Abstract
A Ppy/Ag-ZnO catalyst was successfully synthesized at room temperature using a novel, green methodology. It involves a mechanically assisted metathesis reaction. The Ppy/Ag-ZnO catalyst was analyzed via X-ray diffraction Technique (XRD), Thermogravimetric analysis (TGA), Differential scanning calorimetry (DSC), Fourier Transform Infrared (FTIR), Scanning Electron Microscopy (SEM), UV–visible spectroscopy, Brunauer–Emmett–Teller (BET), and zeta potential. Debye Scherrer’s calculation suggested a crystallite size of 2.30 nm for Ppy/Ag-ZnO nanocomposite. SEM confirmed the production of aggregated particles with an average size of 2.65 μm, endorsing the -ve zeta potential value (−6.78 mV) due to the presence of Van der Waals forces among the particles of Ppy/Ag-ZnO. DSC confirms that the strong interfacial interaction between Ag-ZnO and the polar segments of Ppy is responsible for the higher Tg (107 °C) and Tm (270 °C) in Ppy/Ag-ZnO. The surface area and average pore size of Ppy/Ag-ZnO catalyst were determined to be 47.08 cm3/g and 21.72 Å, respectively. Methyl orange (MO) was used as a probe in a photocatalytic reaction of fabricated material, which demonstrated exceptional efficiency, exhibiting a removal rate of 91.11% with a rate constant of 0.028 min−1. Photocatalytic degradation of MO was shown to follow pseudo-first-order kinetics.
1. Introduction
Pollution is a significant threat to the environment worldwide due to advancements in industrialization, including paper, leather, tannery, pharmaceutical, ink, food, and rubber industries [1,2]. Water bodies are primarily threatened by enhanced industrialization. In fabric-forming factories, complex dyes are broadly utilized. Due to their stability and solubility in water, these dyes can largely contaminate water and become toxic to the water environment and human beings [3,4]. Dyes in polluted water behave like a barrier in dissolving oxygen and block the way sunlight penetrates water bodies, reducing photosynthesis and harming water animals. Several conventional techniques have been used to reduce water contamination caused by organic dyes, but these methods fail to degrade effluents completely. Numerous physical and chemical processes with less harm have also been applied, such as reverse osmosis, ion exchange, adsorption, filtration, and coagulation. Hence, using semiconductor metal or metal oxides for photocatalytic degradation of dyes is propitious for wastewater treatment compared to other methods [5,6,7].
Polypyrrole, a conducting polymer, is conductive due to delocalized holes with positive charges in its oxidized framework, a π-electron conjugated system. The cationic polymer matrix accommodates the electrolytic anions to attain the neutral charge. These anions are also necessary for the polymer chain’s redox characteristics and charge conduction. Due to the position interchange of single and double bonds in the π-conjugated system, the polypyrrole’s π-conjugation produces disturbed energy states. Thus, polypyrrole contains the nondegenerate ground state. The doping approach can also transfer the polypyrrole from insulating to conducting [8]. Its oxidation potential is approximately 0.8 V, which gives it exceptional stability even when subjected to high temperatures and various environmental factors [9]. Furthermore, it demonstrates outstanding optoelectrical characteristics and can dissolve in water [10]. Ppy’s distinctive features make it suitable for various industries, including rechargeable batteries, supercapacitors, and sensors [11]. This adaptable polymer is used in a broad range of applications, including supercapacitors [12,13], adsorbents [14,15,16], anti-corrosion coatings [17], gas sensors [18], energy storage [19], and antibacterial agents [20]. Ppy and its modified materials have proven efficient in removing pollutants from wastewater [17,21,22].
Zinc oxide (ZnO) is an n-type semiconductor with exceptional electrical and photocatalytic characteristics, with a band energy of 3.37 eV. ZnO NPs have a higher exciton binding energy of 60 meV [23]. In addition, ZnO NPs are good choices for UV screening processes due to their minimal toxic characteristics and excellent chemical stability [24,25,26]. The ability of a substance to use light to catalyze a reaction relies on its band energy and its efficacy in creating electron-hole pairs. Their wide band gap restricts the application of ZnO NPs alone for photocatalysis, quick recombination of photo-excited electrons, and primary absorbance in the UV light spectrum. Various methods can be used to overcome these restrictions. One technique is to produce a heterojunction by introducing different metals or metal oxides into ZnO or adjusting its structure to create hybrid materials, which can modify the band gap and decrease the recombination speed of charge carriers [11,27,28,29]. Since most ZnO surface sites are positively charged, negatively charged biomolecules can be bound to its surface. Doping can enhance the electrochemical and optoelectronic properties [8,30].
ZnO doped with silver (Ag) exhibits exceptional efficacy in degrading organic pollutants through photocatalysis [31]. Incorporating Ag into the catalyst improves the degradation of dyes and boosts light absorption for photocatalytic reactions. Additionally, the stability of the catalyst is increased with the addition of Ag loading [32]. Hfijen, I.H. et al. focused on employing primarily sol–gel, mechanochemical, and environmentally friendly methods to synthesize ZnO NPs doped with Ag or selenium. The high surface-to-volume ratio, small size, antimicrobial activity, and semiconducting characteristics of these NPs have led them to be used as photocatalytic and antibacterial agents. Nevertheless, ZnO NPs also show restrictions in specific characteristics. By doping ZnO NPs with Ag, overcoming these restrictions and boosting their use appears feasible [33].
Ersoz.E et al. used the electrospinning method to generate ZnO nanofiber photocatalysts doped with Ag. The photocatalytic degradation of MB (methyl blue), MO (methyl orange), and RhB (rhodamine B) under exposure to ultraviolet light was performed to determine the photocatalytic capabilities of the prepared fibres. After 120 min of UV light radiation exposure, it was discovered that the photodegradation rates of RhB and MO dye solutions were 66% and 51%, respectively. MB was fully degraded after 75 min [34]. Fard. R. et al. reported a simple, green, and environmentally friendly method of synthesizing Ag-doped ZnO NPs utilizing Crataegus monogyna extract and used to remove MO and BV 10 (basic violet 10) dyes. The findings demonstrated that when exposed to UV and sunlight, prepared Ag-ZnO NPs destroyed MO and basic violet 10 by 89.8% and 75.3%, and by 94.2% and 84.7%, respectively. When exposed to UV and sunlight radiations, the Ag-modified ZnO NPs performed better than the pure ZnO NPs in terms of catalytic activity towards organic contaminants [35].
In addition, introducing Ppy in Ag-ZnO NPs considerably improves their conductivity, stability, photocatalytic capabilities, and adsorption properties [36]. Tailored structural designs with specific functionalities can be achieved by preparing nanostructures based on polymers. These modifications result from the characteristics of the material components, which are influenced by the incorporation of polymers, leading to synergistic effects. The advantages of polymer-based nanostructures include the ability to customize the structural design according to specific requirements. Ppy/Ag-ZnO catalysts have garnered considerable attention in the literature for their efficacy in removing various dyes, such as rhodamine B [1], methylene blue, rose Bengal [2], and malachite green [3].
The synthetic procedure in the literature about the synthesis of Ppy/Ag-ZnO is a purely chemical method involving multiple steps, hazardous chemicals, toxic solvents, and unfriendly by-products [8,16,20,37,38,39,40]. Great importance is placed on developing simple, low-temperature, and environmentally friendly methods for preparing ZnO and Zn-based compounds. Various methods, including microwave [41] and mechanochemical techniques [42], have successfully synthesized ZnO NPs. Mechanochemical processing is a milling process involving a metathesis reaction that has been carried out to find the required product. In a metathesis reaction, atomic or ionic species are exchanged between reactants, forming stable products. The primary driving force behind this reaction is a substantial change in enthalpy [43,44]. The favourable formation of stable salt byproducts (alkaline or alkaline–earth halides and nitrates) is through metathesis reactions. The synthesis of ZnO or Zn-based heterostructures from the milled precursor typically contains an in situ generated matrix salt (NaCl) and a Zn compound (ZnCO3). It requires a subsequent heat treatment of over 600 °C [42,45,46]. Other methods of Ag/ZnO preparations that utilize ZnO and AgNO3 as starting materials have been found to require lengthy milling periods [47,48].
In recent work, a remarkably straightforward and eco-friendly approach for synthesizing nanosized ZnO and Ag-ZnO heterostructures was presented using a mechanically assisted metathesis reaction. The metathesis reaction has two functions: initially, it produces LiNO3/NaNO3, high-lattice energy byproducts that drive the reaction in situ; secondly, it crystallizes ZnO and Ag-ZnO directly in the milling media. In the end, Ppy/Ag-ZnO catalyst was produced by ball milling Ag-ZnO with Ppy polymer. One benefit of this simple process is that it is an eco-friendly green method that uses low-cost chemicals without any solvent and produces a high yield. In addition, the catalytic potential of the prepared composite was also evaluated for the degradation of MO dye at various conditions like pH, different catalyst amounts, dye concentration and temperature.
2. Results and Discussion
2.1. XRD Analysis
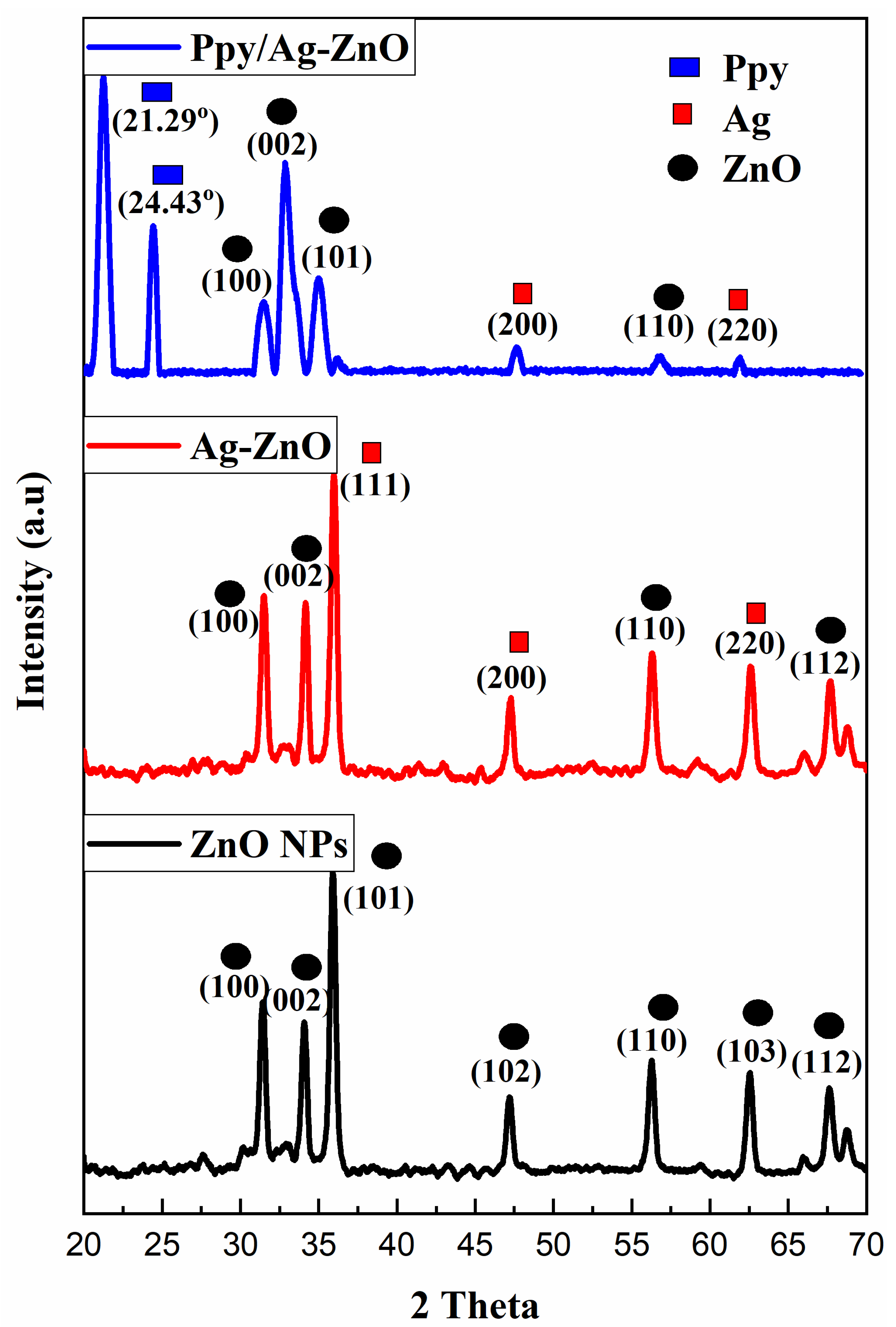
The XRD images for ZnO, Ag-ZnO, and Ppy/Ag-ZnO catalysts are depicted in Figure 1. Sharp diffraction peaks were obtained for ZnO NPs, which suggested the crystalline nature of the synthesized particles and demonstrated the hexagonal wurtzite structure for ZnO NPs with the data card number (JCPDS file No. 36-1451). The diffracted angles examined for ZnO NPs are at 31.45°, 34.10°, 35.91°, 42.29°, 56.21°, 62.48°, and 67.60° corresponding to lattice planes (100), (002), (101), (102), (110), (103), and (112), respectively [15]. For ZnO NPs, a crystallite size of 2.22 nm was obtained, which was calculated using Debye-Scherrer’s equation.
where D = crystallite size (nm), k = Scherrer’s constant is equal to 0.98, λ = wavelength of X-rays equal to 0.15418 nm, β = Full width at half maxima (FWHM), and θ = angle of diffraction.
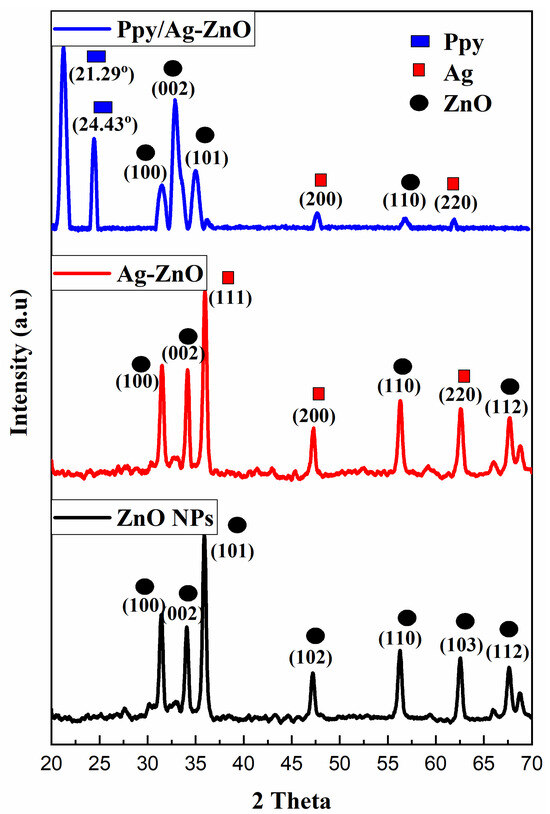
Figure 1.
