Synthesis and Antimicrobial Activity of Chalcone-Derived 1,4-Dihydropyridine Derivatives Using Magnetic Fe2O3@SiO2 as Highly Efficient Nanocatalyst
Abstract
1. Introduction
2. Result and Discussion
2.1. Synthesis of Fe2O3@SiO2 Nanocatalyst
2.2. Characterization of Fe2O3@SiO2 Nanoparticles
2.2.1. FT-IR Characterization
2.2.2. DLS and Zeta-Potential Characterization
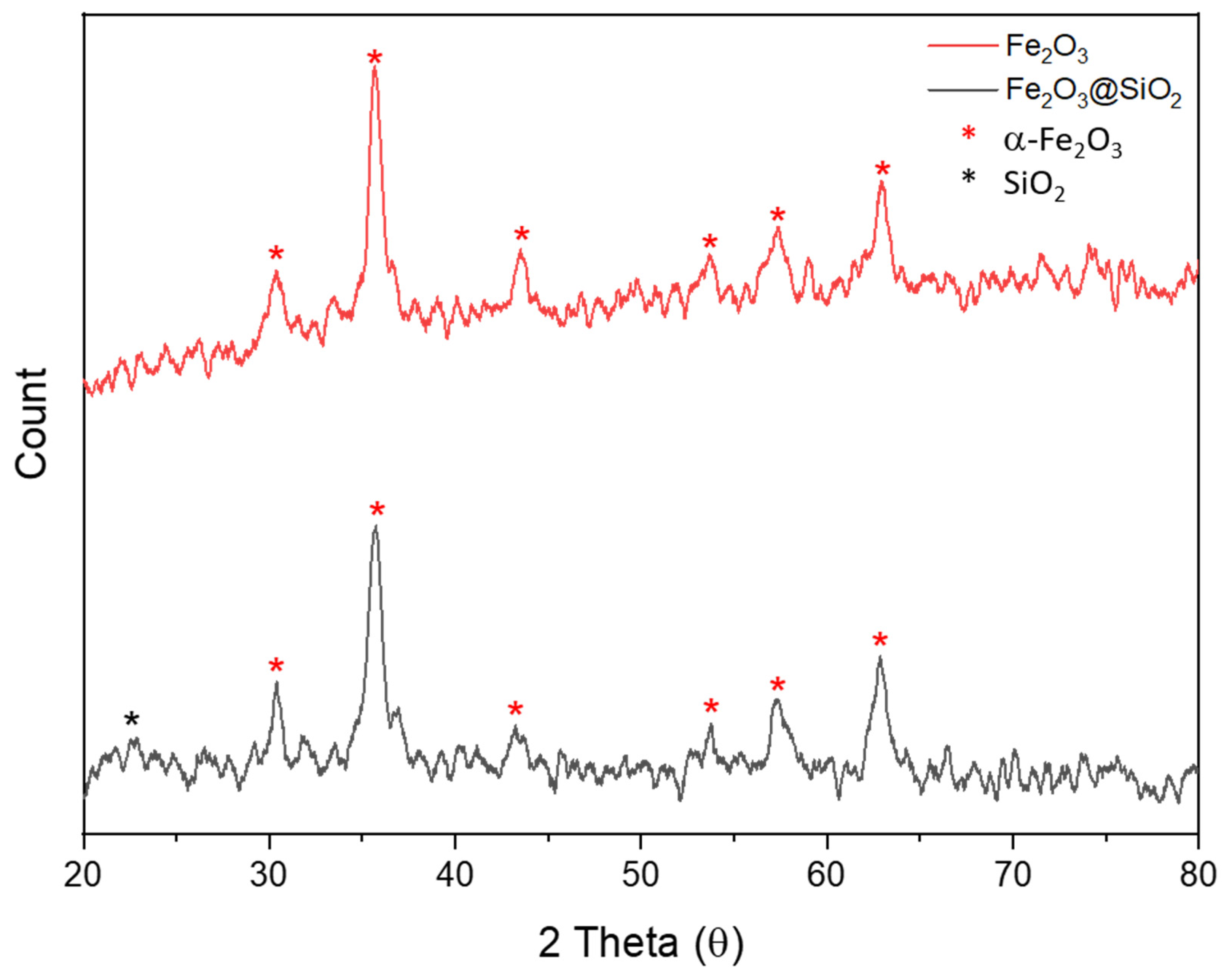
2.2.3. XRD Characterization
2.2.4. SEM-EDS Characterization
2.2.5. VSM Characterization
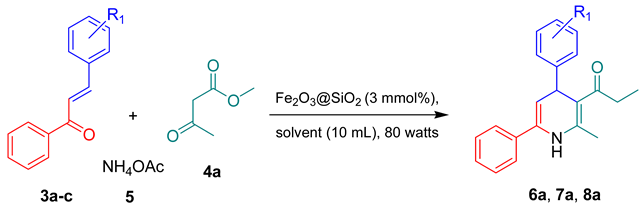
2.3. Optimization of Conditions for Synthesis of Chalcone-Derived 1,4-Dihydropyridine Derivative
2.3.1. Optimization of Amount of Fe2O3@SiO2 Nanocatalyst
2.3.2. Optimization of Solvent
2.3.3. Optimization of Microwave Reactor Power
2.3.4. Reusability of Fe2O3@SiO2 Nanocatalyst
2.4. Characterization of Methyl-2-methyl-4,6-diphenyl-1,4-dihydropyridine-3-carboxylate (6a)
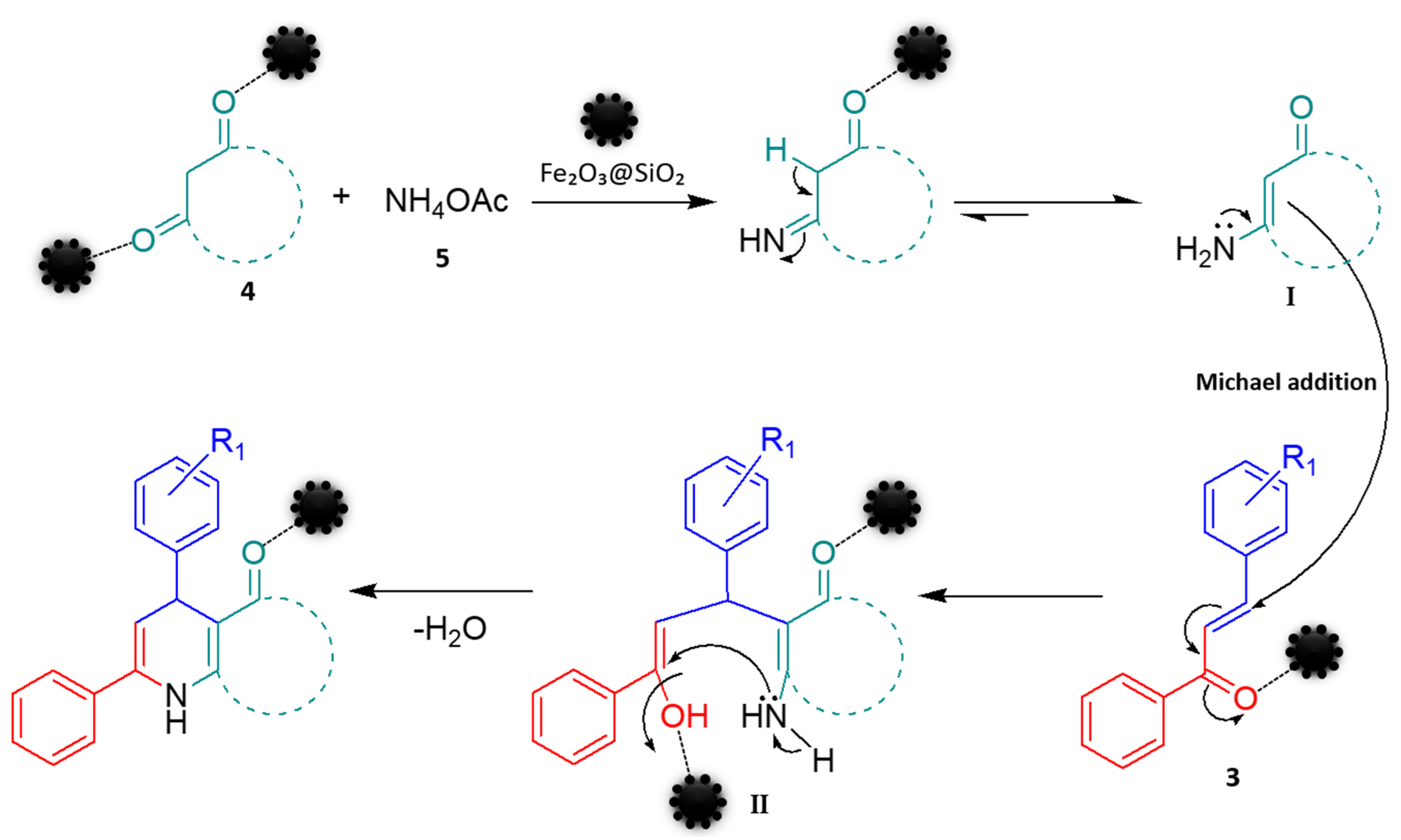
2.5. Plausible Mechanism for Synthesis of Chalcone-Derived 1,4-Dihydropyridine Derivatives
2.6. Antimicrobial Activity
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Synthesis of Fe2O3@SiO2 Nanocatalyst
3.2.1. Synthesis of Magnetic Fe2O3 Nanoparticles
3.2.2. Fabrication of Magnetic Fe2O3 Nanoparticles with SiO2
3.3. General Procedure for Claisen–Schmidt Condensation for Synthesis of Chalcone Derivatives
