Advances in the Enzymatic Synthesis of Nucleoside-5′-Triphosphates and Their Analogs
Abstract
1. Background
1.1. Nucleotides in Metabolism and Industrial Applications
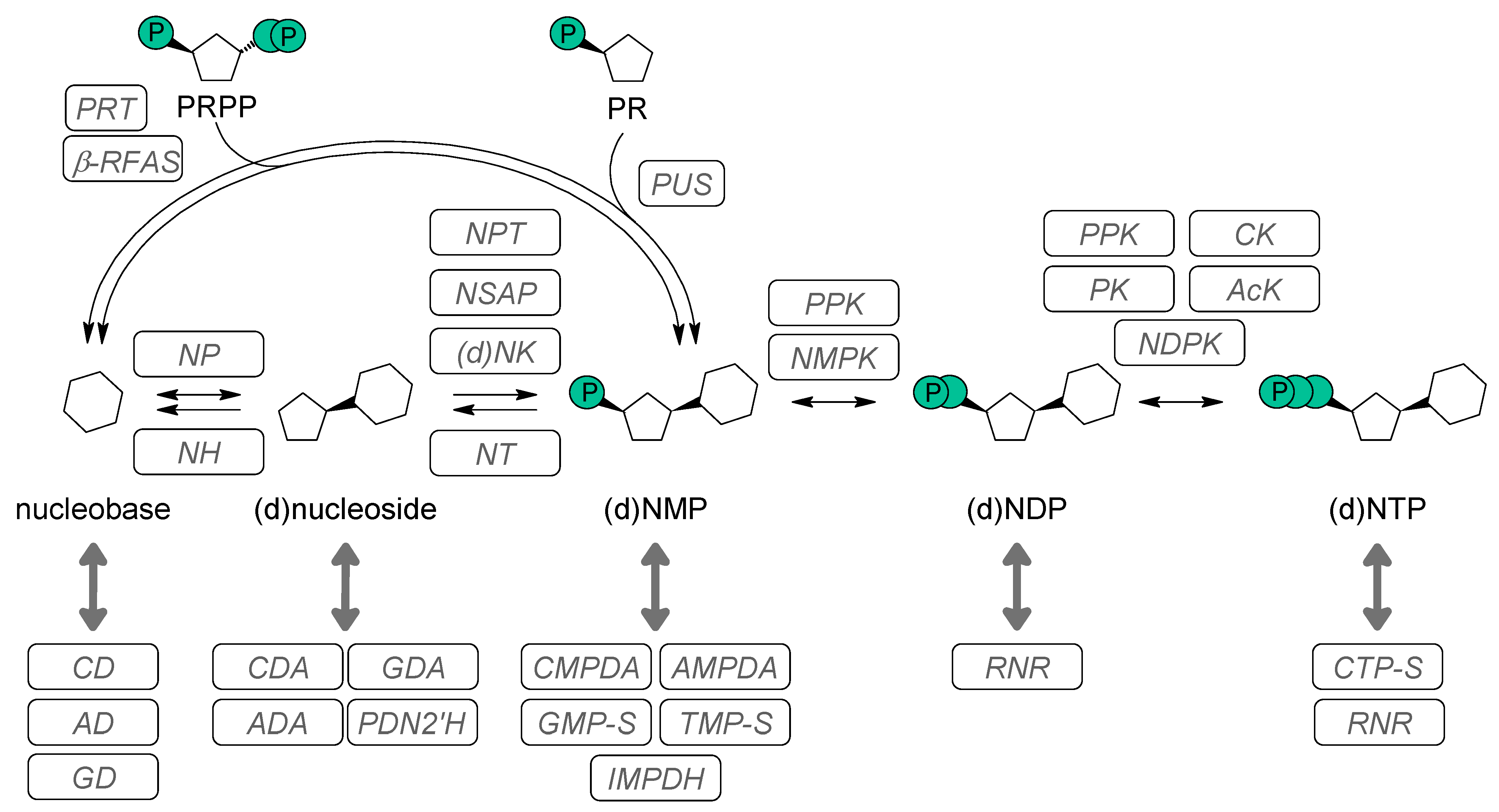
1.2. Enzymes Involved in Nucleotide Metabolism
1.2.1. Enzymes Involved in the Synthesis of Natural and Modified 5′-NMPs
1.2.2. Enzymes Synthesizing 5′-NDPs and Their Analogs
1.2.3. Enzymatic 5´-NTP Synthesis
1.2.4. Conversion of 5′-NMPs and 5′-NDPs to 5′-NTPs by Polyphosphate Kinases
1.3. Chemical Synthesis of NTPs and Their Analogs
2. Enzymatic Cascade Reactions to Produce Natural and Modified 5′-NTPs
2.1. Cascades Starting from Nucleosides and Using Nucleoside and NMP Kinases
2.2. Biocatalytic Synthesis of 5′-NTPs Starting from Nucleobases and Using NMP Kinases
2.3. Biocatalytic Synthesis of 5′-NTPs Using Polyphosphate Kinases
2.4. Other Interesting Approaches to Produce 5′-NTPs
3. Importance of ATP-Regeneration Systems in the Synthesis of 5′-NTPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AcK | acetate kinase |
| AD | adenine deaminase |
| ADA | adenosine deaminase |
| ADP | adenosine-5′-diphosphate |
| AjPPK | Acinetobacter johnsonii PPK |
| AMP | adenosine-5′-monophosphate |
| AMPDA | AMP deaminase |
| AprT | adenine PRT |
| AtADK | Arabidopsis thaliana adenosine kinase |
| ATP | adenosine-5′-triphosphate |
| ß-RFAS | 4-(D-ribofuranosyl)aminobenzene synthases |
| 5-BrU | 5-bromo uracil |
| 5-Br-dUTP | 5-bromo dUTP |
| CD | cytosine deaminase |
| CDA | cytidine deaminase |
| CDP | cytidine-5′-diphosphate |
| CK | creatine kinase |
| 6-Cl-P | 6-chloro purine |
| 2,6-Cl-P | 2,6-dichloropurine |
| 6-Cl-dPTP | 6-chloro purine 5′-triphosphate |
| 2,6-Cl-dPTP | 2,6-dichloropurine 5′-triphosphate |
| CMP | cytidine-5′-monophosphate |
| CMPDA | CMP deaminase |
| CTP | cytidine-5′-triphosphate |
| CTP-S | CTP synthase |
| 5-FU | 5-fluoro uracil |
| 5-F-dUTP | 5-fluoro dUTP |
| 2,6-D | 2,6-diamino purine |
| 5′-deoxyNTP/dNTP | deoxynucleoside-5′-triphosphate |
| DmdNK | deoxynucleoside kinase of Drosophila melanogaster |
| dNK | deoxynucleoside kinase |
| 2,6-dPTP | 2,6-diamino purine 5′-triphosphate |
| EcAPT | Escherichia coli adenine PRT |
| EcAPT | Escherichia coli hypoxanthine PRT |
| GD | guanine deaminase |
| GDA | guanosine deaminase |
| GDP | guanosine-5′-diphosphate |
| glyD | guanine PRT |
| GMP | guanosine-5′-monophosphate |
| GMPK | guanosine kinase |
| GMP-S | GMP synthase |
| GTP | guanosine-5′-triphosphate |
| IMP | inosine-5′-monophosphate |
| IMPDH | IMP dehydrogenase |
| LhPPK | Lampropedia hyalina PPK |
| MjNK | Methanocaldococcus jannaschii NK |
| MrPPK | Meiothermus ruber wildtype PPK |
| 5′-NDP | nucleoside-5′-diphosphate |
| NDPK | NDP kinases |
| NdT | nucleoside 2′-deoxyribosyltransferase |
| NH | nucleoside hydrolase |
| NK | nucleoside kinase |
| 5′-NMP | nucleoside-5′-monophosphate |
| NMPK | NMP kinase |
| NP | nucleoside phosphorylase |
| NPT | nucleoside phosphotransferase |
| NSAP | nonspecific acid phosphatase |
| NT | 5′-nucleotidase |
| 5′-NTP | nucleoside-5′-triphosphate |
| PCR | polymerase chain reaction |
| PDN2′H | pyrimidine nucleoside 2′-hydroxylase |
| PK | pyruvate kinase |
| ppGpp | guanosine tetraphosphate |
| PpnN | nucleosidase PpnN |
| PPK | polyphosphate kinase |
| pppGpp | guanosine pentaphosphate |
| PR | ribose-5′-phosphate |
| PRPP | 5-phospho-D-ribosyl-α-1-pyrophosphate |
| PrsA | PRPP synthase |
| PRT | phosphoribosyl transferase |
| PUS | pseudouridylate synthase |
| RbsK | ribokinase |
| RNR | ribonucleotide reductase |
| ScADK | Saccharomyces cerevisiae adenosine kinase |
| SlPPK | Sulfurovum lithotrophicum PPK |
| SmPPK | Sinorhizobium meliloti PPK |
