Photocatalytic Degradation of Pollutants in Air Streams Using Luminous Textiles Under Ultraviolet Light Illumination: A Pilot-Scale Remediation Study
Abstract
1. Introduction
2. Results
2.1. Degradation of Butyraldehyde
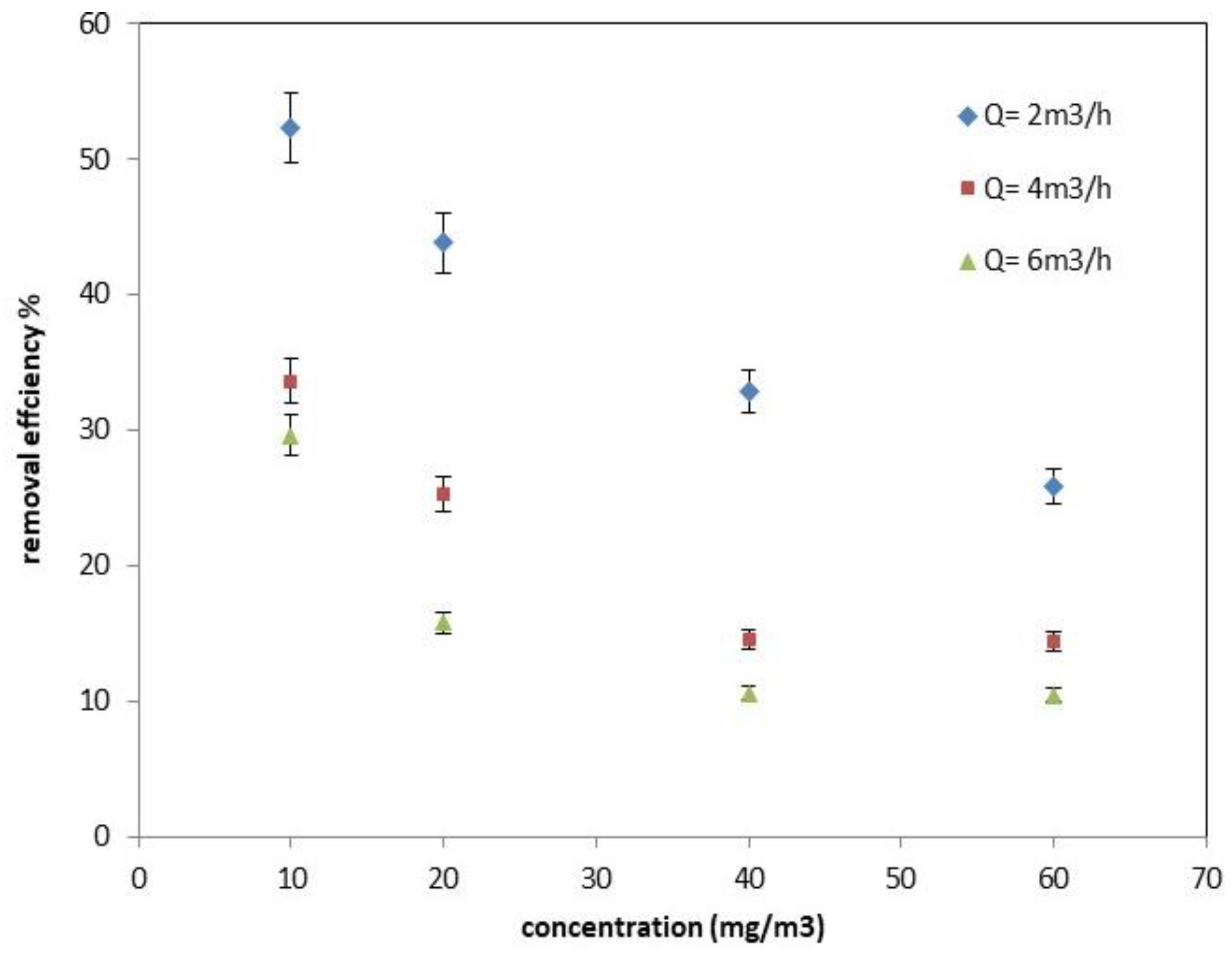
2.1.1. Effect of Inlet Concentration and Flow Rate on the Photocatalytic Process Efficiency
| Catalyst | VOC | Operating Parameters | Studied Parameters | Optimal Results | Ref. |
|---|---|---|---|---|---|
| TiO2-deposited polyester (PES) and TiO2-deposited glass-fiber tissue (GFT) | Butyraldehyde | Batch reactor, UV intensity = 9 W, RH = 40–50%, and T = 20 °C | Inlet concentration, RH, Q, and residence time | 90% removal in 30 min on GFT. BUTY removal on GFT was about twice as fast as PES | [34] |
| Commercialized TiO2 (P25, PC500, and UV100) | Toluene; methyl ethyl ketone | Continuous reactor, UV at 5 mw/cm2, and T = 20 °C | Inlet concentration, Q, RH, and UV | Between 72% and 100% degradation | [32] |
| TiO2/diatomite | Formaldehyde, acetone, and p-xylene | Continuous reactor, RH = 20%, I = 5 w/m2. Composite dosage = 3.76 mg/cm2, light intensity = 1.33 mW/cm2, RH = 0%, and Q = 1 l.mn−1 | Pollutant inlet concentration, RH, and Q, | >90% of degradation for a concentration of 10 ppm | [36] |
| TiO2 coating on GFT | Ammonia and butyraldehyde | Continuous reactor, UV lamp = 9 W, T = 20 °C, Q = 1 m3/h, and RH = 50% | Inlet concentration, RH, and flow rate | High selectivity for photodegradation at a rate of 72% | [35] |
| TiO2-incorporated KIT6 | Ethylene and propylene | A mixture of UVA (320–400 nm) and UVB (290–320 nm), 100 ppm, and RH 60% | Pollutant conversion | 90% conversion for 30% TiO2 KIT6 | [37] |
| Glass-fiber tissue (GFT) | Butyraldehyde and dimethyl disulfide (DMDS) | Continuous reactor, UV intensity = 9 W, T = 20 °C, and Q = 4 m3/h | Inlet concentration and flow rate | Around 70% removal | [38] |
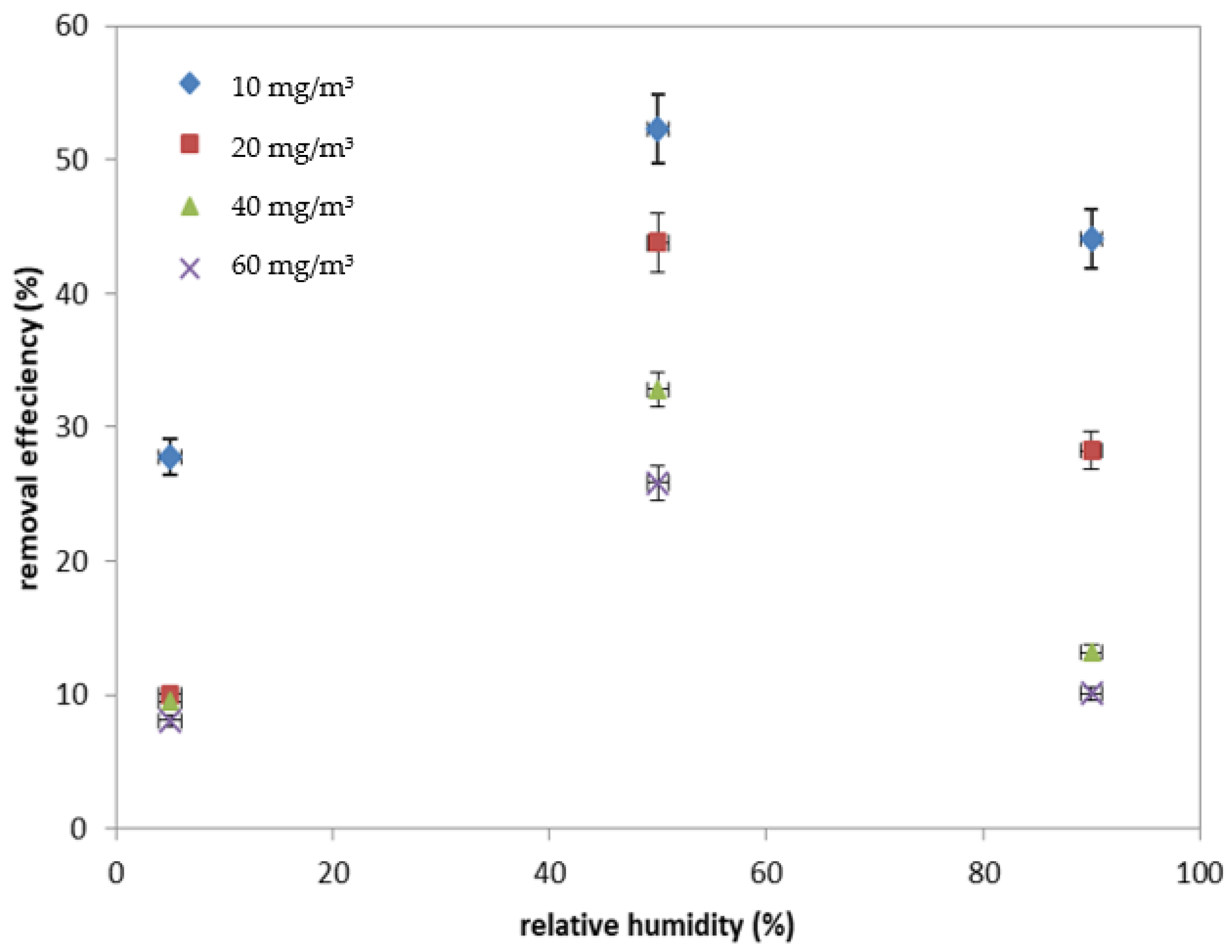
2.1.2. Effect of VOC Inlet Concentration and Relative Humidity on the Photocatalytic Process Efficiency
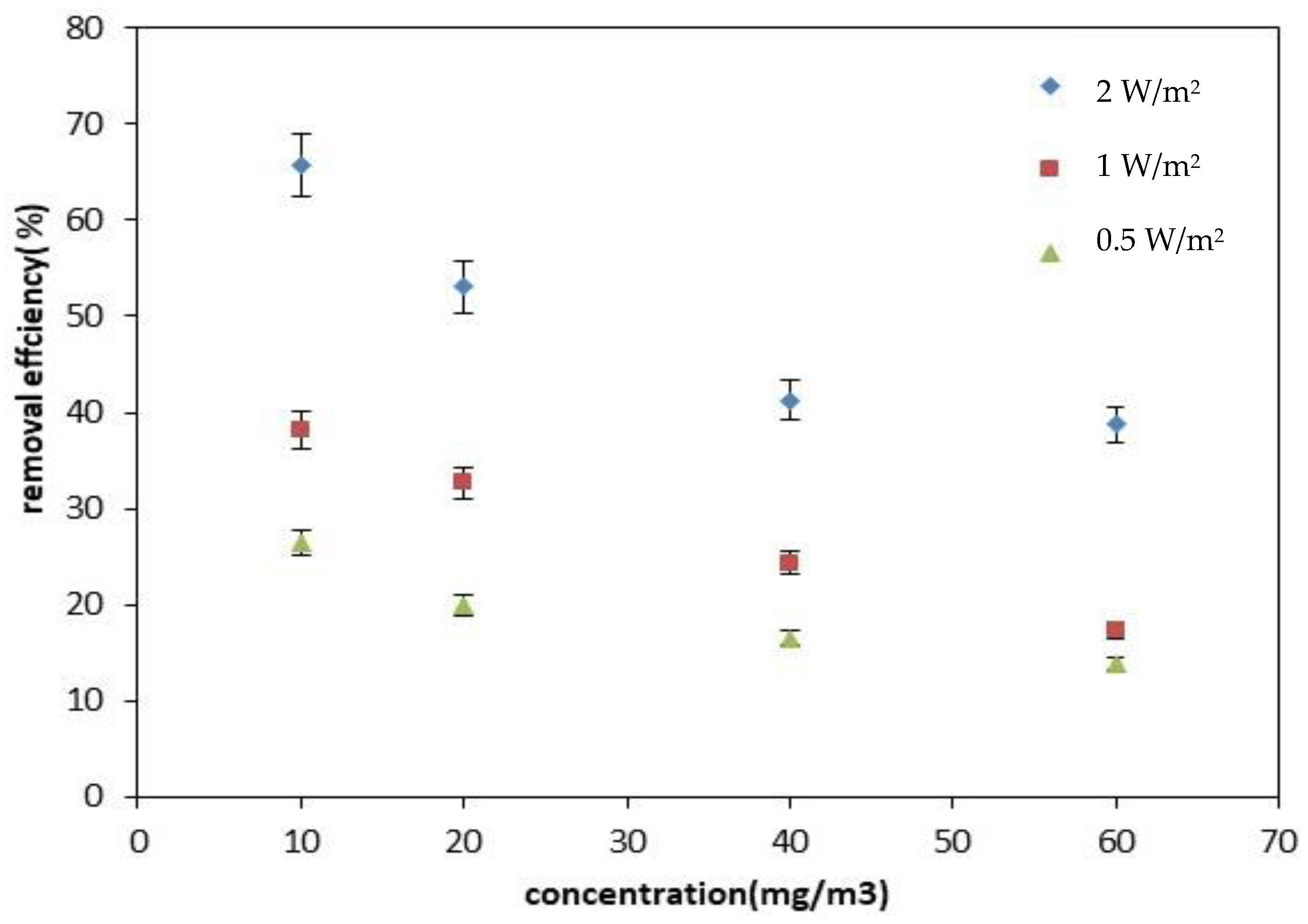
2.1.3. Effect of Pollutant Concentration and UV Radiation Intensity on the Photocatalytic Removal Efficiency
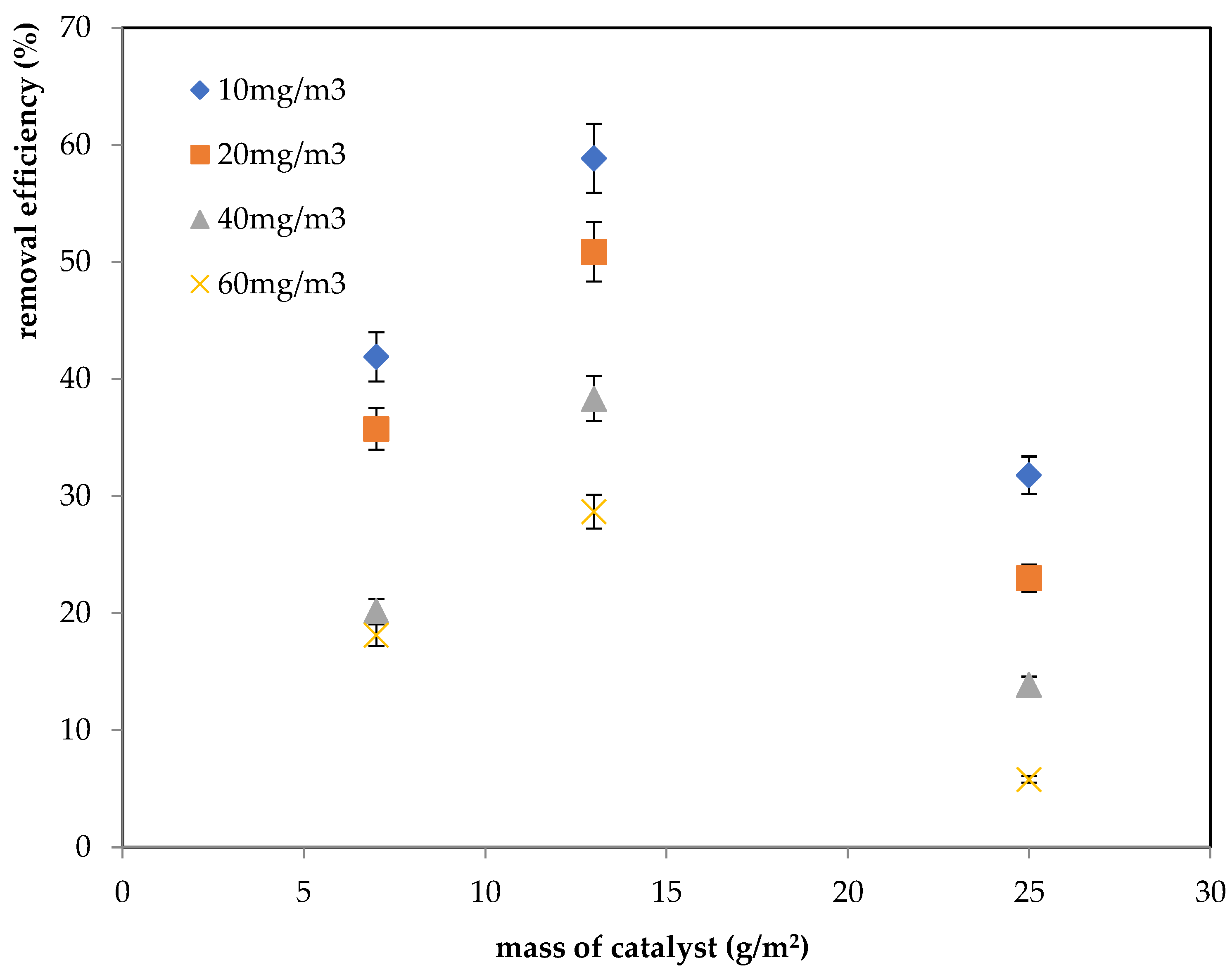
2.1.4. Effect of Inlet Concentration and TiO2 Mass on the Photocatalytic Process
