Novel AlCo2O4/MWCNTs Nanocomposites for Efficient Degradation of Reactive Yellow 160 Dye: Characterization, Photocatalytic Efficiency, and Reusability
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
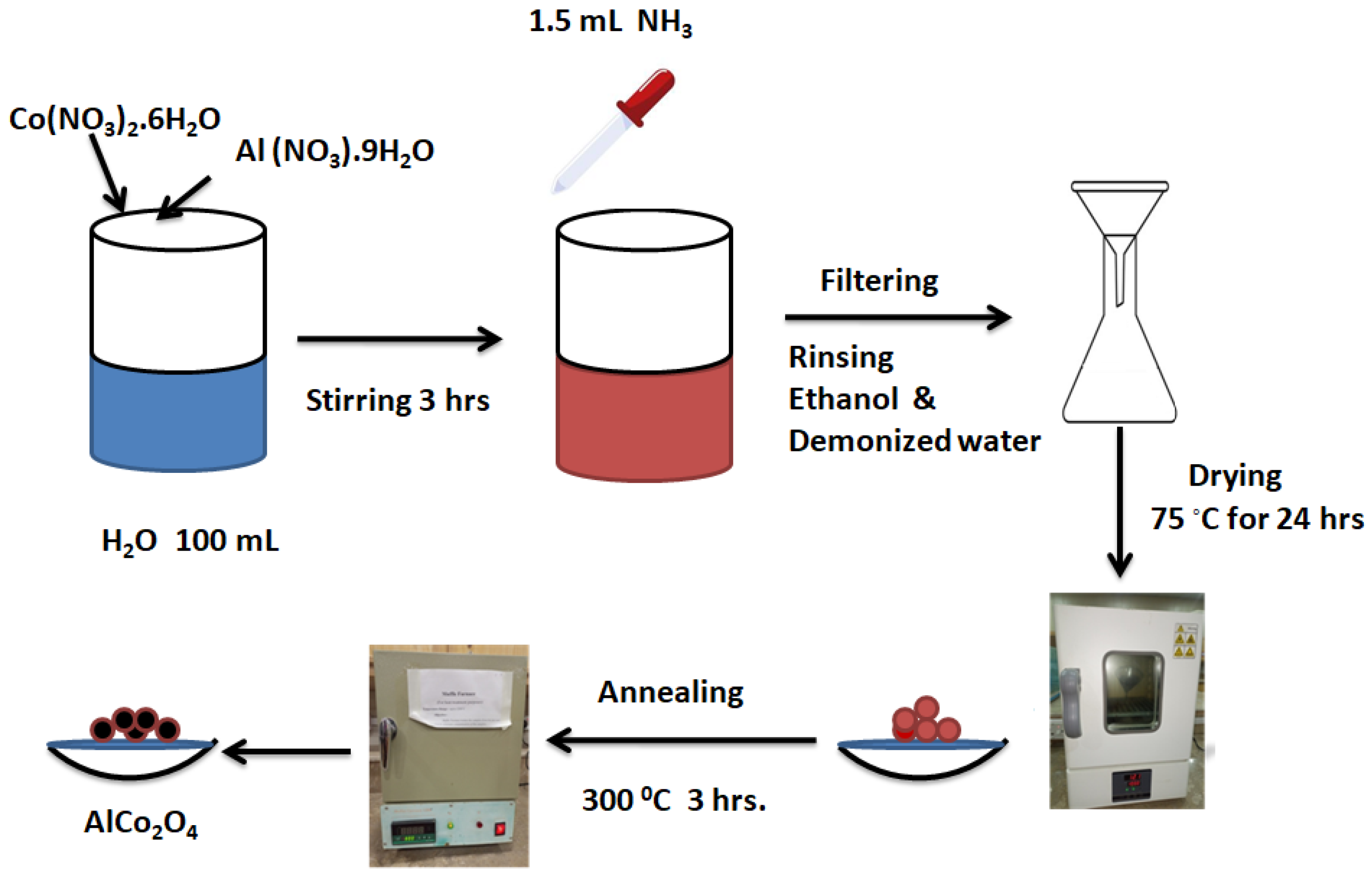
2.2. Synthesis of Spinal AlCo2O4 Nanocomposites
2.3. Synthesis of AlCo2O4 Anchored Multiwalled Carbon Nanotubes
2.4. Instrumentation
3. Results and Discussion
3.1. Compositional Analysis
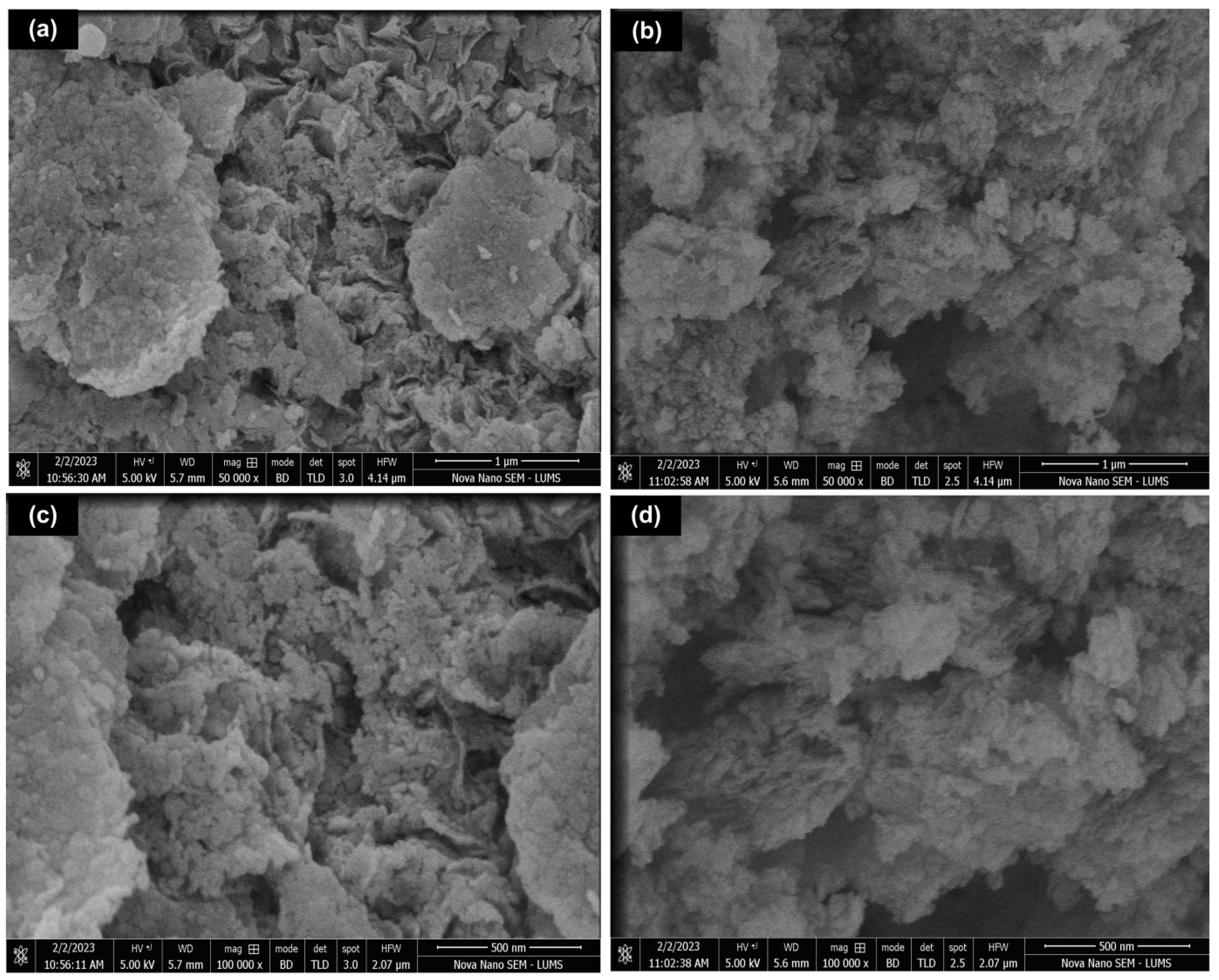
3.2. Morphological Analysis
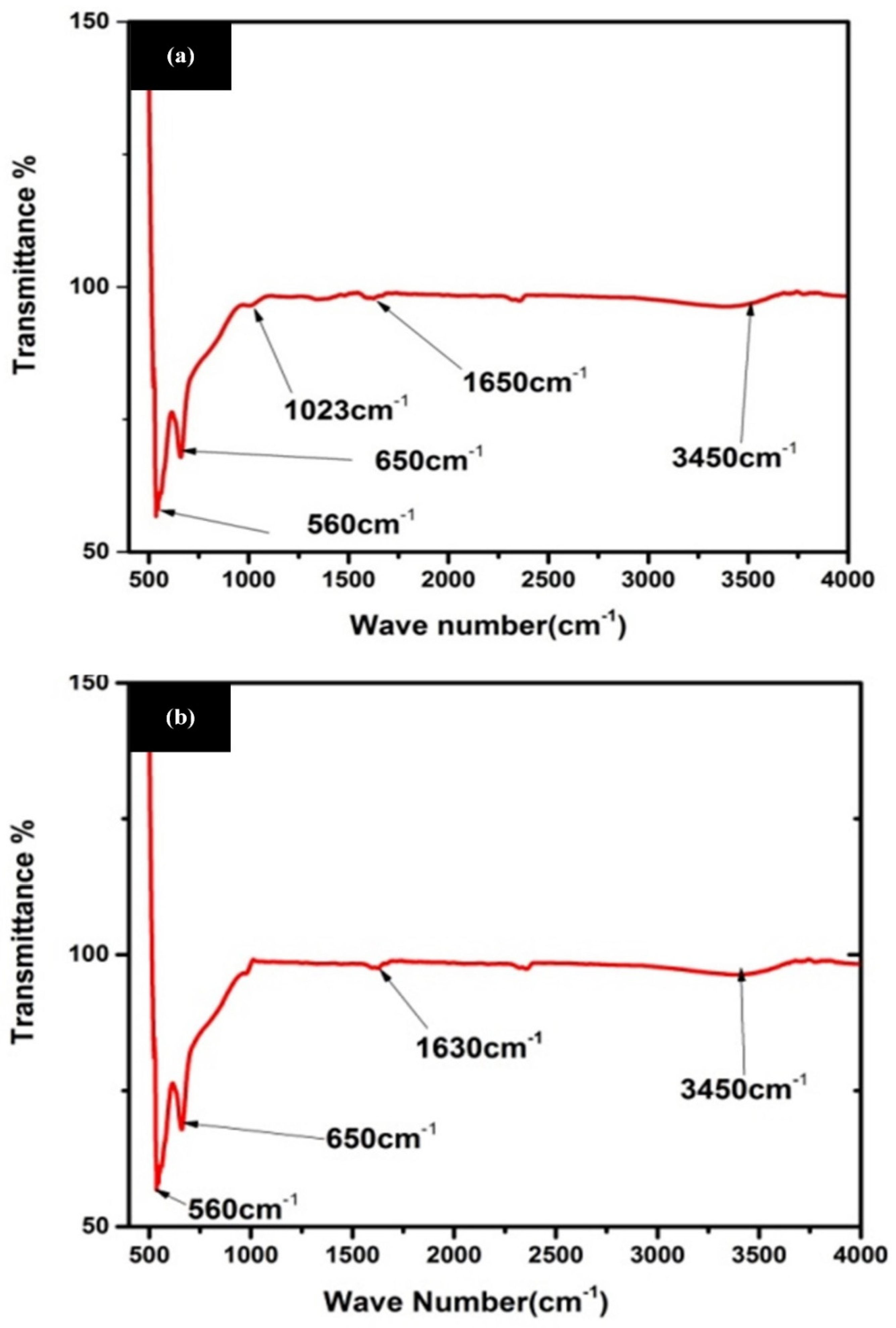
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
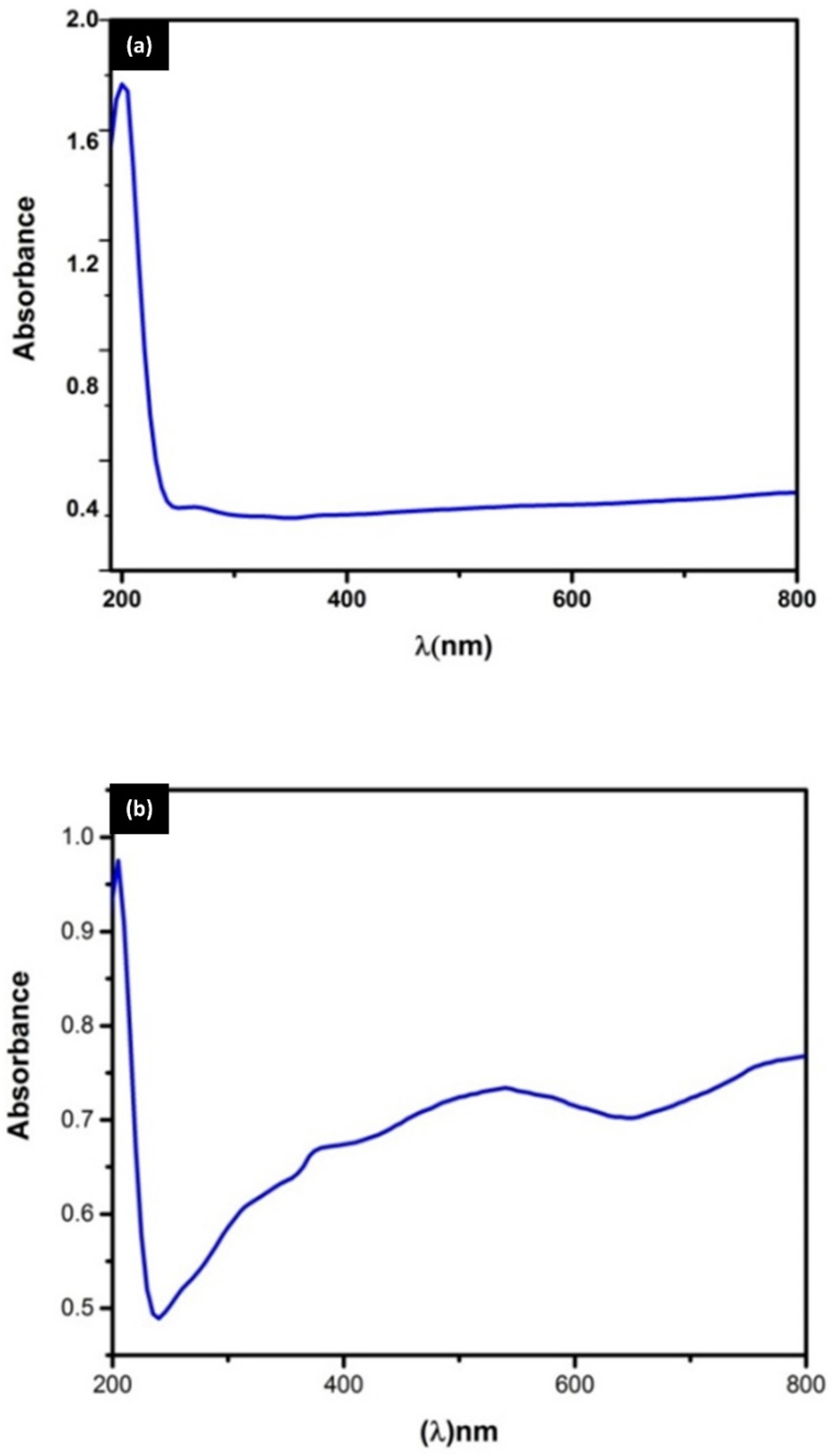
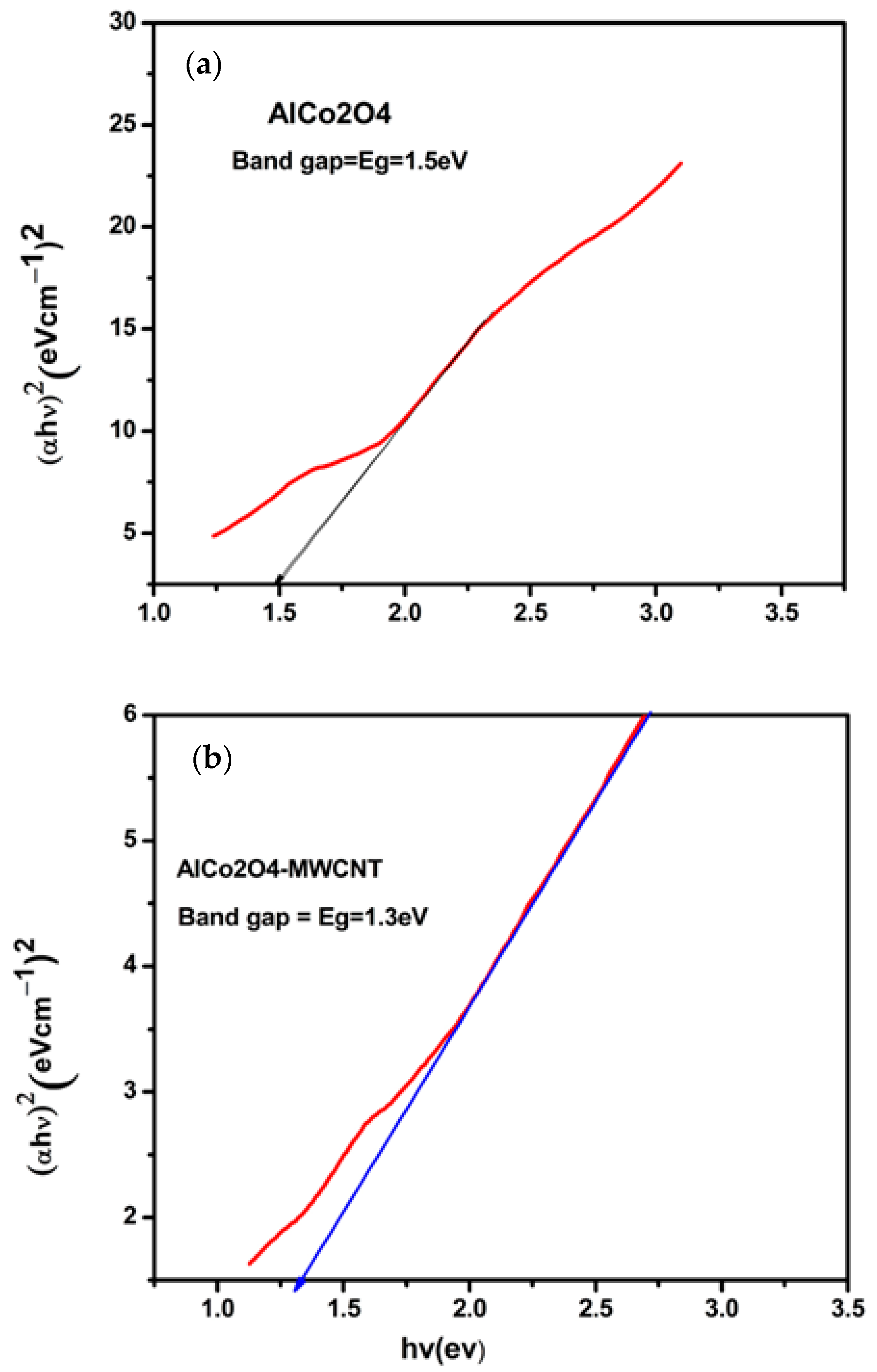
3.4. UV–Visible Spectroscopy
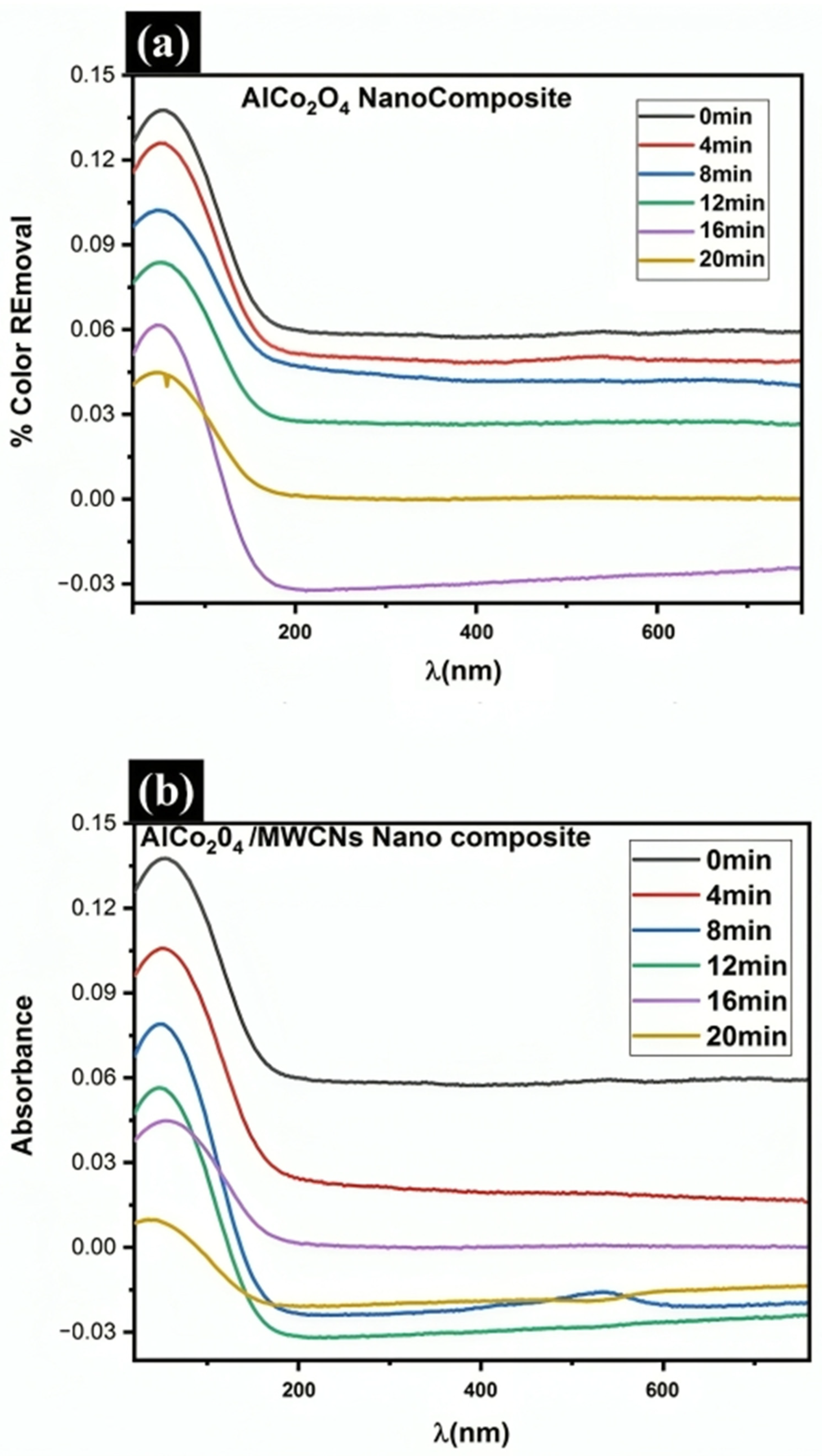
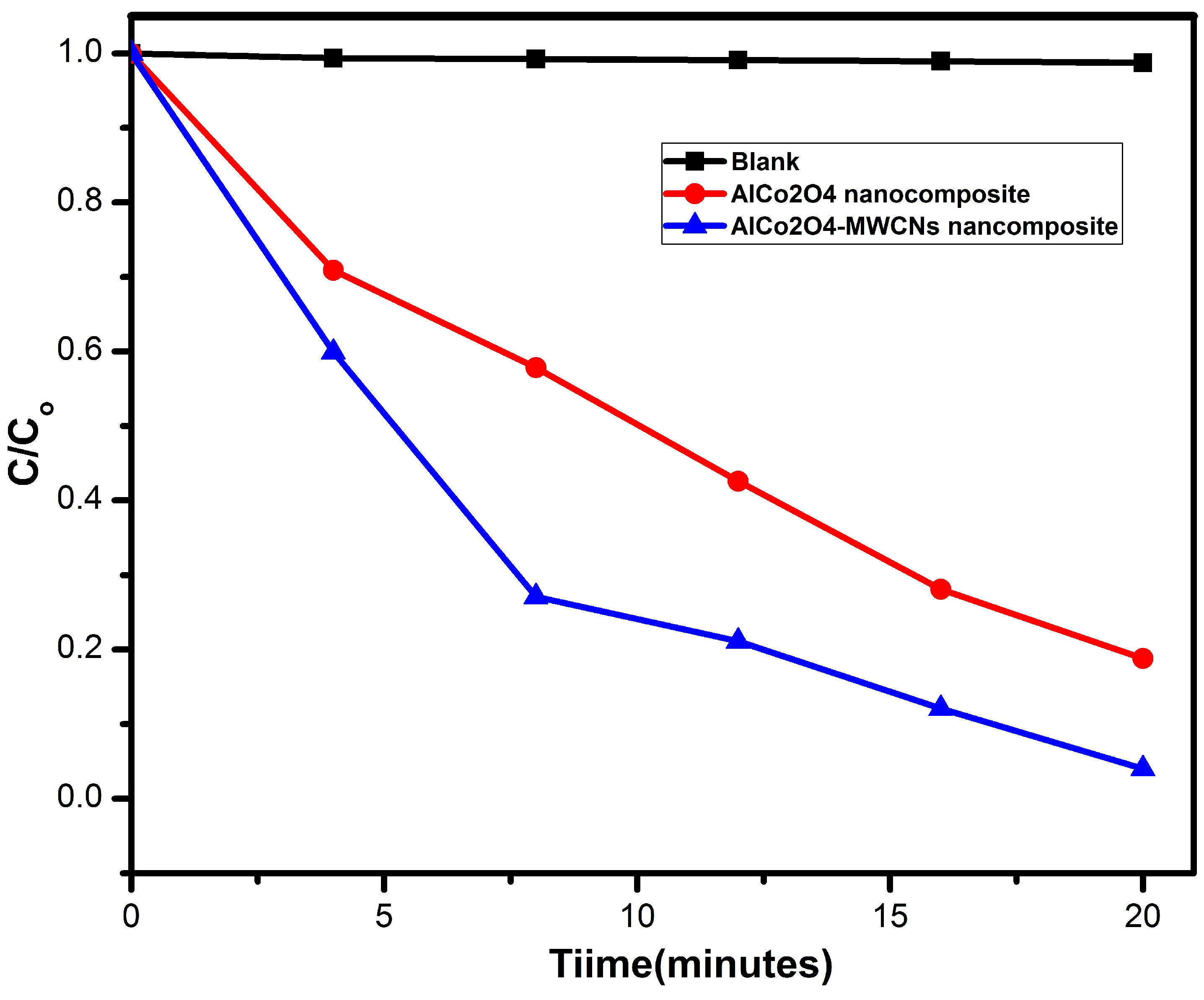
3.5. Photocatalytic Activity
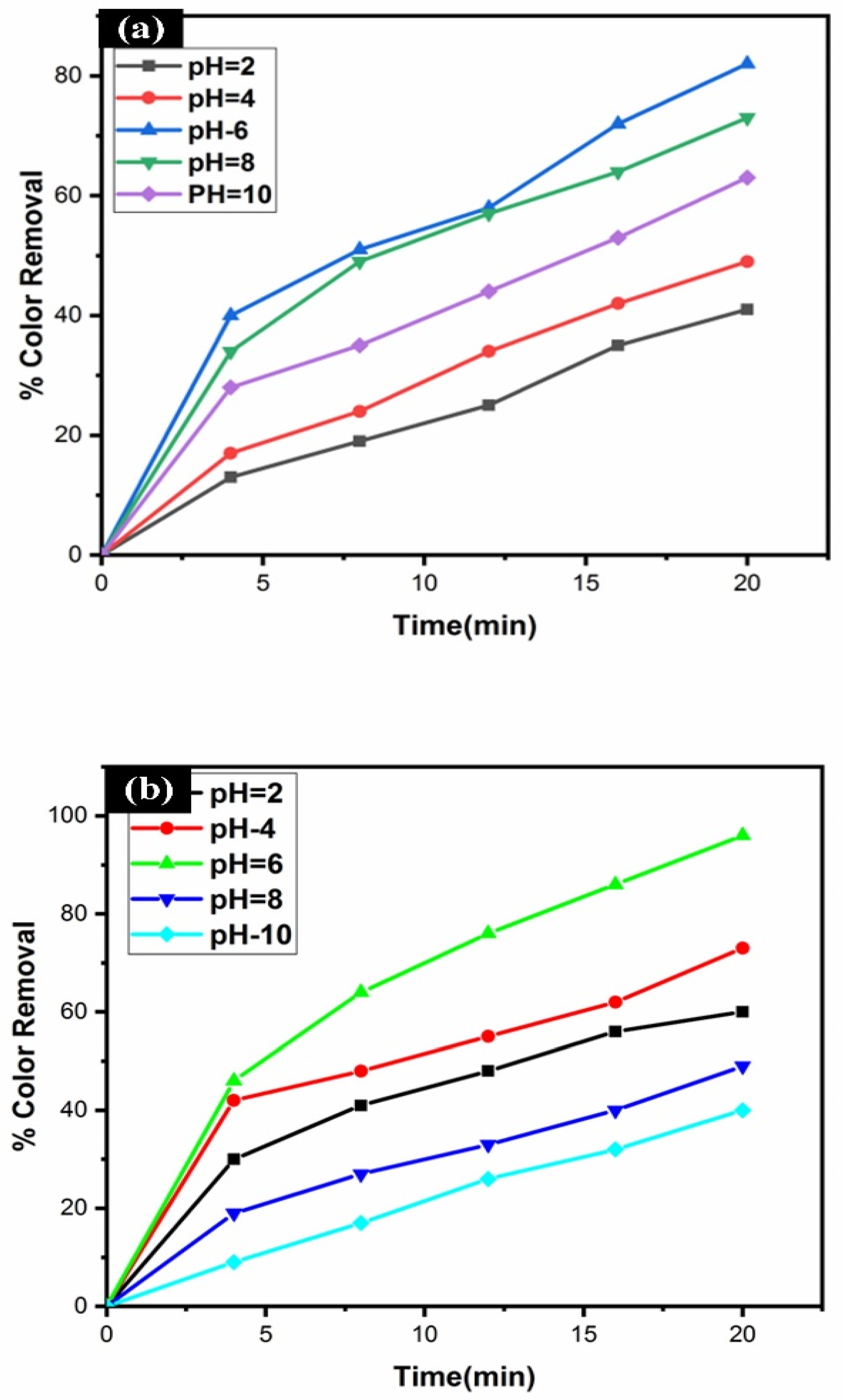
3.6. Effect of pH
3.7. Effect of Temperature
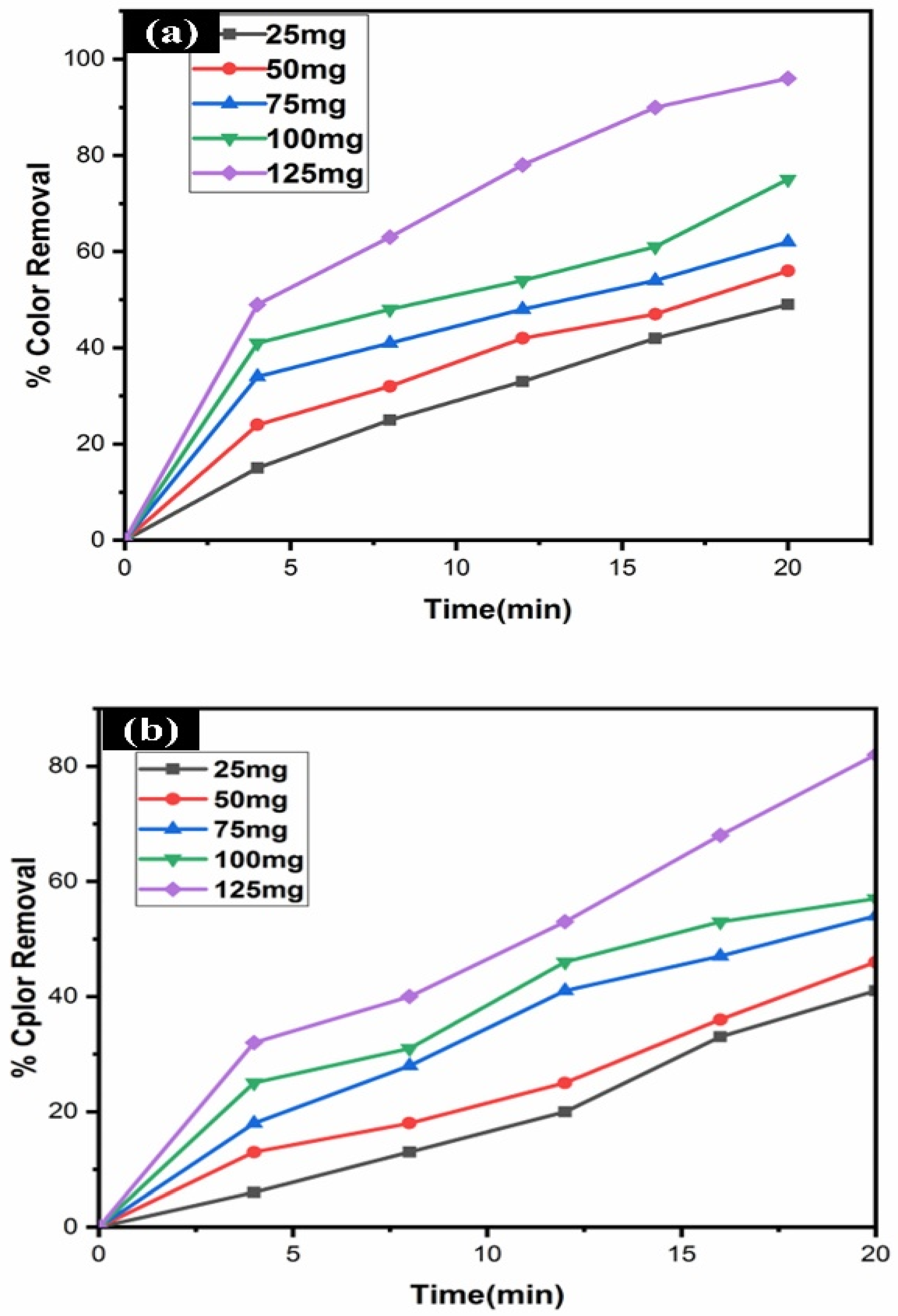
3.8. Effect of Catalyst Concentration
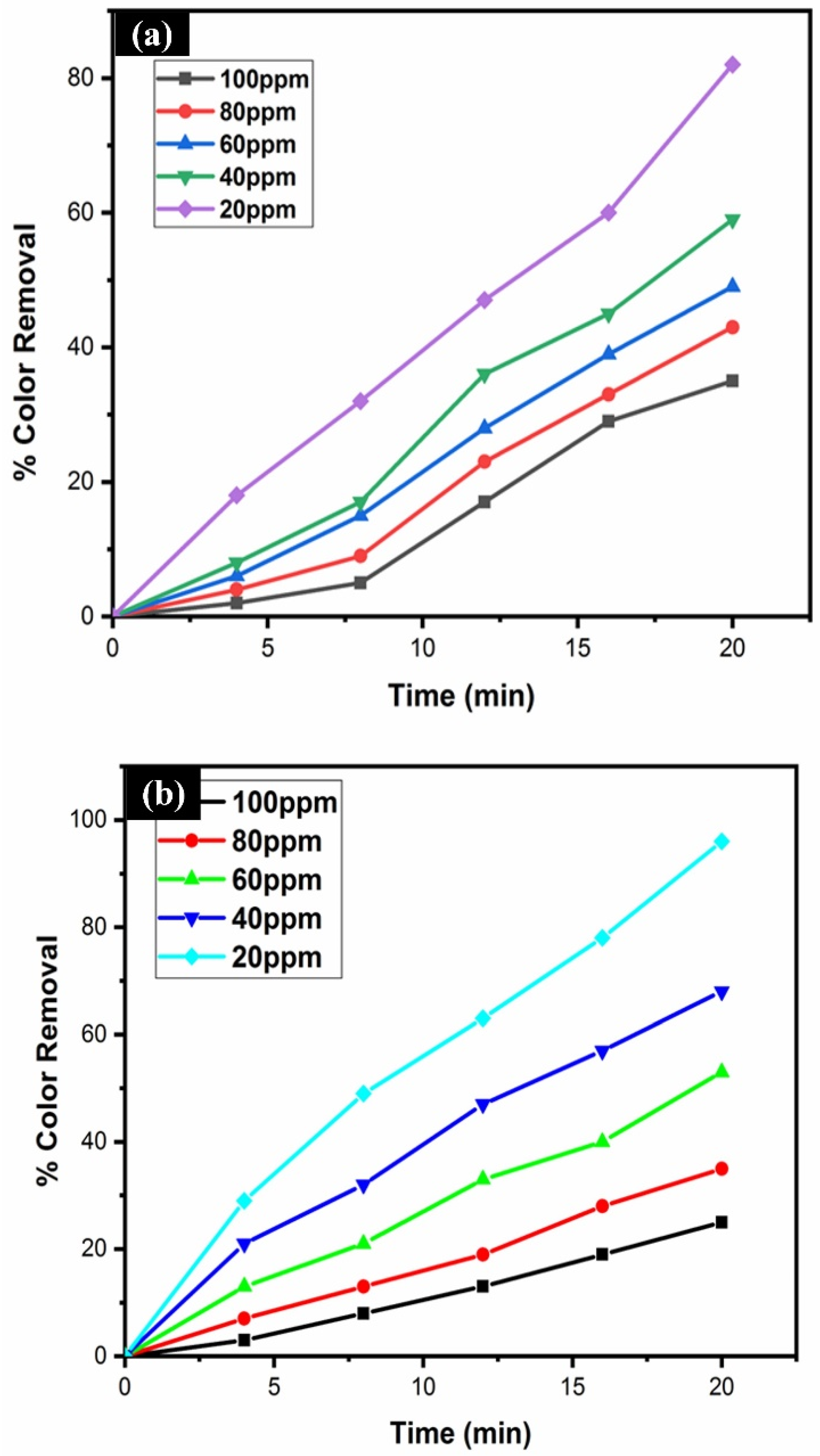
3.9. Effect of Dye Concentration
3.10. Quenching with Hydroxyl Radical Scavenger
3.11. Kinetics
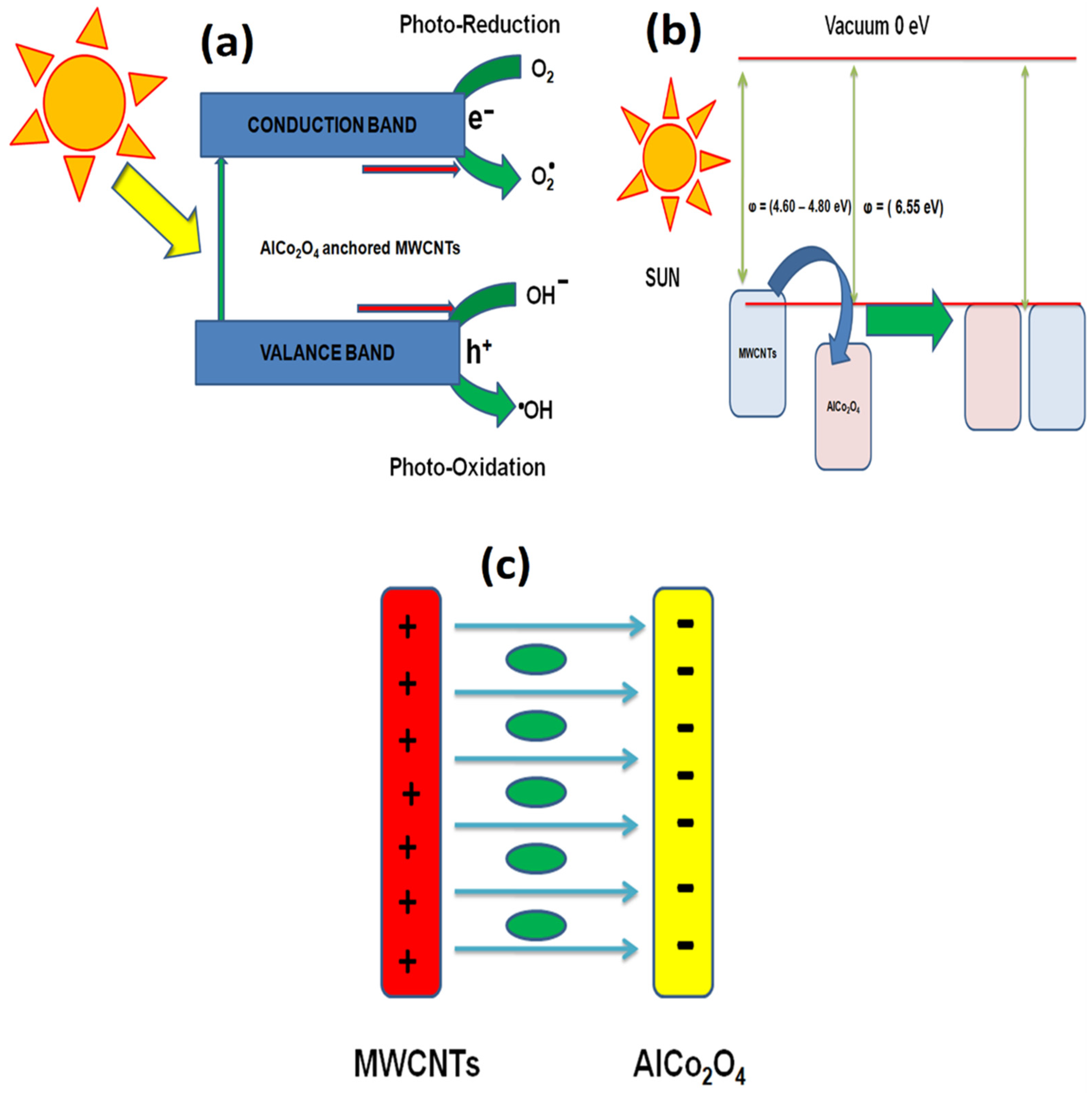
3.12. Proposed Mechanism
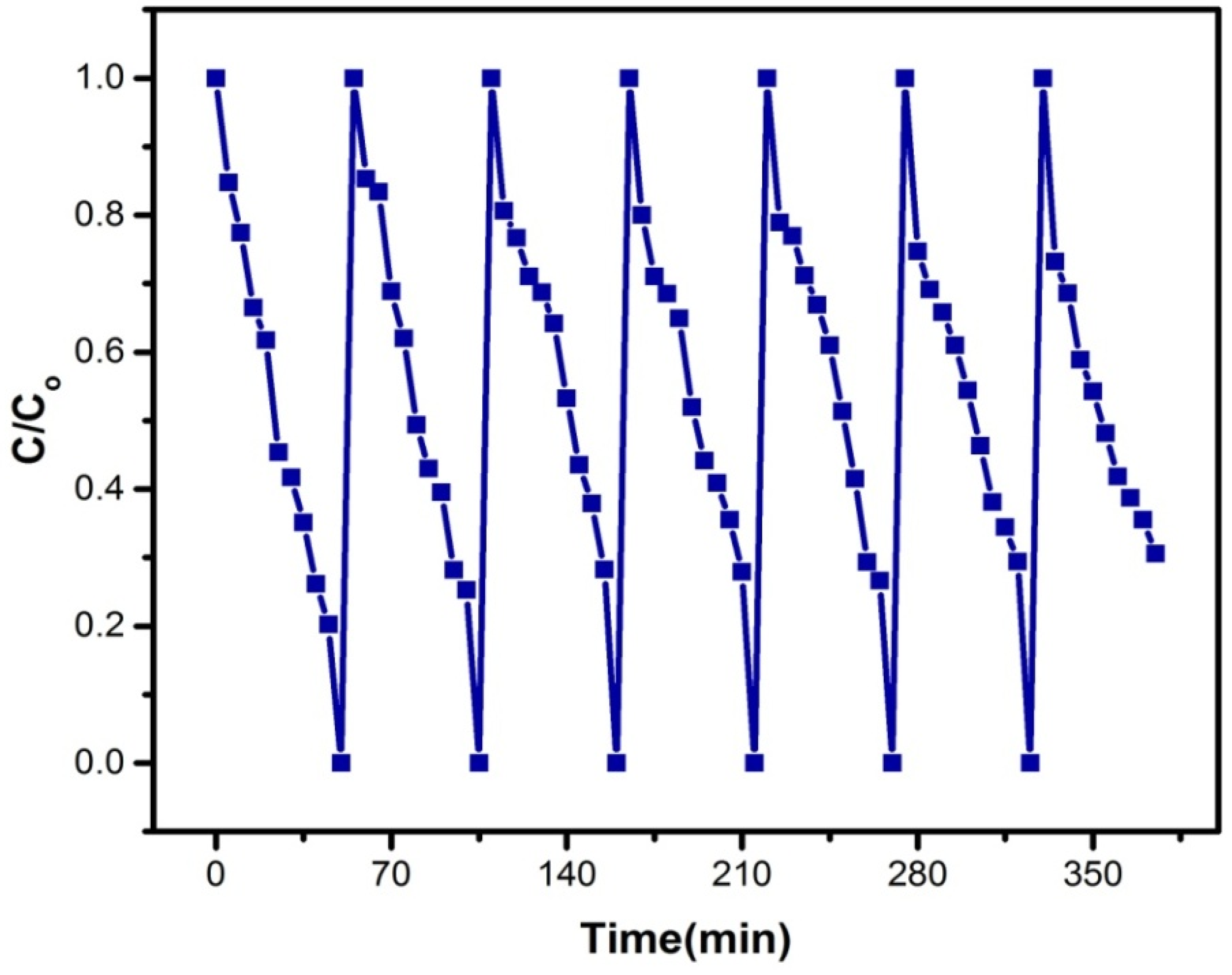
3.13. Reusability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, P. Unlocking Policy Effects: Water Resources Management Plans and Urban Water Pollution. J. Environ. Manag. 2024, 365, 121642. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cheng, J.; Deng, Y. Study on Agricultural Water Resource Utilization Efficiency under the Constraint of Carbon Emission and Water Pollution. Environ. Res. 2024, 253, 119142. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.; Yalcinkaya, F.; Altıok, E.; Pietrelli, A.; Nastro, R.A.; Lovecchio, N.; Ieropoulos, I.A.; Tsipa, A. The Role of Remote Sensing in the Evolution of Water Pollution Detection and Monitoring: A Comprehensive Review. Phys. Chem. Earth 2024, 136, 103712. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, N.; Chu, C.; Xiao, Y.; Niu, R.; Shao, C. Health Impact Assessment of the Surface Water Pollution in China. Sci. Total Environ. 2024, 933, 173040. [Google Scholar] [CrossRef]
