Abstract
Interaction of [Sn(OtBu)4] with the acid 2,2′-diphenylgylcine, Ph2C(X)CO2H (X = NH2), affords the complex {Sn[Ph2C(NH2)(CO2)]4}·2MeCN (1·2MeCN) after work-up, whereas when X = OH (benzilic acid), the complex {Sn[Ph2C(O)(CO2)]2(CH3CO2H)2} (2) is isolated. In 1·2MeCN, the four 2,2′-diphenylglycinate ligands adopt three different coordination modes (two N,O-chelates, an O,O-chelate, and a monodentate carboxylate ligand), whilst in 2, two cis-O,O-chelate ligands are present along with two acetic acid ligands, the latter being derived from hydrolysis of acetonitrile. Both 1 and 2 have been screened as catalysts for the ring opening polymerization of ε-caprolactone and δ-valerolactone; for comparison, the commercial catalyst [Sn(Oct)2], where Oct = 2-ethylhexanoate, and the precursor [Sn(OtBu)4] have been screened under similar conditions. The products were of low to high molecular weight for PCL and low to moderate molecular weight for PVL, with wide Ð values, and they comprised several types of polymer families, including OH-terminated, OH/OMe-terminated, and cyclic polymers. For both monomers, kinetic profiles indicated that [Sn(Oct)2] outperformed 1, 2, and [Sn(OtBu)4], though under certain conditions, 1 and 2 afforded high-molecular weight products with better control.
1. Introduction
Petroleum-based plastics continue to be essential for a variety of everyday applications; however, the issues associated with global plastic pollution are driving the search for more environmentally friendly materials [1,2,3]. With this in mind, much research has focused on the ring opening polymerization (ROP) of cyclic esters. This process typically employs a catalyst, which can be either metal- or organic-based [4,5,6,7,8,9,10,11,12], and in the former case, the active species tends to be either a metal alkoxide or carboxylate. The other ancillary ligands bound to the catalytic metal centre also play a crucial role in controlling the local sterics and electronics of the system and can also greatly influence other properties such as solubility. Commercially, the catalyst of choice is tin octanoate, [Sn(Oct)2], which was selected given its high catalytic activity (high reaction and conversion rates), ability to afford high-molecular weight products, and relatively low cost, despite the cytotoxicity associated with tin compounds [13]. There is considerable interest in the development of new tin-based catalysts for the ROP of cyclic esters [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. For example, Limwanich et al. have recently investigated the microwave-assisted ROP of ε-caprolactone under solvent-free conditions using n-butyltin(IV) chlorides [36]. We have been investigating the coordination chemistry of the acids Ph2C(X)CO2H, where X = NH2 or OH [37,38,39,40,41,42,43,44,45], given their ability to impart high crystallinity on subsequent products [46]. Given this, we have extended our studies of these acids to tin chemistry and report herein two products arising from the interaction of Ph2C(X)CO2H, X = NH2, OH, with [Sn(OtBu)4], Chart 1. [Sn(OtBu)4] was chosen as the entry point, despite the increased toxicity associated with Sn(IV), as solid Sn(II) alkoxides tend to suffer from solubility issues [32]. The tin products 1 and 2 have been screened for their ROP capability with the cyclic esters ε-caprolactone (ε-CL) and δ-valerolactone (δ-VL). Results are compared against the commercial catalyst [Sn(Oct)2], where Oct = 2-ethylhexanoate, and the precursor [Sn(OtBu)4], which have been screened under the same conditions.
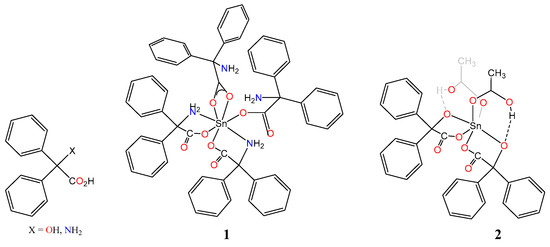
Chart 1.
The acids Ph2C(X)CO2H (X = OH, NH2) and the complexes 1 and 2.
2. Results and Discussion
2.1. Diphenylglycine
Reaction of [Sn(OtBu)4] with Ph2C(NH2)(CO2H), dpgH, in refluxing toluene afforded the complex {Sn[Ph2C(NH2)(CO2)]4}∙2MeCN (1∙2MeCN) after work-up (extraction into MeCN). Single crystals suitable for X-ray diffraction were grown from a saturated MeCN solution on standing for 48h at 0 °C. The molecular structure is shown in Figure 1, with selected bond lengths and angles given in the caption; an alternative view of 1∙2MeCN is given in the Supplementary Materials (Figure S1). The complex crystallises in the centrosymmetric space group P1 with a single, discrete tin complex in the asymmetric unit. The central Sn(VI) ion is seven-coordinate; four dpg− anions are bound at the tin centre, but there are three different coordination modes. The first two dpg anions form five-membered chelates through the carboxylate and amine group (O1, N1 and O3, N2). This can be classified using the Harris notation [47] as a [1.011] binding mode. The third ligand binds through a chelating carboxylate (atoms O5 and O6) in mode [1.110]. The final ligand binds through a single oxygen of the carboxylate (O7). The different coordination modes are readily apparent from the carboxylate bond lengths. The assignment was greatly aided by excellent-quality difference Fourier maps which allowed for H-atoms to be identified. Hydrogens attached to carbon were fitted with a riding model; those attached to nitrogen were refined freely, subject to restraints that all N-H distances be the same with a standard deviation of 0.03 Å, and bond angles were similarly restrained. For the five-membered chelates, the C-O bond lengths are 1.308(3) and 1.216(3) Å for C1 and 1.298(3) and 1.216(3) Å for C15. The chelating carboxylate centred on C29 has C-O bond lengths of 1.282(3) and 1.245(3) Å. The strictly monodentate carboxylate centred on C43 has C-O bond lengths of 1.302(3) and 1.220(3) Å. There is very minor disorder in the position of one of the phenyl groups (two orientations in the ratio 0.569:0.431(15)), but this was modelled conservatively using standard techniques, involving bond length restraints for equivalent atoms in different disorder components. In addition to the tin complex, the asymmetric unit contains two well-resolved molecules of acetonitrile which act as hydrogen bond acceptors to two NH2 groups of the complex, forming a hydrogen-bonding motif [48]. The phenyl rings are orientated in a propellor-like fashion, as noted for a number of benzilate complexes [49].