PXRD Pattern for ZnO NPs, Ag-ZnO, and Ppy/Ag-ZnO catalysts.
XRD data demonstrated the successful doping of Ag on ZnO NPs. Diffracted angles having 2θ values 31.47°, 34.13°, 36.08°, 47.29°, 56.37°, 62.6°, and 67.77° exhibiting lattice plane (100), (002), (111), (200), (110), (220), and (112), respectively, for Ag-ZnO in which diffracted angles 36.08° (111), 47.29° (200), and 62.6° (220) were assigned to Ag with Ag file No. 04-0783. A crystallite size of 3.34 nm was obtained [16].
XRD data for Ppy/Ag-ZnO catalyst are shown in Figure 1, where ZnO NPs have 2θ values at 31.53°, 32.93°, 35.02°, and 56.77°, and Ag have diffracted angles at 47.70° and 61.90° correlated to lattice planes (100), (002), (101), (110), (200), and (220), respectively. Diffraction peaks at 21.29° and 24.43° were assigned to Ppy. Debye Scherrer’s calculation suggested a crystallite size of 2.30 nm [16]. The d-line spacing of 13.05 nm was investigated for Ppy/Ag-ZnO using the following formula.
where d is line spacing, λ is wavelength equal to 0.15418 nm, and θ is diffracted angle.
2.2. Thermogravimetric Analysis (TGA/DTG) and Differential Scanning Calorimetry (DSC)
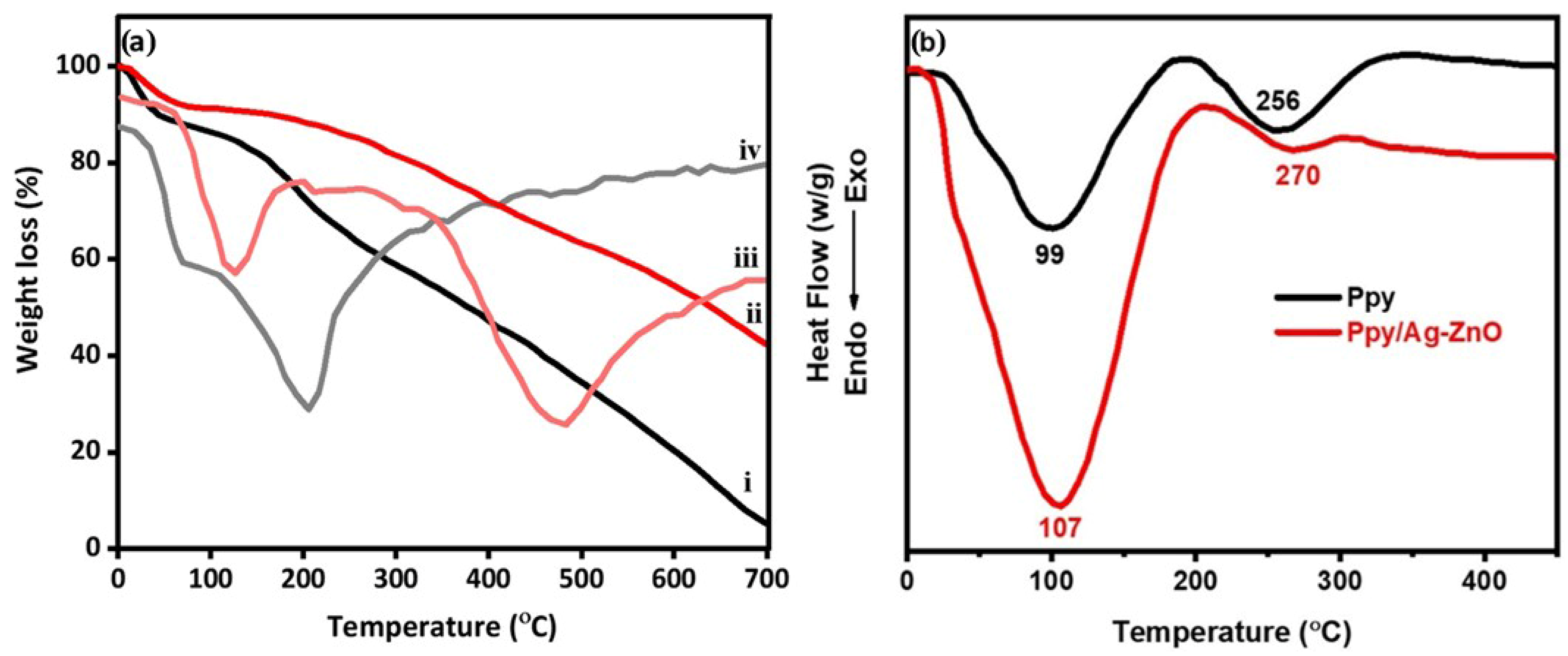
Analyzing the thermal behaviour of the milled samples can provide further evidence of a successful reaction. It has been observed that the thermal stability of Ppy/Ag-ZnO is greater than that of pure Ppy. The TGA curve (Figure 2a) showed that the Ppy and Ppy/Ag-ZnO undergo two stages of weight loss. The initial decomposition of both samples occurs at around 100 °C, corresponding to the evolution of water and low molecular weight oligomers. The final decomposition at 160 °C is attributed to the degradation of the Ppy unit. The second decomposition temperature of Ppy/Ag-ZnO increased by 40 °C compared to Ppy. The higher thermal stability of Ppy/Ag-ZnO is due to the increased intermolecular interaction between the Ag-ZnO and the polymer chain. It is also evident from the data that the char residue of pure Ppy at 700 °C is only 4.9%, while for Ppy/Ag-ZnO, the char residue is 43.8%. The higher amount of char residues indicates better thermal stability of the composites. The char layers act as a barrier by slowing the emission of volatile degradation products. The increased char residue of Ppy/Ag-ZnO indicates better flame resistance of the catalyst [33]. The DTG analysis of Ppy reveals two distinct weight loss peaks. The first peak occurs around 100 °C, indicating water loss and unreacted monomers. The second peak, which emerges above 150 °C, is associated with the decomposition of Ppy. In the case of the Ppy/Ag-ZnO composite, water loss begins at temperatures exceeding 100 °C, while the decomposition of Ppy initiates at 330 °C and persists until 600 °C. The integration of Ppy with Ag-ZnO significantly boosts its thermal stability compared to Ppy alone (Figure 2a).
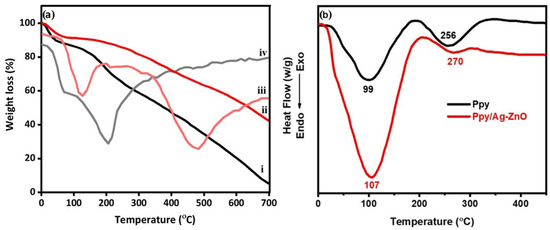
Figure 2.
(a) TGA/DTG (i, iv: Ppy; ii, iii: Ppy/Ag-ZnO) and (b) DSC curves of Ppy and Ppy/Ag-ZnO.
Figure 2b compares the DSC profile of Ppy and Ppy/Ag-ZnO. The results indicate that the glass transition temperature (Tg) and melting temperature (Tm) of Ppy/Ag-ZnO are significantly higher than those of pure Ppy. The values of Tg and Tm depend on several factors, including the mobility of the polymer chain, the polarity of the polymer and filler particles, free volume, and the interaction between the filler and polymer chain. Ppy exhibits a broad endothermic dip at 99 °C and a weak endothermic peak at 256 °C, corresponding to its Tg and Tm, respectively. The Tg value of Ppy/Ag-ZnO is centred at 107 °C, and the Tm value is obtained at 270 °C, which is significantly higher than that of pure Ppy. The strong interfacial interaction between Ag-ZnO and the polar segments of Ppy is responsible for the higher Tg and Tm values of Ppy/Ag-ZnO. The synergetic interaction between the filler and polymer chain reduces the mobility of the macromolecular chain. Additionally, the volume of immobilized or bound polymer increases due to the short inter-particle distance upon loading of Ag-ZnO, which results in high Tg and Tm values. The absence of additional thermal events related to precursor salts or by-products in Equation (1) indicates the complete participation of the precursor salt in the metathesis reaction and the successful removal of by-products (LiOH or NaOH) during multiple washings with water.
2.3. FTIR Study
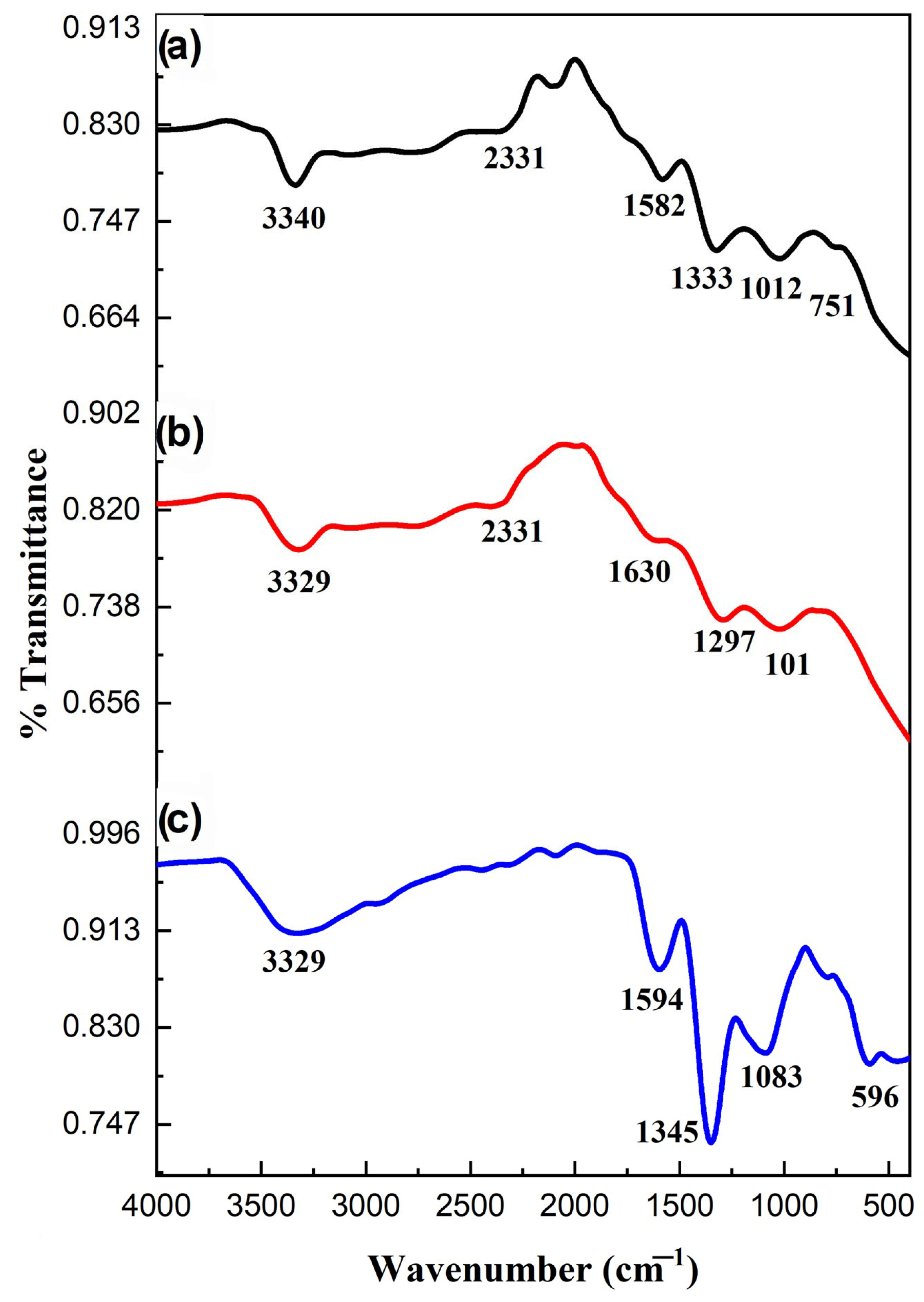
FTIR spectra of prepared samples are depicted in Figure 3. Ag-ZnO NPs peaked at 3329 cm−1, which can be attributed to the adsorbed water molecules on their surface. The metallic stretching frequency associated with the Ag-ZnO was displayed from 670 to 400 cm−1. CN stretching vibrations of Ppy were positioned at 1345 and 1594 cm−1. The =CH vibrations of Ppy were responsible for the bands in the 850–1000 cm−1 area. These variations in vibrational frequencies validated the polymerization of pyrrole, which is consistent with the findings of the previous investigation. The distinctive stretching frequencies of Ppy with the vibrations of metal oxide nanoparticles were visible in the FTIR spectrum of Ppy/Ag-ZnO (Figure 3c). The composite spectrum has a prominent Ag-doped ZnO peak at 596 cm−1. The addition of Ag-ZnO NPs caused a minor shift in the absorption frequencies of composite materials. The shift in absorption bands indicated the intermolecular attraction of the nanoparticles and the electronegative nitrogen of Ppy. Therefore, it is verified by the FTIR spectra that the nanoparticles are securely incorporated into the Ppy [20].
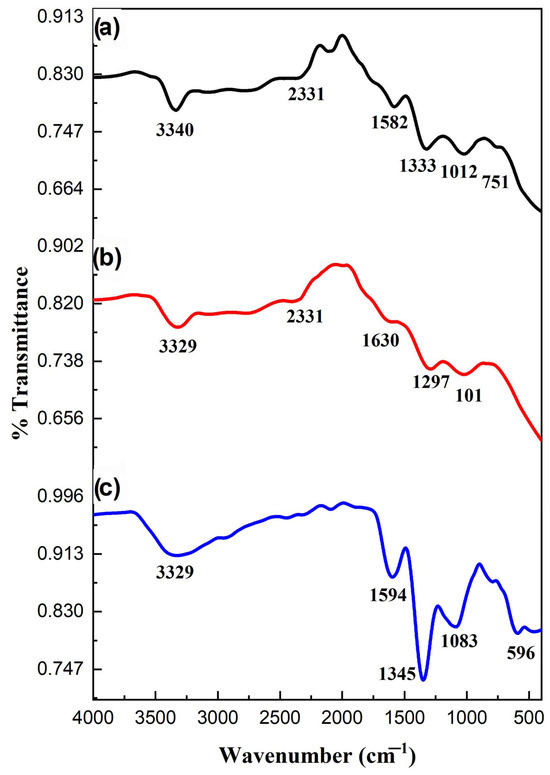
Figure 3.
FTIR of (a) ZnO NPs, (b) Ag-ZnO, and (c) Ppy/Ag-ZnO catalyst.
