A Mini-Review of Recent Progress in Zeolite-Based Catalysts for Photocatalytic or Photothermal Environmental Pollutant Treatment
Abstract
:1. Introduction
2. Principles of Photocatalysis and Photothermal Catalysis
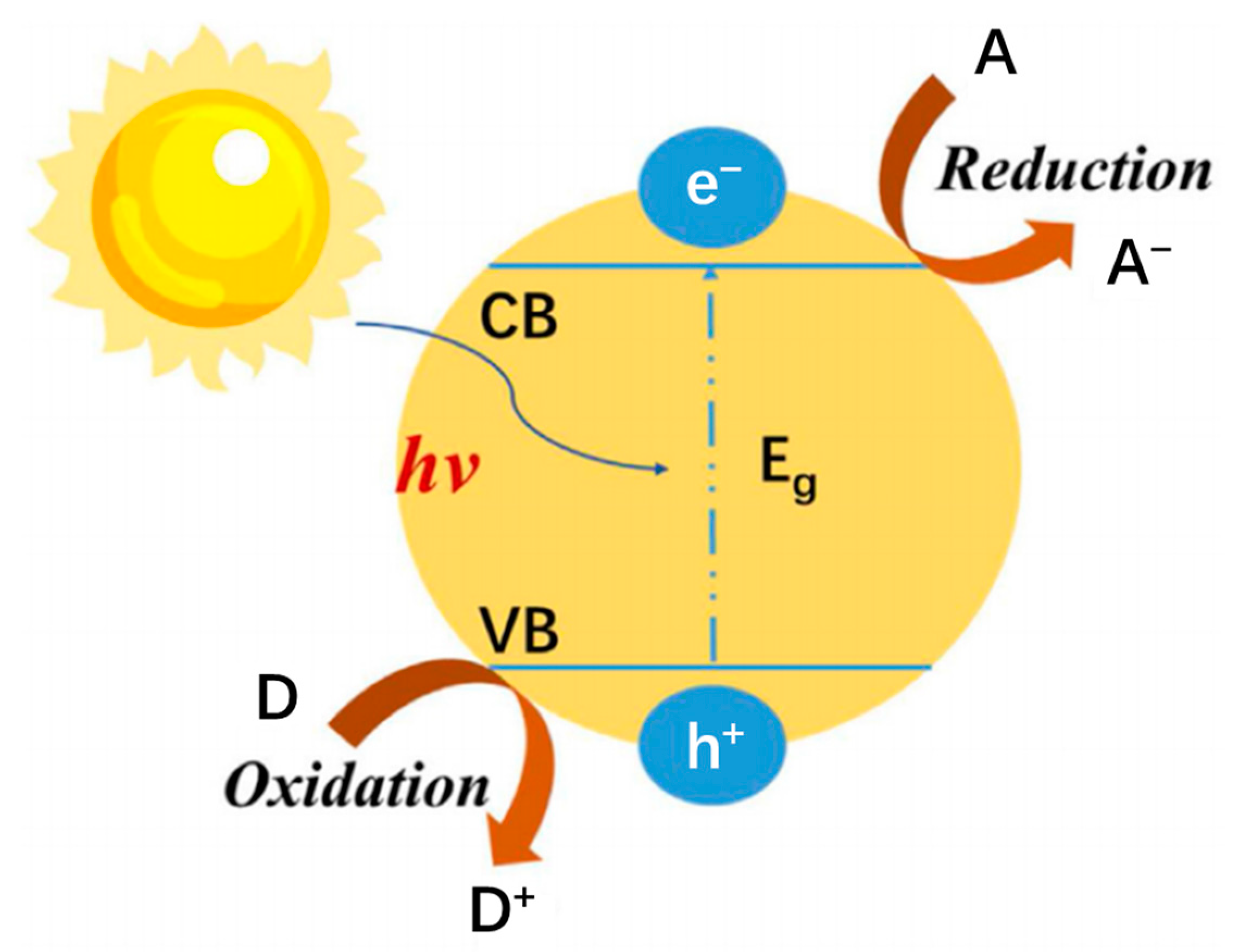
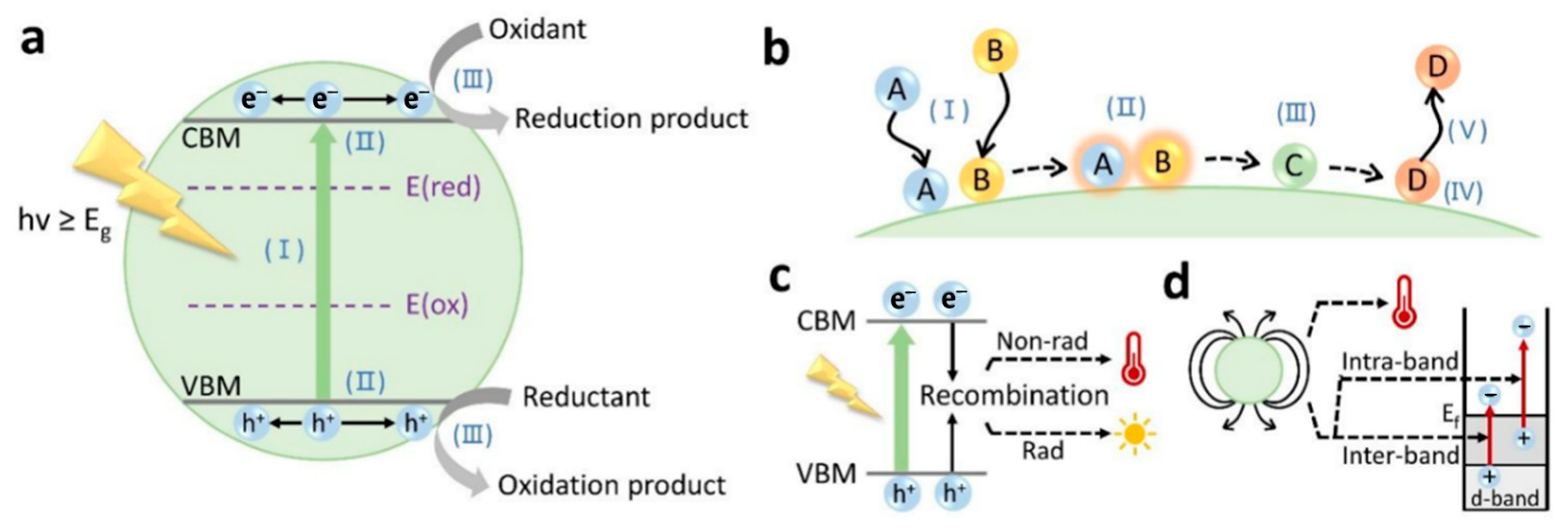
2.1. Principle of Photocatalysis
2.2. Principle of Photothermal Catalysis
3. Modification Strategies for Zeolite Catalysts
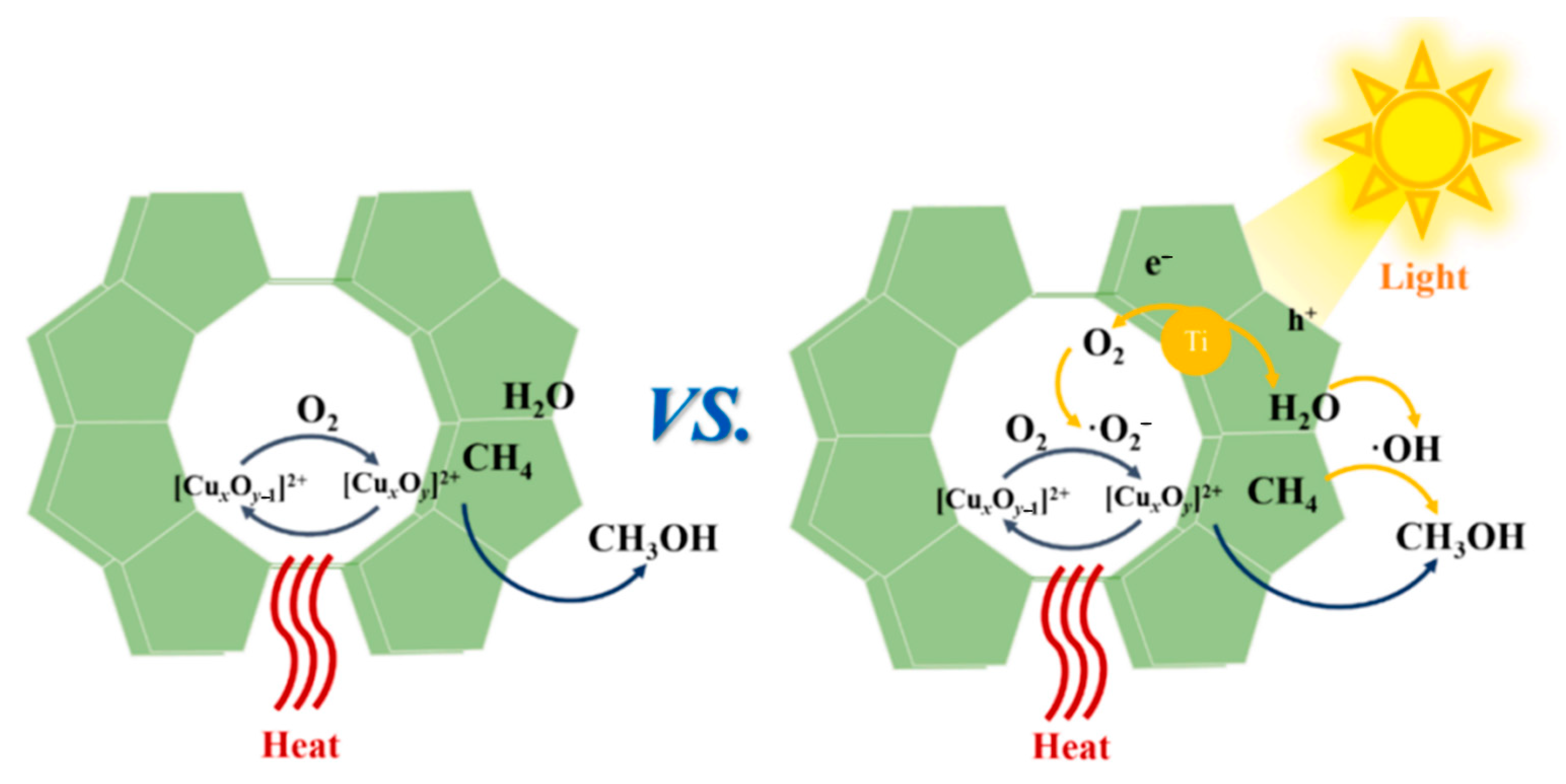
3.1. Construction of Bifunctional Catalysts
3.2. Elemental Doping
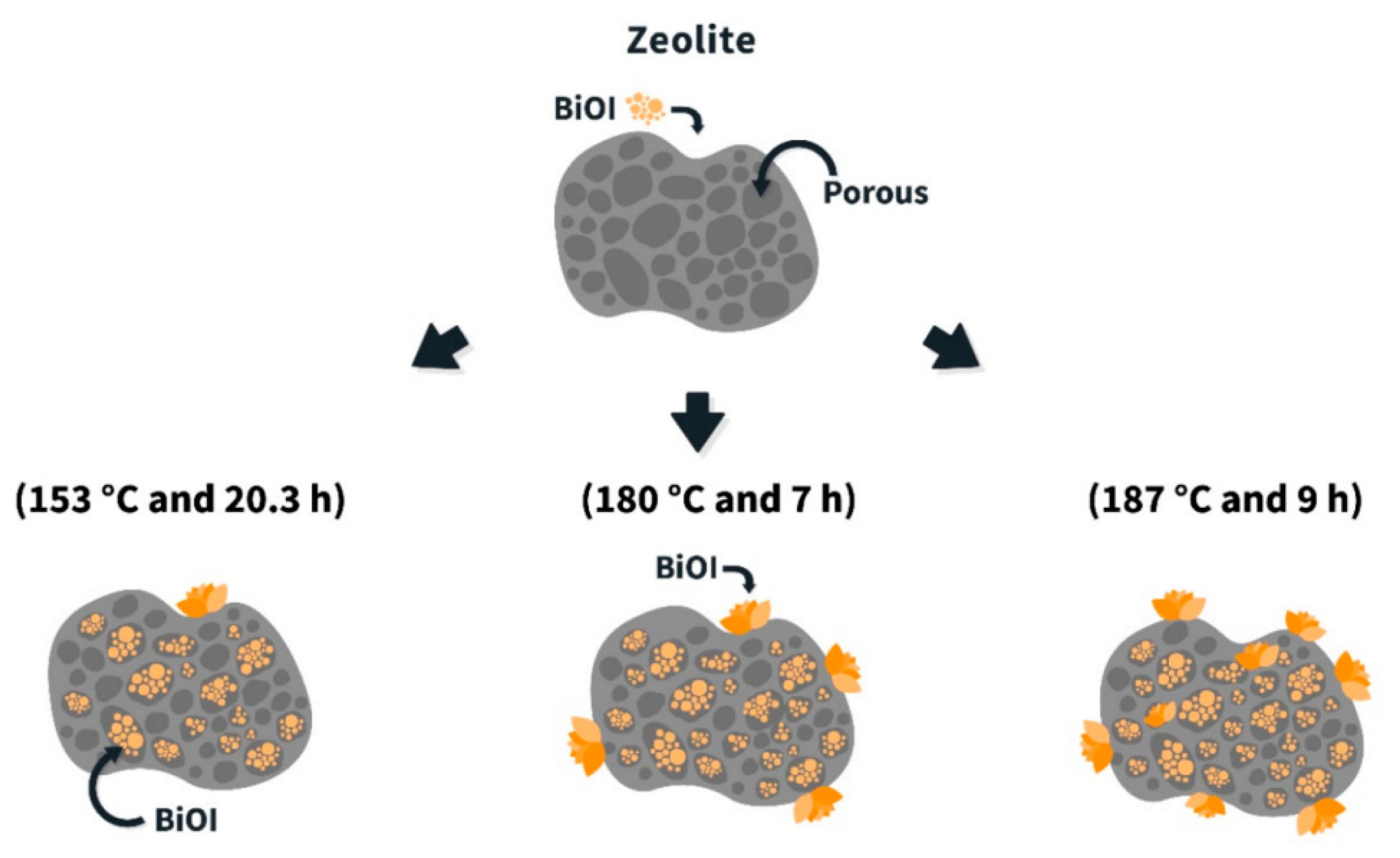
3.3. Structural Modification of Catalysts
4. Advances in Zeolite-Based Catalysts for Photocatalytic/Photothermal Pollutant Treatment
4.1. Photocatalytic and Photothermal Catalytic Degradation of VOCs
| Catalyst | VOC | VOC Concentration | Catalyst Mass (mg) | Temperature (°C) | Optical Density (mW/cm2) | Water Vapor (vol.%) | Conversion (%) | CO2 Selectivity (%) | SBET (m2/g) | Average Pore Size (nm) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.93%Pt-mTiO2/USY | Toluene | 200 | 30 | 243 | 490 | 5 | 86.6 | 74.5 | 487.6 | 2.3 | [34] |
