Intermetallic Fe2Mo Nanoparticles on Hierarchical Nanoporous Copper for Efficient Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Results and Discussion
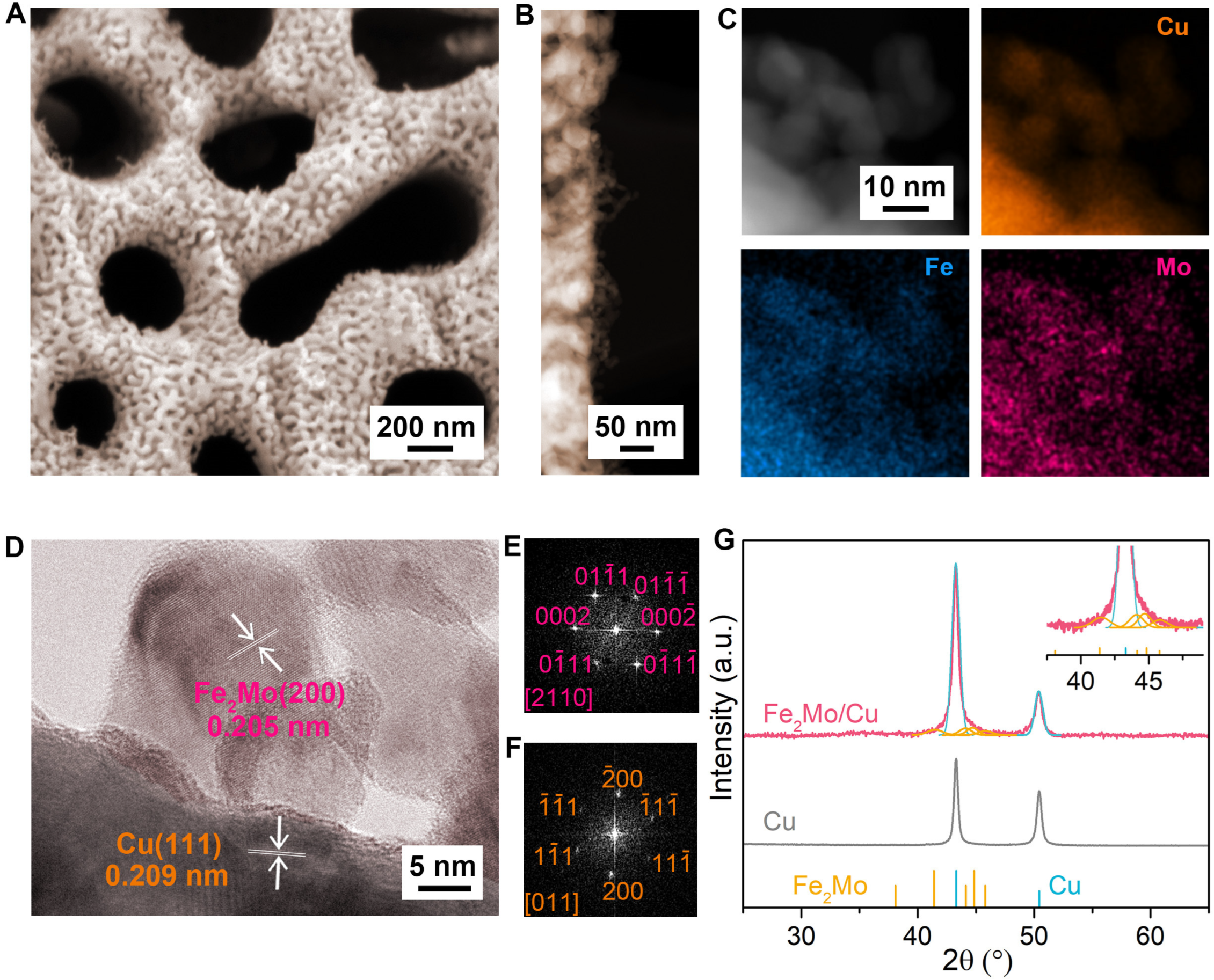
2.1. Synthesis and Microstructural Characterization of Nanoporous Fe2Mo/Cu Electrode
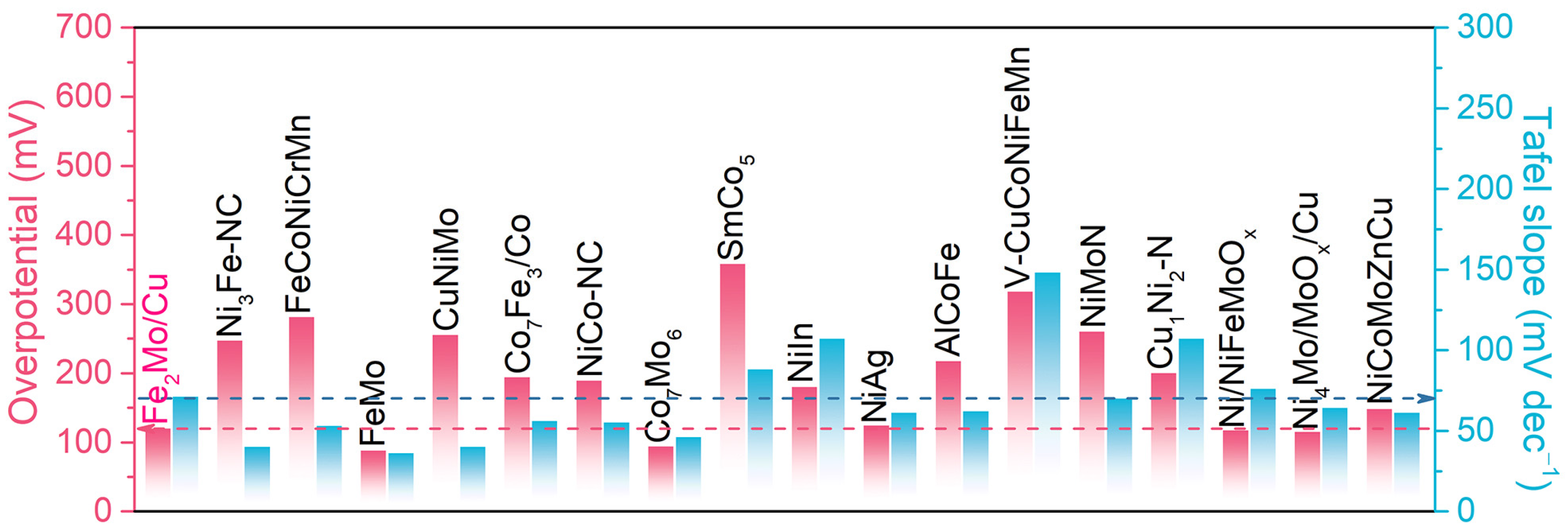
2.2. Electrochemical Properties of Nanoporous Fe2Mo/Cu Electrode
3. Materials and Methods
3.1. Preparation of Nanoporous Electrocatalysts
3.2. Physicochemical Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.H.; Shao, Z.P.; Lim, J. Non-Precious-Metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef]
- Guan, D.Q.; Wang, B.W.; Zhang, J.G.; Shi, R.; Jiao, K.; Li, L.C.; Wang, Y.; Xie, B.; Zhang, Q.W.; Yu, J.; et al. Hydrogen society: From present to future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Markovic, N.M. Interfacing electrochemistry. Nat. Mater. 2013, 12, 101–102. [Google Scholar] [CrossRef]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef]
- Glenk, G.; Reichelstein, S. Economics of converting renewable power to hydrogen. Nat. Energy 2019, 4, 216–222. [Google Scholar] [CrossRef]
- Yang, Y.X.; Li, P.; Zheng, X.B.; Sun, W.P.; Dou, S.X.; Ma, T.Y.; Pan, H.G. Anion-exchange membrane water electrolyzers and fuel cells. Chem. Soc. Rev. 2022, 51, 9620–9693. [Google Scholar] [CrossRef]
- Abbasi, R.; Setzler, B.P.; Lin, S.S.; Wang, J.H.; Zhao, Y.; Xu, H.; Pivovar, B.; Tian, B.Y.; Chen, X.; Wu, G.; et al. A roadmap to low-cost hydrogen with hydroxide exchange membrane electrolyzers. Adv. Mater. 2019, 31, 1805876. [Google Scholar] [CrossRef]
- Li, D.G.; Park, E.J.; Zhu, W.L.; Shi, Q.R.; Zhou, Y.; Tian, H.Y.; Lin, Y.H.; Serov, A.; Zulevi, B.; Baca, E.D.; et al. Highly quaternized polystyrene ionomers for high performance anion exchange membrane water electrolysers. Nat. Energy 2020, 5, 378–385. [Google Scholar] [CrossRef]
- Wang, N.; Song, S.Z.; Wu, W.T.; Deng, Z.F.; Tang, C. Bridging laboratory electrocatalysts with industrially relevant alkaline water electrolyzers. Adv. Energy Mater. 2024, 14, 2303451. [Google Scholar] [CrossRef]
- Lagadec, M.F.; Grimaud, A. Water electrolysers with closed and open electrochemical systems. Nat. Mater. 2020, 19, 1140–1150. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, Y.H.; Xu, H.; Martinez, A.; Chen, K.S. PEM fuel cell and electrolysis cell technologies and hydrogen infrastructure development—A review. Energy Environ. Sci. 2022, 15, 2288–2328. [Google Scholar] [CrossRef]