2.4. Morphology Analysis
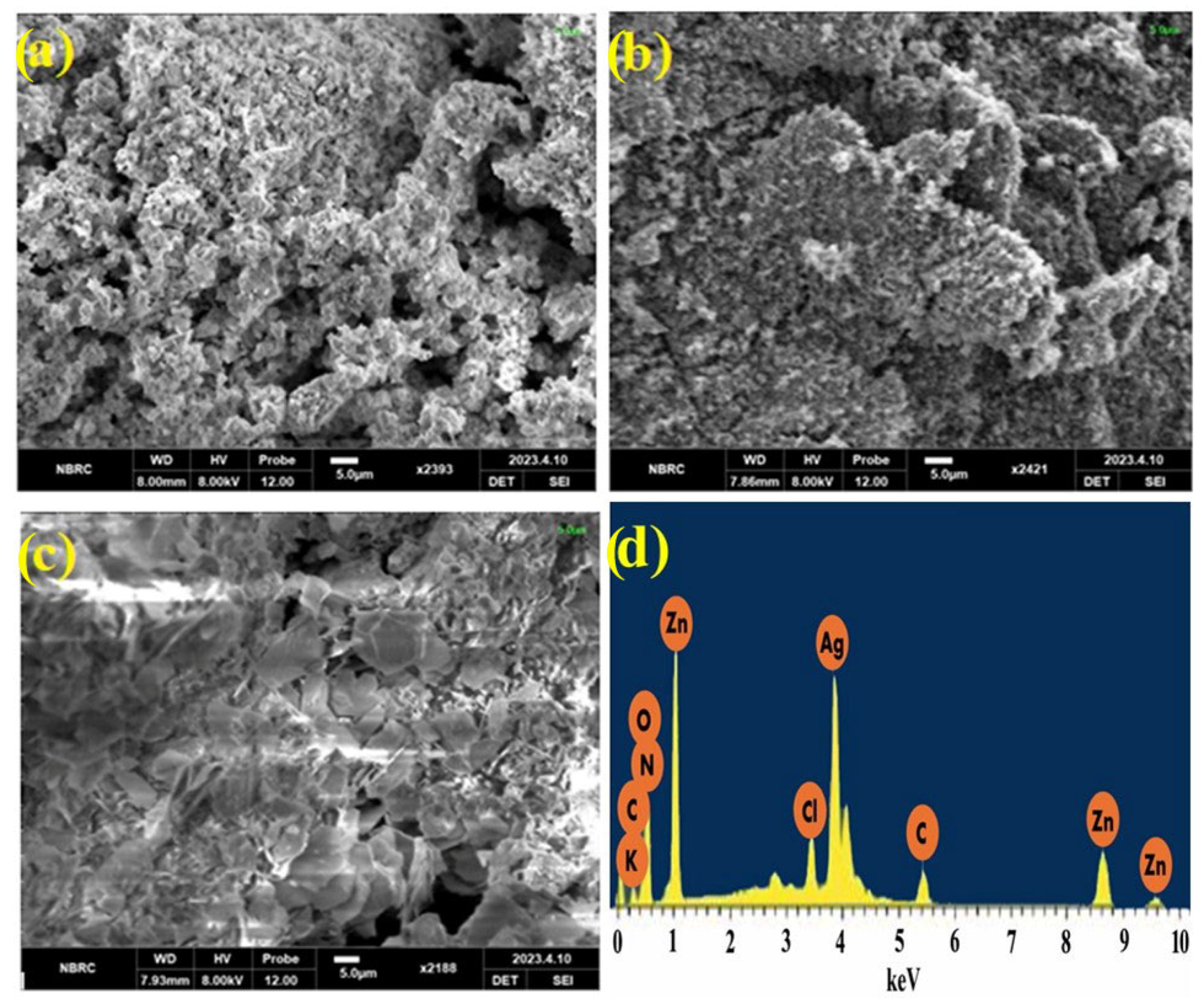
Morphological characterization of prepared nanomaterials ZnO, Ag-ZnO, and Ppy/Ag-ZnO catalysts was investigated through SEM images in Figure 4a–c. SEM micrographs reveal that particles aggregate to form microspheres due to Van der Waal forces. The average grain size of prepared Ag-ZnO NPs was 1.56 μm, shorter than ZnO NPs because of the introduction of Ag ions. The distribution of nanoparticles became narrow because of Ag loading on ZnO NPs. SEM image of the fabricated photocatalyst Ppy/Ag-ZnO catalyst (Figure 4c) showed the lumps of particles with deformity in the polymerized matrix, having an average grain size of 2.65 μm. It is evident from the surface morphology of the nanocomposite that the particles are thoroughly incorporated into the polymer with uniformly shaped particles and few vacancies. The adequate interfacial adhesion between the nanomaterials gave the composite its uniformity. Photocatalysts with higher surface-to-volume ratios perform the photocatalytic activity with greater efficacy [18,19,20]. EDX spectrum (Figure 4d) confirms the presence of Ag and ZnO in the Ppy/Ag-ZnO catalyst.
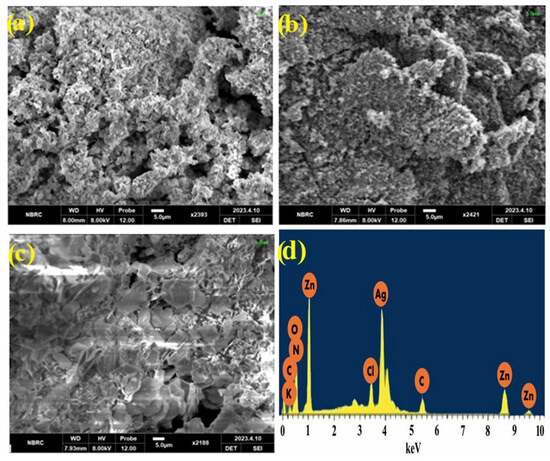
Figure 4.
SEM images for (a) ZnO NPs, (b) Ag-ZnO, (c) Ppy/Ag-ZnO catalyst, and (d) EDX of Ppy/Ag-ZnO.
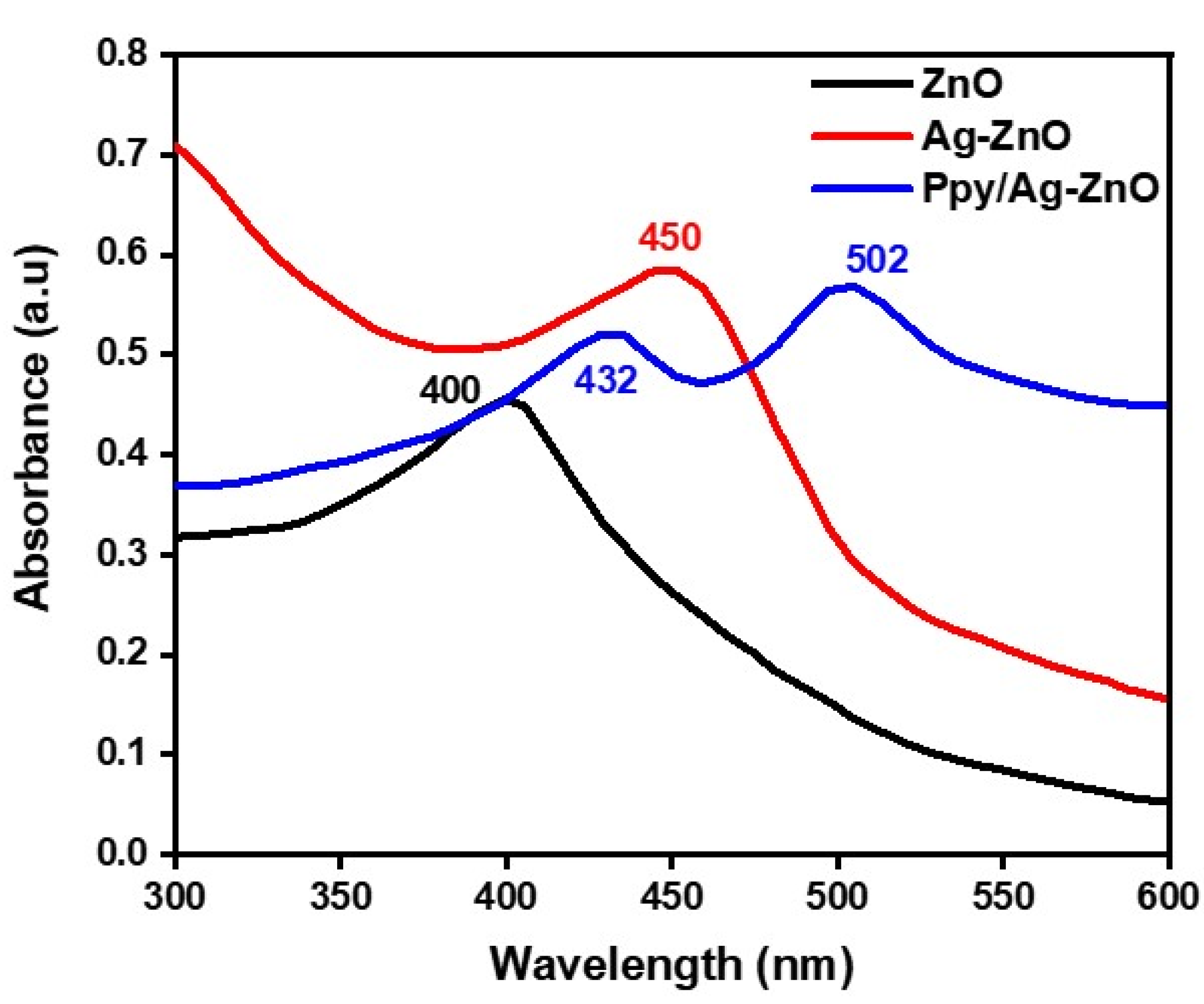
2.5. UV Visible Spectroscopy
UV–visible spectroscopy was used to examine absorption bands to determine the lambda maximum for prepared samples, as displayed in Figure 5. A UV–visible graph ranging from 300 to 600 nm was obtained. The formation of ZnO NPs by the reduction of Zinc to Zn (0) was confirmed by the absorption band at 400 nm. The absorption band obtained at 450 nm was attributed to the doping of Ag upon ZnO NPs. Ag-ZnO NPs displayed bands at higher wavelengths due to surface plasmon absorption characteristics. Confirmation of Ag-ZnO NPs formation was also made by changing the colour from dark brown to black. Another absorption band appeared at 432 and 502 nm, respectively, assigned to Ppy and Ag-ZnO NPs. It confirmed the formation of a Ppy/Ag-ZnO catalyst [17,21].
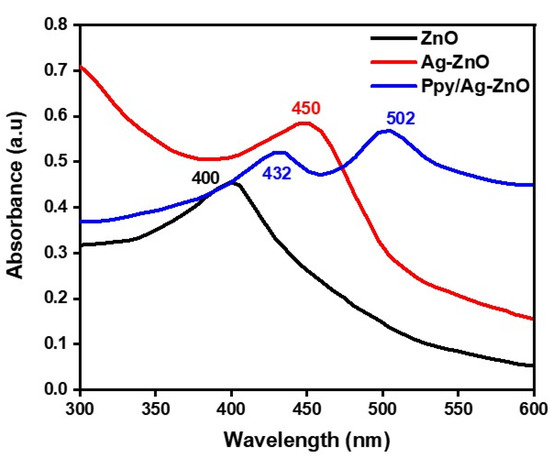
Figure 5.
The UV–visible spectrum of ZnO NPs, Ag-ZnO, and Ppy/Ag-ZnO catalysts.
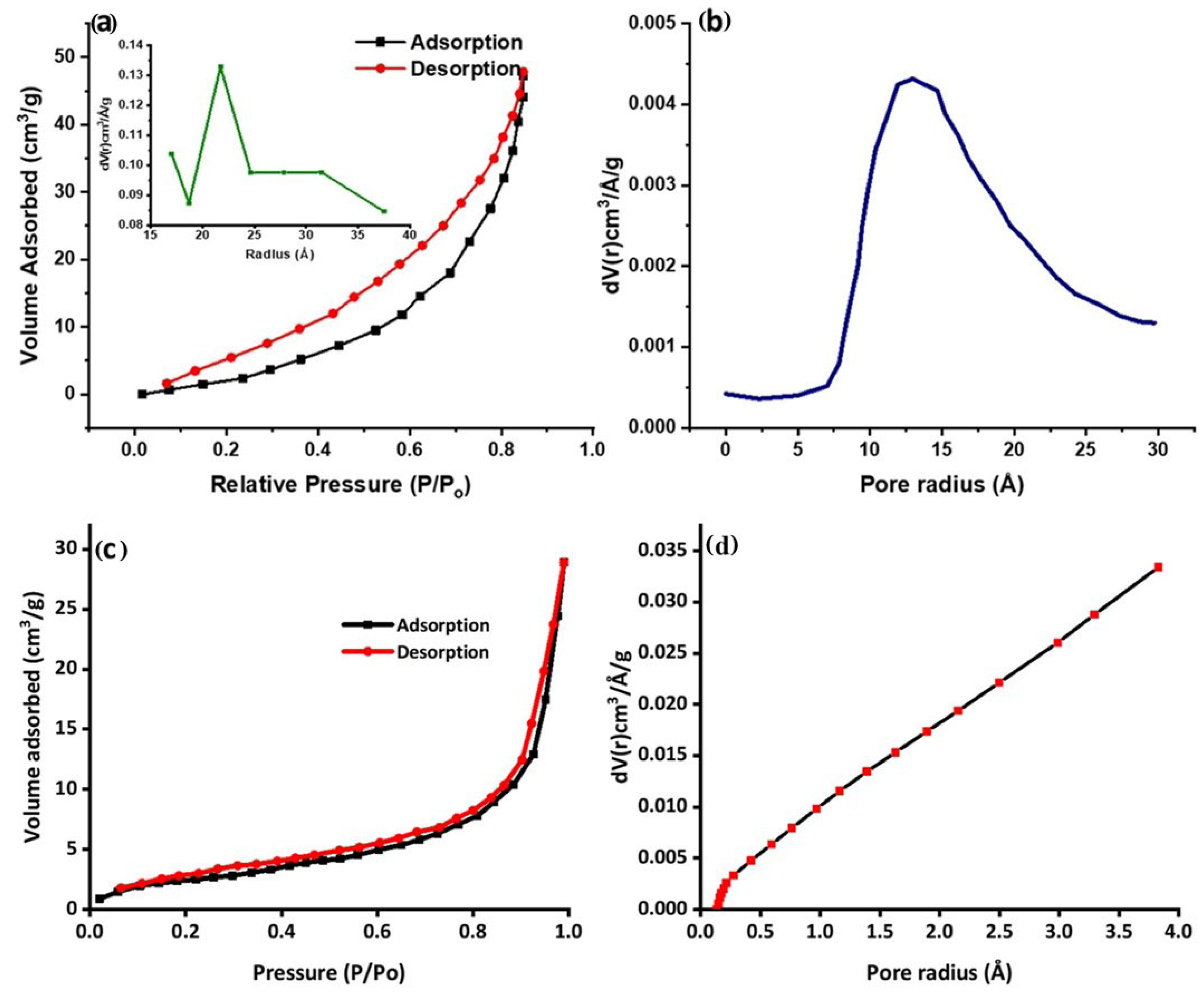
2.6. BET Characterization
The surface characteristics of the prepared nanocomposites were studied using BET analysis. The porousness and specific surface area for the reactants to interact with the catalysts strongly correlate with the photocatalytic capability. The hysteresis loop with high relative pressure (P/Po) ranges between 0 and 1, determined by analysis isotherms, depicted in Figure 6a. Before absorption, the Ppy/Ag-ZnO catalyst plot exhibits a quick absorption of N2 in the low-pressure region. Consistent high adsorption with more significant pressures causes hysteresis, suggesting different compositional pore designs. Porous nanomaterials, which give the reaction porosity, more active sites, and a quick charge–discharge rate, are highly helpful in photocatalytic degradation performance [22,49].