| TMP | thymidine-5′-monophosphate |
| TMP-S | TMP synthase |
| TVNrdJm | RNR of Thermus virus TV74-23 |
| UDP | uridine-5′-diphosphate |
| UK | uridine kinase |
| UMP | uridine-5′-monophosphate |
| UraP | uracil PRT |
| UTP | uridine-5′-triphosphate |
| YeiN | pseudouridylate synthase |
References
- Berg, J.; Tymoczko, J.; Stryer, L. Biochemistry; W. H. Freeman Publishing: New York, NY, USA, 2002; p. 1050. [Google Scholar]
- Roy, B.; Depaix, A.; Périgaud, C.; Peyrottes, S. Recent Trends in Nucleotide Synthesis. Chem. Rev. 2016, 116, 7854–7897. [Google Scholar] [CrossRef] [PubMed]
- Traut, T.W. Physiological Concentrations of Purines and Pyrimidines. Mol. Cell Biochem. 1994, 140, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; Burnstock, G. THE DOUBLE LIFE OF ATP. Sci. Am. 2009, 301, 84. [Google Scholar] [CrossRef]
- Andexer, J.N.; Richter, M. Emerging Enzymes for ATP Regeneration in Biocatalytic Processes. ChemBioChem 2015, 16, 380–386. [Google Scholar] [CrossRef]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.-Y. Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Boraston, A.B. (P)PpGpp and the Stringent Response: An Emerging Threat to Antibiotic Therapy. ACS Infect. Dis. 2019, 5, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, F.; Maertens, J.; Beauprez, J.; Soetaert, W.; De Mey, M. Biotechnological Advances in UDP-Sugar Based Glycosylation of Small Molecules. Biotechnol. Adv. 2015, 33, 288–302. [Google Scholar] [CrossRef]
- Wagner, G.K.; Pesnot, T.; Field, R.A. A Survey of Chemical Methods for Sugar-Nucleotide Synthesis. Nat. Prod. Rep. 2009, 26, 1172–1194. [Google Scholar] [CrossRef]
- Hollenstein, M. Nucleoside Triphosphates—Building Blocks for the Modification of Nucleic Acids. Molecules 2012, 17, 13569–13591. [Google Scholar] [CrossRef]
- Jordheim, L.P.; Durantel, D.; Zoulim, F.; Dumontet, C. Advances in the Development of Nucleoside and Nucleotide Analogues for Cancer and Viral Diseases. Nat. Rev. Drug Discov. 2013, 12, 447–464. [Google Scholar] [CrossRef]
- Espinasse, A.; Lembke, H.K.; Cao, A.A.; Carlson, E.E. Modified Nucleoside Triphosphates in Bacterial Research for in Vitro and Live-Cell Applications. RSC Chem. Biol. 2020, 1, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.L.Y.; Schiess, G.H.A.; Pâ, C.; Miranda, M.; Weber, G.; Astakhova, K. Pseudouridine and N1-Methylpseudouridine as Potent Nucleotide Analogues for RNA Therapy and Vaccine Development. Chem. Biol. 2024, 5, 418. [Google Scholar] [CrossRef] [PubMed]
- Van Rompay, A.R.; Johansson, M.; Karlsson, A. Phosphorylation of Nucleosides and Nucleoside Analogs by Mammalian Nucleoside Monophosphate Kinases. Pharmacol. Ther. 2000, 87, 189–198. [Google Scholar] [CrossRef]
- Tsesmetzis, N.; Paulin, C.B.J.; Rudd, S.G.; Herold, N. Nucleobase and Nucleoside Analogues: Resistance and Re-Sensitisation at the Level of Pharmacokinetics, Pharmacodynamics and Metabolism. Cancers 2018, 10, 240. [Google Scholar] [CrossRef]
- Gollnest, T.; de Oliveira, T.D.; Rath, A.; Hauber, I.; Schols, D.; Balzarini, J.; Meier, C. Membrane-Permeable Triphosphate Prodrugs of Nucleoside Analogues. Angew. Chemie 2016, 128, 5341–5344. [Google Scholar] [CrossRef]
- Rachwalak, M.; Romanowska, J.; Sobkowski, M.; Stawinski, J. Nucleoside Di-and Triphosphates as a New Generation of Anti-HIV Pronucleotides. Chemical and Biological Aspects. Appl. Sci. 2021, 11, 2248. [Google Scholar] [CrossRef]
- Ricca, E.; Brucher, B.; Schrittwieser, J.H. Multi-Enzymatic Cascade Reactions: Overview and Perspectives. Adv. Synth. Catal. 2011, 353, 2239–2262. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Dong, Y. Lipid Nanoparticle-MRNA Formulations for Therapeutic Applications. Acc. Chem. Res. 2021, 54, 4283–4293. [Google Scholar] [CrossRef]