- Jiao, S.L.; Fu, X.W.; Wang, S.Y.; Zhao, Y. Perfecting electrocatalysts via imperfections: Towards the large-scale deployment of water electrocatalysis technology. Energy Environ. Sci. 2021, 14, 1722–1770. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Duan, Y.; Feng, X.Y.; Yu, X.X.; Gao, M.R.; Yu, S.H. Clean and affordable hydrogen fuel from alkaline water splitting: Past, recent progress, and future prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.S.; Zhao, P.X.; Lee, L.Y.S.; Wong, K.Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef]
- Cui, W.G.; Gao, F.; Na, G.Q.; Wang, X.Q.; Li, Z.L.; Yang, Y.X.; Niu, Z.Q.; Qu, Y.Q.; Wang, D.S.; Pan, H.G. Insights into the pH effect on hydrogen electrocatalysis. Chem. Soc. Rev. 2024, 53, 10253–10311. [Google Scholar] [CrossRef]
- Strmcnik, D.; Lopes, P.P.; Genorio, B.; Stamenkovic, V.R.; Markovic, N.M. Design principles for hydrogen evolution reaction catalyst materials. Nano Energy 2016, 29, 29–36. [Google Scholar] [CrossRef]
- Ledezma-Yanez, I.; Wallace, W.D.Z.; Sebastián-Pascual, P.; Climent, V.; Feliu, J.M.; Koper, M.T.M. Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nat. Energy 2017, 2, 17031. [Google Scholar] [CrossRef]
- Tian, X.Y.; Zhao, P.C.; Sheng, W.C. Hydrogen evolution and oxidation: Mechanistic studies and material advances. Adv. Mater. 2019, 31, 1808066. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chorkendorff, I. Considerations for the scaling-up of water splitting catalysts. Nat. Energy 2019, 4, 430–433. [Google Scholar] [CrossRef]
- Zhang, B.X.; Wang, J.M.; Liu, G.M.; Weiss, C.M.; Liu, D.Q.; Chen, Y.P.; Xia, L.X.; Zhou, P.; Gao, M.X.; Liu, Y.F.; et al. A strongly coupled Ru-CrOx cluster-cluster heterostructure for efficient alkaline hydrogen electrocatalysis. Nat. Catal. 2024, 7, 441–451. [Google Scholar] [CrossRef]
- Oener, S.Z.; Foster, M.J.; Boettcher, S.W. Accelerating water dissociation in bipolar membranes and for electrocatalysis. Science 2022, 369, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wei, W.R.; Ding, S.Q.; Wu, L.; Qin, H.Y.; Yuan, X.X. A Multi-site synergistic effect in high-entropy alloy for efficient hydrogen evolution. Adv. Funct. Mater. 2025, 35, 2414554. