Abstract
The shift toward sustainable energy sources is essential to curb greenhouse gas emissions and satisfy energy demands. Among renewable options, carbon-based materials—such as agricultural residues and municipal solid waste—provide a dual advantage by generating energy and fuels while also reducing landfill waste. A notable innovation is transforming plastic waste into methane-rich streams via catalytic hydrogasification, a process in which carbon-based feedstocks interact with hydrogen using a selective catalyst. In this study, a structured catalyst was developed, characterized, and tested for converting plastic waste samples. The thermal degradation properties of plastic waste were first studied using thermogravimetric analysis. The catalyst was prepared using an Oxygen Bonded Silicon Carbide (OBSiC) open-cell foam as the carrier, coated with γ-Al2O3-based washcoat, CeO2, and Ni layers. It was characterized in terms of specific surface area, coating adhesion, pore distribution, acidity, and the strength of its active sites. Experimental tests revealed that a hydrogen-enriched atmosphere significantly enhances CH4 formation. Specifically, during catalytic hydrogasification, methane selectivity reached approximately 59%, compared to 6.7%, 13.7%, and 7.8% observed during pyrolysis, catalyzed pyrolysis, and non-catalyzed hydrogasification tests, respectively. This study presents a novel and effective approach for converting plastic waste using a structured catalyst, a method rarely explored in literature.
1. Introduction
The rapid economic development and rising standards of living have contributed to the depletion of traditional fossil fuel resources, whose intensive use has exacerbated environmental pollution [1]. At the same time, global energy demand continues to rise, driven by population growth [2]. Consequently, exploring new sustainable energy solutions is crucial to mitigate greenhouse gas emissions and meet growing energy needs [3,4]. However, renewable sources remain economically uncompetitive in many cases, and their widespread adoption will depend on supportive energy market structures and national transition policies. In this context, carbon-based materials and biomass—such as organic waste from agriculture and industry, woody residues from forest harvesting, municipal solid waste, and plastic waste—offer a viable alternative for energy production [5], providing a dual advantage by generating energy and fuels while also reducing landfill waste.
Plastic is an exceptional material with numerous applications due to its low cost, lightweight, durability, chemical resistance, and ease of processing. Commonly used polymers include thermoplastics such as polyethylene (PE), polypropylene (PP) and polyvinyl chloride (PVC), with a global production of 125 million tons per year, 69 million tons per year and 39 million tons per year, respectively. In 2020, global plastic production reached 367 million tons [6], expected to rise to 1.1 billion tons by 2050 [7]. As a result, plastic waste is expected to increase significantly, with projections indicating that approximately 12 billion tons will be disposed of in landfills by 2050 [8]. Currently, of the 8600 million tons of plastic ever produced, 59% has been discarded, either ending up in landfills or accumulating in the natural environment, including oceans, while only 17% has been recycled or incinerated for energy recovery [8]. In response, European governments have increasingly focused on improving waste collection and conversion technologies, aiming for a “zero landfilling” scenario. One way to ensure the value recovery of landfill waste is by converting it into energy, fuels, or chemicals. Several techniques can be employed for this purpose. These conversion processes are generally categorized into three types: (i) thermochemical (using heat to break down feedstocks into smaller fragments), (ii) biological, and (iii) physical conversion [5].
Among thermochemical processes, hydrogasification stands out as an intriguing alternative.
Hydrogasification (Figure 1) is a peculiar gasification in which a hydrogen-enriched atmosphere is used to treat a carbon-based fuel. The first implementation of coal hydrogasification dates to the 1930s when Germany, the United States and Great Britain employed this technology to address shortages of gas and liquid fuels. Over the following years, hydrogasification was integrated into broader gasification processes (such as steam reforming and partial oxidation), notably to produce high-octane gasoline from coal via the Fischer–Tropsch process [9]. As such, coal hydrogasification is a well-established technology. In recent years, this approach has been explored as a potential energy storage solution [9] and is increasingly considered as a strategy for producing high-value substitute natural gas (SNG), a methane-rich gas mixture, from low-energy and low-economic-value materials, such as wood biomass, agricultural residues, and plastic waste, and with a thermal efficiency of 80% and in the absence of oxygen [10]. The energy content of methane can be highlighted by comparing its combustion with that of carbon, as shown in Equations (1) and (2):
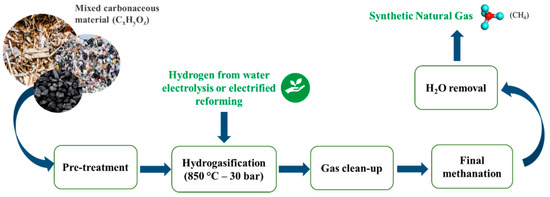
Figure 1.
Scheme of the basic process of hydrogasification.
The hydrogasification process includes several reactions depending on the assumptions (both thermodynamics and kinetics) and on the characteristics of the feedstocks chosen. The general reaction of the process is reported in Equation (3).
The reaction of carbon hydrogasification is reported in Equation (4):
Considering the composition of coal/carbonaceous materials (CaHbOcNdSe), which includes carbon and other elements (hydrogen, oxygen, nitrogen, sulfur, etc.), a realistic reaction scheme of the coal hydrogasification process consists of three steps: (a) the drying process of the original feedstock; (b) the pyrolysis of the dried feedstock which generates volatiles (mainly CO, CO2, CH4, C6H6, N2, H2S, SO2, H2, and H2O) separating them for the char (solid carbon), and (c) a series of gasification reactions of both char and volatiles [10,11]. The temperature reached by the hydro-gasification reactor ranges from 800 °C to 1000 °C. Along with the reaction of hydrogasification, the other main reactions that involve char gasification are carbon steam reforming (Equation (5)), where the carbon reacts with the vapor from the drying step [10], and the Boudouard equilibrium (Equation (6)) [10]:
The volatiles can react in the following reactions (Equation from (7) to (11)) [9]:
The reducing environment allows for the increase in the formation of methane at the expense of CO and CO2. The water–gas shift reaction (Equation (7)) controls the presence of carbon monoxide, which is a by-product of the hydrogasification process. Indeed, when the temperature is above 300 °C, the equilibrium is shifted towards CO and water production. Therefore, a successive step of CO methanation is necessary to further lower the CO concentration Equation (12) in the product stream, even if the excess of hydrogen avoids the formation of heavier hydrocarbons and reduces the CO formation.