Figure 1.
View of molecular structure of {Sn[Ph2C(NH2)(CO2)]4}∙2MeCN (1∙2MeCN), drawn as 50% probability ellipsoids. For clarity, unbound solvent molecules have been omitted. Selected bond lengths (Å) and angles (°): Sn1—O1 2.0576(16), Sn1—O3 2.0923(18), Sn1—O5 2.1461(18), Sn1—O7 2.0687(16), Sn1—N1 2.2244(19), Sn—N2 2.2208(19); O1—Sn—N1 75.59(6), O3—Sn—N2 75.26(7), O5—Sn—O6 56.54(6), O1—Sn—O7 161.00(7).
There are no intramolecular hydrogen bonds. There is extensive N-H∙∙∙N hydrogen bonding between adjacent molecules, but surprisingly, there are no N-H∙∙∙O interactions. Most notably, adjacent molecules related by the inversion centre form an embrace through N-H∙∙∙N hydrogen bonds. There is also evidence for C-H∙∙∙O interactions between adjacent molecules.
2.2. Benzilic Acid
Similar use of benzilic acid led to the complex {Sn[Ph2C(O)(CO2)]2(CH3CO2H)2} after work-up (MeCN) (2). Single crystals suitable for X-ray diffraction were grown from a saturated MeCN solution, standing for 48h at 0 °C. The molecular structure is shown in Figure 2, with selected bond lengths and angles given in the caption; an alternative view of 2 is given in the Supplementary Materials (Figure S2). The complex crystallises in the non-centric space group I-42d, with one half a complex in the asymmetric unit. There is minor disorder in the position of one of the phenyl groups (two orientations in the ratio 0.55:0.45(5)), but this was modelled conservatively using standard techniques, involving bond length restraints for equivalent atoms in different disorder components. Each Sn is six coordinate, and two doubly-deprotonated benzilic acid ligands form five-membered chelates to the Sn in a cis fashion. The remaining two coordination sites are completed by the carbonyl oxygen of acetic acid (C=O distance for the binding oxygen is 1.26(2) Å and for the C-OH, the C-O distance is 1.34(2) Å). The O-H group is not deprotonated but forms an intramolecular hydrogen bond to the carbonyl of the benzilic acid with motif . The crystal as a whole was found to contain a single enantiomer (Flack parameter 0.05(3)). The acetic acid ligand is thought to arise via the hydrolysis of MeCN; such a process usually occurs in the presence of an acid or base [50,51,52].
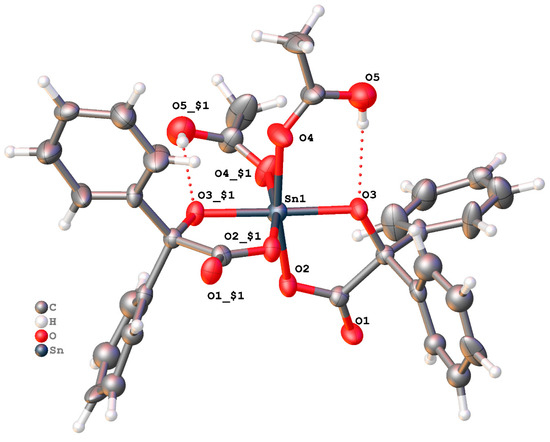
Figure 2.
View of molecular structure of {Sn[Ph2C(O)(CO2)]2(CH3CO2H)2}, drawn as 30% probability ellipsoids. For clarity, minor disorder is not shown. Symmetry operation used to generate equivalent atoms: x, 1.5−y, 1.25−z. Selected bond lengths (Å) and angles (°): Sn1—O2 2.044(10), Sn1—O3 1.965(8), Sn1—O4 2.073(10); O2—Sn—O3 81.6(4), O2—Sn—O4 169.7(4).
There is no included solvent in the structure despite the fact that four pockets of approximately 3.6% of the cell volume are present.
1H NMR spectra for 1 and 2 are provided in the Supplementary Materials (Figures S3 and S4).
3. Ring Opening Polymerization (ROP)
3.1. Ring Opening Polymerization of ε-Caprolactone (ε-CL)
Complexes 1 and 2 have been screened for their ability to act as catalysts for the ROP of ε-caprolactone (ε-CL), and the results are presented in Table 1. Results for 1 and 2 are compared with the industrially employed catalyst Sn(Oct)2. For 1, the ratio of [ε-CL]:[Sn] was varied between 100:1 and 1000:1 at 130 °C over 24 h under N2 or air. Complexes 1 and 2 were found to be active under these polymerization conditions with similar monomer conversions (≥90%, except for entry 4 at 81%), affording polymers with moderate to relatively high molecular weights, with 1 under N2 (entry 3, Table 1) affording the highest at ca. 48,750 Da, albeit with poor control (Ð = 3.81); selected GPC traces are given in the Supplementary Materials (Figures S5–S11). Interestingly, consistent with the wide Ð values, the MALDI-TOF spectra revealed several families of products, including OH-terminated polymers and cyclic polymers (e.g., Figure 3, Figure 4, Figure 5 and Figure 6). There was evidence of transesterification, and all observed Mn values were significantly lower than the calculated values. The polymers obtained using [Sn(Oct)2] consistently gave higher molecular weights than those obtained using 1 and 2 under the same conditions. MALDI-ToF spectra for the PCL obtained via the use of [Sn(Oct)2] revealed the products to be mostly linear polymers with H/OH end groups (e.g., see Figure 6). At ambient temperature (15 °C), all complexes exhibited little or no activity.

Table 1.
The ROP of ε-CL over 24 h catalysed by 1, 2 [Sn(Oct)2], and [Sn(OtBu)4)].
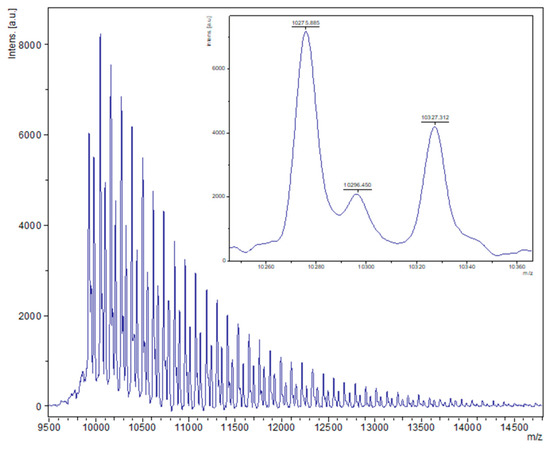
Figure 3.