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.C.; Uchimura, M.; Paulikas, A.P.; Stamenkovic, V.; Markovic, N.M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science 2011, 334, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.S.; Dutta, S.; Hong, Y.R.; Okello, O.F.N.; Im, H.; Ahn, S.; Choi, S.Y.; Han, J.W.; Ryu, S.; Lee, I.S. Harmonious heterointerfaces formed on 2D-Pt nanodendrites by facet-respective stepwise metal deposition for enhanced hydrogen evolution reaction. Angew. Chem. Int. Ed. 2023, 62, e202307816. [Google Scholar] [CrossRef]
- Xu, X.M.; Zhong, Y.J.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z.P. Grain boundary engineering: An emerging pathway toward efficient electrocatalysis. InfoMat 2024, 6, e12608. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Klingenhof, M.; Gao, C.L.; Koketsu, T.; Weiser, G.; Pi, Y.C.; Liu, S.H.; Sui, L.J.; Hou, J.R.; Li, J.Y.; et al. Facilitating alkaline hydrogen evolution reaction on the hetero-interfaced Ru/RuO2 through Pt single atoms doping. Nat. Commun. 2024, 15, 1447. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Mao, C.H.; Tian, J.Y.; Xia, M.Q.; Yang, L.J.; Wang, X.Z.; Wu, Q.; Hu, Z. Correlation between heteroatom coordination and hydrogen evolution for single-site Pt on carbon-based nanocages. Angew. Chem. Int. Ed. 2024, 63, e202401304. [Google Scholar] [CrossRef]
- Liu, K.; Yang, H.; Jiang, Y.L.; Liu, Z.J.; Zhang, S.M.; Zhang, Z.X.; Qiao, Z.; Lu, Y.M.; Cheng, T.; Terasaki, O.; et al. Coherent hexagonal platinum skin on nickel nanocrystals for enhanced hydrogen evolution activity. Nat. Commun. 2023, 14, 2424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.W.; Wang, J.P.; Guo, L.F.; Li, H.D.; Xiao, W.P.; Xu, G.R.; Chen, D.H.; Li, C.X.; Du, Y.M.; Ding, H.; et al. Microwave-assisted PtRu alloying on defective tungsten oxide: A pathway to improved hydroxyl dynamics for highly-efficient hydrogen evolution reaction. Adv. Energy Mater. 2024, 14, 2402372. [Google Scholar] [CrossRef]
- Xu, X.M.; Shao, Z.P.; Jiang, S.P. High-entropy materials for water electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Sun, H.M.; Yan, Z.H.; Liu, F.M.; Xu, W.C.; Cheng, F.Y.; Chen, J. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef]
- Xiong, H.; Zhuang, R.; Cheng, B.; Liu, D.K.; Du, Y.X.; Wang, H.Y.; Liu, Y.; Xu, F.; Wang, H.Q. Self-supported metallic alkaline hydrogen evolution electrocatalysts tolerant for ampere-level current densities. Adv. Energy Mater. 2025, 15, 2404077. [Google Scholar] [CrossRef]