- (E)-1,3-diphenylprop-2-en-1-one (3a): Yield 94%, 0.196 g, White crystalline solid, mp 56–57 °C. IR spectrum, υ, cm−1: 3077, 3026 (aromatic C-H), 2926, 2868 (methylene C-H), 1662 (C=O), 1595 (C=C), 1206, 1076, (C-O). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 8.03–7.41 (12H, m, Ar-H). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 190.6, 144.8, 138.2, 134.9, 132.8, 130.5, 128.9, 128.6, 128.5, 128.4, 122.1. Mass spectrum, m/z: 209 [M + 1]. Anal. calcd. for C15H12O: C, 86.51; H, 5.81. Found: C, 86.39; H, 5.73.
- (E)-3-(3-nitrophenyl)-1-phenylprop-2-en-1-one (3b): Yield 89%, 0.226 g, Lime yellow crystalline solid, mp 143–145 °C. IR spectrum, υ, cm−1: 3088, 3071 (aromatic C-H), 2871 (methylene C-H), 1659, 1593, 1526, 1349, 1217, 1080. 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 8.51–7.52 (11H, m, Ar-H). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 189.6, 148.7, 141.6, 137.6, 136.6, 134.3, 133.3, 130.0, 128.8, 128.7, 128.6, 124.6, 122.3. Mass spectrum, m/z: 254 [M + 1]. Anal. calcd. for C15H11NO3: C, 71.14; H, 4.38; N, 5.59. Found: C, 71.10; H, 4.27; N, 5.56.
- (E)-3-(4-hydroxyphenyl)-1-phenylprop-2-en-1-one (3c): Yield 84%, 0.214 g, White crystalline solid, mp 185–187 °C. IR spectrum, υ, cm−1 (CDCl3, 500 MHz): 3126 (O-H), 2973 (aromatic C-H), 2842 (methylene C-H), 1644 (C=O), 1595 (C=C), 1206, 1076, (C-O). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 8.02–6.93 (11H, m, Ar-H), 3.88 (3H, s, OCH3). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 189.9, 164.5, 159.6, 144.0, 131.0, 130.1, 127.5, 119.5, 115.3, 114.4, 55.42. Mass spectrum, m/z: 255 [M + 1]. Anal. calcd. for C16H14O3: C, 75.57; H, 5.59. Found: C, 75.49; H, 5.51.
