SiO2@Fe(III)-Based Metal–Organic Framework Core–Shell Microspheres for Water-Purification-Based Photo-Fenton Processes
Abstract
1. Introduction
2. Results and Discussion
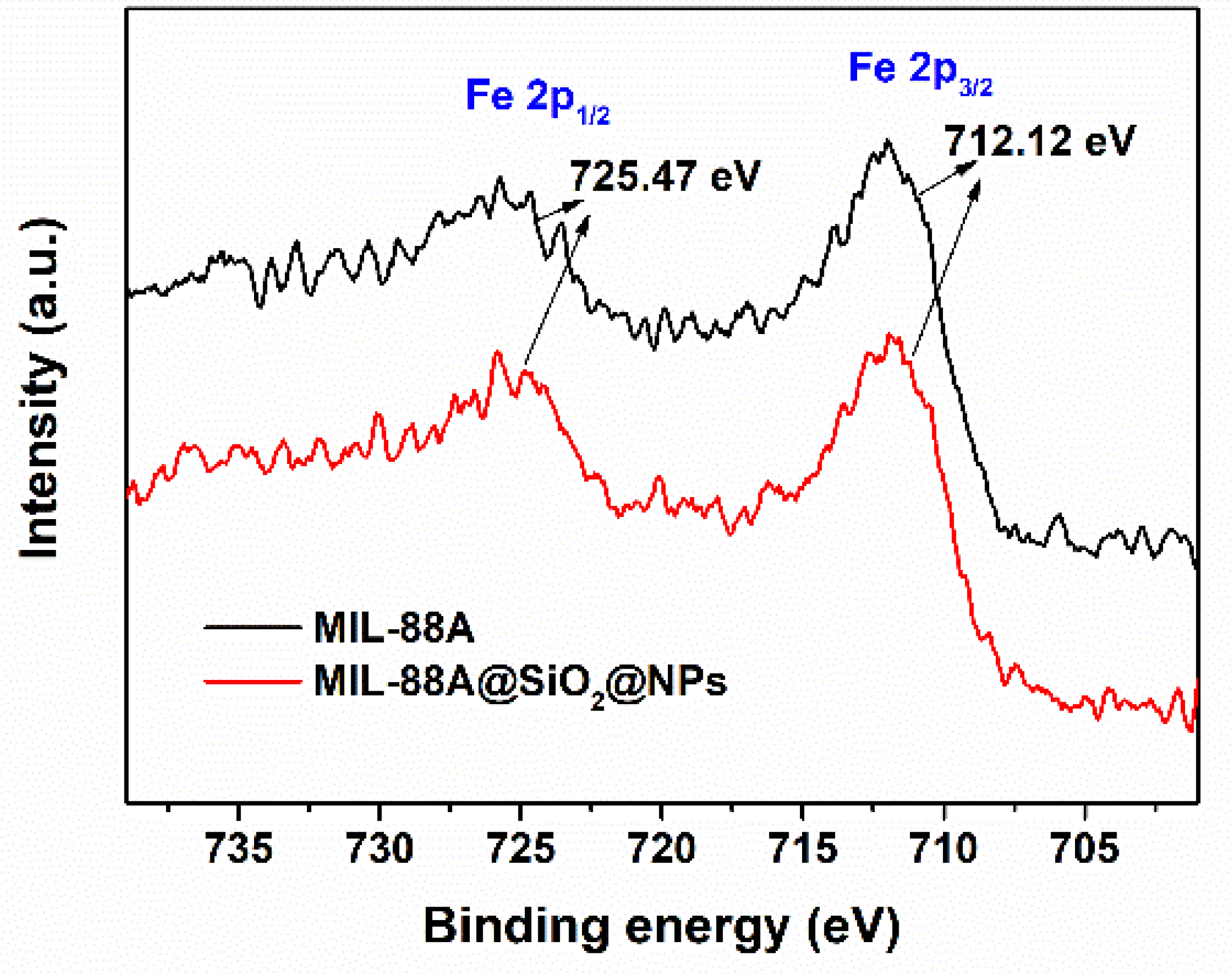
2.1. Synthesis and Structural Characterization of the Core–Shell Catalyst
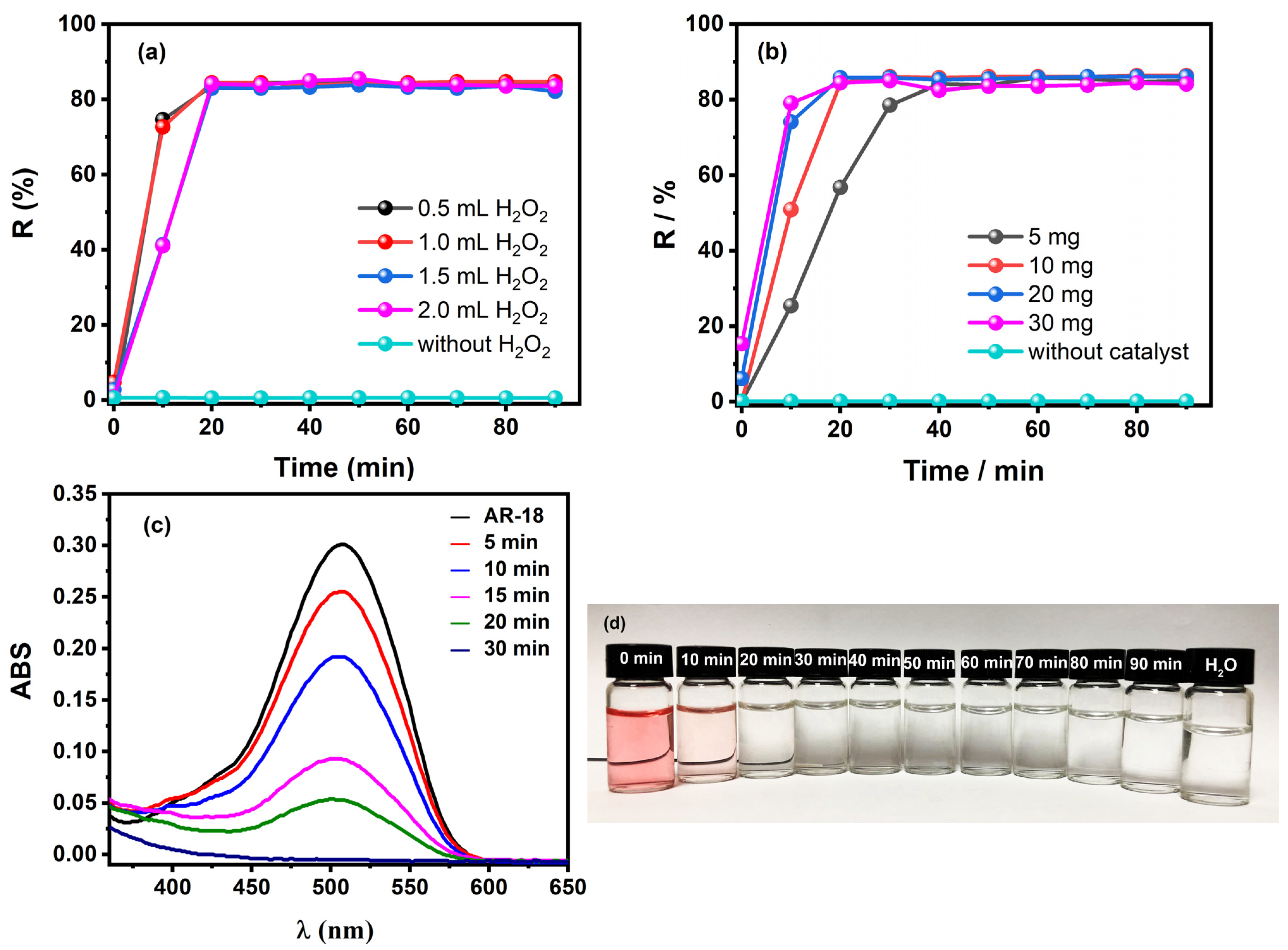
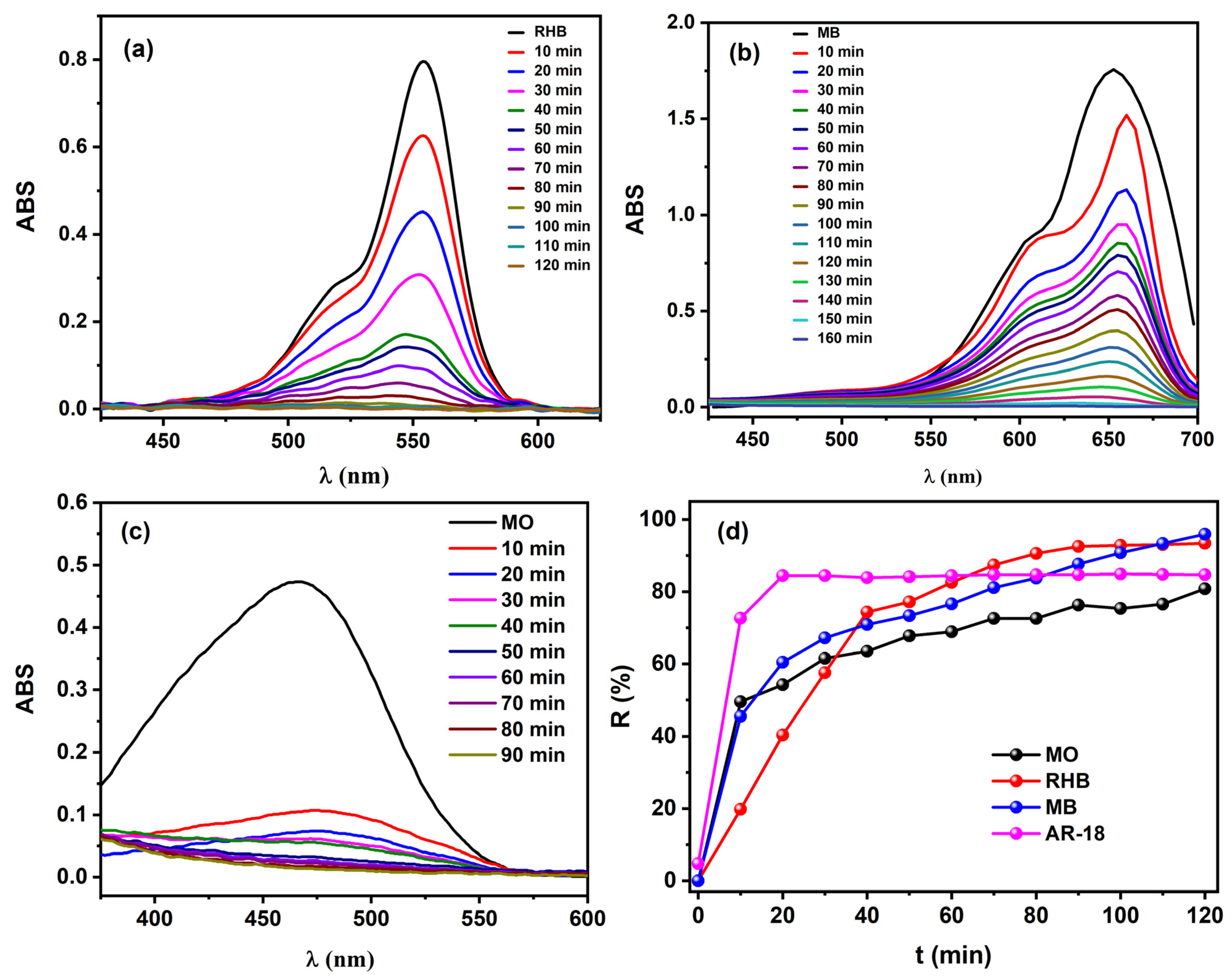
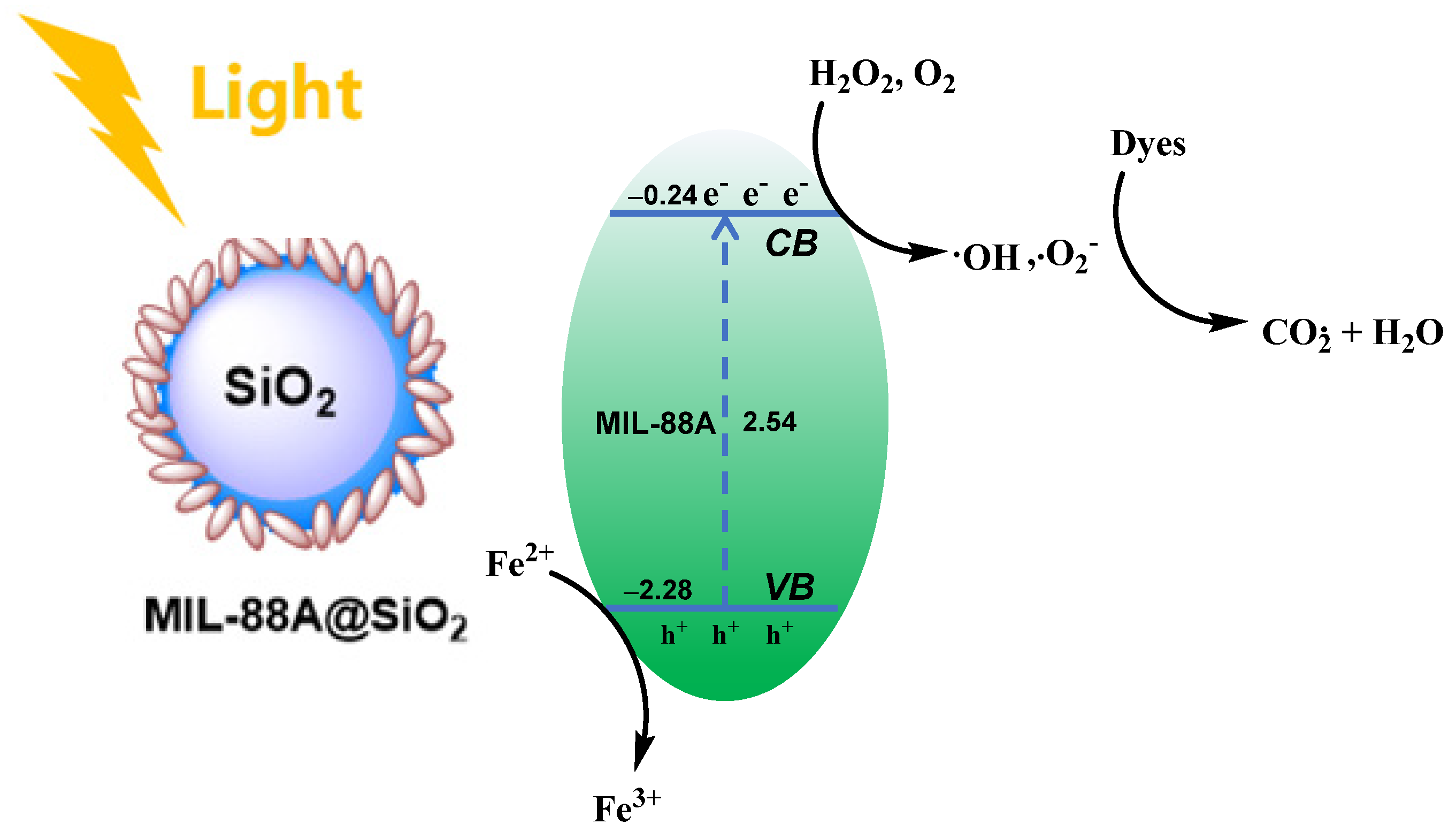
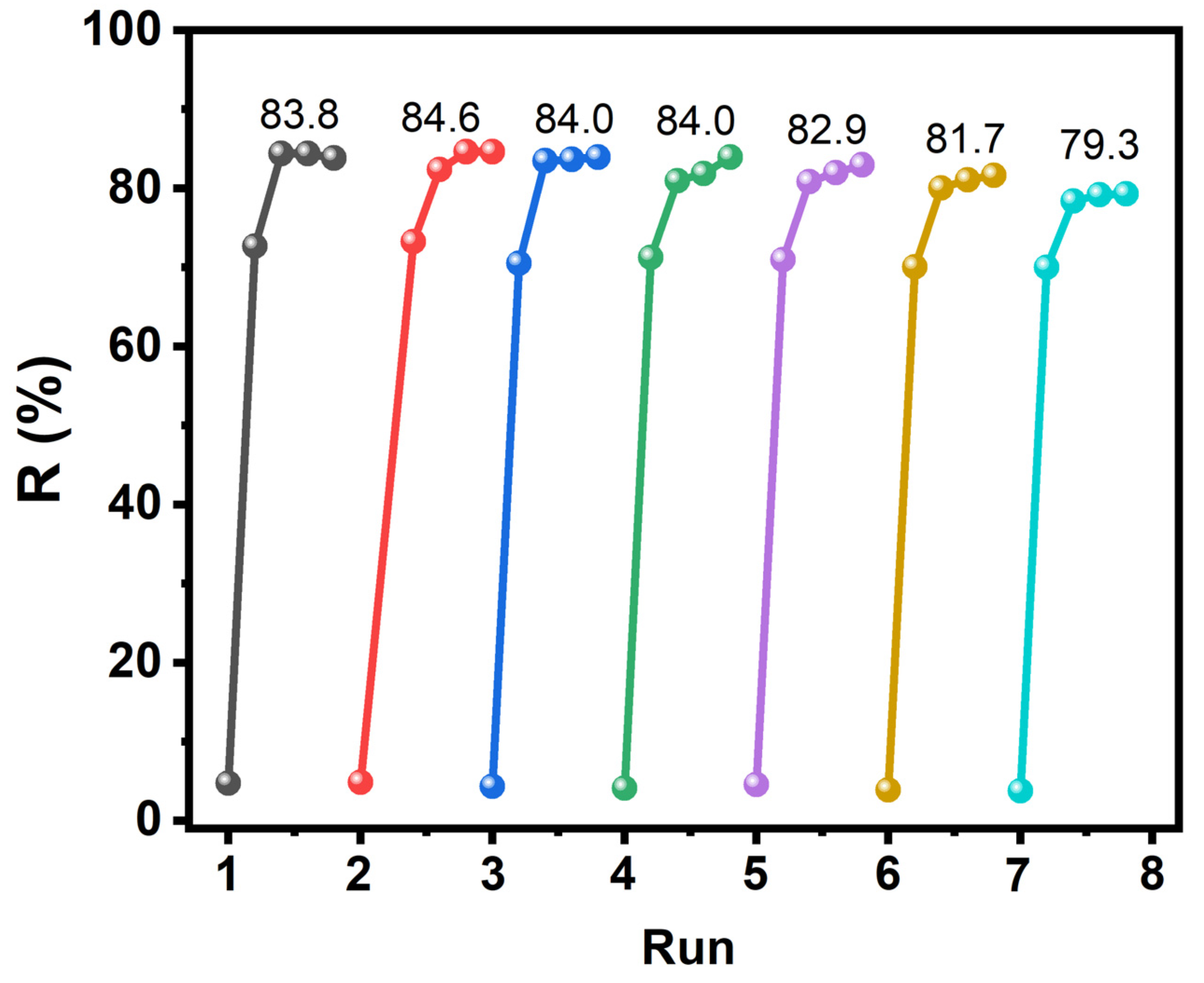
2.2. Catalytic Performance of the Heterogeneous Catalyst
3. Experimental Section
3.1. Characterization
3.2. Synthesis of Carboxylate-Terminated SiO2 Microspheres
3.3. Preparation of the Core–Shell MIL-88A@ SiO2
3.4. General Procedure for the Photo-Fenton Processes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghosh, A.; Nayak, A.K.; Pal, A. Nano-Particle-Mediated Wastewater Treatment: A Review. Curr. Pollut. Rep. 2017, 3, 17–30. [Google Scholar] [CrossRef]
- Che, H.; Che, G.; Dong, H.; Hu, W.; Hu, H.; Liu, C.; Li, C. Fabrication of Z-scheme Bi3O4Cl/g-C3N4 2D/2D heterojunctions with enhanced interfacial charge separation and photocatalytic degradation various organic pollutants activity. Appl. Surf. Sci. 2018, 455, 705–716. [Google Scholar] [CrossRef]
