Abstract
Piezocatalysis is a promising technology for converting mechanical energy to chemical energy. Two-dimensional (2D) piezoelectric materials, with their large surface area, high charge mobility, and good flexibility, are among the most promising candidates in piezocatalysis. In this work, for the first time, we report Bi2O2Se nanosheets (NSs) with an average thickness of ~8 nm and a lateral size of ~160 nm for efficient piezocatalytic H2O2 production from water and oxygen under mechanical force induced by ultrasonication. The Bi2O2Se NSs achieved a high H2O2 production rate of 1033.8 μmol/g/h using ethanol as the sacrificial agent, significantly surpassing that of its bulk-sheet counterpart. Our results provide a novel potential 2D piezocatalytic material and offer valuable guidance for the design and development of high-efficiency H2O2 production driven by mechanical energy from water.
1. Introduction
Hydrogen peroxide (H2O2) is widely recognized for its advantages, such as its high oxidizing ability and environmental friendliness, making it valuable for applications in various areas, including disinfection, wastewater treatment, and green chemistry [1,2,3,4,5,6]. However, the primary production method of the anthraquinone process remains highly energy-intensive and environmentally detrimental due to its reliance on toxic solvents and complex, multi-step processes. As a result, there has been a growing interest in alternative, more sustainable pathways for H2O2 production. Notable green approaches include photocatalysis and electrocatalysis technologies [7,8,9,10,11,12,13,14,15,16,17,18,19]. Photocatalysis relies on the energy from light, while electrocatalysis utilizes electrical energy. Despite their promising promise, these methods are often hindered by the need for complex, expensive catalysts, which significantly limits their practical applications [20,21,22,23,24]. Therefore, developing cost-effective, scalable, and environmentally benign catalytic systems remains a critical goal for advancing H2O2 production in a more sustainable and efficient manner.
Piezocatalysis, using mechanical stress or pressure to drive catalytic reactions, is an emerging and promising approach to convert mechanical energy into chemical energy [25,26]. This method relies on piezoelectric materials, which generate electric charges when subjected to mechanical stress, to drive various chemical redox reactions (e.g., H2O2 production) [27,28,29,30,31,32,33,34,35,36]. Unlike the energy-intensive anthraquinone process, piezocatalysis operates under mild conditions and utilizes mechanical energy, such as water flow and ultrasonic vibration, to drive the reaction, making it a promising, attractive option for decentralized or on-demand H2O2 production [37,38]. Among all piezocatalytic materials, two-dimensional (2D) piezoelectrics are considered among the most promising candidates due to their ultrathin layered structure, high surface area, abundance of active sites, and piezoelectric response resulting from crystal noncentrosymmetry, such as 2D transition metal chalcogenides (TMCs) (e.g., MoS2 and ZnS) [39,40,41].
Bi2O2Se, as a novel 2D material, which belongs to the family of layered bismuth oxychalcogenides and features a distinctive anisotropic structure and tunable electronic properties, has garnered significant attention [42,43]. Due to its unique combination of an ultrathin layered structure, piezoelectric response, high charge mobility (>20,000 cm2/V·s) [44], and excellent chemical stability, 2D piezoelectric Bi2O2Se is a very promising potential piezocatalyst. Its intrinsic piezoelectricity arises from the lack of inversion symmetry in its crystal structure [45,46,47], which enables the generation of electric charges under mechanical stress—a key attribute for piezocatalytic applications. Herein, for the first time, we report 2D Bi2O2Se NSs as an efficient piezocatalyst for producing H2O2 from water and oxygen, driven by ultrasonic mechanical vibrations.
2. Results and Discussions
2.1. Morphology and Crystal Structure
X-ray diffraction (XRD) analysis was performed to investigate the crystal structure of the Bi2O2Se powder samples. As shown in Figure 1a, the diffraction peaks observed at 23.966°, 29.257°, 31.738°, 32.520°, 44.369°, 46.635°, 53.110°, and 57.636° correspond to the (101), (004), (103), (110), (114), (200), (211), and (213) crystal planes of tetragonal Bi2O2Se (JCPDS Card No. 25-1463), respectively, which align well with previously reported results [48]. Additionally, the bulk sheet crystals (BSs) display a pure tetragonal Bi2O2Se phase, which is consistent with the simulated patterns and NSs sample. This confirms that the synthesized Bi2O2Se material crystallizes without detectable impurity phases. The broader full width at half maximum (FWHM) observed for the nanosheets further validates the successful synthesis of a two-dimensional structure.

