Two-Step Tandem Synthesis of Coumarin Derivatives Containing Bioamide Skeleton Catalyzed by Lipozyme TL IM from Thermomyces lanuginosus in Sustainable Continuous-Flow Microreactors
Abstract
1. Introduction
2. Results
2.1. Synthesis of Intermediate Coumarin Carboxylic Acid Methyl Ester Derivatives
2.2. Effect of Reaction Solvent and Catalyst
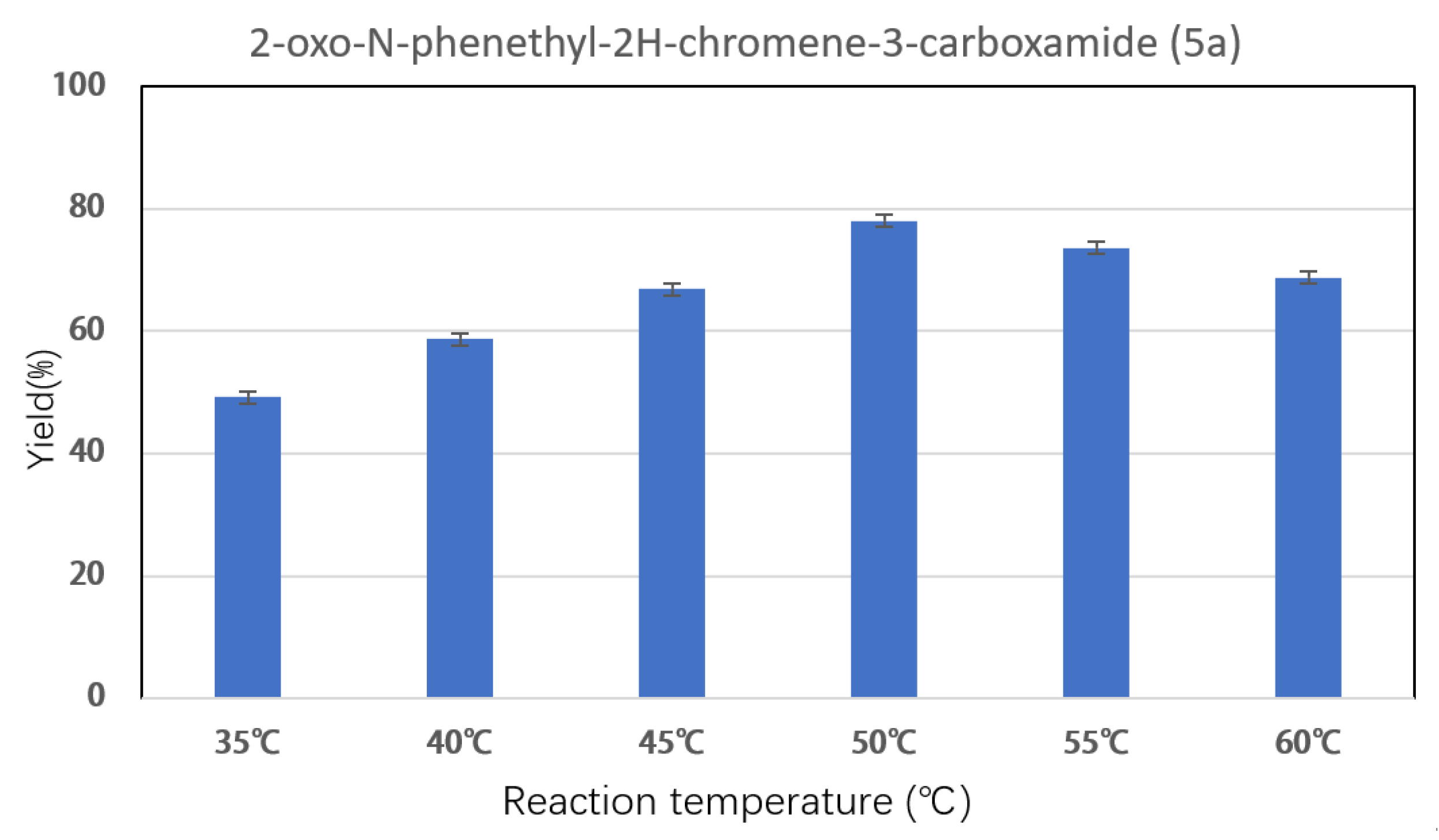
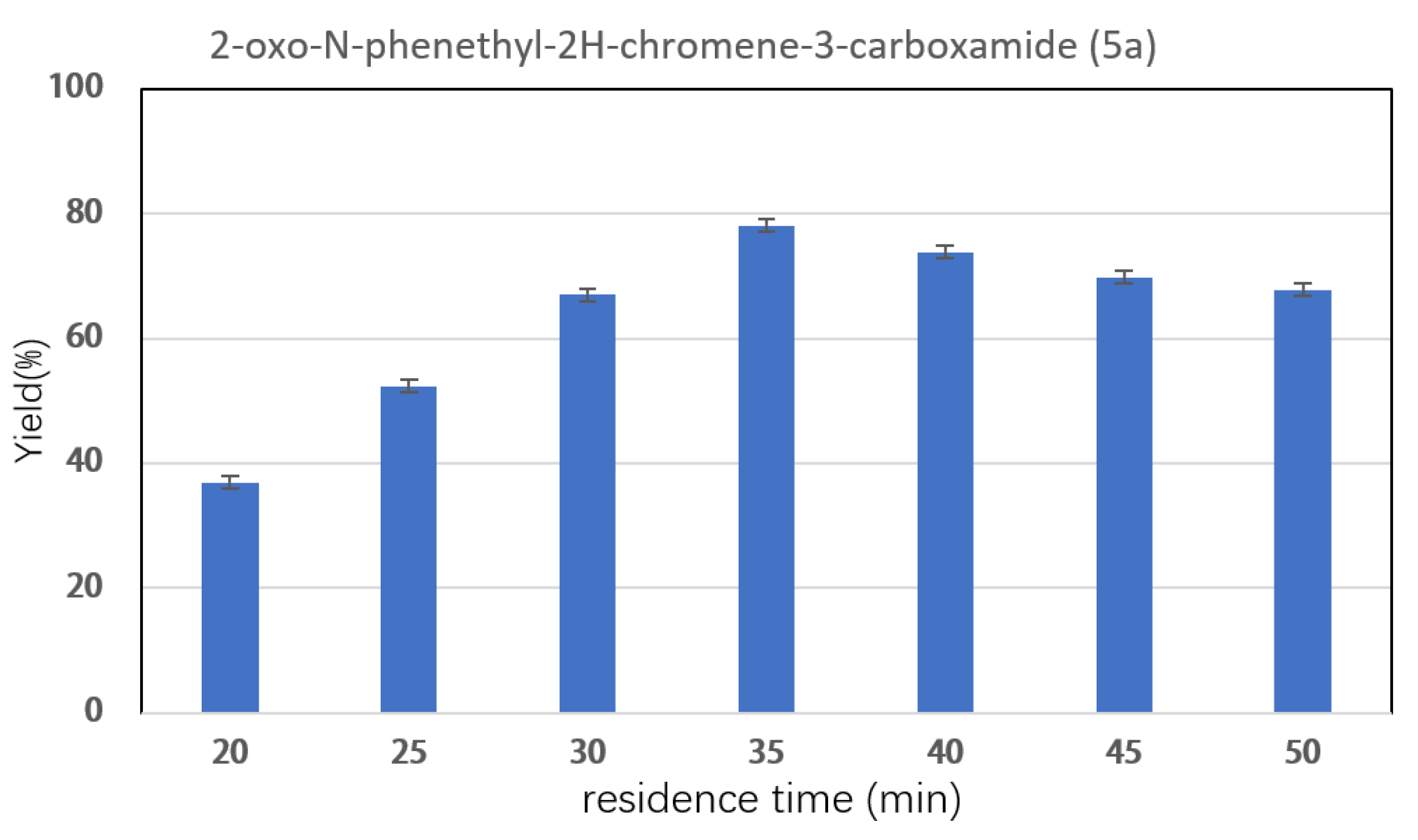
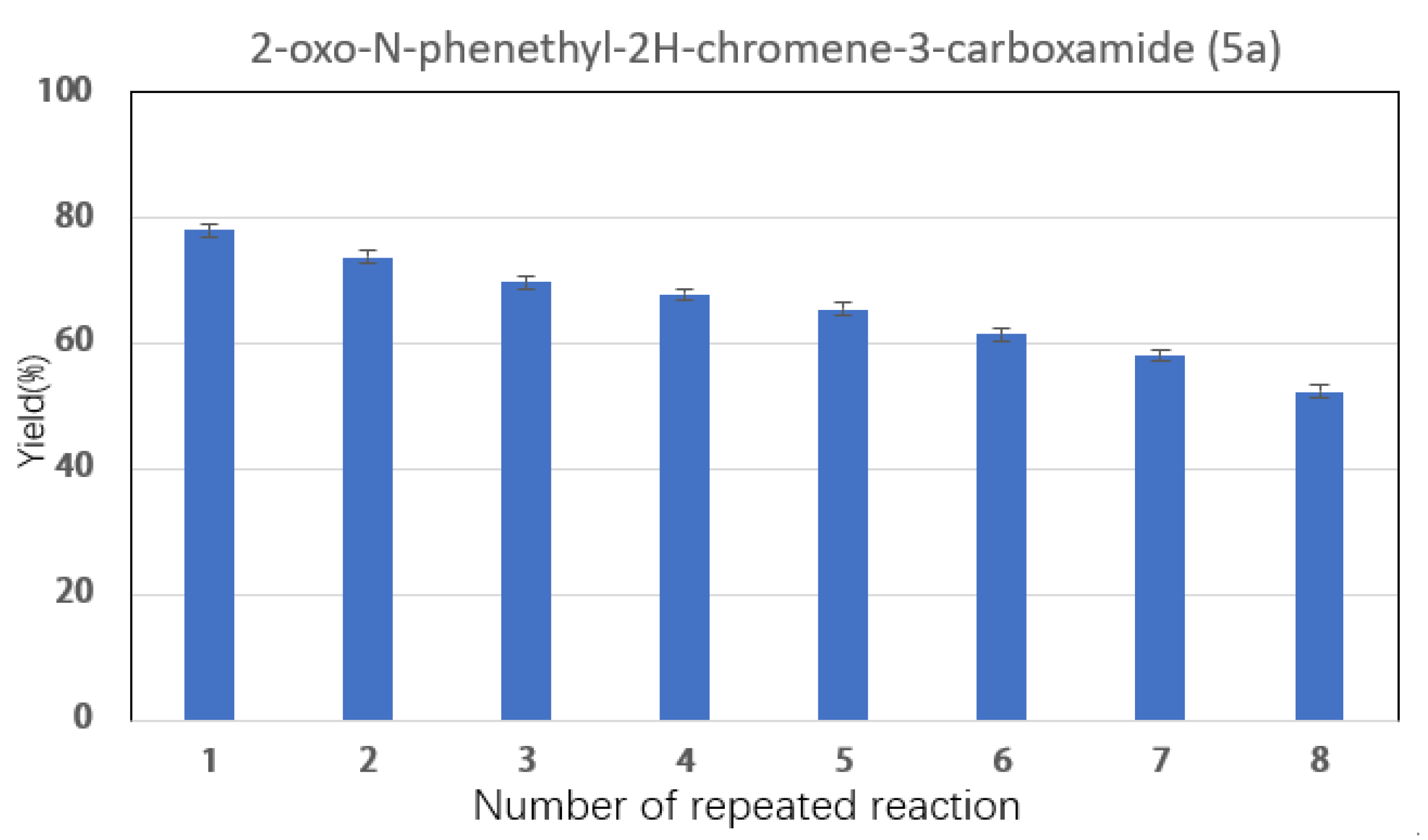
2.3. Effect of Reaction Parameters on Synthesis of Coumarin Bioamide Derivatives
3. Materials and Methods
3.1. Materials
3.2. Experimental Setup and Experiment Conditions
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yadav, A.K.; Shrestha, R.M.; Yadav, P.N. Anticancer mechanism of coumarin-based derivatives. Eur. J. Med. Chem. 2024, 267, 116179. [Google Scholar] [CrossRef] [PubMed]
- Dandriyal, J.; Singla, R.; Kumar, M.; Jaitak, V. Recent developments of C-4 substituted coumarin derivatives as anticancer agents. Eur. J. Med. Chem. 2016, 119, 141–168. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.L.; Zhang, Z.W.; Ravindar, L.; Rakesh, K.P. Antibacterial activities with the structure-activity relationship of coumarin derivatives. Eur. J. Med. Chem. 2020, 207, 112832. [Google Scholar] [CrossRef] [PubMed]
- Hussein, D.M.; Al-Juboory, S.B.; Mahmood, A.A.R. Synthesis, Characterization and Antibacterial Evaluation With Computational Study of New Schiff Bases Derived from 7-Hydroxy-4-Methyl Coumarin. Orient. J. Chem. 2017, 33, 768–782. [Google Scholar] [CrossRef][Green Version]
- Elias, R.; Benhamou, R.I.; Jaber, Q.Z.; Dorot, O.; Zada, S.L.; Oved, K.; Pichinuk, E.; Fridman, M. Antifungal activity, mode of action variability, and subcellular distribution of coumarin-based antifungal azoles. Eur. J. Med. Chem. 2019, 179, 779–790. [Google Scholar] [CrossRef]