At the end of the process, the final product is SNG (synthetic natural gas), which consists of methane with hydrogen plus minor impurities. The presence of hydrogen in methane up to 28 vol% is safe to be used with properly serviced existing domestic appliances [12].
This work aims to employ the hydrogasification process to convert plastic waste into a methane-enriched gaseous stream. A literature review of the state of the art revealed that few studies have explored the conversion of plastics using this process. Most research has focused on coal feedstocks, with pyrolysis and gasification being the more commonly used methods for plastic waste conversion. Moreover, the use of a catalyst is crucial to maximizing the selectivity towards methane.
Among the various catalysts investigated in the literature, those based on iron group metals and alkaline/alkaline earth metals strike the best balance between performance and cost. The excellent catalytic activity of transition metals is primarily attributed to their ability to adsorb and dissociate H2 on the catalyst surface and weaken the C–C bonds by interacting with carbon in the char. However, iron group metals are more prone to deactivation at high temperatures due to coke formation, sulfur poisoning, and sintering during hydrogasification [13]. This issue can be mitigated by introducing a promoter into the catalyst formulation, with calcium being the most promising option [14,15,16]. Calcium can modify the catalytic behavior of iron group metals and enhance their performance to levels comparable to more expensive metals like Rh or Pt [15]. Additionally, calcium delays sintering at high temperatures, protects Fe/Co/Ni from sulfur poisoning, and prevents further sintering [17,18]. The addition of calcium prevented cobalt agglomeration, enhancing its penetration into the carbon matrix and thus accelerated gasification [19,20], leading to enhanced CH4 and tar yields and significantly improved gasification reactivity of the resulting char [21]. Haga et al. [14,15] showed that the bimetallic catalyst 5wt%Co-5wt%Ca exhibited higher activity than Ni-Ca and Fe-Ca in the hydrogasification of bituminous coal, primarily due to its ability to break C–C bonds. Similarly, Zhang et al. [16] found that the catalyst with 5wt% Fe–1wt% Ca exhibited the highest CH4 yield, reaching 53.4% compared to 7.76% with a non-catalytic system. This research demonstrated that a catalyst co-loaded with Fe and Ca improves the reactivity of uncatalyzed char by increasing the dispersion of iron group metals, removing sulfur, and promoting the reduction of metallic iron and carburization. The results indicate that both calcium (Ca) alone and metallic iron (Fe) alone exhibit low catalytic activity, but their interaction results in significantly enhanced activity [22].
Other works also investigated the effect of copper composite Cu-Ni-Ca catalysts, which are active, recoverable, and less inclined to sintering; however, they have pollution issues [23,24].
Another possibility is to use alkaline and alkaline earth-based compounds which exhibit high activity, even surpassing that of transition metals and remarkable selectivity toward CH4 production. However, their interaction with the inherent mineral matter in coal or char can lead to the formation of water-insoluble compounds, such as KAlSiO4, which are difficult to recover [25]. This results in catalyst loss and increases operational costs.
In this work, we investigated the possibility of effectively adding value to plastic waste (supplied by ENEA—National Agency for New Technologies, Energy and Sustainable Development) through an innovative hydrogasification process employing a structured catalyst. This approach presents a promising avenue for giving plastic waste a new life, thereby reducing its environmental impact.
2. Results and Discussion
2.1. Plastic Waste Characterization
As reported by different papers, the composition of plastic solid waste is complex, and also contains paper waste [26], which is derived from poor collecting and recycling management, or from the nature of the plastic packaging (many products use a combination of paper and plastic in their packaging) whose components are difficult to separate and recycle. The plastic waste sample, mainly constituted of PE and PP with a fraction of paper waste, has been characterized using thermogravimetric analysis coupled with mass spectrometry in order to evaluate the temperature range at which the plastics experience weight loss under pyrolysis conditions. The results of the TGA are reported in Figure 2a. The analysis in an inert atmosphere has shown that under pyrolysis conditions, the plastics degradation occurs in the range of 120–600 °C with a maximum weight loss at 430 °C, corresponding to the pyrolysis of 90% of the sample. No ash content is displayed at the end of the analysis. The results are coherent with the characterization made by Xuan et al. [27], who reported the peak temperatures for PE and PP at 450 °C and 430 °C, respectively. Among the gases generated from the degradation, methane, as well as hydrocarbons with two, three, and four carbon atoms (C2–C4) and aromatics, were observed, as reported in Figure 2b, which shows the intensity of the signals revealed by mass spectrometry coupled with the thermal balance.