MALDI-ToF spectrum of PCL obtained from entry 4, Table 1 (1, 1000:1, melt, air). The main families are (i) chain polymer (terminated by 2 OH groups) as potassium adducts [M = 17 (OH) + 1(H) + n × 114.14 (CL) + 39.1 (K+)], e.g., for n = 90, calc. 10,329.7 obsv. 10,327.3; (ii) cyclic polymers as the sodium adducts [M = n × 114.14(CL) + 22.99 (Na+)], e.g., calc. 10,295.6, n = 90, obsv. 10,296.5.
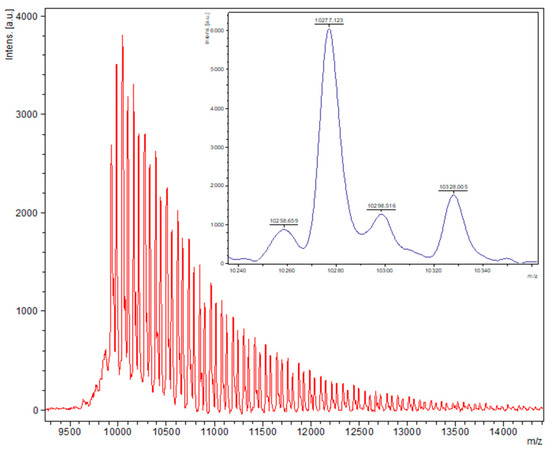
Figure 4.
MALDI-ToF spectrum of PCL obtained from entry 7, Table 1 (1, 500:1 melt, N2). The main families are (i) chain polymer (terminated by 2 OH groups) as potassium adducts [M = 17 (OH) + 1(H) + n × 114.14 (CL) + 39.1 (K+)], e.g., for n = 90, calc. 10,329.7 obsv. 10,328.0; (ii) cyclic polymers as the sodium adducts [M = n × 114.14(CL) + 22.99 (Na+)], e.g., calc. 10,295.6, n = 90, obsv. 10,298.5.
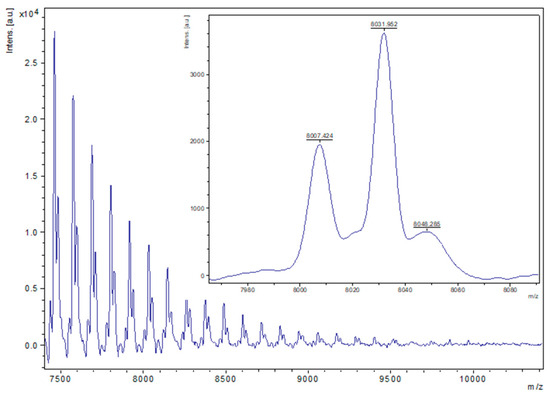
Figure 5.
MALDI-ToF spectrum of PCL obtained from entry 16, Table 1 (2, 500:1 melt, air). The main families are (i) chain polymer (terminated by 2 OH groups, i.e., HO(C6H10O2)H) [M = 17 (OH) + 1(H) + n × 114.14 (CL) + 22.99 (Na+)], e.g., for n = 70, calc. 8007.8 obsv. 8007.4; (ii) chain polymer (terminated by 2 OH groups) as sodium adducts [M = 17 (OH) + 1(H) + n × 114.14 (CL) + 22.99 (Na+)], e.g., for n = 70, calc. 8030.8 obsv. 8032.0. (iii) A minor family can be assigned to chain polymers terminated by OMe/OH end groups as sodium adducts [M = 31 (OMe) + 1(H) + n × 114.14 (CL) + 22.99 (Na+)], e.g., calc. 8044.8, n = 70, obsv. 8048.3.
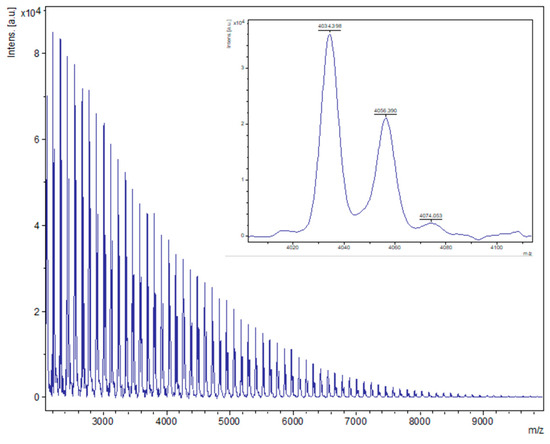
Figure 6.
MALDI-ToF spectrum of PCL obtained from entry 22, Table 1 ([Sn(Oct)2], 500:1 melt, air). The main family is a chain polymer (terminated by 2 OH groups) as sodium adducts [M = 17 (OH) + 1(H) + n × 114.14 (CL) + 22.99 (Na+)], e.g., for n = 35, calc. 4034.4 obsv. 4034.4; n = 70, calc. 8030.8 obsv. 8029.3.
A kinetic study (Figure 7) conducted using 500:1 ([ε-CL]:[cat]) at 110 °C revealed the rate trend [Sn(Oct)2] > 1 > 2 > [Sn(OtBu)4]. Both [Sn(Oct)2] and 1 are rather sluggish over the first 15 h (about 25 h for Sn(OtBu)4), consistent with a structural change under these conditions to a more active species. For the individual kinetic traces, see Figures S12–S15 in the Supplementary Materials.
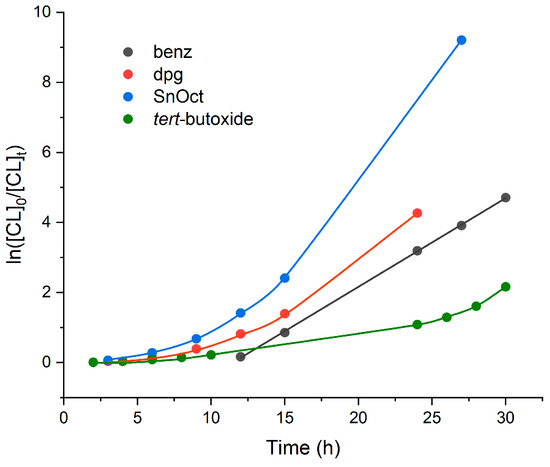
Figure 7.
