Catal. 2023, 13, 5272–5284. [Google Scholar]
- Wang, J.; Wang, S. A critical review on graphitic carbon nitride (g-C3N4)-based materials: Preparation, modification and environmental application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar]
- Guo, J.-P.; Liu, F.-J.; Bie, L.-L.; Si, X.-G.; Li, Y.-H.; Song, P.; Liu, N.; Zhao, Y.-P.; Huang, Z.-X.; Cao, J.-P.; et al. Selective cleavage of C–O bond in lignin and lignin model compounds over iron/nitrogen co-doped carbon supported Ni catalyst. Fuel 2022, 316, 123338. [Google Scholar] [CrossRef]
- Cai, F.; Guo, Y.; Ibrahim, J.J.; Zhang, J.; Sun, Y. A highly active and stable Pd/MoC catalyst for hydrogen production from methanol decomposition. Appl. Catal. B Environ. 2021, 299, 120648. [Google Scholar]
- Zhang, P.; Liu, Y.; Liang, T.; Ang, E.H.; Zhang, X.; Ma, F.; Dai, Z. Nitrogen-doped carbon wrapped Co-Mo2C dual Mott–Schottky nanosheets with large porosity for efficient water electrolysis. Appl. Catal. B Environ. 2021, 284, 119738. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, R.; Huang, L.; Zhou, D.; Tang, F.; Liu, P. Understanding the reaction route of selectively converting furfural to furan over the alkali-induced Co-Mo2C heterostructure. Chem. Eng. J. 2023, 466, 143237. [Google Scholar]
- Ribeiro, L.S.; Delgado, J.J.; Órfão, J.J.M.; Pereira, M.F.R. Carbon supported Ru-Ni bimetallic catalysts for the enhanced one-pot conversion of cellulose to sorbitol. Appl. Catal. B Environ. 2017, 217, 265–274. [Google Scholar]
- Ma, L.; Zhang, G.; Dong, Y.; Dou, S.; Meng, Q.; Yan, P.; Liu, L.; Kong, X. Hydrodeoxygenation of lignin derivatives over Ni-Re bimetallic catalyst supported on mesoporous carbon sphere. J. Environ. Chem. Eng. 2023, 11, 110215. [Google Scholar] [CrossRef]
- Wang, H.-T.; Li, Z.-K.; Yan, H.-L.; Lei, Z.-P.; Yan, J.-C.; Ren, S.-B.; Wang, Z.-C.; Kang, S.-G.; Shui, H.-F. Catalytic hydrogenolysis of lignin and model compounds over highly dispersed Ni-Ru/Al2O3 without additional H2. Fuel 2022, 326, 125027. [Google Scholar] [CrossRef]
- He, D.; Xu, J.; Guo, Y.; Yu, M.; Wang, Q.; Zhou, J.; Wang, X. RuNi nanoparticles embedded in N-doped carbon nanofibers as a bimetallic catalyst for the hydrogenolysis of peanut shell lignin. Fuel Process. Technol. 2022, 238, 107519. [Google Scholar] [CrossRef]
- Yang, C.; Fu, L.; Zhu, R.; Liu, Z. Influence of cobalt species on the catalytic performance of Co–N-C/SiO2 for ethylbenzene oxidation. Phys. Chem. Chem. Phys. 2016, 18, 4635–4642. [Google Scholar] [CrossRef]
- Guo, D.; Wang, S.; Feng, J.; Pan, H. Selective hydrogenation of diphenyl ethers over NiCo bimetallic catalyst. Mol. Catal. 2023, 546, 113215. [Google Scholar]
- Xie, J.-X.; Zhao, Y.-P.; Cao, J.-P.; Li, Q.; Jiang, W.; Zhao, L.; Zhao, X.-Y.; Qiu, L.-L. Catalytic Hydrolysis/Hydrogenolysis of Lignin-Derived Aryl Ethers over Bimetallic Pd-Ni Systems: The Directional Regulation of Reaction Pathways. ACS Sustain. Chem. Eng. 2023, 11, 12724–12738. [Google Scholar] [CrossRef]