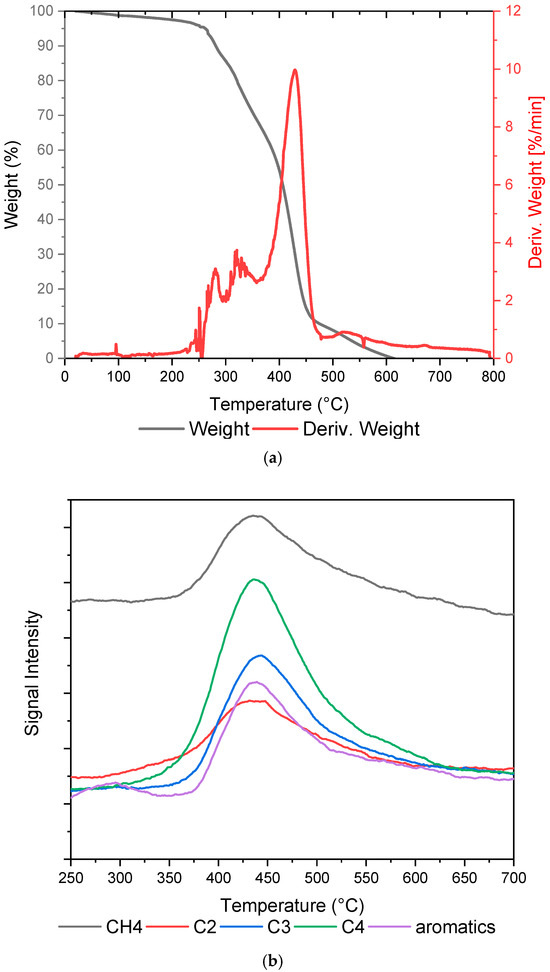
Figure 2.
Results of the (a) TGA and (b) MS performed on the plastic waste.
2.2. Catalyst Characterization
2.2.1. Porosity and Pore Size Distribution
The Hg intrusion porosimetry analysis (Figure 3) has shown that the samples have a micropores and mesopores distribution. By analyzing the experimental data in more detail, it is possible to evidence that the bare foam (blue curve) has a porous distribution centered at 1 μm, while smaller pores of 0.01 μm result from the addition of washcoat and active species (red curve). Moreover, the curves relevant to specific volume evidence the presence of smaller pores in the catalytic sample, as proved by the increasing slope of the left part of the dotted red curve.
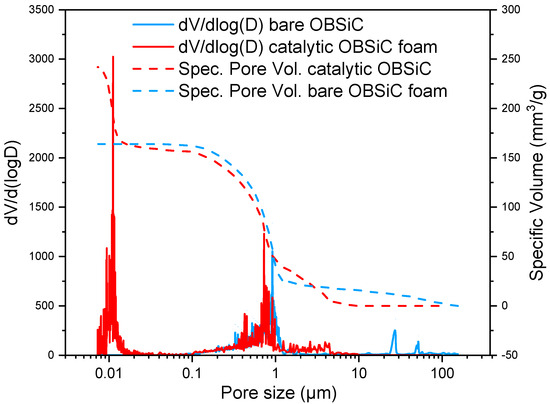
Figure 3.
Results of the Hg intrusion porosimetry analysis.
2.2.2. Textural Properties
Figure 4a,b show the isotherms resulting from the N2 physisorption tests on the bare and Ni-catalyzed OBSiC foams. According to the IUPAC classification, these isotherms are IV(a) type ones, which are typical of mesoporous materials, with a hysteresis of type H2b indicative of pore-blocking affected desorption [28,29]. When comparing the shapes and intensity of the two curves, it is evident that the catalyzed foam not only has an increased mesoporous structure and pore volume (which goes from 0.002 mL∙g−1 for the bare foam to 0.062 mL∙g−1 for the catalyzed one), but also an increased microporous fraction as the height of the curve at lower values of relative P/P0 increases from 0.1 to 3.4 cc∙g−1. Moreover, the BET method has been used for the evaluation of the Specific Superficial Area (SSA), which ranges from 0.8 to 21.5 m2∙g−1 for the bare and final catalytic foam, respectively. This significant increase highlights the positive impact of the deposition of active phases, which was found to be homogeneously distributed on the surface of the carrier, as illustrated in Figure 5, which also shows the results of the SEM-EXD analysis.
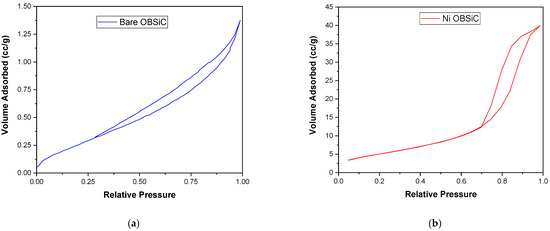
Figure 4.
N2 at 77K adsorption–desorption isotherms for (a) the bare OBSiC foam and (b) the catalytic foam.
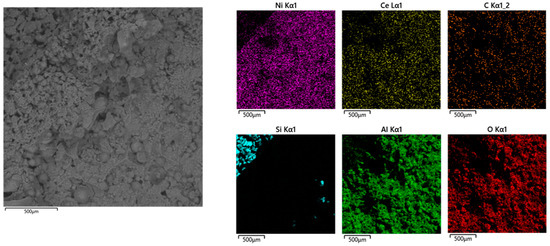
Figure 5.
SEM-EDX analysis results for the catalytic OBSiC foam.
The results of the adherence test are reported in Figure 6. The data show that the maximum weight loss has been maintained below 10% (the value for the last cycle is approximately 1.83%), thus showing that the final catalyst has excellent resistance to mechanical stress and good coating adhesion.
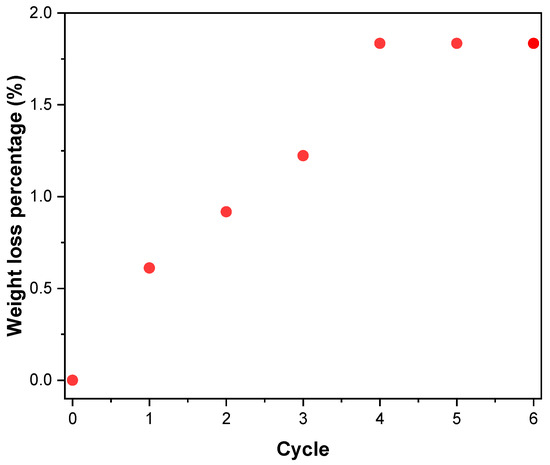
Figure 6.
Results of the ultrasound adherence test.
The weight loss caused by the ultrasonic cleaning is calculated with Equation (13), where the weight of the carriers was not considered.