Kinetic runs using [ε-CL]:[cat] = 500:1 at 110 °C in toluene.
We note that during metal-free studies, we observed that benzilic acid was active for the ROP of ε-CL with near quantitative conversions when using high-catalyst loadings (20:1) at 150 °C over 24 h, whereas for diphenylglycine, conversions were ≤5% at 150 °C over 24 h, with or without BnOH present [41].
3.2. Ring Opening Polymerization of δ-Valerolactone (δ-VL)
Based on the ε-CL results, the complexes were screened for the ROP of δ-VL using the ratio of [VL]:[catalyst] of 500:1 (Table 2). All complexes were found to be active under these polymerization conditions with monomer conversions (≥77%), affording relatively low to moderate-molecular weight polymers; selected GPC traces are given in the Supplementary Materials (Figures S16–S23). Even at ambient temperature, 1, 2, Sn(Oct)2, and [Sn(OtBu)4] were capable of the ROP of δ-VL with good conversions. This behaviour contrasts with previous ROP studies, where more robust conditions are usually needed for the ROP of δ-VL versus ε-Cl [38,39], and it is inconsistent with the thermodynamic parameters for these lactones [55].

Table 2.
The ROP of δ-VL over 24 h catalysed by 1, 2 [Sn(Oct)2], and [Sn(OtBu)4)].
1H NMR and mass spectra of the PVL again indicated that the products contained both linear and cyclic species. The MALDI-TOF spectra revealed several families of products, including H/OH- and H/OMe-terminated polymers and cyclic polymers (e.g., Figure 8, Figure 9 and Figure 10; expansions are given as inserts). As for PCL, there was evidence of transesterification, and all observed PVL Mn values were significantly lower than the calculated values.
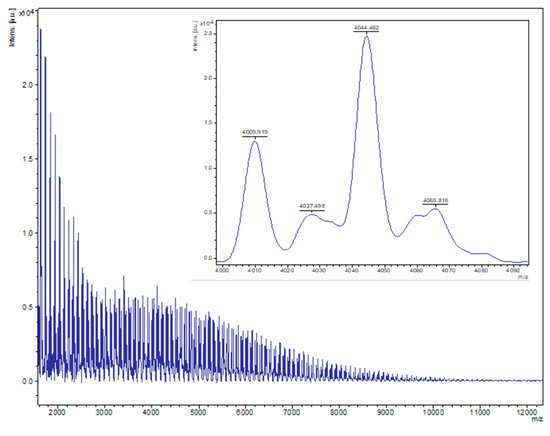
Figure 8.
MALDI-ToF spectrum of PVL obtained from entry 3, Table 2 (1, 500:1, toluene, N2). The main family is composed of chain polymers (terminated by 2 OH groups) as sodium adducts [M = 17 (OH) + 1(H) + n × 114.14 (CL) + 22.99 (Na+)], e.g., for n = 40, calc. 4046.6, obsv. 4044.5.
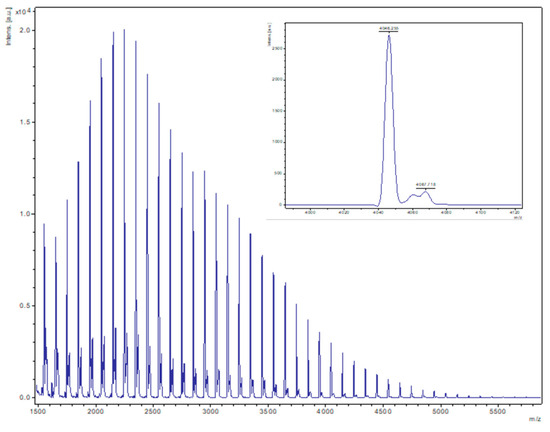
Figure 9.
MALDI-ToF spectrum of PVL obtained from entry 10, Table 2 (2, 500:1 melt, N2). The main family is composed of chain polymers (terminated by 2 OH groups) as sodium adducts [M = 17 (OH) + 1(H) + n × 114.14 (CL) + 22.99 (Na+)], e.g., for n = 40, calc. 4046.6 obsv. 4046.3.
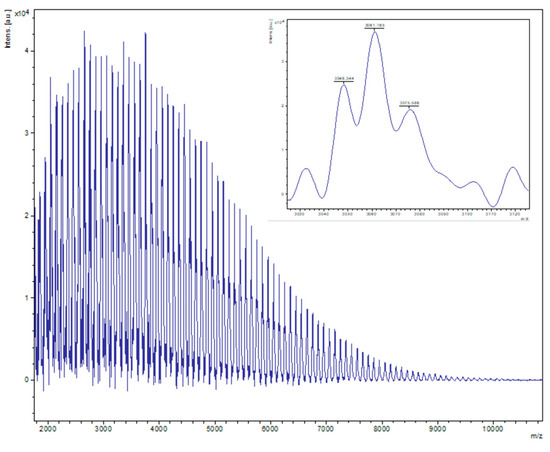
Figure 10.
PVL obtained from entry 10, Table 2 (2, 500:1 melt, air). The main family is composed of chain polymers (terminated by OH/OMe groups) as sodium adducts [M = 17 (OH) + 1(H) + n × 114.14 (CL) + 22.99 (Na+)], e.g., for n = 30, calc. 3059.2, obsv. 3061.2.
A kinetic study (Figure 11) conducted using 500:1 ([ε-CL]:[cat]) at 110 °C revealed the rate trend [Sn(Oct)2] > 1 > [Sn(OtBu)4] > 2. In this case, sluggish behaviour is only observed for 1 over about 12 h and, as in the case of ε-CL, for Sn(OtBu)4 over about 25 h. For the individual kinetic traces, see Figures S24–S27 in the Supplementary Materials.

Figure 11.
Kinetic runs using [δ-VL]:[cat] = 500:1 at 110 °C in toluene.
4. TGA Measurements
The stability of the complexes at the polymerization temperature was checked by TGA. The runs indicated that both systems were stable, and in the case of 1∙2MeCN, only solvent of crystallization (MeCN) was lost (with calc./obsv. values of ~7%) (see Figure 12).
