Polyol Formation of Silver@Metal Oxides Nanohybrid for Photocatalytic and Antibacterial Performance
Abstract
1. Introduction
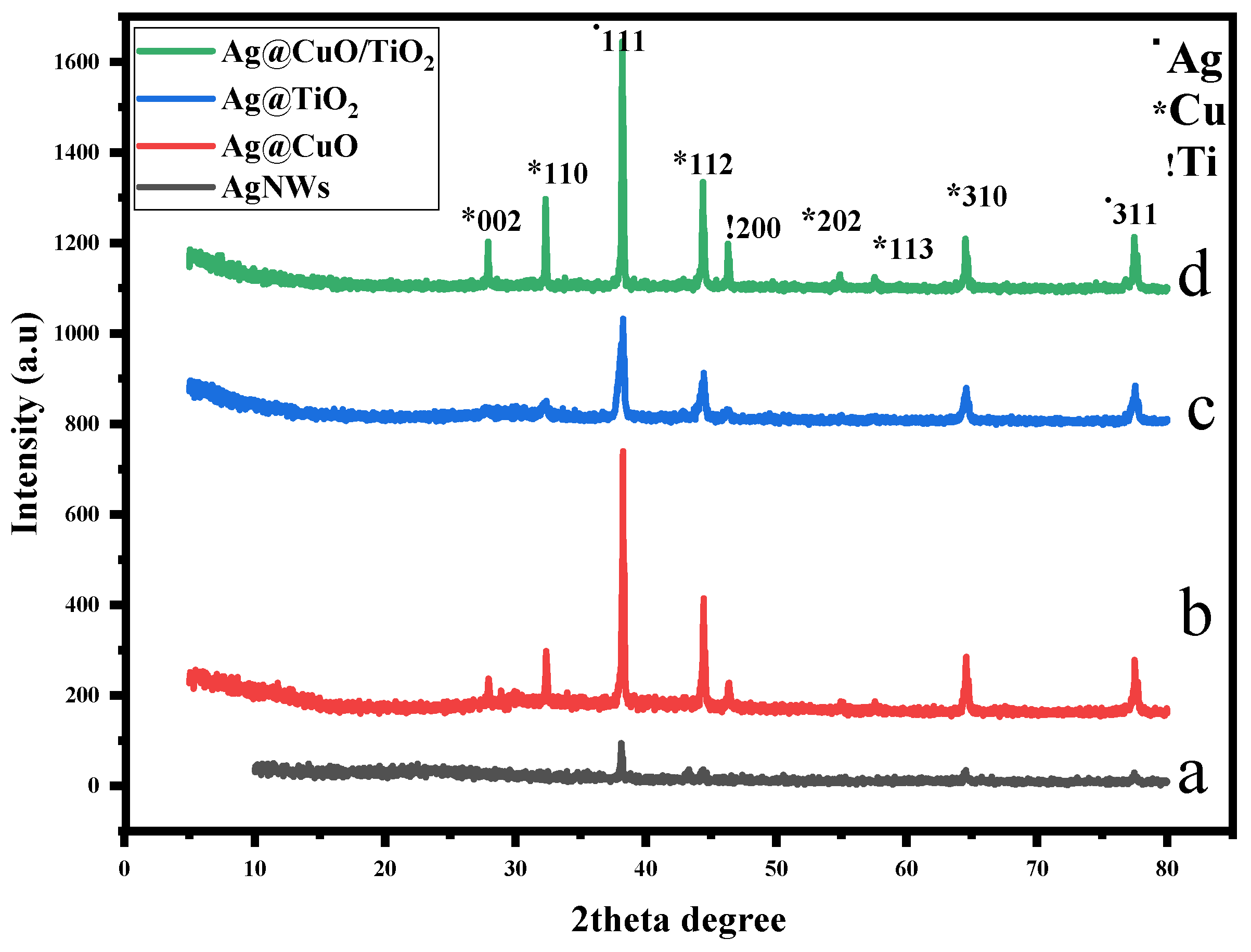
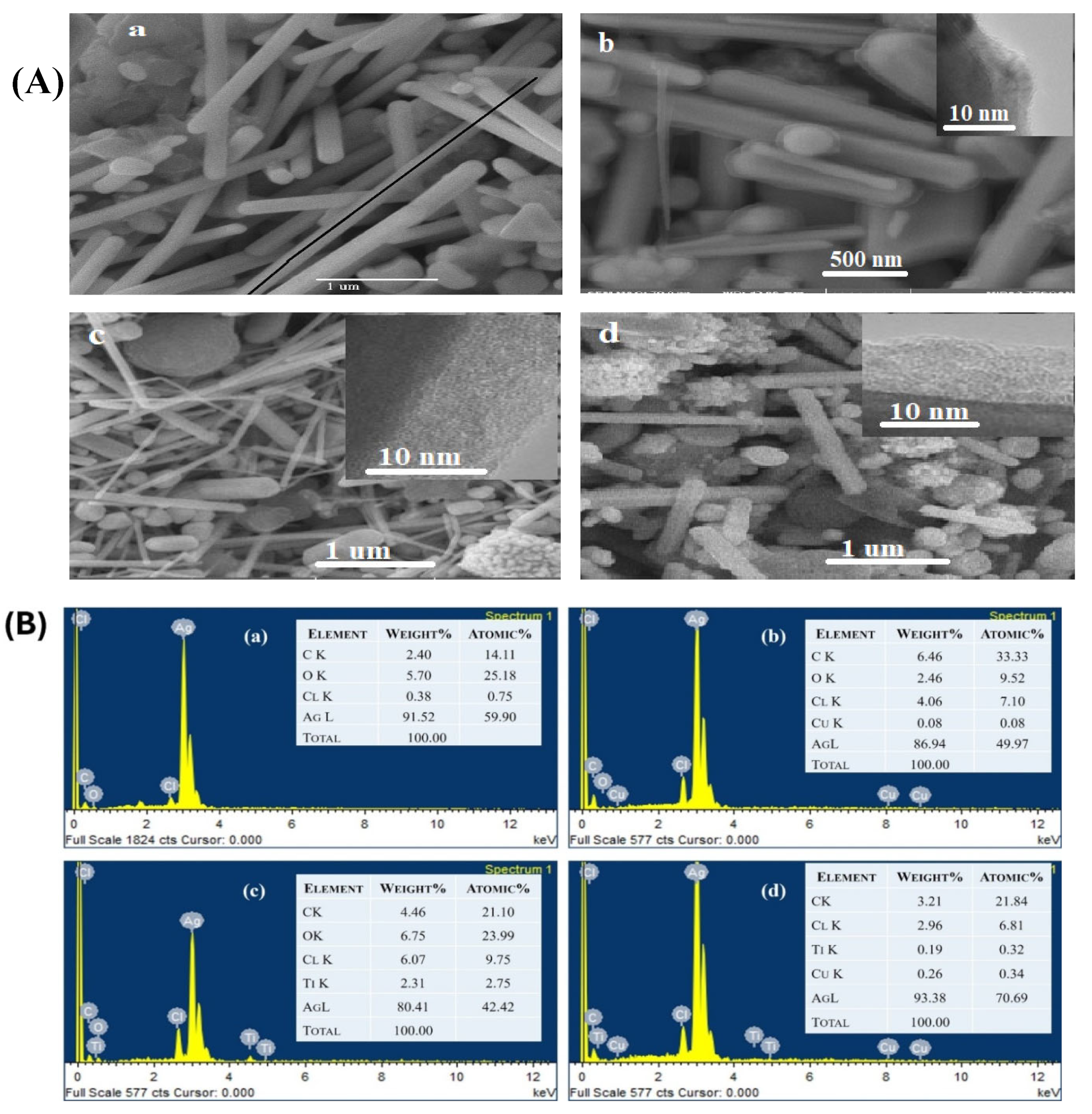
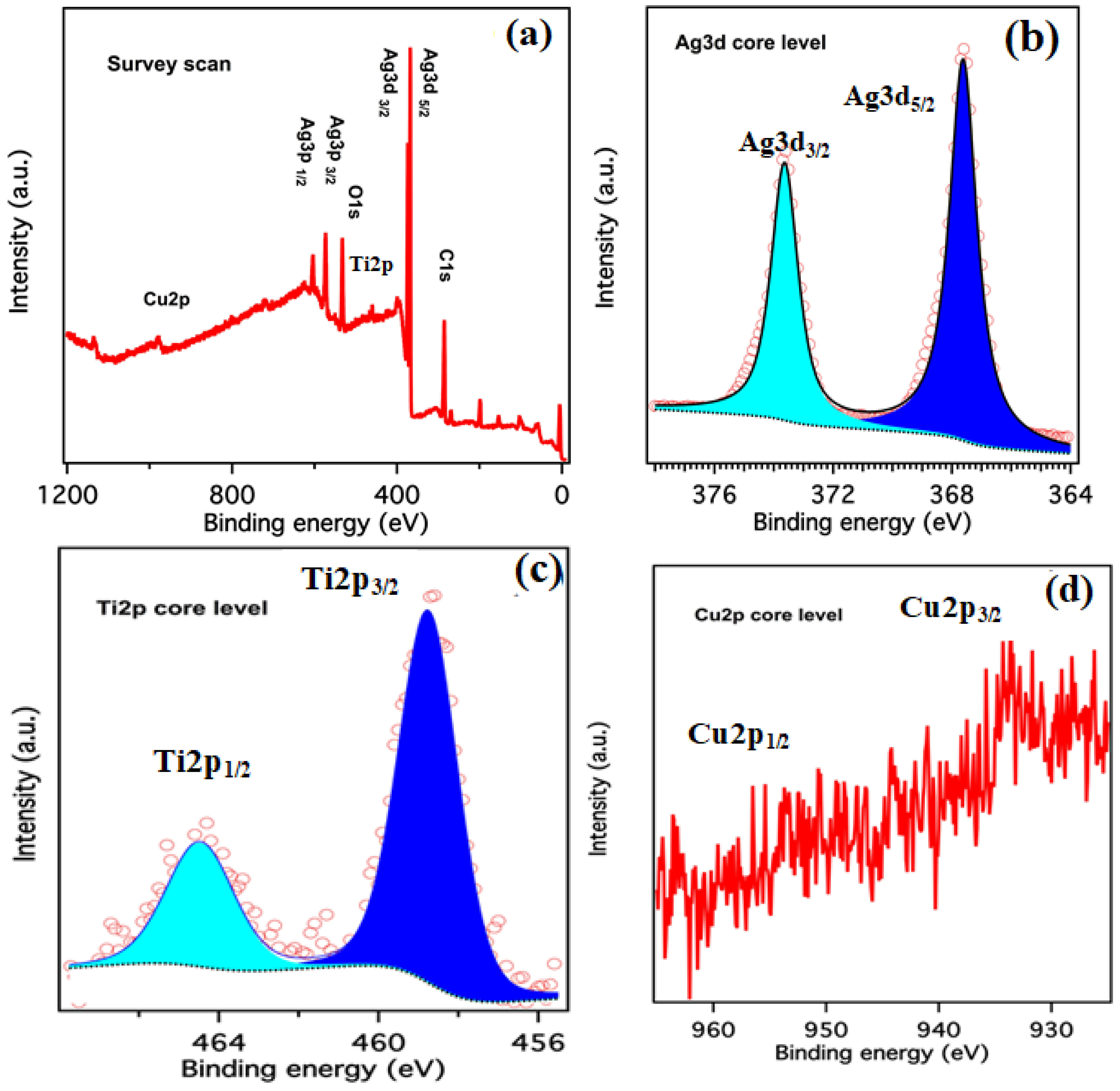
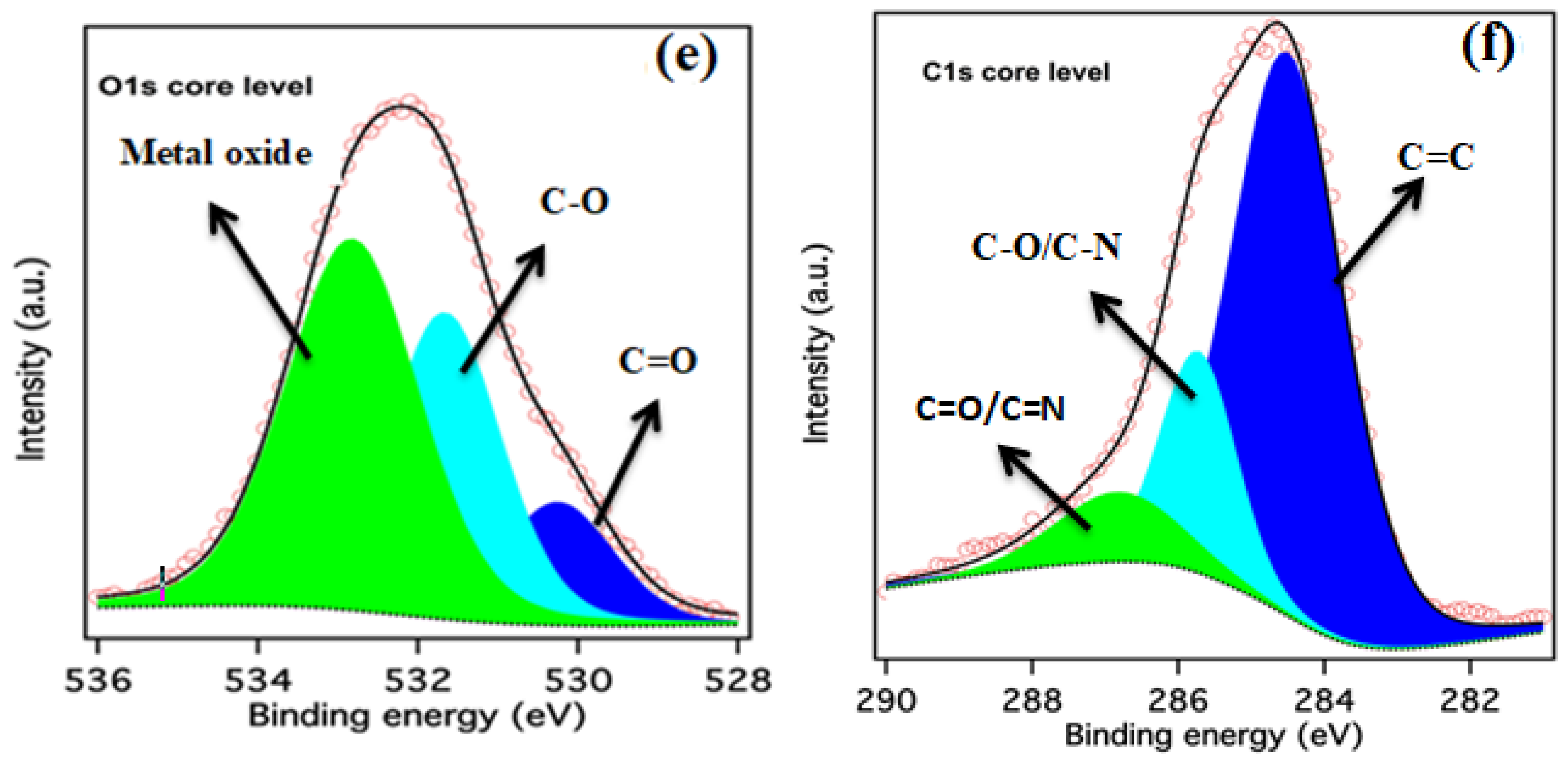
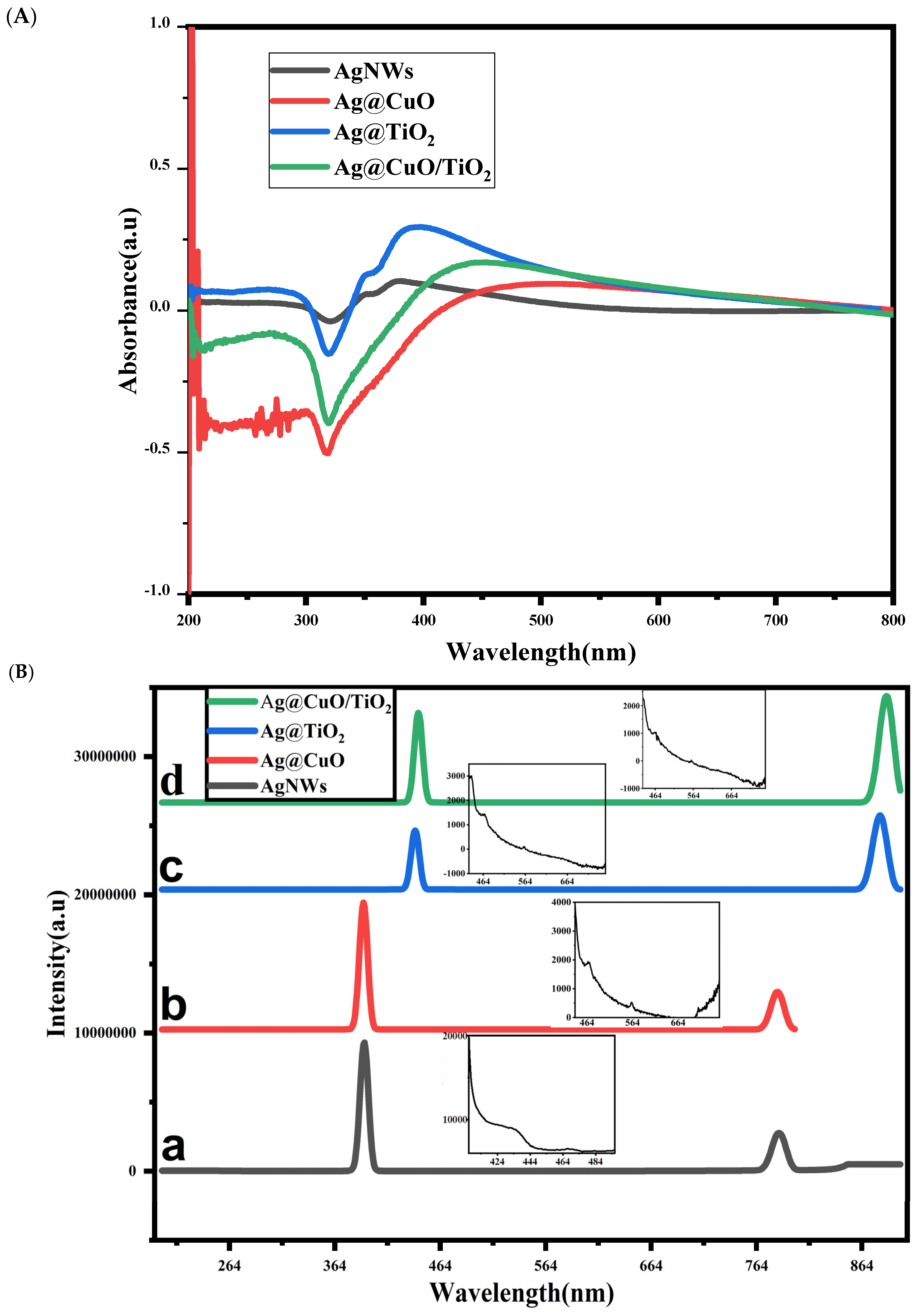
2. Results and Discussion
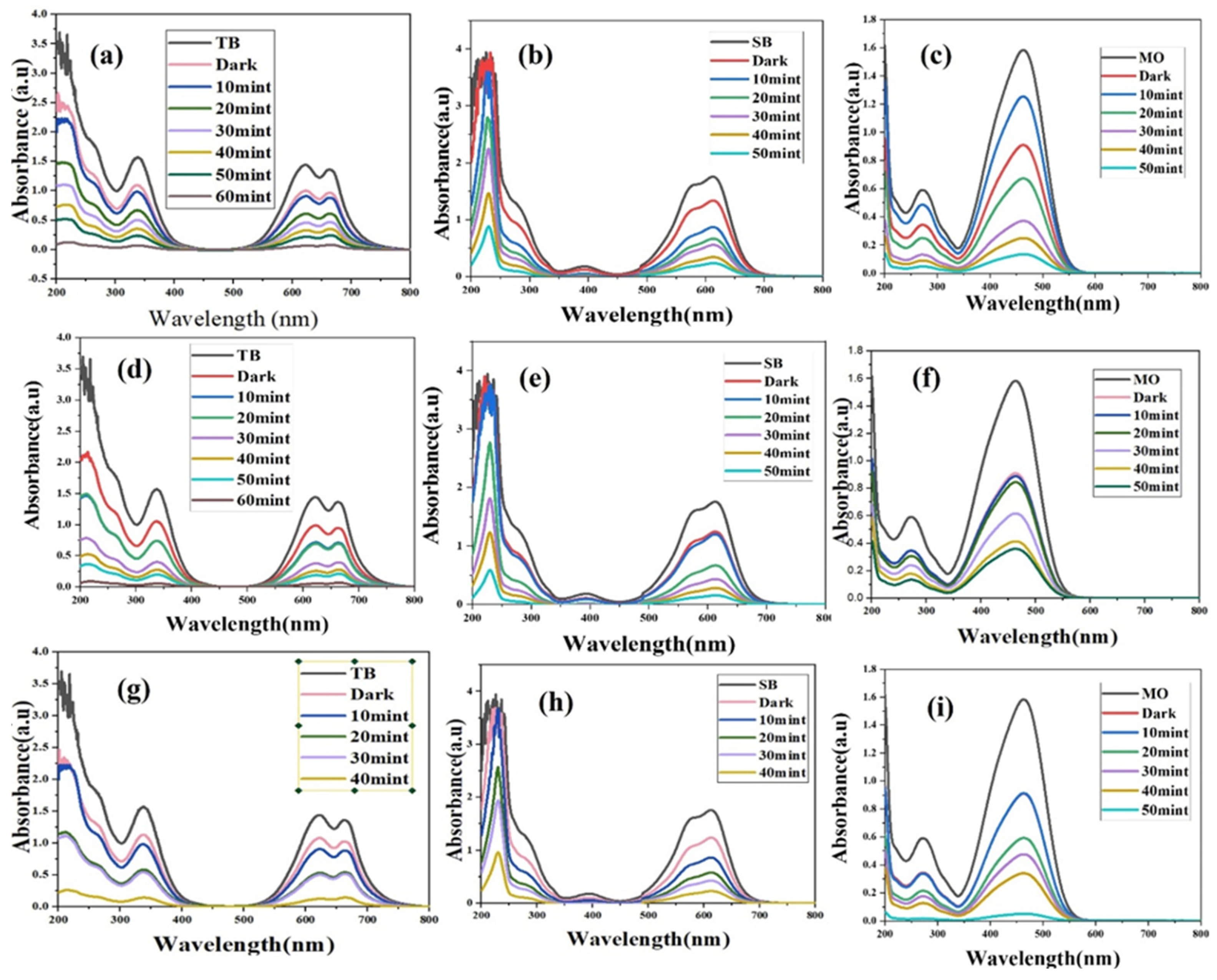
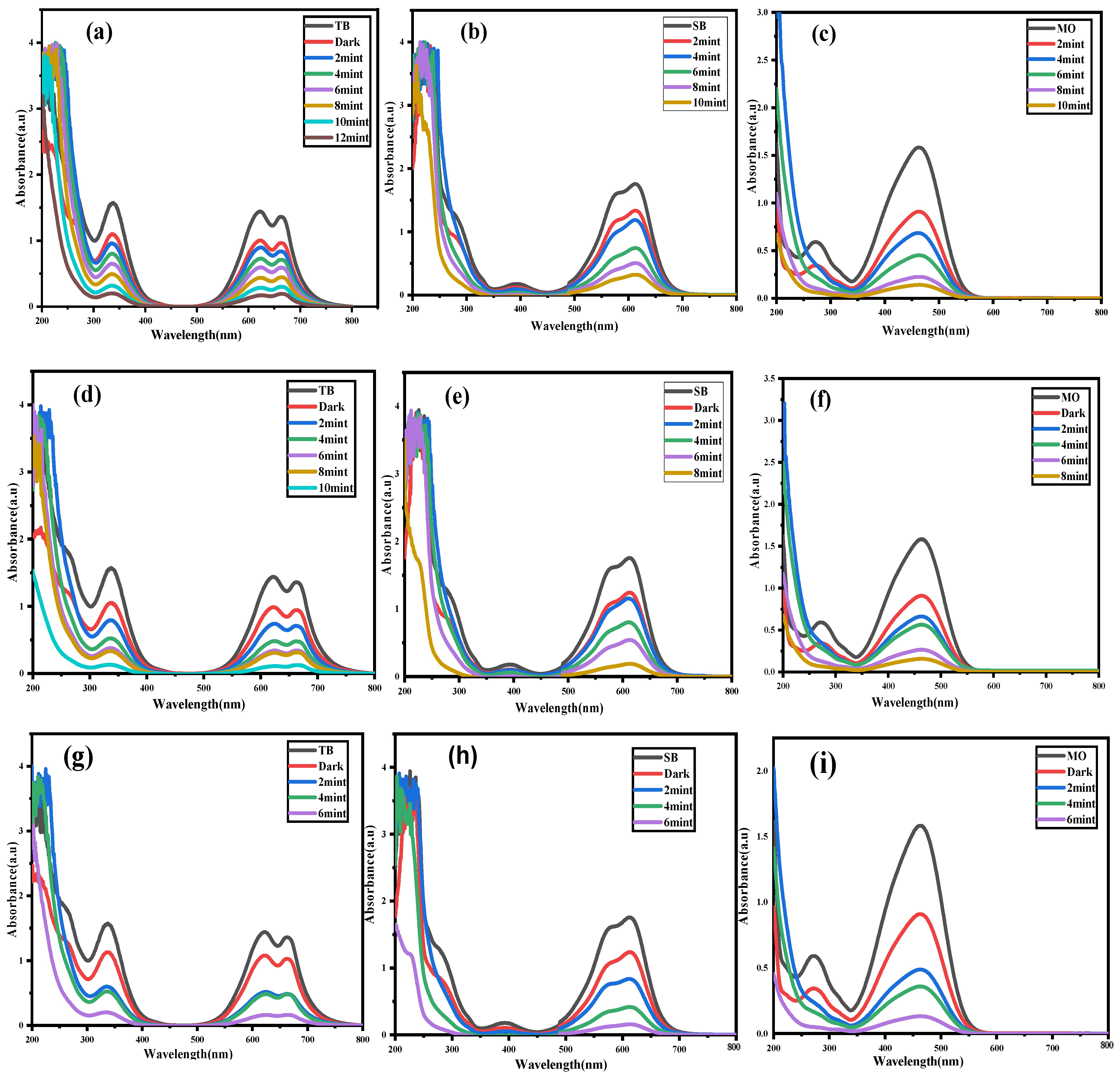
2.1. Photocatalytic Activities
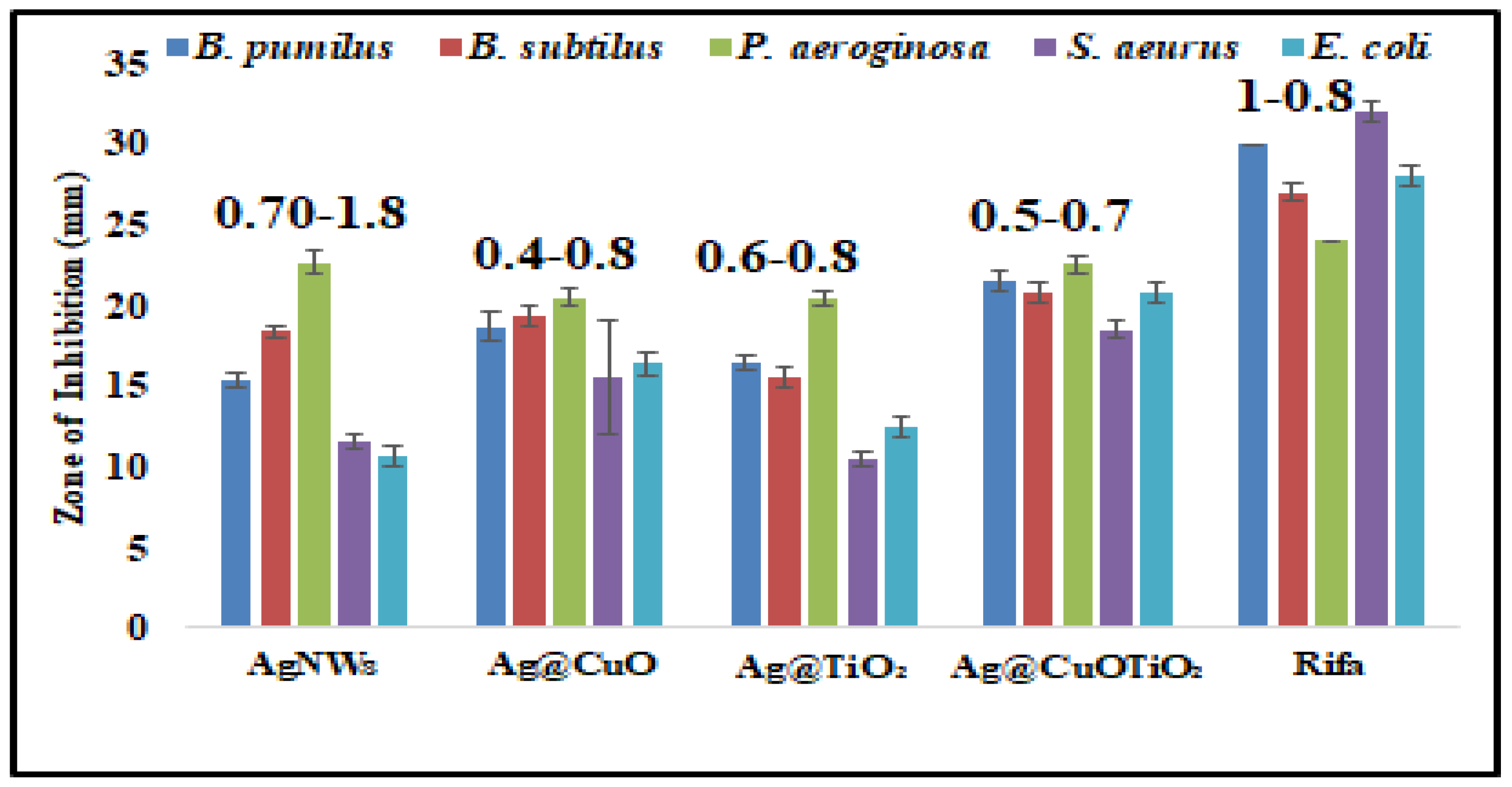
2.2. Antibacterial Activities
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation and Coating of Ag NWs
3.3. Catalytic Activities
3.4. Antibacterial Activities
3.5. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Wang, H.; Wu, C.; Huang, Y.; Sun, F.; Lin, N.; Soomro, A.M.; Zhong, Z.; Yang, X.; Chen, X.; Kang, J.; et al. One-pot synthesis of superfine core–shell Cu@ metal nanowires for highly tenacious transparent LED dimmer. ACS Appl. Mater. Interfaces 2016, 8, 28709–28717. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kim, S.; Ma, X.; Byrley, P.; Yu, N.; Liu, Q.; Sun, X.; Xu, D.; Peng, S.; Hartel, M.C.; et al. Ultrathin-shell epitaxial Ag@ Au core-shell nanowires for high-performance and chemically-stable electronic, optical, and mechanical devices. Nano Res. 2021, 14, 4294–4303. [Google Scholar] [CrossRef]
- Kang, H.; Kim, J.S.; Choi, S.-R.; Kim, Y.-H.; Kim, D.H.; Kim, J.-G.; Lee, T.-W.; Cho, J.H. Electroplated core–shell nanowire network electrodes for highly efficient organic light-emitting diodes. Nano Converg. 2022, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-P.; Shan, S.; Zang, S.-Q.; Zhong, C.-J. Dynamic core–shell and alloy structures of multimetallic nanomaterials and their catalytic synergies. Acc. Chem. Res. 2020, 53, 2913–2924. [Google Scholar] [CrossRef]