Figure 1.
(a) XRD patterns of Bi2O2Se NSs and BCs. SEM images of (b) Bi2O2Se NSs and (c) BCs. (d) EDS mapping images of Bi2O2Se NSs.
The scanning electron microscopy (SEM) image (Figure 1b) reveals that the powder sample exhibits a sheet-like morphology, with lateral dimensions up to several hundred nanometers and thicknesses of few nanometers. On the contrary, the BSs sample displays a thick-sheet morphology (Figure 1c). Energy-dispersive spectroscopy (EDS) mapping images (Figure 1d) of the NSs sample further demonstrate the homogeneous distribution of Bi, O, and Se elements. High-resolution transmission electron microscopy (HRTEM) was further employed to analyze the microstructure and crystallinity of the Bi2O2Se NSs (Figure 2). As shown in Figure 2b, the Bi2O2Se material has a 2D sheet-like structure, which is consistent with the SEM characterizations. Selected area electron diffraction (SAED) analysis (Figure 2c) confirmed the single crystalline nature of the Bi2O2Se NSs. The lattice fringes observed in Figure 3a reveal an interatomic spacing of 2.9 Å, corresponding to the (004) crystal plane of Bi2O2Se, in agreement with the XRD patterns and previously reported data [47,48]. Furthermore, the EDS mapping images in Figure 2d confirm the uniform distribution of elements within the Bi2O2Se NSs. Taking these results together, 2D Bi2O2Se ultrathin NSs were successfully synthesized.

Figure 2.
(a) HRTEM image, (b) TEM image, (c) SAED patterns, and (d) EDS mapping images of Bi2O2Se NSs.

Figure 3.
XPS spectra of Bi2O2Se NSs: (a) survey scan, (b) Bi 4f, (c) O 1s, and (d) Se 3d.
The chemical states of elements (Bi, O, and Se) in the 2D Bi2O2Se NSs were characterized by X-ray photoelectron spectroscopy (XPS), as shown in Figure 3. All spectra were corrected using the C 1s peak at 284.4 eV as the reference. The peaks at ~159 eV and 164 eV correspond to the characteristic Bi 4f7/2 and Bi 4f5/2 peaks, respectively (Figure 3b). The peaks at ~529 eV and 531 eV are attributed to the O 1s signals from metal oxides (Figure 3c). The Se 3d peak appears at ~52.3 eV (Figure 3d), showing a slight shift compared to the standard peak, indicating strong interactions between Se and Bi. These XPS results further confirm the successful synthesis of Bi2O2Se.
2.2. Piezocatalytic Activity Performance
The piezocatalysis H2O2 production activity was tested under mechanical force provided by ultrasonic vibration. Figure 4a displays the produced H2O2 amount over different Bi2O2Se samples operating under ultrasonication (100 W and 45 kHz), using ethanol as the sacrificial agent. Among the samples, Bi2O2Se NSs demonstrated the highest H2O2 production yield, with a production rate of 1033.8 μmol/g/h, significantly surpassing the 803.1 μmol/g/h achieved by the Bi2O2Se BSs. Moreover, compared with previous catalysts (Table 1), Bi2O2Se NSs demonstrate comparable piezocatalytic performance, showing promising potential. This enhanced performance can be attributed to the ultrathin nature of Bi2O2Se NSs, which possess a greater number of surface-active sites and are more easily deformed under mechanical stress.

Figure 4.
(a) The yield of H2O2 over different Bi2O2Se samples under ultrasonic vibration. (b) The yield of H2O2 over Bi2O2Se NSs under different ultrasonication frequencies. (c) Cycling piezocatalytic experiments and (d) XRD patterns before and after tests.

Table 1.
Comparison of piezocatalytic performance for H2O2 production with reported representative piezocatalysts.
The effects of ultrasonic frequency on the piezocatalytic performance of Bi2O2Se NSs were further analyzed. As shown in Figure 4b, the Bi2O2Se NSs exhibited better H2O2 production performance at 35 kHz compared to 53 kHz, demonstrating that piezocatalytic efficiency is closely related to the resonance frequency of the Bi2O2Se nanomaterials. Furthermore, the stability of the Bi2O2Se NSs material was also tested. As shown in Figure 4c, after five cycles, the piezocatalytic performance almost remained unchanged, and the XRD pattern showed negligible changes in the crystalline phase before and after cycling catalytic tests (Figure 4d), confirming that the Bi2O2Se 2D piezoelectric materials exhibit excellent catalytic stability under piezocatalytic reaction conditions.