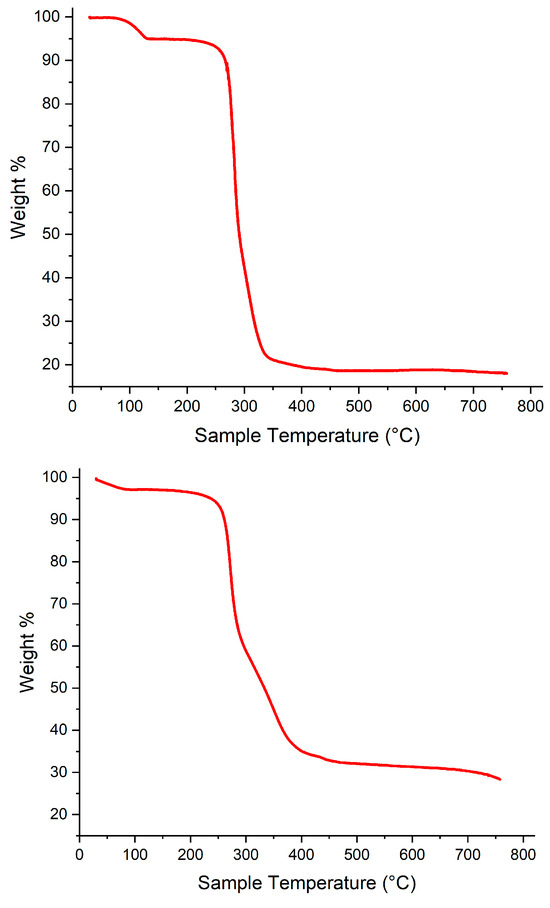
Figure 12.
TGAs of top 1∙2MeCN and bottom 2.
5. Materials and Methods
All manipulations were carried out under an atmosphere of nitrogen using standard Schlenk line and cannula techniques or a conventional N2-filled glove box. Solvents were refluxed over the appropriate drying agents and distilled and degassed prior to use, i.e., toluene was refluxed over Na; acetonitrile was refluxed over calcium hydride. These were purchased from commercial sources and used directly. ε-Caprolactone (Fisher Scientific, Loughborough, UK) and δ-valerolactone (Sigma Aldrich, Gillingham, UK) were dried over CaH2 and then distilled. Tin tert-butoxide (Sigma Aldrich, UK) was stored under nitrogen in a dry box. 2,2′-diphenylglycine (Sigma Aldrich, UK) and benzilic acid (Sigma Aldrich, UK) were dried under vacuum at 80 °C for 4 h prior to use. Elemental analyses were performed at the London Metropolitan University or Xi’an Rare Metal Materials Research Institute Co., Ltd. (Xi’an, China). FTIR spectra (nujol mulls, KBr windows) were recorded on a Nicolet Avatar 360 FT-IR spectrometer. 1H NMR spectra were recorded at 400.2 MHz on a JEOL ECZ 400S spectrometer (Peabody, MA, USA), with TMS δH = 0 as the internal standard or residual protic solvent; chemical shifts are given in ppm (δ). Matrix-Assisted Laser Desorption/Ionization–Time of Flight (MALDI-TOF) mass spectrometry was performed on a Bruker III smart beam in linear mode. MALDI-TOF mass spectra were acquired by averaging at least 100 laser shots. Molecular weights were calculated from the experimental traces using the OmniSEC software (Malvern Panalytical Ltd., Malvern, UK, v11.35). For the TGA runs, data were collected on a PerkinElmer TGA 400 (Shelton, CT, USA) using PyrisTM software (v11.0) and a rate of 10 °C per min over the 30 °C to 800 °C under N2. Sample weights were typically between 3 and 5 mg.
5.1. Synthesis of {Sn[Ph2C(NH2)(CO2)]4}∙2MeCN (1∙2MeCN)
Ph2C(NH2)CO2H (1.00 g, 4.40 mmol) and Sn(OtBu)4 (0.90 g (0.85 mL), 2.20 mmol) were refluxed in toluene (20 mL) for 12 h. On cooling, the volatiles were removed in vacuo, and the residue was extracted into warm MeCN (30 mL). Removal of the MeCN afforded a white solid. Yield: 1.02 g, 84% (based on dpgH). C56H48N4O8Sn∙2MeCN requires C 65.17, H 4.92, N 7.60%. Found C 65.26, H 4.89, N 7.71%. IR: 3406bw, 3185bw, 2357w, 2336w, 1958w, 1867w, 1659m, 1575s, 1489m, 1403s, 1318m, 1277m, 1261s, 1209w, 1169m, 1158m, 1094s, 1029s, 941w, 918w, 895m, 803s, 764m, 721m, 699s, 676w, 638m. M.S. 1023 (M+–2MeCN). 1H NMR (CDCl3) δ: 7.21 (bs, 40H, arylH), 3.45 (bs, 8H, NH2), 1.96 (s, 6H, MeCN).
5.2. Synthesis of {Sn[Ph2C(O)(CO2)]2(CH3CO2H)2} (2)
Ph2C(OH)CO2H (1.00 g, 4.38 mmol) and Sn(OtBu)4 (0.60 g, 1.46 mmol) were refluxed in toluene (20 mL) for 12 h. On cooling, the volatiles were removed in vacuo, and the residue was extracted into warm MeCN (30 mL). Removal of the MeCN afforded a white solid. Yield: 0.92 g, 91% (based on Sn). C32H28O10Sn requires C 55.60, H 4.08%. Found C 55.14, H 3.93%. IR: 1957w, 1881w, 1810w, 1650s, 1560s, 1300s, 1281s, 1210m, 1164s, 1085m, 1047s, 1029s, 1002m, 985m, 940w, 906m, 822m, 783m, 758m, 723s, 698m, 621w. M.S. 404 (M+—acetic acid—doubly deprotonated benzilic acid). 1H NMR (CDCl3) δ: 7.63 (dd, 1H, J 8.0 Hz, J’ 2.4 Hz, arylH), 7.51–7.41 (overlapping m, 7H, arylH), 7.33 (bm, 8H, arylH), 7.17 (bm, 2H, arylH), 7.05 (bm, 2H, arylH), 1.85 (bs, 2H, OH), 1.26 (s, 6H, Me).