- Feng, H.; Schyns, J.F.; Krol, M.S.; Yang, M.; Su, H.; Liu, Y.; Lv, Y.; Zhang, X.; Yang, K.; Che, Y. Water Pollution Scenarios and Response Options for China. Sci. Total Environ. 2024, 914, 169807. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Zafar, M.; Javed, F.; Yasar, A.; Akram, A.; Shabbir, S.; Qi, F. Catalytic Ozonation for the Removal of Reactive Black 5 (RB-5) Dye Using Zeolites Modified with CuMn2O4/GC3N4 in a Synergic Electro Flocculation-Catalytic Ozonation Process. Water Sci. Technol. 2021, 84, 1943–1953. [Google Scholar] [CrossRef]
- Cerqueira, A.; Russo, C.; Marques, M.R.C. Electroflocculation for Textile Wastewater Treatment. Braz. J. Chem. Eng. 2009, 26, 659–668. [Google Scholar] [CrossRef]
- Daghrir, R.; Gherrou, A.; Noel, I.; Seyhi, B. Hybrid Process Combining Electrocoagulation, Electroreduction, and Ozonation Processes for the Treatment of Grey Wastewater in Batch Mode. J. Environ. Eng. 2016, 142, 1–13. [Google Scholar] [CrossRef]
- Barathi, S.; Gitanjali, J.; Rathinasamy, G.; Sabapathi, N.; Aruljothi, K.N.; Lee, J.; Kandasamy, S. Recent Trends in Polycyclic Aromatic Hydrocarbons Pollution Distribution and Counteracting Bio-Remediation Strategies. Chemosphere 2023, 337, 139396. [Google Scholar] [CrossRef]
- Bogler, A.; Lin, S.; Bar-Zeev, E. Biofouling of Membrane Distillation, Forward Osmosis and Pressure Retarded Osmosis: Principles, Impacts and Future Directions. J. Memb. Sci. 2017, 542, 378–398. [Google Scholar] [CrossRef]
- Saeed, A.; Sharif, M.; Iqbal, M. Application Potential of Grapefruit Peel as Dye Sorbent: Kinetics, Equilibrium and Mechanism of Crystal Violet Adsorption. J. Hazard. Mater. 2010, 179, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Saghanejhad Tehrani, M.; Zare-Dorabei, R. Highly Efficient Simultaneous Ultrasonic-Assisted Adsorption of Methylene Blue And Rhodamine B onto Metal Organic Framework MIL-68(Al): Central Composite Design Optimization. RSC Adv. 2016, 6, 27416–27425. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Raashid, M.; Akram, A.; Kazmi, M.; Farman, S. Removal of Methylene Blue Dye from Aqueous Solutions by Adsorption in Combination with Ozonation on Iron Loaded Sodium Zeolite: Role of Adsorption. Desalin. Water Treat. 