2.3. Mechanism Analysis
To further investigate the mechanism of piezocatalytic H2O2 production, the influence of reaction atmospheres and scavengers on piezocatalytic activity was investigated, as presented in Figure 5. Almost no H2O2 was produced under a N2 atmosphere, which is in sharp contrast with that under O2 and air. This suggests that the piezocatalytic synthesis of H2O2 follows an oxygen reduction reaction (ORR) pathway. The addition of hole scavenger EDTA-2Na resulted in an increased H2O2 yield, while the introduction of Ag⁺ inhibited the piezocatalytic activity, indicating that electrons play a dominant role. Additionally, with the addition of BQ, the H2O2 yield significantly decreased, suggesting that superoxide anion (•O2−) is an important intermediate in the generation of H2O2. Based on these results, we consider that the mechanism of piezocatalytic H2O2 production over Bi2O2Se is through an ORR involving a two-electron transfer process. The piezocatalytic H2O2 yield decreased without using ethanol. This is possibly because hydroxyl radicals (•OH) compete with O2 to gain electrons and form OH−. As a hydroxyl radical scavenger, ethanol consumed the •OH radicals in the system during the reaction, allowing more electrons to participate in the ORR, thus enhancing the H2O2 synthesis efficiency.
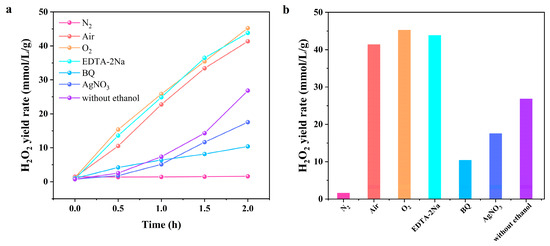
Figure 5.
(a) Piezocatalytic tests of Bi2O2Se NSs under different atmospheres and sacrificial agents. (b) The corresponding reaction rates.
The piezo-electrochemical tests were conducted to further elucidate the charge transport characteristics of the Bi2O2Se piezoelectric materials. As shown in Figure 6a, at a voltage of 0.05 V, the transient piezo-electrochemical current density of the Bi2O2Se NSs was approximately twice that of the BSs. Additionally, as shown in Figure 6b, the Bi2O2Se NSs exhibited smaller impedance compared to that of BSs. These results indicate that the carrier recombination rate in the NSs is lower than that of BSs, allowing more carriers to migrate to the material’s surface and participate in catalytic reactions.

Figure 6.
(a) Transient piezo-electrochemical current and (b) impedance of different Bi2O2Se materials. (c) Mott–Schottky plots of Bi2O2Se NSs. (d) Energy band structure and piezocatalytic H2O2 production mechanism.
The band structure of the material is crucial in the piezocatalytic process. The Mott–Schottky plots (Figure 6c) were measured to calculate the band energy position of the Bi2O2Se NSs. According to the intercept of the Mott–Schottky plots, the flat band potential (Efb) was determined to be at −0.33 eV (vs. NHE) for SnSe NSs. As a result, the conduction band minimum (CBM) edge was estimated at −0.43 eV (~0.1 eV above the Efb). By combining the bandgap Eg (~1.76 eV) estimated from the UV–visible DRS spectra, the valence band maximum (VBM) was determined to be at 1.33 eV.
Based on the experimental results above, a possible mechanism for mechanically driven piezocatalytic H2O2 evolution over Bi2O2Se NSs is proposed. The band diagrams of the Bi2O2Se NSs are illustrated in Figure 6d. Based on the analysis of the material’s band structure and active radicals, the possible charge transfer processes in the piezocatalytic reaction system can be explained as follows. Under the external ultrasound, Bi2O2Se NSs undergo elastic deformation, generating a piezoelectric effect. This effect leads to the formation of a piezoelectric field within the material, promoting the separation of charge carriers on the two opposite sides of the Bi2O2Se NSs. Since the CBM of the material is more negative than the standard redox potential of the O2/•O2− couple, the electrons in the conduction band combine with dissolved O2 to generate superoxide anions (•O2−). These •O2− ions then further combine with electrons to produce H2O2. On the other hand, holes on the VBM will react with the sacrificial agent ethanol to facilitate the piezocatalytic reaction process. The overall reaction pathway follows an oxygen reduction reaction through a two-electron transfer mechanism.