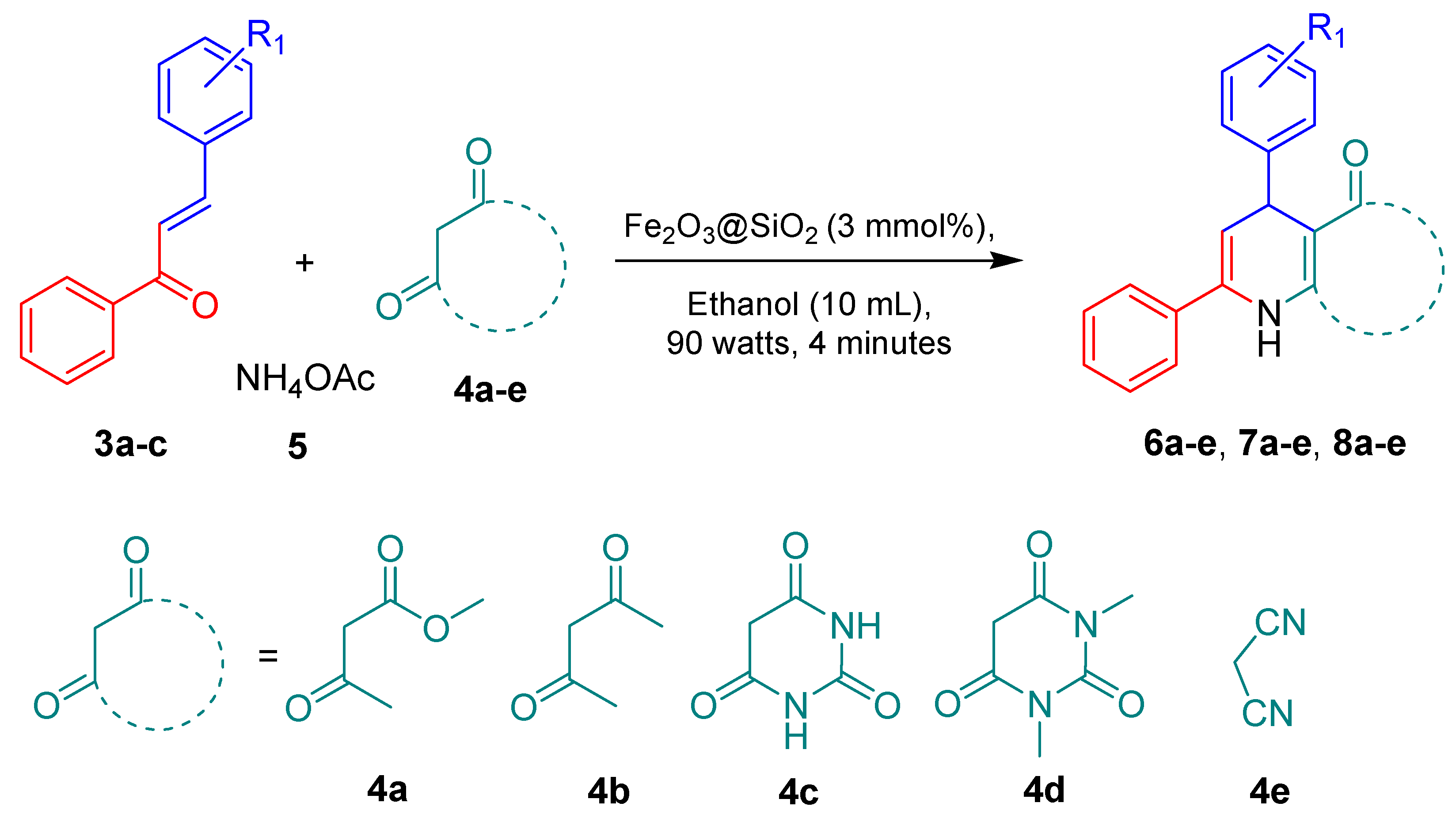
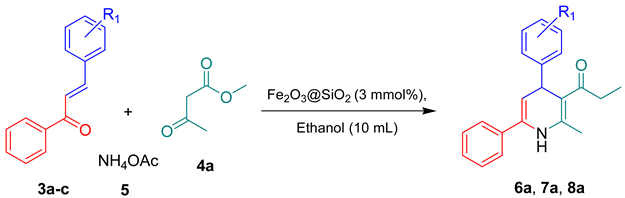
3.4. General Procedure for the Synthesis of 1,4-Dihydropyridine Derivatives from Chalcones Using Fe2O3@SiO2 Nanocatalyst
- Methyl 2-methyl-4,6-diphenyl-1,4-dihydropyridine-3-carboxylate (6a): Yield 94%, 0.287 g, White crystalline solid, mp 156–157 °C. IR spectrum, υ, cm−1: 3441 (N-H), 3106 (aromatic C-H), 3031 (COOH), 2943 (methyl C-H), 1668 (C=O), 1595 (C=C), 1206, 1081, (C-O). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 11.13 (1H, s, NH), 8.01–7.47 (10H, m, Ar-H), 4.72–4.71 (1H, H-5), 4.35–4.32 (1H, H-4), 3.54 (3H, s, COOCH3), 2.26 (3H, s, CH3). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 173.2, 151.6, 142.4, 141.7, 135.1, 129.6, 128.8, 128.1, 127.2, 126.4, 124.9, 106.8, 94.5, 55.1, 47.6, 19.2. Mass spectrum, m/z: 306 [M + 1]. Anal. calcd. for C20H19NO2: C, 78.66; H, 6.27: N, 4.59. Found: C, 78.59; H, 6.22: N, 4.56.
- 1-(2-methyl-4,6-diphenyl-1,4-dihydropyridin-3-yl)ethenone (6b): Yield 92%, 0.281 g, White crystalline solid, mp 164–166 °C. IR spectrum, υ, cm−1: 3452 (N-H), 3096, 3078 (aromatic C-H), 2926, 2868 (methylene C-H), 1661 (C=O), 1615 (C=C). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 11.17 (1H, s, NH), 7.84–7.27 (10H, m, Ar-H), 4.88–4.86 (1H, H-5), 4.63–4.61 (1H, H-4), 2.47 (3H, s, COCH3), 1.90 (3H, s, CH3). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 194.8, 153.1, 143.0, 141.1, 134.8, 129.8, 129.2, 128.7, 128.1, 127.5, 126.0, 112.9, 96.1, 42.3, 29.7, 18.8. Mass spectrum, m/z: 290 [M + 1]. Anal. calcd. for C20H19NO: C, 83.01; H, 6.62: N, 4.84. Found: C, 82.97; H, 6.53: N, 4.81.
- 5,7-diphenylpyrido[2,3-d]pyrimidine-2,4(1H,3H,5H,8H)-dione (6c): Yield 96%, 0.295 g, Pale crystalline solid, mp 271–272 °C. IR spectrum, υ, cm−1: 3449 (N-H), 3086, 3023 (aromatic C-H), 2912 (methylene C-H), 1696 (C=O), 1589 (C=C), 1462 (C-N). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 11.15 (1H, s, NH), 10.0 (1H, s, NH), 7.89 (1H, s, NH), 7.65–7.17 (10H, m, Ar-H), 4.86–4.84 (1H, H-5), 4.70–4.68 (1H, H-4). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 167.3, 155.8, 152.8, 142.8, 141.3, 135.2, 129.6, 129.0, 128.7, 128.1, 127.2, 125.9, 94.9, 82.3, 43.7. Mass spectrum, m/z: 318 [M + 1]. Anal. calcd. for C19H15N3O2: C, 71.91; H, 4.76: N, 13.24. Found: C, 71.88; H, 4.70: N, 13.20.
- 1,3-dimethyl-5,7-diphenylpyrido[2,3-d]pyrimidine-2,4(1H,3H,5H,8H)-dione (6d): Yield 93%, 0.345 g, Pale crystalline solid, mp 263–265 °C. IR spectrum, υ, cm−1: 3465 (N-H), 3087, 3063 (aromatic C-H), 2983, 2920 (methylene C-H), 1682 (C=O), 1597 (C=C), 1454 (C-N). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 11.10 (1H, s, NH), 7.69–7.24 (10H, m, Ar-H), 4.86–4.84 (1H, H-5), 4.57–4.55 (1H, H-4), 2.90 (3H, s, CH3), 2.83 (3H, s, CH3). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 165.9, 153.2, 149.6, 142.5, 140.8, 135.1, 129.7, 129.3, 128.6, 128.0, 126.4, 125.7, 92.6, 80.9, 42.8, 32.4, 29.9. Mass spectrum, m/z: 346 [M + 1]. Anal. calcd. for C21H19N3O2: C, 73.03; H, 5.54; N, 12.1. Found: C, 72.95; H, 5.48; N, 11.9.