2021, 237, 302–306. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Y.; Chen, F.; Yao, F.; Sun, J.; Wang, S.; Yi, K.; Hou, L.; Li, X.; Wang, D. Recent Advances in Photo-Activated Sulfate Radical-Advanced Oxidation Process (SR-AOP) for Refractory Organic Pollutants Removal in Water. Chem. Eng. J. 2019, 378, 122149. [Google Scholar] [CrossRef]
- Yang, R.; Fan, Y.; Zhang, Y.; Mei, L.; Zhu, R.; Qin, J.; Hu, J.; Chen, Z.; Hau Ng, Y.; Voiry, D.; et al. 2D Transition Metal Dichalcogenides for Photocatalysis. Angew. Chem.-Int. Ed. 2023, 62, 1–29. [Google Scholar] [CrossRef]
- Krishnan, A.; Swarnalal, A.; Das, D.; Krishnan, M.; Saji, V.S.; Shibli, S.M.A. A Review on Transition Metal Oxides Based Photocatalysts for Degradation of Synthetic Organic Pollutants. J. Environ. Sci. 2024, 139, 389–417. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Batoo, K.M.; Khursheed, H.; Nadeem, M.S.; Hussain, S.; Ponraj, J.; Koc, M.; Iqbal, F. Oxygen Vacancies Generation in CeO2 via Y/Nd Co-Doping with Accelerated Charge Separation by Decorating on rGO Sheets for Sunlight-Driven Photodegradation of Hazardous Dyes. Ceram. Int. 2024, 50, 11486–11499. [Google Scholar] [CrossRef]
- Babu, N.; Devadathan, D.; Sebastian, A.; Vidhya, B. Photocatalytic Study of Cobalt Aluminate Nano-Particles Synthesised by Solution Combustion Method. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Tongchoo, P.; Intachai, S.; Pankam, P.; Suppaso, C.; Khaorapapong, N. The Usage of CoAl-Layered Double Oxide for Removal of Toxic Dye from Aqueous Solution. J. Met. Mater. Miner. 2020, 30, 45–50. [Google Scholar] [CrossRef]
- Abishad, P.; Jayashankar, M.; Hezam, A.; Srinath, B.S.; Kurkure, N.V.; Barbuddhe, S.B.; Rawool, D.B.; Vergis, J. Synthesis and Characterization of Nano-Cobalt Aluminium Oxide as a Potential Antioxidant, Biocidal and Photocatalytic Disinfectant Against multi Drug-Resistant Pathogens of Public Health Significance. Nano-Struct. Nano-Objects 2024, 37, 101112. [Google Scholar] [CrossRef]
- Aljohania, M.M.; Masoudb, E.M.; Mohamed, N.M.; Nassar, M.Y. Cobalt Aluminate/Carbon Nanocomposite via an auto-Combustion Method: An Efficient Photocatalyst for Photocatalytic Degradation of Organic Dyes from Aqueous Media. Int. J. Environ. Anal. Chem. 2021, 103, 7979–7999. [Google Scholar] [CrossRef]
