Abstract
Coal fly ash and acid mine drainage are significant environmental issues in South Africa, causing storage constraints and impacting water quality. This study explores the use of coal fly ash and acid mine drainage in preparing zeolite HBEA-supported Fe catalysts. The Na-BEA parent catalysts were synthesised hydrothermally using coal fly ash as a feedstock. The Fe was loaded upon the H-BEA form zeolite using liquid-phase ion exchange or wet impregnation, using Fe-rich acid mine drainage as the metal precursor. The ion-exchanged Fe-BEA catalysts exhibited excellent activity, with the highest selectivity achieved over the 25 AHW after 0.5 h on stream. The study also found that when impregnation was used to load Fe onto the zeolite support, other metals present in the AMD affected the overall activity, with Mn, Ca, Mg, and Na decreasing conversion and selectivity, while Ni had a promoting effect. This study demonstrates that green solid acid catalysts with high catalytic activity can be prepared using two waste materials, coal fly ash and acid mine drainage. To the best of our knowledge, we are reporting for the first time the use of acid mine drainage as a metal precursor in Fe-BEA catalyst preparation.
1. Introduction
Coal incineration for electric power generation produces vast amounts of coal fly ash (CFA) as a waste by-product. When coal is heated to very high temperatures (in the range 1400–1700 °C), the ultra-fine grey-coloured ash component that is carried up with the flue gas stream and gathered by electrostatic precipitators is what is referred to as CFA [1,2,3]. CFA is composed of unburnt carbon, metal oxides (Si, Fe, Ca, and Al), and other inorganic elements. Depending on the chemical composition, CFA is categorised into Class F and Class C in accordance with the maximum and minimum wt.% of SiO2, Al2O3, Fe2O3, and SO3 [4,5]. Based on the ASTM C618 ash classification, CFA containing > 70% wt.% of SiO2 + Al2O3 + Fe2O3 is defined as Class F, while CFA having a wt.% in the range of 50–70% is defined as Class C [6]. Globally, annual generation of CFA waste is estimated to be around 750 Mt while in South Africa it is around 34.4 Mt [7,8]. With the increase of energy demand and the affordability of coal as a source of energy, the production of CFA worldwide continues unabated.
Waste CFA is a contributing factor in air, water, and soil pollution, which all lead to both human health concerns and numerous geo-environmental concerns. Thus, disposal of CFA in landfills can hamper entire ecological cycles if not well managed. The elemental composition of CFA shows that it contains a wide range of elements below atomic number 92, and these elements are considered major sources of air, water, and soil pollution. These metals include As, Be, B, Cd, Cr, Co, Pb, Mn, Hg, Se, Sr, Tl, and V in varying concentrations [9,10]. CFA has also been associated with several physiological and health issues, including cancer, anaemia, gastroenteritis, and dermatitis [11]. In addition to these health and environmental issues, CFA also poses storage constraints at power stations, and to alleviate these issues, CFA utilisation is a rational consideration. Globally, of the CFA produced from coal-fired thermal power stations, only 25–30% is utilised in different sectors [12]. In South Africa, only 7% of the produced CFA is utilised and most of the power stations are running out of ash storage space. CFA beneficiation may also trigger a reduction in power production costs, which could filter through to the consumer in terms of electricity pricing [7]. There are several known CFA applications, some of which include its use as soil amelioration; in the concrete, ceramic, and construction industry, in catalysis or as structural fillers; or for valuable metal recovery, stabilisation/solidification, and zeolite synthesis [5,13,14,15].
The major presence of Al and Si in CFA makes it a suitable feedstock in the synthesis of zeolites. Numerous studies report on the synthesis of zeolites from CFA [4,16,17,18,19,20]. Zeolites are three-dimensional tetrahedral aluminosilicate minerals. They possess diverse pore structures and have applications across many fields. The most commonly employed synthesis routes of zeolites from CFA use a one- or two-step hydrothermal method, alkaline sintering, or microwave- or ultrasonic-assisted hydrothermal methods [21]. Zeolites are composed of a framework of interconnected silica (SiO4) and alumina (AlO4) tetrahedra that form channels and cavities within the crystal structure. This results in a porous material having high surface area and acidity, properties which consequently enable zeolites to be applied in numerous fields including medicine, cosmetics, agriculture, biotechnology, waste management, pollution control, ion exchange, adsorption, catalysis, and water treatment [22,23]. The ability of zeolites to ion exchange and to act as adsorbents has seen them being applied in the removal of toxic metals from wastewaters including acid mine drainage [22,24,25,26,27].
Acid mine drainage (AMD) is the largest environmental problem posed by mining activities. AMD pollution originates from complex oxidation interactions that occur under ambient conditions in abandoned and active mines following the oxidation of sulphide-bearing minerals such as pyrites. The chemical reactions leading to AMD formation occur in the presence of oxygen, water, and sulphate-oxidising bacteria (thiobaccillus ferrooxidans) [4,28]. In South Africa, former and present gold and coal mines are the major sources of AMD. Apart from reducing the pH of the waters, the generated AMD dissolves acid-soluble toxic metals from tailings and deposits them into soils and water via numerous mechanisms. Consequently, the soil and water quality deteriorate, endangering ecological systems and plant life [29,30]. To deal with this issue, numerous AMD remediation technologies have been developed and adopted, which include chemical neutralisation, precipitation, adsorption, ion exchange, membrane separation processes, and bioremediation [31,32,33,34,35,36]. Since most of the AMD remediation technologies available are costly, there has been growing advocacy towards sustainable remediation technologies alongside AMD beneficiation. It is in this light that AMD has been applied in activation of pyrite and chalcopyrite flotation, regulating pH, leaching of copper-bearing rock, and recovery of valuable resources such as clean water, sulphuric acid, and metals [37,38,39]. When zeolites are employed in the treatment of AMD, the removal of metal ions progresses via ion exchange and chemisorption [26].
Commercial iron-containing zeolites have proved to be versatile catalysts and have been applied in both liquid-phase and gas-phase reaction transformations. Some of the reactions include oxidation of benzene to phenol, dehydrative aromatisation of furans to aromatics, selective catalytic reduction of nitrogen oxides, hydrogenation of CO2 into hydrocarbons, and Fenton and photo-Fenton oxidation [40,41,42,43,44]. Another reaction in which Fe-zeolite catalysts have been used is the Friedel–Crafts (FC) alkylation reaction. The FC alkylation reaction is a classic electrophilic substitution reaction often used in the preparation of alkyl-substituted benzenes [4,45]. The FC alkylation of benzene is a fundamental reaction transformation in the fine chemicals, petroleum, plastic, and detergent industries. In detergent production, zeolites are mostly used in the preparation of linear alkylbenzene sulphonates (LABS) [46]. The preferred use of LABS in detergent manufacture is based on their unique characteristics such as water solubility, dispersibility, and surface activity [47]. In addition, they are also biodegradable, and this has seen them replacing branched alkylbenzenes in detergent synthesis [48].
Conventional catalysts that are used in the FC alkylation reaction include AlCl3, FeCl3, BF3, BeCl2, TiCl4, SbCl5, or SnCl4. In certain instances, strong Brønsted acids including sulfuric acid and hydrofluoric acid or super acids such as HF•SbF5 and HSO3F•SbF5 have been used [49]. These ranges of catalysts present product separation constraints, and in the case of strong acids, they can be corrosive to reaction vessels and have negative effects if they find their way into the environment. The use of metal-loaded zeolites as solid acid catalysts in the FC alkylation reaction will avoid these challenges. Another advantage of using zeolite catalysts is their shape selectivity, which is absent in the traditional FC alkylation catalysts [50] The Friedel–Crafts alkylation of benzene using t-butyl chloride progresses in accordance with the following Scheme 1:
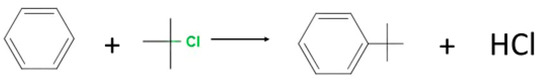
Scheme 1.
Alkylation of benzene with t-butyl chloride.
The preparation of t-butyl benzene from the FC alkylation of benzene using t-butyl chloride produces a mixture of the monoalkylated product (t-butyl chloride), dialkylated products (1,3-di-t-butylbenzene and 1,4-di-t-butylbenzene), and a trialkylate (1,3,5-tri-t-butylbenzene) [50]. Thus, if the objective is to synthesise a linear alkylbenzene, an ideal catalyst should have good selectivity towards the monoalkylate. In systems where shape selectivity is absent, the production of the monoalkylated product is less favoured, since the monoalkylated product is more active than the benzene and thus susceptible to further alkylation to form dialkylates or even the trialkylate. There has been a marked advancement in the use of green solid acid catalysts in the FC alkylation reaction, and metal-loaded zeolites are ideal candidates. The synthesis of zeolite HBEA from CFA and its utilisation in the removal of metal ions from AMD, resulting in a metal-loaded zeolite which can be further used as a solid acid catalyst, presents beneficiation of two waste materials (CFA and AMD) and may provide a sustainable green catalyst for various applications. To study the feasibility of the Fe-zeolite catalysts generated by using CFA as feedstock for zeolite synthesis and using AMD as a metal precursor in the catalyst synthesis, the Friedel–Crafts (FC) alkylation of benzene with t-butyl benzene was used as a probe reaction.