- Yao, M.; Wang, B.; Sun, B.; Luo, L.; Chen, Y.; Wang, J.; Wang, N.; Komarneni, S.; Niu, X.; Hu, W. Rational design of self-supported Cu@ WC core-shell mesoporous nanowires for pH-universal hydrogen evolution reaction. Appl. Catal. B Environ. 2021, 280, 119451. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Z. Multifunctional nanoparticle@ MOF core–shell nanostructures. Adv. Mater. 2013, 25, 5819–5825. [Google Scholar] [CrossRef]
- Arabzadeh, A.; Salimi, A. One dimensional CdS nanowire@ TiO2 nanoparticles core-shell as high performance photocatalyst for fast degradation of dye pollutants under visible and sunlight irradiation. J. Colloid Interface Sci. 2016, 479, 43–54. [Google Scholar] [CrossRef]
- Choubrac, L.; Bär, M.; Kozina, X.; Félix, R.; Wilks, R.G.; Brammertz, G.; Levcenko, S.; Arzel, L.; Barreau, N.; Harel, S.; et al. Sn substitution by Ge: Strategies to overcome the open-circuit voltage deficit of kesterite solar cells. ACS Appl. Energy Mater. 2020, 3, 5830–5839. [Google Scholar] [CrossRef]
- Shin, J.C.; Kim, K.H.; Yu, K.J.; Hu, H.; Yin, L.; Ning, C.-Z.; Rogers, J.A.; Zuo, J.-M.; Li, X. InxGa1-x As nanowires on silicon: One-dimensional heterogeneous epitaxy, bandgap engineering, and photovoltaics. Nano Lett. 2011, 11, 4831–4838. [Google Scholar] [CrossRef]
- Han, L.; Zhan, W.; Liang, X.; Zhang, W.; Huang, R.; Chen, R.; Ni, H. In-situ generation Cu2O/CuO core-shell heterostructure based on copper oxide nanowires with enhanced visible-light photocatalytic antibacterial activity. Ceram. Int. 2022, 48, 22018–22030. [Google Scholar] [CrossRef]
- El-Toni, A.M.; Habila, M.A.; Labis, J.P.; Alothman, Z.A.; Alhoshan, M.; Elzatahry, A.A.; Zhang, F. Design, synthesis and applications of core–shell, hollow core, and nanorattle multifunctional nanostructures. Nanoscale 2016, 8, 2510–2531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wada, H. Laser ablation in liquids for nanomaterial synthesis and applications. In Handbook of Laser Micro- and Nano-Engineering; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–35. [Google Scholar]