| CuOx-CeO2−x-STO/USY | Toluene | 200 | 30 | 227 | 700 | 10 | 86.2 | 76.6 | 404.6 | 5.4 | [49] |
| 20%CuOx-WOx/mTiO2−x-USY | Toluene | 200 | 30 | 235 | 500 | 5 | 90.4 | 82 | 380.8 | 7.99 | [50] |
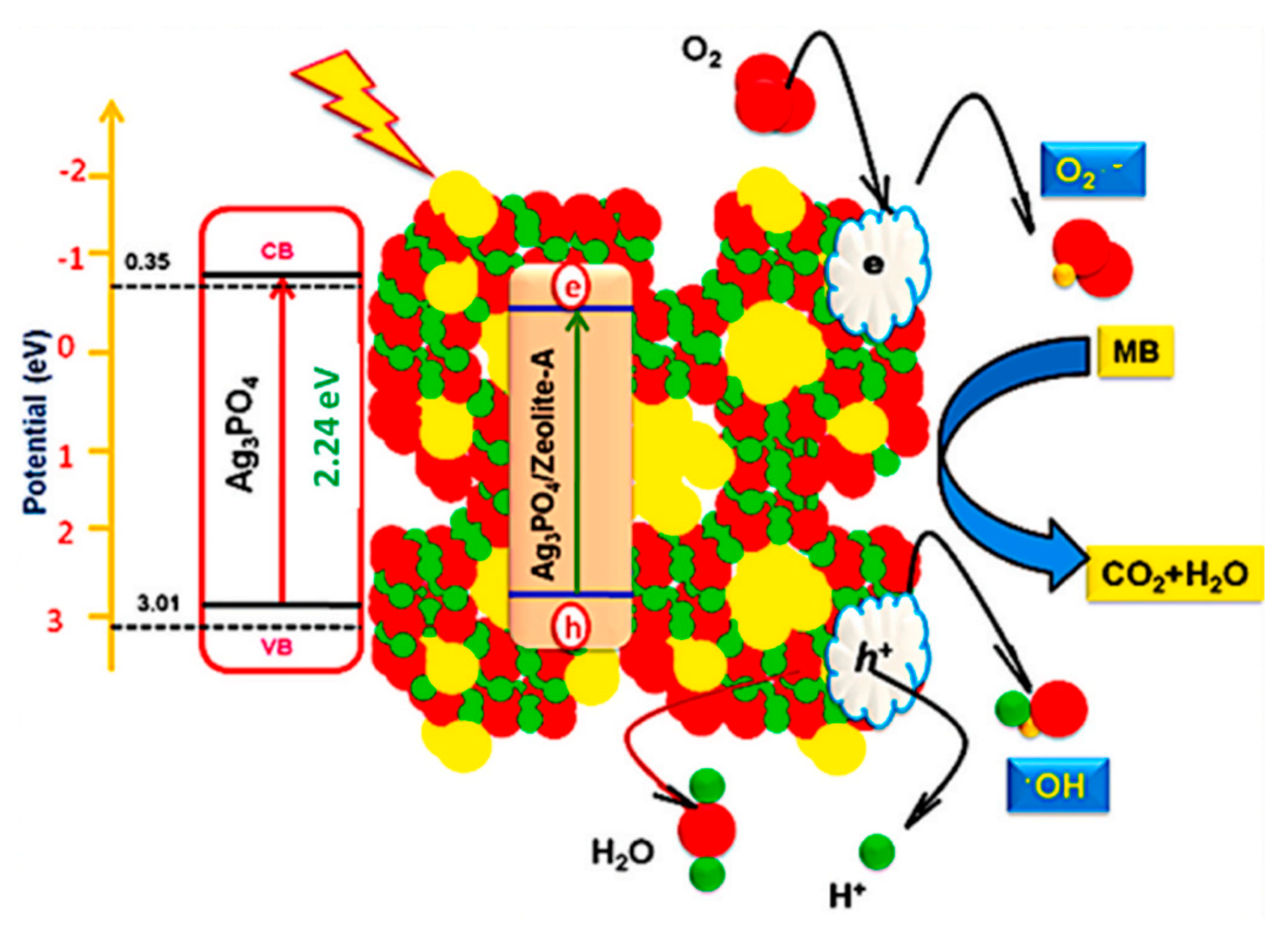
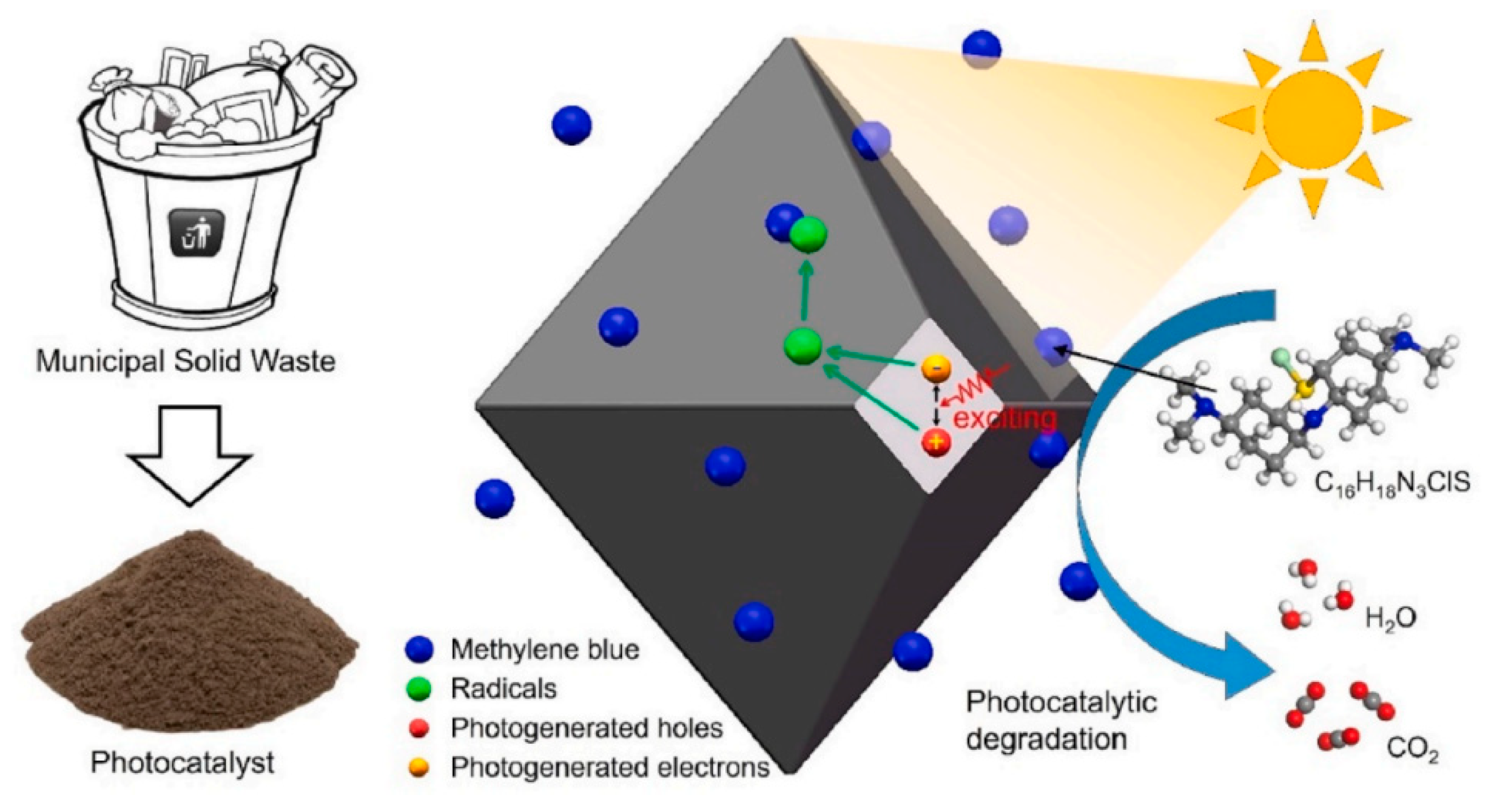
4.2. Application of Photocatalysis and Photothermal Catalysis in Water Treatment
4.3. Secondary Pollution
5. Conclusions and Future Prospects
- (1)
- More reliable zeolite-based catalysts with high catalytic performance need to be developed. In order to promote the application of zeolite-based catalysts, catalysts with high catalytic activity and stability are still scarce. By optimizing the structure of zeolite, element doping, or constructing composite materials, the light absorption, electron transfer, and structural stability of catalysts can be further improved in their practical application for pollutant treatment and energy conversion. In addition, the regeneration and recycling of zeolite is also important to realize the sustainability of environmental treatment.