- Simijonovic, D.M.; Milenkovic, D.A.; Avdovic, E.H.; Milanovic, Z.B.; Antonijevic, M.R.; Amic, A.D.; Dolicanin, Z.; Markovic, Z.S. Coumarin N-Acylhydrazone Derivatives: Green Synthesis and Antioxidant Potential—Experimental and Theoretical Study. Antioxidants 2023, 12, 1858. [Google Scholar] [CrossRef]
- Dabhi, R.C.; Sharma, V.S.; Arya, P.S.; Patel, U.P.; Shrivastav, P.S.; Maru, J.J. Coumarin functionalized dimeric mesogens for promising anticoagulant activity: Tuning of liquid crystalline property. J. Mol. Struct. 2023, 1283, 135336. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. Coumarin-containing hybrids and their anticancer activities. Eur. J. Med. Chem. 2019, 181, 111587. [Google Scholar] [CrossRef]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef]
- Chen, L.Z.; Sun, W.W.; Bo, L.; Wang, J.Q.; Xiu, C.; Tang, W.J.; Shi, J.B.; Zhou, H.P.; Liu, X.H. New arylpyrazoline-coumarins: Synthesis and anti-inflammatory activity. Eur. J. Med. Chem. 2017, 138, 170–181. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, Y.; Wu, X.; Tu, X.; Wang, G.X. Synthesis and biological evaluation of coumarin derivatives containing imidazole skeleton as potential antibacterial agents. Eur. J. Med. Chem. 2018, 143, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.L.; Ke, X.; Liu, M. Coumarin–triazole Hybrids and Their Biological Activities. J. Heterocycl. Chem. 2018, 55, 791–802. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B.; Singh, N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012, 20, 1175–1180. [Google Scholar] [CrossRef]
- Khoobi, M.; Alipour, M.; Sakhteman, A.; Nadri, H.; Moradi, A.; Ghandi, M.; Emami, S.; Foroumadi, A.; Shafiee, A. Design, synthesis, biological evaluation and docking study of 5-oxo-4,5-dihydropyrano[3,2-c]chromene derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors. Eur. J. Med. Chem. 2013, 68, 260–269. [Google Scholar] [CrossRef]