- Xie, A.; Dai, J.; Cui, J.; Lang, J.; Wei, M.; Dai, X.-H.; Li, C.; Yan, Y. Novel graphene oxide–confined nanospace directed synthesis of glucose-based porous carbon nanosheets with enhanced adsorption performance. ACS Sustain. Chem. Eng. 2017, 5, 11566–11576. [Google Scholar] [CrossRef]
- Xie, A.; Dai, J.; Chen, X.; Ma, P.; He, J.; Li, C.; Zhou, Z.; Yan, Y. Ultrahigh adsorption of typical antibiotics onto novel hierarchical porous carbons derived from renewable lignin via halloysite nanotubes-template and in-situ activation. Chem. Eng. J. 2016, 304, 609–620. [Google Scholar] [CrossRef]
- Che, H.; Liu, C.; Hu, W.; Hu, H.; Li, J.; Dou, J.; Shi, W.; Li, C.; Dong, H. NGQD active sites as effective collectors of charge carriers for improving the photocatalytic performance of Z-scheme g-C3N4/Bi2WO6heterojunctions. Catal. Sci. Technol. 2018, 8, 622–631. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Angelidaki, I.; Zhang, Y. Bio-electro-Fenton processes for wastewater treatment: Advances and prospects. Chem. Eng. J. 2018, 354, 492–506. [Google Scholar] [CrossRef]
- Sudmalis, D.; Da Silva, P.; Temmink, H.; Bijmans, M.; Pereira, M. Biological treatment of produced water coupled with recovery of neutral lipids. Water Res. 2018, 147, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Avilés, P.; Keller, A.A. Incidence of metal-based nanoparticles in the conventional wastewater treatment process. Water Res. 2021, 189, 116603. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Simultaneous activation and magnetization toward facile preparation of auricularia-based magnetic porous carbon for efficient removal of tetracycline. J. Alloy. Compd. 2019, 784, 76–87. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Goh, P.S.; Ismail, A.F.; Ng, B.C.; Abdullah, M.S. Recent Progresses of Forward Osmosis Membranes Formulation and Design for Wastewater Treatment. Water 2019, 11, 2043. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Yuan, X.; Zhang, J.; Chew, J.W. Metal-organic framework membranes for wastewater treatment and water regeneration. Coordination Chem. Rev. 2020, 404, 213116. [Google Scholar] [CrossRef]
- Reshmy, R.; Thomas, D.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Binod, P.; Pugazhendhi, A.; Sirohi, R.; Tarafdar, A.; et al. Potential of nanocellulose for wastewater treatment. Chemosphere 2021, 281, 130738. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, S.; Sun, Y.; Xiong, T.; Dong, F.; Zhang, W. Bi metal sphere/graphene oxide nanohybrids with enhanced direct plasmonic photocatalysis. Appl. Catal. B Environ. 2017, 214, 148–157. [Google Scholar] [CrossRef]
- Zhang, X.W.; Wang, F.; Wang, C.C.; Wang, P.; Fu, H.; Zhao, C. Photocatalysis activation of peroxodisulfate over the supported Fe3O4 catalyst derived from MIL-88A(Fe) for efficient tetracycline hydrochloride degradation. Chem. Eng. J. 2021, 426, 131927. [Google Scholar] [CrossRef]
- Dang, T.T.; Do, V.M.; Trinh, V.T. Nano-Catalysts in Ozone-Based Advanced Oxidation Processes for Wastewater Treatment. Curr. Pollut. Rep. 2020, 6, 217–229. [Google Scholar] [CrossRef]
- Rajput, H.; Kwon, E.E.; Younis, S.A.; Weon, S.; Jeon, T.H.; Choi, W.; Kim, K.H. Photoelectrocatalysis as a high-de platform for pulping wastewater treatment and energy production. Chem. Eng. J. 2021, 412, 128612. [Google Scholar] [CrossRef]
- Jain, B.; Singh, A.K.; Kim, H.; Lichtfouse, E.; Sharma, V.K. Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ. Chem. Lett. 2018, 16, 947–967. [Google Scholar] [CrossRef]
- Meyerstein, D. Re-examining Fenton and Fenton-like reactions. Nat. Rev. Chem. 2021, 5, 595–597. [Google Scholar] [CrossRef]
- Lu, Z.; Cao, X.; Wei, H.; Huo, W.; Wang, Q.; Li, K. Strong enhancement effect of bisulfite on MIL-68(Fe)-catalyzed Fenton-like reaction for organic pollutants degradation. Appl. Surf. Sci. 2021, 542, 148631. [Google Scholar] [CrossRef]