Lastly, CO2-TPD characterization has been used to evaluate the acidity/basicity properties of fresh and used catalysts. The results are reported in Figure 7. Ewald et al. [30] reported that Ni/γAl2O3 catalysts can show three different peaks: peak in the range 50–250 °C is related to the weakly adsorbed species; peak in the range 250–700 °C is related to species on medium basic sites; peak in the range 700–900 °C is related to strongly adsorbed species. The graph of the fresh catalyst shows three different CO2 desorption peaks, the first at 95 °C attributed to the weak basic sites, the second at approximately 480 °C and the third at 630 °C attributed to the medium basic sites, proving a good interaction between active species and support. The spent catalyst shows a peak at 200 °C and the peak at high temperatures shifts at approximately 500 °C. Moreover, the intensity of the peaks in the spent catalyst is lower, suggesting a decreasing acidity behavior.
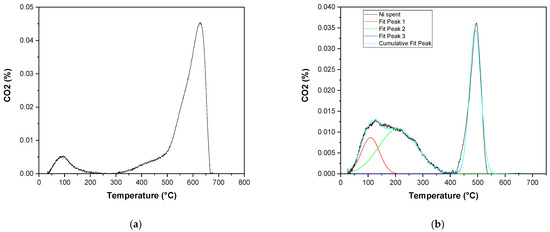
Figure 7.
CO2-TPD curves for the (a) fresh and (b) spent catalyst.
Table 1 summarizes the results of the CO2-TPD in terms of the maximum temperature of each deconvoluted peak and its integrated area (μmol∙g−1 CO2 per gram of sample) as well as the total basicity. These results clearly indicate the decreased ability of the spent catalyst to adsorb CO2 on its surface at high temperatures, while an enhanced capacity to interact with the catalyst surface at lower temperatures. The fresh catalyst is able to adsorb CO2 on strong basic sites at elevated temperatures and can lead to higher reaction rates and prevent carbon deposition.

Table 1.
Quantitative CO2-TPD results.
2.3. Experimental Test Results
The plastic waste has been converted under different conditions to better understand the role of the catalyst and the hydrogen in the reactant stream.
The catalyst was activated by a Temperature Programmed Reduction (TPR), feeding to the reactor a reducing stream consisting of 5 vol% H2 in Ar with a total flowrate of 500 NmL·min−1 and raising the temperature from room temperature up to 900 °C with a heating rate of 10 °C min−1. No plastic waste was introduced into the reactor during the catalyst activation to avoid pyrolysis during the heating process.
The pyrolysis tests conducted in an inert atmosphere provided insights into the decomposition process of mixed plastics that make up the plastic waste. Subsequently, a structured catalyst was employed to assess the potential for enhancing methane yield without introducing additional reactants under inert conditions. Furthermore, the inclusion of hydrogen in the feed stream enabled a deeper understanding of its role in both catalytic and non-catalytic hydrogasification tests.
The results are discussed in the next section and have been used to understand a global reaction mechanism for the degradation of mixed plastics. The reaction system is indeed complex, and a first qualitative analysis has been carried out.
The composition of the gaseous stream produced when the plastic waste is heated in an inert atmosphere (pyrolysis) is reported in Figure 8a. First, the gases released from the solid matrix are hydrogen and carbon dioxide, beginning at approximately 200 °C. As the temperature rises, carbon monoxide, methane, and hydrocarbons containing 2 (C2), 3 (C3), and 4 (C4) carbon atoms are subsequently emitted. The decomposition of the solid mainly generates hydrogen, which can facilitate the hydrogenation of heavy hydrocarbons into short-chain gases, such as methane.
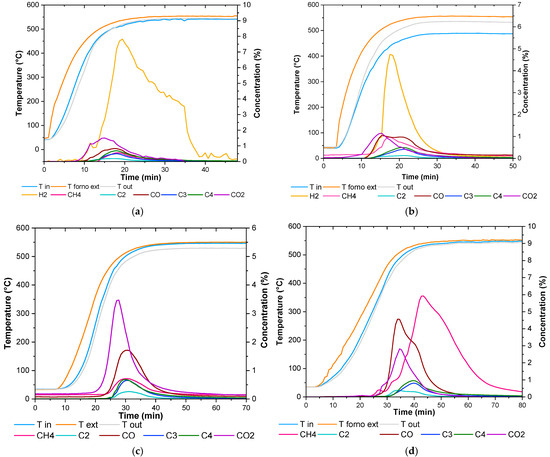
Figure 8.
Results of experimental test under (a) pyrolysis, (b) catalytic pyrolysis, (c) hydrogasification, and (d) catalytic hydrogasification condition.
The introduction of the catalyst (Figure 8b) leads to increased production of hydrogen and carbon monoxide, while the trends for C2, C3, and C4 hydrocarbons remain consistent with those observed in the previous non catalytic pyrolysis test. No clear evidence is provided regarding the catalyst’s role in the hydrogenation of C2, C3, and C4 into methane. The effect of hydrogen can be observed in Figure 8c, in which the data relevant to the non-catalytic hydrogasification process have been reported: an increased formation of both carbon dioxide and carbon monoxide occurred.
Finally, catalytic hydrogasification (Figure 8d) results in an increased formation of methane, although hydrocarbons with a higher number of carbon atoms are still present. This can be attributed to the limited contact time between the gaseous volatiles and the catalyst, which is insufficient for the complete breakdown of hydrocarbon molecules into smaller ones. Future work will be dedicated to investigating this hypothesis and elucidating the complete reaction mechanism [27,31].
The evaluation of methane selectivity and other gaseous species was conducted to better assess the potential of catalyzed hydrogasification for methane production.
The selectivity is calculated as:
where
As shown in Figure 9, methane selectivity reached approximately 59% in the case of Ni-catalyzed hydrogasification, a significantly higher value compared to the other tests (6.7% for pyrolysis, 13.7% for catalyzed pyrolysis, and 7.8% for non-catalytic hydrogasification). These findings highlight the crucial role of the catalyst in steering the reaction pathway, as CO2 remains the dominant product in both non-catalytic pyrolysis and hydrogasification.