- Geng, W.; Li, W.; Liu, L.; Liu, J.; Liu, L.; Kong, X. Facile assembly of Cu-Cu2O/N-reduced graphene oxide nanocomposites for efficient synthesis of 2-methylfuran. Fuel 2020, 259, 116267. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Zhang, C.; Liu, Y.; Xu, M.; Xue, Y.; Liu, L.; Leung, M.K.H. Bimetallic Mo–Co nanoparticles anchored on nitrogen-doped carbon for enhanced electrochemical nitrogen fixation. J. Mater. Chem. A 2020, 8, 9091–9098. [Google Scholar]
- Jiang, S.; Shu, R.; Wang, A.; Deng, Z.; Xiao, Y.; Li, J.; Meng, Q.; Zhang, Q. Efficient hydrodeoxygenation of lignin-derived phenolic compounds under acid-free conditions over carbon-supported NiMo catalysts. Green Chem. 2024, 26, 9330–9345. [Google Scholar]
- Ouyang, T.; Ye, Y.Q.; Wu, C.Y.; Xiao, K.; Liu, Z.Q. Heterostructures Composed of N-Doped Carbon Nanotubes Encapsulating Cobalt and beta-Mo(2) C Nanoparticles as Bifunctional Electrodes for Water Splitting. Angew. Chem. Int. Ed. Engl. 2019, 58, 4923–4928. [Google Scholar]
- Huang, H.; Kong, L.; Liu, M.; He, J.; Shuang, W.; Xu, Y.; Bu, X.-H. Constructing bifunctional Co/MoC@N-C catalyst via an in-situ encapsulation strategy for efficient oxygen electrocatalysis. J. Energy Chem. 2021, 59, 538–546. [Google Scholar]
- Xia, Z.; Niu, L.; Wu, Q.; An, Y.; Bai, G. Construction of isolated Co–Nx and dual Con–CoNx sites for the regulation of hydrogenation and hydrodeoxygenation selectivity of biomass-derived chemicals. Green Chem. 2023, 25, 9313–9321. [Google Scholar]
- Song, F.; Du, K.; Yang, H.; Luo, Q.; Liu, Y.; Qiang, Q.; Ding, Y.; An, Q.; Li, C. Depolymerization of lignin over heterogeneous Co–NC catalyst. J. Environ. Sci. 2025, 152, 654–663. [Google Scholar]
- Song, Q.-L.; Zhao, Y.-P.; Wu, F.-P.; Li, G.-S.; Fan, X.; Wang, R.-Y.; Cao, J.-P.; Wei, X.-Y. Selective hydrogenolysis of lignin-derived aryl ethers over Co/C@N catalysts. Renew. Energy 2020, 148, 729–738. [Google Scholar]
- Zhang, X.; Wang, J.; Guo, T.; Liu, T.; Wu, Z.; Cavallo, L.; Cao, Z.; Wang, D. Structure and phase regulation in MoxC (α-MoC1-x/β-Mo2C) to enhance hydrogen evolution. Appl. Catal. B Environ. 2019, 247, 78–85. [Google Scholar]
- Wu, P.; Lu, G.; Cai, C. Cobalt–molybdenum synergistic catalysis for the hydrogenolysis of terephthalate-based polyesters. Green Chem. 2021, 23, 8666–8672. [Google Scholar]
- Pan, L.F.; Li, Y.H.; Yang, S.; Liu, P.F.; Yu, M.Q.; Yang, H.G. Molybdenum carbide stabilized on graphene with high electrocatalytic activity for hydrogen evolution reaction. Chem. Commun. 2014, 50, 13135–13137. [Google Scholar]
- Huang, Y.; Gong, Q.; Song, X.; Feng, K.; Nie, K.; Zhao, F.; Wang, Y.; Zeng, M.; Zhong, J.; Li, Y. Mo2C Nanoparticles Dispersed on Hierarchical Carbon Microflowers for Efficient Electrocatalytic Hydrogen Evolution. ACS Nano 2016, 10, 11337–11343. [Google Scholar]
