Preparation of Novel ACE Inhibitory Peptides from Skimmed Goat Milk Hydrolyzed by Multi-Enzymes: Process Optimization, Purification, and Identification
Abstract
1. Introduction
2. Results
2.1. Response Surface Optimization of Multi-Enzymatic Hydrolysis of SGM
2.2. ACE Inhibitory Activity of SGM Fractions
2.3. Molecular Weight Distribution of ACE Inhibitory Peptides
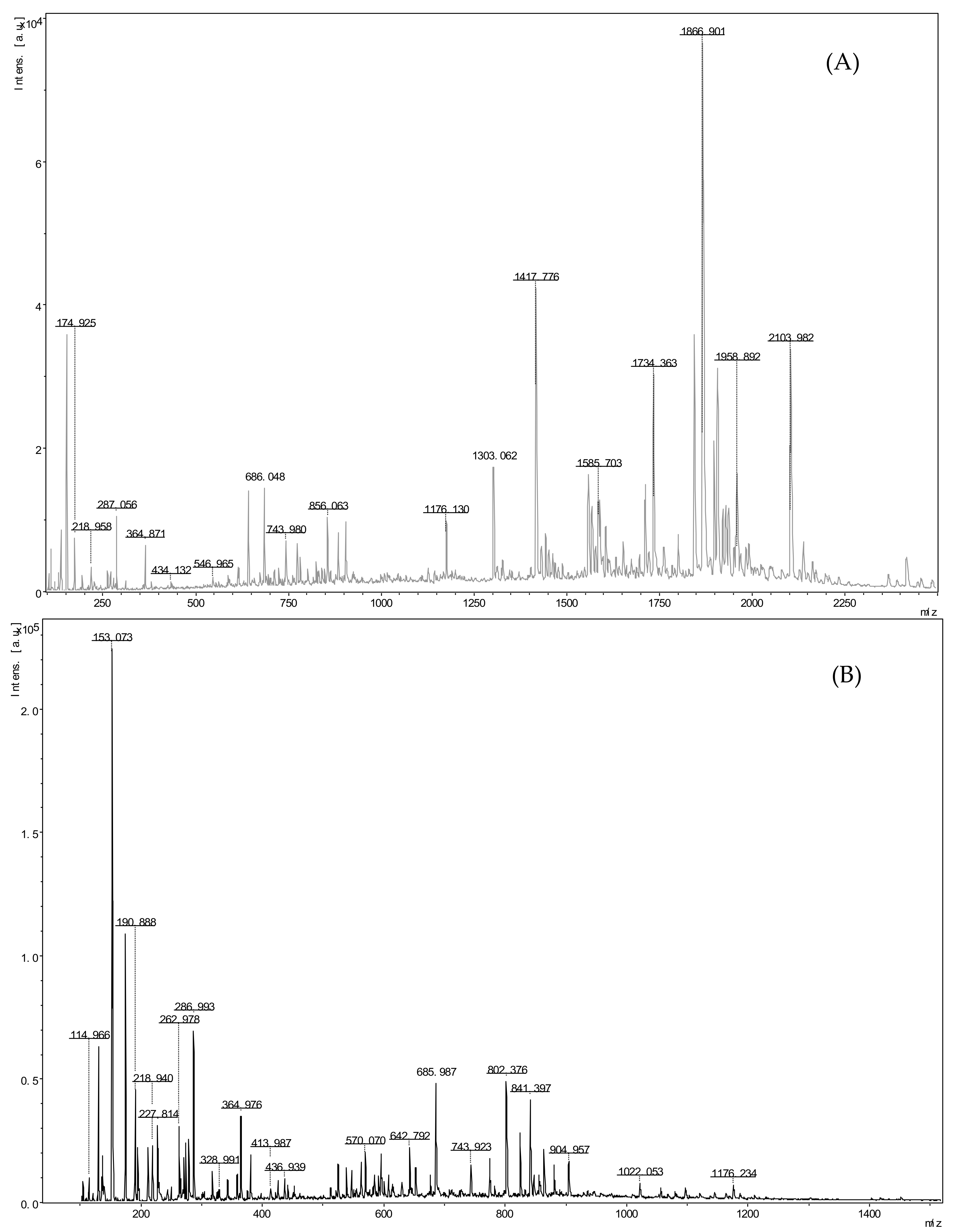
2.4. Identification of ACE Inhibitory Peptide
3. Materials and Methods
3.1. Materials and Reagents
3.2. Methods
3.2.1. Preparation of ACE Inhibitory Peptide in SGM
3.2.2. Response Surface Experimental Design
3.2.3. Isolation and Purification of ACE Inhibitory Peptide in SGM
3.2.4. Identification of ACE Inhibitory Peptides
3.2.5. Determination of Proteolysis Degree
3.2.6. Determination of ACE Inhibitory Rate
3.2.7. Determination of Peptides Content
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ding, Q.; Sheikh, A.R.; Chen, Q.; Hu, Y.; Hu, N.; Su, X.; Luo, L.; Ma, H.; He, R. Understanding the Mechanism for the Structure-Activity Relationship of Food-Derived ACEI Peptides. Food Rev. Int. 2021, 39, 1751–1769. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates peptides from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, F.; Wu, N.; Shuang, Q. Research progress of ACE inhibiting peptide. China Brew. 2020, 39, 6–11. [Google Scholar]
- Olalere, O.A.; Yap, P.-G.; Gan, C.-Y. Comprehensive review on some food-derived bioactive peptides with anti-hypertension therapeutic potential for angiotensin-converting enzyme (ACE) inhibition. J. Proteins Proteom. 2023, 14, 129–161. [Google Scholar] [CrossRef]
- Hui, L.; Ying, L.; Ke, D.; Tao, H. Preparation and Function Assessment of Antihypertensive Peptides from Food. Food Nutr. China 2015, 21, 26–30. [Google Scholar]
- Xu, Z.; Wu, C.; Sun-Waterhouse, D.; Zhao, T.; Waterhouse, G.I.N.; Zhao, M.; Su, G. Identification of post-digestion angiotensin-I converting enzyme ACE inhibitory peptides from soybean protein Isolate: Their production conditions and in silico molecular docking with ACE. Food Chem. 2021, 345, 128855. [Google Scholar] [CrossRef]
- Shao, B.; Huang, X.; Xu, M.; Cheng, D.; Li, X.; Li, M. Peptides isolated from black soybean synergistically inhibit the activity of angiotensin converting enzyme ACE. J. Funct. Foods 2023, 106, 105604. [Google Scholar] [CrossRef]
- Li, J.; Hu, H.; Chen, X.; Zhu, H.; Zhang, W.; Tai, Z.; Yu, X.; He, Q. A novel ACE inhibitory peptide from Douchi hydrolysate: Stability, inhibition mechanism, and antihypertensive potential in spontaneously hypertensive rats. Food Chem. 2024, 460 Pt 3, 140734. [Google Scholar] [CrossRef]