2. Results and Discussion
2.1. Characterisation
The elemental composition of the AMD used as metal precursor is presented in Table 1.

Table 1.
Elemental composition of the acid mine drainage used as metal precursor (showing major elements only).
The elemental compositions of the AMD metal-loaded HBEA prepared via liquid-phase ion exchange and wet impregnation with AMD are presented in Table 2 and Table 3, respectively. It should be noted that using ICP-OES, the Si/Al ratio of the pristine HBEA was 19.2. The Si/Al ratio can give an idea of how many exchange sites are available per gram of zeolite, assuming that all the framework positions occupied by Al are Brønsted acid exchange sites and accessible to the cations or metals being exchanged. The prepared metal-loaded zeolite catalysts were acid digested and elemental analysis carried out using ICP-OES. For the ion-exchanged samples, only the wt.% of Fe as determined by ICP-OES analysis is presented. In the case of the ion-exchanged samples, Fe was the only element detected in significant quantities, despite the fact that the AMD sample applied contained other metal ions. This may be ascribed to the relatively higher concentration of Fe in the AMD and also preferential selectivity of the zeolite towards Fe relative to other metal ions during ion exchange. The theoretical metal wt.% loading for the ion-exchanged samples was calculated based on the respective amounts of metal concentration in the volume of AMD used to prepare the respective catalyst, and the actual Fe wt.% loading obtained corresponded closely to the theoretical value. In the case of the wet-impregnated samples, a slightly higher amount of Fe was loaded compared to the ion exchange protocol, but wet impregnation simultaneously loaded Ni and various cations onto the support in amounts related to the volume of AMD used. Thus, the wet impregnation metal loading approach ensured that all the metals present in the AMD were present on the support, either occupying exchange sites or adsorbed to the surface of the support, and only the most abundant metals are presented in Table 3.

Table 2.
Elemental composition of the ion-exchanged HBEA prepared using AMD.

Table 3.
Elemental composition of the wet-impregnated HBEA prepared using AMD as precursor (the theoretical Fe wt.% is the same as for the ion-exchanged counterparts).
In related studies [24,51] in which metal ions were removed from a multi-element solution via ion exchange using zeolites, it was found that Fe was more preferred. The selectivity sequence when clinoptilolite was used to remove metal ions from a multi-component solution was Fe3+ > Zn2+ > Cu2+ > Mn2+ [51]. When faujasite was used, the selectivity followed the order Fe > As > Pb > Zn > Cu > Ni > Cr [24].
The hydrothermal synthesis protocol produced the desired Na-BEA form, which was converted to HBEA by ion exchange using ammonium nitrate followed by calcination in air to obtain the H-form zeolite. The XRD diffractograms for the metal-loaded zeolite HBEA are presented in Figure 1a,b. Both diffractograms show the characteristic peaks of HBEA at 2θ = 7.8, 22.4, and 25.3 [52,53]. The intensity of the HBEA characteristic peaks in the metal-loaded zeolite and in the pristine HBEA are almost identical, indicative of the fact that the zeolite crystallinity was not altered by either metal loading approaches employed. This further confirms that the metals did not incorporate into the zeolite framework; thus, the Fe existed as isolated ion-exchanged metal centres or as well-dispersed FeOx particles. There were no peaks due to any metal oxides that were detected on the samples prepared via ion exchange. However, with the wet impregnation samples, additional peaks were observed at 2θ = 28.6, 32, 36.3, 40.8, 49.4, and 52.2, and these peaks were assigned to anhydrite CaSO4. During impregnation, some of the metals in AMD, such as Fe2+, Mn2+, Cu2+, and Zn2+, attach to the exchange sites more preferably relative to Ca2+ [54]. This results in a solution that has a high concentration of Ca, which continuously increases, since the technique (wet impregnation) involves decreasing the precursor volume by evaporation with time. So, eventually, the Ca2+ precipitates as CaSO4, which may be placed either on the surface of the zeolite or in the pore system. From the diffractograms in Figure 1b, it can be seen that as the metal loading increases, there is a corresponding increase in the peak intensities assigned to CaSO4. In related studies in which Fe (the most abundant metal in the AMD) was loaded onto zeolite BEA via ion exchange and incipient wetness impregnation using commercial salts, comparable results were obtained, and samples having Fe wt.% < 10% did not exhibit characteristic peaks due to the well-known short range crystallographic order of Fe2O3 crystallites [53,55,56,57].
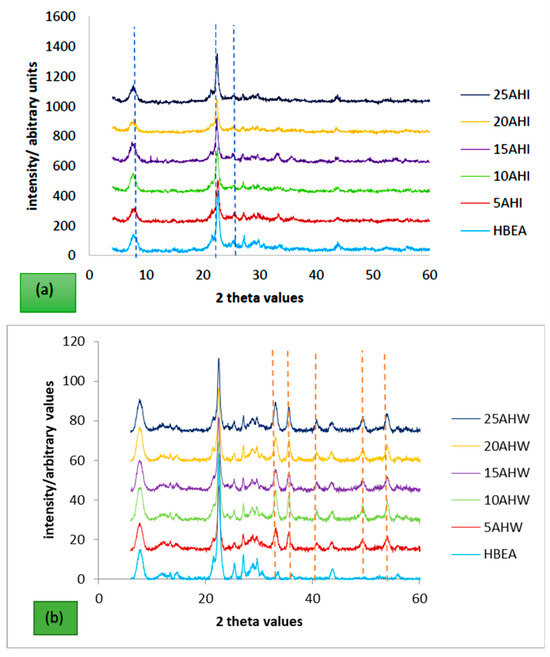
Figure 1.
XRD profiles of the HBEA-supported metal catalyst prepared via (a) ion exchange (b) wet impregnation using AMD as metal precursor (orange dotted lines represent peaks due to anhydrite CaSO4 and blue dotted lines represent characteristic peaks of BEA).
The micrographs for the 25 AHI and 25 AHW are presented in Figure 2, and they both confirm that the crystalline habit of the HBEA was preserved upon metal loading via the two approaches. The outcome is in line with the results from XRD analysis presented in Figure 1. In related studies [55,58] in which commercial HBEA was loaded with Fe using commercial salts, similar micrographs were obtained. The SAED analysis further confirmed that the metal oxides on the catalysts were nanophased polycrystallites. This concurs with the XRD diffractograms, which did not exhibit any characteristic peaks of large metal crystallites.
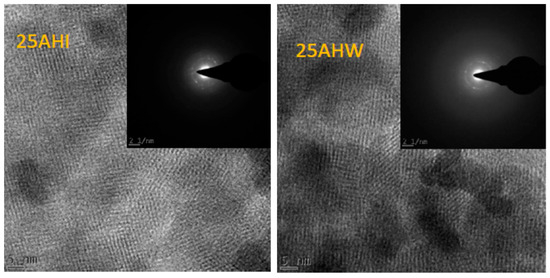
Figure 2.
HRTEM micrographs and SAED images for 25 AHI and 25 AHW.
The surface areas and micropore areas of the catalysts are presented in Table 4. Surface area determination was performed using the Brunauer–Emmett–Teller (BET) equation and the pore size distribution was determined using the Barrett–Joyner–Halenda (BJH) equation. There was a systematic decrease in the surface area and micropore area that correlated with increasing metal wt.% loading. The 25 AHW had more metals loaded onto it than the 25 AHI, as confirmed by the ICP-OES elemental analysis. Consistent observations were made in related studies in which HBEA-supported Fe was prepared using commercial feedstocks [59,60]. The decrease in micropore surface area with increasing metal loading, particularly in the case of 25 AHW, is also indicative that the metals were hosted in the micropores, reducing the micropore surface area proportionately to the metal loading. The parent zeolite HBEA micropore and mesopore surface area was not altered much upon metal loading using ion exchange, indicating that the metal did not disrupt the micropore or mesopore structure, whereas in the case of wet impregnation both microporous and mesoporous surface area was reduced substantially. However, the mesopore size range of the 25 AHI was from 28.1–118.1 Å, while that of the 25 AHW was 26.8–115.6 Å, indicating a suitable porosity for the benzene (ring diameter of 60.6 Å) to be hosted in the mesopore structure.

Table 4.
Nitrogen adsorption surface area and pore size of the HBEA-supported metal catalysts.