- Imranullah, M.; Hussain, T.; Ahmad, R.; Shuaib, U.; Shakir, I. Spinel Nickel Cobaltite Nanoflakes Anchored Multiwalled Carbon Nanotubes Driven Photocatalyst for Highly Efficient Degradation of Organic Pollutants Using Natural Sunlight Irradiation. Ceram. Int. 2022, 48, 313–319. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Carbon Nanotube–Metal Oxide Nanocomposites: Fabrication, Properties and Applications. Chem. Eng. J. 2016, 302, 344–367. [Google Scholar] [CrossRef]
- Chinnappan, A.; Baskar, C.; Kim, H.; Ramakrishna, S. Carbon Nanotube Hybrid Nanostructures: Future Generation Conducting Materials. J. Mater. Chem. A 2016, 4, 9347–9361. [Google Scholar] [CrossRef]
- Wang, W.; Ding, M.; Lu, C.; Ni, Y.; Xu, Z. A Study on Upconversion UV-vis-NIR Responsive Photocatalytic Activity and Mechanisms of Hexagonal Phase NaYF4: Yb3+,Tm3+@TiO2 Core-Shell Structured Photocatalyst. Appl. Catal. B Environ. 2014, 144, 379–385. [Google Scholar] [CrossRef]
- Lal, B.; Singh, R.N.; Singh, N.K. Synthesis and Electrocatalytic Properties of Ni-Substituted Co3O4 for Oxygen Evolution in Alkaline Medium. J. New Mater. Electrochem. Syst. 2018, 21, 163–170. [Google Scholar] [CrossRef]
- Abbasi, Z.; Haghighi, M.; Fatehifar, E.; Saedy, S. Synthesis and Physicochemical Characterizations of Nanostructured Pt/Al2O3-CeO2 Catalysts for Total Oxidation of VOCs. J. Hazard. Mater. 2011, 186, 1445–1454. [Google Scholar] [CrossRef]
- Imranullah, M.; Hussain, T.; Ahmad, R.; Ahmad, S.; Shakir, I. Stable and Highly Efficient Natural Sunlight Driven Photo-Degradation of Organic Pollutants Using Hierarchical Porous Flower-like Spinel Nickel Cobaltite Nanoflakes. Ceram. Int. 2021, 47, 15408–15414. [Google Scholar] [CrossRef]
- Mergen, Ö.B.; Arda, E. Determination of Optical Band Gap Energies of CS/MWCNT Bio-Nanocomposites by Tauc and ASF Methods. Synth. Met. 2020, 269, 116539. [Google Scholar] [CrossRef]
- Qusti, A.H. Fabrication and Characterization of ZnO/MWCNTs with Enhanced Photocatalytic Activity. Asian J. Chem. 2014, 26, 70–73. [Google Scholar] [CrossRef]