- Cao, J.; Xiang, B.; Dou, B.; Hu, J.; Zhang, L.; Kang, X.; Lyu, M.; Wang, S. Novel Angiotensin-Converting Enzyme-Inhibitory Peptides Obtained from Trichiurus lepturus: Preparation, Identification and Potential Antihypertensive Mechanism. Biomolecules 2024, 14, 581. [Google Scholar] [CrossRef]
- Gisela, C.A.; Fidel, T.; Leticia, M. Effect of thermal pretreatment and gastrointestinal digestion on the bioactivity of dry-cured ham bone enzymatic hydrolyzates. Food Res. Int. 2024, 188, 114513. [Google Scholar]
- Wang, S.; Zhang, L.; Wang, H.; Liu, J.; Hu, Y.; Tu, Z. Angiotensin converting enzyme ACE inhibitory peptide from the tuna Thunnus thynnus muscle: Screening, interaction mechanism and stability. Int. J. Biol. Macromol. 2024, 279, 135469. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.H.; Jeanette, O.; Cristian, D.G.; Osman, A.; Hamad, E. Angiotensin I-converting enzyme inhibitory activity and antioxidant capacity of bioactive peptides derived from enzymatic hydrolysis of buffalo milk proteins. Int. Dairy. J. 2017, 66, 91–98. [Google Scholar]
- Ibrahim, H.R.; Ahmed, A.S.; Miyata, T. Novel angiotensin-converting enzyme inhibitory peptides from caseins and whey proteins of goat milk. J. Adv. Res. 2017, 8, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.A.; Mustafa, Ç. Comparative analysis of milk casein hydrolysates from cow, water buffalo, goat and sheep: Effects of flavourzyme and neutrase on formation of bioactive peptides. J. Food Meas. Charact. 2025, 19, 341–355. [Google Scholar]
- Wei, G.; Wang, T.; Li, Y.; He, R.; Huang, A.; Wang, X. Identification, structural characterization, and molecular dynamic simulation of ACE inhibitory peptides in whey hydrolysates from Chinese Rushan cheese by-product. Food Chem. X 2024, 21, 101211. [Google Scholar] [CrossRef]
- Liu, K.; Gao, Z.; Li, Q.; Zhang, H. Identification and mechanistic study of four novel ACE inhibitory peptides from maize germ protein hydrolysates. LWT 2023, 186, 115254. [Google Scholar] [CrossRef]
- Bougatef, H.; Sila, A.; Bougatef, A.; Martínez-Alvarez, O. Protein Hydrolysis as a way to Valorise Squid-Processing byproducts: Obtaining and identification of ACE, DPP-IV and PEP inhibitory peptides. Mar. Drugs 2024, 22, 156. [Google Scholar] [CrossRef]
- Pires, C.; Leitão, M.; Sapatinha, M.; Gonçalves, A.; Oliveira, H.; Nunes, M.L.; Teixeira, B.; Mendes, R.; Camacho, C.; Machado, M. Protein Hydrolysates from Salmon Heads and Cape Hake By-Products: Comparing Enzymatic Method with Subcritical Water Extraction on Bioactivity Properties. Foods 2024, 13, 15. [Google Scholar] [CrossRef]