- Asadipour, A.; Alipour, M.; Jafari, M.; Khoobi, M.; Emami, S.; Nadri, H.; Sakhteman, A.; Moradi, A.; Sheibani, V.; Homayouni Moghadam, F.; et al. Novel coumarin-3-carboxamides bearing N-benzylpiperidine moiety as potent acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2013, 70, 623–630. [Google Scholar] [CrossRef]
- Razavi, S.F.; Khoobi, M.; Nadri, H.; Sakhteman, A.; Moradi, A.; Emami, S.; Foroumadi, A.; Shafiee, A. Synthesis and evaluation of 4-substituted coumarins as novel acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2013, 64, 252–259. [Google Scholar] [CrossRef]
- Alipour, M.; Khoobi, M.; Moradi, A.; Nadri, H.; Homayouni Moghadam, F.; Emami, S.; Hasanpour, Z.; Foroumadi, A.; Shafiee, A. Synthesis and anti-cholinesterase activity of new 7-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2014, 82, 536–544. [Google Scholar] [CrossRef]
- Bagheri, S.M.; Khoobi, M.; Nadri, H.; Moradi, A.; Emami, S.; Jalili-Baleh, L.; Jafarpour, F.; Homayouni Moghadam, F.; Foroumadi, A.; Shafiee, A. Synthesis and anticholinergic activity of 4-hydroxycoumarin derivatives containing substituted benzyl-1,2,3-triazole moiety. Chem. Biol. Drug Des. 2015, 86, 1215–1220. [Google Scholar] [CrossRef]
- Ghanei-Nasab, S.; Khoobi, M.; Hadizadeh, F.; Marjani, A.; Moradi, A.; Nadri, H.; Emami, S.; Foroumadi, A.; Shafiee, A. Synthesis and anticholinesterase activity of coumarin-3-carboxamides bearing tryptamine moiety. Eur. J. Med. Chem. 2016, 121, 40–46. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.W.; Shi, Y.; Song, Z.G.; Jin, Y.H.; Liu, Z.Q. Design and synthesis of coumarin-3-acylamino derivatives to scavenge radicals and to protect DNA. Eur. J. Med. Chem. 2014, 84, 1–7. [Google Scholar] [CrossRef]