2.2. Catalytic Activity
The alkylation of benzene using t-butyl chloride progresses in accordance with Scheme 1. It should, however, be noted that the reaction is accompanied by formation of other by-products alongside the t-butyl benzene. Generally, alkylation reactions are associated with transalkylation polymerisation and polyalkylation. The extent to which other by-products are formed can be controlled by the catalysts’ selectivity and also by the reactant molar ratios. In this study an excess of benzene was used, which enhances selectivity towards the monoalkylated product, since the monoalkylated product is relatively more active than benzene [50]. Use of an excess of the alkyl halide will enhance polyalkylation [61]. Thus, when targeting the production of the monoalkylated product, an excess of benzene and the exploitation of the selective zeolite pore system can greatly influence selectivity.
2.3. Conversion of t-Butyl Chloride and Selectivity of t-Butyl Benzene
To evaluate the activity of the prepared catalysts, conversion of t-butyl chloride was evaluated over 5 h time on stream. The t-butyl chloride conversion profiles presented in Figure 3 show rapid increase initially, followed by a steady state over the time on stream of 5 h, characteristic of an autocatalytic reaction [62].
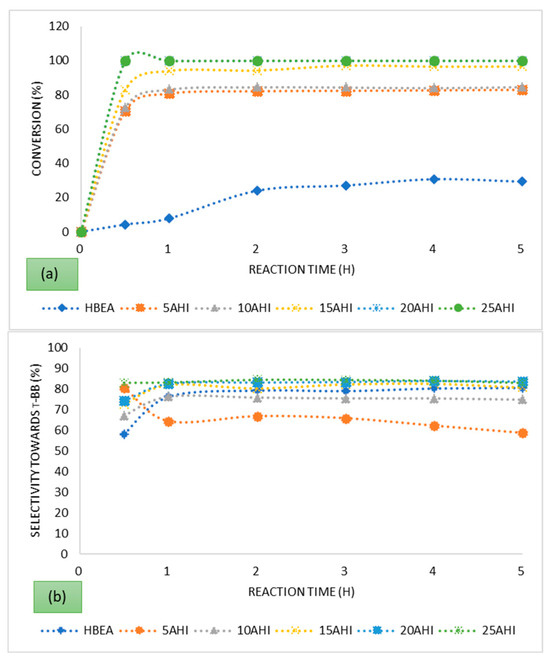
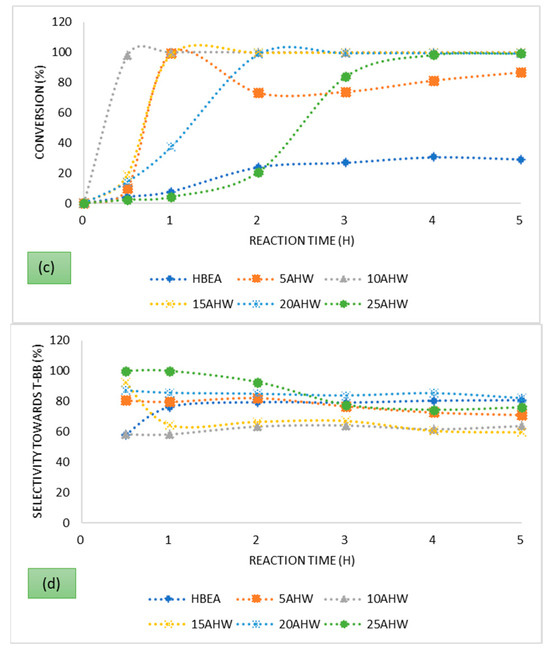
Figure 3.
Conversion of t-butyl chloride and selectivity of t-butyl benzene over the series of HBEA-supported metals prepared via (a,b) ion exchange and (c,d) wet impregnation using AMD as metal precursor.
The outcomes, presented in Figure 3, show that fly ash-based HBEA loaded with Fe from AMD can be used in the preparation of metal-loaded catalysts for the FC alkylation reaction. The parent HBEA had the lowest conversion, and its activity was associated with the presence of Brønsted acid sites as it was in the HBEA form, while the metal-loaded zeolites’ activity was based on the synergism of both Lewis and Brønsted acidity. The Brønsted sites affect the carbonium ion formation, while the deprotonation occurs on the Lewis acid sites to form the alkylated product [63]. The activation of the benzene nucleus on the Fe oxide species, having redox function, in close proximity with the zeolite protons contributed to the high activity [4,62]. The highest conversion achieved over the parent zeolite HBEA was 30.7% after 4 h on stream, while the catalysts having the highest Fe wt.% loading of 3.49% achieved a conversion of 100% within the first 30 min. With the ion-exchanged catalysts, the conversion increased with increasing metal wt.% loading, but this was not the case with the catalysts prepared via wet impregnation. Despite having the highest metal wt.% loading, being prepared via wet impregnation, the 25 AHW’s activity was not significant based on conversion of the t-butyl chloride. In the case of catalysts having lower metal loadings such as the 10 AHW and the 15 AHW, conversion of 100% was achieved within the first hour. Based on the XRD analysis, the wet-impregnated catalysts were characterised by the significant presence of anhydrite CaSO4. This may pose diffusional constraints if the CaSO4 forms inside the pore channels of the zeolite, as is evident from the reduced micropore surface area compared to the ion-exchanged samples. Furthermore, the wet impregnation metal loading approach ensures that all the metals present in the AMD will eventually end up as being part of the resultant catalyst. Thus, some of the metals may have a negative or positive catalytic effect on the reaction. The 10 AHI and the 10 AHW had comparable Fe wt.% loadings, but the latter managed to attain a conversion of 100% after only 30 min while the former only attained a conversion of 84.65% after 1 h on stream. This is indicative of the fact that despite diffusional constraints associated with the formation of the CaSO4, some of the metals in the AMD had a positive/promoting catalytic effect. It is reported that in the alkylation of benzene, metal dispersion on the support has a significant influence on the catalytic activity. It has been revealed that nanoparticle-phased iron oxide activates the benzene, forming C6H5–H (C6H5δ−…Hδ+) species which are more reactive than benzene [64]. The XRD and HRTEM analyses confirmed that the metal ions were well dispersed on the support for the series prepared via both IE and WI.
The FC reaction is accompanied by the liberation of HCl, and it is a mineral acid that also enhances the activity, but it may compromise the selectivity if it occurs outside the pore system of the zeolite. In a related study by Choudhary et al., in which the alkylation of benzene by benzyl chloride over H-ZSM-5 modified by Fe and Ga was conducted, it was found that the presence of chlorine activated the reaction in the initial reaction stages, resulting in high conversions [62]. Work by Arata and Hino, in which the alkylation of benzene with various alkylating agents was conducted, concluded that the iron chloride which is formed on the surface of the iron oxide by the reaction with HCl is a catalytically active species for alkylation [65]. The trends presented in Figure 3 are similar to those that have been observed in earlier studies involving the alkylation of benzene [62,66].
2.4. Friedel–Crafts Alkylation Selectivity and Product Distribution
The Friedel–Crafts alkylation of benzene using t-butyl chloride over traditional catalysts tends to produce polyalkylated products. However, the use of acid catalysts tends to favour the formation of the monoalkylated product (tertiary butyl benzene). If conventional catalysts are used polyalkylates can be produced, and these are; 1,3-di-tertiary butyl benzene, I,4-di-butyl benzene and the 1,3,5-tri-butyl benzene. Scheme 2 shows the possible products of the Friedel-Crafts alkylation of benzene with tertiary butyl chloride.
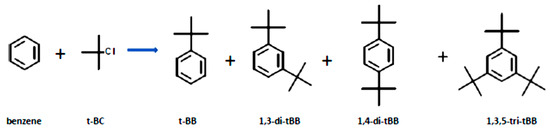
Scheme 2.
Main products of the alkylation of benzene with t-butyl chloride over Fe-BEA catalysts.
Figure 4 presents the product distribution over the parent HBEA and the catalysts with the highest metal wt.% loading for each of the respective loading approaches employed (25 AHI and 25 AHW). These two catalysts had the highest metal wt.% loading for the ion-exchanged series and the wet-impregnated series, respectively.