- 2-nitro-4,6-diphenyl-1,4-dihydropyridine-3-carbonitrile (6e): Yield 95%, 0.335 g, White crystalline solid, mp 219–222 °C. IR spectrum, υ, cm−1: 3441 (N-H), 3078, 3061 (aromatic C-H), 2869 (methylene C-H), 2250 (C≡N), 1597 (C=C). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 11.21 (1H, s, NH), 7.75–7.19 (10H, m, Ar-H), 6.59 (s, 1H, NH2), 4.83–4.81 (1H, H-5), 4.60–4.58 (1H, H-4). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 191.6, 148.5, 142.2, 140.0, 133.6, 129.6, 128.9, 128.3, 127.8, 126.0, 125.2, 102.7, 91.6, 41.8. Mass spectrum, m/z: 336 [M + 1]. Anal. calcd. for C24H17NO: C, 85.94; H, 5.11: N, 4.18. Found: C, 85.90; H, 5.02: N, 4.16.
- Methyl 2-methyl-4-(3-nitrophenyl)-6-phenyl-1,4-dihydropyridine-3-carboxylate (7a): Yield 94%, 0.350 g, Lemon crystalline solid, mp 133–134 °C. IR spectrum, υ, cm−1: 3436 (N-H), 3101 (aromatic C-H), 3045 (COOH), 2951 (methyl C-H), 1667 (C=O), 1648 (N-O), 1590 (C=C), 1202, (C-O). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 11.33 (1H, s, NH), 7.80–7.12 (9H, m, Ar-H), 4.69–4.68 (1H, d, H-5), 4.29–4.27 (1H, H-4), 3.55 (3H, s, COOCH3), 2.36 (3H, s, CH3). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 172.3, 152.1, 148.4, 145.9, 142.4, 135.2, 133.7, 129.8, 128.8, 128.2, 124.1, 122.9, 120.7, 105.2, 93.3, 54.7, 46.3, 18.8. Mass spectrum, m/z: 351 [M + 1]. Anal. calcd. for C20H18N2O4: C, 68.56; H, 5.18: N, 8.00. Found: C, 68.50; H, 5.12: N, 7.98.
- 1-(2-methyl-4-(3-nitrophenyl)-6-phenyl-1,4-dihydropyridin-3-yl)ethenone (7b): Yield 97%, 0.334 g, Lemon crystalline solid, mp 151–153 °C. IR spectrum, υ, cm−1: 3447 (N-H), 3091, 3083 (aromatic C-H), 2931, 2883 (methylene C-H), 1656 (C=O), 1640 (N-O), 1611 (C=C). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 11.12 (1H, s, NH), 7.92–7.23 (9H, m, Ar-H), 4.88–4.86 (1H, d, H-5), 4.57–4.55 (1H, H-4), 2.40 (3H, s, COCH3), 1.78 (3H, s, CH3). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 193.5, 150.3, 148.2, 144.8, 142.3, 135.9, 134.6, 130.6, 129.6, 128.4, 126.3, 123.7, 118.9, 110.3, 94.8, 43.3, 28.1, 16.7. Mass spectrum, m/z: 335 [M + 1]. Anal. calcd. for C20H18N2O3: C, 71.84; H, 5.43: N, 8.38. Found: C, 71.77; H, 5.40: N, 8.34.
- 5-(3-nitrophenyl)-7-phenylpyrido[2,3-d]pyrimidine-2,4(1H,3H,5H,8H)-dione (7c): Yield 92%, 0.314 g, Pale crystalline solid, mp 239–241 °C. IR spectrum, υ, cm−1: 3425 (N-H), 3092, 3044 (aromatic C-H), 2903 (methylene C-H), 1682 (C=O), 1591 (C=C), 1478 (C-N). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 11.11 (1H, s, NH), 10.25 (1H, s, NH), 8.56 (1H, s, NH), 8.01–7.32 (9H, m, Ar-H), 4.80–4.78 (1H, H-5), 4.62–4.60 (1H, H-4). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 166.2, 154.1, 151.4, 148.3, 144.5, 142.2, 134.6, 136.8, 129.7, 128.9, 127.6, 126.1, 123.6, 119.6, 91.8, 80.9, 42.4. Mass spectrum, m/z: 363 [M + 1]. Anal. calcd. for C15H14N4O4: C, 62.98; H, 3.89: N, 15.46. Found: C, 62.91; H, 3.78: N, 15.43.
- 1,3-dimethyl-5-(3-nitrophenyl)-7-phenylpyrido[2,3-d]pyrimidine-2,4(1H,3H,5H,8H)-dione (7d): Yield 91%, 0.391 g, Pale crystalline solid, mp 236–238 °C. IR spectrum, υ, cm−1: 3451 (N-H), 3084, 3057 (aromatic C-H), 2978, 2926 (methylene C-H), 1688 (C=O), 1639 (N-O), 1590 (C=C), 1447 (C-N). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 11.14 (1H, s, NH), 7.95–7.42 (9H, m, Ar-H), 4.79–4.77 (1H, H-5), 4.57–4.56 (1H, H-4), 2.94 (3H, s, CH3), 2.91 (3H, s, CH3). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 166.1, 152.7, 149.3, 148.0, 144.4, 141.1, 136.4, 134.8, 130.2, 129.4, 122.9, 128.1, 125.7, 122.9, 120.2, 92.8, 81.3, 42.4, 32.1, 29.5. Mass spectrum, m/z: 391 [M + 1]. Anal. calcd. for C21H18N4O4: C, 64.61; H, 4.65: N, 14.35. Found: C, 64.54; H, 4.58: N, 14.30.
- 2-nitro-4-(3-nitrophenyl)-6-phenyl-1,4-dihydropyridine-3-carbonitrile (7e): Yield 94%, 0.381 g, Lemon crystalline solid, mp 208–210 °C. IR spectrum, υ, cm−1: 3423 (N-H), 3081, 3057 (aromatic C-H), 2853 (methylene C-H), 1714 (C=O), 2251.3 (C≡N), 1589 (C=C). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 11.22 (1H, s, NH), 7.95–7.14 (9H, m, Ar-H), 6.89 (s, 1H, NH2), 4.78–4.76 (1H, H-5), 4.61–4.59 (1H, H-4). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 190.9, 148.7, 147.1, 143.7, 141.2, 136.1, 135.3, 129.6, 128.2, 129.4, 119.5, 127.8, 125.7, 123.8, 105.0, 92.3, 40.3. Mass spectrum, m/z: 381 [M + 1]. Anal. calcd. for C24H16N2O3: C, 75.78; H, 4.24: N, 7.36. Found: C, 75.71; H, 4.17: N, 7.32.
- Methyl 4-(4-methoxyphenyl)-2-methyl-6-phenyl-1,4-dihydropyridine-3-carboxylate (8a): Yield 90%, 0.336 g, White crystalline solid, mp 172–173 °C. IR spectrum, υ, cm−1: 3434 (N-H), 3102 (aromatic C-H), 3038 (COOH), 2940 (methyl C-H), 1657 (C=O), 1650 (N-O), 1591 (C=C), 1211, 1093, (C-O). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 11.13 (1H, s, NH), 8.01–7.47 (9H, m, Ar-H), 4.73–4.71 (1H, d, H-5), 4.35–4.432 (1H, d, H-4), 3.54 (3H, s, OCH3), 2.51 (3H, s, COOCH3), 2.26 (3H, s, CH3). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 169.8, 157.6, 155.9, 150.4, 141.2, 135.0, 131.7, 127.9, 126.7, 117.3, 115.1, 105.8, 92.1, 56.6, 51.3, 17.5. Mass spectrum, m/z: 336 [M + 1]. Anal. calcd. for C21H21NO3: C, 71.78; H, 5.02: N, 3.99. Found: C, 71.69; H, 4.95: N, 3.94.