- Sohrabi, M.R.; Khavaran, A.; Shariati, S. Removal of Carmoisine edible dye by Fenton and photo Fenton processes using Taguchi orthogonal array design. Arab. J. Chem. 2017, 10, S3523–S3531. [Google Scholar] [CrossRef]
- Belloa, M.M.; Ramana, A.A.A.; Asghar, A. A review on approaches for addressing the limitations of Fentonoxidation for recalcitrant wastewater treatment. Process Saf. Environ. Prot. 2019, 126, 119–140. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhua, J.; Zhue, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A Review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Mendoza-Cortes, J.L.; O’Keeffe, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1257. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S.-I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334. [Google Scholar] [CrossRef] [PubMed]
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Cen, W.; Li, Q.; Li, J.; Lu, Y.; Wang, H.; Wu, Z. A mild one-step method for enhancing optical absorption of amine-functionalized metal-organic frameworks. Appl. Catal. B Environ. 2018, 227, 190–197. [Google Scholar] [CrossRef]
- Yang, H.; He, X.-W.; Wang, F.; Kang, Y.; Zhang, J. Doping copper into ZIF-67 for enhancing gas uptake capacity and visible-light-driven photocatalytic degradation of organic dye. J. Mater. Chem. 2012, 22, 21849–21851. [Google Scholar] [CrossRef]
- Roy, D.; Neogi, S.; De, S. Mechanistic investigation of photocatalytic degradation of Bisphenol-A using MIL-88A(Fe)/MoS2 Z-scheme heterojunction composite assisted peroxymonosulfate activation. Chem. Eng. J. 2022, 428, 131028. [Google Scholar] [CrossRef]
- Liu, N.; Huang, W.; Zhang, X.; Tang, L.; Wang, L.; Wang, Y.; Wu, M. Ultrathin graphene oxide encapsulated in uniform MIL-88A(Fe) for enhanced visible light-driven photodegradation of RhB. Appl. Catal. B Environ. 2018, 221, 119–128. [Google Scholar] [CrossRef]
- Xue, B.; Du, L.; Jin, J.; Meng, H.; Mi, J. In situ growth of MIL-88A into polyacrylate and its application in highly efficient photocatalytic degradation of organic pollutants in water. Appl. Surf. Sci. 2021, 564, 150404. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef] [PubMed]
- Pascanu, V.; Miera, G.G.; Inge, A.K.; Martín-Matute, B. Metal–Organic Frameworks as Catalysts for Organic Synthesis: A Critical Perspective. J. Am. Chem. Soc. 2019, 141, 7223–7234. [Google Scholar] [CrossRef]