- Awale, S.; Okada, T.; Dibwe, D.F.; Maruyama, T.; Takahara, S.; Okada, T.; Endo, S.; Toyooka, N. Design and synthesis of functionalized coumarins as potential anti-austerity agents that eliminates cancer cells’ tolerance to nutrition starvation. Bioorg. Med. Chem. Lett. 2019, 29, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Hooda, V.; Gahlaut, A.; Gothwal, A.; Hooda, V. Enzymatic biosensors for the quantification of biogenic amines: A literature update. Crit. Rev. Biotechnol. 2020, 40, 1–14. [Google Scholar] [CrossRef]
- Onal, A.; Tekkeli, S.E.; Onal, C. A review of the liquid chromatographic methods for the determination of biogenic amines in foods. Food Chem. 2013, 138, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Ramani, D.; De Bandt, J.P.; Cynober, L. Aliphatic polyamines in physiology and diseases. Clin. Nutr. 2014, 33, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Macreadie, I.G. Tyramine and amyloid beta 42: A toxic synergy. Alzheimer’s Dement. 2020, 8, 145. [Google Scholar] [CrossRef]
- Petchey, M.R.; Grogan, G. Enzyme-Catalysed Synthesis of Secondary and Tertiary Amides. Adv. Synth. Catal. 2019, 361, 3895–3914. [Google Scholar] [CrossRef]
- Barak, D.S.; Batra, S. Direct Access to Amides from Nitro-Compounds via Aminocarbonylation and Amidation Reactions: A Minireview. Chem. Rec. 2021, 21, 4059–4087. [Google Scholar] [CrossRef]
- Zheng, Y.-L.; Newman, S.G. Methyl Esters as Cross-Coupling Electrophiles: Direct Synthesis of Amide Bonds. ACS Catal. 2019, 9, 4426–4433. [Google Scholar] [CrossRef]
- Bryan, M.C.; Dunn, P.J.; Entwistle, D.; Gallou, F.; Koenig, S.G.; Hayler, J.D.; Hickey, M.R.; Hughes, S.; Kopach, M.E.; Moine, G.; et al. Key Green Chemistry research areas from a pharmaceutical manufacturers’ perspective revisited. Green Chem. 2018, 20, 5082–5103. [Google Scholar] [CrossRef]