- (2)
- The role of zeolite in enhancing adsorption capacity needs to be further investigated to improve the catalytic performance, stability, and product selectivity. Improving the surface properties and pore structure of zeolites can help to enhance the adsorption capacity, which is beneficial to improving catalytic stability and selectivity for specific pollutants, thereby realizing environmental purification goals.
- (3)
- The mechanisms and pathways of pollutant degradation over zeolite-based catalysts should be further investigated. Although the reaction systems show good catalytic activity, understanding the degradation process, electron transfer mechanism, and detailed reaction pathway is beneficial to catalyst structure design in photocatalysis and photothermal catalysis.
- (4)
- Zeolite catalysts always show a narrow light absorption range and low utilization efficiency of sunlight, which limits their catalytic activity in practical applications. The regulation of band structure and enhancement of light absorption ability are beneficial to improving the generation of photogenerated charge and light-to-heat conversion ability.
- (5)
- The fast recombination of photogenerated charge is a serious problem that limits the photocatalytic and photothermal catalytic activity. The improvement of charge separation efficiency is important for practical applications of zeolite-based catalysts.
- (6)
- Studies of in situ characterizations during photocatalytic or photothermal reactions are still scarce, which restricts the in-depth understanding of the complex mechanisms in the reactions. Designing a suitable multifunctional in situ reaction cell is also important to reveal the key active species in catalytic reactions. This will help to promote the practical application of zeolite-based catalysts in photocatalytic or photothermal technologies.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, G.; Yang, J.; Duan, X.; Farnood, R.; Yang, C.; Yang, J.; Liu, W.; Liu, Q. Recent Developments and Challenges in Zeolite-Based Composite Photocatalysts for Environmental Applications. Chem. Eng. J. 2021, 417, 129209. [Google Scholar] [CrossRef]
- Dai, L.; Lu, Q.; Zhou, H.; Shen, F.; Liu, Z.; Zhu, W.; Huang, H. Tuning Oxygenated Functional Groups on Biochar for Water Pollution Control: A Critical Review. J. Hazard. Mater. 2021, 420, 126547. [Google Scholar] [CrossRef]
- Ma, R.; Sun, J.; Li, D.H.; Wei, J.J. Review of Synergistic Photo-Thermo-Catalysis: Mechanisms, Materials and Applications. Int. J. Hydrogen Energy 2020, 45, 30288–30324. [Google Scholar] [CrossRef]
- Rashtbari, S.; Dehghan, G.; Marefat, A.; Khataee, S.; Khataee, A. Proficient Sonophotocatalytic Degradation of Organic Pollutants Using Co3O4/TiO2 Nanocomposite Immobilized on Zeolite: Optimization, and Artificial Neural Network Modeling. Ultrason. Sonochem. 2024, 102, 106740. [Google Scholar] [CrossRef] [PubMed]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.-L.; Akhtar, F. Adsorption of Heavy Metals on Natural Zeolites: A Review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhong, Q.; Xu, P.; Huang, H.; Yang, F.; Cao, M.; He, L.; Zhang, Q.; Chen, J. Solar Thermal Catalysis for Sustainable and Efficient Polyester Upcycling. Matter 2022, 5, 1305–1317. [Google Scholar] [CrossRef]
- Pu, Y.; Xie, X.; Jiang, W.; Yang, L.; Jiang, X.; Yao, L. Low-Temperature Selective Catalytic Reduction of NOx with NH3 over Zeolite Catalysts: A Review. Chin. Chem. Lett. 2020, 31, 2549–2555. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, D.; Huang, G.; Li, D.; Zheng, Y. Research and Application Development of Catalytic Redox Technology for Zeolite-Based Catalysts. Catalysts 2023, 13, 1197. [Google Scholar] [CrossRef]
- Yu, Q.; Cheng, H.; Tang, X.; Yi, H.; Ren, X.; Li, Z. Progress in the Synthesis of Small-Pore Zeolites for Purifying NOx from Motor Vehicle Exhaust. J. Clean. Prod. 2022, 381, 135119. [Google Scholar] [CrossRef]
- Zhu, Z.-J.; He, Z.-H.; Tian, Y.; Wang, S.-W.; Sun, Y.-C.; Wang, K.; Wang, W.; Zhang, Z.-F.; Liu, J.; Liu, Z.-T. Mass-Transfer Enhancement in the CO2 Oxidative Dehydrogenation of Propane over GaN Supported on Zeolite Nanosheets with a Short b -Axis and Hierarchical Pores. ACS Catal. 2024, 14, 10376–10391. [Google Scholar] [CrossRef]
- Liaquat, I.; Munir, R.; Abbasi, N.A.; Sadia, B.; Muneer, A.; Younas, F.; Sardar, M.F.; Zahid, M.; Noreen, S. Exploring Zeolite-Based Composites in Adsorption and Photocatalysis for Toxic Wastewater Treatment: Preparation, Mechanisms, and Future Perspectives. Environ. Pollut. 2024, 349, 123922. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Tamizhdurai, P.; Mangesh, V.L.; Ragupathi, C.; Santhana Krishnan, P.; Ramesh, A. Recent Advances in the Synthesis and Applications of Mordenite Zeolite—Review. RSC Adv. 2021, 11, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Baur, R.; Krishna, R. The Effectiveness Factor for Zeolite Catalysed Reactions. Catal. Today 2005, 105, 173–179. [Google Scholar] [CrossRef]