- Yan, Y.C.; Du, J.S.S.; Gilroy, K.D.; Yang, D.R.; Xia, Y.N.; Zhang, H. Intermetallic nanocrystals: Syntheses and catalytic applications. Adv. Mater. 2017, 29, 1605997. [Google Scholar] [CrossRef]
- Song, R.R.; Han, J.H.; Okugawa, M.; Belosludov, R.; Wada, T.; Jiang, J.; Wei, D.X.; Kudo, A.; Tian, Y.; Chen, M.W.; et al. Ultrafine nanoporous intermetallic catalysts by high-temperature liquid metal dealloying for electrochemical hydrogen production. Nat. Commun. 2022, 13, 5157. [Google Scholar] [CrossRef]
- Kim, H.Y.; Joo, S.H. Recent advances in nanostructured intermetallic electrocatalysts for renewable energy conversion reactions. J. Mater. Chem. A 2020, 8, 8195–8217. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Mofarah, S.S.; Shen, X.J.; Bartlett, S.A.; Koshy, P.; Sorrell, C.C.; Sun, H.Y.; Pozo-Gonzalo, C.; Dastafkan, K.; et al. Stacking fault-enriched MoNi4/MoO2 enables high-performance hydrogen evolution. Adv. Mater. 2024, 36, 2402156. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Liu, P.; Liao, Z.Q.; Liu, S.H.; Zhuang, X.D.; Chen, M.W.; Zschech, E.; Feng, X.L. Efficient hydrogen production on MoNi4 electrocatalysts with fast water dissociation kinetics. Nat. Commun. 2017, 8, 15437. [Google Scholar] [CrossRef]
- Shi, H.; Dai, T.Y.; Sun, X.Y.; Zhou, Z.L.; Zeng, S.P.; Wang, T.H.; Han, G.F.; Wen, Z.; Fang, Q.R.; Lang, X.Y.; et al. Dual-intermetallic heterostructure on hierarchical nanoporous metal for highly efficient alkaline hydrogen electrocatalysis. Adv. Mater. 2024, 36, 2406711. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Yang, T.; Sun, L.G.; Zhao, Y.L.; Li, W.P.; Luan, J.H.; Lyu, F.C.; Zhang, L.C.; Kruzic, J.J.; Kai, J.J.; et al. A novel multinary intermetallic as an active electrocatalyst for hydrogen evolution. Adv. Mater. 2020, 32, 2000385. [Google Scholar] [CrossRef] [PubMed]
- Menezes, P.W.; Panda, C.; Garai, S.; Walter, C.; Guiet, A.; Driess, M. Structurally ordered intermetallic cobalt stannide nanocrystals for high-performance electrocatalytic overall water-splitting. Angew. Chem. Int. Ed. 2018, 57, 15237–15242. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, Y.T.; Yao, R.Q.; Wan, W.B.; Ge, X.; Zhang, W.; Wen, Z.; Lang, X.Y.; Zheng, W.T.; Jiang, Q. Spontaneously separated intermetallic Co3Mo from nanoporous copper as versatile electrocatalysts for highly efficient water splitting. Nat. Commun. 2020, 11, 2940. [Google Scholar] [CrossRef]