- Dalaka, E.; Stefos, G.C.; Politis, I.; Theodorou, G. Evaluation of In Vitro Antihypertensive and Anti-Inflammatory Properties of Dairy By-Products. Appl. Sci. 2024, 14, 6885. [Google Scholar] [CrossRef]
- Tang, H.; Wang, C.; Cao, S.; Wang, F. Novel angiotensin I-converting enzyme ACE inhibitory peptides from walnut protein isolate: Separation, identification and molecular docking study. J. Food Biochem. 2022, 46, e14411. [Google Scholar] [CrossRef]
- Pan, D.; Cao, J.; Guo, H.; Zhao, B. Studies on purification and the molecular mechanism of a novel ACE inhibitory peptide from whey protein hydrolysate. Food Chem. 2012, 130, 121–126. [Google Scholar] [CrossRef]
- Rahimi, M.; Ghaffari, S.M.; Salami, M.; Mousavy, S.J.; Niasari-Naslaji, A.; Jahanbani, R.; Yousefinejad, S.; Khalesi, M.; Moosavi-Movahedi, A.A. ACE-inhibitory and radical scavenging activities of bioactive peptides obtained from camel milk casein hydrolysis with proteinase K. Dairy Sci. Technol. 2016, 96, 489–499. [Google Scholar] [CrossRef]
- Daraksha, I.; Singh, S.M.; Sameena, Z.; Shilpa, V.; Ashutosh; Sunita, M. In silico identification of antidiabetic and hypotensive potential bioactive peptides from the sheep milk proteins—A molecular docking study. J. Food Biochem. 2022, 46, e14137. [Google Scholar]
- Sandra, Z.B.; Uriel, S.V.J.; Antonio, C.L.G.; Luis, D.R.D.; Humberto, G.G.J. Hydrolysates from ultrafiltrated double–cream cheese whey: Enzymatic hydrolysis, antioxidant, and ACE–inhibitory activities and peptide characterization. J. Food Process. Preserv. 2021, 45, e15790. [Google Scholar]
- Singh, S.M.; Daraksha, I.; Shilpa, V.; Suman, K.; Sunita, M. In vitro biosafety and bioactivity assessment of the goat milk protein derived hydrolysates peptides. J. Food Saf. 2023, 43, e13061. [Google Scholar]
- Li, X.; Feng, C.; Hong, H.; Zhang, Y.; Luo, Z.; Wang, Q.; Luo, Y.; Tan, Y. Novel ACE inhibitory peptides derived from whey protein hydrolysates: Identification and molecular docking analysis. Food Biosci. 2022, 48, 101737. [Google Scholar] [CrossRef]
- Zapata, B.S.; Gil, G.J.; Stefano, S.; Tullia, T. Bioactivity and peptide profile of whey protein hydrolysates obtained from Colombian double-cream cheese production and their products after gastrointestinal digestion. LWT 2021, 145, 111334. [Google Scholar]