- Manna, M.; Sen, S. A Mechanistic Evaluation for Total Removal of Toxic Hexavalent and Trivalent Chromium from Water by Oxygen Vacancy-Engineered Nanocomposite. J. Environ. Chem. Eng. 2024, 12, 111755. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Hushmandrad, S. Solar Photodecolorization of Methylene Blue by CuO/X Zeolite as a Heterogeneous Catalyst. Appl. Catal. A Gen. 2010, 388, 149–159. [Google Scholar] [CrossRef]
- Umejuru, E.C.; Mashifana, T.; Kandjou, V.; Amani-Beni, M.; Sadeghifar, H.; Fayazi, M.; Karimi-Maleh, H.; Sithole, N.T. Application of Zeolite Based Nanocomposites for Wastewater Remediation: Evaluating Newer and Environmentally Benign Approaches. Environ. Res. 2023, 231, 116073. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, T.; Miyazawa, T.; Shimura, K.; Hirata, S. Jet Fuel Synthesis from Fischer–Tropsch Product under Mild Hydrocracking Conditions Using Pt-Loaded Catalysts. Chem. Eng. J. 2015, 263, 178–185. [Google Scholar] [CrossRef]
- Jaime-Acuña, O.E.; Villavicencio, H.; Díaz-Hernández, J.A.; Petranovskii, V.; Herrera, M.; Raymond-Herrera, O. Atomic and Electronic Structure of Quaternary CdxZnySδOγ Nanoparticles Grown on Mordenite. Chem. Mater. 2014, 26, 6152–6159. [Google Scholar] [CrossRef]
- Rutkowska, M.; Pacia, I.; Basąg, S.; Kowalczyk, A.; Piwowarska, Z.; Duda, M.; Tarach, K.A.; Góra-Marek, K.; Michalik, M.; Díaz, U.; et al. Catalytic Performance of Commercial Cu-ZSM-5 Zeolite Modified by Desilication in NH3-SCR and NH3-SCO Processes. Microporous Mesoporous Mater. 2017, 246, 193–206. [Google Scholar] [CrossRef]
- Yadav, V.K.; Choudhary, N.; Tirth, V.; Kalasariya, H.; Gnanamoorthy, G.; Algahtani, A.; Yadav, K.K.; Soni, S.; Islam, S.; Yadav, S.; et al. A Short Review on the Utilization of Incense Sticks Ash as an Emerging and Overlooked Material for the Synthesis of Zeolites. Crystals 2021, 11, 1255. [Google Scholar] [CrossRef]
- Frising, T.; Leflaive, P. Extraframework Cation Distributions in X and Y Faujasite Zeolites: A Review. Microporous Mesoporous Mater. 2008, 114, 27–63. [Google Scholar] [CrossRef]
- Chen, L.-H.; Sun, M.-H.; Wang, Z.; Yang, W.; Xie, Z.; Su, B.-L. Hierarchically Structured Zeolites: From Design to Application. Chem. Rev. 2020, 120, 11194–11294. [Google Scholar] [CrossRef]
- Gao, W.; Tang, X.; Yi, H.; Jiang, S.; Yu, Q.; Xie, X.; Zhuang, R. Mesoporous Molecular Sieve-Based Materials for Catalytic Oxidation of VOC: A Review. J. Environ. Sci. 2023, 125, 112–134. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Razavi, Z.; Mirghaffari, N.; Alemrajabi, A.A.; Davar, F.; Soleimani, M. Adsorption and Photocatalytic Removal of SO2 Using Natural and Synthetic Zeolites-Supported TiO2 in a Solar Parabolic Trough Collector. J. Clean. Prod. 2021, 310, 127376. [Google Scholar] [CrossRef]
- Shi, M.; Meng, X. Photothermal Catalysis: From Principles to Applications. Int. J. Hydrogen Energy 2023, 48, 34659–34676. [Google Scholar] [CrossRef]
- Fang, S.; Hu, Y.H. Thermo-Photo Catalysis: A Whole Greater than the Sum of Its Parts. Chem. Soc. Rev. 2022, 51, 3609–3647. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Fan, Y.; Dong, X.; Zeng, H.; Ma, X.; Fu, Y. Study on Preparation of UV-CDs/Zeolite-4A/TiO2 Composite Photocatalyst Coupled with Ultraviolet-Irradiation and Their Application of Photocatalytic Degradation of Dyes. J. Environ. Manag. 2024, 354, 120342. [Google Scholar] [CrossRef]
- Tang, J.; Fu, M.; Mao, Y.; Yang, S.; Lin, W.; Yu, Y.; Song, S. Decorating {0 0 1} TiO2 Nanosheets on Hydrophobic NaY Zeolite: An Efficient Deactivation-Resistant Photocatalyst for Gaseous Toluene Removal. Chem. Eng. J. 2023, 472, 144883. [Google Scholar] [CrossRef]
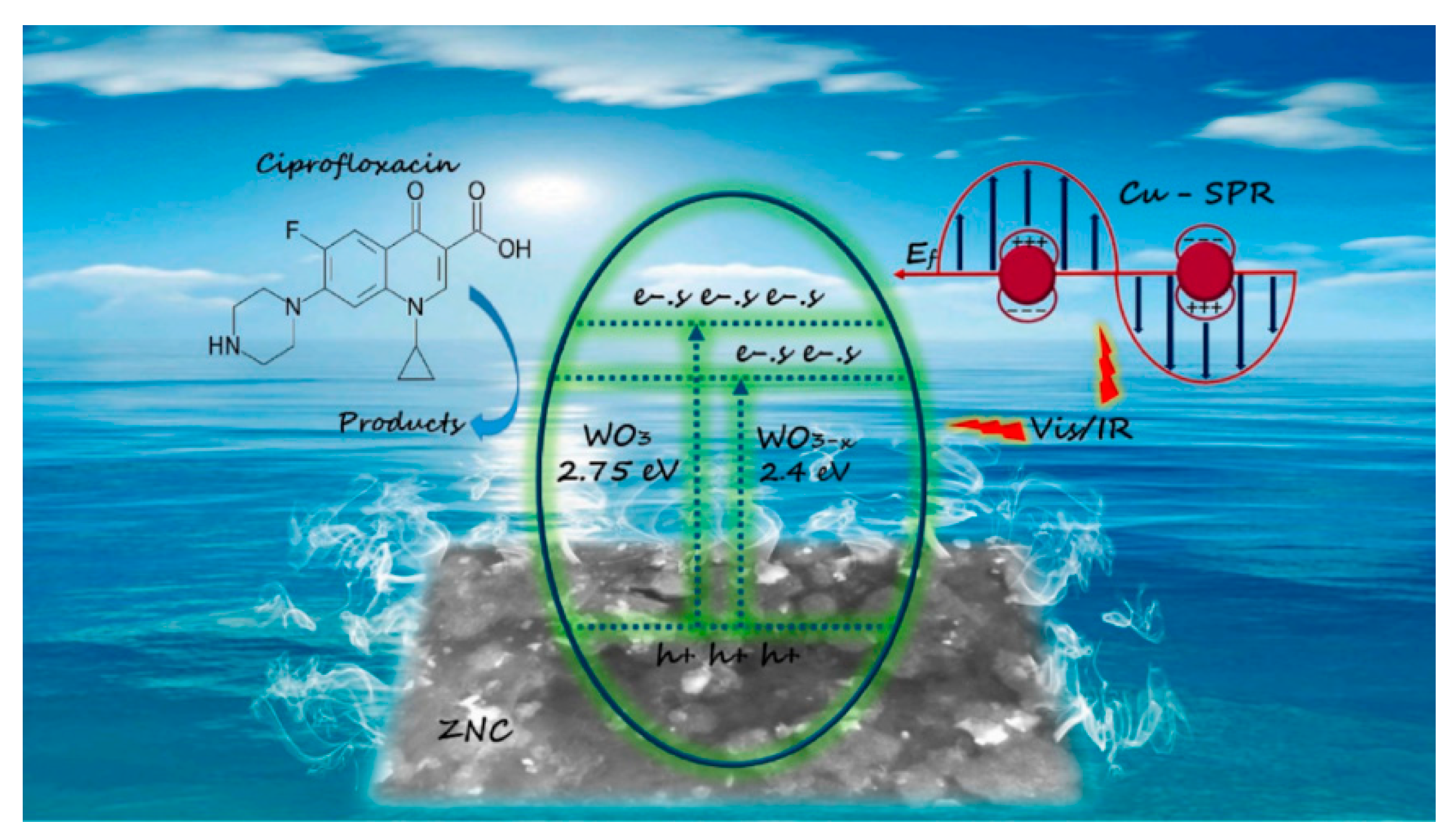
- Zakaria, H.; Li, Y.; Wei, H.; Fathy, M.M.; Zhang, C. Noble Metal-Free Plasmonic Cu/WO3-x@ZNC Bi-Functional Composite for Ciprofloxacin Photocatalytic Degradation and Photothermal Water Evaporation via Visible-Infrared Exposure. Chemosphere 2024, 364, 143309. [Google Scholar] [CrossRef] [PubMed]
- Sacco, O.; Vaiano, V.; Matarangolo, M. ZnO Supported on Zeolite Pellets as Efficient Catalytic System for the Removal of Caffeine by Adsorption and Photocatalysis. Sep. Purif. Technol. 2018, 193, 303–310. [Google Scholar] [CrossRef]
- Hu, G.; Duan, X.; Yang, J.; Yang, C.; Liu, Q.; Ren, S.; Li, J.; Teng, L.; Liu, W. A Novel Conversion of Ti-Bearing Blast Furnace Slag into Ti-Containing Zeolites: Comparison Study between FAU and MFI Type Zeolites. Adv. Powder Technol. 2022, 33, 103559. [Google Scholar] [CrossRef]
- Ke, X.; Ribbens, S.; Fan, Y.; Liu, H.; Cool, P.; Yang, D.; Zhu, H. Integrating Efficient Filtration and Visible-Light Photocatalysis by Loading Ag-Doped Zeolite Y Particles on Filtration Membrane of Alumina Nanofibers. J. Membr. Sci. 2011, 375, 69–74. [Google Scholar] [CrossRef]