- Li, W.X.; Liu, Y.; Azam, A.; Liu, Y.C.; Yang, J.; Wang, D.Y.; Sorrell, C.C.; Zhao, C.; Li, S. Unlocking efficiency: Minimizing energy loss in electrocatalysts for water splitting. Adv. Mater. 2024, 36, 2404658. [Google Scholar] [CrossRef]
- Du, Z.M.; Guo, C.P.; Li, C.R.; Zhang, W.J. Thermodynamic description of the Al-Mo and Al-Fe-Mo systems. J. Phase Equilib. Diffus. 2009, 30, 487–501. [Google Scholar] [CrossRef]
- Guillermet, A.F. The Fe-Mo (iron-molybdenum) system. Bull. Alloy Phase Diagr. 1982, 3, 359–367. [Google Scholar] [CrossRef]
- Yan, J.Y.; Liu, P.; Li, J.W.; Huang, H.; Tong, S.F.; Song, W.B. A bioinspired Fe/Mo bimetallic nitride catalyst for efficient electrochemical ammonia synthesis and Zn-nitrate battery. Chem. Eng. J. 2024, 498, 155108. [Google Scholar] [CrossRef]
- Li, S.B.; Hou, Y.Y.; Feng, G.; Li, Q.C.; Zhai, H.; Hua, Q.F.; Hu, R.M.; Xu, M.; Zhang, C.X.; Huang, Z.Q.; et al. High-entropy alloy nanoflower array electrodes with optimizable reaction pathways for low-voltage hydrogen production at industrial-grade current density. Adv. Mater. 2025, 37, 2416200. [Google Scholar] [CrossRef]
- Li, T.F.; Luo, G.; Liu, K.H.; Li, X.; Sun, D.M.; Xu, L.; Li, Y.F.; Tang, Y.W. Encapsulation of Ni3Fe nanoparticles in N-doped carbon nanotube–grafted carbon nanofibers as high-efficiency hydrogen evolution electrocatalysts. Adv. Funct. Mater. 2018, 28, 1805828. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Mao, J.N.; Zhou, H.; Xing, L.L.; Qian, S.S.; Yuan, J.L.; Mei, B.B.; Wei, Z.X.; Zhao, S.L.; Tang, Y.H.; et al. Coordination shell dependent activity of CuCo diatomic catalysts for oxygen reduction, oxygen evolution, and hydrogen evolution reaction. Adv. Funct. Mater. 2024, 34, 2311664. [Google Scholar] [CrossRef]
- Zhu, H.; Sun, S.H.; Hao, J.C.; Zhuang, Z.C.; Zhang, S.G.; Wang, T.D.; Kang, Q.; Lu, S.L.; Wang, X.F.; Lai, F.L.; et al. A high-entropy atomic environment converts inactive to active sites for electrocatalysis. Energy Environ. Sci. 2023, 16, 619–628. [Google Scholar] [CrossRef]