- Singh, P.; Vandemeulebroucke, A.; Li, J.; Schulenburg, C.; Fortunato, G.; Kohen, A.; Hilvert, D.; Cheatum, C.M. Evolution of the Chemical Step in Enzyme Catalysis. ACS Catal. 2021, 11, 6726–6732. [Google Scholar] [CrossRef]
- Itoh, T.; Hanefeld, U. Enzyme catalysis in organic synthesis. Green Chem. 2017, 19, 331–332. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Lubberink, M.; Finnigan, W.; Flitsch, S.L. Biocatalytic amide bond formation. Green Chem. 2023, 25, 2958–2970. [Google Scholar] [CrossRef]
- Pan, X.; Wang, L.; Ye, J.; Qin, S.; He, B. Efficient synthesis of beta-lactam antibiotics with very low product hydrolysis by a mutant Providencia rettgeri penicillin G acylase. Appl. Microbiol. Biotechnol. 2018, 102, 1749–1758. [Google Scholar] [CrossRef]
- Yousefi-Mokri, M.; Sharafi, A.; Rezaei, S.; Sadeghian-Abadi, S.; Imanparast, S.; Mogharabi-Manzari, M.; Amanzadeh, Y.; Faramarzi, M.A. Enzymatic hydrolysis of inulin by an immobilized extremophilic inulinase from the halophile bacterium Alkalibacillus filiformis. Carbohydr. Res. 2019, 483, 107746. [Google Scholar] [CrossRef]
- Salehipour, M.; Rezaei, S.; Yazdani, M.; Mogharabi-Manzari, M. Recent advances in preparation of polymer hydrogel composites and their applications in enzyme immobilization. Polym. Bull. 2023, 80, 5861–5896. [Google Scholar] [CrossRef]
- Ismail, A.R.; Baek, K.H. Lipase immobilization with support materials, preparation techniques, and applications: Present and future aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef]
- Kumar, A.; Gudiukaite, R.; Gricajeva, A.; Sadauskas, M.; Malunavicius, V.; Kamyab, H.; Sharma, S.; Sharma, T.; Pant, D. Microbial lipolytic enzymes–promising energy-efficient biocatalysts in bioremediation. Energy 2020, 192, 116674. [Google Scholar] [CrossRef]