- Ji, P.; Feng, X.; Oliveres, P.; Li, Z.; Murakami, A.; Wang, C.; Lin, W. Strongly Lewis Acidic Metal–Organic Frameworks for Continuous Flow Catalysis. J. Am. Chem. Soc. 2019, 141, 14878–14888. [Google Scholar] [CrossRef] [PubMed]
- Serre, C.; Mellot-Draznieks, C.; Surblé, S.; Audebrand, N.; Filinchuk, Y.; Férey, G. Role of Solvent-Host Interactions That Lead to Very Large Swelling of Hybrid Frameworks. Science 2007, 315, 1828–1831. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Li, X.; Xie, Y.; He, J.; Yu, J.; Song, Y. The MIL-88A-Derived Fe3O4-Carbon Hierarchical Nanocomposites for Electrochemical Sensing. Sci. Rep. 2015, 5, 14341. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Cui, J.; Yang, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Graphene oxide/Fe(III)-based metal-organic framework membrane for enhanced water purification based on synergistic separation and photo-Fenton processes. Appl. Catal. B Environ. 2020, 264, 118548. [Google Scholar] [CrossRef]
- Fu, H.; Song, X.-X.; Wu, L.; Zhao, C.; Wang, P.; Wang, C.-C. Room-temperature preparation of MIL-88A as a heterogeneous photo-Fenton catalyst for degradation of rhodamine B and bisphenol a under visible light. Mater. Res. Bull. 2020, 125, 110806. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, Z.; Gao, J.; Tong, P.; Liu, W.; Zhang, L. Metal–organic framework UiO-66 modified magnetite@silicacore–shell magnetic microspheres for magnetic solid-phaseextraction of domoic acid from shellfish samples. J. Chromatogr. A 2015, 1400, 10–18. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Zhu, Y.; Cheng, T.; Liu, G.; Tan, C. SiO2@Fe(III)-Based Metal–Organic Framework Core–Shell Microspheres for Water-Purification-Based Photo-Fenton Processes. Catalysts 2025, 15, 23. https://doi.org/10.3390/catal15010023
Liu K, Zhu Y, Cheng T, Liu G, Tan C. SiO2@Fe(III)-Based Metal–Organic Framework Core–Shell Microspheres for Water-Purification-Based Photo-Fenton Processes. Catalysts. 2025; 15(1):23. https://doi.org/10.3390/catal15010023
Chicago/Turabian StyleLiu, Kaihong, Yuanli Zhu, Tanyu Cheng, Guohua Liu, and Chunxia Tan. 2025. "SiO2@Fe(III)-Based Metal–Organic Framework Core–Shell Microspheres for Water-Purification-Based Photo-Fenton Processes" Catalysts 15, no. 1: 23. https://doi.org/10.3390/catal15010023
APA StyleLiu, K., Zhu, Y., Cheng, T., Liu, G., & Tan, C. (2025). SiO2@Fe(III)-Based Metal–Organic Framework Core–Shell Microspheres for Water-Purification-Based Photo-Fenton Processes. Catalysts, 15(1), 23. https://doi.org/10.3390/catal15010023