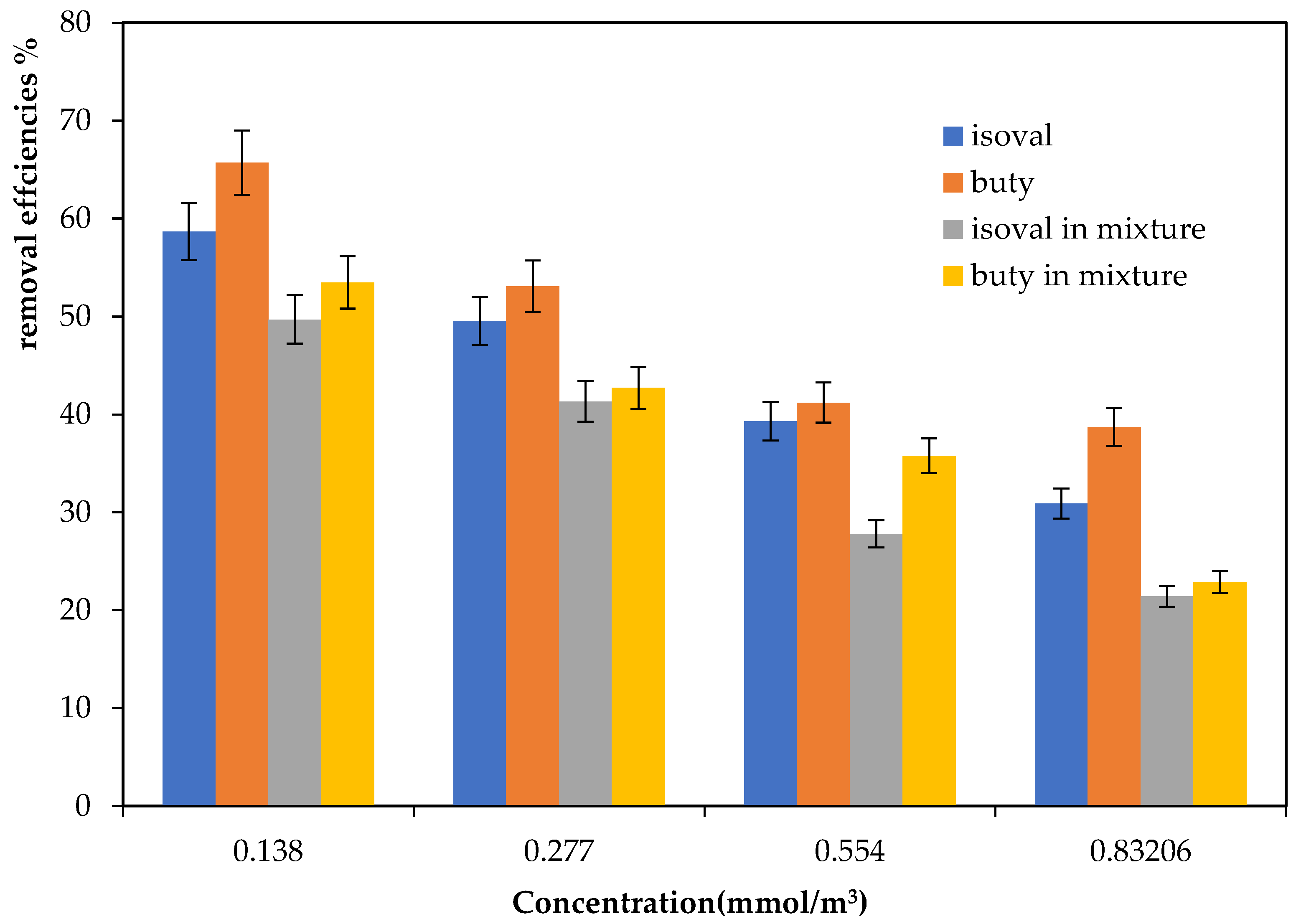
2.2. Degradation of a Mixture of Compounds
2.3. CO2 Selectivity
2.4. Kinetic Modeling of the Degradation with the Contribution of Mass Transfer in the Planar Reactor
3. Materials and Methods
3.1. Reagents
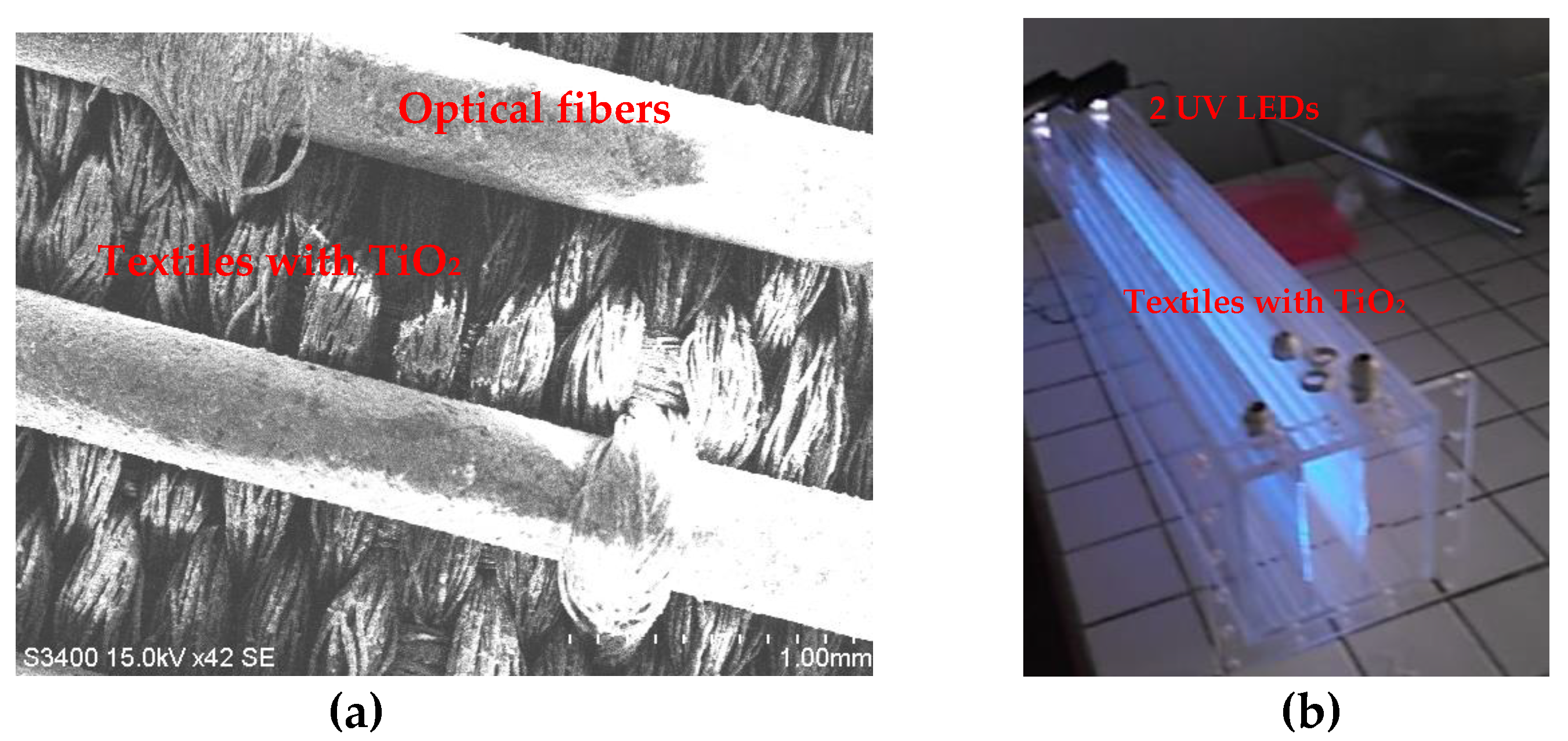
3.2. Luminous Textiles
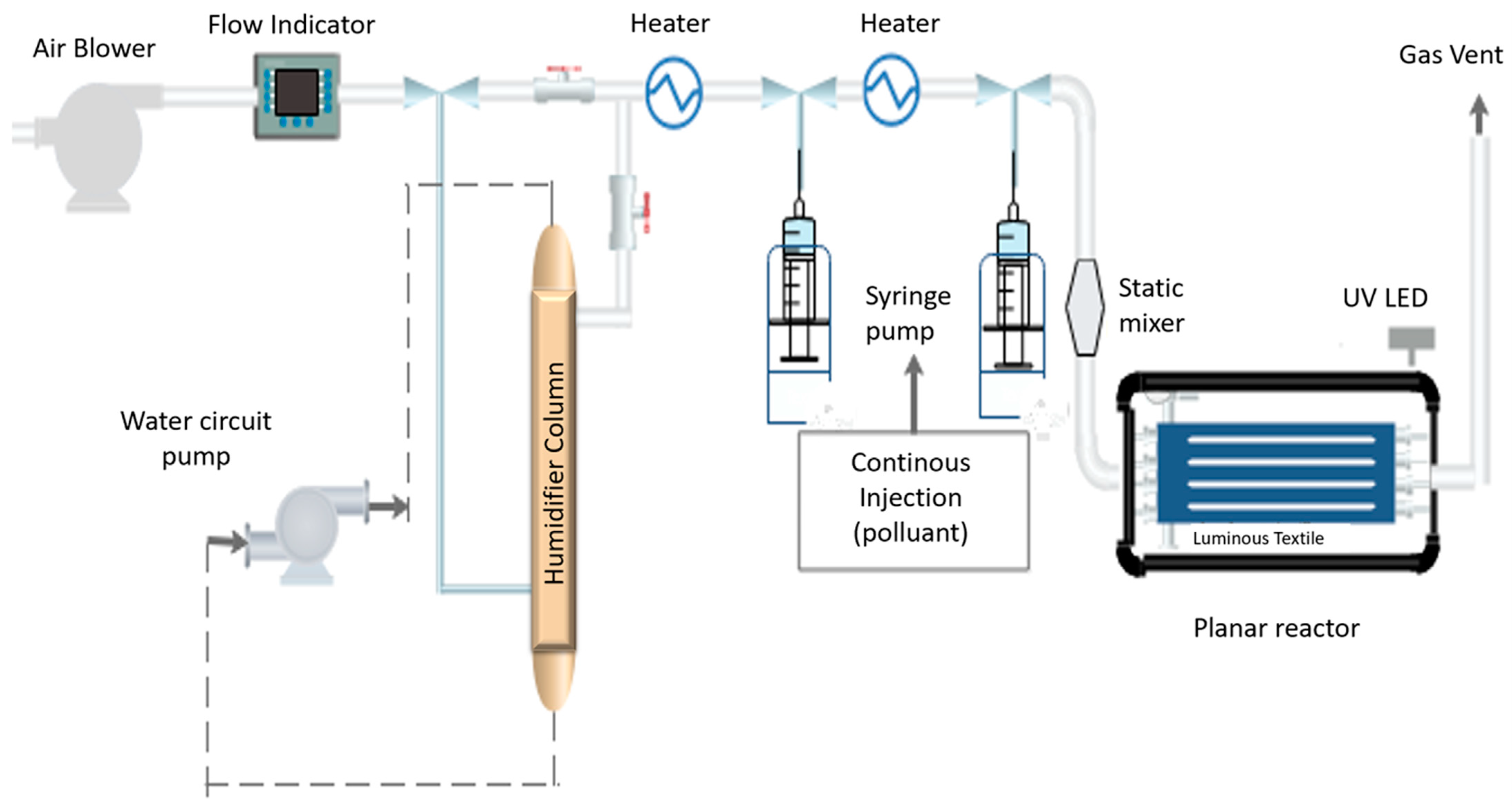
3.3. Experimental Setup
3.4. Apparatus and Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Edo, G.I.; Itoje-akpokiniovo, L.O.; Obasohan, P.; Ikpekoro, V.O.; Samuel, P.O.; Jikah, A.N.; Nosu, L.C.; Ekokotu, H.A.; Ugbune, U.; Oghroro, E.E.A.; et al. Impact of environmental pollution from human activities on water, air quality and climate change. Ecol. Front. 2024, 44, 874–889. [Google Scholar] [CrossRef]
- Lee, S.; Hung, R.; Bennett, J.W. An Overview of Fungal Volatile Organic Compounds (VOCs); Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Lavaine, E.; Majerus, P.; Treich, N. Health, air pollution, and animal agriculture. Rev. Agric. Food Environ. Stud. 2020, 101, 517–528. [Google Scholar] [CrossRef]
- Fetisov, V.; Gonopolsky, A.M.; Davardoost, H.; Ghanbari, A.R.; Mohammadi, A.H. Regulation and impact of VOC and CO2 emissions on low-carbon energy systems resilient to climate change: A case study on an environmental issue in the oil and gas industry. Energy Sci. Eng. 2023, 11, 1516–1535. [Google Scholar] [CrossRef]
- Kiani, S.S.; Faiz, Y.; Farooq, A.; Ahmad, M.; Irfan, N.; Nawaz, M.; Bibi, S. Synthesis and adsorption behavior of activated carbon impregnated with ASZM-TEDA for purification of contaminated air. Diam. Relat. Mater. 2020, 108, 107916. [Google Scholar] [CrossRef]
- Masi, M.; Nissim, W.G.; Pandolfi, C.; Azzarello, E.; Mancuso, S. Modelling botanical biofiltration of indoor air streams contaminated by volatile organic compounds. J. Hazard. Mater. 2022, 422, 126875. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, Y.; Wang, Y.; Wu, S.; Yang, C. Volatile organic compound removal via biofiltration: Influences, challenges, and strategies. Chem. Eng. J. 2023, 471, 144420. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Tan, Z.; Yu, H. Multivariate modeling of intrinsic kinetics for gas-solid heterogeneous photocatalytic reaction: A general method for different pollutant-photocatalyst systems. Chem. Eng. J. 2024, 479, 147651. [Google Scholar] [CrossRef]