- 1-(4-(4-hydroxyphenyl)-2-methyl-6-phenyl-1,4-dihydropyridin-3-yl)ethenone (8b): Yield 93%, 0.320 g, White crystalline solid, mp 187–188 °C. IR spectrum, υ, cm−1: 3452 (N-H), 3090, 3074 (aromatic C-H), 2922, 2873 (methylene C-H), 1664 (C=O), 1645 (N-O), 1622 (C=C). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 11.13 (1H, s, NH), 7.20–6.61 (9H, m, Ar-H), 4.78–4.76 (1H, d, Hz, H-5), 4.42–4.40 (1H, H-4), 3.69 (3H, s, OCH3), 2.23 (3H, s, OCH3), 1.81 (3H, s, CH3). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 197.2, 157.8, 156.1, 149.7, 140.9, 135.6, 130.7, 128.2, 127.0, 116.5, 114.8, 113.6, 93.4, 57.1, 42.7, 28.5, 16.8. Mass spectrum, m/z: 320 [M + 1]. Anal. calcd. for C21H21NO2: C, 75.20; H, 6.31: N, 4.18. Found: C, 75.16; H, 6.24: N, 4.15.
- 5-(4-methoxyphenyl)-7-phenylpyrido[2,3-d]pyrimidine-2,4(1H,3H,5H,8H)-dione (8c): Yield 95%, 0.348 g, Lemon crystalline solid, mp 286–288 °C. IR spectrum, υ, cm−1: 3440 (N-H), 3082, 3027 (aromatic C-H), 2919 (methylene C-H), 1691 (C=O), 1583 (C=C), 1455 (C-N). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 11.02 (1H, s, NH), 8.47 (1H, s, NH), 7.87 (1H, s, NH), 7.21–6.63 (9H, m, Ar-H), 4.82–4.80 (1H, H-5), 4.58–4.56 (1H, H-4), 3.74 (3H, s, OCH3). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 164.9, 157.0, 155.2, 153.4, 151.7, 141.4, 135.8, 130.6, 127.9, 126.2, 117.1, 115.7, 93.0, 79.9, 56.7, 41.2. Mass spectrum, m/z: 348 [M + 1]. Anal. calcd. for C20H17N3O3: C, 66.11; H, 4.72: N, 11.56. Found: C, 66.03; H, 4.67: N, 11.51.
- 5-(4-methoxyphenyl)-1,3-dimethyl-7-phenylpyrido[2,3-d]pyrimidine-2,4(1H,3H,5H,8H)-dione (8d): Yield 96%, 0.376 g, Lemon crystalline solid, mp 289–291 °C. IR spectrum, υ, cm−1: 3452 (N-H), 3092, 3056 (aromatic C-H), 2971, 2934 (methylene C-H), 1685 (C=O), 1590 (C=C), 1448 (C-N). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 11.16 (1H, s, NH), 7.15–6.69 (9H, m, Ar-H), 4.74–4.72 (1H, H-5), 4.50–4.48 (1H, H-4), 3.67 (3H, s, OCH3), 2.85 (3H, s, CH3), 2.80 (3H, s, CH3). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 163.5, 157.8, 156.7, 152.0, 148.4, 140.9, 134.7, 132.4, 129.8, 127.3, 116.6, 113.7, 92.3, 80.4, 56.2, 41.6, 31.6, 28.9. Mass spectrum, m/z: 375 [M + 1]. Anal. calcd. for C22H21N3O3: C, 67.51; H, 5.41: N, 10.74. Found: C, 67.43; H, 5.34: N, 10.68.
- 4-(4-methoxyphenyl)-2-nitro-6-phenyl-1,4-dihydropyridine-3-carbonitrile (8e): Yield 93%, 0.333 g, White crystalline solid, mp 235–237 °C. IR spectrum, υ, cm−1: 3437 (N-H), 3082, 3066 (aromatic C-H), 2863 (methylene C-H), 2251.6 (C≡N), 1592 (C=C). 1H NMR spectrum, δ, ppm (CDCl3, 500 MHz): 11.25 (1H, s, NH), 7.18–6.62 (9H, m, Ar-H), 6.89 (s, 1H, NH2), 4.82–4.80 (1H, H-5), 4.59–4.57 (1H, H-4), 3.51 (3H, s, OCH3). 13C NMR spectrum, δ, ppm (CDCl3, 125 MHz): 193.1, 158.4, 157.1, 146.7, 141.2, 143.7, 141.2, 135.6, 131.3, 128.0, 126.5, 116.1, 114.4, 101.9, 94.1, 55.6, 41.2. Mass spectrum, m/z: 334 [M + 1]. Anal. calcd. for C19H15N3O3: C, 78.72; H, 5.02: N, 3.67. Found: C, 78.65; H, 4.95: N, 3.62.