- Johansson, N.G.; Eriksson, S. Structure-Activity Relationships for Phosphorylation of Nucleoside Analogs to Monophosphates by Nucleoside Kinases. Acta Biochim. Polonica. 1996, 43, 143–160. [Google Scholar] [CrossRef]
- Zhang, Y.E.; Bærentsen, R.L.; Fuhrer, T.; Sauer, U.; Gerdes, K.; Brodersen, D.E. (P)PpGpp Regulates a Bacterial Nucleosidase by an Allosteric Two-Domain Switch. Mol. Cell 2019, 74, 1239–1249.e4. [Google Scholar] [CrossRef]
- Deville-Bonne, D.; El Amri, C.; Meyer, P.; Chen, Y.; Agrofoglio, L.A.; Janin, J. Human and Viral Nucleoside/Nucleotide Kinases Involved in Antiviral Drug Activation: Structural and Catalytic Properties. Antiviral Res. 2010, 86, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, S.; Munch-Petersen, B.; Johansson, K.; Ecklund, H. Structure and Function of Cellular Deoxyribonucleoside Kinases. Cell Mol. Life Sci. 2002, 59, 1327–1346. [Google Scholar] [CrossRef] [PubMed]
- Haynie, S.L.; Whitesides, G.M. Preparation of a Mixture of Nucleoside Triphosphates Suitable for Use in Synthesis of Nucleotide Phosphate Sugars from Ribonucleic Acid Using Nuclease P1, a Mixture of Nucleoside Monophosphokinases and Acetate Kinase. Appl. Biochem. Biotechnol. 1990, 23, 205–220. [Google Scholar] [CrossRef]
- Del Arco, J.; Fernandez-Lucas, J. Purine and Pyrimidine Phosphoribosyltransferases: A Versatile Tool for Enzymatic Synthesis of Nucleoside-5’-Monophosphates. Curr. Pharm. Des. 2017, 23, 6898–6912. [Google Scholar] [CrossRef]
- Ribar, A.; Pfeiffer, M.; Correspondence, B.N. Phosphorylation-Condensation Cascade for Biocatalytic Synthesis of C-Nucleosides. Chem. Catal. 2024, 4, 101127. [Google Scholar] [CrossRef]
- Bennett, L.L.; Allan, W.; Arnett, G.; Shealy, Y.F.; Shewach, D.S.; Mason, W.S.; Fourel, I.; Parker, W.B. Metabolism in Human Cells of the D and L Enantiomers of the Carbocyclic Analog of 2’-Deoxyguanosine: Substrate Activity with Deoxycytidine Kinase, Mitochondrial Deoxyguanosine Kinase, and 5’-Nucleotidase. Antimicrob. Agents Chemother. 1998, 42, 1045–1051. [Google Scholar] [CrossRef]
- Van Rompay, A.R.; Johansson, M.; Karlsson, A. Substrate Specificity and Phosphorylation of Antiviral and Anticancer Nucleoside Analogues by Human Deoxyribonucleoside Kinases and Ribonucleoside Kinases. Pharmacol. Ther. 2003, 100, 119–139. [Google Scholar] [CrossRef]
- Long, M.C.; Shaddix, S.C.; Moukha-Chafiq, O.; Maddry, J.A.; Nagy, L.; Parker, W.B. Structure-Activity Relationship for Adenosine Kinase from Mycobacterium Tuberculosis. II. Modifications to the Ribofuranosyl moiety. Biochem. Pharmacol. 2008, 75, 1588–1600. [Google Scholar] [CrossRef][Green Version]
- Arnfors, L.; Hansen, T.; Schönheit, P.; Ladenstein, R.; Meining, W. Structure of Methanocaldococcus jannaschii Nucleoside Kinase: An Archaeal Member of the Ribokinase Family. Acta Crystallogr. Sect. D Biol. Crystallogr. 2006, 62, 1085–1097. [Google Scholar] [CrossRef]
- Elkin, S.R.; Kumar, A.; Price, C.W.; Columbus, L. A Broad Specificity Nucleoside Kinase from Thermoplasma acidophilum. Proteins Struct. Funct. Bioinform. 2013, 81, 568–582. [Google Scholar] [CrossRef]
- Piškur, J.; Sandrini, M.P.B.; Knecht, W.; Munch-Petersen, B. Animal Deoxyribonucleoside Kinases: ‘Forward’ and ‘Retrograde’ Evolution of Their Substrate Specificity. FEBS Lett. 2004, 560, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.; Ramaswamy, S.; Ljungcrantz, C.; Knecht, W.; Piskur, J.; Munch-Petersen, B.; Eriksson, S.; Eklund, H. Structural Basis for Substrate Specificities of Cellular Deoxyribonucleoside Kinases. Nat. Struct. Biol. 2001, 8, 616–620. [Google Scholar] [CrossRef]