- Hashempour, Y.; Mortezazadeh, F.; Rezaei, S.; Salehipour, M.; Gholami-Borujeni, F.; Ebrahimnejad, P.; Mogharabi-Manzari, M. Co-immobilization of laccase and zinc oxide nanoparticles onto bacterial cellulose to achieve synergistic effect of photo and enzymatic catalysis for biodegradation of favipiravir. Int. J. Biol. Macromol. 2025, 292, 139288. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Continuous flow reactors: A perspective. Green Chem. 2012, 14, 38–54. [Google Scholar] [CrossRef]
- Porcar, R.; Sans, V.; Ríos-Lombardía, N.; Gotor-Fernández, V.; Gotor, V.; Burguete, M.I.; García-Verdugo, E.; Luis, S.V. Stereoselective Chemoenzymatic Synthesis of Enantiopure 2-(1H-imidazol-yl)cycloalkanols under Continuous Flow Conditions. ACS Catal. 2012, 2, 1976–1983. [Google Scholar] [CrossRef]
- Britton, J.; Majumdar, S.; Weiss, G.A. Continuous flow biocatalysis. Chem. Soc. Rev. 2018, 47, 5891–5918. [Google Scholar] [CrossRef] [PubMed]
- Porta, R.; Benaglia, M.; Puglisi, A. Flow Chemistry: Recent Developments in the Synthesis of Pharmaceutical Products. Org. Process Res. Dev. 2015, 20, 2–25. [Google Scholar] [CrossRef]
- Holtze, C.; Boehling, R. Batch or flow chemistry?—A current industrial opinion on process selection. Curr. Opin. Chem. Eng. 2022, 36, 100798. [Google Scholar] [CrossRef]
- Robert, S.; Bertolla, C.; Masereel, B.; Dogné, J.M.; Pochet, L. Novel 3-carboxamide-coumarins as potent and selective FXIIa inhibitors. J. Med. Chem. 2008, 51, 3077–3080. [Google Scholar] [CrossRef]



 | |||||
| Entry | R1 | R2 | R3 | Product | Yield b (%) |
|---|---|---|---|---|---|
| 1 | H | H | H | 3a | 86.5 ± 1.7 |
| 2 | CH3 | H | H | 3b | 95.5 ± 1.5 |
| 3 | Cl | H | H | 3c | 82.6 ± 0.8 |
| 4 | H | CH3 | H | 3d | 93.1 ± 1.3 |
| 5 | H | H | CH3 | 3e | 89.3 ± 0.5 |
 | ||||
|---|---|---|---|---|
| Entry | Solvent | Catalysts | Log p | Yield b(%) |
| 1 | Methanol | None | −0.76 | n.d. |
| 2 | Methanol | Lipozyme TL IM | −0.76 | 77.8 ± 1.1 |