5.3. ROP of ɛ-Caprolactone (ε-CL) and δ-Valerolactone (δ-VL)
The pre-catalyst (0.010 mmol) was added to a Schlenk tube in the glovebox at room temperature. For ROPs in solution, toluene (5 mL) was added. The appropriate amount of ε-CL (or δ-VL) was added, and the reaction mixture was then placed into a sand bath pre-heated at 130 °C and heated for the prescribed time (24 h) under either N2 or air. The polymerization mixture was quenched on addition of an excess of glacial acetic acid (0.2 mL) in methanol (50 mL). The resultant polymer was then collected on filter paper and dried in vacuo. GPC (in THF) were used to determine molecular weights (Mn and Ð) of the polymer products.
5.4. Kinetic Studies
The polymerizations were carried out at 110 °C in toluene (2 mL) using 0.010 mmol of complex. The molar ratio of monomer to initiator to co-catalyst was fixed at 500:1, and at appropriate time intervals, 0.5 μL aliquots were removed (under N2) and were quenched with wet CDCl3. The percent conversion of monomer to polymer was determined using 1H NMR spectroscopy.
5.5. X-Ray Crystallography
In both cases, crystals suitable for an X-ray diffraction study were grown from a saturated MeCN solution at 0 °C. Single crystal X-ray diffraction data were collected by the UK National Crystallography Service (NCS, Southampton, UK) using a Rigaku Oxford Diffraction diffractometer operating with a rotating anode X-ray generator and HyPix 6000HE detector (Neu-Isenberg, Germany). Table 3 contains basic crystallographic data and refinements details. It happens that one structure was collected using a Cu source and one with a Mo source; this normally reflects which instrument is available at the NCS at the time the sample is studied. There are not technical reasons for the different choice of sources. Samples were mounted on Mitegen loops and held at 100 K using an Oxford Cryosystems nitrogen gas cryostream. Both structures were solved and refined routinely [56,57,58]. H atoms were included in a riding model; Uiso(H) was set to 120% of that of the carrier atoms except for OH, NH3, and CH3 (150%). Further details are presented in Table 2. CCDC 2410598-9 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures (accessed on 10 January 2025).

Table 3.
Crystallographic data for 1·2MeCN and 2.
6. Conclusions
In conclusion, the use of the acids Ph2C(X)CO2H on reaction with tin tert-butoxide afforded the complexes {Sn[Ph2C(NH2)(CO2)]4}·2MeCN (1·2MeCN) for X = NH2 or the complex {Sn[Ph2C(O)(CO2)]2(CH3CO2H)2} (2) for X = OH. These tin-based systems are active as catalysts for the ROP of ε-caprolactone and δ-valerolactone when employed in solution (toluene) or as melts under either air or N2. The products are of low to high molecular weight for PCL (1150–48,750 Da) and low to moderate molecular weight for PVL (1630–7720 Da), generally with broad Ð (1.48–3.81 for PCL; 1.29–3.24 for PVL). A number of families were evident in the MALDI-ToF mass spectra, with polymers present assigned to those terminated with H/OH, H/OMe, and cyclic polymers. Kinetic profiles indicated that [Sn(Oct)2] outperformed 1 and 2, though under certain conditions, 1 and 2 afforded high-molecular weight products with better control.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15030261/s1, Figure S1. An alternative view of 1∙2MeCN. Figure S2. An alternative view of 2. Figures S3 and S4. 1H NMR spectra of 1∙2MeCN and 2. Figures S5–S11. Selected gpc traces for PCL. Figures S12–S15. Kinetic profiles for PCL formation using 1, 2, [Sn(Oct)2] and [Sn(OtBu)4]. Figures S16–S23. Selected gpc traces for PVL. Figures S24–S27. Kinetic profiles for PVL formation using 1, 2, [Sn(Oct)2] and [Sn(OtBu)4].
Author Contributions
T.J.P.: Investigation, writing—review and editing. C.R.: Conceptualization, writing—original draft, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by UKRI Creative Circular Plastic grant (EP/S025537/1).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
CR thanks the EPSRC for support (grant no. EP/S025537/1). The EPSRC National Crystallographic Service Centre at Southampton University is thanked for data collection of 1·2MeCN and 2.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Thomas, C.M. Stereocontrolled ring-opening polymerization of cyclic esters: Synthesis of new polyester microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef]
- Arbaoui, A.; Redshaw, C. Metal Catalysts for ε-caprolactone polymerisation. Polym. Chem. 2010, 1, 801–826. [Google Scholar] [CrossRef]
- Sarazin, Y.; Carpentier, J.-F. Discrete Cationic Complexes for Ring-Opening Polymerization Catalysis of Cyclic Esters and Epoxides. Chem. Rev. 2015, 115, 3564–3614. [Google Scholar] [CrossRef]
- Redshaw, C. Use of Metal Catalysts Bearing Schiff Base Macrocycles for the Ring Opening Polymerization (ROP) of Cyclic Esters. Catalysts 2017, 7, 165. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, D.; Zhang, W.; Solan, G.A.; Ma, Y.; Sun, W.-H. Recent progress in the application of group 1, 2 & 13 metal complexes as catalysts for the ring opening polymerization of cyclic esters. Inorg. Chem. Front. 2019, 6, 2619–2652. [Google Scholar] [CrossRef]
- Lyubov, D.M.; Tolpygin, A.O.; Trifonov, A.A. Rare-earth metal complexes as catalysts for ring-opening polymerization of cyclic esters. Coord. Chem. Rev. 2019, 392, 83–145. [Google Scholar] [CrossRef]
- Santoro, O.; Redshaw, C. Use of Titanium Complexes Bearing Diphenolate or Calix[n]arene Ligands in α-Olefin Polymerization and the ROP of Cyclic Esters. Catalysts 2020, 10, 210. [Google Scholar] [CrossRef]
- Santoro, O.; Zhang, X.; Redshaw, C. Synthesis of biodegradable polymers: A review on the use of Schiff-base metal complexes as catalysts for the Ring Opening Polymerization (ROP) of cyclic esters. Catalysts 2020, 10, 800. [Google Scholar] [CrossRef]
- Munzeiwa, W.A.; Omondi, B.O.; Nyamori, V.O. Perspective into ring-opening polymerization of ε-caprolactone and lactides: Effect of, ligand, catalyst structure and system dynamics, on catalytic activity and polymer properties. Polym. Bull. 2024, 81, 9419–9464. [Google Scholar] [CrossRef]
- Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB0308694.htm (accessed on 14 January 2025).