- Munch-Petersen, B.; Piskur, J.; Søndergaard, L. Four Deoxynucleoside Kinase Activities from Drosophila Melanogaster Are Contained within a Single Monomeric Enzyme, a New Multifunctional Deoxynucleoside Kinase. J. Biol. Chem. 1998, 273, 3926–3931. [Google Scholar] [CrossRef]
- Mikkelsen, N.E.; Munch-Petersen, B.; Eklund, H. Structural Studies of Nucleoside Analog and Feedback Inhibitor Binding to Drosophila Melanogaster Multisubstrate Deoxyribonucleoside Kinase. FEBS J. 2008, 275, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Serra, I.; Conti, S.; Piškur, J.; Clausen, A.R.; Munch-Petersen, B.; Terreni, M.; Ubiali, D. Immobilized Drosophila melanogaster Deoxyribonucleoside Kinase (DmdNK) as a High Performing Biocatalyst for the Synthesis of Purine Arabinonucleotides. Adv. Synth. Catal. 2014, 356, 563–570. [Google Scholar] [CrossRef]
- Arnér, E.S.J.; Eriksson, S. Mammalian Deoxyribonucleoside Kinases. Pharmacol. Ther. 1995, 67, 155–186. [Google Scholar] [CrossRef]
- Welin, M. Nordlund, Understanding Specificity in Metabolic Pathways-Structural Biology of Human Nucleotide Metabolism. Biochem. Biophys. Res. Commun. 2010, 396, 157–163. [Google Scholar] [CrossRef]
- Médici, R.; Garaycoechea, J.I.; Valino, A.L.; Pereira, C.A.; Lewkowicz, E.S.; Iribarren, A.M. A Comparative Study on Phosphotransferase Activity of Acid Phosphatases from Raoultella planticola and Enterobacter aerogenes on Nucleosides, Sugars, and Related Compounds. Appl. Microbiol. Biotechnol. 2014, 98, 3013–3022. [Google Scholar] [CrossRef]
- Valino, A.L.; Iribarren, A.M.; Lewkowicz, E. New Biocatalysts for One Pot Multistep Enzymatic Synthesis of Pyrimidine Nucleoside Diphosphates from Readily Available Reagents. J. Mol. Catal. B Enzym. 2015, 114, 58–64. [Google Scholar] [CrossRef]
- Zinchenko, I.; Barai, V.N.; Zalashko, L.M.; Poopeiko, N.E.; Sivets, G.G.; a Mikhailopulo, I.; Pricota, I. Enzymatic Synthesis of Nucleoside 5’-Mono and -Triphosphates. FEBS Lett. 1990, 260, 254–256. [Google Scholar] [CrossRef]
- Tasnádi, G.; Jud, W.; Hall, M.; Baldenius, K.; Ditrich, K.; Faber, K. Evaluation of Natural and Synthetic Phosphate Donors for the Improved Enzymatic Synthesis of Phosphate Monoesters. Adv. Synth. Catal. 2018, 360, 2394–2401. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Mihara, Y.; Yamada, H. A Novel Selective Nucleoside Phosphorylating Enzyme from Morganella morganii. J. Biosci. Bioeng. 1999, 87, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Zhang, L.; Sun, L.H.; Li, X.J.; Wan, N.W.; Zheng, Y.G. Enzymatic Production of 5′-Inosinic Acid by a Newly Synthesised Acid Phosphatase/Phosphotransferase. Food Chem. 2012, 134, 948–956. [Google Scholar] [CrossRef]
- Mihara, Y.; Utagawa, T.; Yamada, H.; Asano, Y. Phosphorylation of Nucleosides by the Mutated Acid Phosphatase from Morganella morganii. Appl. Environ. Microbiol. 2000, 66, 2811–2816. [Google Scholar] [CrossRef]
- Yuan, H.; fan Jia, Z.; hua He, J.; guang Fan, X.; Chen, N. Production of 5′-Inosinic Acid by Whole-Cell Biocatalyst Expressing a Mutated Acid Phosphatase/Phosphotransferase. In Lecture Notes in Electrical Engineering; Springer: Berlin/Heidelberg, Germany, 2018; Volume 444, pp. 605–614. [Google Scholar]
- Okuyama, K.; Shibuya, S.; Hamamoto, T.; Noguchi, T. Enzymatic Synthesis of 2′-Deoxyguanosine with Nucleoside Deoxyribosyltransferase-II. Biosci. Biotechnol. Biochem. 2003, 67, 989–995. [Google Scholar] [CrossRef]
- Genz, F.; Friedrich, F.; Lönarz, C.; Einsle, O.; Jung, M.; Mu, M.; Fessner, N.D. Identification and Characterization of Pyrimidine Nucleoside 2′-Hydroxylase. ACS Catal. 2024, 15, 3611–3618. [Google Scholar] [CrossRef]