| 3 | Methanol | Novozym® 435 | −0.76 | 55.2 ± 0.3 |
| 4 | DMSO | Lipozyme TL IM | −1.3 | n.d. |
| 5 | DMSO | Novozym® 435 | −1.3 | n.d. |
| 6 | Acetonitrile | Lipozyme TL IM | −0.33 | 42.5 ± 0.8 |
| 7 | Acetonitrile | Novozym® 435 | −0.33 | n.d. |
| 8 | tert-amyl alcohol | Lipozyme TL IM | 0.60 | 63.3 ± 1.5 |
| 9 | tert-amyl alcohol | Novozym® 435 | 0.60 | 40.7 ± 0.6 |
| 10 | Isopropanol | Lipozyme TL IM | 0.39 | 32.3 ± 0.5 |
| 11 | Isopropanol | Novozym® 435 | 0.39 | n.d. |
| 12 | acetone | Lipozyme TL IM | −0.23 | 36.5 ± 0.7 |
| 13 | acetone | Novozym® 435 | −0.23 | n.d. |
 | |||
|---|---|---|---|
| Entry | Method | STY (g L−1 h−1) | Yield b (%) |
| 1 | A | 195.8 | 77.8 ± 0.7 |
| 2 | B | 0.84 | 61.3 ± 1.1 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | R4 | Product | Yield b (%) |
| 1 | H | H | H |  | 5a | 77.8 ± 0.7 |
| 2 | H | H | H |  | 5b | 72.1 ± 1.1 |
| 3 | H | H | H |  | 5c | 77.9 ± 0.9 |
| 4 | H | H | H |  | 5d | 68.1 ± 0.4 |
| 5 | CH3 | H | H | 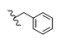 | 5e | 84.5 ± 1.3 |
| 6 | CH3 | H | H | 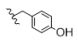 | 5f | 87.1 ± 0.5 |
| 7 | CH3 | H | H | 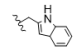 | 5g | 82.8 ± 0.7 |
| 8 | CH3 | H | H |  | 5h | 79.3 ± 0.3 |
| 9 | Cl | H | H | 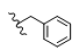 | 5i | 65.4 ± 0.8 |
| 10 | Cl | H | H | 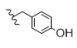 | 5j | 62.7 ± 0.1 |
| 11 | Cl | H | H | 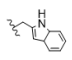 | 5k | <5 |
| 12 | Cl | H | H | 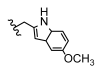 | 5l | 63.6 ± 1.8 |
| 13 | H | CH3 | H |  | 5m | 83.2 ± 0.6 |
| 14 | H | CH3 | H | 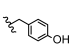 | 5n | 85.7 ± 1.2 |
| 15 | H | CH3 | H | 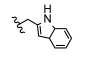 | 5o | 79.9 ± 0.4 |
| 16 | H | CH3 | H |  | 5p | 80.2 ± 0.2 |