- Tomić, J.; Malinović, N. Titanium dioxide photocatalyst: Present situation and future approaches. AIDASCO Rev. 2023, 1, 26–30. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Spray-Deposited TiO2 Layers on Aluminum Foil for Sustainable Water Remediation. Crystals 2024, 14, 875. [Google Scholar] [CrossRef]
- Ghosh, R.; Sahu, R.P.; Ganguly, R.; Zhitomirsky, I.; Puri, I.K. Photocatalytic activity of electrophoretically deposited TiO2 and ZnO nanoparticles on fog harvesting meshes. Ceram. Int. 2020, 46, 3777–3785. [Google Scholar] [CrossRef]
- Jaison, A.; Mohan, A.; Lee, Y.C. Recent Developments in Photocatalytic Nanotechnology for Purifying Air Polluted with Volatile Organic Compounds: Effect of Operating Parameters and Catalyst Deactivation. Catalysts 2023, 13, 407. [Google Scholar] [CrossRef]
- Chen, Z.; Pan, M.; Cheng, C.; Luo, J.; Deng, X. Water disinfection: Advances in photocatalysis and piezo/triboelectric catalysis with progressively enhanced energy utilization. SusMat 2024, 4, e232. [Google Scholar] [CrossRef]
- Dhiflaoui, H.; Hajjaji, M.A.; Hajjaji, A.; Khezami, L.; Karrech, A.; Bessais, B.; Larbi, A.B.C.; Amlouk, M. Enhanced Interfacial Adhesion of TiO2 Nanotubes Decorated With Ag Silver Nanoparticles Prepared by Photo-Reduction Process. J. Tribol. 2023, 145, 091106. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Chen, A.; Pu, W.; Gong, J. Influence of yolk-shell Au@TiO2 structure induced photocatalytic activity towards gaseous pollutant degradation under visible light. Appl. Catal. B Environ. 2019, 251, 57–65. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, H.; Zhang, P. Isolated Pt single atomic sites anchored on nanoporous TiO2 film for highly efficient photocatalytic degradation of low concentration toluene. J. Hazard. Mater. 2020, 388, 121746. [Google Scholar] [CrossRef]
- Udayabhanu; Lakshmana Reddy, N.; Shankar, M.V.; Sharma, S.C.; Nagaraju, G. One-pot synthesis of Cu–TiO2/CuO nanocomposite: Application to photocatalysis for enhanced H2 production, dye degradation & detoxification of Cr (VI). Int. J. Hydrogen Energy 2020, 45, 7813–7828. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, B.; Li, Q.; Tian, C.; Ma, L.; Li, Z. The Application of Bi-Doped TiO2 for the Photocatalytic Oxidation of Formaldehyde. Cryst. Res. Technol. 2022, 57, 2100231. [Google Scholar] [CrossRef]