- Rai, P. Plasmonic noble metal@ metal oxide core–shell nanoparticles for dye-sensitized solar cell applications. Sustain. Energy Fuels 2019, 3, 63–91. [Google Scholar] [CrossRef]
- Fiévet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.-Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef]
- Li, G.; Tang, Z. Noble metal nanoparticle@ metal oxide core/yolk–shell nanostructures as catalysts: Recent progress and perspective. Nanoscale 2014, 6, 3995–4011. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, A. Recent advances in the synthesis and application of photocatalytic metal–metal oxide core–shell nanoparticles for environmental remediation and their recycling process. RSC Adv. 2016, 6, 83589–83612. [Google Scholar] [CrossRef]
- Baranowska-Korczyc, A.; Mackiewicz, E.; Ranoszek-Soliwoda, K.; Grobelny, J.; Celichowski, G. Core/shell Ag/SnO2 nanowires for visible light photocatalysis. Catalysts 2021, 12, 30. [Google Scholar] [CrossRef]
- Yadav, A.K.; Jain, C.; Malik, D. Toxic characterization of textile dyes and effluents in relation to human health hazards. J. Sustain. Environ. Res. 2014, 3, 95–102. [Google Scholar]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Senapati, S.; Srivastava, S.K.; Singh, S.B.; Mishra, H.N. Magnetic Ni/Ag core–shell nanostructure from prickly Ni nanowire precursor and its catalytic and antibacterial activity. J. Mater. Chem. 2012, 22, 6899–6906. [Google Scholar] [CrossRef]
- Sundaram, M.; Kalpana, S.; Sivaganesan, V.; Nandhakumar, E. Studies on the catalytic activity of CuO/TiO2/ZnO ternary nanocomposites prepared via one step hydrothermal green approach. Mater. Res. Express 2019, 6, 125043. [Google Scholar]