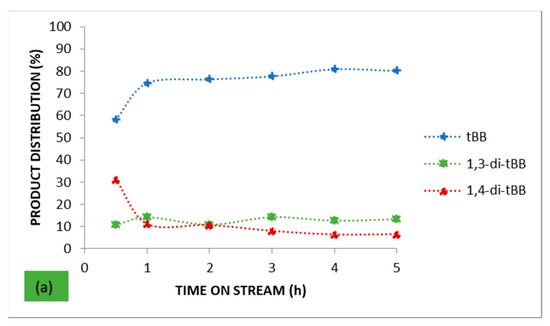
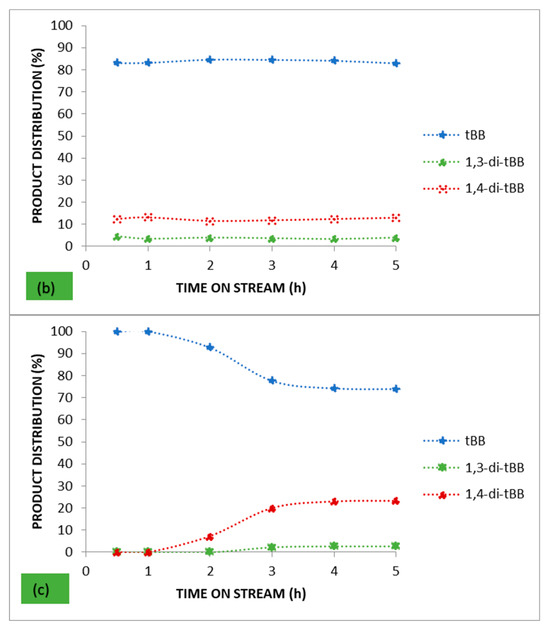
Figure 4.
Product distribution for benzene alkylation with t-butyl chloride over (a) the parent HBEA, (b) 25 AHI, and (c) 25 AHW.
The alkylation of benzene by t-butyl chloride gives numerous reaction products: the monoalkylated product (t-butylbenzene), the di-alkylated products (1.2-di-t-butyl benzene, 1.3-di-t-butyl benzene, and 1,4-di-t-butyl benzene), and the trialkylated product (1,3,5-tri-tert-butylbenzene). Reactant molar ratios and the catalyst’s selectivity play a major role in influencing the ratios of the reaction products. In this study the target product was the monoalkylated product, and from the profiles presented in Figure 4, good selectivity was obtained over the parent HBEA (80.6% after 5 h on stream) despite its low conversion. The highest selectivity was obtained over the 25 AHI, which achieved a selectivity of <82% throughout the entire 5 h time on stream. After 1 h on stream, all the catalysts prepared via ion exchange maintained an almost constant selectivity, contrary to the results obtained with their wet-impregnated counterparts. It can be said that the metal-loaded catalysts prepared via ion exchange presented comparable selectivity percentages with the parent HBEA, and this confirms that the selectivity was mostly governed by the zeolite pore system rather than the metal wt.% loading, since low metal wt.% were used. The slight differences observed may be attributed to the slight variation of the Lewis acid and Brønsted acid sites in the catalyst, and this may have influenced the selectivity [63].
On the other hand, there was a noticeable variation in the selectivity percentages obtained over the series of catalysts prepared via wet impregnation. Both the lower micropore surface area and the presence of other metals and cations in various proportions upon the wet-impregnated support affected the general conversion and selectivity of these catalysts. The highest selectivity obtained was 100% over the 25 AHW after 30 min on stream. However, at this point the corresponding conversion over the catalysts was low at only 2.37%, but as the conversion increased the selectivity decreased. Nur et al. studied the effect of the Lewis acid:Brønsted acid ratio on the alkylation of resorcinol over Ga-BEA and reported that there was an optimum ratio where both conversion and selectivity were at a maximum [63]. Their outcome is consistent with the observation made in this study concerning the ion-exchanged catalysts. However, the same cannot be said about the catalysts prepared via wet impregnation.
In order of relative abundance, the product mixture obtained over all the metal-loaded catalysts after achieving steady state was composed of t-butylbenzene > 1,4-di-t-butylbenzene (para isomer) > 1,3-di-t-butylbenzene. The product distribution was different, though, with the parent HBEA, which had more of the meta isomer relative to the para isomer when steady state was established. This is a confirmation that the metal Lewis acidity brought about by the inclusion of the metal(s) impacted the selectivity/product distribution.
The yields obtained over the ion-exchanged and wet-impregnated AMD-based catalysts are presented in Figure 5. Yield was calculated in accordance with the equation:
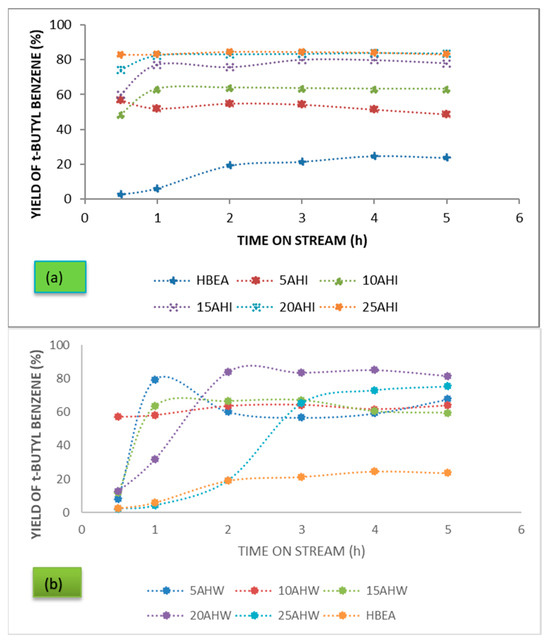
Figure 5.
Product yield of t-butyl benzene over the series of HBEA-supported metals prepared using AMD as metal precursor via (a) ion exchange and (b) wet impregnation.
In a reaction in which there are numerous by-products, the catalytic activity is best described by considering the yield of the desired product. The trends presented in Figure 5 depict the yield of t-butyl benzene obtained over the series of catalysts tested. Considering the catalysts prepared using ion exchange with AMD, it can be seen that the yield was positively correlated with the metal wt.% loading obtained. The yield obtained over the parent HBEA material was only 24.7% after 4 h on stream. The highest yield obtained was 84.6% over the 25 AHI (Fe wt.% loading of 3.49) after 2 h on stream. With the wet-impregnated samples, the highest yield of 85.08% was obtained over the 20 AHW (Fe wt.% loading of 2.62) after 4 h on stream. Although the 25 AHW had the highest metal wt.% loading, its conversion was lower in comparison to that of the 20 AHW. Since yield is calculated from conversion and selectivity, the factors governing the two parameters consequently affect the overall yield obtained over the respective catalyst.
From the trends in Figure 4 it can be concluded that when steady state was established the product distribution remained almost constant, maintaining an equilibrium mixture. However, establishment of steady state was different from catalyst to catalyst. The ion-exchanged catalysts were generally much quicker to reach steady state. This can be attributed to the good metal dispersion associated with the metal loading approach, as shown by the XRD and BET analysis. Furthermore, apart from Fe, the ion-exchanged catalysts did not have significant quantities of any of the other metals or cations present in the AMD. This minimised the effects they may have had on the overall catalytic activity. It should also be remembered that the ion-exchanged series of catalysts was composed of Fe as the major metal present with negligible amounts of other metals, while their wet-impregnated counterparts supported all the metals in the AMD precursor used. Some of these metals impacted on the overall activity, i.e., conversion, selectivity, and product distribution, and consequently the product yield. These additional metals may have had promoting and demoting effects on the activity.
Thus, in a further set of experiments, pure salts were used to load the HBEA, and the major metals detected were tested to evaluate the metals’ impact on the catalytic activity.
2.5. Effect of Various Metals in AMD on the FC Alkylation Reaction
The metals Fe, Mn, Mg, Na, Ca, and Ni were loaded sequentially upon the parent HBEA via wet impregnation using pure commercial metal sulphates following the same order. This was done in order to evaluate and understand the effect on the catalytic activity of each respective metal that was present in significant quantities on the support. The effect of each metal addition was evaluated based on the conversion, selectivity, and yield obtained. The chosen wt.% loading of each respective metal was determined by the respective proportion of metal observed in the catalysts 10 AHW. The actual metal wt.% loadings achieved as determined by ICP-OES are presented in Table 5. The conversion of t-butyl chloride and selectivity towards t-butyl benzene are presented in Figure 6. The conversion plot for t-butyl chloride is only shows the first 2 h, after which most data points started to cluster.

Table 5.
Elemental composition of the metal-loaded HBEA catalysts prepared via sequential wet impregnation.
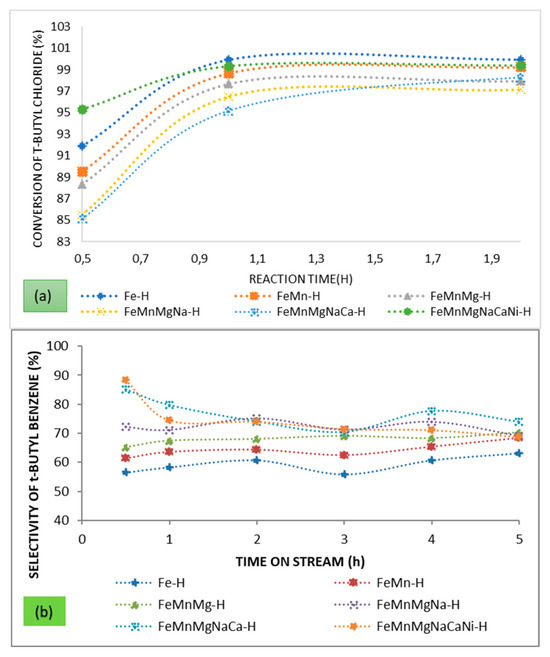
Figure 6.