- Kowalski, A.; Duda, A.; Penczek, S. Kinetics and mechanism of cyclic esters polymerization initiated with Tin(II) Octoate, 1. Polymerization of ε-caprolactone. Macromol. Rapid Commun. 1998, 19, 567–572. [Google Scholar] [CrossRef]
- Kricheldof, H.R.; Kreiser-Saunder, I.; Sticker, A. Polylactones 48. SnOct2-initiated polymerizations of lactide: A mechanistic study. Macromolecules 2000, 33, 702–709. [Google Scholar] [CrossRef]
- Duda, A.; Penczek, S.; Kowalski, A.; Libiszowski, J. Polymerizations of ε-caprolactone and L,L-dilactide initiated with tin octoate and tin butoxide a comparison. Macromol. Symp. 2000, 153, 41–53. [Google Scholar] [CrossRef]
- Byers, J.A.; Biernesser, A.B.; Delle Chiaie, K.R.; Kaur, A.; Kehl, J.A. Catalytic Systems for the Production of Poly(lactic acid). In Synthesis, Structure and Properties of Poly(lactic acid); Springer: Cham, Switzerland, 2017; p. 67. [Google Scholar]
- Kricheldorf, H.R.; Lee, S.R. Polylactones. 35. Macrocyclic and Stereoselective Polymerization of .beta.-D,L-Butyrolactone with Cyclic Dibutyltin Initiators. Macromolecules 1995, 28, 6718–6725. [Google Scholar] [CrossRef]
- Finne, A.; Albertsson, A.C. Controlled Synthesis of Star-Shaped l-Lactide Polymers Using New Spirocyclic Tin Initiators. Biomacromolecules 2002, 3, 684–690. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Fechner, B. Polylactones. 59. Biodegradable Networks via Ring-Expansion Polymerization of Lactones and Lactides with a Spirocyclic Tin Initiator. Biomacromolecules 2002, 3, 691–695. [Google Scholar] [CrossRef]
- Storey, R.F.; Mullen, B.D.; Dsai, G.S.; Sherman, J.W.; Tang, C.N. Soluble tin(II) macroinitiator adducts for the controlled ring-opening polymerization of lactones and cyclic carbonates. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3434–3442. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Fechner, B. Polylactones. LVIII. Star-Shaped Polylactones with Functional End Groups via Ring-Expansion Polymerization with a Spiro initiator. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 1047–1057. [Google Scholar] [CrossRef]
- Odelius, K.; Finne, A.; Albertsson, A.C. Versatile and controlled synthesis of resorbable star-shaped polymers using a spirocyclic tin initiator—Reaction optimization and kinetics. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 596–605. [Google Scholar] [CrossRef]
- Odelius, K.; Albertsson, A.C. Precision synthesis of microstructures in star-shaped copolymers of ϵ-caprolactone, L-lactide, and 1,5-dioxepan-2-one. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 1249–1264. [Google Scholar] [CrossRef]
- Ma, W.-A.; Wang, Z.-X. Synthesis and characterisation of aluminium (III) and tin (II) complexes bearing quinoline-based N,N,O-tridentate ligands and their catalysis in the ring-opening polymerisation of ε-caprolactone. Dalton Trans. 2011, 40, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Phomphrai, K.; Pongchan-o, C.; Thumrongpatanaraks, W.; Sangtrirutnugul, P.; Kongsaeree, P.; Pohmakotr, M. Synthesis of high-molecular-weight poly(ε-caprolactone) catalyzed by highly active bis(amidinate) tin(II) complexes. Dalton Trans. 2011, 40, 2157–2159. [Google Scholar] [CrossRef]
- Piromjitpong, P.; Ratanapanee, P.; Thumrongpatanaraks, W.; Kongsaeree, P.; Phomphrai, K. Synthesis of cyclic polylactide catalysed by bis(salicylaldiminato)tin(II) complexes. Dalton Trans. 2012, 41, 12704–12710. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, K.M.; Jang, J.; Kim, E.; Chung, J.S.; Do, Y.; Yoon, S.C.; Park, S.Y. Characteristics of silica-supported tin(II) methoxide catalysts for ring-opening polymerization (ROP) of L-lactide. J. Mol. Cat. A Chem. 2014, 385, 68–72. [Google Scholar] [CrossRef]
- Limwanich, W.; Khunmanee, S.; Kungwan, N.; Punyodom, W.; Meepowpan, P. Effect of tributyltin alkoxides chain length on the ring-opening polymerization of ε-caprolactone: Kinetics studies by non-isothermal DSC. Thermochim. Acta 2015, 599, 1–7. [Google Scholar] [CrossRef]
- Punyodom, W.; Limwanich, W.; Meepowpan, P. Tin(II) n-butyl L-lactate as novel initiator for the ring-opening polymerization of epsilon-caprolactone: Kinetics and aggregation equilibrium analysis by non-isothermal DSC. Thermochim. Acta 2017, 655, 337–343. [Google Scholar] [CrossRef]
- Praban, S.; Yimthachote, S.; Kiriratnikom, J.; Chotchatchawankul, S.; Tantirungrotechai, J.; Phomphrai, K. Synthesis and characterizations of bis(phenoxy)-amine tin(II) complexes for ring-opening polymerization of lactide. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2104–2112. [Google Scholar] [CrossRef]
- Sriyai, M.; Chaiwon, T.; Molloy, R.; Meepowpan, P.; Punyodom, W. Efficiency of liquid tin(II) n-alkoxide initiators in the ring-opening polymerization of l-lactide: Kinetic studies by non-isothermal differential scanning calorimetry. RSC Adv. 2020, 10, 43566–43578. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Weidner, S.M. ROP of L-lactide and ε-caprolactone catalyzed by tin(ii) and tin(iv) acetates–switching from COOH terminated linear chains to cycles. J. Polym. Sci. 2021, 59, 439–450. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Weidner, S.M. Syntheses of polylactides by means of tin catalysts. Polym. Chem. 2022, 13, 1618–1647. [Google Scholar] [CrossRef]