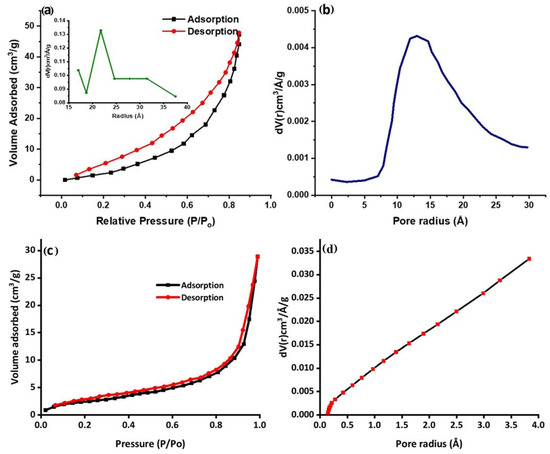
Figure 6.
(a) Nitrogen adsorb–desorb isotherm for Ppy/Ag-ZnO (inset: BJH pore size distribution), (b) DA pore radius, (c) Nitrogen adsorb–desorb isotherm for Ppy, and (d) DA pore radius.
The associated Barrett–Joyner–Halenda (BJH) pore size distribution plot for Ppy/Ag-ZnO is displayed in the inset of Figure 6a. The surface area and average pore size of the synthesized Ppy/Ag-ZnO catalyst were determined to be 47.08 cm3/g and 21.72 Å, respectively. The surface area and average pore radius of Ppy were determined to be 11.83 cm3/g and 2.72 Å, as shown in Figure 6c,d. While the Ppy/Ag-ZnO pore radius was determined to be 13.02 Å based on the Dubinin–Astakhov (DA) graph, shown in Figure 6b. The difference in surface area and porosity values indicates the successful formation of Ppy/Ag-ZnO and confers the superior catalytic properties of the composite.
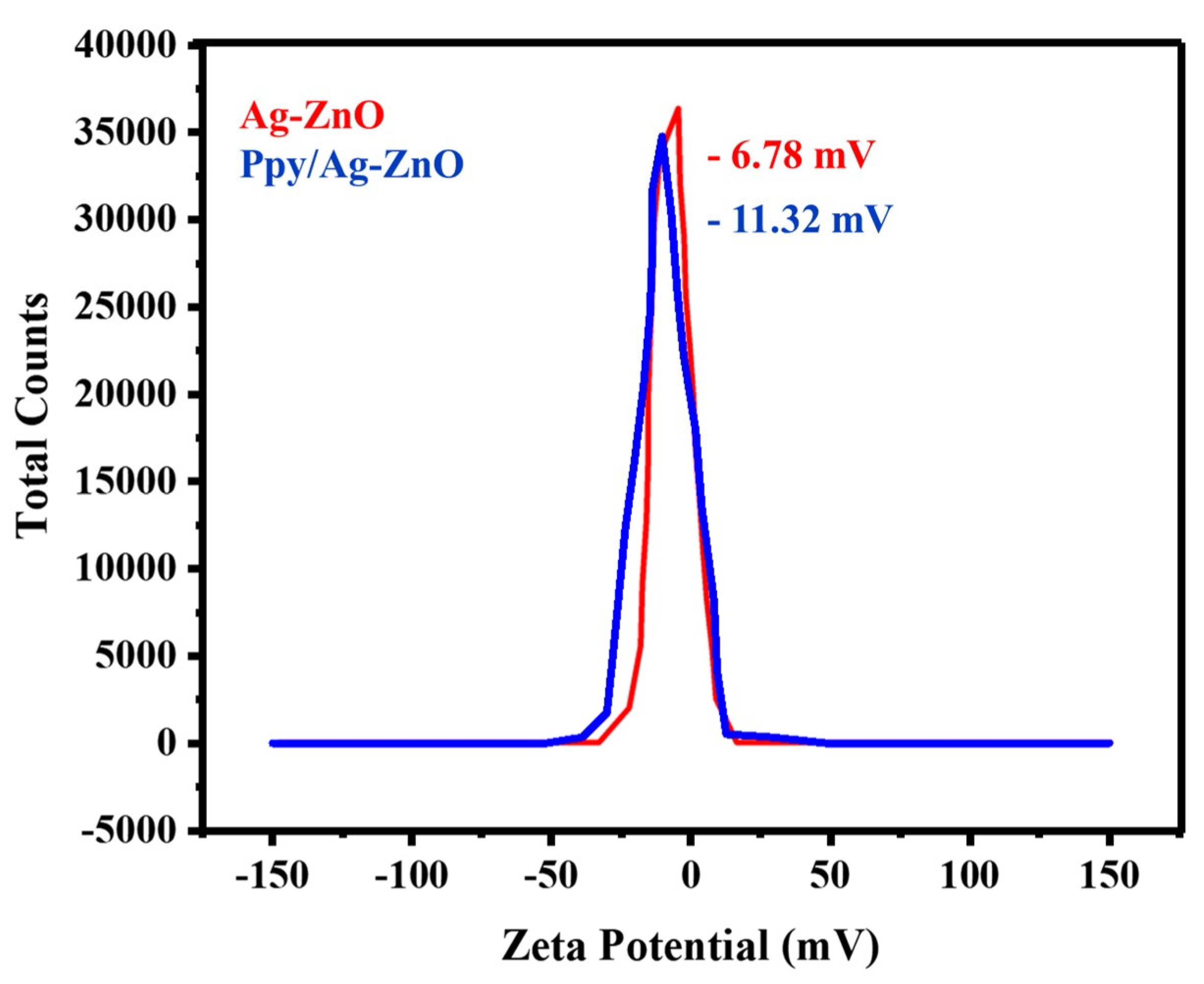
2.7. Zeta Potential (ZP)
Zeta potential measurements were used to determine the stability of the Ppy/Ag-ZnO catalyst, as shown in Figure 7. The zeta potential determines the electrostatic repulsion between the particles according to their strength and inclination to combine. The fabricated catalyst demonstrated a steady dispersion of the Ag/ZnO and Ppy/Ag-ZnO catalyst with a zeta potential value of −6.78 mV and −11.32 mV. After adding polypyrrole, the ZP value becomes more negative, indicating the redox state of Ppy/Ag-ZnO [40]. The presence of negatively charged dispersing particles is responsible for the negative zeta potential. Particles with lower zeta potential values could cause aggregation because of Van der Waals forces. Overall, negative zeta potential indicates that the Ppy/Ag-ZnO catalyst is appealing for many applications that demand stable and distributed nanomaterials [50].
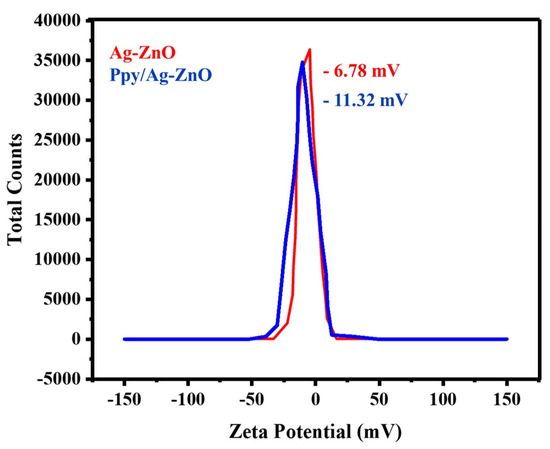
Figure 7.
Zeta potential plot for Ag-ZnO and Ppy/Ag-ZnO.
2.8. Evaluation of Photocatalytic Activity
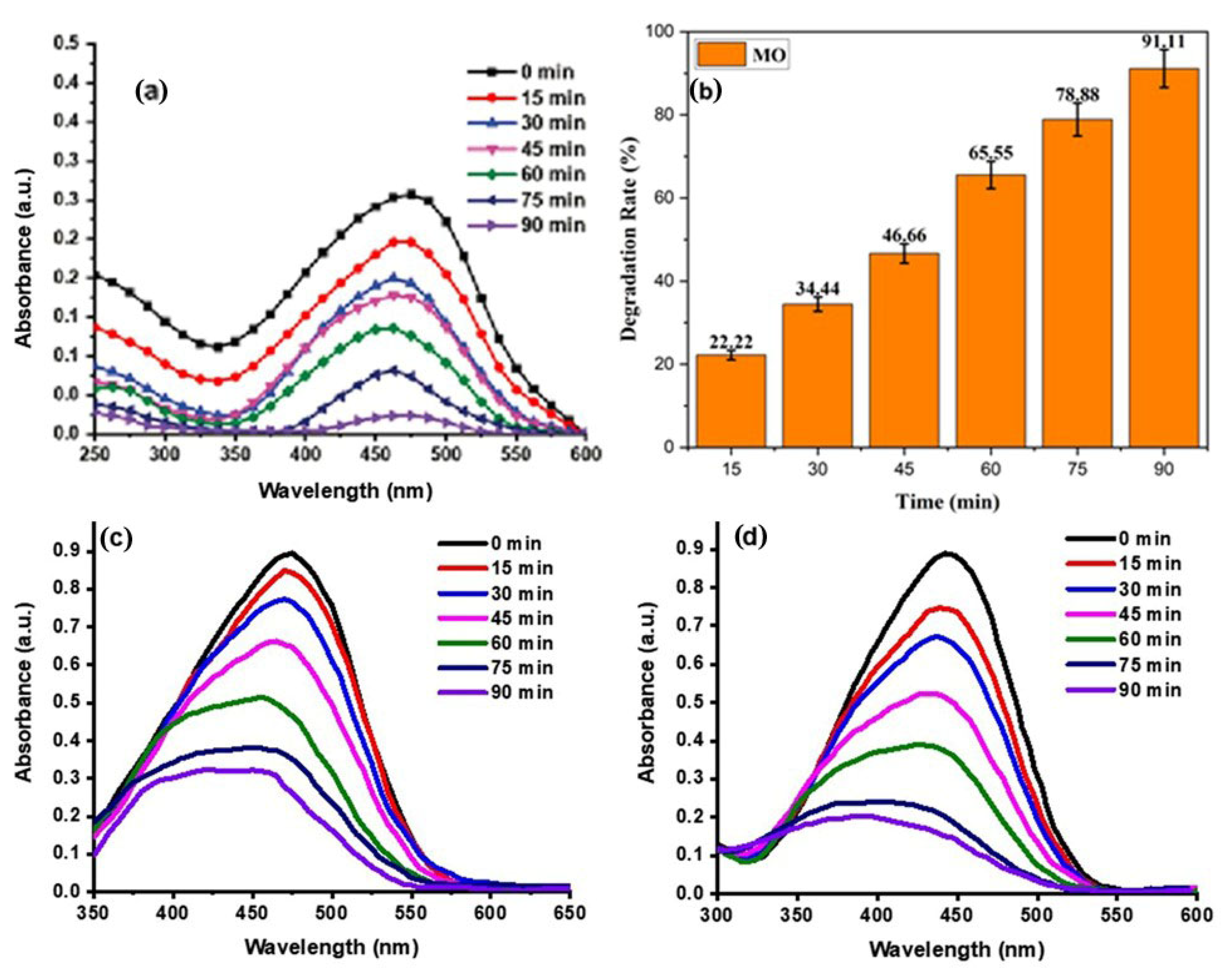
MO destabilization in the presence of an effective Ppy/Ag-ZnO catalyst was examined under natural sunlight for 90 min and manifested in Figure 8. The degradation of MO dye was confirmed by the solution’s colour, which became faint and colourless. A 91.11% degradation rate was obtained after 90 min of reaction. The rate constant for this reaction was 0.028 min−1. (For comparative analysis, the MO degradation potential of ZnO and Ag-ZnO was determined, which was found to be 67.25 and 82.63%, respectively (See Table 1 and Figure 8b,c). The bar graph and kinetic study are given in Supplementary Materials Figures S1 and S2.
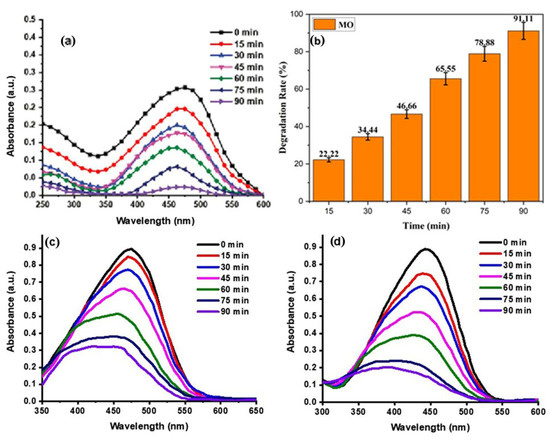
Figure 8.
Degradation of MO dye by Ppy/Ag-ZnO (a,b), ZnO (c), and Ag-ZnO (d) catalysts.

Table 1.
Crystallographic parameters of the samples.
2.8.1. Kinetic Studies
The kinetic behaviour of the photocatalyst was further investigated using the following equation [51] to determine the reaction rate of the Ppy/Ag-ZnO catalyst. The findings are presented in Figure 9.