- Panayiotou, C.; Solaroli, N.; Johansson, M.; Karlsson, A. Evidence of an Intact N-Terminal Translocation Sequence of Human Mitochondrial Adenylate Kinase 4. Int. J. Biochem. Cell Biol. 2010, 42, 62–69. [Google Scholar] [CrossRef]
- Nucleoside Triphospates and Their Analogs. Chemistry, Biotechnology, and Biological Applications, 1st ed.; Vaghefi, M., Ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Teplyakov, A.; Sebastiao, P.; Obmolova, G.; Perrakis, A.; Brush, G.S.; Bessman, M.J.; Wilson, K.S. Crystal Structure of Bacteriophage T4 Deoxynucleotide Kinase with Its Substrates DGMP and ATP. EMBO J. 1996, 15, 3487–3497. [Google Scholar] [CrossRef]
- Caillat, C.; Topalis, D.; Agrofoglio, L.A.; Pochet, S.; Balzarini, J.; Deville-Bonne, D.; Meyer, P. Crystal Structure of Poxvirus Thymidylate Kinase: An Unexpected Dimerization Has Implications for Antiviral Therapy. Proc. Natl. Acad. Sci. USA 2008, 105, 16900–16905. [Google Scholar] [CrossRef]
- Torrents, E.; Aloy, P.; Gibert, I.; Rodríguez-Trelles, F. Ribonucleotide Reductases: Divergent Evolution of an Ancient Enzyme. J. Mol. Evol. 2002, 55, 138–152. [Google Scholar] [CrossRef]
- Gonin, P.; Xu, Y.; Milon, L.; Dabernat, S.; Morr, M.; Kumar, R.; Lacombe, M.L.; Janin, J.; Lascu, I. Catalytic Mechanism of Nucleoside Diphosphate Kinase Investigated Using Nucleotide Analogues, Viscosity Effects, and x-Ray Crystallography. Biochemistry 1999, 38, 7265–7272. [Google Scholar] [CrossRef] [PubMed]
- Cherfils, J.; Moréra, S.; Janin, J.; Lascu, I.; Véron, M. X-Ray Structure of Nucleoside Diphosphate Kinase Complexed with Thymidine Diphosphate and Mg2+ at 2-Å Resolution. Biochemistry 1994, 33, 9062–9069. [Google Scholar] [CrossRef]
- Eckstein, F.; Goody, R.S. Synthesis and Properties of Diastereoisomers of Adenosine 5′-(O-1-Thiotriphosphate) and Adenosine 5′-(O-2-Thiotriphosphate). Biochemistry 1976, 15, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Peliska, J.A.; O’Leary, M.H. Sulfuryl Transfer Catalyzed by Phosphokinases. Biochemistry 1991, 30, 1049–1057. [Google Scholar] [CrossRef]
- Kamiya, H.; Kasai, H. Preparation of 8-Hydroxy-DGTP and 2-Hydroxy-DATP by a Phosphate Transfer Reaction by Nucleoside-Diphosphate Kinase. Nucleosides Nucleotides 1999, 18, 307–310. [Google Scholar] [CrossRef]
- Wu, W.; Bergstrom, D.E.; Davisson, V.J. A Combination Chemical and Enzymatic Approach for the Preparation of Azole Carboxamide Nucleoside Triphosphate. J. Org. Chem. 2003, 68, 3860–3865. [Google Scholar] [CrossRef]
- Imazawa, M.; Eckstein, F. Synthesis of Sugar-Modified Nucleoside 5’-Triphosphates with Partially Purified Nucleotide Kinases from Calf Thymus. Biochim. Biophys. Acta 1979, 570, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Bao, J.; Gu, X.; Xin, X.; Chen, C.; Ryu, D.D.Y. Substrate Promiscuity of Pyruvate Kinase on Various Deoxynucleoside Diphosphates for Synthesis of Deoxynucleoside Triphosphates. Enzyme Microb. Technol. 2008, 43, 455–459. [Google Scholar] [CrossRef]
- Kim, M.J.; Whitesides, G.M. Enzyme-Catalyzed Synthesis of Nucleoside Triphosphates from Nucleoside Monophosphates–ATP from AMP and Ribavirin 5’-Triphosphate from Ribavirin 5’-Monophosphate. Appl. Biochem. Biotechnol. 1987, 16, 95–108. [Google Scholar] [CrossRef]
- Cardeilhac, T.; Cohen, S.S. Some Metabolic Properties of Nucleotides of 1-Beta-D-Arabinofuranosylcytosine. Cancer Res. 1964, 24, 1595–1603. [Google Scholar]
- Da Costa, C.P.; Fedor, M.J.; Scott, L.G. 8-Azaguanine Reporter of Purine Ionization States in Structured RNAs. J. Am. Chem. Soc. 2007, 129, 3426–3432. [Google Scholar] [CrossRef] [PubMed]