- Novák, M.; Milasheuskaya, Y.; Srb, M.; Podzimek, S.; Bouška, M.; Jambor, R. Synthesis of star-shaped poly(lactide)s, poly(valerolactone)s and poly(caprolactone)s via ROP catalysed by N-donor tin(II) cations and comparison of their wetting properties with linear analogues. RSC Adv. 2024, 14, 23273–23285. [Google Scholar] [CrossRef] [PubMed]
- Funfuenha, W.; Punyodom, W.; Meepowpan, P.; Limwanich, W. Microwave-assisted solvent-free ring-opening polymerization of ε-caprolactone initiated by n-butyltin(IV) chlorides. Polym. Bull. 2024, 81, 475–490. [Google Scholar] [CrossRef]
- Prior, T.J.; Burke, B.P.; Roberts, D.P.; Archibald, S.J.; Stasiuk, G.; Higham, L.J.; Redshaw, C. Water-Soluble Rhenium Phosphine Complexes Incorporating the Ph2C(X) Motif (X = O−, NH−): Structural and Cytotoxicity Studies. Inorg. Chem. 2020, 59, 2367–2378. [Google Scholar] [CrossRef]
- Al-Khafaji, Y.F.; Elsegood, M.R.J.; Frese, J.W.A.; Redshaw, C. Ring opening polymerization of lactides and lactones by multimetallic alkyl zinc complexes derived from the acids Ph2C(X)CO2H (X = OH, NH2). RSC Adv. 2017, 7, 4510–4517. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, K.-Q.; Al-Khafaji, Y.F.; Mo, S.; Prior, T.J.; Elsegood, M.R.J.; Redshaw, C. Organoaluminium complexes derived from anilines or Schiff bases for the ring-opening polymerization of ε-caprolactone, δ-valerolactone and rac-lactide. Eur. J. Inorg. Chem. 2017, 2017, 1951–1965. [Google Scholar] [CrossRef]
- Al-Khafaji, Y.F.; Prior, T.J.; Horsburgh, L.; Elsegood, M.R.J.; Redshaw, C. Ring opening polymerization of lactides and lactones by multimetallic lithium complexes derived from the acids Ph2C(X)CO2H (X = OH, NH2). Chem. Select 2017, 2, 759–768. [Google Scholar] [CrossRef]
- Collins, J.; Santoro, O.; Prior, T.J.; Chen, K.; Redshaw, C. Rare-earth metal complexes derived from the acids Ph2C(X)CO2H (X = OH, NH2): Structural and ring opening polymerization (ROP) studies. J. Mol. Struct. 2021, 1224, 129083. [Google Scholar] [CrossRef]
- Alshamrani, A.F.A.; Santoro, O.; Ounsworth, S.; Prior, T.J.; Stasiuk, G.J.; Redshaw, C. Synthesis, characterisation and ROP catalytic evaluation of Cu(II) complexes bearing 2,2′-diphenylglycine-derived moieties. Polyhedron 2021, 195, 114977. [Google Scholar] [CrossRef]
- Zhang, X.; Prior, T.J.; Chen, K.; Santoro, O.; Redshaw, C. Ring opening polymerization of lactides and lactones by multimetallic titanium complexes derived from the acids Ph2C(X)CO2H (X = OH, NH2). Catalysts 2022, 12, 935. [Google Scholar] [CrossRef]
- Zhang, X.; Prior, T.J.; Redshaw, C. Niobium and Tantalum complexes derived from the acids Ph2C(X)CO2H (X = OH, NH2): Synthesis, structure and ROP capability. New J. Chem. 2022, 46, 14146–14154. [Google Scholar] [CrossRef]
- Glenister, M.A.; Frese, J.W.A.; Elsegood, M.R.J.; Canaj, A.B.; Brechin, E.; Redshaw, C. Reaction of Ph2C(X)(CO2H) (X = OH, NH2) with [VO(OR)3] (R = Et, nPr): Structure, magnetic susceptibility and ROP capability. Dalton Trans. 2024, 53, 5351–5355. [Google Scholar] [CrossRef]
- Braun, M. The “Magic” Diarylhydroxymethyl Group. Angew. Chem. 1996, 35, 519–522. [Google Scholar] [CrossRef]
- Coxall, R.A.; Harris, S.G.; Henderson, D.K.; Parsons, S.; Tasker, P.A.; Winpenny, R.E.P. Inter-ligand reactions: In situ formation of new polydentate ligands. J. Chem. Soc. Dalton Trans. 2000, 2349–2356. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C. Graph-Set Analysis of Hydrogen-Bond Patterns in Organic Crystals. Acta Cryst. 1990, B46, 256–262. [Google Scholar] [CrossRef]
- Delgado, G.E.; Mora, A.J.; Ramírez, B.; Díaz de Delgado, G.; Cisterna, J.; Cádenas, A.; Brito, I. Synthesis, Crystal Structure and Hirshfeld Surface Analysis of a new Coordination Polymer: Strontium Benzilate. J. Chil. Chem. Soc. 2021, 66, 5081–5085. [Google Scholar] [CrossRef]
- March, J. Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 2nd ed.; McGraw-Hill, Inc.: New York, NY, USA, 1977. [Google Scholar]
- Parris, C.L.; Christenson, R.M. N-Alkylation of Nitriles with Benzyl Alcohol, Related Alcohols, and Glycols. J. Org. Chem. 1960, 25, 331. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, X.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. Mechanism of TBD-catalyzed hydrolysis of acetonitrile. J. Mol. Struct Theochem. 2009, 911, 40–45. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wang, F.-C.; Ko, B.-T.; Yu, T.-L.; Lin, C.-C. Ring-Opening Polymerization of ε-Caprolactone and L-Lactide Using Aluminum Thiolates as Initiator. Macromolecules 2001, 34, 356–361. [Google Scholar] [CrossRef]
- Save, M.; Schappacher, M.; Soum, A. Controlled Ring-Opening Polymerization of Lactones and Lactides Initiated by Lanthanum Isopropoxide, 1. General Aspects and Kinetics. Macromol. Chem. Phys. 2002, 203, 889–899. [Google Scholar] [CrossRef]
- Dubois, P.; Coulembier, O.; Raquez, J.-M. (Eds.) Handbook of Ring Opening Polymerization; Wiley-VCH: Hoboken, NJ, USA, 2009; p. 594. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).