3. Experimental Section
3.1. Synthesis of Bi2O2Se NSs
Bi2O2Se nanosheets were synthesized via a hydrothermal method [48]. The typical procedure is as follows (Scheme 1). First, 1.48 g of Bi(NO₃)₃·5H₂O (≥98%, Aladdin, Shanghai, China) is dissolved in 30 mL of deionized water and stirred for 30 min. Then, 1.39 g of sodium citrate (98%, Aladdin, China) is added, and the mixture is stirred for 30 min to obtain solution A. Separately, 2.664 g of PVP (AR, Aladdin, China) is dissolved in 30 mL of deionized water, followed by the sequential addition of 0.60 g of NaOH (≥98%, Aladdin, China) and 0.148 g of selenourea (98%, Merck, Darmstadt, Germany), with continuous stirring for 30 min to obtain solution B. The two solutions are mixed and stirred for 30 min and transferred to a Teflon-lined autoclave. The autoclave is placed in an oven and heated at 200 °C for 24 h. After the reaction, the product is washed with DI water and ethanol to remove impurities. Finally, the obtained product is vacuum-dried at 60 °C for 12 h to obtain the Bi2O2Se nanosheets. The bulk Bi2O2Se sheets (BSs) were purchased commercially.

Scheme 1.
Schematic of preparation process of 2D Bi2O2Se NSs.
3.2. Material Characterization
The crystal structure was analyzed by X-ray diffraction (XRD, Rigaku D/max-2550 VB, Rigaku Corporation, Akishima, Japan) using a Cu Kα radiation source (λ = 0.154 nm, 45 kV). The morphology and microstructure were investigated by both field emission scanning electron microscopy (FESEM, Zeiss Merlin, Carl Zeiss AG, Jena, Germany) and transmission electron microscopy (TEM, Talos F200i, Thermo Fisher Scientifi, Waltham, MA, USA). The surface electronic states of elements were characterized by X-ray photoelectron spectroscopy (XPS, ESCALAB 250 Xi, Thermo Fisher Scientifi, USA). The binding energy was calibrated with the C1s peak at 284.6 eV. The diffuse reflectance spectroscopy (DRS) was measured using a UV–vis-NIR spectrophotometer (Lambda 1050, PerkinElmer, Waltham, MA, USA) equipped with an integrating sphere.
3.3. Piezocatalytic Activity Tests
The piezocatalytic tests were conducted in a glass beaker containing 50 mL ethanol solution (10%), which was bubbled with O2 for 30 min prior to the reaction. In each test, 10 mg of the powder catalyst was added to the solution. Mechanical force was provided by an ultrasonic cleaner (Branson 2800, Branson Ultrasonics, Fremont, CA, USA). The reaction solution was kept at room temperature using a water-cooling system. At an interval of 30 min, 1 mL of the reaction solution was taken and filtrated. The concentration of H2O2 was determined using the classic Iodometric method [54]. Two solutions were prepared. Solution A consisted of 0.4 M potassium iodide, 0.06 M sodium hydroxide, and 0.1 mM ammonium molybdate, while Solution B was 0.1 M potassium hydrogen phthalate. For each measurement, 1 mL of the reaction solution was filtered and mixed with 0.5 mL solution A and 0.5 mL solution B. The resulting mixture was analyzed using a UV–vis spectrophotometer, measuring the characteristic absorption peak at 350 nm.
3.4. Piezo-Electrochemical Measurements
Piezo-electrochemical measurements were performed using an electrochemical workstation (CHI 760E, Shanghai Chenhua, Instrument Co., Ltd., Shanghai, China) in a standard three-electrode system with Bi2O2Se powder-coated conductive Cu as the working electrode, a Pt plate as the counter electrode, and Ag/AgCl as the reference electrode. A 0.1 M Na2SO4 deoxygenated solution (pH = 7 ± 0.2) was used as the electrolyte. The vibration was performed by 100 W ultrasonication excitation under a frequency of 45 kHz. The piezo-electrochemical current was measured at a potential of 0.05 V. The electrochemical impedance spectra (EIS) measurements were performed with a bias potential of −0.4 V. The Mott–Schottky plots were measured at −1.0 V, at frequencies of 500, 1000, and 1500 Hz, respectively.