3.5. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davarpanah, J.; Ghahremani, M.; Najafi, O. Synthesis of 1,4-Dihydropyridine and Polyhydroquinoline Derivatives via Hantzsch Reaction Using Nicotinic Acid as a Green and Reusable Catalyst. J. Mol. Struct. 2019, 1177, 525–535. [Google Scholar] [CrossRef]
- Bhaskaruni, S.V.H.S.; Maddila, S.; Van Zyl, W.E.; Jonnalagadda, S.B. Four-Component Fusion Protocol with NiO/ZrO2 as a Robust Recyclable Catalyst for Novel 1,4-Dihydropyridines. ACS Omega 2019, 4, 21187–21196. [Google Scholar] [CrossRef] [PubMed]
- Evdokimov, N.M.; Kireev, A.S.; Yakovenko, A.A.; Antipin, M.Y.; Magedov, I.V.; Kornienko, A. One-Step Synthesis of Heterocyclic Privileged Medicinal Scaffolds by a Multicomponent Reaction of Malononitrile with Aldehydes and Thiols. J. Org. Chem. 2007, 72, 3443–3453. [Google Scholar] [CrossRef] [PubMed]
- Evdokimov, N.M.; Magedov, I.V.; Kireev, A.S.; Kornienko, A. One-Step, Three-Component Synthesis of Pyridines and 1,4-Dihydropyridines with Manifold Medicinal Utility. Org. Lett. 2006, 8, 899–902. [Google Scholar] [CrossRef]
- Trivedi, A.; Dodiya, D.; Dholariya, B.; Kataria, V.; Bhuva, V.; Shah, V. Synthesis and Biological Evaluation of Some Novel 1,4-Dihydropyridines as Potential Antitubercular Agents. Chem. Biol. Drug Des. 2011, 78, 881–886. [Google Scholar] [CrossRef]
- Malhi, D.S.; Kaur, M.; Sohal, H.S. Effect of Substitutions on 1,4-Dihdropyridines to Achieve Potential Anti-Microbial Drugs: A Review. ChemistrySelect 2019, 4, 11321–11336. [Google Scholar] [CrossRef]
- Abdel-Mohsen, H.T.; Ragab, F.A.F.; Ramla, M.M.; El Diwani, H.I. Novel Benzimidazole–Pyrimidine Conjugates as Potent Antitumor Agents. Eur. J. Med. Chem. 2010, 45, 2336–2344. [Google Scholar] [CrossRef]
- Sirisha, K.; Achaiah, G.; Reddy, V.M. Facile Synthesis and Antibacterial, Antitubercular, and Anticancer Activities of Novel 1,4-Dihydropyridines. Arch. Pharm. 2010, 343, 342–352. [Google Scholar] [CrossRef]
- Zhou, B.; Xing, C. Diverse Molecular Targets for Chalcones with Varied Bioactivities. Med. Chem. 2015, 5, 388–404. [Google Scholar] [CrossRef]
- Martha, R.; Gutierrez, P.; Muñiz-ramirez, A.; Sauceda, J.V. Review: The Potential of Chalcones as a Source of Drugs. Afr. J. Pharm. Pharmacol. 2015, 9, 237–257. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, F.; Xiao, B.; Li, X.; Peng, C.; Peng, F. Metochalcone Induces Senescence-Associated Secretory Phenotype via JAK2/STAT3 Pathway in Breast Cancer. Oncol. Res. 2024, 32, 943–953. [Google Scholar] [CrossRef]
- Lin, S.; Chen, Y.; Li, H.; Liu, J.; Liu, S. Design, Synthesis, and Evaluation of Amphiphilic Sofalcone Derivatives as Potent Gram-Positive Antibacterial Agents. Eur. J. Med. Chem. 2020, 202, 112596. [Google Scholar] [CrossRef]
- Yadav, V.R.; Prasad, S.; Sung, B.; Aggarwal, B.B. The Role of Chalcones in Suppression of NF-ΚB-Mediated Inflammation and Cancer. Int. Immunopharmacol. 2011, 11, 295–309. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Darweesh, A.F.; Elwahy, A.H.M.; Abdelhamid, I.A. Synthesis, Characterization and Antitumor Activity of Novel Tetrapodal 1,4-Dihydropyridines: P53 Induction, Cell Cycle Arrest and Low Damage Effect on Normal Cells Induced by Genotoxic Factor H2O2. RSC Adv. 2016, 6, 40900–40910. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, J.; Wang, W.; Liu, H.; Guo, H. Synthesis of Heterocyclic Compounds through Nucleophilic Phosphine Catalysis. Chem. Commun. 2020, 56, 15235–15281. [Google Scholar] [CrossRef] [PubMed]
- Rucins, M.; Plotniece, A.; Bernotiene, E.; Tsai, W.B.; Sobolev, A. Recent Approaches to Chiral 1,4-Dihydropyridines and Their Fused Analogues. Catalysts 2020, 10, 1019. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, R.A.; Sharma, S.; Kumar, M.; Bhatia, G. Synthesis and Biological Evaluation of N-Aryl-1,4-Dihydropyridines as Novel Antidyslipidemic and Antioxidant Agents. Eur. J. Med. Chem. 2010, 45, 501–509. [Google Scholar] [CrossRef]
- Sutarni, Y.D.; Purwono, B.; Kunarti, E.S.; Mardjan, M.I.D. Fe/ZSM-5-Catalyzed-Synthesis of 1,4-Dihydropyridines under Ultrasound Irradiation and Their Antioxidant Activities. Sains Malaysiana 2023, 52, 1189–1202. [Google Scholar] [CrossRef]
- De Luca, M.; Ioele, G.; Ragno, G. 1,4-Dihydropyridine Antihypertensive Drugs: Recent Advances in Photostabilization Strategies. Pharmaceutics 2019, 11, 85. [Google Scholar] [CrossRef]
- Maheswara, M.; Siddaiah, V.; Rao, Y.K.; Tzeng, Y.M.; Sridhar, C. A Simple and Efficient One-Pot Synthesis of 1,4-Dihydropyridines Using Heterogeneous Catalyst under Solvent-Free Conditions. J. Mol. Catal. A Chem. 2006, 260, 179–180. [Google Scholar] [CrossRef]
- Kalhor, S.; Yarie, M.; Rezaeivala, M.; Zolfigol, M.A. Novel Magnetic Nanoparticles with Morpholine Tags as Multirole Catalyst for Synthesis of Hexahydroquinolines and 2-Amino-4,6-Diphenylnicotinonitriles through Vinylogous Anomeric-Based Oxidation. Res. Chem. Intermed. 2019, 45, 3453–3480. [Google Scholar] [CrossRef]
- Parthiban, A.; Makam, P. 1,4-Dihydropyridine: Synthetic Advances, Medicinal and Insecticidal Properties. RSC Adv. 2022, 12, 29253–29290. [Google Scholar] [CrossRef]