Effect of various metals on (a) the conversion of t-butyl chloride and (b) the selectivity towards t-butyl benzene.
The Fe-H is comparable with respect to Fe wt.% loading to the 10 AHI and 10 AHW, but the conversions obtained over these catalysts after 30 min on stream are 91.9%, 72.5%, and 97.9%, respectively. Higher activity over the Fe-H can be attributed to the fact that a pure metal salt precursor was used. Although the 10 AHI showed Fe as the major metal present, there were traces of other metals which were not presented in the elemental analysis data, and these metals could be responsible for the lower activity of the catalyst. Already, this outcome is testimony that some metals which were present in the AMD did have a positive catalytic effect on the conversion of t-butyl chloride. As Mn, Mg, Na, and Ca were added, there was a corresponding decline in the conversion, signifying that these metals and cations have a negative catalytic effect on the reaction. The addition of Ni increased the conversion, and it can be concluded that Ni had a promoting effect on the reaction. Hlatywayo, investigated the use of Fe/Mn supported on HBEA and MCM-41 on the alkylation of benzene with an alkyl halide, reporting that Mn is not active in the FC alkylation reaction, and the same observation of decreasing activity was noticed when a bimetallic Fe/Mn catalyst was evaluated here [67]. The addition of alkali and alkali earth metals to zeolites reduces their acidity, and thus the decrease in conversion upon addition of Mg, Na, and Ca is an expected outcome. These metals reduce acidity by occupying the exchange sites or by masking both the Lewis and Brønsted acid sites. Emana and Chand, found that the addition of Mg did not improve the conversion of benzene with ethanol over Mg-modified ZSM-5 [68]. He at al. found that the presence of Na significantly decreased the conversion of benzyl chloride over Fe-Na-modified MCM-41 [69]. Dong et al. found that Na was not an active metal for the alkylation reaction. The outcomes in this study confirm these previous findings [70].
The addition of Ni significantly increased the conversion from 85.1% to 95.3% after 0.5 h on stream. This outcome is indicative of the promoting effect of Ni towards the conversion of t-butyl chloride. Several studies attest to the catalytic activity of Ni in the Friedel–Crafts alkylation reaction. Sebti et al. reported on the use of Ni supported on hydroxyapatite in the alkylation of benzene with benzyl chloride [71]. In a related study, Ali et al. reported on the use of Ni supported on zirconia in the alkylation of benzene with benzyl chloride [72]. The higher conversion observed over the Fe/Ni system in the present study may be attributed not only to the fact that these metals are active for the reaction under study but also to the fact that when they are immobilised together on the support there is metal-to-metal charge transfer, which further enhances the activity [73].
The lowest selectivity after 0.5 h on stream was obtained over the monometallic Fe catalyst. As the metals were added to the Fe catalyst, starting with Mn, it was found that the selectivity increased from 56.6% to 61.3%. The addition of Mg, Na, and Ca resulted in improvement in the selectivity from 56.6 to 85.1% after 0.5 h time on stream. These metals reduce the acid strength and rather impose basicity [4,74]. Alkali metals and alkaline earth metals reduce the number of acid sites by masking them and consequently reducing further oligomerisation [68]. Mn suffers partial reduction of the Mn2O3/MnO4, which may result in the decrease in acid strength, consequently affecting product selectivity [75]. The addition of Ni further enhanced the selectivity towards formation of the monoalkylated product in the initial stages of the reaction. The selectivity after 0.5 h on stream increased from 85.1 to 88.4%. However, with prolonged reaction times, the selectivity of the catalyst decreases. This may be attributed to the high alkylating activity of the Ni, which may result in further alkylation, forming dialkylated products. Hlatywayo reported, upon investigating the activity of supported Fe-Mn bimetallic catalysts on the FC alkylation reaction, that Mn does enhance selectivity [67]. A study by Ko and Huang, in which the alkylation of ethylbenzene with methanol was performed over modified HZSM-5, showed that Ca and Mg reduced the conversion while concomitantly increasing selectivity [76]. These outcomes were attributed to the diminution of both the strong acid sites and the pore size and micropore surface area of zeolites.
3. Experimental Details
3.1. Acid-Assisted Silica Extraction from CFA
The CFA used in the study was collected from a power station in Mpumalanga, South Africa. A ratio of 1:1.2 of CFA to NaOH was fused at 550 °C for 1.5 h. The fused ash was then mixed with water at a solid-to-liquid ratio of 1:5, stirred for 2 h, and filtered. The pH of the solution was adjusted to 10 by adding H2SO4, and it is at this point that the silica precipitated and was filtered off. The silica was dried overnight at 70 °C and then treated with a saturated solution of oxalic acid at a solid-to-liquid ratio of 1:10, at a temperature of 80 °C for 6 h. The resultant solid (silica) was then used in the synthesis of zeolite HBEA.
3.2. Synthesis of Zeolite HBEA
The synthesis gel was prepared by mixing 2.85 g of the fly ash-based silica, 0.15 g of NaOH (Kimix, SA, Durban, South Africa), and 6.35 g of tetramethylammonium hydroxide (Sigma Aldrich, St. Louis, MO, USA) with 3.65 g of deionised water. The mixture was then aged for 30 min. at room temperature and transferred into a 100 mL Teflon-lined stainless-steel autoclave. The mixture was subjected to hydrothermal treatment at a temperature of 140 °C for 72 h. The resultant solid material was washed several times with deionised water and then filtered, dried overnight at 70 °C, and calcined at 550 °C in air for 3 h to remove the template. The resultant product was the Na-BEA form, which was then converted to HBEA by ion exchange using a solution of 2.0 M NH4NO3 followed by calcination in air to obtain the H-form zeolite.
3.3. Metal Loading via Liquid-Phase Ion Exchange and Wet Impregnation
The AMD used in the study was collected from a coal mine shaft in Mpumalanga, South Africa. Different AMD volumes (having Fe concentration of 4166.6 ppm) were made up to 25 mL with distilled water and contacted with a gram of zeolite. The mixture was stirred under reflux at 80 °C for 24 h over a silicon oil bath. The sample was then washed with 500 mL of deionised water and filtered, resulting in ion-exchanged HBEA with various metal wt.%. The metal-loaded zeolite BEA was dried in air at 100 °C for 10 h, followed by calcination in air at 550 °C for 4 h, to covert the loaded metal to oxides. When either ferrous sulphate or ferric sulphate is used as Fe solution precursor and exchanged onto the zeolite, calcination in air at 550 °C converts the Fe species to Fe3+ species via oxidation [77]. For wet-impregnated catalysts, the same liquid-to-solid ratio and metal solution precursors applied for ion exchange were used. The respective metal precursor and support were placed into a 150 mL round-bottomed flask and mounted on a rotary evaporator. The metal loading was performed under vacuum for 24 h at a temperature of 80 °C, after which all the water had evaporated, leaving behind a dry solid residue. The solid material was dried and calcined under the same conditions as for the catalyst prepared via ion exchange.
3.4. Catalyst Coding
The catalyst coding for the HBEA-supported metal catalysts prepared via ion excnge and wet impregnation is presented in Table 6.

Table 6.
Catalyst coding for HBEA-supported metal catalysts prepared via ion exchange (IE) and wet impregnation (WI). Typically, a code such as 5 AHI corresponds to a catalyst prepared using 5 mL of the AMD prepared via ion exchange while 5 AHW is the corresponding catalyst prepared via wet impregnation.
3.5. Catalyst Characterisation
The elementary compositions of the catalysts were determined by ICP-OES (Varian 710-ES series) and their surface areas by the N2 adsorption method (Autosorb-1-C Chemisorption-Physisorption Analyzer, Quantachrome Instruments, Giangarlo Scientific Co., Inc., Pittsburgh, PA, USA). X-ray diffraction patterns were obtained via a Philips X-pert pro MPD X-ray diffractometer using Cu-K radiation at 40 kV and 40 mA. The analysis was conducted between 4° and 60° 2θ. The crystallinity and morphology were determined using HRTEM (Tecnai G2 F20 X-Twin, transmission electron micrsope, Thermo Fisher Scientific Inc., Waltham, MA, USA).