4. Conclusions
We synthesized 2D Bi2O2Se nanosheets and investigated their piezocatalytic performance for H2O2 production under ultrasonic mechanical vibration. The results demonstrated that the morphology of the materials significantly impacts the piezocatalytic activity of Bi2O2Se, and the Bi2O2Se nanosheets achieved a high H2O2 production rate of 1033.8 μmol/g/h. The piezocatalytic H2O2 production activity was also dependent on the frequency of ultrasound and atmosphere. The free charge carriers generated under mechanical stress drive the ORR, leading to efficient H2O2 synthesis via a two-electron transfer pathway. This study demonstrates the promising application potential of Bi2O2Se nanomaterials in piezocatalysis, providing valuable insights for advancing the development of efficient 2D piezocatalysts. Prospectively, further advancements in scalable synthesis techniques for Bi2O2Se nanosheets and enhanced catalytic efficiency will pave the way for industrial applications.
Author Contributions
Conceptualization, S.L.; materials synthesis and catalytic tests, X.L.; writing—original draft preparation, X.Z. and S.L.; writing—review and editing, and supervision, Y.L.; funding acquisition, S.L and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by financial aid from the National Natural Science Foundation of China (Grant No. 22075126 and 52172187) and the Guangdong–Macao joint funding program for science and technology innovation (2022A0505020025).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Hydrogen Peroxide: A Key Chemical for Today’s Sustainable Development. ChemSusChem 2016, 9, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.C.; Pangotra, D.; Vieira, L.; Csepei, L.-I.; Sieber, V.; Wang, L.; de León, C.P.; Walsh, F.C. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 2019, 3, 442–458. [Google Scholar] [CrossRef]
- Dowling, J.A.; Rinaldi, K.Z.; Ruggles, T.H.; Davis, S.J.; Yuan, M.; Tong, F.; Lewis, N.S.; Caldeira, K. Role of Long-Duration Energy Storage in Variable Renewable Electricity Systems. Joule 2020, 4, 1907–1928. [Google Scholar] [CrossRef]
- Esan, O.C.; Shi, X.; Pan, Z.; Liu, Y.; Huo, X.; An, L.; Zhao, T.S. A high-performance H2O2-based fuel cell for air-free applications. J. Power Sources 2022, 548, 232114. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen Peroxide Synthesis: An Outlook beyond the Anthraquinone Process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef]
- Fink, A.G.; Delima, R.S.; Rousseau, A.R.; Hunt, C.; LeSage, N.E.; Huang, A.; Stolar, M.; Berlinguette, C.P. Indirect H2O2 synthesis without H2. Nat. Commun. 2024, 15, 766. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, C.; Bian, G.; Xu, J.; Dong, Y.; Zhang, Y.; Lou, Y.; Liu, W.; Zhu, Y. H2O2 generation from O2 and H2O on a near-infrared absorbing porphyrin supramolecular photocatalyst. Nat. Energy 2023, 8, 361–371. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Yu, H.; Položij, M.; Guo, Y.; Sum, T.C.; Heine, T.; Jiang, D. Linkage-engineered donor–acceptor covalent organic frameworks for optimal photosynthesis of hydrogen peroxide from water and air. Nat. Catal. 2024, 7, 195–206. [Google Scholar] [CrossRef]
- Teng, Z.; Zhang, Q.; Yang, H.; Kato, K.; Yang, W.; Lu, Y.-R.; Liu, S.; Wang, C.; Yamakata, A.; Su, C.; et al. Atomically dispersed antimony on carbon nitride for the artificial photosynthesis of hydrogen peroxide. Nat. Catal. 2021, 4, 374–384. [Google Scholar] [CrossRef]