- Indumathi, T.; Suriyaprakash, J.; Alarfaj, A.A.; Hirad, A.H.; Jaganathan, R.; Mathanmohun, M. Synergistic effects of CuO/TiO2-chitosan-farnesol nanocomposites: Synthesis, characterization, antimicrobial, and anticancer activities on melanoma cells SK-MEL-3. J. Basic Microbiol. 2024, 64, 2300505. [Google Scholar] [CrossRef] [PubMed]
- Kerli, S.; Kavgacı, M.; Soğuksu, A.K.; Avar, B. Photocatalytic degradation of methylene blue, rhodamine-B, and malachite green by Ag@ ZnO/TiO2. Braz. J. Phys. 2022, 52, 22. [Google Scholar] [CrossRef]
- Idrees, S.; Ahmad, Z.; Akhtar, T.; Choudhary, M.A.; Rafiq, M.A.; Mehmood, A. Facile chemical strategy to synthesize Ag@ polypyrrole microarrays and investigating its anisotropic effect on polymer conductivity. Mater. Sci.-Pol. 2019, 37, 563–569. [Google Scholar] [CrossRef]
- Yang, X.; Fu, H.; Zhang, L.; An, X.; Xiong, S.; Jiang, X.; Yu, A. Enhanced gas sensing performance based on the fabrication of polycrystalline Ag@ TiO2 core-shell nanowires. Sens. Actuators B Chem. 2019, 286, 483–492. [Google Scholar] [CrossRef]
- Sharif, S.; Ahmad, Z.; Hoskins, C.; Choudhary, M.A.; Mehmood, A. Ag nanostructure morphologies and physicochemical properties dictated by the polyols used in the synthesis. J. Nano Res. 2022, 76, 93–106. [Google Scholar] [CrossRef]
- Yamashita, M. Next generation multifunctional nano-science of advanced metal complexes with quantum effect and nonlinearity. Bull. Chem. Soc. Jpn. 2021, 94, 209–264. [Google Scholar] [CrossRef]
- Ma, H.R.; Chen, X.Y.; Li, J.W.; Chang, C.T.; Wang, G.; Li, H.; Wang, X.M.; Li, R.W. Fe-based amorphous coating with high corrosion and wear resistance. Surf. Eng. 2017, 33, 56–62. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Asefa, T.; Guo, H.; Biradar, A.V.; Peng, D.-L.; Zboril, R.; Varma, R.S. Core–shell nanoparticles: Synthesis and applications in catalysis and electrocatalysis. Chem. Soc. Rev. 2015, 44, 7540–7590. [Google Scholar] [CrossRef]