3.6. Catalyst Activation
A catalyst sample of 320 mg was weighed and transferred into a 50 mL round-bottomed flask. To activate the catalyst, the flask was placed in an oven and heated to 250 °C for a period of 2 h. The catalyst was allowed to cool to room temperature before the reactants were added in accordance with the following catalytic evaluation procedure.
3.7. Catalyst Testing
A volume of 10 mL of benzene was added together with 1 mL of t-butyl chloride and 1 mL of nonane (internal standard) into a 50 mL round-bottomed flask containing the activated catalyst. The mixture was stirred under reflux over a silicon oil bath at a temperature of 45 °C, and sample aliquots were taken at 0.5, 1, 2, 3, 4, and 5 h. Samples were analysed using a GC (Agilent technologies 7890A Wilmington, DE, USA) equipped with FID and HP-5 (phenylmethylsilicon) capillary column.
3.8. Effect of Various Metals on the Catalytic Activity
The evaluation of various metals in the AMD on the catalytic activity was investigated by preparing metal-loaded CFA-based HBEA catalysts using AR-grade metal sulphates as a control. The catalysts were prepared via wet impregnation by sequential impregnation. The various metals were added to the HBEA in accordance with the proportion in the catalyst 10 AHW. The metals were added in the order Fe, Mn, Mg, Na, Ca, and Ni (the metal selection was based on the fact that these were the metals present in significant quantities in the AMD used). The catalyst coding for this set of catalysts is presented in Table 7. Characterisation of these catalysts was not performed, since they were prepared using commercial salts and the aim was to investigate the effects of the respective metals on the catalytic activity.

Table 7.
Coding for fly ash-based HBEA-supported catalysts used in the investigation of various metals on the catalytic activity in the FC alkylation reaction.
4. Conclusions
The study has shown that solid acid BEA zeolite catalysts can be generated from waste CFA, and waste AMD can be used as a metal precursor in catalyst preparation. In the Friedel–Crafts alkylation of benzene with t-butyl chloride, these novel catalysts gave satisfactory results, comparable to and in some instances better than activity obtained over catalysts prepared using commercial salts. In addition, the study has provided an avenue for the beneficiation of two waste materials (CFA and AMD). Metal loading on the CFA-based zeolite HBEA via conventional liquid-phase ion exchange and wet impregnation using AMD as a metal precursor proved to be effective, resulting in good metal dispersion without zeolite framework disruption. The conversion of t-butyl chloride, selectivity towards formation of t-butyl benzene, and the yield of t-butyl benzene obtained over the AMD-based ion-exchanged HBEA catalyst was positively correlated with the Fe wt.% loading. On the other hand, the wet-impregnated catalysts did not exhibit a perfectly positive correlation, and this can be ascribed to the formation of significant quantities of anhydrite CaSO4 during wet impregnation with AMD. Furthermore, the wet-impregnated catalysts were polymetallic, being composed of all the metals available in the sample AMD precursor solution. Apart from Fe, which was the main active component, the presence of Ni in the wet-impregnated samples had a positive effect on the conversion and selectivity, while Mn, Ca, Mg, and Na had a negative effect on the conversion of t-butyl chloride but a positive effect on the selectivity towards formation of the monoalkylated product (t-butyl benzene). The use of AMD as a metal precursor in the synthesis of Fe-loaded zeolite is promising; thus, there is a need to further explore the activity of the aforementioned catalysts in other reactions that are based on Fe-zeolite systems. Furthermore, it is recommended to study the stability and reusability of these catalysts in future before their full industrial application can be considered.
Author Contributions
T.H., L.P. and B.L. conceived and planned the experiments. T.H. carried out the experiments. L.P. provided the necessary resources. All the authors contributed to the interpretation of the results. T.H. took the lead in writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Environmental and Nanoscience Group, University of the Western Cape.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.
Acknowledgments
We would like to acknowledge the Environmental and Nanoscience Group at the University of the Western Cape for administrative and technical support as well as funding the research that enabled us to collect the data for this paper. The article is based on a broader study for a doctoral degree thesis published as “Coal fly ash and acid mine drainage based, heterogeneous Fe catalysts for the Friedel-Crafts alkylation reaction”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nyale, S.M.; Babajide, O.O.; Birch, G.D.; Böke, N.; Petrik, L.F. Synthesis and characterization of coal fly ash-based foamed geopolymer. Procedia Environ. Sci. 2013, 18, 722–730. [Google Scholar] [CrossRef]
- Böke, N.; Birch, G.D.; Nyale, S.M.; Petrik, L.F. New synthesis method for the production of coal fly ash-based foamed geopolymers. Constr. Build. Mater. 2015, 75, 189–199. [Google Scholar] [CrossRef]
- Imoisili, P.E.; Jen, T.-C. Synthesis and characterization of amorphous nano silica from South African coal fly ash. Mater. Today Proc. 2023, 105, 21–26. [Google Scholar] [CrossRef]
- Hlatywayo, T. Coal Fly Ash and Acid Mine Drainage Based, Heterogeneous Fe Catalysts for the Friedel-Crafts Alkylation Reaction. Ph.D. Thesis, Chemistry Department, University of the Western Cape, Cape Town, South Africa, 2020. Available online: https://uwcscholar.uwc.ac.za/items/eb0c759b-cfcd-4180-9e81-c2d3a5e339ae (accessed on 7 January 2025).
- Alterary, S.S.; Marei, N.H. Fly ash properties, characterization, and applications: A review. J. King Saud Univ.-Sci. 2021, 33, 101536. [Google Scholar] [CrossRef]
- Bhatt, A.; Priyadarshini, S.; Mohanakrishnan, A.A.; Abri, A.; Sattler, M.; Techapaphawit, S. Physical, chemical, and geotechnical properties of coal fly ash: A global review. Case Stud. Constr. Mater. 2019, 11, e00263. [Google Scholar] [CrossRef]
- Reynolds-Clausen, K.; Singh, N. South Africa’s Power Producer’s Revised Coal Ash Strategy and Implementation Progress. Coal Combustion and Gasification Products 11. In Proceedings of the WOCA Conference, Lexington, KY, USA, 9–11 May 2018; Available online: http://www.flyash.info (accessed on 7 January 2025).
- Perämäki, S.E.; Tiihonen, A.J.; Väisänen, A.O. Occurrence and recovery potential of rare earth elements in Finnish peat and biomass combustion fly ash. J. Geochem. Explor. 2019, 201, 71–78. [Google Scholar] [CrossRef]
- Khan, I.; Umar, R. Environmental risk assessment of coal fly ash on soil and groundwater quality, Aligarh, India. Groundw. Sustain. Dev. 2019, 8, 346–357. [Google Scholar] [CrossRef]
- Yadav, V.K.; Gacem, A.; Choudhary, N.; Rai, A.; Kumar, P.; Yadav, K.K.; Abbas, M.; Khedher, N.B.; Awwad, N.S.; Barik, D.; et al. Status of Coal-Based Thermal Power Plants, Coal Fly Ash Production, Utilization in India and Their Emerging Applications. Minerals 2022, 12, 1503. [Google Scholar] [CrossRef]
- Ghazali, N.; Muthusamy, K.; Wan Ahmad, S. Utilization of Fly Ash in Construction. IOP Conf. Ser. Mater. Sci. Eng. 2019, 601, 012023. [Google Scholar] [CrossRef]