- Mergen, Ö.B. Effect of MWCNT Addition on the Optical Band Gap of PVA/CS Transient Biocomposites. J. Compos. Mater. 2021, 55, 4347–4359. [Google Scholar] [CrossRef]
- Phin, H.Y.; Ong, Y.T.; Sin, J.C. Effect of Carbon Nanotubes Loading on the Photocatalytic Activity of Zinc Oxide/Carbon Nanotubes Photocatalyst Synthesized via a Modified Sol-Gel Method. J. Environ. Chem. Eng. 2020, 8, 103222. [Google Scholar] [CrossRef]
- Navidpour, A.H.; Abbasi, S.; Li, D.; Mojiri, A.; Zhou, J.L. Investigation of Advanced Oxidation Process in the Presence of TiO2 Semiconductor as Photocatalyst: Property, Principle, Kinetic Analysis, and Photocatalytic Activity. Catalysts 2023, 13, 232. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukrullah, S.; Yasin Naz, M.; Ullah, S.; Ali Assiri, M. Designing and Modification of Bismuth Oxyhalides BiOX (X = Cl, Br and I) Photocatalysts for Improved Photocatalytic Performance. J. Ind. Eng. Chem. 2022, 105, 1–33. [Google Scholar] [CrossRef]
- Khodakov, A.Y. Fischer-Tropsch Synthesis: Relations between Structure of Cobalt Catalysts and Their Catalytic Performance. Catal. Today 2009, 144, 251–257. [Google Scholar] [CrossRef]
- Kiran, S.; Rafique, M.A.; Iqbal, S.; Nosheen, S.; Naz, S.; Rasheed, A. Synthesis of Nickel Nanoparticles Using Citrullus Colocynthis Stem Extract for Remediation of Reactive Yellow 160 Dye. Environ. Sci. Pollut. Res. 2020, 27, 32998–33007. [Google Scholar] [CrossRef]
- Keskin, C.S.; Keskin, S.Y.; Topcu, M.C. Simultaneous Biosorption of Acid Violet and Reactive Yellow Dyes by Cladosporium Cladosporioides. Clean Technol. Environ. Policy 2024, 26, 3469–3480. [Google Scholar] [CrossRef]
- Mustafa, G.; Munir, R.; Sadia, B.; Younas, F.; Sayed, M.; Muneer, A.; Sardar, M.F.; Albasher, G.; Noreen, S. Synthesis of Polymeric Ferrite Composites (Ni-CoFe2O4/Chitosan, Zn-NiFe2O4/Starch, Co-NiZnFe2O4/Polyaniline, Ni doped CrZnFe2O4/Alginate, and Cr doped ZnCoFe2O4/PVA) for the Removal of Reactive Golden Yellow-160 Dye from Wastewater. J. Environ. Chem. Eng. 2024, 12, 112581. [Google Scholar] [CrossRef]
- Yasin, M.; Saeed, M.; Muneer, M.; Usman, M.; Haq, A.U.; Sadia, M.; Altaf, M. Development of Bi2O3-ZnO Heterostructure for Enhanced Photodegradation of Rhodamine B and Reactive Yellow Dyes. Surf. Interfaces 2022, 30, 101846. [Google Scholar] [CrossRef]