- Lin, K.; Zhang, L.; Han, X.; Meng, Z.; Zhang, J.; Wu, Y.; Cheng, D. Quantitative Structure-Activity Relationship Modeling Coupled with Molecular Docking Analysis in Screening of Angiotensin I-Converting Enzyme Inhibitory Peptides from Qula Casein Hydrolysates Obtained by Two-Enzyme Combination Hydrolysis. J. Agric. Food Chem. 2018, 66, 3221–3228. [Google Scholar] [CrossRef]
- Shanmugam, V.P.; Kapila, S.; Kemgang, T.S.; Reddi, S.; Kapila, R.; Muthukumar, S.; Rajesh, D. Isolation and Characterization of Angiotensin Converting Enzyme Inhibitory Peptide from Buffalo Casein. Int. J. Pept. Res. Ther. 2021, 27, 1481–1491. [Google Scholar] [CrossRef]
- Ugwu, C.P.; Abarshi, M.M.; Mada, S.B.; Sanusi, B.; Nzelibe, H.C. Camel and Horse Milk Casein Hydrolysates Exhibit Angiotensin Converting Enzyme Inhibitory and Antioxidative Effects In Vitro and In Silico. Int. J. Pept. Res. Ther. 2019, 25, 1595–1604. [Google Scholar] [CrossRef]
- Shu, G.; Huang, J.; Bao, C.; Meng, J.; Chen, H.; Cao, J. Effect of Different Proteases on the Degree of Hydrolysis and Angiotensin I-Converting Enzyme-Inhibitory Activity in Goat and Cow Milk. Biomolecules 2018, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Chen, H.; Chen, L.; Cao, J.; Meng, J. Comparison of ACE inhibitory activity in skimmed goat and cow milk hydrolyzed by alcalase, flavourzyme, neutral protease and proteinase K. Acta Univ. Cibiniensis Ser. E Food Technol. 2016, 20, 77–84. [Google Scholar] [CrossRef]
- Bao, C. Research on the Preparation of Ace Inhibitory Peptides from Skim Milk by the Multi-Enzymatic Hydrolysis. Master’s Thesis, Shaanxi University of Science and Technology, Xi’an, China, 2017. [Google Scholar]
- Zhao, Y.; Li, B.; Ma, J.; Dong, S.; Liu, Z.; Zeng, M. Purification and Synthesis of ACE Inhibitory Peptide from Acaudina molpadioidea Protein Hydrolysate. Chem. J. Chin. Univ. 2012, 33, 308–312. [Google Scholar]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Protein; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Lu, W.; Ren, G.; Song, J. Determination of Content of Peptides in Protein Hydrolysates. Food Sci. 2005, 26, 169–171. [Google Scholar]
- Gao, D.; Zhang, F.; Ma, Z.; Chen, S.; Ding, G.; Tian, X.; Feng, R. Isolation and identification of the angiotensin-I converting enzyme ACE inhibitory peptides derived from cottonseed protein: Optimization of hydrolysis conditions. Int. J. Food Prop. 2019, 22, 1296–1309. [Google Scholar] [CrossRef]
- Li, Y.; Yang, N.; Shi, F.; Ye, F.; Huang, J. Isolation and identification of angiotensin converting enzyme inhibitory peptides from Tartary buckwheat albumin Isolation and identification of ACE inhibitory peptides from Tartary buckwheat albumin. J. Sci. Food Agric. 2023, 103, 5019–5027. [Google Scholar] [CrossRef]