- Elimian, E.A.; Zhang, M.; Chen, J.; Jia, H.; Sun, Y.; He, J. Construction of Pt-mTiO2/USY Multifunctional Catalyst Enriched with Oxygen Vacancies for the Enhanced Light-Driven Photothermocatalytic Degradation of Toluene. Appl. Catal. B Environ. 2022, 307, 121203. [Google Scholar] [CrossRef]
- Alakhras, F.; Alhajri, E.; Haounati, R.; Ouachtak, H.; Addi, A.A.; Saleh, T.A. A Comparative Study of Photocatalytic Degradation of Rhodamine B Using Natural-Based Zeolite Composites. Surf. Interfaces 2020, 20, 100611. [Google Scholar] [CrossRef]
- Vaiano, V.; Sannino, D. UV Light Driven Selective Oxidation of Cyclohexane in Gaseous Phase Using Mo-Functionalized Zeolites. Surfaces 2019, 2, 546–559. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Z.; Cui, T.; Li, J.; Zhang, J.; Chen, T.; Wang, X.; Liang, X. Photocatalysis of Two-Dimensional Honeycomb-like ZnO Nanowalls on Zeolite. Chem. Eng. J. 2014, 235, 257–263. [Google Scholar] [CrossRef]
- Znad, H.; Abbas, K.; Hena, S.; Awual, M.R. Synthesis a Novel Multilamellar Mesoporous TiO2/ZSM-5 for Photo-Catalytic Degradation of Methyl Orange Dye in Aqueous Media. J. Environ. Chem. Eng. 2018, 6, 218–227. [Google Scholar] [CrossRef]
- Fernández-Catalá, J.; Sánchez-Rubio, M.; Navlani-García, M.; Berenguer-Murcia, Á.; Cazorla-Amorós, D. Synthesis of TiO2/Nanozeolite Composites for Highly Efficient Photocatalytic Oxidation of Propene in the Gas Phase. ACS Omega 2020, 5, 31323–31331. [Google Scholar] [CrossRef] [PubMed]
- Suárez, S. Silicalite-1 Synthesized with Geothermal and Ludox Colloidal Silica and Corresponding TiO2/Silicalite-1 Hybrid Photocatalysts for VOC Oxidation. Microporous Mesoporous Mater. 2020, 302, 110202. [Google Scholar] [CrossRef]
- Ko, S.; Pekarovic, J.; Fleming, P.D.; Ari-Gur, P. High Performance Nano-Titania Photocatalytic Paper Composite. Part I: Experimental Design Study for TiO2 Composite Sheet Using a Natural Zeolite Microparticle System and Its Photocatalytic Property. Mater. Sci. Eng. B 2010, 166, 127–131. [Google Scholar] [CrossRef]
- Yan, G.; Long, J.; Wang, X.; Li, Z.; Fu, X. Photoactive Sites in Commercial HZSM-5 Zeolite with Iron Impurities: An UV Raman Study. Comp. Rend. Chim. 2007, 11, 114–119. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Liu, M.; Ge, H.; Zha, W.; Wei, Y.; Fei, E.; Zhang, Z.; Long, J.; Sa, R.; et al. Understanding Structure-Function Relationships in HZSM-5 Zeolite Catalysts for Photocatalytic Oxidation of Isopropyl Alcohol. J. Catal. 2019, 377, 322–331. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.; Park, H.-J.; Park, S.; Kim, J.Y.; Jeong, Y.W.; Yang, H.H.; Choi, Y.; Yeom, M.; Song, D.; et al. Practical Scale Evaluation of a Photocatalytic Air Purifier Equipped with a Titania-Zeolite Composite Bead Filter for VOC Removal and Viral Inactivation. Environ. Res. 2022, 204, 112036. [Google Scholar] [CrossRef]
- Kovalevskiy, N.S.; Lyulyukin, M.N.; Selishchev, D.S.; Kozlov, D.V. Analysis of Air Photocatalytic Purification Using a Total Hazard Index: Effect of the Composite TiO2/Zeolite Photocatalyst. J. Hazard. Mater. 2018, 358, 302–309. [Google Scholar] [CrossRef]
- Fan, Y.; Lyu, C.; Song, W.; Hao, M. Synthesis of Tungsten Iron-Oxide Zeolite Composites and Their Catalytic Degradation in VOC from Rubber Powder Modified Asphalt. Alex. Eng. J. 2024, 94, 212–218. [Google Scholar] [CrossRef]
- Jansson, I.; Suárez, S.; Garcia-Garcia, F.J.; Sánchez, B. Zeolite–TiO2 Hybrid Composites for Pollutant Degradation in Gas Phase. Appl. Catal. B Environ. 2015, 178, 100–107. [Google Scholar] [CrossRef]
- Ma, R.; Chen, W.; Wang, L.; Yi, X.; Xiao, Y.; Gao, X.; Zhang, J.; Tang, X.; Yang, C.; Meng, X.; et al. N-Oxyl Radicals Trapped on Zeolite Surface Accelerate Photocatalysis. ACS Catal. 2019, 9, 10448–10453. [Google Scholar] [CrossRef]
- Agbovhimen Elimian, E.; Zhang, M.; Li, Q.; Sun, Y.; He, J.; Jia, H. Tailoring CuO-CeO2 Active Sites on Strontium Titanate/Ultra-Stable Y Zeolite Catalyst for Photothermal Catalytic Oxidation of Toluene. Sep. Purif. Technol. 2024, 340, 126771. [Google Scholar] [CrossRef]
- Elimian, E.A.; Zhang, M.; Li, Q.; Chen, J.; Sun, Y.; Jia, H.; He, J. Light-Driven Photothermal Catalytic Oxidation of Toluene over CuOx-WOx/mTiO2−x-USY: Revealing CuOx-WOx Synergy. Appl. Catal. B Environ. 2023, 331, 122702. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, J.; Xie, Z.; Liang, M.; Chen, S. Removal of Gaseous Toluene by the Combination of Photocatalytic Oxidation under Complex Light Irradiation of UV and Visible Light and Biological Process. J. Hazard. Mater. 2010, 177, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Sastre, F.; Fornés, V.; Corma, A.; García, H. Selective, Room-Temperature Transformation of Methane to C1 Oxygenates by Deep UV Photolysis over Zeolites. J. Am. Chem. Soc. 2011, 133, 17257–17261. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.-C.; Zhang, R.-X.; Ren, M.; Zhao, J.-X.; Gao, Z.-H.; Liu, L.; Zhang, Z.-X.; Zuo, Z.-J. Effect of the Preparation Method on Cu-MOR/g-C3N4 for Direct Methanol Synthesis from Methane Oxidation by Photothermal Catalysis. Catalysts 2023, 13, 868. [Google Scholar] [CrossRef]