- Tan, H.; Zhou, P.; Liu, M.; Zhang, Q.; Liu, F.; Guo, H.; Zhou, Y.; Chen, Y.; Zeng, L.; Gu, L.; et al. Photocatalysis of water into hydrogen peroxide over an atomic Ga-N5 site. Nat. Synth. 2023, 2, 557–563. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Takii, T.; Hagi, T.; Mori, S.; Kofuji, Y.; Kitagawa, Y.; Tanaka, S.; Ichikawa, S.; Hirai, T. Resorcinol–formaldehyde resins as metal-free semiconductor photocatalysts for solar-to-hydrogen peroxide energy conversion. Nat. Mater. 2019, 18, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Lv, W.; Ge, X.; Huang, X.; Hu, Q.; Song, K.; Liu, Q.; Xie, H.; Wu, B.; Yuan, J. Electron-Deficient Engineering in Large-Conjugate-Heptazine Framework to Effectively Shuttle Hot Electrons for Efficient Photocatalytic H2O2 Production. Adv. Funct. Mater. 2025, 35, 2414193. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Sang, Z.; Tan, H.; Ye, N.; Sun, C.; Sun, Z.; Luo, M.; Guo, S. Enhanced hydrogen peroxide photosynthesis in covalent organic frameworks through induced asymmetric electron distribution. Nat. Synth. 2025, 4, 134–141. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, G.; Siahrostami, S.; Chen, Z.; Liu, K.; Xie, J.; Liao, L.; Wu, T.; Lin, D.; Liu, Y.; et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 2018, 1, 156–162. [Google Scholar] [CrossRef]
- Xia, C.; Xia, Y.; Zhu, P.; Fan, L.; Wang, H. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 2019, 366, 226–231. [Google Scholar] [CrossRef]
- Jung, E.; Shin, H.; Lee, B.-H.; Efremov, V.; Lee, S.; Lee, H.S.; Kim, J.; Antink, W.H.; Park, S.; Lee, K.-S.; et al. Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 2020, 19, 436–442. [Google Scholar] [CrossRef]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef]
- Jiang, Y.; Ni, P.; Chen, C.; Lu, Y.; Yang, P.; Kong, B.; Fisher, A.; Wang, X. Selective Electrochemical H2O2 Production through Two-Electron Oxygen Electrochemistry. Adv. Energy Mater. 2018, 8, 1801909. [Google Scholar] [CrossRef]
- Sun, X.; Yang, J.; Zeng, X.; Guo, L.; Bie, C.; Wang, Z.; Sun, K.; Sahu, A.K.; Tebyetekerwa, M.; Rufford, T.E.; et al. Pairing Oxygen Reduction and Water Oxidation for Dual−pathway H2O2 Production. Angew. Chem. Int. Ed. 2024, 63, e202414417. [Google Scholar] [CrossRef]
- Sun, P.; Mo, Z.; Zhang, J.; Wu, G.; Miao, Z.; Zhong, K.; Wei, Y.; Jia, C.; Chen, Z.; Xu, H. Cyano-rich carbon nitride with tunable n → π* electronic transition for enhanced broad-spectrum photocatalytic H2O2 production. Chem. Eng. J. 2023, 478, 147337. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.; Yu, X.; Sun, L.; Li, J.; Yang, J.; Liu, Q. Precise regulation of built-in electric field over NH2-MIL-125-Ti/WO3-x S-scheme heterojunction for achieving simultaneous formation of CO and H2O2 from CO2 and H2O. Chem. Eng. J. 2023, 466, 143129. [Google Scholar] [CrossRef]
- Zhou, C.; Song, Y.; Wang, Z.; Liu, J.; Sun, P.; Mo, Z.; Yi, J.; Zhai, L. Cyano-rich porous carbon nitride nanosheets for enhanced photocatalytic H2O2 production. J. Environ. Chem. Eng. 2023, 11, 110138. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Sun, L.; Jiang, H.; Liu, Y.; Liu, Q.; She, X.; Tang, H. Se–Se bonds induced highly metallic 1T’ MoSe2.3 nanosheets cocatalysts towards boosted H2O2 photosynthesis over NH2-MIL-125 derived TiO2 nanotablets. Chem. Eng. J. 2023, 477, 146945. [Google Scholar] [CrossRef]
- Deng, D.; Wang, J.; Wang, M.; Wang, Y.; Jiang, J.; Chen, Y.; Bai, Y.; Wu, Q.; Lei, Y. Accelerated O2 adsorption and stabilized *OOH for electrocatalytic H2O2 production. J. Mater. Sci. Technol. 2025, 227, 76–81. [Google Scholar] [CrossRef]