- Mathapati, M.; Amate, K.; Prasad, D.; Jayavardhana, M.L.; Raju, T.H. A review on fly ash utilization. Mater. Today Proc. 2022, 50, 1535–1540. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Gilbert, C.; Ayanda, O.S.; Fatoba, O.O.; Madzivire, G.; Petrik, L.F. A Novel Method of Using Iron Nanoparticles from Coal Fly Ash or Ferric Chloride for Acid Mine Drainage Remediation. In Mine Water and the Environment; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Darmansyah, D.; You, S.-J.; Wang, Y.-F. Advancements of coal fly ash and its prospective implications for sustainable materials in Southeast Asian countries: A review. Renew. Sustain. Energy Rev. 2023, 188, 113895. [Google Scholar] [CrossRef]
- Missengue, R.M.; Losch, P.; Sedres, G.; Musyoka, N.M.; Fatoba, O.O.; Louis, B.; Pale, P.; Petrik, L.F. Transformation of South African coal fly ash into ZSM-5 zeolite and its application as an MTO catalyst. Comptes Rendus Chim. 2017, 20, 78–86. [Google Scholar] [CrossRef]
- Petrik, L.F.; Missengue, R.N.M.; Ameh, A.E.; Hlatywayo, T. Process for Production of High Silica Content Zeolite from Fly Ash. Patent WO/2017221192, 28 December 2017. [Google Scholar]
- Fan, Y.; Huang, R.; Liu, Q.; Cao, Q.; Guo, R. Synthesis of zeolite A from fly ash and its application in the slow release of urea. Waste Manag. 2023, 158, 47–55. [Google Scholar] [CrossRef]
- Lankapati, H.M.; Lathiya, D.R.; Choudhary, L.; Dalai, A.K.; Maheria, K.C. Modification and characterization of Mordenite zeolite derived from waste coal fly ash and its application as a heterogeneous catalyst for the n-butyl levulinate synthesis. Catal. Commun. 2023, 183, 106772. [Google Scholar] [CrossRef]
- Yao, Q.; Peng, Y.; Chen, M.; Wang, Y.; Ding, J.; Ma, B.; Wang, Q.; Lu, S. One-step high efficiency synthesis of zeolite from fly ash by mechanochemical method as a low-cost adsorbent for cadmium removal. J. Environ. Chem. Eng. 2024, 12, 111877. [Google Scholar] [CrossRef]
- Eren, S.; Türk, F.N.; Arslanoğlu, H. Synthesis of zeolite from industrial wastes: A review on characterization and heavy metal and dye removal. Environ. Sci. Pollut. Res. 2024, 31, 41791. [Google Scholar] [CrossRef] [PubMed]
- Buzukashvili, S.; Sommerville, R.; Hu, W.; Brooks, O.; Kökkılı, O.; Ouzilleau, P.; Rowson, N.A.; Waters, K.E. Zeolite synthesis from coal fly ash and its application to heavy metals remediation from water contaminated with Pb, Cu, Zn and Ni ions. Miner. Eng. 2024, 209, 108619. [Google Scholar] [CrossRef]
- Senila, M.; Cadar, O. Modification of natural zeolites and their applications for heavy metal removal from polluted environments: Challenges, recent advances, and perspectives. Heliyon 2024, 10, e25303. [Google Scholar] [CrossRef] [PubMed]
- Ríos, C.A.; Williams, C.D.; Roberts, C.L. Removal of heavy metals from acid mine drainage (AMD) using coal fly ash, natural clinker and synthetic zeolites. J. Hazard. Mater. 2008, 156, 23–35. [Google Scholar] [CrossRef]
- Motsi, T.; Rowson, N.A.; Simmons, M.J.H. Kinetic studies of the removal of heavy metals from acid mine drainage by natural zeolite. Int. J. Miner. Process. 2011, 101, 42–49. [Google Scholar] [CrossRef]
- Silva, D.; Weber, C.; Oliveira, C. Neutralization and uptake of pollutant cations from acid mine drainage (amd) using limestones and zeolites in a pilot-scale passive treatment system. Miner. Eng. 2021, 170, 107000. [Google Scholar] [CrossRef]
- Gaikwad, R.W.; Sonawane, A.V.; Hakke, V.S.; Sonawane, S.H.; Gaikwad, M.S.; Lakhera, S.K.; Venu, B.G.; Warade, A.R.; Urgunde, A.B.; Sapkal, V.S. Application of apophyllite and thomsonite natural zeolite as modified adsorbents for the removal of zinc from acid mine drainage. Chemosphere 2024, 350, 141095. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Beauclair, N.; Maaza, M.; Mokrani, T.; Ambushe, A.A.; Seopela, M.P.; Msagati, A.T. Acid mine drainage treatment and metals recovery by means of selective precipitation using magnesium oxide (MgO): An experimental study. Groundw. Sustain. Dev. 2024, 25, 101151. [Google Scholar] [CrossRef]
- Humphries, M.S.; McCarthy, T.S.; Letitia, P. Attenuation of pollution arising from acid mine drainage by a natural wetland on the Witwatersrand. S. Afr. J. Sci 2017, 113, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shabalala, A.N.; Ekolu, S.O. Quality of water recovered by treating acid mine drainage using pervious concrete adsorbent. Water SA 2019, 45, 4. [Google Scholar] [CrossRef]
- Feng, D.; Aldrich, C.; Tan, H. Treatment of acid mine water by use of heavy metal precipitation and ion exchange. Miner. Eng. 2000, 13, 623–642. [Google Scholar] [CrossRef]
- Luptakova, A.; Kusnierova, M. Bioremediation of acid mine drainage contaminated by SRB. Hydrometallurgy 2005, 77, 97–102. [Google Scholar] [CrossRef]
- Gitari, W.; Petrik, L.; Ajayi, A.S. Treatment of Acid Mine Drainage with Coal Fly Ash: Exploring the Solution Chemistry and Product Water Quality. In Coal Fly Ash Beneficiation: Treatment of Acid Mine Drainage with Coal Fly Ash; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Pereira, T.C.B.; dos Santos, K.B.; Lautert-Dutra, W.; Teodoro, L.D.; de Almeida, V.O.; Weiler, J.; Schneider, I.A.H.; Bogo, M.R. Acid mine drainage (AMD) treatment by neutralization: Evaluation of physical-chemical performance and ecotoxicological effects on zebrafish (Danio rerio) development. Chemosphere 2020, 253, 126665. [Google Scholar] [CrossRef] [PubMed]
- Levio-Raiman, M.; Briceño, G.; Schalchli, H.; Bornhardt, C.; Diez, M.C. Alternative treatment for metal ions removal from acid mine drainage using an organic biomixture as a low cost adsorbent. Environ. Technol. Innov. 2021, 24, 101853. [Google Scholar] [CrossRef]
- Chimanlal, I.; Nthunya, L.N.; Richards, H. Resource recovery from acid mine drainage in membrane distillation crystallization. Front. Membr. Sci. Technol. 2023, 2, 1247276. [Google Scholar] [CrossRef]
- Alegbe, M.J.; Ayanda, O.S.; Ndungu, P.; Nechaev, A.; Fatoba, O.O.; Petrik, L.F. Physicochemical Characteristics of Acid Mine Drainage, Simultaneous Remediation and use as Feedstock for Value Added Products. J. Environ. Chem. Eng. 2019, 7, 103097. [Google Scholar] [CrossRef]
- Madzivire, G.; Maleka, R.M.; Tekere, M.; Petrik, L.F. Cradle to cradle solution to problematic waste materials from mine and coal power station: Acid mine drainage, coal fly ash and carbon dioxide. J. Water Process Eng. 2019, 30, 100474. [Google Scholar] [CrossRef]
- Yuan, J.; Ding, Z.; Bi, Y.; Li, J.; Wen, S.; Bai, S. Resource Utilization of Acid Mine Drainage (AMD): A Review. Water 2022, 14, 2385. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, X.; Hensen, E.J.M. Stable Fe/ZSM-5 nanosheet zeolite catalysts for the oxidation of benzene to phenol. Am. Chem. Soc. Catal. 2017, 7, 2709–2719. [Google Scholar] [CrossRef] [PubMed]
- Patet, R.E.; Koehle, M.; Lobo, R.F.; Caratzoulas, S.; Vlachos, D.G. General acid-type catalysis in the dehydrative aromatization of furans to aromatics in H-[Al]-BEA, H-[Fe]-BEA, H-[Ga]-BEA, and H-[B]-BEA Zeolites. J. Phys. Chem. C 2017, 121, 13666–13679. [Google Scholar] [CrossRef]
- Ingole, A.K.; Dixit, D.; Dingare, S.V. A review on Selective Catalytic Reduction technique for diesel engine exhaust after treatment. Int. J. Curr. Eng. Technol. 2017, 7, 206–210. [Google Scholar]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Velichkova, F.; Delmas, H.; Julcour, C.; Koumanova, B. Heterogeneous fenton and photo-fenton oxidation for paracetamol removal using iron containing ZSM-5 zeolite as catalyst. Am. Inst. Chem. Eng. J. 2016, 63, 669–679. [Google Scholar] [CrossRef]
- Olah, G.A.; Molnar, A. Hydrocarbon Chemistry, 2nd ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2003; pp. 230–232. [Google Scholar]