- Wan, J.P.; Liu, Y. Recent Advances in New Multicomponent Synthesis of Structurally Diversified 1,4-Dihydropyridines. RSC Adv. 2012, 2, 9763–9777. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, S.K. Synthesis, Utility and Medicinal Importance of 1,2- & 1,4-Dihydropyridines. RSC Adv. 2017, 7, 2682–2732. [Google Scholar] [CrossRef]
- Moradi, S.; Moradian, M.; Naeimi, H. Efficient One-Pot Synthesis of 1,4-Dihydropyridines Catalyzed by Magnetic MnFe2O4 Nanoparticles. Acta Chim. Slov. 2022, 69, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Koukabi, N.; Kolvari, E.; Khazaei, A.; Zolfigol, M.A.; Shirmardi-Shaghasemi, B.; Khavasi, H.R. Hantzsch Reaction on Free Nano-Fe2O3 Catalyst: Excellent Reactivity Combined with Facile Catalyst Recovery and Recyclability. Chem. Commun. 2011, 47, 9230–9232. [Google Scholar] [CrossRef]
- Li, J.; He, P.; Yu, C. DPTA-Catalyzed One-Pot Regioselective Synthesis of Polysubstituted Pyridines and 1,4-Dihydropyridines. Tetrahedron 2012, 68, 4138–4144. [Google Scholar] [CrossRef]
- Olejníková, P.; Švorc, L.; Olšovská, D.; Panáková, A.; Vihonská, Z.; Kovaryová, K.; Marchalín, Š. Antimicrobial Activity of Novel C2-Substituted 1,4-Dihydropyridine Analogues. Sci. Pharm. 2014, 82, 221–232. [Google Scholar] [CrossRef]
- Sobhanardakani, S.; Jafari, A.; Zandipak, R.; Meidanchi, A. Removal of Heavy Metal (Hg(II) and Cr(VI)) Ions from Aqueous Solutions Using Fe2O3@SiO2 Thin Films as a Novel Adsorbent. Process Saf. Environ. Prot. 2018, 120, 348–357. [Google Scholar] [CrossRef]
- Shahabadi, N.; Zendehcheshm, S.; Jamshidi, D.; Khademi, F.; Soltani, L. Green Synthesis of γ-Fe2O3@SiO2 Magnetic Nanoparticles Functionalized with Penciclovir Drug: Antiproliferative Effect, and Nucleic Acids (DNA and RNA) Interaction. J. Taibah Univ. Sci. 2024, 18, 2357820. [Google Scholar] [CrossRef]
- Hjiri, M.; Zahmouli, N.; Khouzami, K.; Mir, L.E.; Aida, M.S.; Moulaee, K.; Lemine, O.M.; Leonardi, S.G.; Neri, G. A Comparison of NO2 Sensing Characteristics of α- and γ-Iron Oxide-Based Solid-State Gas Sensors. Appl. Phys. A Mater. Sci. Process. 2020, 126, 788. [Google Scholar] [CrossRef]
- Drbohlavova, J.; Hrdy, R.; Adam, V.; Kizek, R.; Schneeweiss, O.; Hubalek, J. Preparation and Properties of Various Magnetic Nanoparticles. Sensors 2009, 9, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Zhao, R.; Luo, D.; Dempsey, E.; Wang, X.; Iqbal, M.Z.; Kong, X. Magnetic Hydroxyapatite Nanocomposites: The Advances from Synthesis to Biomedical Applications. Mater. Des. 2021, 197, 109269. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Ngila, J.C.; Ndungu, P.G.; Nomngongo, P.N. Ultrasound Assisted Adsorptive Removal of Cr, Cu, Al, Ba, Zn, Ni, Mn, Co, and Ti from Seawater Using Fe2O3-SiO2-PAN Nanocomposite: Equilibrium Kinetics. J. Mar. Sci. Eng. 2019, 7, 133. [Google Scholar] [CrossRef]
- El-Attar, H.G.; Salem, M.A.; Ibrahim, S.A.; Bakr, E.A. Highly Efficient and Recyclable Novel Spindles Fe2O3@SiO2/In2O3 Nanomagnetic Catalyst Designed for Green Synthesis of Azomethine Compounds. Res. Chem. Intermed. 2023, 49, 469–489. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, Y.; Tuersun, T.; Yu, Y.; Zhi, J. Simple Preparation and Highly Selective Detection of Silver Ions Using an Electrochemical Sensor Based on Sulfur-Doped Graphene and a 3,3′,5,5′-Tetramethylbenzidine Composite Modified Electrode. Analyst 2018, 143, 2076–2082. [Google Scholar] [CrossRef]
- Cai, X.; Yang, C.; Chen, L.; Li, R.; Du, C. The Dissolution Mechanism, Molecular Dynamics Simulation and Thermodynamic Analysis of Nirmatrelvir (Treatment of COVID-19) in Aqueous Ethanol Mixtures. J. Mol. Liq. 2023, 391, 123256. [Google Scholar] [CrossRef]
- Zondervan, R.; Kulzer, F.; Berkhout, G.C.G.; Orrit, M. Local Viscosity of Supercooled Glycerol near Tg Probed by Rotational Diffusion of Ensembles and Single Dye Molecules. Proc. Natl. Acad. Sci. USA 2007, 104, 12628–12633. [Google Scholar] [CrossRef]
- Rusdan, N.A.; Timmiati, S.N.; Isahak, W.N.; Yaakob, Z.; Lim, K.L.; Khaidar, D. Recent Application of Core-Shell Nanostructured Catalysts for CO2 Thermocatalytic Conversion Processes. Nanomaterials 2022, 12, 3877. [Google Scholar] [CrossRef]
- Raup, D.E.A.; Cardinal-David, B.; Holte, D.; Scheidt, K.A. Cooperative Catalysis by Carbenes and Lewis Acids in a Highly Stereoselective Route to Gamma-Lactams. Nat. Chem. 2010, 2, 766–771. [Google Scholar] [CrossRef]
- Kumar, A.; Rout, L.; Achary, L.S.K.; Mohanty, S.K.; Nayak, P.S.; Barik, B. Solvent Free Synthesis of Chalcones over Graphene Oxide-Supported MnO2 Catalysts Synthesized via Combustion Route. Mater. Chem. Phys. 2021, 259, 124019. [Google Scholar] [CrossRef]
- Vaishali; Sharma, S.; Sharma, P.; Das, D.K.; Vashistha, V.K.; Dhiman, J.; Sharma, R.; Kumar, R.; vir Singh, M.; Kumar, Y. Magnetic Nanoparticle-Catalysed Synthesis of Quinoline Derivatives: A Green and Sustainable Method. Heliyon 2024, 10, e40451. [Google Scholar] [CrossRef] [PubMed]
- Curti, C.; Gellis, A.; Vanelle, P. Synthesis of α,β-Unsaturated Ketones as Chalcone Analogues via a SRN1 Mechanism. Molecules 2007, 12, 797–804. [Google Scholar] [CrossRef]
- Hennessy, M.C.; O’Sullivan, T.P. Recent Advances in the Transesterification of β-Keto Esters. RSC Adv. 2021, 11, 22859–22920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Niu, Y.; Li, Y.; Li, Y.; Zhao, J. Preparation and Thermal Stability of the Spindle α-Fe2O3@SiO2 Core–Shell Nanoparticles. J. Solid State Chem. 2014, 211, 69–74. [Google Scholar] [CrossRef]