- Lyu, F.C.; Zeng, S.S.; Jia, Z.; Ma, F.X.; Sun, L.G.; Cheng, L.Z.; Pan, J.; Bao, Y.; Mao, Z.Y.; Bu, Y.; et al. Two-dimensional mineral hydrogel-derived single atoms-anchored heterostructures for ultrastable hydrogen evolution. Nat. Commun. 2022, 13, 6249. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, H.Y.; Zhang, L.F.; Xu, X.N.; Cheng, H.L.; Yan, C.L.; Qian, T. Evolution of grain boundaries promoted hydrogen production for industrial-grade current density. Adv. Mater. 2024, 36, 2313156. [Google Scholar] [CrossRef]
- Tan, X.Y.; Geng, S.Z.; Ji, Y.J.; Shao, Q.; Zhu, T.; Wang, P.T.; Li, Y.Y.; Huang, X.Q. Closest packing polymorphism interfaced metastable transition metal for efficient hydrogen evolution. Adv. Mater. 2020, 32, 2002857. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, X.Q.; Nie, Y.; Wang, R.H.; Zou, J.L. Electronic-state modulation of metallic Co-assisted Co7Fe3 alloy heterostructure for highly efficient and stable overall water splitting. Adv. Sci. 2023, 10, 2301961. [Google Scholar] [CrossRef]
- Kumar, A.; Bui, V.Q.; Lee, J.S.; Wang, L.L.; Jadhav, A.R.; Liu, X.H.; Shao, X.D.; Liu, Y.; Yu, J.M.; Hwang, Y.; et al. Moving beyond bimetallic-alloy to single-atom dimer atomic-interface for all-pH hydrogen evolution. Nat. Commun. 2021, 12, 6766. [Google Scholar] [CrossRef]
- Chen, Z.L.; Mebs, S.; Mondal, I.; Yang, H.Y.; Dau, H.; Kang, Z.H.; Haumann, M.; Ghosh, S.; Cen, W.L.; Driess, M.; et al. Hydrogen-induced disproportionation of samarium-cobalt intermetallics enabling promoted hydrogen evolution reaction activity and durability in alkaline media. Adv. Funct. Mater. 2024, 34, 2402699. [Google Scholar] [CrossRef]
- Mondal, S.; Dutta, S.; Mal, S.; Pati, S.K.; Bhattacharyya, S. Lattice mismatch guided nickel-indium heterogeneous alloy electrocatalysts for promoting the alkaline hydrogen evolution. Angew. Chem. Int. Ed. 2023, 62, e202301269. [Google Scholar] [CrossRef]
- Majee, R.; Kumar, A.; Das, T.; Chakraborty, S.; Bhattacharyya, S. Tweaking nickel with minimal silver in a heterogeneous alloy of decahedral geometry to deliver platinum-like hydrogen evolution activity. Angew. Chem. Int. Ed. 2020, 59, 2881–2889. [Google Scholar] [CrossRef]
- Manivelan, N.; Piao, J.J.; Kim, J.; Lee, S.; Kim, Y.; Soundharrajan, V.; Son, M.K.; Humayun, A.; Ganji, M.D.; Ko, H.; et al. Unveiling the aluminum doping effects of in-situ transmogrified dual-LDH heterostructure and its fermi-level alignment to water splitting potentials. Adv. Energy Mater. 2025, 15, 2403889. [Google Scholar] [CrossRef]