- Prieto, P.; Nistor, V.; Nouneh, K.; Oyama, M.; Abd-Lefdil, M.; Díaz, R. XPS study of silver, nickel and bimetallic silver–nickel nanoparticles prepared by seed-mediated growth. Appl. Surf. Sci. 2012, 258, 8807–8813. [Google Scholar] [CrossRef]
- Chi, M.; Sun, X.; Lozano-Blanco, G.; Tatarchuk, B.J. XPS and FTIR investigations of the transient photocatalytic decomposition of surface carbon contaminants from anatase TiO2 in UHV starved water/oxygen environments. Appl. Surf. Sci. 2021, 570, 151147. [Google Scholar] [CrossRef]
- Ivanova, T.; Maslakov, K.; Sidorov, A.; Kiskin, M.; Linko, R.; Savilov, S.; Lunin, V.; Eremenko, I. XPS detection of unusual Cu (II) to Cu (I) transition on the surface of complexes with redox-active ligands. J. Electron Spectrosc. Relat. Phenom. 2020, 238, 146878. [Google Scholar] [CrossRef]
- Zahoor, A.; Qiu, T.; Zhang, J.; Li, X. Synthesis and characterization of Ag@ polycarbazole nanoparticles and their novel optical behavior. J. Mater. Sci. 2009, 44, 6054–6059. [Google Scholar] [CrossRef]
- Sharif, S.; Ahmed, T.; Ahmad, Z.; Choudhary, M.A.; Arshad, M. Synthesis and characterization of Ag@ Ni co-axial nanocables and their fluorescent and catalytic properties. Sci. Eng. Compos. Mater. 2024, 31, 20240010. [Google Scholar] [CrossRef]
- Arya, S.; Singh, A.; Kour, R. Comparative study of CuO, CuO@ Ag and CuO@ Ag: La nanoparticles for their photosensing properties. Mater. Res. Express 2019, 6, 116313. [Google Scholar] [CrossRef]
- Adekoya, J.; Dare, E.; Mesubi, M.; Nejo, A.A.; Swart, H.; Revaprasadu, N. Synthesis of polyol based Ag/Pd nanocomposites for applications in catalysis. Results Phys. 2014, 4, 12–19. [Google Scholar] [CrossRef]