- Mahapatra, S.D.; Mohapatra, P.C.; Aria, A.I.; Christie, G.; Mishra, Y.K.; Hofmann, S.; Thakur, V.K. Piezoelectric Materials for Energy Harvesting and Sensing Applications: Roadmap for Future Smart Materials. Adv. Sci. 2021, 8, 2100864. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, J.; Lee, C.; Lee, S.-W.; Lin, L. A comprehensive review on piezoelectric energy harvesting technology: Materials, mechanisms, and applications. Appl. Phys. Rev. 2018, 5, 041306. [Google Scholar] [CrossRef]
- Xue, X.; Zang, W.; Deng, P.; Wang, Q.; Xing, L.; Zhang, Y.; Wang, Z.L. Piezo-potential enhanced photocatalytic degradation of organic dye using ZnO nanowires. Nano Energy 2015, 13, 414–422. [Google Scholar] [CrossRef]
- Hong, K.-S.; Xu, H.; Konishi, H.; Li, X. Direct Water Splitting Through Vibrating Piezoelectric Microfibers in Water. J. Phys. Chem. Lett. 2010, 1, 997–1002. [Google Scholar] [CrossRef]
- Starr, M.B.; Shi, J.; Wang, X. Piezopotential-Driven Redox Reactions at the Surface of Piezoelectric Materials. Angew. Chem. Int. Ed. 2012, 51, 5962–5966. [Google Scholar] [CrossRef]
- Tan, C.F.; Ong, W.L.; Ho, G.W. Self-biased hybrid piezoelectric-photoelectrochemical cell with photocatalytic functionalities. ACS Nano 2015, 9, 7661–7670. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Z.; Zhao, J.; Zhang, Z.; Li, X.; Zhang, J. Recent Advances of Ferro-, Piezo-, and Pyroelectric Nanomaterials for Catalytic Applications. ACS Appl. Nano Mater. 2020, 3, 1063–1079. [Google Scholar] [CrossRef]
- Starr, M.B.; Wang, X. Fundamental Analysis of Piezocatalysis Process on the Surfaces of Strained Piezoelectric Materials. Sci. Rep. 2013, 3, 2160. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sang, Y.; Chang, S.; Huang, X.; Zhang, Y.; Yang, R.; Jiang, H.; Liu, H.; Wang, Z.L. Enhanced Ferroelectric-Nanocrystal-Based Hybrid Photocatalysis by Ultrasonic-Wave-Generated Piezophototronic Effect. Nano Lett. 2015, 15, 2372–2379. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Z.; Liu, M.; Liu, X.; Huang, W.; Sun, S.; Jiang, Y.; Liu, Y.; Zhang, J.; Zhang, Z. Remarkably enhanced photocatalytic performance of Au/AgNbO3 heterostructures by coupling piezotronic with plasmonic effects. Nano Energy 2022, 95, 107031. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, Y.; Ye, J.; Zhou, X.; Zhou, X.; Zhao, Y.; Feng, K.; Luo, H.; Zhang, D.; Bowen, C. Retrievable Hierarchically Porous Ferroelectric Ceramics for “Greening” the Piezo-Catalysis Process. Adv. Funct. Mater. 2024, 34, 2311091. [Google Scholar] [CrossRef]
- Zhang, Y.; Khanbareh, H.; Dunn, S.; Bowen, C.R.; Gong, H.; Duy, N.P.H.; Phuong, P.T.T. High Efficiency Water Splitting using Ultrasound Coupled to a BaTiO3 Nanofluid. Adv. Sci. 2022, 9, 2105248. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Xu, J.; Tang, W.; Wang, Z.L.; Fan, F.R. Contact-electro-catalysis for Direct Synthesis of H2O2 under Ambient Conditions. Angew. Chem. Int. Ed. 2023, 62, e202300604. [Google Scholar] [CrossRef]
- Ran, M.; Du, B.; Liu, W.; Liang, Z.; Liang, L.; Zhang, Y.; Zeng, L.; Xing, M. Dynamic defects boost in-situ H2O2 piezocatalysis for water cleanup. Proc. Natl. Acad. Sci. USA 2024, 121, e2317435121. [Google Scholar] [CrossRef]
- Jin, C.-C.; Liu, D.-M.; Zhang, L.-X. An Emerging Family of Piezocatalysts: 2D Piezoelectric Materials. Small 2023, 19, 2303586. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, L.; Wang, W.; Li, X.; Zhang, Y.; Shao, D. Enhanced H2 evolution based on ultrasound-assisted piezo-catalysis of modified MoS2. J. Mater. Chem. A 2018, 6, 11909–11915. [Google Scholar] [CrossRef]