- Shoshani, I.; Boudou, V.; Pierra, C.; Gosselin, G.; Johnson, R.A. Enzymatic Synthesis of Unlabeled and Beta-(32)P-Labeled Beta-L-2’, 3’-Dideoxyadenosine-5’-Triphosphate as a Potent Inhibitor of Adenylyl Cyclases and Its Use as Reversible Binding Ligand. J. Biol. Chem. 1999, 274, 34735–34741. [Google Scholar] [CrossRef][Green Version]
- Scott, L.G.; Geierstanger, B.H.; Williamson, J.R.; Hennig, M. Enzymatic Synthesis and 19 F NMR Studies of 2-Fluoroadenine-Substituted RNA. J. Am. Chem. Soc. 2004, 126, 11776–11777. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Inouye, M. Adenylate Kinase Complements Nucleoside Diphosphate Kinase Deficiency in Nucleotide Metabolism. Proc. Natl. Acad. Sci. USA 1996, 93, 5720–5725. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Conserva, F.; Panayiotou, C.; Karlsson, A.; Solaroli, N. The Human Adenylate Kinase 9 Is a Nucleoside Mono- and Diphosphate Kinase. Int. J. Biochem. Cell Biol. 2013, 45, 925–931. [Google Scholar] [CrossRef]
- Pospísilová, H.; Sebela, M.; Novák, O.; Frébort, I. Hydrolytic Cleavage of N6-Substituted Adenine Derivatives by Eukaryotic Adenine and Adenosine Deaminases. Biosci. Rep. 2008, 28, 335–347. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.H.P.J. Enzymatic Regeneration and Conservation of ATP: Challenges and Opportunities. Crit. Rev. Biotechnol. 2020, 41, 16–33. [Google Scholar] [CrossRef]
- Tavanti, M.; Hosford, J.; Lloyd, R.C.; Brown, M.J.B. ATP Regeneration by a Single Polyphosphate Kinase Powers Multigram-Scale Aldehyde Synthesis in Vitro. Green. Chem. 2021, 23, 828–837. [Google Scholar] [CrossRef]
- Motomura, K.; Hirota, R.; Okada, M.; Ikeda, T.; Ishida, T.; Kuroda, A. A New Subfamily of Polyphosphate Kinase 2 (Class III PPK2) Catalyzes Both Nucleoside Monophosphate Phosphorylation and Nucleoside Diphosphate Phosphorylation. Appl. Environ. Microbiol. 2014, 80, 2602–2608. [Google Scholar] [CrossRef]
- Mordhorst, S.; Andexer, J.N. Round, Round We Go–Strategies for Enzymatic Cofactor Regeneration. Nat. Prod. Rep. 2020, 37, 1316–1333. [Google Scholar] [CrossRef]
- Kornberg, S.R. Adenosine Triphosphate Synthesis from Polyphosphate by an Enzyme from Escherichia Coli. BBA Biochim. Biophys. Acta 1957, 26, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Shiba, T. Use of Escherichia Coli Polyphosphate Kinase for Oligosaccharide Synthesis. Biosci. Biotechnol. Biochem. 1998, 62, 1594–1596. [Google Scholar] [CrossRef]
- Kuroda, A.; Kornberg, A. Polyphosphate Kinase as a Nucleoside Diphosphate Kinase in Escherichia Coli and Pseudomonas Aeruginosa. Proc. Natl. Acad. Sci. USA 1997, 94, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Hildenbrand, J.C.; Teleki, A.; Jendrossek, D. A Universal Polyphosphate Kinase: PPK2c of Ralstonia Eutropha Accepts Purine and Pyrimidine Nucleotides Including Uridine Diphosphate. Appl. Microbiol. Biotechnol. 2020, 104, 6659–6667. [Google Scholar] [CrossRef] [PubMed]
- Kulmer, S.T.; Gutmann, A.; Lemmerer, M.; Nidetzky, B. Biocatalytic Cascade of Polyphosphate Kinase and Sucrose Synthase for Synthesis of Nucleotide-Activated Derivatives of Glucose. Adv. Synth. Catal. 2017, 359, 292–301. [Google Scholar] [CrossRef]
- Benčić, P.; Keppler, M.; Kuge, M.; Qiu, D.; Schütte, L.M.; Häner, M.; Strack, K.; Jessen, H.J.; Andexer, J.N.; Loenarz, C. Non-Canonical Nucleosides: Biomimetic Triphosphorylation, Incorporation into MRNA and Effects on Translation and Structure. FEBS J. 2023, 290, 4899–4920. [Google Scholar] [CrossRef]
- Querengässer, T.; Wenzlaff, J.; Loderer, C. Broadening the Substrate Scope of a Polyphosphate Kinase for Canonical and Non-Canonical Nucleotides. ChemCatChem 2024, 16, e202400181. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Kato, T.; Takenishi, T. A Novel Method for Phosphorylation of Nucleosides to 5′-Nucleotides. Tetrahedron Lett. 1967, 50, 5065–5068. [Google Scholar] [CrossRef]