- Nanda, A.; Kaur, N.; Kaur, M.; Husain, F.M.; Han, H.; Bhowmik, P.K.; Sohal, H.S. Synthesis and Antimicrobial Activity of (E)-1-Aryl-2-(1H-Tetrazol-5-Yl)Acrylonitrile Derivatives via [3+2] Cycloaddition Reaction Using Reusable Heterogeneous Nanocatalyst under Microwave Irradiation. Molecules 2024, 29, 4339. [Google Scholar] [CrossRef]
- Elkanzi, N.A.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef]






 | |||||
|---|---|---|---|---|---|
| Entry | Fe2O3@SiO2 (mmol%) | Time (minutes) | Yield of 6a a,* (%) | Yield of 7a a,* (%) | Yield of 8a a,* (%) |
| 1 | 0 | 10 | 51 | 49 | 54 |
| 2 | 1 | 8 | 74 | 72 | 70 |
| 3 | 2 | 7 | 89 | 85 | 87 |
| 4 | 3 | 6 | 93 | 92 | 94 |
| 5 | 3 | 8 | 91 | 90 | 91 |
| 6 | 3 | 10 | 90 | 89 | 90 |
| 7 | 4 | 6 | 92 | 91 | 94 |
| 8 | 5 | 6 | 92 | 92 | 93 |
 | |||||
|---|---|---|---|---|---|
| Entry | Solvent | Time (Minutes) | Yield of 6a a,* (%) | Yield of 7a a,* (%) | Yield of 8a a,* (%) |
| 1 | Polyethylene glycol | 20 | 70 | 68 | 69 |
| 2 | Ethylene glycol | 20 | 72 | 74 | 73 |
| 3 | Water | 15 | 35 | 36 | 38 |
| 4 | Acetonitrile | 14 | 70 | 72 | 68 |
| 5 | Glycerol | 15 | 90 | 91 | 89 |
| 6 | Ethanol | 7 | 94 | 95 | 93 |
| 7 | Methanol | 11 | 82 | 80 | 84 |
| 8 | DMSO | 30 | 30 | 32 | 34 |
| 9 | DMF | 25 | 55 | 55 | 56 |
| 10 | No solvent | 30 | - | - | - |
 | |||||
|---|---|---|---|---|---|
| Entry | Watts | Time (minutes) | Yield of 6a a,* (%) | Yield of 7a a,* (%) | Yield of 8a a,* (%) |
| 1 | 75 | 15 | 79 | 77 | 80 |
| 2 | 80 | 12 | 81 | 80 | 82 |
| 3 | 85 | 7 | 92 | 91 | 90 |
| 4 | 90 | 4 | 98 | 96 | 98 |
| 5 | 95 | 4 | 96 | 97 | 95 |
| 6 | 100 | 5 | 93 | 91 | 92 |
 | ||||
|---|---|---|---|---|
| Entry | Run | Yield for 6a a,* (%) | Yield for 7a a,* (%) | Yield for 8a a,* (%) |
| 1 | Fresh | 98 | 96 | 98 |
| 2 | 1 | 97 | 95 | 97 |
| 3 | 2 | 97 | 94 | 96 |
| 4 | 3 | 96 | 94 | 96 |
| 5 | 4 | 95 | 93 | 95 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Chalcone-Derived 1,4-DHPs Derivatives | Rf | Yield (%) | Melting Point (°C) | Reference | |
| Lit | Exp | |||||
| 1 | 6a | 0.78 | 94 | 153–155 | 156–157 | [13] |
| 2 | 6b | 0.79 | 92 | 162–164 | 164–166 | [20] |
| 3 | 6c | 0.75 | 96 | 270–271 | 271–272 | [13] |
| 4 | 6d | 0.73 | 93 | 260–261 | 263–265 | [28] |
| 5 | 6e | 0.71 | 95 | 202–203 | 204–205 | [28] |
| 6 | 7a | 0.66 | 94 | 131–133 | 133–134 | [13] |
| 7 | 7b | 0.67 | 97 | 150–152 | 151–153 | [20] |
| 8 | 7c | 0.64 | 92 | 239–240 | 239–241 | [28] |
| 9 | 7d | 0.63 | 91 | 234–235 | 236–238 | [20] |
| 10 | 7e | 0.62 | 94 | 210–211 | 202–204 | [28] |
| 11 | 8a | 0.67 | 90 | 170–171 | 172–173 | [20] |
| 12 | 8b | 0.68 | 93 | 185–187 | 187–188 | [13] |
| 13 | 8c | 0.62 | 95 | 284–286 | 286–288 | [28] |
| 14 | 8d | 0.61 | 96 | 288–290 | 289–291 | [20] |
| 15 | 8e | 0.59 | 93 | 212–214 | 215–216 | [20] |
| Chalcone-Derived 1,4-DPHs Derivatives | Gram (+ve) Bacteria | Gram (-ve) Bacteria | Fungi | |||||
|---|---|---|---|---|---|---|---|---|
| B. Subtilis | S. Pyogenes | E. Coli | K. Pneumonia | S. Aureus | A. Janus | A. Niger | A. Sclerotiorum | |
| 6a | 128 | 46 | 64 | 128 | 32 | 64 | 128 | – |
| 6b | 128 | 64 | 64 | 32 | 64 | 128 | 128 | 128 |
| 6c | 32 | 32 | 64 | 64 | 64 | 32 | – | 128 |
| 6d | 128 | 64 | 64 | 128 | 128 | 64 | 128 | 64 |
| 6e | 128 | 128 | – | 128 | – | 128 | 128 | 128 |
| 7a | 64 | 64 | 128 | 64 | 32 | 32 | 64 | 128 |
| 7b | 64 | 32 | 32 | 32 | 64 | 64 | 3 | 32 |
| 7c | 8 | 16 | 16 | 8 | 8 | 16 | 8 | 16 |
| 7d | 32 | 8 | 8 | 8 | 32 | 8 | 32 | 8 |
| 7e | 16 | 32 | 8 | 32 | 16 | 32 | 8 | 16 |
| 8a | 32 | 16 | 16 | 16 | 32 | 16 | 8 | 16 |
| 8b | 32 | 16 | 16 | 16 | 32 | 16 | 8 | 16 |
| 8c | 4 | 4 | 8 | 4 | 4 | 4 | 8 | 2 |
| 8d | 8 | 16 | 16 | 32 | 16 | 16 | 16 | 16 |
| 8e | 32 | 32 | 32 | 16 | 16 | 16 | 32 | 8 |
| Amoxicillin | 4 | 4 | 4 | 4 | 4 | – | – | – |
| Fluconazole | – | – | – | – | – | 2 | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malhi, D.S.; Kaur, N.; Kaur, M.; Han, H.; Bhowmik, P.K.; Husain, F.M.; Sohal, H.S.; Verma, M. Synthesis and Antimicrobial Activity of Chalcone-Derived 1,4-Dihydropyridine Derivatives Using Magnetic Fe2O3@SiO2 as Highly Efficient Nanocatalyst. Catalysts 2025, 15, 281. https://doi.org/10.3390/catal15030281
Malhi DS, Kaur N, Kaur M, Han H, Bhowmik PK, Husain FM, Sohal HS, Verma M. Synthesis and Antimicrobial Activity of Chalcone-Derived 1,4-Dihydropyridine Derivatives Using Magnetic Fe2O3@SiO2 as Highly Efficient Nanocatalyst. Catalysts. 2025; 15(3):281. https://doi.org/10.3390/catal15030281
Chicago/Turabian StyleMalhi, Dharambeer Singh, Navneet Kaur, Manvinder Kaur, Haesook Han, Pradip K. Bhowmik, Fohad Mabood Husain, Harvinder Singh Sohal, and Meenakshi Verma. 2025. "Synthesis and Antimicrobial Activity of Chalcone-Derived 1,4-Dihydropyridine Derivatives Using Magnetic Fe2O3@SiO2 as Highly Efficient Nanocatalyst" Catalysts 15, no. 3: 281. https://doi.org/10.3390/catal15030281
APA StyleMalhi, D. S., Kaur, N., Kaur, M., Han, H., Bhowmik, P. K., Husain, F. M., Sohal, H. S., & Verma, M. (2025). Synthesis and Antimicrobial Activity of Chalcone-Derived 1,4-Dihydropyridine Derivatives Using Magnetic Fe2O3@SiO2 as Highly Efficient Nanocatalyst. Catalysts, 15(3), 281. https://doi.org/10.3390/catal15030281