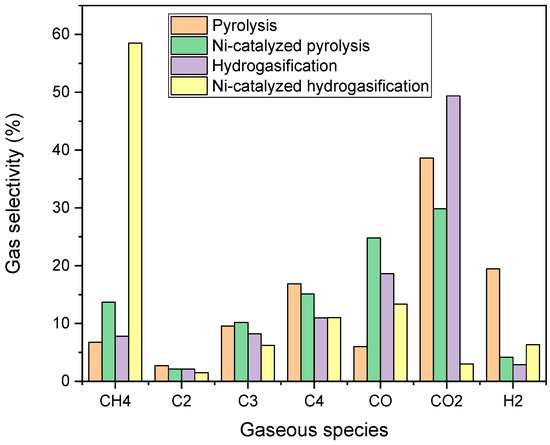
Figure 9.
Gas Selectivity in the experimental test.
Gaseous species are not the only products of the reaction; solid and liquid residues can also be recovered from the plastic waste conversion. The liquid residue is collected in a trap located in the cooling bath after the reactor, while the solid residue, primarily ash and char, is retrieved directly from the reactor. The weights and corresponding percentages of these residues are provided in Table 2. These values demonstrate that the addition of the catalyst significantly improved plastic waste gasification. Furthermore, the catalyst facilitates the gasification of the liquid residue, driving the conversion of plastic waste into solid and gaseous products. Specifically, the liquid residue decreased to 5.50% in the case of Ni-catalyzed hydrogasification, while gas production rose to 76.45%.

Table 2.
Amount of the liquid and solid residues compared to the gaseous fraction.
2.4. Reaction Mechanism
The reported curves align with the reaction mechanisms described in the literature, where polymeric macromolecules undergo a series of dissociation steps culminating in methane formation. As reported by Xuan et al. [27], during the pyrolysis process the polymer dissociation happens up to the polymer monomer and then, the monomer converts to gas molecule. According to Hong et al. [31], the PE dissociation begins with C-C bond breaking in a random position. A break in a different C-C bond results in some shorter chains. Then, after the initial C-C bond fracture of the polymer, a large number of monomers appear by the terminal dissociation. Finally, intramolecular and intermolecular dehydrogenation of the monomer occurs (with the highest energy barrier), and gas molecules are generated.
When a Ni-catalyst is added to the system, the methane formation is boosted up. Nickel is known to have an intermediate affinity for hydrogen and carbon, allowing it to effectively adsorb both species on its surface. This adsorption facilitates the breaking of existing bonds (C=O) and the formation of new bonds (C-H), thus promoting the hydrogenation reaction towards methane. Thus, the Ni-catalyzed catalyst was expected to give good methane selectivity in the process. Moreover, γ-alumina has a high surface area, which allows for good dispersion of nickel particles, resulting in a greater number of active sites and, consequently, greater catalytic activity; Lewis acidic sites, which can interact with basic species such as CO2, facilitating the adsorption and activation of CO2 on the catalyst surface [32,33]. It is known that the medium basic sites of the catalyst are the more active for the hydrogenation to methane [34,35]. The CO2-TPD curve for the fresh catalyst has the largest peak in the medium basic sites range, proving the good activity of the catalyst.
This preliminary study will serve as a foundation for more in-depth investigations aimed at precisely determining the reaction mechanism.
3. Materials and Methods
3.1. Plastic Waste Matrix
The plastic waste (Figure 10) has been provided by ENEA Centro Ricerche Casaccia, Rome, Italy. The sample mainly contains PE (polyethylene) and PP (polypropylene). The elemental analysis, provided by ENEA, is reported in Table 3.

Figure 10.
Plastic waste used in the experimental test supplied by ENEA.

Table 3.
Elemental Analysis of the Plastic Waste Sample.
A thermogravimetric analysis coupled with a mass spectrometry analysis has been performed to evaluate the weight loss and species obtained from the depolymerization of the plastics. The analysis was performed using a Q600 thermogravimetric balance coupled with a mass spectrometer (TG-MS) (TA instruments, New Castle, DE, USA) to analyze the gases released during heating. This setup enables the determination of both the temperature at which mass loss occurs, and the molecular structures involved. The analysis was carried out in a nitrogen atmosphere (50 mL·min−1), with a heating ramp of 10 °C·min−1 up to 800 °C, followed by an isothermal hold at 800 °C for 1 h.
3.2. Carrier for the Structured Catalyst
The OB (oxygen-bonded) SiC foams used for the structured catalyst have a diameter of 6 cm and a height of 2 cm. The choice of using a structured catalyst is based on several key advantages. These include a high gas/solid contact surface per unit volume, which improves reaction efficiency, and low-pressure drops, even at high flow rates, which supports smoother operation. Additionally, the structured catalyst minimizes mass transfer resistance, enhancing the overall reaction process.
The material used for the carrier has excellent thermal conductivity, helping to reduce thermal gradients within the foam structure. This leads to improved thermal control, ensuring a more uniform temperature distribution during catalytic processes, which is crucial for maintaining reaction efficiency and stability.
3.3. Preparation of the Structured Catalyst
The preparation procedure (Figure 11) for the structured catalysts involved two main phases, dip-coating and wet impregnation, to deposit the washcoat and active species, respectively.
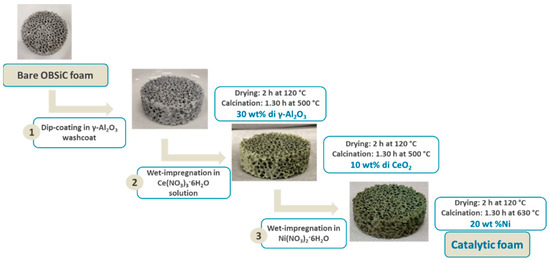
Figure 11.