- Ivanchina, E.; Ivashkina, E.; Dolganova, I.; Dolganov, I.; Krutey, A. Application of mathematical modeling for optimization of linear alkylbenzenes sulphonation modes in film reactor. Procedia Eng. 2016, 152, 73–80. [Google Scholar] [CrossRef]
- Shi, H.; Zhu, W. Removal of free acids from heavy alkylbenzene sulfonic acid by a novel three-step method. Chem. Eng. Process.-Process Intensif. 2023, 193, 109531. [Google Scholar] [CrossRef]
- Shokria, A.; Karimia, S. A review in linear alkylbenzene (LAB) production processes in the petrochemical industry. Russ. J. Appl. Chem. 2021, 94, 1546–1559. [Google Scholar] [CrossRef]
- Rueping, M.; Nachtsheim, B.J. A review of new developments in the Friedel-Crafts alkylation—From green chemistry to asymmetric catalysis. Beilstein J. Org. Chem. 2010, 6, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bidart, A.M.; Borges, A.P.; Nogueira, L.; Lachter, E.R.; Mota, C.J.A. Iron-Exchanged Zeolite as Effective Catalysts for Friedel–Crafts Alkylation with Alkyl Halides. Catal. Lett. 2001, 75, 155–157. [Google Scholar] [CrossRef]
- Motsi, T.; Rowson, N.A.; Simmons, M.J.H. Adsorption of heavy metals from acid mine drainage by natural zeolite. Int. J. Miner. Process. 2009, 92, 42–48. [Google Scholar] [CrossRef]
- Kosri, C.; Deekamwong, K.; Sophiphun, O.; Osakoo, N.; Chanlek, N.; Fo¨ttinger, K.; Wittayakun, J. Comparison of Fe/HBEA catalysts from incipient wetness impregnation with various loading on phenol hydroxylation. React. Kinet. Mech. Catal. 2017, 121, 751–761. [Google Scholar] [CrossRef]
- Wu, R.; Liu, N.; Dai, C.; Xu, R.; Wang, N.; Yu, G.; Chen, B. Collaborative Purification of Tert-Butanol and N2O over Fe/Co-Zeolite Catalysts. Int. J. Environ. Res. Public Health 2023, 20, 4902. [Google Scholar] [CrossRef] [PubMed]
- Moreno, N.; Querol, X.; Ayora, C.; Pereira, C.F.; Janssen-Jurkovicová, M. Utilization of zeolites synthesized from coal fly ash for the purification of acid mine waters. Environ. Sci. Technol. 2001, 35, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Balle, P.; Geiger, B.; Kureti, S. Selective catalytic reduction of NOx by NH3 on Fe/HBEA zeolite catalysts in oxygen-rich exhaust. Appl. Catalysis. B Environ. 2009, 85, 109–119. [Google Scholar] [CrossRef]
- Sophiphun, O.; Föttinger, K.; Loiha, S.; Neramittagapong, A.; Prayoonpokarach, S.; Rupprechter, G.; Wittayakun, J. Properties and catalytic performance in phenol hydroxylation of iron on zeolite beta prepared by different methods. React. Kinet. Mech. Catal. 2015, 116, 549–561. [Google Scholar] [CrossRef]
- Yan, P.; Kennedy, E.; Stockenhuber, M. Hydrodeoxygenation of guaiacol over BEA supported bimetallic Ni-Fe catalysts with varied impregnation sequence. J. Catal. 2021, 404, 1–11. [Google Scholar] [CrossRef]
- Andrade, M.A.; Ansari, L.M.; Pombeiro, A.J.; Carvalho, A.P.; Martins, A.; Martins, L.M. Fe@Hierarchical BEA Zeolite Catalyst for MW-Assisted Alcohol Oxidation Reaction: A Greener Approach. Catalysts 2020, 10, 1029. [Google Scholar] [CrossRef]
- Miskolczi, N.; Juzsakova, T.; Sója, J. Preparation and application of metal loaded ZSM-5 and Y-zeolite catalysts for thermo-catalytic pyrolysis of real end of life vehicle plastics waste. J. Energy Inst. 2019, 92, 118–127. [Google Scholar] [CrossRef]
- Ahmed, M.H.M.; Muraza, O.; Jamil, A.K.; Shafei, E.N.; Yamani, Z.H.; Choi, K.H. Steam catalytic cracking of n-dodecane over Ni and Ni/Co bimetallic catalyst supported on hierarchical BEA zeolite. Energy 2017, 31, 5482–5490. [Google Scholar] [CrossRef]
- Barclay, L.; Betts, E. The tertiarybutylbenzenes: I. Alkylation of 1,4-di-t-butylbenzene with t-butyl chloride. Can. J. Chem. 2011, 33, 672–678. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Jana, S.K.; Kiran, B.P. Alkylation of benzene by benzyl chloride over H-ZSM-5 zeolite with its framework Al completely or partially substituted by Fe or Ga. Catal. Lett. 1999, 59, 217–219. [Google Scholar] [CrossRef]
- Nur, H.; Ramli, Z.; Efendi, J.; Rahman, A.N.A.; Chandren, S.; Yuan, L.S. Synergistic role of Lewis and Brönsted acidities in Friedel–Crafts alkylation of resorcinol over gallium-zeolite beta. Catal. Commun. 2011, 12, 822–825. [Google Scholar] [CrossRef]
- Lin, T.; Zhang, X.; Li, R.; Bai, T.; Yang, Y.S. Synergistic catalysis of isolated Fe3+ and Fe2O3 on FeOx/HZSM-5 catalysts for Friedel–Crafts benzylation of benzene. Chin. Chem. Lett. 2011, 22, 639–642. [Google Scholar] [CrossRef]
- Arata, K.; Hino, M. High catalytic activity of iron oxide for benzylation, t-butylation, and acetylation of toluene with benzyl, t-butyl, and acetyl chlorides. Chem. Lett. 1980, 9, 1479–1480. [Google Scholar] [CrossRef]
- Vinu, A.; Sawant, D.P.; Ariga, K.; Hartmann, M.; Halligudi, S.B. Benzylation of benzene and other aromatics by benzyl chloride over mesoporous AlSBA-15 catalysts. Microporous Mesoporous Mater. 2005, 80, 195–203. [Google Scholar] [CrossRef]
- Hlatywayo, T. Supported Metal Catalysts for Friedel-Crafts Alkylation. Master’s Thesis, Chemistry Department, University of The Western Cape, Cape Town, South Africa, 2014. Available online: https://uwcscholar.uwc.ac.za/items/a2b0c1f7-9a95-49dc-b67a-b07214864521 (accessed on 7 January 2025).
- Emana, A.N.; Chand, S. Alkylation of benzene with ethanol over modified HZSM-5 zeolite catalysts. Appl. Petrochem. Res. 2015, 5, 121–134. [Google Scholar] [CrossRef]
- He, N.; Bao, S.; Xu, Q. Fe-containing mesoporous molecular sieves materials: Very active Friedel-Crafts alkylation catalysts. Appl. Catal. A Gen. 1998, 169, 29–36. [Google Scholar] [CrossRef]
- Dong, P.; Li, Z.; Wang, X.; Yun, H.; Li, G. The alkylation reaction of benzene with methanol to produce toluene: Y-C and Y-CCs catalyst. Green Chem. Lett. Rev. 2018, 11, 158–164. [Google Scholar] [CrossRef]
- Sebti, S.; Tahir, R.; Nazih, R.; Boulaajaj, S. Comparison of different Lewis acid supported on hydroxyapatite as new catalysts of Friedel–Crafts alkylation. Appl. Catal. A Gen. 2001, 218, 25–30. [Google Scholar] [CrossRef]
- Ali, T.T.; Narasimharao, K.; Ahmed, N.S.; Basahel, S.; Al-Thabaiti, S.; Mokhtar, M. Nanosized iron and nickel oxide zirconia supported catalysts forbenzylation of benzene: Role of metal oxide support interaction. Appl. Catal. A Gen. 2014, 486, 19–31. [Google Scholar] [CrossRef]
- Shen, S.; Chen, J.; Koodali, R.T.; Hu, Y.; Xiao, Q.; Zhou, J.; Wang, X.; Guo, L. Activation of MCM-41 mesoporous silica by transition-metal incorporation for photocatalytic hydrogen production. Appl. Catal. B Environ. 2014, 150–151, 138–146. [Google Scholar] [CrossRef]
- Marakatti, V.S.; Rao, P.V.C.; Choudary, N.V.; Ganesh, G.S.; Shah, G.; Maradur, S.P.; Halgeri, A.B.; Shanbhag, G.V.; Ravishankar, R. Influence of Alkaline Earth Cation Exchanged X-Zeolites Towards Ortho-Selectivity in Alkylation of Aromatics: Hard-Soft-Acid-Base Concept. Adv. PorousMater. 2014, 2, 221–229. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Huo, W.; Jia, M.; Jing, X.; Yang, P.; Yang, Z.; Liub, G.; Zhang, W. Vapor phase ortho-selective alkylation of phenol with methanol over silica–manganese mixed oxide catalysts. Chem. Eng. J. 2012, 181–182, 630–635. [Google Scholar] [CrossRef]
- Ko, A.-N.; Huang, C.S. Alkylation of Ethylbenzene with Methanol on HY, HM and HZSM-5 Zeolite. J. Chin. Chem. Soc. 1993, 40, 345–350. [Google Scholar] [CrossRef]
- Li, Q.; Wu, A.; Zhang, M.; Li, J.; Cao, J.; Li, H.; Jiang, Y. Study on the Influence of Calcination Temperature of Iron Vitriol on the Coloration of Ancient Chinese Traditional Iron Red Overglaze Color. Materials 2023, 17, 2800. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).