- Rathi, H.; Jeice, A.R. Visible Light Photocatalytic Dye Degradation, Antimicrobial Activities of Green Synthesized Ag/TiO2 Nanoparticles. Chem. Phys. Impact 2024, 8, 100537. [Google Scholar] [CrossRef]
- Kim, C.-M.; Chowdhury, M.F.; Im, H.R.; Cho, K.; Am, J. NiAlFe LTH /MoS2 p-n Junction Heterostructure Composite as an Effective Visible-Light-Driven Photocatalyst for Enhanced Degradation of Organic Dye under High Alkaline Conditions. Chemosphere 2024, 358, 142094. [Google Scholar] [CrossRef] [PubMed]
- Naz, A.; Bibi, I.; Majid, F.; Dahshan, A.; Jilani, K.; Taj, B.; Ghafoor, A.; Nazeer, Z.; Alzahrani, F.M.; Iqbal, M. Cu and Fe Doped NiCo2O4/g-C3N4 Nanocomposite Ferroelectric, Magnetic, Dielectric and Optical Properties: Visible Light-Driven Photocatalytic Degradation of RhB and CR Dyes. Diam. Relat. Mater. 2024, 141, 110592. [Google Scholar] [CrossRef]
- Mirzaeifard, Z.; Shariatinia, Z. Economical, One-Pot, and Green Synthesis of Plant-Based Carbon Quantum Dots for Efficient Visible-Light Photocatalytic Dye Degradation and Water Purification. J. Taiwan Inst. Chem. Eng. 2024, 163, 105655. [Google Scholar] [CrossRef]
- Bhava, A.; Shenoy, U.S.; Bhat, D.K. Silver Doped Barium Titanate Nanoparticles for Enhanced Visible Light Photocatalytic Degradation of Dyes. Environ. Pollut. 2024, 344, 123430. [Google Scholar] [CrossRef]
- Jia, Z.; Miao, J.; Lu, H.B.; Habibi, D.; Zhang, W.C.; Zhang, L.C. Photocatalytic Degradation and Absorption Kinetics of Cibacron Brilliant Yellow 3G-P by Nanosized ZnO Catalyst under Simulated Solar Light. J. Taiwan Inst. Chem. Eng. 2016, 60, 267–274. [Google Scholar] [CrossRef]
- Mahde, B.W.; Radia, N.D.; Jasim, L.S.; Jamel, H.O. Synthesis and Characterization of Polyacrylamide Hydrogel for the Controlled Release of Aspirin. J. Pharm. Sci. Res. 2018, 10, 2850–2854. [Google Scholar]
- Soares, S.F.; Fernandes, T.; Sacramento, M.; Trindade, T.; Daniel-da-Silva, A.L. Magnetic Quaternary Chitosan Hybrid Nanoparticles for the Efficient Uptake of Diclofenac from Water. Carbohydr. Polym. 2019, 203, 35–44. [Google Scholar] [CrossRef]