Preparation of the structured catalyst.
The washcoat slurry was made by dissolving 1 wt% of methyl cellulose in water. Then, 20 wt% of an alumina/pseudoboehmite mixture (ratio 10:3) was dispersed into this solution, with the pH adjusted to 3 using nitric acid (HNO3) and stirred vigorously. The carriers were dipped into the slurry, and the excess was removed by centrifuging at 1500 rpm for 15 min.
Following this, the coated foams were heat-treated in a muffle furnace at 120 °C for 2 h, with a temperature ramp of 10 °C·min−1, followed by further heating at 400 °C for 1 h. This process was repeated until the desired washcoat loading (30 wt% relative to the carrier weight) was achieved.
The ceria promoter was added by multiple wet impregnation steps using an aqueous Ce(NO3)3 solution (precursor: Ce(NO3)3·6H3O), alternating with heat treatments at 120 °C for 2 h and at 450 °C for 1 h, until a 10 wt% loading (relative to the washcoat) was reached. Finally, 20 wt% of nickel (relative to the weight of the previous coatings) was deposited by similar wet impregnation steps using Ni(NO3)2 solution, followed by heating at 120 °C for 2 h and then at 850 °C for 1 h. Before the tests, the catalyst has been activated using a stream of H2 (5 vol%) in Ar to convert the nickel oxide (NiO) in the active species, which is metallic nickel.
3.4. Characterization Techniques
Different physical–chemical analysis techniques were used to characterize the catalysts. The adherence of the catalytic layer to the carriers was evaluated by performing an ultrasound adherence test with an ultrasonic bath CP104 (EIA S.p.A.): the samples, immersed in a beaker containing 25 mL of petroleum ether, were exposed to different cycles of 5 min, applying the 60% of rated power at 25 °C. Each ultrasonic cycle was alternated with a step of drying at 120 °C for 1 h, after which the samples were cooled, and their weight loss was evaluated.
The porosity and pore size distribution were evaluated by Hg penetration technique, with a “PASCAL 140” and “PASCAL 240” (Thermo Fisher Scientific, Como, Italy). N2 physisorption at −196 °C, by means of Novatouch LX4 (Anton Paar Italia S.r.l, Rivoli, Italy), was used for determining the adsorption–desorption isotherms, the specific surface areas (SSA) applying Brunauer–Emmett–Teller (BET) method, and the mesopores characterization through Barrett–Joyner–Halenda (BJH) method. The dispersion of the active species on the carrier was checked with a Scanning Electron Microscope (SEM) Philips Mod. XL30 coupled to an Energy Dispersive X-Ray analyzer (EDX mod. INCA Energy 350, Oxford Instruments, Abingdon, UK).
Temperature Programmed Reduction (TPR) analysis was performed by feeding to the reactor a reducing stream consisting of 5% H2 in Ar with a total flowrate of 500 NmL·min−1 and raising the temperature from room temperature up to 900 °C with a heating rate of 10 °C·min−1. The catalyst acidity and basicity were measured by CO2 temperature programmed desorption (CO2-TPD). After the H2-TPR test, the catalysts underwent an aging process under a 10 vol% of H2 in an Ar gas mixture (flow rate of 100 NmL·min−1·gcat −1) at 580 °C for 5 h, to simulate the catalysts condition during the reaction experiments. The samples were then treated in 10 vol% of CO2 in an Ar gas mixture at 50 °C for 1 h followed by an Ar purge. The TPD was performed by flowing Ar in the temperature range of 25–680 °C (heating ramp of 10 °C min−1).
3.5. Experimental Plant and Test
The experimental plant used for testing the catalysts is reported in Figure 12a and has three different sections.
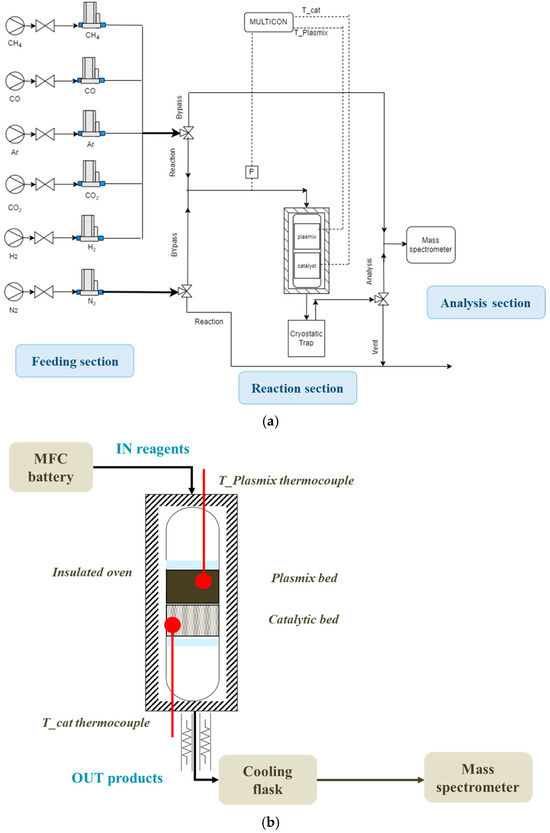
Figure 12.
Scheme of (a) experimental setup and (b) reaction section.
- Feeding section: A battery of mass flow controllers (MFCs) is used for the regulation of flow rates of the different gases used. In particular, the gases are H2 and Ar for the reaction and CO and CO2 for the mass spectrometer calibration; N2 is used for the cooling step of the reactor.
- Reaction section: The reactor has a diameter of 1.5 cm and a length of 40 cm and is arranged in an oven which heats up to the reaction temperature. Two K-type thermocouples, located one on the plastic waste bed and one on the catalyst bed, are used to measure the temperatures; a pressure transducer measures the pressure upstream of the reactor. The gaseous products pass through a tube heated up to 200 °C and then undergo quick coaling in a flask dipped into a refrigerant liquid at −15 °C. This step allows for the condensation of the heavier hydrocarbons produced by the reaction. A detailed representation of this section is reported in Figure 12b.