- Noureen, L.; Wang, Q.; Humayun, M.; Shah, W.A.; Xu, Q.; Wang, X. Recent advances in structural engineering of photocatalysts for environmental remediation. Environ. Res. 2023, 219, 115084. [Google Scholar] [CrossRef]
- Azami, M.S.; Jalil, A.A.; Hassan, N.S.; Hussain, I.; Fauzi, A.A.; Aziz, M.A.A. Green carbonaceous material—fibrous silica-titania composite photocatalysts for enhanced degradation of toxic 2-chlorophenol. J. Hazard. Mater. 2021, 414, 125524. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Balasankar, A.; Arokiyaraj, S.; Sumathi, P.; Hwan Oh, T. Multi-usable titanium dioxide and poly(vinylidene fluoride) composite foam photocatalyst for degradation of organic pollutants. Appl. Surf. Sci. 2023, 609, 155264. [Google Scholar] [CrossRef]
- Kwon, M.; Kim, J.; Kim, J. Photocatalytic activity and filtration performance of hybrid tio2-cellulose acetate nanofibers for air filter applications. Polymers 2021, 13, 1331. [Google Scholar] [CrossRef] [PubMed]
- Amor, N.; Tayyab Noman, M.; Petru, M.; Sebastian, N.; Balram, D. Machining performance of TiO2 embedded-glass fiber reinforced composites with snake optimizer. Meas. J. Int. Meas. Confed. 2024, 227, 114253. [Google Scholar] [CrossRef]
- Ma, X.; Wang, H.; Chen, Y.; Fu, L.; Zhou, J.; Zhang, L.; Xing, Z.; Zhang, Q.; Xia, L. Application of Ag@g-C3N4/TiO2 cotton fabric flexible substrate with dual functionality: Photocatalytic reusability and SERS signal amplification for food safety detection. Appl. Surf. Sci. 2024, 661, 160068. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, X.; Tebyetekerwa, M.; Wang, Z.; Bie, C.; Sun, X.; Marriam, I.; Zhang, X. Engineering 2D Photocatalysts for Solar Hydrogen Peroxide Production. Adv. Energy Mater. 2024, 14, 2400740. [Google Scholar] [CrossRef]
- O’Neal Tugaoen, H.; Garcia-Segura, S.; Hristovski, K.; Westerhoff, P. Compact light-emitting diode optical fiber immobilized TiO2 reactor for photocatalytic water treatment. Sci. Total Environ. 2018, 613–614, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Wu, C.C.; Rahman, K.H.; Chen, K.C. A study on photodegradation of trichloroethylene using an optical fiber coated with different photocatalysts. Mater. Sci. Semicond. Process 2023, 163, 107538. [Google Scholar] [CrossRef]