- Sivanantham, A.; Lee, H.; Hwang, S.W.; Lee, H.U.; Cho, S.B.; Ahn, B.; Cho, I.S. Complementary functions of vanadium in boosting electrocatalytic activity of CuCoNiFeMn high-entropy alloy for water splitting. Adv. Funct. Mater. 2023, 33, 2301153. [Google Scholar] [CrossRef]
- Li, Y.; Wei, X.F.; Chen, L.S.; Shi, J.L.; He, M.Y. Nickel-molybdenum nitride nanoplate electrocatalysts for concurrent electrolytic hydrogen and formate productions. Nat. Commun. 2019, 10, 5335. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Xu, L.; Huang, F.Z.; Qu, L.B.; Li, J.T.; Owusu, K.A.; Liu, Z.A.; Lin, Z.F.; Xiang, B.H.; Liu, X.; et al. Copper–nickel nitride nanosheets as efficient bifunctional catalysts for hydrazine-assisted electrolytic hydrogen production. Adv. Energy Mater. 2019, 9, 1900390. [Google Scholar] [CrossRef]
- Li, Y.K.; Zhang, G.; Lu, W.T.; Cao, F.F. Amorphous Ni–Fe–Mo suboxides coupled with Ni network as porous nanoplate array on nickel foam: A highly efficient and durable bifunctional electrode for overall water splitting. Adv. Sci. 2020, 7, 1902034. [Google Scholar] [CrossRef]
- An, Y.M.; Long, X.; Ma, M.; Hu, J.; Lin, H.; Zhou, D.; Xing, Z.; Huang, B.L.; Yang, S.H. One-step controllable synthesis of catalytic Ni4Mo/MoOx/Cu nanointerfaces for highly efficient water reduction. Adv. Energy Mater. 2019, 9, 1901454. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.-L.; Liu, Y.; Wang, Y.; Zeng, S.-P.; Shi, H.; Lang, X.-Y.; Jiang, Q. Intermetallic Fe2Mo Nanoparticles on Hierarchical Nanoporous Copper for Efficient Hydrogen Evolution Reaction. Catalysts 2025, 15, 278. https://doi.org/10.3390/catal15030278
Zhou Z-L, Liu Y, Wang Y, Zeng S-P, Shi H, Lang X-Y, Jiang Q. Intermetallic Fe2Mo Nanoparticles on Hierarchical Nanoporous Copper for Efficient Hydrogen Evolution Reaction. Catalysts. 2025; 15(3):278. https://doi.org/10.3390/catal15030278
Chicago/Turabian StyleZhou, Zhi-Lan, Yang Liu, Ying Wang, Shu-Pei Zeng, Hang Shi, Xing-You Lang, and Qing Jiang. 2025. "Intermetallic Fe2Mo Nanoparticles on Hierarchical Nanoporous Copper for Efficient Hydrogen Evolution Reaction" Catalysts 15, no. 3: 278. https://doi.org/10.3390/catal15030278
APA StyleZhou, Z.-L., Liu, Y., Wang, Y., Zeng, S.-P., Shi, H., Lang, X.-Y., & Jiang, Q. (2025). Intermetallic Fe2Mo Nanoparticles on Hierarchical Nanoporous Copper for Efficient Hydrogen Evolution Reaction. Catalysts, 15(3), 278. https://doi.org/10.3390/catal15030278