| 17 | H | H | CH3 | 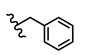 | 5q | 80.3 ± 1.1 |
| 18 | H | H | CH3 | 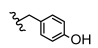 | 5r | 74.1 ± 0.8 |
| 19 | H | H | CH3 |  | 5s | 70.8 ± 0.5 |
| 20 | H | H | CH3 | 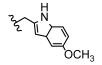 | 5t | 75.2 ± 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, L.-H.; Lin, H.; Fu, G.-N.; Huang, Z.-H.; Chen, Y.-M.; Xie, H.-J.; Yan, B.-L.; Xue, M.-M.; Zhang, A.-Y.; Wang, L.; et al. Two-Step Tandem Synthesis of Coumarin Derivatives Containing Bioamide Skeleton Catalyzed by Lipozyme TL IM from Thermomyces lanuginosus in Sustainable Continuous-Flow Microreactors. Catalysts 2025, 15, 268. https://doi.org/10.3390/catal15030268
Du L-H, Lin H, Fu G-N, Huang Z-H, Chen Y-M, Xie H-J, Yan B-L, Xue M-M, Zhang A-Y, Wang L, et al. Two-Step Tandem Synthesis of Coumarin Derivatives Containing Bioamide Skeleton Catalyzed by Lipozyme TL IM from Thermomyces lanuginosus in Sustainable Continuous-Flow Microreactors. Catalysts. 2025; 15(3):268. https://doi.org/10.3390/catal15030268
Chicago/Turabian StyleDu, Li-Hua, Hang Lin, Guo-Neng Fu, Zong-Hao Huang, Yi-Min Chen, Han-Jia Xie, Bing-Lin Yan, Miao-Miao Xue, Ao-Ying Zhang, Lin Wang, and et al. 2025. "Two-Step Tandem Synthesis of Coumarin Derivatives Containing Bioamide Skeleton Catalyzed by Lipozyme TL IM from Thermomyces lanuginosus in Sustainable Continuous-Flow Microreactors" Catalysts 15, no. 3: 268. https://doi.org/10.3390/catal15030268
APA StyleDu, L.-H., Lin, H., Fu, G.-N., Huang, Z.-H., Chen, Y.-M., Xie, H.-J., Yan, B.-L., Xue, M.-M., Zhang, A.-Y., Wang, L., & Luo, X.-P. (2025). Two-Step Tandem Synthesis of Coumarin Derivatives Containing Bioamide Skeleton Catalyzed by Lipozyme TL IM from Thermomyces lanuginosus in Sustainable Continuous-Flow Microreactors. Catalysts, 15(3), 268. https://doi.org/10.3390/catal15030268