- Behineh, E.S.; Solaimany Nazar, A.R.; Farhadian, M.; Karimi-Alavijeh, H.R. Embedded ZnO nanorod array/TiO2/GO coated optical fiber in a photocatalytic microreactor for Cefixime degradation: Diffused or focused light sources effect. J. Ind. Eng. Chem. 2024, 130, 623–637. [Google Scholar] [CrossRef]
- Shojaei, A.; Ghafourian, H.; Yadegarian, L.; Lari, K.; Sadatipour, M.T. Removal of volatile organic compounds (VOCs) from waste air stream using ozone assisted zinc oxide (ZnO) nanoparticles coated on zeolite. J. Environ. Heal. Sci. Eng. 2021, 19, 771–780. [Google Scholar] [CrossRef]
- Xu, P.; Ding, C.; Li, Z.; Yu, R.; Cui, H.; Gao, S. Photocatalytic degradation of air pollutant by modified nano titanium oxide (TiO2)in a fluidized bed photoreactor: Optimizing and kinetic modeling. Chemosphere 2023, 319, 137995. [Google Scholar] [CrossRef]
- Verma, S.; Vikrant, K.; Kim, K.H. Optimization of process variables for the concurrent removal of aliphatic and aromatic volatile organic compounds over a copper impregnated titanium dioxide photocatalyst. Environ. Sci. Nano 2023, 10, 2035–2052. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.S. Photocatalytic degradation of VOCs on various commercial titanium dioxides: Impact of operating parameters on removal efficiency and by-products generation. Build. Environ. 2018, 138, 275–282. [Google Scholar] [CrossRef]
- Elfalleh, W.; Assadi, A.A.; Bouzaza, A.; Wolbert, D.; Kiwi, J.; Rtimi, S. Innovative and stable TiO2 supported catalytic surfaces removing aldehydes under UV-light irradiation. J. Photochem. Photobiol. A Chem. 2017, 343, 96–102. [Google Scholar] [CrossRef]
- Abou Saoud, W.; Assadi, A.A.; Guiza, M.; Bouzaza, A.; Aboussaoud, W.; Soutrel, I.; Ouederni, A.; Wolbert, D.; Rtimi, S. Abatement of ammonia and butyraldehyde under non-thermal plasma and photocatalysis: Oxidation processes for the removal of mixture pollutants at pilot scale. Chem. Eng. J. 2018, 344, 165–172. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Hashisho, Z.; Sun, Z.; Zheng, S.; Zhong, L. Adsorption and photocatalytic degradation performances of TiO2/diatomite composite for volatile organic compounds: Effects of key parameters. Appl. Surf. Sci. 2020, 525, 2400740. [Google Scholar] [CrossRef]