- Shen, W.; Suen, D.W.-S.; Sze, E.T.-P.; Chen, X.; Liang, C.; Tsang, C.-W. Co–MoCx supported on N-doped CNTs for efficient hydrogen evolution reaction under alkaline medium conditions. New J. Chem. 2023, 47, 21024–21032. [Google Scholar]
- Liu, K.; Cao, Y.; Yang, S.; Wu, C.; Zhang, Z.; Zhang, Q.; Zhang, H. Molybdenum Carbide-Promoted Cobalt as an Efficient Catalyst for Selective Hydrogenation. Ind. Eng. Chem. Res. 2020, 59, 14267–14277. [Google Scholar]
- Zhang, G.; Ma, L.; Dong, Y.; Dou, S.; Kong, X. In situ construction of 3D NiMo bimetallic catalysts anchored on dendritic mesoporous silica for the upgrading of biomass derivatives. J. Colloid Interface Sci. 2023, 647, 188–200. [Google Scholar] [CrossRef]
- Chen, M.; Li, H.; Wang, Y.; Tang, Z.; Dai, W.; Li, C.; Yang, Z.; Wang, J. Lignin depolymerization for aromatic compounds over Ni-Ce/biochar catalyst under aqueous-phase glycerol. Appl. Energy 2023, 332, 120489. [Google Scholar] [CrossRef]
- Fu, Z.-P.; Zhao, Y.-P.; Wu, F.-P.; Xie, J.-X.; Qiu, L.-L.; Xiao, J.; Liang, J.; Bai, Y.-H.; Liu, F.-J.; Cao, J.-P. Selective hydrogenolysis of C–O bonds in lignin model compounds and Kraft lignin over highly efficient NixCoyAl catalysts. Mol. Catal. 2023, 547, 113334. [Google Scholar]
- Li, L.; Huang, Z.; Shu, F.; Gao, Y.; Long, J. Hydrodeoxygenation of heavy lignin bio-oil to oxygenated fuel catalyzed by CuxNiy/MgO. Fuel 2024, 357, 129805. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, X.; Wang, L.; Liang, J.; Wei, X. Selective hydrogenolysis of aryl ethers over a nitrogen-doped porous carbon supported Ni–CeO2 catalyst at low temperature. Catal. Sci. Technol. 2021, 11, 3241–3250. [Google Scholar]
- Zhang, J.; Su, Z.; Wu, Z.; Wang, P.; Xiao, F.-S. Basic carrier promoted Pt-catalyzed hydrogenolysis of alkaline lignin. Catal. Today 2021, 365, 193–198. [Google Scholar] [CrossRef]
- Torr, K.M.; van de Pas, D.J.; Cazeils, E.; Suckling, I.D. Mild hydrogenolysis of in-situ and isolated Pinus radiata lignins. Bioresour. Technol. 2011, 102, 7608–7611. [Google Scholar] [PubMed]
- Zhou, L.; Zhu, Z.; Luo, B.; He, Y.; Shu, R.; Tian, Z.; Wang, C. Bimetallic NiCo catalyzed enzymatic hydrolysis lignin hydrogenolysis to produce aromatic monomers. Fuel 2023, 333, 126357. [Google Scholar]
- Galkin, M.V.; Smit, A.T.; Subbotina, E.; Artemenko, K.A.; Bergquist, J.; Huijgen, W.J.; Samec, J.S. Hydrogen-free catalytic fractionation of woody biomass. ChemSusChem 2016, 9, 3280–3287. [Google Scholar]
- Zhang, K.; Jiang, J.; Liu, Z.; Ye, J.; Tao, R.; Xu, H.; Xie, J.; Yang, J.; Zhao, J.; Zhang, N.; et al. Catalytic Hydrogenolysis of Lignin into Propenyl-monophenol over Ru Single Atoms Supported on CeO2 with Rich Oxygen Vacancies. ACS Catal. 2024, 14, 16115–16126. [Google Scholar]
- Cheng, C.; Zhao, H.; Yang, Y.; Shen, D.; Jiang, X. Fe-promoted Ni nanocatalysts for hydrogenolysis of Klason lignin to monophenols. Biomass Bioenergy 2024, 191, 107449. [Google Scholar]