- Wang, W.; Hao, J.; Liu, L.; Zhang, Z.; Huang, W.; Zuo, Z. Photothermal Catalytic Methane Oxidation to Methanol on Cu/Ti-ZSM-5. Fuel 2024, 376, 132720. [Google Scholar] [CrossRef]
- Preparation and Enhanced Visible-Light Photo-Catalytic Dye Degradation Activity of NiZY Composites. Biointerface Res. Appl. Chem 2022, 13, 72. [CrossRef]
- Agustina, T.E.; Prajawita, A.U.; Sinaga, S.A.S. The Effect of pH on Synthesis of ZnO-Natural Zeolite Nanocomposite by Co-Precipitation Method. IOP Conf. Ser. Mater. Sci. Eng. 2020, 845, 012049. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Younesi, H.; Zinatizadeh, A.A. Preparation, Characterization and Photocatalytic Application of TiO2/Fe-ZSM-5 Nanocomposite for the Treatment of Petroleum Refinery Wastewater: Optimization of Process Parameters by Response Surface Methodology. Chemosphere 2016, 159, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Fukahori, S.; Fujiwara, T. Modeling of Sulfonamide Antibiotic Removal by TiO2/High-Silica Zeolite HSZ-385 Composite. J. Hazard. Mater. 2014, 272, 1–9. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, Y.; Ridder, D.J.D.; Zhai, J.; Wei, Y.; Deng, H. Advantages of TiO2/5A Composite Catalyst for Photocatalytic Degradation of Antibiotic Oxytetracycline in Aqueous Solution: Comparison between TiO2 and TiO2/5A Composite System. Chem. Eng. J. 2014, 248, 280–289. [Google Scholar] [CrossRef]
- Ramírez-Aparicio, J.; Samaniego-Benítez, J.E.; Ramírez-Bon, R. TiO2-Chabazite Semiconductor Composites for Photocatalytic Degradation of Rhodamine under Sunlight Irradiation. Sol. Energy 2016, 139, 258–265. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, W.; Hao, Z.; Zou, L. A Study on the Synergistic Adsorptive and Photocatalytic Activities of TiO2−xNx/Beta Composite Catalysts under Visible Light Irradiation. Chem. Eng. J. 2010, 165, 301–309. [Google Scholar] [CrossRef]
- Malakootian, M.; Pourshaban-Mazandarani, M.; Hossaini, H.; Ehrampoush, M.H. Preparation and Characterization of TiO2 Incorporated 13X Molecular Sieves for Photocatalytic Removal of Acetaminophen from Aqueous Solutions. Process Saf. Environ. Prot. 2016, 104, 334–345. [Google Scholar] [CrossRef]
- Halder, S.; Chou, F.-C.; Wan, T.-K.; Tsai, Y.-T.; Cheng, P.-C.; Lin, Y.-C. Enhanced Photodegradation of Pentafluoropropionic Acid (PFPA) via TiO2-Synthetic Zeolite Composites: A Sustainable Approach for Effective Defluorination. J. Water Process Eng. 2024, 62, 105296. [Google Scholar] [CrossRef]
- Hu, T.; Li, J.; Wang, L.; Wang, H.; Zhang, Z.; Jiang, W.; Xue, C. ZnO/ZnFe2O4/Zeolite Composite Catalyst for Peroxymonosulfate Oxidation and Photocatalysis. Mater. Lett. 2023, 330, 133310. [Google Scholar] [CrossRef]
- Sodha, V.; Bandyopadhyay, M.; Gaur, R.; Bandyopadhyay, R.; Shahabuddin, S. Synthesis and Application of ZSM-5/Graphene Composite for Photocatalytic Degradation of Industrial Dyes. Adv. Nat. Sci. Nanosci. Nanotechnol. 2024, 15, 015006. [Google Scholar] [CrossRef]
- Guesh, K.J.; López-Muñoz, M.; Márquez-Álvarez, C.; Chebude, Y.; Diaz, I. Ethiopian Natural Zeolites for Photocatalysis. Bull. Chem. Soc. Eth. 2015, 29, 431. [Google Scholar] [CrossRef]
- Subtil, G.W.; Vicentini, J.C.M.; De Oliveira, D.M.; De Castro-Hoshino, L.V.; Yassue-Cordeiro, P.H.; Vicentino, R.C.; Scaliante, M.H.N.O. The Influence of Different Zeolitic Supports on Hydrogen Production and Waste Degradation. Can. J. Chem. Eng. 2023, 101, 1345–1357. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Y.; Qiu, Q.; Long, L.; Liu, X.; Lin, S.; Jiang, X. Zeolite NaP1 Synthesized from Municipal Solid Waste Incineration Fly Ash for Photocatalytic Degradation of Methylene Blue. Environ. Res. 2023, 218, 114873. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, X.; Zheng, S.; Sun, Z.; Liu, S.; Li, H. Influence of Calcination Temperature on the Structural, Adsorption and Photocatalytic Properties of TiO2 Nanoparticles Supported on Natural Zeolite. Powder Technol. 2015, 274, 88–97. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Salimi, Z. Solar Photocatalytic Degradation of O-Phenylenediamine by Heterogeneous CuO/X Zeolite Catalyst. Desalination 2011, 280, 281–287. [Google Scholar] [CrossRef]
- Shankar, M.V.; Anandan, S.; Venkatachalam, N.; Arabindoo, B.; Murugesan, V. Fine Route for an Efficient Removal of 2,4-Dichlorophenoxyacetic Acid (2,4-D) by Zeolite-Supported TiO2. Chemosphere 2006, 63, 1014–1021. [Google Scholar] [CrossRef]
- Rakanović, M.; Vukojević, A.; Savanović, M.M.; Armaković, S.; Pelemiš, S.; Živić, F.; Sladojević, S.; Armaković, S.J. Zeolites as Adsorbents and Photocatalysts for Removal of Dyes from the Aqueous Environment. Molecules 2022, 27, 6582. [Google Scholar] [CrossRef]