- Hussain, M.; Akhter, P.; Iqbal, J.; Ali, Z.; Yang, W.; Shehzad, N.; Majeed, K.; Sheikh, R.; Amjad, U.E.S.; Russo, N. VOCs photocatalytic abatement using nanostructured titania-silica catalysts. J. Environ. Chem. Eng. 2017, 5, 3100–3107. [Google Scholar] [CrossRef]
- Abou Saoud, W.; Assadi, A.A.; Guiza, M.; Bouzaza, A.; Aboussaoud, W.; Ouederni, A.; Soutrel, I.; Wolbert, D.; Rtimi, S. Study of synergetic effect, catalytic poisoning and regeneration using dielectric barrier discharge and photocatalysis in a continuous reactor: Abatement of pollutants in air mixture system. Appl. Catal. B Environ. 2017, 213, 53–61. [Google Scholar] [CrossRef]
- Payan, A.; Charchi Aghdam, N.; Soltan, J. Novel insight into the effect of relative humidity on the acetone photocatalytic decomposition using Ag−CeO2 under vacuum ultraviolet illumination: Catalytic synergies, performance, and mechanistic interpretation. J. Photochem. Photobiol. A Chem. 2023, 443, 114847. [Google Scholar] [CrossRef]
- Jin, H.; Lee, T.M.; Choi, H.; Kim, K.S. Effects of process variables for NO conversion by double-layered photocatalytic mortar with TiO2 nanoparticles. J. Ind. Eng. Chem. 2023, 117, 461–472. [Google Scholar] [CrossRef]
- Zhong, L.; Brancho, J.J.; Batterman, S.; Bartlett, B.M.; Godwin, C. Experimental and modeling study of visible light responsive photocatalytic oxidation (PCO) materials for toluene degradation. Appl. Catal. B Environ. 2017, 216, 122–132. [Google Scholar] [CrossRef]
- Masresha, G.; Jabasingh, S.A.; Kebede, S.; Doo-Arhin, D.; Assefa, M. A review of prospects and challenges of photocatalytic decomposition of volatile organic compounds (VOCs) under humid environment. Can. J. Chem. Eng. 2023, 101, 6905–6918. [Google Scholar] [CrossRef]
- Tu, L.N.Q.; Nhan, N.V.H.; Van Dung, N.; An, N.T.; Long, N.Q. Enhanced photocatalytic performance and moisture tolerance of nano-sized Me/TiO2–zeolite Y (Me=Au, Pd) for gaseous toluene removal: Activity and mechanistic investigation. J. Nanoparticle Res. 2019, 21, 194. [Google Scholar] [CrossRef]
- Zhang, J.; Vikrant, K.; Kim, K.-H.; Dong, F.; Won Chung, M.; Weon, S. Unveiling the Collective Effects of Moisture and Oxygen on the Photocatalytic Degradation of m-Xylene using a Titanium Dioxide Supported Platinum Catalyst. Chem. Eng. J. 2022, 439, 135747. [Google Scholar] [CrossRef]