- Hossain, M.A.; Saelee, T.; Tulaphol, S.; Rahaman, M.S.; Phung, T.K.; Maihom, T.; Praserthdam, P.; Praserthdam, S.; Yelle, D.J.; Sathitsuksanoh, N. Catalytic Hydrogenolysis of Lignin into Phenolics by Internal Hydrogen over Ru Catalyst. ChemCatChem 2022, 14, e202200549. [Google Scholar]
- Yan, N.; Zhao, C.; Dyson, P.J.; Wang, C.; Liu, L.T.; Kou, Y. Selective degradation of wood lignin over noble-metal catalysts in a two-step process. ChemSusChem 2008, 1, 626–629. [Google Scholar]
- Zhang, J.-W.; Lu, G.-P.; Cai, C. Self-hydrogen transfer hydrogenolysis of β–O–4 linkages in lignin catalyzed by MIL-100(Fe) supported Pd–Ni BMNPs. Green Chem. 2017, 19, 4538–4543. [Google Scholar]
- Zhai, Y.; Li, C.; Xu, G.; Ma, Y.; Liu, X.; Zhang, Y. Depolymerization of lignin via a non-precious Ni–Fe alloy catalyst supported on activated carbon. Green Chem. 2017, 19, 1895–1903. [Google Scholar] [CrossRef]
- Luo, B.; Tian, Z.; Shu, R.; Wang, C.; Chen, Y.; Liu, J.; Liao, Y. Highly stable biochar-encapsulated CoTi@BC nanocatalysts for lignin hydrogenolysis. J. Catal. 2025, 442, 115914. [Google Scholar] [CrossRef]


 | |||||
| Entry | Catalysts | Conversion (%) | Product Yield (%) | ||
|---|---|---|---|---|---|
| 2 | 3 | 4 | |||
| 1 | Blank | 0 | 0 | 0 | 0 |
| 2 | NC | 0 | 0 | 0 | 0 |
| 3 | MoC@NC | 68.4 | 37.8 | 29.2 | 17.7 |
| 4 | Co0.2MoxC@NC | 84.3 | 43.8 | 38.5 | 0 |
| 5 | Co0.8MoxC@NC | 90.4 | 42.3 | 36.1 | 0 |
| 6 | Co1MoxC@NC | 99.9 | 49.9 | 41.2 | 0 |
| 7 | Co1.5MoxC@NC | 92.6 | 44.5 | 36.2 | 0 |
| 8 | Co2MoxC@NC | 87.9 | 39.5 | 32.1 | 0 |
| 9 | Co@NC | 84.8 | 32.8 | 28.6 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Cao, C.; Chang, K.; Zhao, Y.; Hua, D.; Sun, L.; Yang, S.; Dong, Z.; Li, T. Efficient Hydrogenolysis of Lignin into Aromatic Monomers over N-Doped Carbon Supported Co and Dual-Phase MoxC Nanoparticles. Catalysts 2025, 15, 297. https://doi.org/10.3390/catal15040297
Chen L, Cao C, Chang K, Zhao Y, Hua D, Sun L, Yang S, Dong Z, Li T. Efficient Hydrogenolysis of Lignin into Aromatic Monomers over N-Doped Carbon Supported Co and Dual-Phase MoxC Nanoparticles. Catalysts. 2025; 15(4):297. https://doi.org/10.3390/catal15040297
Chicago/Turabian StyleChen, Lei, Chuanxin Cao, Kai Chang, Yuying Zhao, Dongliang Hua, Laizhi Sun, Shuangxia Yang, Zhiguo Dong, and Tianjin Li. 2025. "Efficient Hydrogenolysis of Lignin into Aromatic Monomers over N-Doped Carbon Supported Co and Dual-Phase MoxC Nanoparticles" Catalysts 15, no. 4: 297. https://doi.org/10.3390/catal15040297
APA StyleChen, L., Cao, C., Chang, K., Zhao, Y., Hua, D., Sun, L., Yang, S., Dong, Z., & Li, T. (2025). Efficient Hydrogenolysis of Lignin into Aromatic Monomers over N-Doped Carbon Supported Co and Dual-Phase MoxC Nanoparticles. Catalysts, 15(4), 297. https://doi.org/10.3390/catal15040297