- Ludwig, J.; Eckstein, F. Rapid and Efficient Synthesis of Nucleoside 5′-O-(1-Thiotriphosphates), 5′-Triphosphates and 2′,3′-Cyclophosphorothioates Using 2-Chloro-4H-1,3,2-Benzodioxaphosphorin-4-One. J. Org. Chem. 1989, 54, 631–635. [Google Scholar] [CrossRef]
- Kovács, T.; Ötvös, L. Simple Synthesis of 5-Vinyl- and 5-Ethynyl-2′-Deoxyuridine-5′-Triphosphates. Tetrahedron Lett. 1988, 29, 4525–4528. [Google Scholar] [CrossRef]
- Gillerman, I.; Fischer, B. An Improved One-Pot Synthesis of Nucleoside 5′-Triphosphate Analogues. Nucleosides Nucleotides Nucleic Acids 2010, 29, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Caton-Williams, J.; Smith, M.; Carrasco, N.; Huang, Z. Protection-Free One-Pot Synthesis of 2 0-Deoxynucleoside 5 0-Triphosphates and DNA Polymerization. Org. Lett. 2011, 4, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Kore, A.; Srinivasan, B. Recent Advances in the Syntheses of Nucleoside Triphosphates. Curr. Org. Synth. 2014, 10, 903–934. [Google Scholar] [CrossRef]
- Burgess, K.; Cook, D. Syntheses of Nucleoside Triphosphates. Chem. Rev. 2000, 100, 2047–2059. [Google Scholar] [CrossRef]
- Johnson, D.C.; Widlanski, T.S. Overview of the Synthesis of Nucleoside Phosphates and Polyphosphates. Curr. Protoc. Nucleic Acid. Chem. 2004, 15, 13.1.1–13.1.31. [Google Scholar] [CrossRef]
- Rozzell, J.D. Commercial Scale Biocatalysis: Myths and Realities. Bioorg Med. Chem. 1999, 7, 2253–2261. [Google Scholar] [CrossRef]
- Fehlau, M.; Kaspar, F.; Hellendahl, K.F.; Schollmeyer, J.; Neubauer, P.; Wagner, A. Modular Enzymatic Cascade Synthesis of Nucleotides Using a (d)ATP Regeneration System. Front. Bioeng. Biotechnol. 2020, 8, 854. [Google Scholar]
- Baughn, R.L.; Adalsteinsson, O.; Whitesides, G.M. Large-Scale Enzyme-Catalyzed Synthesis of ATP from Adenosine and Acetyl Phosphate. Regeneration of ATP from AMP. J. Am. Chem. Soc. 1978, 100, 304–306. [Google Scholar] [CrossRef]
- Hennig, M.; Scott, L.G.; Sperling, E.; Bermel, W.; Williamson, J.R. Synthesis of 5-Fluoropyrimidine Nucleotides as Sensitive NMR Probes of RNA Structure. J. Am. Chem. Soc. 2007, 129, 14911–14921. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Nidetzky, B. Biocatalytic Cascade Transformations for the Synthesis of C-Nucleosides and N-Nucleoside Analogs. Curr. Opin. Biotechnol. 2023, 79, 102873. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Nidetzky, B. Reverse C-Glycosidase Reaction Provides C-Nucleotide Building Blocks of Xenobiotic Nucleic Acids. Nat. Commun. 2020, 11, 6270. [Google Scholar] [CrossRef] [PubMed]
- Frisch, J.; Maršić, T.; Loderer, C. A Novel One-Pot Enzyme Cascade for the Biosynthesis of Cladribine Triphosphate. Biomolecules 2021, 11, 346. [Google Scholar] [CrossRef]
- Eltoukhy, L.; Loderer, C. A Multi-Enzyme Cascade for the Biosynthesis of AICA Ribonucleoside Di- and Triphosphate. ChemBioChem 2022, 23, e202100596. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, Z.; Ning, X.; Xu, W.; Li, Z. In Vitro Biosynthesis of ATP from Adenosine and Polyphosphate. Bioresour. Bioprocess. 2021, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Nikel, P.; Andexer, J.N.; Lütz, S.; Rosenthal, K. A Multi-Enzyme Cascade Reaction for the Production of 2′3′-CGAMP. Biomolecules 2021, 11, 590. [Google Scholar] [CrossRef]
- Becker, M.; Nowak, I.; Hildebrand, K.; Lütz, S.; Rosenthal, K. Development of a Multi-Enzyme Cascade for 2′3′-CGAMP Synthesis from Nucleosides. Catal. Sci. Technol. 2024, 14, 3335–3345. [Google Scholar] [CrossRef]