- Blancon, J.; Nie, W.; Neukirch, A.J.; Gupta, G.; Tretiak, S.; Cognet, L.; Mohite, A.D.; Crochet, J.J. The effects of electronic impurities and electron–hole recombination dynamics on large-grain organic–inorganic perovskite photovoltaic efficiencies. Adv. Funct. Mater. 2016, 26, 4283–4292. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, A. Photocatalytic oxidation of pollutant dyes in wastewater by TiO2 and ZnO nano-materials—A mini-review. Nanosci. Technol. Mank. 2014, 36–72. [Google Scholar]
- Ahmad, Z.; Maqsood, M.; Mehmood, M.; Ahmad, M.J.; Choudhary, M.A. Synthesis and characterization of pure and nano-Ag impregnated chitosan beads and determination of catalytic activities of nano-Ag. Bull. Chem. React. Eng. Catal. 2017, 12, 127–135. [Google Scholar] [CrossRef]
- Hu, H.; Liu, P.; Cao, S.; You, L.; Zhang, N.; Xi, J.; Guo, S.; Zhou, K. Single Metal Atoms Anchored on N-Doped Holey Graphene as Efficient Dual-Active-Component Catalysts for Nitroarene Reduction. Adv. Funct. Mater. 2024, 34, 2307162. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Hegazey, R.; El-Azabawy, R.E. Efficient removal of methylene blue dye from aqueous media using Fe/Si, Cr/Si, Ni/Si, and Zn/Si amorphous novel adsorbents. J. Mater. Res. Technol. 2019, 8, 5301–5313. [Google Scholar] [CrossRef]
- Mantasha, I.; Hussain, S.; Ahmad, M.; Shahid, M. Two dimensional (2D) molecular frameworks for rapid and selective adsorption of hazardous aromatic dyes from aqueous phase. Sep. Purif. Technol. 2020, 238, 116413. [Google Scholar] [CrossRef]