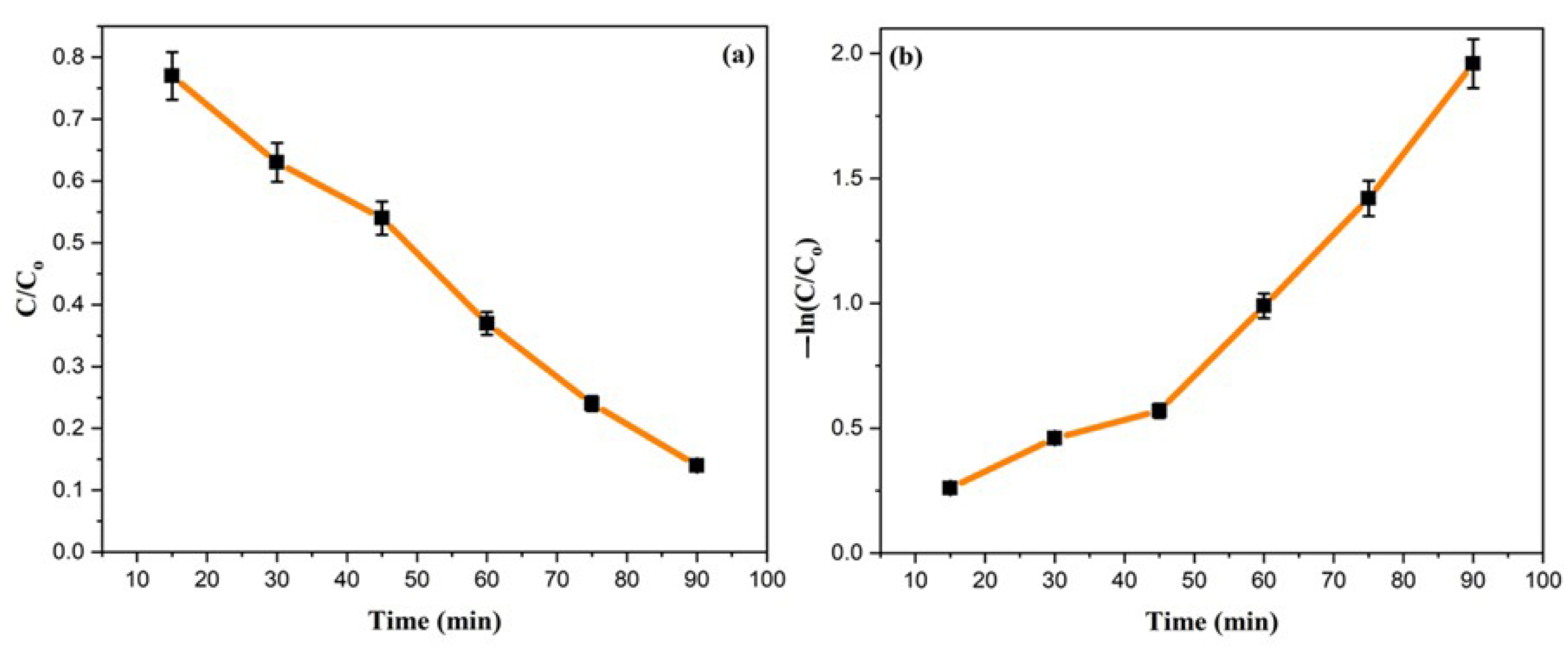
where Ao and At are the absorbance of MO dye at time “0” and time “t”, respectively, k is the rate constant, and t is reaction time. A linear graph is obtained between C/Co vs. time, determining the pseudo-first-order kinetic behaviour for photocatalytic degradation of the MO dye. The degradation reaction of MO is determined to have a reaction rate constant of 0.028 min−1.
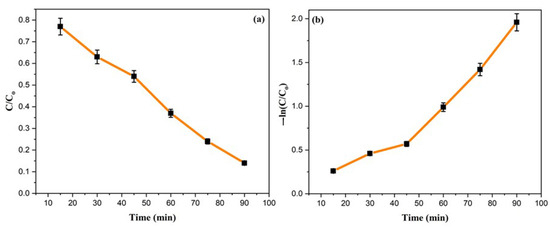
Figure 9.
Kinetic graphs for MO degradation (a) C/Co vs. time and (b) −ln (C/Co) vs. time.
2.8.2. Mechanism
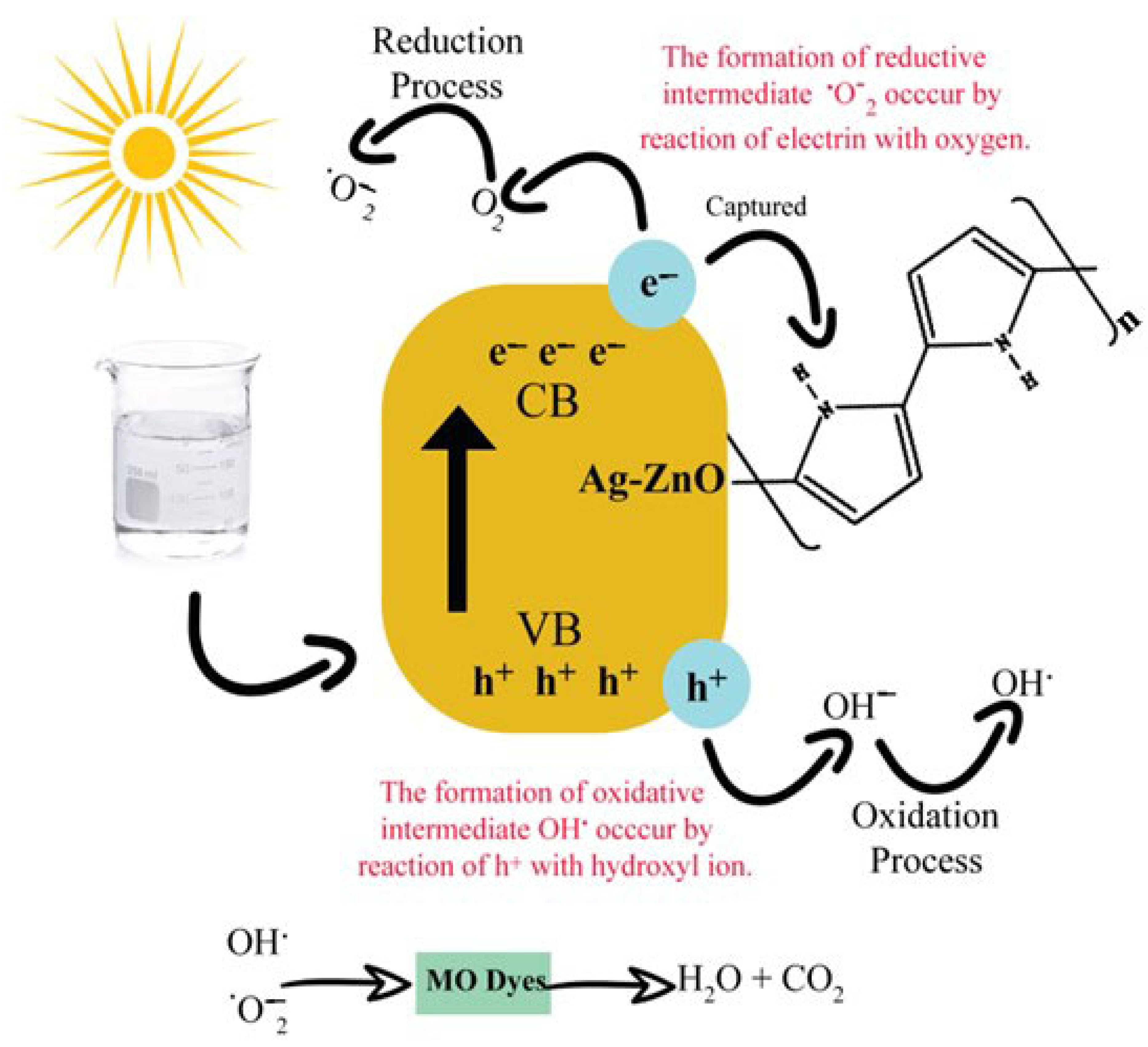
Photocatalysis of prepared polymer-based Ag-ZnO NPs was performed using MO dye from a visible tungsten filament source. The photocatalytic oxidation method was employed to destabilize MO. The reaction was initiated under light irradiation and generated prominent radicals for degradation. The primary mechanism involved the transition of electrons from the valence band (VB) to the conduction band (CB) when the dye solution is stirred under light irradiation. Therefore, the enhanced electron moves from VB of ZnO to CB of polypyrrole. A hole (h+VB) is produced after electrons jump from VB to CB. Light-generated electrons and holes are oxidizing agents that create radicals and degrade MO dye. Electron reacts with molecular oxygen in solution and converts it into superoxide radical anion. In contrast, a positive hole converts the hydroxyl ion to a hydroxyl radical. The following reactions take place during photocatalysis [2].
Ppy/Ag-ZnO nano-catalysts were found to have higher charge separation capacity of electron-hole pairs, which supported the increased efficiency of their photocatalytic activity. Additionally, their inhibition of recombination rates increased the electron–hole pairs involved in the photodegradation system. When electrons transfer from VB to CB, their lifetime in the space–charge zone allows the resulting electron–hole pair to participate in chemical processes.
Additionally, by adding suitable quenchers, such as 1,4-benzoquinone (BQ), a superoxide radical (O2−) scavenger, and isopropanol, an OH* radical scavenger, degradation efficiency decreases, which confirms the role of superoxide and hydroxyl radicals in degradation. Figure 10 gives a diagrammatic representation of the mechanism for the degradation of MO by Ppy/Ag-ZnO.
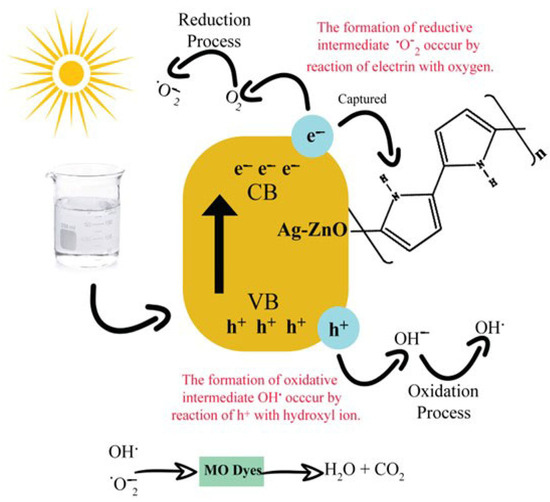
Figure 10.
The mechanism for the degradation of MO [2].
2.8.3. Factors Affecting the Degradation of MO
pH
A solution’s pH significantly impacts the catalytic removal of MO dye because it regulates the adsorption of dye molecules to the photocatalyst’s surface. The pH of the solution also affects the charge on the photocatalyst surface, particle accumulation, and the positions of the conduction and valence band formation, which can change the catalytic capacity and involve the removal rate [52].
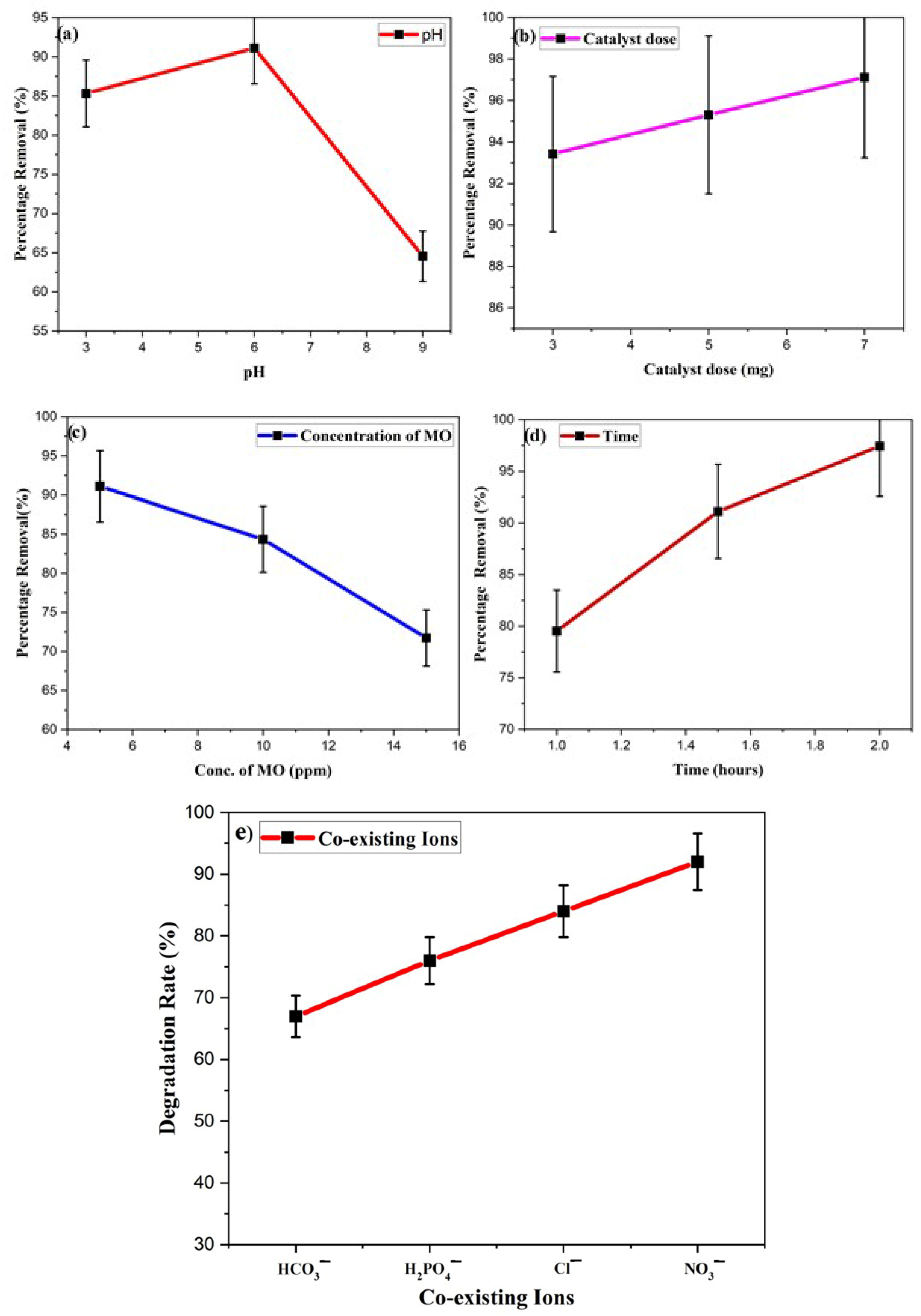
Therefore, the degradation studies were carried out at three different pH levels (3,6,9) by taking the same amount of the catalyst. The acidic and basic pH levels were checked by adding various concentrated HCl and NaOH concentrations. The results demonstrate that the removal capacity was in mildly acidic pH and steadily declined in the basic medium, as depicted in Figure 11a. The results revealed that at pH 6, MO dyestuff was eliminated in 90 min with an efficiency of 91.11%. As pH increased to pH 9, the efficiency decreased to 64.54%. The strong electrostatic interactions between the positively charged surface of the photocatalyst and the negatively charged MO dye raise the removal rate at pH 6. Decreased degradation efficiency was observed as a repulsive attraction worked between the Ppy/Ag-ZnO catalyst and the MO dyestuff, which reduced dye adsorption at the catalyst surface. The decrease in OH• content at higher pH also reduced the degradation effectiveness [53].