- Kuriakose, S.; Choudhary, V.; Satpati, B.; Mohapatra, S. Enhanced Photocatalytic Activity of Ag-ZnO Hybrid Plasmonic Nanostructures Prepared by a Facile Wet Chemical Method. Beilstein J. Nanotechnol. 2014, 5, 639–650. [Google Scholar] [CrossRef]
- Raashid, M.; Kazmi, M.; Ikhlaq, A.; Iqbal, T.; Sulaiman, M.; Shakeel, A. Degradation of Aqueous Confidor® Pesticide by Simultaneous TiO2 Photocatalysis and Fe-Zeolite Catalytic Ozonation. Water 2021, 13, 3327. [Google Scholar] [CrossRef]
- Habeeb Alshamsi, H.A.; Hussein, B.S. Hydrothermal Preparation of Silver Doping Zinc Oxide Nanoparticles: Studys, Characterization and Photocatalytic Activity. Orient. J. Chem. 2018, 34, 1898–1907. [Google Scholar] [CrossRef]
- Liu, J.F.; Zhao, Z.S.; Jiang, G. Bin Coating Fe3O4 Magnetic Nanoparticles with Humic Acid for High Efficient Removal of Heavy Metals in Water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef] [PubMed]
- Gümüş, D.; Akbal, F. A Comparative Study of Ozonation, Iron Coated Zeolite Catalyzed Ozonation and Granular Activated Carbon Catalyzed Ozonation of Humic Acid. Chemosphere 2017, 174, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.D.; Nguyen, D.Q.; Do, P.T.; Tran, U.N.P. Kinetics of Photocatalytic Degradation of Organic Compounds: A Mini-Review and New Approach. RSC Adv. 2023, 13, 16915–16925. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1–31. [Google Scholar] [CrossRef]
- Su, W.S.; Leung, T.C.; Chan, C.T. Work Function of Single-Walled and Multiwalled Carbon Nanotubes: First-Principles Study. Phys. Rev. B-Condens. Matter Mater. Phys. 2007, 76, 2–9. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Q.; Wu, X.; Lv, K.; Tang, D.; Li, M. Facile Synthesis of CNTs/CaIn2S4 Composites with Enhanced Visible-Light Photocatalytic Performance. Appl. Surf. Sci. 2017, 391, 565–571. [Google Scholar] [CrossRef]




| Technique Used | Novel Material | % Degradation | Reference |
|---|---|---|---|
| Biosorption | Nickel nanoparticles | 91.4% in 40 min | [36] |
| Biosorption | Cladosporium cladosporioides | ≈99.99% in 60 min | [37] |
| Chemisorption | Polymeric ferrite composites | 94% in 60 min | [38] |
| Photocatalysis (visible light) | Bi2O3-ZnO heterostructure | 91% in 120 min | [39] |