- Analysis section: A mass spectrometer is used to analyze the gas composition of the products in continuous mode. Before each test, a gaseous stream with known composition is sent to calibrate the instrument, allowing the evaluation of the response factors; once this operation has been conducted, it is possible to start the test.
The tests were carried out under the conditions specified in Table 4. The non-catalyzed pyrolysis and hydrogasification tests involved heating 2 g of plastic waste with a heating ramp of 40 °C·min⁻1 in an argon or hydrogen-enriched (80 vol% in Ar) atmosphere, respectively. The catalyzed pyrolysis and hydrogasification were performed using a catalyst/plastic waste ratio of 2/3 and a space velocity of 9000 NmLgas·h−1·gcat−1.

Table 4.
Operative conditions in the experimental test.
4. Conclusions
In this study, the catalytic hydrogasification of plastic waste was investigated and compared with other thermal conversion methods. The plastic waste provided by ENEA was analyzed using thermogravimetric analysis to study its thermal degradation behavior. The results revealed that degradation occurs within the temperature range of 120–600 °C, with the maximum weight loss observed at 430 °C. At this temperature, 90% of the sample undergoes pyrolysis, releasing methane, hydrocarbons with two, three, and four carbon atoms (C2–C4), as well as aromatic compounds. For the catalytic tests, a structured catalyst was prepared using an OBSiC open-cell foam, which was coated with an γ-Al2O3-based washcoat, followed by a CeO2 layer as a promoter and a Ni layer as the active catalytic phase. The prepared catalyst was characterized and tested in an experimental setup under pyrolysis and hydrogasification conditions. The results demonstrate that Ni-catalyzed hydrogasification is a promising method for converting plastics into methane, achieving a methane selectivity of approximately 59% (compared to 6.7%, 13.7%, and 7.8% observed during pyrolysis, catalyzed pyrolysis, and non-catalytic hydrogasification tests, respectively) while also facilitating the gasification of the liquid residue generated during pyrolysis. However, the gas stream also contains heavier hydrocarbons, including ethane, ethylene, propane, propylene, and butane, which can be further converted into methane.
Future work will focus on maximizing gas yield by optimizing reactor design and operating conditions. Additionally, further research will be conducted to identify the most effective catalytic formulation for enhancing methane selectivity.
Author Contributions
Conceptualization, E.S., E.M. and V.P.; methodology, E.S., E.M. and V.P.; software, E.S., E.M. and V.P.; validation, E.S., E.M. and V.P.; formal analysis, E.S., E.M. and V.P.; investigation, E.S.; resources, V.P.; data curation, E.S., E.M. and V.P.; writing—original draft preparation, E.S.; writing—review and editing, E.S. and E.M.; visualization, E.S.; supervision, E.M. and V.P.; project administration, E.S., E.M., A.G. and V.P.; funding acquisition, V.P. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union—NextGenerationEU from the Italian Ministry of Environment and Energy Security, POR H2 AdP MEES/ENEA with involvement of CNR and RSE, PNRR—Mission 2, Component 2, Investment 3.5 "Ricerca e sviluppo sull’idrogeno", CUP: I83C22001170006.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors wish to thankfully acknowledge Roberto Clerici (Sasol Performance Chemicals) for providing alumina used in this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Weldekidan, H.; Strezov, V.; Town, G. Review of solar energy for biofuel extraction. Renew. Sustain. Energy Rev. 2018, 88, 184–192. [Google Scholar] [CrossRef]
- Morales, S.; Miranda, R.; Bustos, D.; Cazares, T.; Tran, H. Solar biomass pyrolysis for the production of bio-fuels and chemical commodities. J. Anal. Appl. Pyrolysis 2014, 109, 65–78. [Google Scholar] [CrossRef]
- Meloni, E.; Iervolino, G.; Ruocco, C.; Renda, S.; Festa, G.; Martino, M.; Palma, V. Electrified Hydrogen Production from Methane for PEM Fuel Cells Feeding: A Review. Energies 2022, 15, 3588. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Iervolino, G.; Ruocco, C.; Renda, S.; Festa, G.; Palma, V. The Route from Green H2 Production through Bioethanol Reforming to CO2 Catalytic Conversion: A Review. Energies 2022, 15, 2383. [Google Scholar] [CrossRef]
- Lee, K.; Jing, Y.; Wang, J.; Yan, N. A unified view on catalytic conversion of biomass and waste plastics. Nat. Res. 2022, 6, 635–652. [Google Scholar] [CrossRef]
- Plastics-the Facts 2019 An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://plasticseurope.org/wp-content/uploads/2021/10/2019-Plastics-the-facts.pdf (accessed on 29 November 2024).
- Wang, Z.; Burra, K.G.; Lei, T.; Gupta, A.K. Co-pyrolysis of waste plastic and solid biomass for synergistic production of biofuels and chemicals-A review. Prog. Energy Combust. Sci. 2021, 84, 100899. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Tosti, S.; Spazzafumo, G.; Capobianco, D.; Buceti, G.; Pozio, A.; Bartucca, S. EU scenarios of renewable coal hydro-gasification for SNG production. Sustain. Energy Technol. Assess. 2016, 16, 43–52. [Google Scholar] [CrossRef]
- Yan, L.; He, B.; Ma, L.; Pei, X.; Wang, C. Chemical equilibrium model and prediction for coal hydrogasification. Int. J. Chem. React. Eng. 2014, 12, 103–112. [Google Scholar] [CrossRef]
- Barbuzza, E.; Buceti, G.; Pozio, A.; Santarelli, M.; Tosti, S. Gasification of wood biomass with renewable hydrogen for the production of synthetic natural gas. Fuel 2019, 242, 520–531. [Google Scholar] [CrossRef]
- Melaina, M.W.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues. 2013. Available online: http://www.osti.gov/bridge (accessed on 29 November 2024).