- Debono, O.; Hequet, V.; Le Coq, L.; Locoge, N.; Thevenet, F. VOC ternary mixture effect on ppb level photocatalytic oxidation: Removal kinetic, reaction intermediates and mineralization. Appl. Catal. B Environ. 2017, 218, 359–369. [Google Scholar] [CrossRef]
- Sun, X.; Li, C.; Yu, B.; Wang, J.; Wang, W. Removal of gaseous volatile organic compounds via vacuum ultraviolet photodegradation: Review and prospect. J. Environ. Sci. 2023, 125, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Munnik, P.; De Jongh, P.E.; De Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Chong, M.C.; Narindri Rara Winayu, B.; Chu, H. Advancement of visible light-driven photocatalytic degradation of dimethyl sulfide by Zn doped rGO/TiO2. Appl. Surf. Sci. 2024, 648, 159048. [Google Scholar] [CrossRef]
- Malayeri, M.; Lee, C.S.; Niu, J.; Zhu, J.; Haghighat, F. Kinetic modeling and reaction mechanism of toluene and by-products in photocatalytic oxidation reactor. Chem. Eng. J. 2022, 427, 131536. [Google Scholar] [CrossRef]
- Seo, B.; Ko, E.H.; Kim, B.; Park, N.K.; Kang, S.B.; Kang, D.; Kim, M. Computational screening-based development in VOC removal catalyst: Methyl ethyl ketone oxidation over Pt/TiO2. Chem. Eng. J. 2023, 452, 139466. [Google Scholar] [CrossRef]
- Malayeri, M.; Lee, C.S.; Haghighat, F.; Klimes, L. Modeling of gas-phase heterogeneous photocatalytic oxidation reactor in the presence of mass transfer limitation and axial dispersion. Chem. Eng. J. 2020, 386, 124013. [Google Scholar] [CrossRef]
- Ješić, D.; Lašič Jurković, D.; Pohar, A.; Suhadolnik, L.; Likozar, B. Engineering photocatalytic and photoelectrocatalytic CO2 reduction reactions: Mechanisms, intrinsic kinetics, mass transfer resistances, reactors and multi-scale modelling simulations. Chem. Eng. J. 2021, 407, 126799. [Google Scholar] [CrossRef]
- Thevenet, F.; Sivachandiran, L.; Guaitella, O.; Barakat, C.; Rousseau, A. Plasma-catalyst coupling for volatile organic compound removal and indoor air treatment: A review. J. Phys. D. Appl. Phys. 2014, 47, 224011. [Google Scholar] [CrossRef]
- Reisener, J.; Reuter, M.A.; Krüger, J. Modelling of the mass transfer in gas-sparged electrolysers with neural nets. Chem. Eng. Sci. 1993, 48, 1089–1101. [Google Scholar] [CrossRef]