| Photocatalysis (visible light) | AlCo2O4/MWCNTs composites | >96% in 20 min | This Study |
| Photocatalyst | Dye | % Degradation | Reference |
|---|---|---|---|
| Ag/TiO2 nanoparticles | Methylene Blue | 94% in 40 min | [40] |
| NiAlFe LTH/MoS2 p-n junction heterostructure | Indigo | ≈99.99% in 100 min | [41] |
| Ni1−xCuxCo2−yFeyO4/g-C3N4 | Congo Red | 94% in 24 min | [42] |
| 10%F-10%B co-doped Carbon Quantum Dots | Rhodamine B | 89.13% in 60 min | [43] |
| Silver doped barium titanate nanoparticles | Eosin Yellow | ≈92% in 40 min 99.3% in 60 min | [44] |
| AlCo2O4/MWCNTs composites | Reactive Yellow RY-160 | >96% in 20 min | This Study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, J.; Ikhlaq, A.; Raashid, M.; Ikhlaq, U.; Qazi, U.Y.; Masood, H.T.; Hussain, T.; Kazmi, M.; Ramzan, N.; Naeem, A.; et al. Novel AlCo2O4/MWCNTs Nanocomposites for Efficient Degradation of Reactive Yellow 160 Dye: Characterization, Photocatalytic Efficiency, and Reusability. Catalysts 2025, 15, 154. https://doi.org/10.3390/catal15020154
Ahmad J, Ikhlaq A, Raashid M, Ikhlaq U, Qazi UY, Masood HT, Hussain T, Kazmi M, Ramzan N, Naeem A, et al. Novel AlCo2O4/MWCNTs Nanocomposites for Efficient Degradation of Reactive Yellow 160 Dye: Characterization, Photocatalytic Efficiency, and Reusability. Catalysts. 2025; 15(2):154. https://doi.org/10.3390/catal15020154
Chicago/Turabian StyleAhmad, Junaid, Amir Ikhlaq, Muhammad Raashid, Uzma Ikhlaq, Umair Yaqub Qazi, Hafiz Tariq Masood, Tousif Hussain, Mohsin Kazmi, Naveed Ramzan, Asma Naeem, and et al. 2025. "Novel AlCo2O4/MWCNTs Nanocomposites for Efficient Degradation of Reactive Yellow 160 Dye: Characterization, Photocatalytic Efficiency, and Reusability" Catalysts 15, no. 2: 154. https://doi.org/10.3390/catal15020154
APA StyleAhmad, J., Ikhlaq, A., Raashid, M., Ikhlaq, U., Qazi, U. Y., Masood, H. T., Hussain, T., Kazmi, M., Ramzan, N., Naeem, A., Aly Hassan, A., Qi, F., & Javaid, R. (2025). Novel AlCo2O4/MWCNTs Nanocomposites for Efficient Degradation of Reactive Yellow 160 Dye: Characterization, Photocatalytic Efficiency, and Reusability. Catalysts, 15(2), 154. https://doi.org/10.3390/catal15020154