- Gong, P.; Li, B.; Kong, X.; Shakeel, M.; Liu, J.; Zuo, S. Hybriding Hierarchical Zeolite with Pt Nanoparticles and Graphene: Ternary Nanocomposites for Efficient Visible-Light Photocatalytic Degradation of Methylene Blue. Microporous Mesoporous Mater. 2018, 260, 180–189. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, H.; Li, Y.; Liu, Z. Photodegradation of Oxytetracycline in Aqueous by 5A and 13X Loaded with TiO2 under UV Irradiation. J. Hazard. Mater. 2010, 176, 884–892. [Google Scholar] [CrossRef]
- De Brites-Nóbrega, F.F.; Polo, A.N.B.; Benedetti, A.M.; Leão, M.M.D.; Slusarski-Santana, V.; Fernandes-Machado, N.R.C. Evaluation of Photocatalytic Activities of Supported Catalysts on NaX Zeolite or Activated Charcoal. J. Hazard. Mater. 2013, 263, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Haq, Z.U.; Pandith, A.H.; Bashir, A.; Nazir, I.; Tomar, R. Enhanced Charge Transfer and Photocatalytic Performance of Cube-Shaped Ag3PO4@Zeolite-A Nanocomposite. Mater. Chem. Phys. 2023, 302, 127701. [Google Scholar] [CrossRef]
- Foroughi, M.; Peighambardoust, S.J.; Ramavandi, B.; Foroutan, R.; Peighambardoust, N.S. Simultaneous Degradation of Methyl Orange and Indigo Carmine Dyes from an Aqueous Solution Using Nanostructured WO3 and CuO Supported on Zeolite 4A. Sep. Purif. Technol. 2024, 344, 127265. [Google Scholar] [CrossRef]
- Nippes, R.P.; Frederichi, D.; Olsen Scaliante, E.M.H.N. Enhanced Photocatalytic Performance under Solar Radiation of ZnO through Hetero-Junction with Iron Functionalized Zeolite. J. Photochem. Photobiol. A Chem. 2021, 418, 113373. [Google Scholar] [CrossRef]
- Kurniawan, T.; Saepurahman; Azis, M.A. Jayanudin Simultaneous Impregnation-Dealumination to Produce SnO2-Hierarchical Zeolite for Methylene Blue Elimination via Adsorption-Photodegradation. Case Stud. Chem. Environ. Eng. 2024, 9, 100613. [Google Scholar] [CrossRef]
- Li, C.J.; Zhang, Y.J.; Chen, H.; He, P.Y.; Zhang, Y.; Meng, Q. Synthesis of Fly Ash Cenospheres-Based Hollow ABW Zeolite for Dye Removal via the Coupling of Adsorption and Photocatalysis. Adv. Powder Technol. 2021, 32, 3436–3446. [Google Scholar] [CrossRef]
- Guaya, D.; Debut, A.; Campoverde, J. A Novel Approach to Waste Recycling and Dye Removal: Lithium-Functionalized Nanoparticle Zeolites. Molecules 2024, 29, 4643. [Google Scholar] [CrossRef]
- Speltini, A.; Maraschi, F.; Sturini, M.; Caratto, V.; Ferretti, M.; Profumo, A. Sorbents Coupled to Solar Light TiO2-Based Photocatalysts for Olive Mill Wastewater Treatment. Int. J. Photoenergy 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Gallegos-Alcaíno, A.; Robles-Araya, N.; Avalos, C.; Alfonso-Alvarez, A.; Rodríguez, C.A.; Valdés, H.; Sánchez-Flores, N.A.; Durán-Alvarez, J.C.; Bizarro, M.; Romero-Salguero, F.J.; et al. Synthesis of BiOI/Mordenite Composites for Photocatalytic Treatment of Organic Pollutants Present in Agro-Industrial Wastewater. Nanomaterials 2022, 12, 1161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, X.; Wei, G.; Su, Z. All-Weather Photothermal-Electrothermal Integrated System for Efficient Solar Steam Generation. Chem. Eng. J. 2023, 458, 141520. [Google Scholar] [CrossRef]

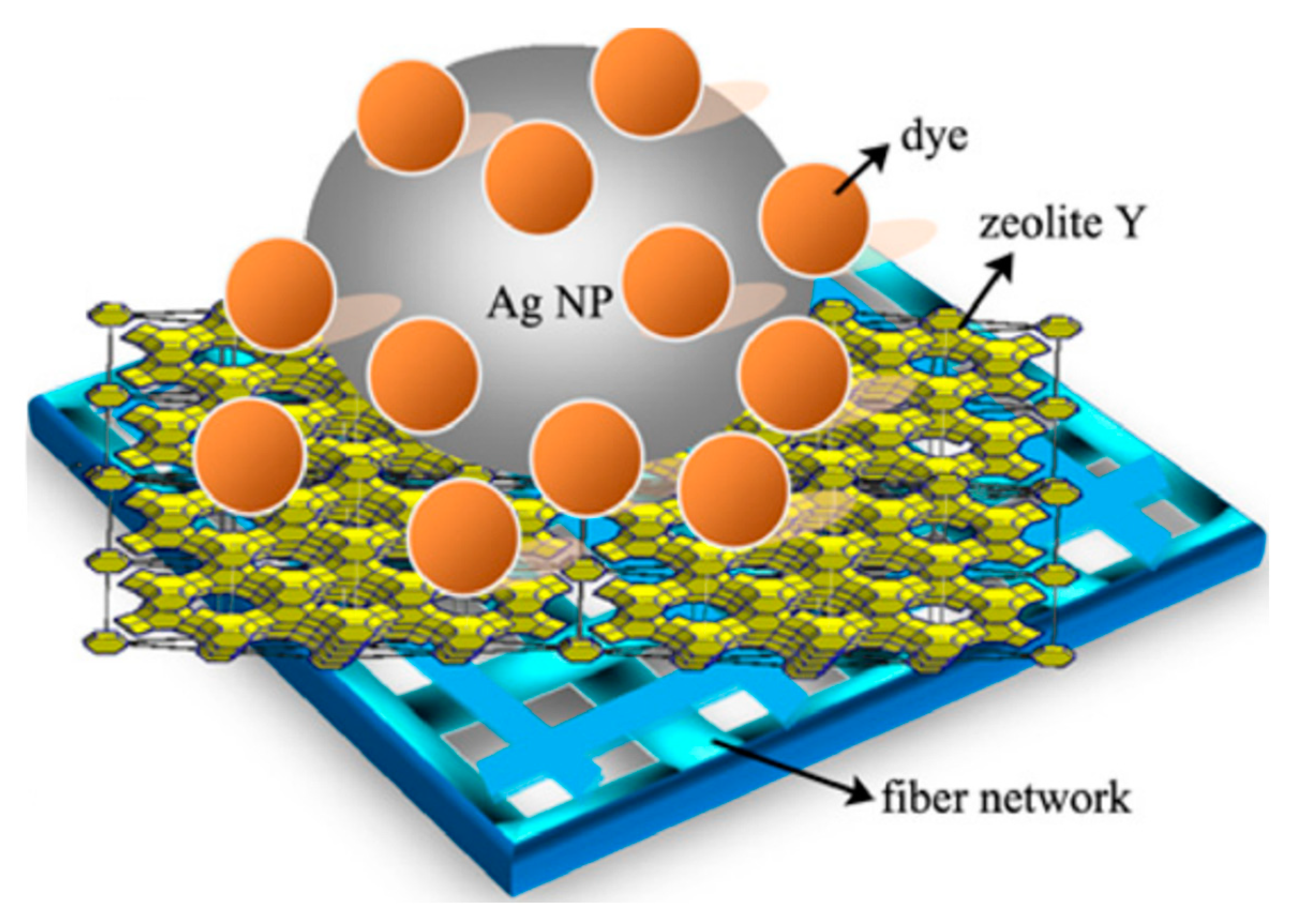
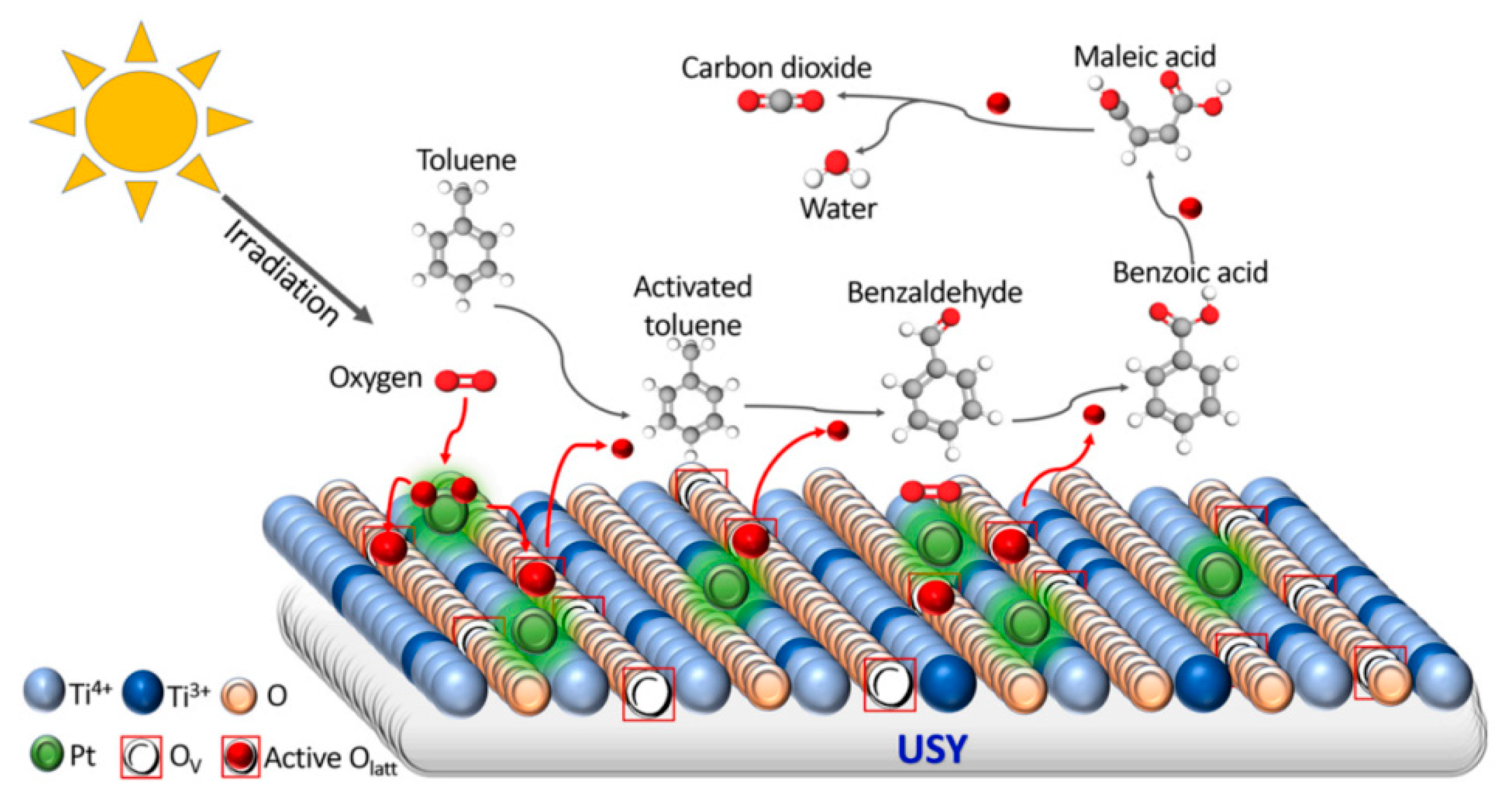
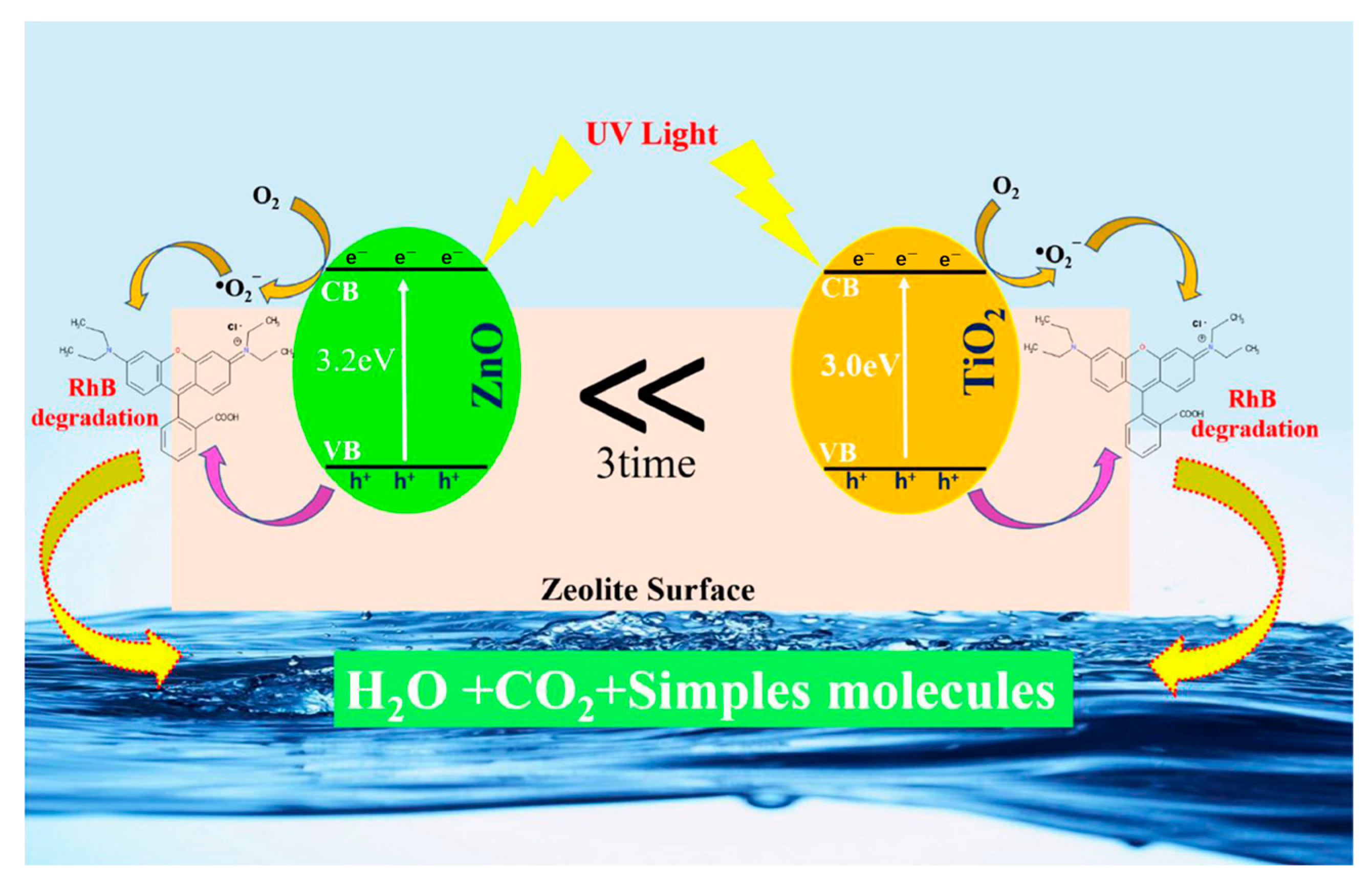
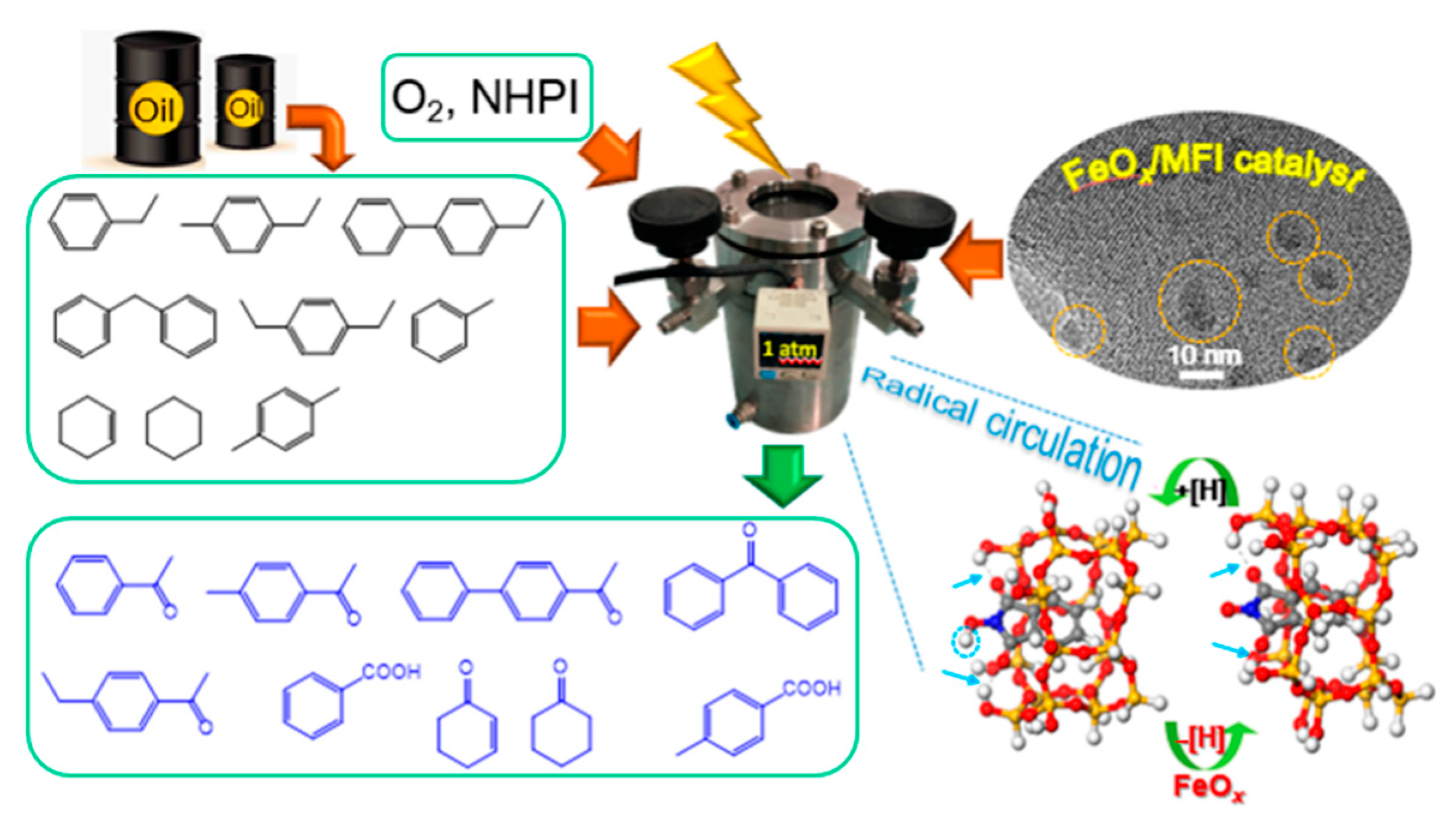


| Types of Zeolite | Structural Characteristics | Crystal Structure | Pore Size (Å) | Silicon to Aluminum Ratio | Type of Pore Channel | Exchange Capacity (meq/g) | Specific Surface Area (m2/g) | Acid Strength |
|---|---|---|---|---|---|---|---|---|
| A-Type Zeolite | Cubic Crystal Structure | LTA Type | 4 | 1:1–3:1 | Cubic Diagonal Channel | 0.3–0.5 | 300–600 | Weakly Acidic |
| X-Type Zeolite | Cubic Crystal Structure | FAU Type | 8–9 | 1:1–2:1 | Octahedral Pore | 3.0–4.5 | 500–900 | Strong Acid |
| Y-Type Zeolite | Cubic Crystal Structure | FAU Type | 7–9 | >2:1 | Octahedral Pore | 0.7–1.2 | 600–1000 | Strong Acid |
| β-Type Zeolite | Hexagonal Crystal Structure | BEA Type | 5.6–6.6 | >10:1 | Double Channel | 1.0–1.5 | 500–800 | Moderate Acidity |
| Mordenite | Monoclinic Crystal Structure | MOR Type | 6.5–7.0 | 5:1–20:1 | Straight and Curved Ducts | 0.8–1.2 | 350–500 | Moderate Acidity |
| ZSM-5 | Monoclinic Crystal Structure | MFI Type | 5.3–5.7 | >10:1 | Straight Channel | 2.5–3.5 | 300–500 | Strong Acid |
| USY | Cubic Crystal Structure | FAU Type | 7.4 | >3:1 | Octahedral Pore | 0.5–3.5 | 400–700 | Strong Acid |
| Catalyst | VOC | VOC Concentration | Catalyst Mass (mg) | Light | Conversion (%) | SBET (m2/g) | Reference |
|---|---|---|---|---|---|---|---|
| TiO2/ZSM-5 | Propene | 100 ppmv | 110 | 8 W/365 nm | 82 | 234 | [39] |
| TiO2/silicalite | Trichloroethylene | 25 ppm | 30 | 8 W | 74 | 396 | [40] |
| TiO2/zeolite | Toluene | 42.5 ppm | — | 4 W/365 nm | 89 | — | [41] |
| FeZSM-5-HT | Ethylene | 1000 ppm | 200 | 4 W/254 nm | 52 | — | [42] |
| HZSM-5 | Isopropyl alcohol | 150 ppm | 200 | 8 W/254 nm | 94 | 308 | [43] |
| TiO2/HZSM-5 | Formaldehyde | 10 ppmv | — | 1.6 W/365 nm | 80 | 78 | [44] |
| TiO2/HZSM-5 | Acetaldehyde | 10 ppmv | — | 1.6 W/365 nm | 50 | 78 | [44] |
| TiO2/HZSM-5 | Acetic acid | 10 ppmv | — | 1.6 W/365 nm | 98 | 78 | [44] |