- Romkes, S.J.P.; Dautzenberg, F.M.; van den Bleek, C.M.; Calis, H.P.A. CFD modelling and experimental validation of particle-to-fluid mass and heat transfer in a packed bed at very low channel to particle diameter ratio. Chem. Eng. J. 2003, 96, 3–13. [Google Scholar] [CrossRef]


| Pollutant | Formula | Diffusivity in the Air (Dm) (cm2/s) |
|---|---|---|
| Butyraldehyde | C4H8O | 0.0892 |
| Isovaleraldehyde | C5H10O | 0.0797 |
| Flow Rate (Q) (m3/h) | Reynolds Number | Mass Transfer Coefficient (km) (m/s) | |
|---|---|---|---|
| Butyraldehyde | Isovaleraldehyde | ||
| 2 | 248 | 7.7 | 7.2 |
| 4 | 497 | 10.9 | 10.1 |
| 6 | 745 | 13.4 | 12.4 |
| Butyraldehyde | Isovaleraldehyde | |
|---|---|---|
| kobs (mmol/(m 3.s)) | 0.018 | 0.081 |
| K (m3/mmol) | 1.159 | 0.246 |
| R2 (%) | 98.6 | 92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelkader, M.; Assadi, A.A.; Guiza, M.; Elfalleh, W.; Khezami, L.; Tahraoui, H.; Baaloudj, O.; Mouni, L.; Zhang, J.; Amrane, A. Photocatalytic Degradation of Pollutants in Air Streams Using Luminous Textiles Under Ultraviolet Light Illumination: A Pilot-Scale Remediation Study. Catalysts 2025, 15, 262. https://doi.org/10.3390/catal15030262
Abdelkader M, Assadi AA, Guiza M, Elfalleh W, Khezami L, Tahraoui H, Baaloudj O, Mouni L, Zhang J, Amrane A. Photocatalytic Degradation of Pollutants in Air Streams Using Luminous Textiles Under Ultraviolet Light Illumination: A Pilot-Scale Remediation Study. Catalysts. 2025; 15(3):262. https://doi.org/10.3390/catal15030262
Chicago/Turabian StyleAbdelkader, Meriem, Amine Aymen Assadi, Monia Guiza, Walid Elfalleh, Lotfi Khezami, Hichem Tahraoui, Oussama Baaloudj, Lotfi Mouni, Jie Zhang, and Abdeltif Amrane. 2025. "Photocatalytic Degradation of Pollutants in Air Streams Using Luminous Textiles Under Ultraviolet Light Illumination: A Pilot-Scale Remediation Study" Catalysts 15, no. 3: 262. https://doi.org/10.3390/catal15030262
APA StyleAbdelkader, M., Assadi, A. A., Guiza, M., Elfalleh, W., Khezami, L., Tahraoui, H., Baaloudj, O., Mouni, L., Zhang, J., & Amrane, A. (2025). Photocatalytic Degradation of Pollutants in Air Streams Using Luminous Textiles Under Ultraviolet Light Illumination: A Pilot-Scale Remediation Study. Catalysts, 15(3), 262. https://doi.org/10.3390/catal15030262