- Mordhorst, S.; Siegrist, J.; Müller, M.; Richter, M.; Andexer, J.N. Catalytic Alkylation Using a Cyclic S-Adenosylmethionine Regeneration System. Angew. Chemie Int. Ed. 2017, 56, 4037–4041. [Google Scholar] [CrossRef]
- Popadić, D.; Mhaindarkar, D.; Dang Thai, M.H.N.; Hailes, H.C.; Mordhorst, S.; Andexer, J.N. A Bicyclic S-Adenosylmethionine Regeneration System Applicable with Different Nucleosides or Nucleotides as Cofactor Building Blocks. RSC Chem. Biol. 2021, 2, 883–891. [Google Scholar] [CrossRef]
- Schultheisz, H.; Szymczyna, B.; Scott, L.; Williamson, J. Pathway Engineered Enzymatic de Novo Purine Nucleotide Synthesis. ACS Chem. Biol. 2008, 5, 313–320. [Google Scholar] [CrossRef]
- Schultheisz, H.L.; Szymczyna, B.R.; Scott, L.G.; Williamson, J.R. Enzymatic de Novo Pyrimidine Nucleotide Synthesis. J. Am. Chem. Soc. 2010, 133, 297–304. [Google Scholar] [CrossRef]
- Kaminski, A.; Labesse, G. Phosphodeoxyribosyltransferases, Designed Enzymes for Deoxyribonucleotides Synthesis. J. Biol. Chem. 2013, 288, 6534–6541. [Google Scholar] [CrossRef]
- Siedentop, R.; Prenzel, T.; Waldvogel, S.R.; Rosenthal, K.; Lütz, S. Reaction Engineering and Comparison of Electroenzymatic and Enzymatic ATP Regeneration Systems. ChemElectroChem 2023, 10, e202300332. [Google Scholar] [CrossRef]
- Chenault, H.K.; Simon, E.S.; Whitesides, G. Cofactor Regeneration for Enzyme-Catalysed Synthesis. Biotechnol. Genet. Eng. Rev. 1988, 6, 221–270. [Google Scholar] [CrossRef]
- Ruccolo, S.; Brito, G.; Christensen, M.; Itoh, T.; Mattern, K.; Stone, K.; Strotman, N.A.; Sun, A.C. Electrochemical Recycling of Adenosine Triphosphate in Biocatalytic Reaction Cascades. J. Am. Chem. Soc. 2022, 144, 22582–22588. [Google Scholar] [CrossRef] [PubMed]
- Ishige, K.; Noguchi, T. Inorganic Polyphosphate Kinase and Adenylate Kinase Participate in the Polyphosphate:AMP Phosphotransferase Activity of Escherichia Coli. Proc. Natl. Acad. Sci. USA 2002, 97, 14168–14171. [Google Scholar] [CrossRef]
- Scism, R.A.; Bachmann, B.O. Five-Component Cascade Synthesis of Nucleotide Analogues in an Engineered Self-Immobilized Enzyme Aggregate. ChemBioChem 2010, 11, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.L.; Hahn, T.M.; Reynolds, K.K.; Shewach, D.S. Kinetic Analysis of Human Deoxycytidine Kinase with the True Phosphate Donor Uridine Triphosphate. Biochemistry 1997, 36, 7540–7547. [Google Scholar] [CrossRef]
- Li, Z.; Ning, X.; Zhao, Y.; Zhang, X.; Xiao, C.; Li, Z. Efficient One-Pot Synthesis of Cytidine 5′-Monophosphate Using an Extremophilic Enzyme Cascade System. J. Agric. Food Chem. 2020, 68, 9188–9194. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Zhu, J. Enzymatic Production of L-Theanine by γ-Glutamylmethylamide Synthetase Coupling with an ATP Regeneration System Based on Polyphosphate Kinase. Process Biochem. 2016, 51, 1458–1463. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fehlau, M.; Westarp, S.; Neubauer, P.; Kurreck, A. Advances in the Enzymatic Synthesis of Nucleoside-5′-Triphosphates and Their Analogs. Catalysts 2025, 15, 270. https://doi.org/10.3390/catal15030270
Fehlau M, Westarp S, Neubauer P, Kurreck A. Advances in the Enzymatic Synthesis of Nucleoside-5′-Triphosphates and Their Analogs. Catalysts. 2025; 15(3):270. https://doi.org/10.3390/catal15030270
Chicago/Turabian StyleFehlau, Maryke, Sarah Westarp, Peter Neubauer, and Anke Kurreck. 2025. "Advances in the Enzymatic Synthesis of Nucleoside-5′-Triphosphates and Their Analogs" Catalysts 15, no. 3: 270. https://doi.org/10.3390/catal15030270
APA StyleFehlau, M., Westarp, S., Neubauer, P., & Kurreck, A. (2025). Advances in the Enzymatic Synthesis of Nucleoside-5′-Triphosphates and Their Analogs. Catalysts, 15(3), 270. https://doi.org/10.3390/catal15030270