- Bathla, A.; Rather, R.A.; Poonia, T.; Pal, B. Morphology dependent photocatalytic activity of CuO/CuO–TiO2 nanocatalyst for degradation of methyl orange under sunlight. J. Nanosci. Nanotechnol. 2020, 20, 3123–3130. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Rajput, J.K. Solar light photocatalytic activity of CuO/TiO2 mixed oxide derived from conjugated azomethine metal complex for degradation of food colorants. J. Mol. Liq. 2022, 366, 120280. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R. Evaluation of UV/H2O2 advanced oxidation process (AOP) for the degradation of diazo dye Reactive Green 19 in aqueous solution. Desalin. Water Treat. 2014, 52, 1571–1577. [Google Scholar] [CrossRef]
- Ren, H.; Wang, S.; Labidi, A.; Pan, B.; Luo, J.; Wang, C. Nanoengineering of ultrathin N-CQDs/Bi2WO6 S-scheme heterojunction for enhanced photodegradation of antibiotics as emerging contaminants: Mechanism insight and toxicity assessment. Sep. Purif. Technol. 2025, 362, 131717. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Jang, Y.H.; Kim, D.H. A study on the mechanism for the interaction of light with noble metal-metal oxide semiconductor nanostructures for various photophysical applications. Chem. Soc. Rev. 2013, 42, 8467–8493. [Google Scholar] [CrossRef]
- Sin, J.-C.; Lam, S.-M.; Zeng, H.; Lin, H.; Li, H.; Huang, L.; Liaw, S.-J.; Mohamed, A.R.; Lim, J.-W. Integrating BaFe2O4 nanoparticles onto N-doped Bi2WO6 microspheres for eminent visible light-driven photocatalytic performance towards aquaculture contaminants and pathogens. Colloids Surf. A Physicochem. Eng. Asp. 2024, 692, 133905. [Google Scholar] [CrossRef]
- Rawat, D.; Mishra, V.; Sharma, R.S. Detoxification of azo dyes in the context of environmental processes. Chemosphere 2016, 155, 591–605. [Google Scholar] [CrossRef]
- Rtimi, S.; Dionysiou, D.D.; Pillai, S.C.; Kiwi, J. Advances in catalytic/photocatalytic bacterial inactivation by nano Ag and Cu coated surfaces and medical devices. Appl. Catal. B Environ. 2019, 240, 291–318. [Google Scholar] [CrossRef]