- Wu, J.M.; Sun, Y.-G.; Chang, W.-E.; Lee, J.-T. Piezoelectricity induced water splitting and formation of hydroxyl radical from active edge sites of MoS2 nanoflowers. Nano Energy 2018, 46, 372–382. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, G.; Wang, Y.; Yang, X.; Wei, T.; Wang, Q.; Liang, J.; Xu, N.; Li, Z.; Zhu, B.; et al. Seed-Induced Vertical Growth of 2D Bi2O2Se Nanoplates by Chemical Vapor Transport. Adv. Funct. Mater. 2019, 29, 1906639. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Ye, S.; Song, J.; Qu, J. Progress Report on Property, Preparation, and Application of Bi2O2Se. Adv. Funct. Mater. 2020, 30, 2004480. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, H.; Meng, M.; Chen, C.; Sun, Y.; Chen, Z.; Dang, W.; Tan, C.; Liu, Y.; Yin, J.; et al. High electron mobility and quantum oscillations in non-encapsulated ultrathin semiconducting Bi2O2Se. Nat. Nanotechnol. 2017, 12, 530–534. [Google Scholar] [CrossRef]
- Zhu, Z.; Yao, X.; Zhao, S.; Lin, X.; Li, W. Giant Modulation of the Electron Mobility in Semiconductor Bi2O2Se via Incipient Ferroelectric Phase Transition. J. Am. Chem. Soc. 2022, 144, 4541–4549. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Y.; Zhang, Y.; Zhang, Z.; Wang, W.; Lai, Z.; Xie, P.; Li, D.; Chen, D.; Quan, Q.; et al. Electrically Switchable Polarization in Bi2O2Se Ferroelectric Semiconductors. Adv. Mater. 2023, 35, 2210854. [Google Scholar] [CrossRef]
- Ghosh, T.; Samanta, M.; Vasdev, A.; Dolui, K.; Ghatak, J.; Das, T.; Sheet, G.; Biswas, K. Ultrathin Free-Standing Nanosheets of Bi2O2Se: Room Temperature Ferroelectricity in Self-Assembled Charged Layered Heterostructure. Nano Lett. 2019, 19, 5703–5709. [Google Scholar] [CrossRef]
- Li, M.-Q.; Dang, L.-Y.; Wang, G.-G.; Li, F.; Han, M.; Wu, Z.-P.; Li, G.-Z.; Liu, Z.; Han, J.-C. Bismuth Oxychalcogenide Nanosheet: Facile Synthesis, Characterization, and Photodetector Application. Adv. Mater. Technol. 2020, 5, 2000180. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Y.; Xie, M.; Li, Z.; Wu, C.; Zhao, H.; Wang, J.; Wang, H.; Yan, Y.; Zhong, N.; et al. Piezo-photocatalysts in the field of energy and environment: Designs, applications, and prospects. Nano Energy 2023, 112, 108508. [Google Scholar] [CrossRef]
- Fu, M.; Luo, J.; Shi, B.; Tu, S.; Wang, Z.; Yu, C.; Ma, Z.; Chen, X.; Li, X. Promoting Piezocatalytic H2O2 Production in Pure Water by Loading Metal-Organic Cage-Modified Gold Nanoparticles on Graphitic Carbon Nitride. Angew. Chem. Int. Ed. 2024, 63, e202316346. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, L.; Xiang, D.; Guo, Z.; Wang, C.; Song, Q. Facile and enlargeable preparation of piezocatalytic CaCO3 for efficient degradation of organic dyes. Enviromental Res. 2025, 267, 120649. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Shan, T.; Deng, L.; Li, M.; Pan, X.; Yang, X.; Zhao, X.; Yang, M.-Q. Facile synthesis of hierarchical CdS nanoflowers for efficient piezocatalytic hydrogen evolution. Dalton Trans. 2023, 52, 13426–13434. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tian, N.; An, Y.; Sun, R.; Zhang, Y.; Huang, H. Morphology Regulation and Oxygen Vacancy Construction Synergistically Boosting the Piezocatalytic Degradation and Pure Water Splitting of SrTiO3. Small 2024, 20, 2407624. [Google Scholar] [CrossRef] [PubMed]
- Tomita, O.; Otsubo, T.; Higashi, M.; Ohtani, B.; Abe, R.J.A.C. Partial oxidation of alcohols on visible-light-responsive WO3 photocatalysts loaded with palladium oxide cocatalyst. ACS Catal. 2016, 6, 1134–1144. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).