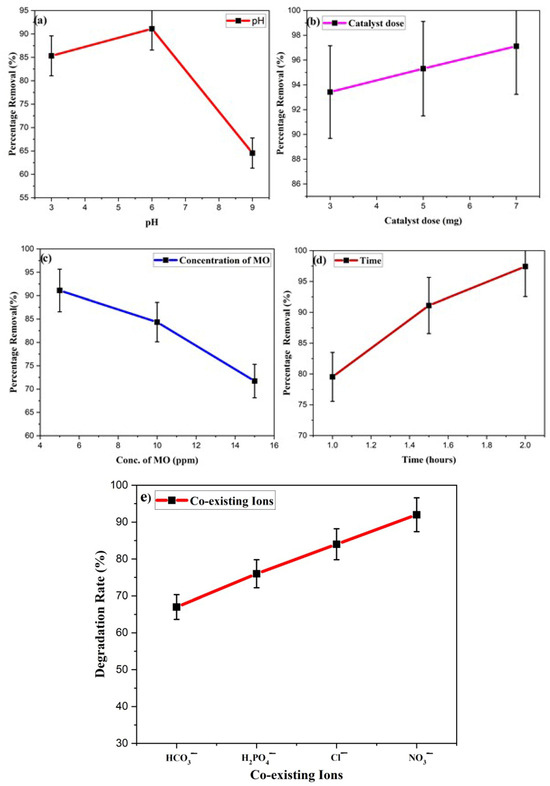
Figure 11.
Effect of various factors on the degradation of MO dye (a) pH, (b) catalyst dose, (c) concentration of MO, (d) Time, and (e) co-existing ions.
Effect of Catalyst Loading
Catalyst Ppy/Ag-ZnO loadings ranging from 3 mg to 7 mg were used to degrade the MO dye by keeping the other conditions (MO conc. 5 ppm, Time 90 min) the same, and the results are shown in Figure 11b. The degradation rate dramatically increased and reached 97.12% as the catalyst concentration was raised for the same concentration of MO dye for 90 min under sunlight irradiation. The development of additional active sites on the photocatalyst surface as the number of catalysts rises was the cause of this improvement in degrading efficiency. According to reports, dye photodegradation occurs in heterogeneous reactions in proportion to the loaded catalyst. It increases as the loaded catalyst concentration rises. However, this increase in degradation rate is only possible within a particular range since, in some circumstances, adding too much catalyst can make the solution appear cloudier and prevent light from penetrating it [54,55].
Effect of MO Dye Amount
MO dye solutions containing concentrations ranging from 5 to 15 ppm were utilized to determine the effect of the dye amount on the degree of degradation. Using 5 ppm of MO resulted in an observation of a 91.11% removal rate. The degradation rate was shown to decrease as the MO concentration was raised. The generation of hole and OH radicals and their interaction with the organic effluents are responsible for the decontamination of contaminants. The lower creation of radicles on the catalyst’s surface is the leading cause of the lower removal rate at a high amount of dye. Pollutant decomposition occurs most rapidly in areas exposed to high levels of light. The production of radicals and degradation rate were reduced when visible light was absorbed by dyestuff instead of a catalyst, which is another potential explanation for the slower degradation rate [56].
Effect of Time
The impact of time on the removal rate of MO was assessed by taking 2 mg of catalyst under bright sunlight for 1, 1.5, and 2 h. The degradation rate was calculated for different time intervals, and the findings regarding the UV–VIS absorption spectra are depicted in Figure 11d. The removal efficiency was relatively high, and degradation reached up to 97.43% after 2 h of reaction under sunlight [57].
Effect of Co-Existing Ions
Numerous dyeing procedures often use large amounts of salts, and the concentration of dissolved inorganic ions in wastewater impacts the effective degradation of dyes. The effects of multiple inorganic ions, including NO3−, Cl−, HCO3−, and H2PO4− frequently present in wastewater, were investigated in a required concentration range. The experimental findings are shown in Figure 11e. The reaction rate constant of MO degradation decreased as the ionic content in the solutions increased. A reduction in radicals might be caused by the high ionic strength. According to one study, bicarbonate anions suppressed hydroxyl radicals more effectively than phosphate despite both substances being effective scavengers of hydroxyl radicals. Additionally, it was discovered that the anions may slow the rate of MO degradation. Their inhibitory effects could be rated from weak to strong in the following order: NO3− < Cl− < H2PO4− < HCO3−. The comparison of degradation of MO dyes by Ppy/Ag-ZnO and other composites is given in Table 2.

Table 2.
Comparative study for degradation of MO dye.
Thermodynamic Parameters for MO Degradation
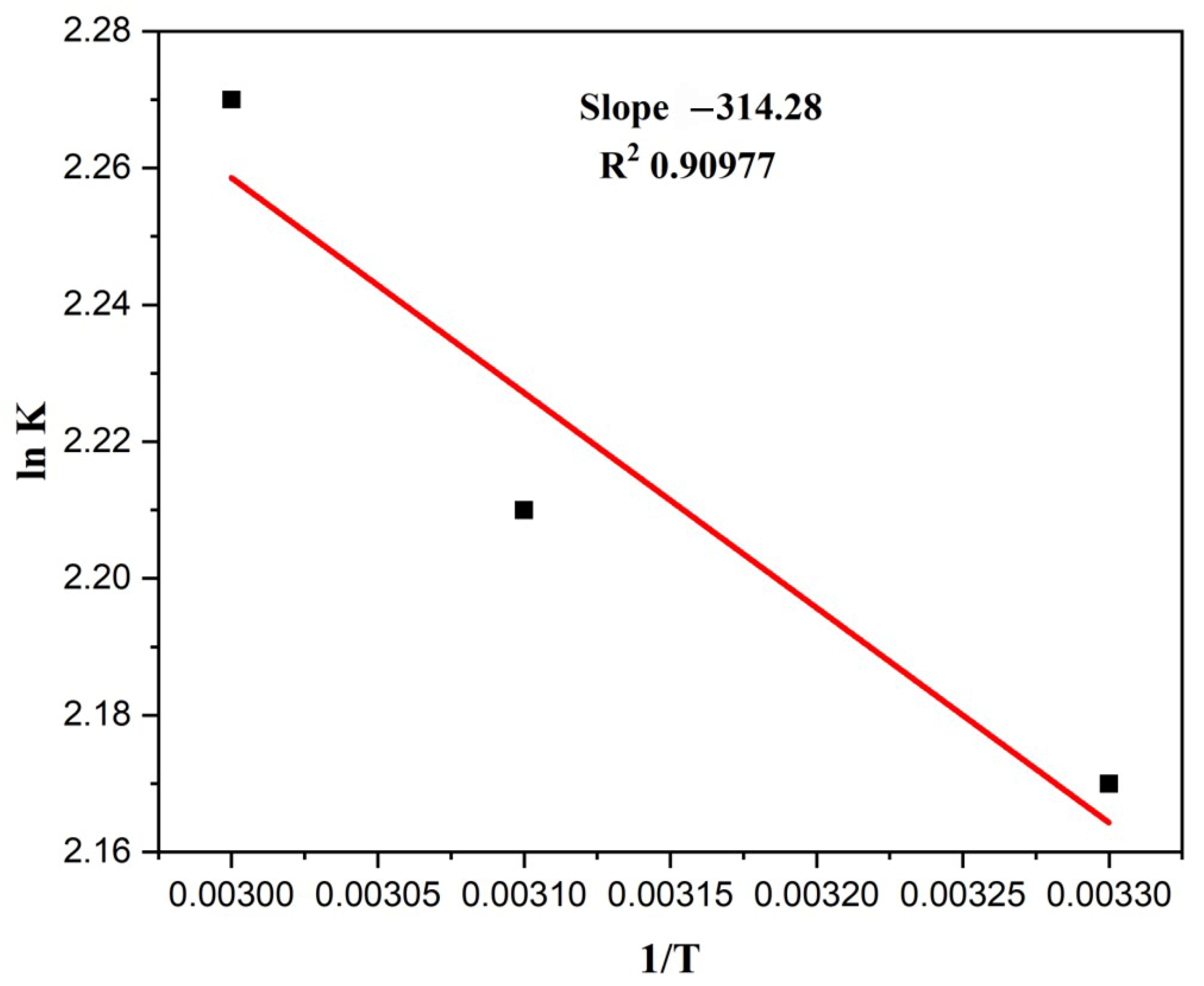
The ∆G°, ∆H°, and ∆S° were determined to comprehend the deterioration’s thermodynamic feasibility and favourability. These variables essentially show how temperature affects degradation. Table 3 contains the computed values for these parameters under various experimental settings. The reaction was discovered to be spontaneous in all the conditions, as the values of ∆G° were negative. A plot of lnk vs. 1/T is given in Figure 12.

Table 3.
Thermodynamic parameters for MO degradation.
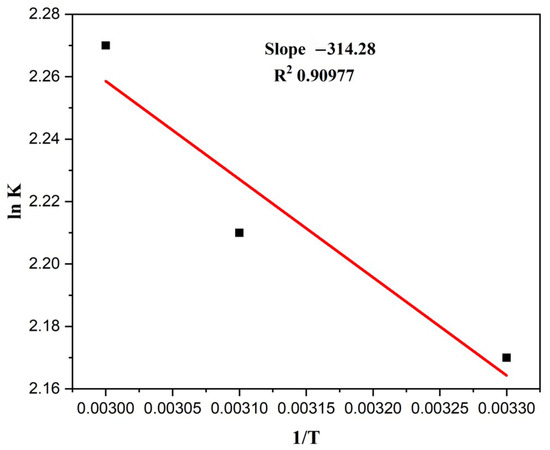
Figure 12.
A plot of lnk vs. 1/T (Alt text: Calculation of slope value (−314.28) and R2 value by plotting a graph between lnK and 1/T).
Thermodynamic studies were carried out at three different temperatures: 303 K, 313 K, and 323 K, with a MO quantity of 5 ppm. The following equations can be used to compute the thermodynamic parameters ∆G°, ∆H°, and ∆S°.
where ∆G° = change in Gibbs free energy (kJ/mol), R = general gas constant having a value of 8.314 (J/molK), T = absolute temperature (K), KL is the equilibrium constant, ∆S° = a change in entropy (kJ/molK), ∆H° = a change in enthalpy (kJ/mol).
The graph was drawn with 1/T on the x-axis and ln kL on the y-axis. Slope and intercept can be used to derive the values of ∆H° and ∆S°. A low value of ∆G° indicates that the process is thermally feasible. In contrast, high values of ∆H° and ∆S° suggest that the reaction is endothermic and increases randomness [57].
Recyclability of Photocatalyst
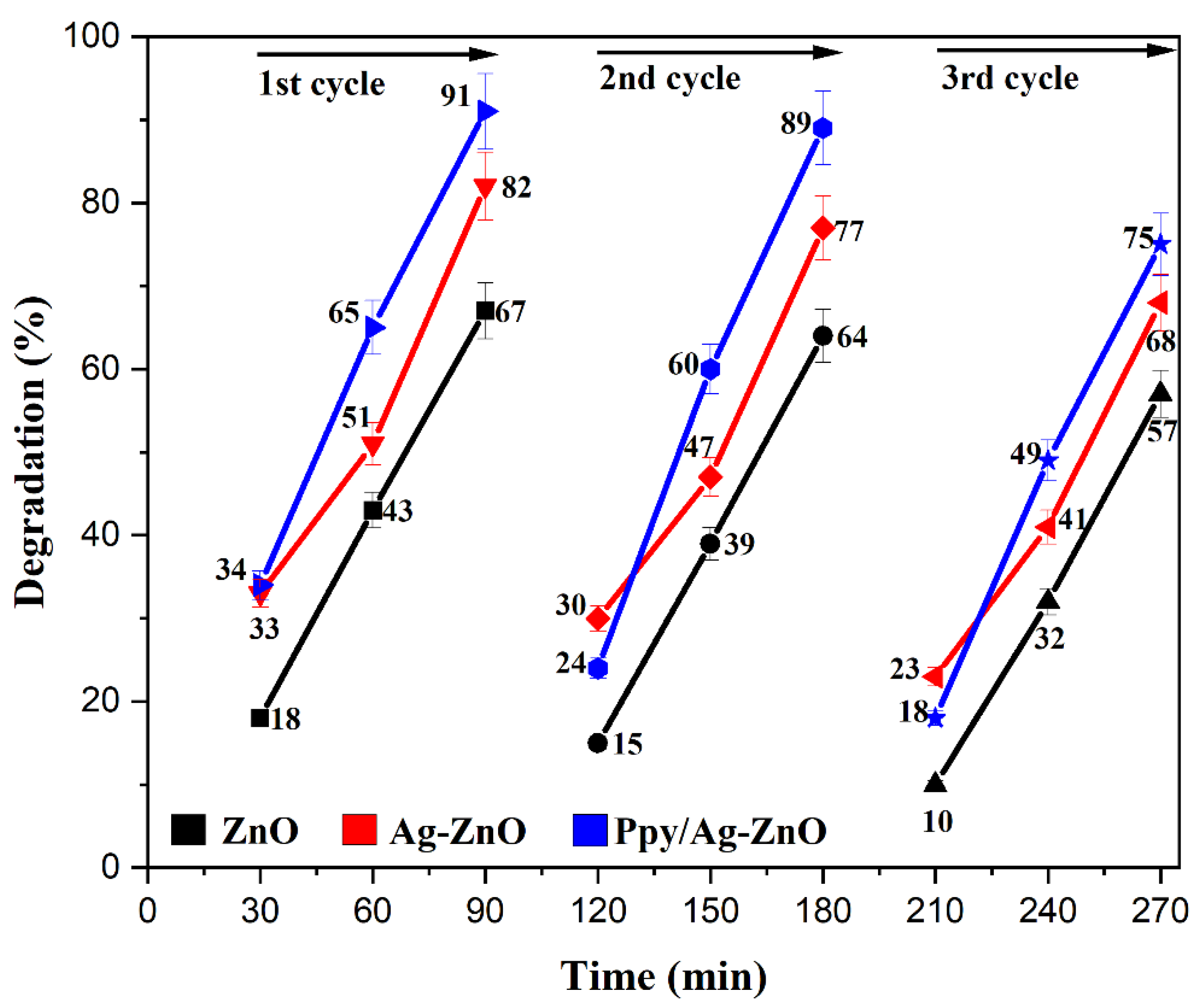
Three continuous degradation cycles of 90 min of the prepared photocatalyst were performed. After the first cycle, the photocatalyst was washed with deionized water and ethanol, dried, and reused for the next cycle. Consequences showed that MO was degraded from 91% to 75%, 82% to 68%, and 67% to 57% after three runs, indicating the acceptable recyclability and stability of Ppy/Ag-ZnO, Ag-ZnO, and ZnO catalysts, respectively, as shown in Figure 13. The FTIR spectrum (Figure S3) of recovered Ppy/Ag-ZnO after MO degradation confirms the catalyst’s structural stability, as no prominent change in peaks was observed. The main reason for the decrease in percentage degradation was the adsorption of intermediate materials on the catalyst’s surface. It decreased the transition of electrons from HOMO to LUMO [70].