- Vaishampayan, A.; Grohmann, E. Antimicrobials functioning through ros-mediated mechanisms: Current insights. Microorganisms 2021, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Vaiwala, R.; Sharma, P.; Ayappa, K.G. Differentiating interactions of antimicrobials with Gram-negative and Gram-positive bacterial cell walls using molecular dynamics simulations. Biointerphases 2022, 17, 061008. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, S.; Harris, M.T.; Barkey, D.P. Polyol silver nanowire synthesis and the outlook for a green process. J. Nanomater. 2020, 2020, 9341983. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azam, J.; Ahmad, Z.; Irfan, A.; Naz, A.; Arshad, M.; Sattar, R.; Raish, M.; Abbasi, B.B.K.; Jardan, Y.A.B. Polyol Formation of Silver@Metal Oxides Nanohybrid for Photocatalytic and Antibacterial Performance. Catalysts 2025, 15, 283. https://doi.org/10.3390/catal15030283
Azam J, Ahmad Z, Irfan A, Naz A, Arshad M, Sattar R, Raish M, Abbasi BBK, Jardan YAB. Polyol Formation of Silver@Metal Oxides Nanohybrid for Photocatalytic and Antibacterial Performance. Catalysts. 2025; 15(3):283. https://doi.org/10.3390/catal15030283
Chicago/Turabian StyleAzam, Jovairya, Zahoor Ahmad, Ali Irfan, Asima Naz, Muhammad Arshad, Rabia Sattar, Mohammad Raish, Bakar Bin Khatab Abbasi, and Yousef A. Bin Jardan. 2025. "Polyol Formation of Silver@Metal Oxides Nanohybrid for Photocatalytic and Antibacterial Performance" Catalysts 15, no. 3: 283. https://doi.org/10.3390/catal15030283
APA StyleAzam, J., Ahmad, Z., Irfan, A., Naz, A., Arshad, M., Sattar, R., Raish, M., Abbasi, B. B. K., & Jardan, Y. A. B. (2025). Polyol Formation of Silver@Metal Oxides Nanohybrid for Photocatalytic and Antibacterial Performance. Catalysts, 15(3), 283. https://doi.org/10.3390/catal15030283