| TiO2/HZSM-5 | Toluene | 10 ppmv | — | 1.6 W/365 nm | 70 | 78 | [44] |
| TiO2/zeolite | Ethanol | 700 ppm | 9 | 100 W/367 nm | 100 | 335 | [45] |
| TiO2/zeolite | Diethyl sulfide | 375 ppm | 9 | 100 W/367 nm | 100 | 335 | [45] |
| 5Fe-10W-NaY | Acetaldehyde | — | 1000 | >400 nm | 80 | 18.4 | [46] |
| 5Fe-10W-NaY | O-xylene | — | 1000 | >400 nm | 76 | 18.4 | [46] |
| TiO2/ZSM-5 | Formaldehyde | 15 ppm | 30 | 8 W | 95 | 422 | [47] |
| TiO2/Zeolite Y | trichloroethylene | 25 ppm | 30 | 8 W | 80 | 776 | [47] |
| TiO2@HYZ | Toluene | 760 ppm | 100 | 300 W | 96.6 | 86.6 | [29] |
| Catalyst | Water Pollutant | Pollutant Concentration (mg/L) | Catalyst Concentration (g/L) | Light | Time (min) | Degradation Rate (%) | SBET (m2/g) | Reference |
|---|---|---|---|---|---|---|---|---|
| CuO/NaX | Methylene blue | 9.6 | 1 | — | 180 | 94 | 412 | [15] |
| NiO/zeolite | Malachite green | 30 | 0.25 | 12 W/460 nm | 250 | 83 | 720 | [55] |
| ZnO-natural zeolite | Procion red | 50 | 4 | 15 W/254 nm | 120 | 75.54 | 134.35 | [56] |
| TiO2/Fe-ZSM-5 | COD | 602 | 2 | 8 W | 240 | 80 | 304.6 | [57] |
| TiO2/HSZ-385 | Sulfamethazine | 10 | 0.2 | 365 nm | 360 | 66.7 | 424.22 | [58] |
| TiO2/5A | Oxytetracycline | 50 | 0.1 | 16 W/254 nm | 210 | 100 | 539.51 | [59] |
| Chabazite-TiO2 | Rhodamine 6G | 14.37 | 0.25 | Sunlight | 90 | 97.9 | — | [60] |
| 10%TiO2−xNx/Beta | Methylene blue | 15 | 0.3 | >460 nm | 175 | 85 | 315.6 | [61] |
| TiO2-HX | Acetaminophen | 1 | 1 | 15 W/245 nm | 120 | 95.45 | — | [62] |
| TiO2-zeolite | Pentafluoropropionic acid | 10 | 0.5 | 16 W/185 nm | 480 | 58.7 | 270 | [63] |
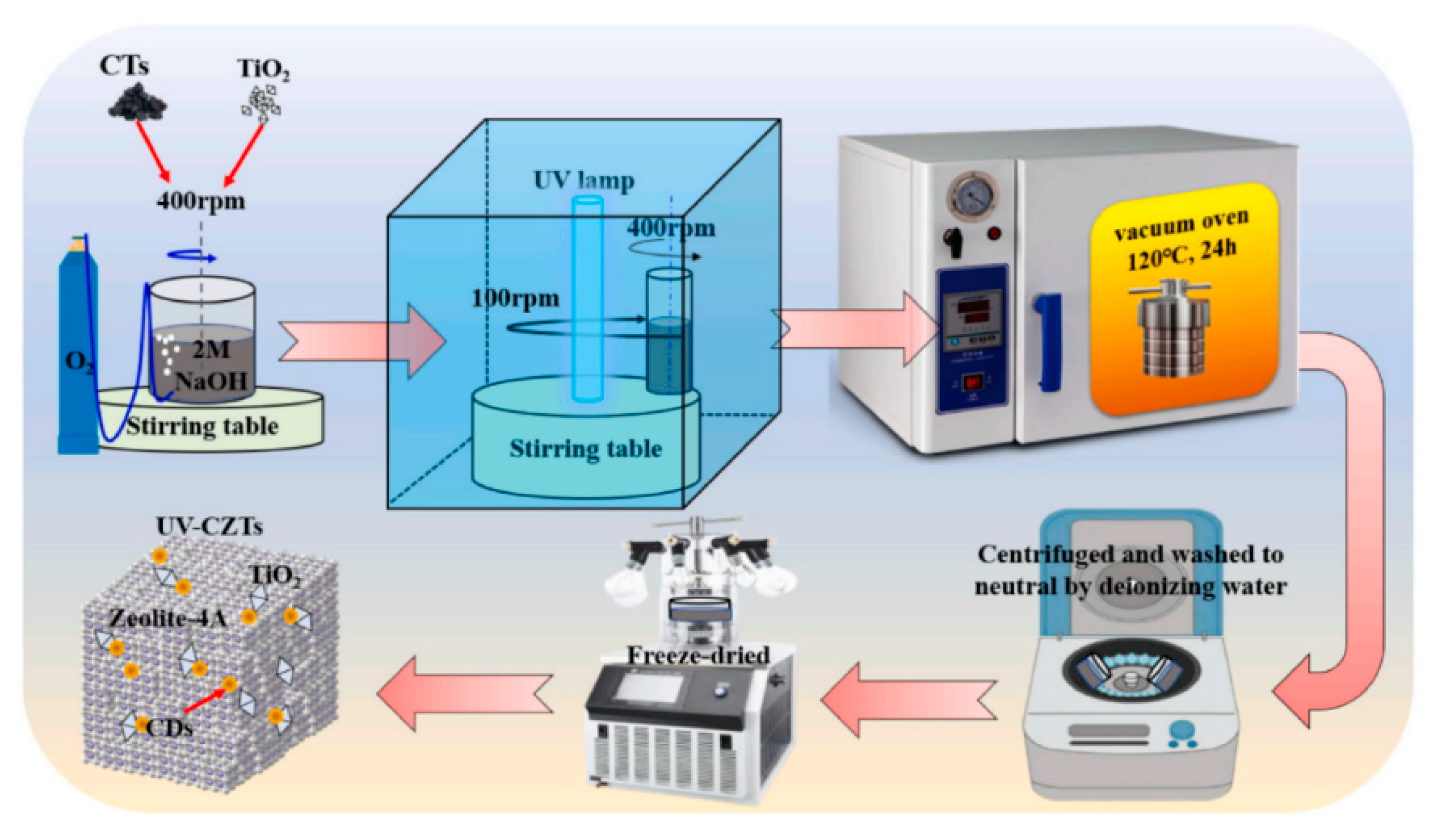
| UV-CDs/zeolite-4A/TiO2 | Methylene blue | 10 | 0.1 | 500 W/365 nm | 60 | 90.63 | 237.55 | [28] |
| ZnO/ZnFe2O4/zeolite | Rhodamine B | 50 | 0.2 | 30 W/395 nm | 60 | 98.55 | 62.97 | [64] |
| ZSM-5/graphene | Methyl orange | 20 | 0.5 | 450 W | 180 | 92 | — | [65] |
| 20%TiO2/ET4 | Methyl orange | 10 | 0.5 | 150 W | 240 | 95 | — | [66] |
| Cu/TiO2/NaY | Reactive blue dye | 10 | 1 | 7 W/254 nm | 240 | 53 | 13.29 | [67] |
| NaP1 | Methylene blue | 100 | 1 | — | 720 | 96 | 24.86 | [68] |
| TiO2/zeolite | Cr(VI) | 25 | 2 | 500 W | 180 | 100 | 53.59 | [69] |
| 10.4%CuO/X zeolite | O-phenylenediamine | 25 | 0.3 | Sunlight | 240 | 90 | — | [70] |
| TiO2/HY | 2,4-D | 200 | 2 | 8 W/254 nm | 300 | 100 | — | [71] |
| ZnO/zeolite | Methylene blue | 10 | 1 | 30 W/365 nm | 180 | 90 | 395 | [37] |
| NH4ZSM-5 | Methylene blue | 1 | 0.5 | 125 W/365 nm | 180 | 77.5 | — | [72] |
| NH4BETA | Rhodamine B | 1 | 0.5 | 125 W/365 nm | 180 | 83.3 | — | [72] |
| RGO@1%Pt/Ti-MFI-NSs | Methylene blue | 220 | 2.5 | 300 W/420 nm | 90 | 99 | — | [73] |
| 15%TiO2/5A | Oxytetracycline | 50 | 0.5 | 16 W/254 nm | 210 | 100 | — | [74] |
| 10%TiO2/13X | Oxytetracycline | 50 | 0.5 | 16 W/254 nm | 210 | 100 | — | [74] |
| 10%ZnO/NaX | Reactive blue 5G | 10 | 1 | 250 W/310–350 nm | 30 | 100 | 239 | [75] |
| Ti-NaZSM-5 | Methyl orange | 10 | 0.33 | 300 W | 90 | 100 | 325.2 | [32] |
| 15%Ag3PO4@Zeolite-A | Methylene blue | 10 | 1 | 300 W | 150 | 100 | — | [76] |
| 4A/WO3/CuO | Methyl orange | 10 | 0.15 | 15 W | 30 | 99.12 | 10.76 | [77] |
| 4A/WO3/CuO | Indigo carmine | 10 | 0.15 | 15 W | 30 | 97.24 | 10.76 | [77] |
| Zeo-TiO2 | Rhodamine B | 5 | 1 | 35 W | 80 | 100 | 172 | [35] |
| Zeo-ZnO | Rhodamine B | 5 | 1 | 35 W | 80 | 81 | 158 | [35] |
| 5%ZnO/FeY | Dichlorophenoxyacetic acid | 18 | 1 | 250 W | 300 | 57 | 523.41 | [78] |
| 5%ZnO/FeY | Dichlorophenoxyacetic acid | 18 | 1 | Sunlight | 300 | 85 | 523.41 | [78] |
| SnO2-hierarchical zeolite | Methylene blue | 40 | 1 | 15 W/254 nm | 120 | 97 | 229 | [79] |
| 6%Co3O4/ABW | Bordeaux dye | 2 | 2 | — | 30 | 90 | 29.45 | [80] |
| TiO2/ZSM-5 | Methyl orange | 20 | 2 | 550 W | 180 | 99.55 | 1151 | [38] |
| MT-ZLSH-Li+ | Methylene blue | 15 | 0.5 | 300–800 nm | 180 | 77 | — | [81] |
| TiO2@Zeolite-Y | Polyphenols | 111 | 1 | 15 W/254 nm | 480 | 77 | 216 | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Xu, L.; Xu, J.; Shen, B. A Mini-Review of Recent Progress in Zeolite-Based Catalysts for Photocatalytic or Photothermal Environmental Pollutant Treatment. Catalysts 2025, 15, 158. https://doi.org/10.3390/catal15020158
Zhang S, Xu L, Xu J, Shen B. A Mini-Review of Recent Progress in Zeolite-Based Catalysts for Photocatalytic or Photothermal Environmental Pollutant Treatment. Catalysts. 2025; 15(2):158. https://doi.org/10.3390/catal15020158
Chicago/Turabian StyleZhang, Shenhao, Le Xu, Jie Xu, and Boxiong Shen. 2025. "A Mini-Review of Recent Progress in Zeolite-Based Catalysts for Photocatalytic or Photothermal Environmental Pollutant Treatment" Catalysts 15, no. 2: 158. https://doi.org/10.3390/catal15020158
APA StyleZhang, S., Xu, L., Xu, J., & Shen, B. (2025). A Mini-Review of Recent Progress in Zeolite-Based Catalysts for Photocatalytic or Photothermal Environmental Pollutant Treatment. Catalysts, 15(2), 158. https://doi.org/10.3390/catal15020158