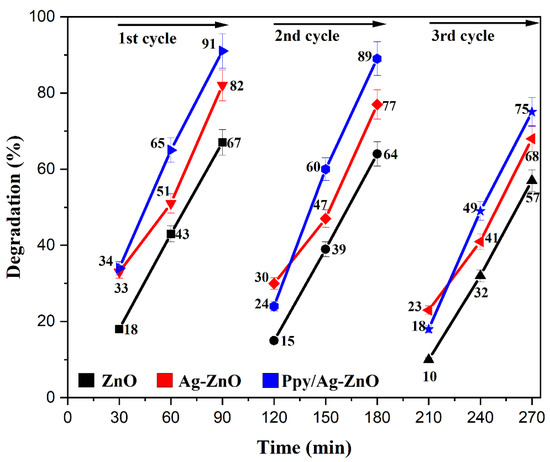
Figure 13.
Recyclability of ZnO, Ag-ZnO, and Ppy/Ag-ZnO photocatalysts.
3. Experiment
3.1. Material
The following materials were used to synthesize the Ppy/Ag-ZnO catalyst: zinc nitrate hexahydrate, 99.99% Sigma Aldrich (Saint Louis, MO, USA); silver nitrate, 99.99% Sigma Aldrich; sodium hydroxide, 99.99% Sigma Aldrich; lithium hydroxide, 99.99% Sigma Aldrich; polypyrrole, 99.99% Sigma Aldrich; and Methyl Orange (MO), 99.99% Sigma Aldrich. Deionized water was used to make the solutions. All compounds were pure and used as received.
3.2. Synthesis of Ppy/Ag-ZnO Catalyst
First step: ZnO NPs were synthesized using a mixture of Zn(NO3)2 and LiOH/NaOH (0.54:0.46 M ratio) in the correct proportions [71]. The mixture was then milled in a planetary ball mill [72] (details are given in the Supporting Information). The amount of reactants was balanced so that there was no excess of zinc nitrate or hydroxides [Zn(NO3)2:LiOH/NaOH molar ratio = 1:2] according to the following Equation.
The sample was dried at 80 °C for 2 h. After that, byproducts (LiNO3/NaNO3) were eliminated by washing them multiple times with water. The ZnO NPs were collected using centrifugation.
Second step: For the Ag-containing samples, 4.50 mol% of AgNO3 was added to the starting mixture in the above Equation, the process was repeated, and the product was labelled as Ag-ZnO NPs.
Third step: For Ppy/Ag-ZnO, the 0.1 M Ppy is mixed with Ag-ZnO (3 wt%) in a planetary ball mill to obtain a Ppy/Ag-ZnO catalyst. The Supporting Information mentions the planetary ball milling process’s specifics.
3.3. Photocatalytic Activity
The mechanochemically synthesized photocatalysts, ZnO, Ag-ZnO, and Ppy/Ag-ZnO, were used to degrade the organic dye, MO. The destabilization of the dyestuff was performed under sunlight irradiation. The experiment was initiated by taking a 5 ppm solution of MO with 2 mg of the prepared catalyst under constant stirring. After establishing adsorption–desorption equilibrium, a few millilitres of the mixture were examined under a UV–visible spectrometer. Absorbance was noted as Ao, and the mixture was stirred under sunlight for 90 min. After every 15 min, a few ml of solution was removed to observe the absorbance [14].
The percentage removal was calculated by using a formula.
Ao is the initial absorbance and At is the final absorbance.
3.4. Characteristic Techniques
Both quantitative and qualitative analysis techniques can investigate prepared polymer-based nanocomposite. The sample was analyzed morphologically to monitor diffraction pattern and crystallite size using Bruker D8 Advance X-Ray Diffractometer (Bruker AXS, Bremen, Germany) with Cu-Kα radiation source with λ = 1.541 Å. The XRD scan range covered 2θ values from 10 to 80°, with a scan rate of 2° per minute and a step size of 0.05°. FTIR measurements were conducted using the conventional KBr pellet method with a Perkin Elmer FTIR spectrometer (PerkinElmer, Norwalk, CT, USA) with 4000–400 cm−1 spectra. The optical study of samples was examined through a Cecil 7500 UV–visible spectrometer (Cecil Instruments Limited, Cambridge, UK) ranging from 200 nm to 800 nm. SEM images for the nanocomposite were characterized via SEM MIRA-III TESCAN (TESCAN, Kohoutovice, Brno, Czech Republic) to discover structural features. Shimadzu’s BET surface area analyzer Gemini 2375 (Micromeritics GEMINI 2375 Surface Area Pore Size Analyzer, 100-240VAC, Norcross, GA, USA) was used to measure the size of the composite both before and after the trial; the pore design, and the specific surface area of the composites. Using the Zetasizer (Nano-ZS, Malvern Instruments, Malvern, UK), produced nanocomposites were tested for stability and surface charge.
4. Conclusions
An attractive mechanochemical technique effectively produced a Ppy/Ag-ZnO catalyst of nanometric diameters at room temperature. In this study, the mechanically activated metathesis successfully synthesized ZnO and Ag-ZnO NPs, which are mechanochemically fabricated with Ppy and used to degrade MO dyestuff up to 91.11% under visible light for 90 min. Factors like pH, catalyst concentration, dye amount, and time were also studied. The designed catalyst’s surface area and average pore size were found to be 47.08 cm3/g and 21.72 Å, respectively. Due to negatively charged scattered particles, the Ppy/Ag-ZnO catalyst displayed a negative zeta potential of −6.78 mV. A crystallite size of 2.30 nm was identified via PXRD analysis. It was found that the decontamination of effluents is significantly influenced by hydroxyl and superoxide radicals. Pseudo-first-order kinetics was followed with a removal rate of 91.11%, having a rate constant of 0.028 min−1. These results show that the mechanochemically synthesized Ppy/Ag-ZnO catalyst is a promising candidate for industrial/bulk preparation (synthetic method-wise) and photocatalytic applications at ambient temperature.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15030284/s1, Figure S1: Degradation of ZnO and Ag-ZnO NPs; Figure S2: Kinetic graphs for MO degradation for ZnO and Ag-ZnO NPs (a) At/Ao vs time and (b) −ln (At/Ao) vs time; Figure S3. FTIR of Ppy/Ag-ZnO after degradation of MO dyes.
Author Contributions
Methodology, M.K.N. and M.B.T.; Software, A.A.A.-G.; Formal analysis, A.A., F.M.H.A., H.M.B. and O.M.A.; Investigation, O.M.A. and M.M.A.; Resources, M.M.A.; Data curation, M.M.A.; Writing—original draft, M.K.N.; Writing—review & editing, M.B.T., M.M.A. and J.F.-G.; Visualization, M.I.K., A.S. and J.F.-G.; Supervision, M.B.T., M.M.A. and J.F.-G.; Project administration, M.B.T., M.M.A. and J.F.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Taif University, Saudi Arabia, project number (TU-DSPP-2024-276) and project HEC-A20.1267 from University College London & IQS-School of Engineering.
Data Availability Statement
All data are present within the manuscript body.
Acknowledgments
The authors appreciate Taif University Saudi Arabia, Higher Education Commission. Pakistan, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Su-perior (project DOIs: 10.54499/LA/P/0008/2020, 10.54499/UIDP/50006/2020, 10.54499/UIDB/50006/2020 and Scientific Employment Stimulus—Institutional Call DOI 10.54499/CEECINST/00102/2018/CP1567/CT0026) and IQS-School of Engineering. Plus, GESPA group are recognized as Consolidated Research Group by the Catalan Government (2021 SGR 00321). They also acknowledge funding from the project HEC-A20.1267.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yousaf, S.; Kousar, T.; Taj, M.B.; Agboola, P.O.; Shakir, I.; Warsi, M.F. Synthesis and characterization of double heterojunction-graphene nano-hybrids for photocatalytic applications. Ceram. Int. 2019, 45, 17806–17817. [Google Scholar] [CrossRef]
- Aroob, S.; Taj, M.B.; Shabbir, S.; Imran, M.; Ahmad, R.H.; Habib, S.; Raheel, A.; Akhtar, M.N.; Ashfaq, M.; Sillanpää, M. In situ biogenic synthesis of CuO nanoparticles over graphene oxide: A potential nanohybrid for water treatment. J. Environ. Chem. Eng. 2021, 9, 105590. [Google Scholar] [CrossRef]
- Reddy, K.R.; Hassan, M.; Gomes, V.G. Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis. Appl. Catal. A Gen. 2015, 489, 1–16. [Google Scholar] [CrossRef]
- Karthik, K.; Raghu, A.; Reddy, K.R.; Ravishankar, R.; Sangeeta, M.; Shetti, N.P.; Reddy, C.V. Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Chemosphere 2022, 287, 132081. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Khan, A.; Kim, K.-H.; Kukkar, D.; Zhang, M. The adsorptive removal of lead ions in aquatic media: Performance comparison between advanced functional materials and conventional materials. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2441–2483. [Google Scholar] [CrossRef]
- Sadiq, H.; Sher, F.; Sehar, S.; Lima, E.C.; Zhang, S.; Iqbal, H.M.; Zafar, F.; Nuhanović, M. Green synthesis of ZnO nanoparticles from Syzygium Cumini leaves extract with robust photocatalysis applications. J. Mol. Liq. 2021, 335, 116567. [Google Scholar] [CrossRef]
- Kannan, K.; Radhika, D.; Sadasivuni, K.K.; Reddy, K.R.; Raghu, A.V. Nanostructured metal oxides and its hybrids for photocatalytic and biomedical applications. Adv. Colloid Interface Sci. 2020, 281, 102178. [Google Scholar] [CrossRef]
- Ara, B.; Gul, K. Label-free immunosensor for detection of hepatitis C (HCV) core antigen using ternary polypyrrole-Ag doped ZnO-exfoliated graphene nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2024, 681, 132709. [Google Scholar]
- Kim, J.; Lee, J.; You, J.; Park, M.-S.; Al Hossain, M.S.; Yamauchi, Y.; Kim, J.H. Conductive polymers for next-generation energy storage systems: Recent progress and new functions. Mater. Horiz. 2016, 3, 517–535. [Google Scholar] [CrossRef]
- Saleh, H.M.; Hassan, A.I. Synthesis and characterization of nanomaterials for application in cost-effective electrochemical devices. Sustainability 2023, 15, 10891. [Google Scholar] [CrossRef]
- Lo, J.-W.; Lien, W.-C.; Lin, C.-A.; He, J.-H. Er-doped ZnO nanorod arrays with enhanced 1540 nm emission by employing Ag island films and high-temperature annealing. ACS Appl. Mater. Interfaces 2011, 3, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.C.; Malik, S.; Gururani, P.J. Utilization of polypyrrole/ZnO nanocomposite in the adsorptive removal of Cu 2+, Pb 2+ and Cd 2+ ions from wastewater. Lett. Appl. NanoBioScience 2021, 10, 2339–2351. [Google Scholar]
- Ponnamma, D.; Cabibihan, J.-J.; Rajan, M.; Pethaiah, S.S.; Deshmukh, K.; Gogoi, J.P.; Pasha, S.K.; Ahamed, M.B.; Krishnegowda, J.; Chandrashekar, B.N.; et al. Synthesis, optimization and applications of ZnO/polymer nanocomposites. Mater. Sci. Eng. C 2019, 98, 1210–1240. [Google Scholar] [CrossRef]
- Taj, M.B.; Alkahtani, M.D.F.; Raheel, A.; Shabbir, S.; Fatima, R.; Aroob, S.; Yahya, R.; Alelwani, W.; Alahmadi, N.; Abualnaja, M.; et al. Bioconjugate synthesis, phytochemical analysis, and optical activity of NiFe2O4 nanoparticles for the removal of ciprofloxacin and Congo red from water. Sci. Rep. 2021, 11, 5439. [Google Scholar] [CrossRef] [PubMed]
- Rakibuddin, M.; Ananthakrishnan, R. Novel nano coordination polymer based synthesis of porous ZnO hexagonal nanodisk for higher gas sorption and photocatalytic activities. Appl. Surf. Sci. 2016, 362, 265–273. [Google Scholar] [CrossRef]
- Sahyar, B.Y.; Kaplan, M.; Ozsoz, M.; Celik, E.; Otles, S. Electrochemical xanthine detection by enzymatic method based on Ag doped ZnO nanoparticles by using polypyrrole. Bioelectrochemistry 2019, 130, 107327. [Google Scholar] [CrossRef]
- Podasca, V.E.; Buruiana, T.; Buruiana, E.C. Photocatalytic degradation of Rhodamine B dye by polymeric films containing ZnO, Ag nanoparticles and polypyrrole. J. Photochem. Photobiol. A Chem. 2019, 371, 188–195. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Xu, M.; Yuan, H.; Chen, Y.; Zhang, J.; Luo, K.; Zhang, J.; You, B. Novel and efficient synthesis of Ag-ZnO nanoparticles for the sunlight-induced photocatalytic degradation. Appl. Surf. Sci. 2019, 476, 632–640. [Google Scholar] [CrossRef]
- Mtavangu, S.G.; Machunda, R.L.; van der Bruggen, B.; Njau, K.N. In situ facile green synthesis of Ag-ZnO nanocomposites using Tetradenia riperia leaf extract and its antimicrobial efficacy on water disinfection. Sci. Rep. 2022, 12, 15359. [Google Scholar] [CrossRef]
- Ramesan, M.T.; Santhi, V. In situ synthesis, characterization, conductivity studies of polypyrrole/silver doped zinc oxide nanocomposites and their application for ammonia gas sensing. J. Mater. Sci. Mater. Electron. 2017, 28, 18804–18814. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Seifi, A. The green synthesis of Ag/ZnO in montmorillonite with enhanced photocatalytic activity. Appl. Surf. Sci. 2016, 386, 33–40. [Google Scholar] [CrossRef]
- Singh, S.; Anil, A.G.; Khasnabis, S.; Kumar, V.; Nath, B.; Adiga, V.; Naik, T.S.K.; Subramanian, S.; Kumar, V.; Singh, J.; et al. Sustainable removal of Cr (VI) using graphene oxide-zinc oxide nanohybrid: Adsorption kinetics, isotherms and thermodynamics. Environ. Res. 2022, 203, 111891. [Google Scholar] [CrossRef] [PubMed]
- Koutavarapu, R.; Babu, B.; Reddy, C.V.; Reddy, I.N.; Reddy, K.R.; Rao, M.; Aminabhavi, T.M.; Cho, M.; Kim, D.; Shim, J. ZnO nanosheets-decorated Bi2WO6 nanolayers as efficient photocatalysts for the removal of toxic environmental pollutants and photoelectrochemical solar water oxidation. J. Environ. Manag. 2020, 265, 110504. [Google Scholar] [CrossRef]