- Murakami, K.; Arai, M.; Shirai, M. Hydrogasification of Loy Yang Brown Coal by Ion-Exchanged Nickel Species. Energy Fuels 2000, 14, 1240–1244. [Google Scholar] [CrossRef]
- Haga, T.; Ozaki, J.-I.; Suzuki, K.; Nishiyama, Y. Role of MgO and CaO Promoters in Ni-Catalyzed Hydrogenation Reactions of CO and Carbon. J. Catal. 1992, 134, 107–117. [Google Scholar] [CrossRef]
- Haga, T.; Nishiyama, Y. Promotion of iron-group catalyst by calcium salts in hydrogasification of carbons at elevated pressures. Ind. Eng. Chem. Res. 1987, 26, 1202–1206. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, H.; Bi, J.; Qu, X.; Yan, S.; Zhang, J.; Zhang, J. The evolution of Fe and Fe-Ca catalysts during char catalytic hydrogasification. Fuel 2019, 257, 116040. [Google Scholar] [CrossRef]
- Yan, S.; Qu, X.; Xia, Z.; Chen, C.; Bi, J. Effect of experimental variables on coal catalytic hydrogasification in a pressurized fluidized bed. Fuel 2022, 307, 121761. [Google Scholar] [CrossRef]
- Qu, X.; Wang, Q.F.; Yan, S.; Feng, J.; Zhang, J.S.; Zhang, R.; Bi, J.C. The behavior of the different catalysts for model carbon hydrogasification. J. Fuel Chem. Technol. 2022, 50, 1–10. [Google Scholar] [CrossRef]
- Qu, X.; Yan, S.; Yan, X.; Zhang, J.; Zheng, Q.; An, Y. Role of cobalt and calcium on coal catalytic hydrogasification in a pressurized fluidized bed. Fuel 2019, 239, 347–356. [Google Scholar] [CrossRef]
- Yan, S.; Bi, J.; Qu, X. The behavior of catalysts in hydrogasification of sub-bituminous coal in pressured fluidized bed. Appl. Energy 2017, 206, 401–412. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, J.; Yan, X.; Pan, D.; Ren, H.; Qu, X. Catalytic coal hydrogasification by cobalt-calcium catalyst in a pressurized fluidized bed: Role of hydropyrolysis and catalysis process. J. Anal. Appl. Pyrolysis 2018, 135, 251–259. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, Z.; Liu, Q. Synergetic catalysis of calcium oxide and iron in hydrogasification of char. Energy Fuels 2017, 31, 198–204. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.; Bi, J.; Zhang, R.; Qu, X.; Zhang, J.; Zhang, J. Investigating the Cu-based catalysts for char catalytic hydrogasification and its recovery. Fuel 2021, 294, 120567. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, F.; Bi, J.; Qu, X.; Yan, S.; Zhang, J.; Zhang, J. Study of Cu-Ni-Ca Composite Catalysts in Catalytic Hydrogasification of Char. Energy Fuels 2019, 33, 9661–9670. [Google Scholar] [CrossRef]
- Skodras, G.; Panagiotidou, S.; Kokorotsikos, P.; Serafidou, M. Potassium catalyzed hydrogasification of low-rank coal for synthetic natural gas production. Open Chem. 2016, 14, 92–102. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, N.; Lv, Y.; Cheng, Y.; Wang, Y.; Liu, Y.; Cobb, K.; Chen, P.; Lei, H.; Ruan, R. Pyrolysis technology for plastic waste recycling: A state-of-the-art review. Prog. Energy Combust. Sci. 2022, 93, 101021. [Google Scholar] [CrossRef]
- Xuan, W.; Yan, S.; Dong, Y. Exploration of Pyrolysis Behaviors of Waste Plastics (Polypropylene Plastic/Polyethylene Plastic/Polystyrene Plastic): Macro-Thermal Kinetics and Micro-Pyrolysis Mechanism. Processes 2023, 11, 2764. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Guillet-Nicolas, R.; García-Martínez, J.; Thommes, M. Recent advances in the textural characterization of hierarchically structured nanoporous materials. R. Soc. Chem. 2017, 46, 389–414. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Ewald, S.; Hinrichsen, O. On the interaction of CO2 with Ni-Al catalysts. Appl. Catal. A Gen. 2019, 580, 71–80. [Google Scholar] [CrossRef]
- Hong, D.; Li, P.; Si, T.; Guo, X. ReaxFF simulations of the synergistic effect mechanisms during co-pyrolysis of coal and polyethylene/polystyrene. Energy 2021, 218, 119553. [Google Scholar] [CrossRef]
- Albeladi, D.; Alsulami, Q.A.; Narasimharao, K. Recent Progress in Nickel and Silica Containing Catalysts for CO2 Hydrogenation to CH4. Catalysts 2023, 13, 1104. [Google Scholar] [CrossRef]
- Lv, C.; Xu, L.; Chen, M.; Cui, Y.; Wen, X.; Li, Y.; Wu, C.E.; Yang, B.; Miao, Z.; Hu, X.; et al. Recent Progresses in Constructing the Highly Efficient Ni Based Catalysts with Advanced Low-Temperature Activity Toward CO2 Methanation. Front. Chem. 2020, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Peng, P.; Sun, T.; Wang, S.; Wang, S. Insight into the reaction route of CO2 methanation: Promotion effect of medium basic sites. Catal. Commun. 2014, 45, 74–78. [Google Scholar] [CrossRef]
- Zhao, K.; Li, Z.; Bian, L. CO2 methanation and co-methanation of CO and CO2 over Mn-promoted Ni/Al2O3 catalysts. Front. Chem. Sci. Eng. 2016, 10, 273–280. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).