- Ansari, A.A.; Lv, R.; Gai, S.; Parchur, A.K.; Solanki, P.R.; Ansari, Z.; Dhayal, M.; Yang, P.; Nazeeruddin, M.; Tavakoli, M.M. ZnO nanostructures–Future frontiers in photocatalysis, solar cells, sensing, supercapacitor, fingerprint technologies, toxicity, and clinical diagnostics. Coord. Chem. Rev. 2024, 515, 215942. [Google Scholar] [CrossRef]
- Ali, S.; Sidra; Asghar, T.; Jan, M.I.; Waqas, M.; Ali, T.; Ullah, R.; Bari, A. Green Synthesis of Novel Rhododendron arboreum-Based Zinc Oxide Nanoparticles for Enhanced Antimicrobial and Photocatalytic Degradation Activities. Catalysts 2024, 14, 337. [Google Scholar] [CrossRef]
- Vasiljevic, Z.; Vunduk, J.; Bartolic, D.; Miskovic, G.; Ognjanovic, M.; Tadic, N.B.; Nikolic, M.V. An Eco-friendly Approach to ZnO NP Synthesis Using Citrus reticulata Blanco Peel/Extract: Characterization and Antibacterial and Photocatalytic Activity. ACS Appl. Bio Mater. 2024, 7, 3014–3032. [Google Scholar] [CrossRef]
- Karthik, K.V.; Reddy, C.V.; Reddy, K.R.; Ravishankar, R.; Sanjeev, G.; Kulkarni, R.V.; Shetti, N.P.; Raghu, A.V. Barium titanate nanostructures for photocatalytic hydrogen generation and photodegradation of chemical pollutants. J. Mater. Sci. Mater. Electron. 2019, 30, 20646–20653. [Google Scholar] [CrossRef]
- Jayakumar, G.; Irudayaraj, A.A.; Raj, A.D. Photocatalytic degradation of methylene blue by nickel oxide nanoparticles. Mater. Today Proc. 2017, 4, 11690–11695. [Google Scholar] [CrossRef]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Nawaz, A.; Farhan, A.; Maqbool, F.; Ahmad, H.; Qayyum, W.; Ghazy, E.; Rahdar, A.; Díez-Pascual, A.M.; Fathi-karkan, S. Zinc Oxide Nanoparticles: Pathways to Micropollutant Adsorption, Dye Removal, and Antibacterial Actions-A Study of Mechanisms, Challenges, and Future Prospects. J. Mol. Struct. 2024, 1312, 138545. [Google Scholar] [CrossRef]
- Khan, F.; Zahid, M.; Bhatti, H.; Jamil, Y. Degradation of persistent organic pollutant using Ag-doped ZnO-ZnS–polyaniline composite as photocatalyst. Int. J. Environ. Sci. Technol. 2023, 20, 4811–4826. [Google Scholar] [CrossRef]
- Wagh, S.S.; Kadam, V.S.; Jagtap, C.V.; Salunkhe, D.B.; Patil, R.S.; Pathan, H.M.; Patole, S.P. Comparative Studies on Synthesis, Characterization and Photocatalytic Activity of Ag Doped ZnO Nanoparticles. ACS Omega 2023, 8, 7779–7790. [Google Scholar] [CrossRef] [PubMed]
- Ifijen, I.H.; Maliki, M.; Anegbe, B. Synthesis, photocatalytic degradation and antibacterial properties of selenium or silver doped zinc oxide nanoparticles: A detailed review. OpenNano 2022, 8, 100082. [Google Scholar] [CrossRef]
- Ersöz, E.; Altintas Yildirim, O. Green synthesis and characterization of Ag-doped ZnO nanofibers for photodegradation of MB, RhB and MO dye molecules. J. Korean Ceram. Soc. 2022, 59, 655–670. [Google Scholar] [CrossRef]
- Fouladi-Fard, R.; Aali, R.; Mohammadi-Aghdam, S.; Mortazavi-derazkola, S. The surface modification of spherical ZnO with Ag nanoparticles: A novel agent, biogenic synthesis, catalytic and antibacterial activities. Arab. J. Chem. 2022, 15, 103658. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, P.S.; Sarkar, D.; Pal, S.; Naskar, M.K.; Chaudhary, Y.S.; Dey, S.; Sinha, C. Z-Scheme Heterostructures Using Band-Gap-Tunable ZnO by Metal Doping and Coupling with Polypyrrole for Enhanced Photocatalytic Water Splitting. ACS Appl. Polym. Mater. 2023, 5, 9918–9930. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Z. A one-pot method to prepare a ZnO/Ag/polypyrrole composite for zinc alkaline secondary batteries. RSC Adv. 2015, 5, 33814–33817. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Z.; Feng, Z.; Xie, X.; Wen, X. A novel ZnO@ Ag@ Polypyrrole hybrid composite evaluated as anode material for zinc-based secondary cell. Sci. Rep. 2016, 6, 24471. [Google Scholar] [CrossRef]
- Elkady, M.F.; Hassan, H.S. Photocatalytic degradation of malachite green dye from aqueous solution using environmentally compatible Ag/ZnO polymeric nanofibers. Polymers 2021, 13, 2033. [Google Scholar] [CrossRef]
- Mohamed, F.; Enaiet Allah, A.; Abu Al-Ola, K.A.; Shaban, M. Design and characterization of a novel ZnO–Ag/polypyrrole core–shell nanocomposite for water bioremediation. Nanomaterials 2021, 11, 1688. [Google Scholar] [CrossRef]
- Gu, F.; You, D.; Wang, Z.; Han, D.; Guo, G. Improvement of gas-sensing property by defect engineering in microwave-assisted synthesized 3D ZnO nanostructures. Sens. Actuators B Chem. 2014, 204, 342–350. [Google Scholar] [CrossRef]
- Ao, W.; Li, J.; Yang, H.; Zeng, X.; Ma, X. Mechanochemical synthesis of zinc oxide nanocrystalline. Powder Technol. 2006, 168, 148–151. [Google Scholar] [CrossRef]
- Mandal, T.K.; Gopalakrishnan, J. From rocksalt to perovskite: A metathesis route for the synthesis of perovskite oxides of current interest. J. Mater. Chem. 2004, 14, 1273–1280. [Google Scholar] [CrossRef]
- Parkin, I. Solid state metathesis reaction for metal borides, silicides, pnictides and chalcogenides: Ionic or elemental pathways. Chem. Soc. Rev. 1996, 25, 199–207. [Google Scholar] [CrossRef]
- Rajesh, D.; Lakshmi, B.V.; Sunandana, C. Two-step synthesis and characterization of ZnO nanoparticles. Phys. B Condens. Matter 2012, 407, 4537–4539. [Google Scholar] [CrossRef]
- Hosseini, S.; Sarsari, I.A.; Kameli, P.; Salamati, H. Effect of Ag doping on structural, optical, and photocatalytic properties of ZnO nanoparticles. J. Alloys Compd. 2015, 640, 408–415. [Google Scholar] [CrossRef]
- Joshi, P.; Rao, V.; Rehani, B.; Pratap, A. Silver-zinc oxide electrical contact materials by mechanochemical synthesis route. Indian J. Pure Appl. Phys. 2007, 45, 9–15. [Google Scholar]
- Choi, Y.I.; Jung, H.J.; Shin, W.G.; Sohn, Y. Band gap-engineered ZnO and Ag/ZnO by ball-milling method and their photocatalytic and Fenton-like photocatalytic activities. Appl. Surf. Sci. 2015, 356, 615–625. [Google Scholar] [CrossRef]
- Dhas, S.D.; Maldar, P.S.; Patil, M.D.; Nagare, A.B.; Waikar, M.R.; Sonkawade, R.G.; Moholkar, A.V. Synthesis of NiO nanoparticles for supercapacitor application as an efficient electrode material. Vacuum 2020, 181, 109646. [Google Scholar] [CrossRef]
- Barzinjy, A.A.; Azeez, H.H. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globulus Labill. leaf extract and zinc nitrate hexahydrate salt. SN Appl. Sci. 2020, 2, 991. [Google Scholar] [CrossRef]
- Aroob, S.; Carabineiro, S.A.C.; Taj, M.B.; Bibi, I.; Raheel, A.; Javed, T.; Yahya, R.; Alelwani, W.; Verpoort, F.; Kamwilaisak, K.; et al. Green Synthesis and Photocatalytic Dye Degradation Activity of CuO Nanoparticles. Catalysts 2023, 13, 502. [Google Scholar] [CrossRef]
- Ahmed, A.; Usman, M.; Yu, B.; Ding, X.; Peng, Q.; Shen, Y.; Cong, H. Efficient photocatalytic degradation of toxic Alizarin yellow R dye from industrial wastewater using biosynthesized Fe nanoparticle and study of factors affecting the degradation rate. J. Photochem. Photobiol. B Biol. 2019, 202, 111682. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, M.; Javanbakht, V. Photocatalytic degradation of cationic and anionic dyes by a novel nanophotocatalyst of TiO2/ZnTiO3/αFe2O3 by ultraviolet light irradiation. Mater. Electron. 2018, 29, 9908–9919. [Google Scholar] [CrossRef]
- Ma, C.M.; Hong, G.B.; Lee, S.C. Facile synthesis of tin dioxide nanoparticles for photocatalytic degradation of Congo red dye in aqueous solution. Catalysts 2020, 10, 792. [Google Scholar] [CrossRef]
- Boruah, P.K.; Borthakur, P.; Darabdhara, G.; Kamaja, C.K.; Karbhal, I.; Shelke, M.V.; Phukan, P.; Saikia, D.; Das, M.R. Sunlight assisted degradation of dye molecules and reduction of toxic Cr (VI) in aqueous medium using magnetically recoverable Fe3O4/reduced graphene oxide nanocomposite. RSC Adv. 2016, 6, 11049–11063. [Google Scholar] [CrossRef]
- Sakarkar, S.; Muthukumran, S.; Jegatheesan, V. Factors affecting the degradation of remazol turquoise blue (RTB) dye by titanium dioxide (TiO2) entrapped photocatalytic membrane. J. Environ. Manag. 2020, 272, 111090. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Karim, M.N.; Nitun, N.A.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic performance assessment of GO and Ag co-synthesized TiO2 nanocomposite for the removal of methyl orange dye under solar irradiation. Environ. Technol. Innov. 2021, 22, 101537. [Google Scholar] [CrossRef]
- Sathish, T.; Chandramohan, D.; Dinesh Kumar, S.; Rajkumar, S.; Vijayan, V. A facile synthesis of Ag/ZnO nanocomposites prepared via novel green mediated route for catalytic activity. Appl. Phys. 2021, 127, 692. [Google Scholar] [CrossRef]
- Bhosale, A.; Kadam, J.; Gade, T.; Sonawane, K.; Garadkar, K. Efficient photodegradation of methyl orange and bactericidal activity of Ag doped ZnO nanoparticles. J. Indian Chem. Soc. 2023, 100, 100920. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Liu, X.; Jin, J.; Meng, H. Polymer-assisted freeze-drying synthesis of Ag-doped ZnO nanoparticles with enhanced photocatalytic activity. Ceram. Int. 2019, 45, 494–502. [Google Scholar] [CrossRef]
- Bhatti, M.A.; Shah, A.A.; Almani, K.F.; Tahira, A.; Chalangar, S.E.; dad Chandio, A.; Nur, O.; Willander, M.; Ibupoto, Z.H. Efficient photo catalysts based on silver doped ZnO nanorods for the photo degradation of methyl orange. Ceram. Int. 2019, 45, 23289–23297. [Google Scholar] [CrossRef]
- Ruchi, N.; Bamne, J.; Singh, N.; Sharma, P.; Singh, P.; Umar, A.; Haque, F.Z. Synthesis of titania/silica nanocomposite for enhanced photodegradation of methylene blue and methyl orange dyes under uv and mercury lights. ES Mater. Manuf. 2022, 16, 78–88. [Google Scholar]
- Narzary, S.; Alamelu, K.; Raja, V.; Ali, B.J. Visible light active, magnetically retrievable Fe3O4@ SiO2@ g-C3N4/TiO2 nanocomposite as efficient photocatalyst for removal of dye pollutants. J. Environ. Chem. Eng. 2020, 8, 104373. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Amira, M.F.; Abouelanwar, M.E.; Salam, M.A. Green synthesis and surface decoration of silver nanoparticles onto δ-FeOOH-Polymeric nanocomposite as efficient nanocatalyst for dyes degradation. J. Environ. Chem. Eng. 2021, 9, 104697. [Google Scholar] [CrossRef]
- Kouhbanani, M.A.J.; Beheshtkhoo, N.; Taghizadeh, S.; Amani, A.M.; Alimardani, V. One-step green synthesis and characterization of iron oxide nanoparticles using aqueous leaf extract of Teucrium polium and their catalytic application in dye degradation. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 015007. [Google Scholar] [CrossRef]
- Barzinjy, A.A.; Hamad, S.M.; Aydın, S.; Ahmed, M.H.; Hussain, F.H.S. Green and eco-friendly synthesis of Nickel oxide nanoparticles and its photocatalytic activity for methyl orange degradation. J. Mater. Sci. Mater. Electron. 2020, 31, 11303–11316. [Google Scholar] [CrossRef]
- Raj, S.; Singh, H.; Trivedi, R.; Soni, V. Biogenic synthesis of AgNPs employing Terminalia arjuna leaf extract and its efficacy towards catalytic degradation of organic dyes. Sci. Rep. 2020, 10, 9616. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Sharma, B.; Garg, V.; Neelratan, P.P.; Kumar, V.; Kumar, D.; Sharma, S.K. Enhanced photocatalytic degradation of methylene blue and methyl orange using biogenic ZnO NPs synthesized via Vachellia nilotica (Babool) leaves extract. Hybrid Adv. 2024, 5, 100160. [Google Scholar] [CrossRef]
- Laksaci, H.; Belhamdi, B.; Khelifi, O.; Khelifi, A.; Trari, M. The use of green synthesized ZnO nanoparticles for the degradation of methyl orange under solar light irradiation. Int. J. Environ. Sci. Technol. 2024, 21, 7227–7236. [Google Scholar] [CrossRef]
- Afzal, M.A.; Javed, M.; Aroob, S.; Javed, T.; Alnoman, M.M.; Alelwani, W.; Bibi, I.; Sharif, M.; Saleem, M.; Rizwan, M.; et al. The Biogenic Synthesis of Bimetallic Ag/ZnO Nanoparticles: A Multifunctional Approach for Methyl Violet Photocatalytic Degradation and the Assessment of Antibacterial, Antioxidant, and Cytotoxicity Properties. Nanomaterials 2023, 13, 2079. [Google Scholar] [CrossRef]
- Mendoza-Mendoza, E.; Nuñez-Briones, A.; García-Cerda, L.; Peralta-Rodríguez, R.; Montes-Luna, A. One-step synthesis of ZnO and Ag/ZnO heterostructures and their photocatalytic activity. Ceram. Int. 2018, 44, 6176–6180. [Google Scholar] [CrossRef]
- Otis, G.; Ejgenberg, M.; Mastai, Y. Solvent-free mechanochemical synthesis of ZnO nanoparticles by high-energy ball milling of ε-Zn(OH)2 crystals. Nanomaterials 2021, 11, 238. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).