- Puspitojati, E.; Indrati, R.; Cahyanto, M.N.; Marsono, Y. Effect of fermentation time on the molecular weight distribution of ACE inhibitory peptide from jack bean tempe. IOP Conf. Ser. Earth Environ. Sci. 2023, 1177, 012026. [Google Scholar] [CrossRef]
- Shao, M.; Wu, H.; Wang, B.; Zhang, X.; Gao, X.; Jiang, M.; Su, R.; Shen, X. Identification and Characterization of Novel ACE Inhibitory and Antioxidant Peptides from Sardina pilchardus Hydrolysate. Foods 2023, 12, 2216. [Google Scholar] [CrossRef]
- Norhameemee, K.; Papassara, S.; Piroonporn, S.; Tanatorn, S.; Onrapak, R.; Kiattawee, C.; Aphichart, K. ACE inhibitory peptides derived from de-fatted lemon basil seeds: Optimization, purification, identification, structure-activity relationship and molecular docking analysis. Food Funct. 2020, 11, 8161–8178. [Google Scholar]
- Liu, L. Hydrolysis of Whey Protein and ACEI Peptide of Hudrolysate. Master’s Thesis, Tianjin University of Science and Technology, Tianjin, China, 2005. [Google Scholar]
- Li, M.; Li, S.; Zhang, Y.; Li, X. Optimization of ACE inhibitory peptides from black soybean by microwave-assisted enzymatic method and study on its stability. LWT 2018, 98, 358–365. [Google Scholar] [CrossRef]
- Cheung, H.S.; Wang, F.L.; Ondetti, M.A.; Sabo, E.F.; Cushman, D.W. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J. Biol. Chem. 1980, 255, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive Peptides from Muscle Sources: Meat and Fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Safavi, M.; Hadizadeh, M. In vitro and in silico studies of novel synthetic ACE-inhibitory peptides derived from Saccharomyces cerevisiae protein hydrolysate. Bioorg. Chem. 2019, 87, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Choung, S.; Cao, G.; Lee, K.W.; Choi, Y.J. In silico investigation of action mechanism of four novel angiotensin-I converting enzyme inhibitory peptides modified with Trp. J. Funct. Foods 2015, 17, 632–639. [Google Scholar] [CrossRef]
- Li, J.; Hu, H.; Chen, F.; Yang, C.; Yang, W.; Pan, Y.; Yu, X.; He, Q. Characterization, mechanisms, structure–activity relationships, and antihypertensive effects of ACE inhibitory peptides: Rapid screening from sufu hydrolysate. Food Funct. 2024, 15, 9224–9234. [Google Scholar] [CrossRef]
- Weber, M.; Burgos, R.; Yus, E.; Yang, J.-S.; Maria, L.-S.; Serrano, L. Impact of C-terminal amino acid composition on protein expression in bacteria. Mol. Syst. Biol. 2020, 16, e9208. [Google Scholar] [CrossRef]
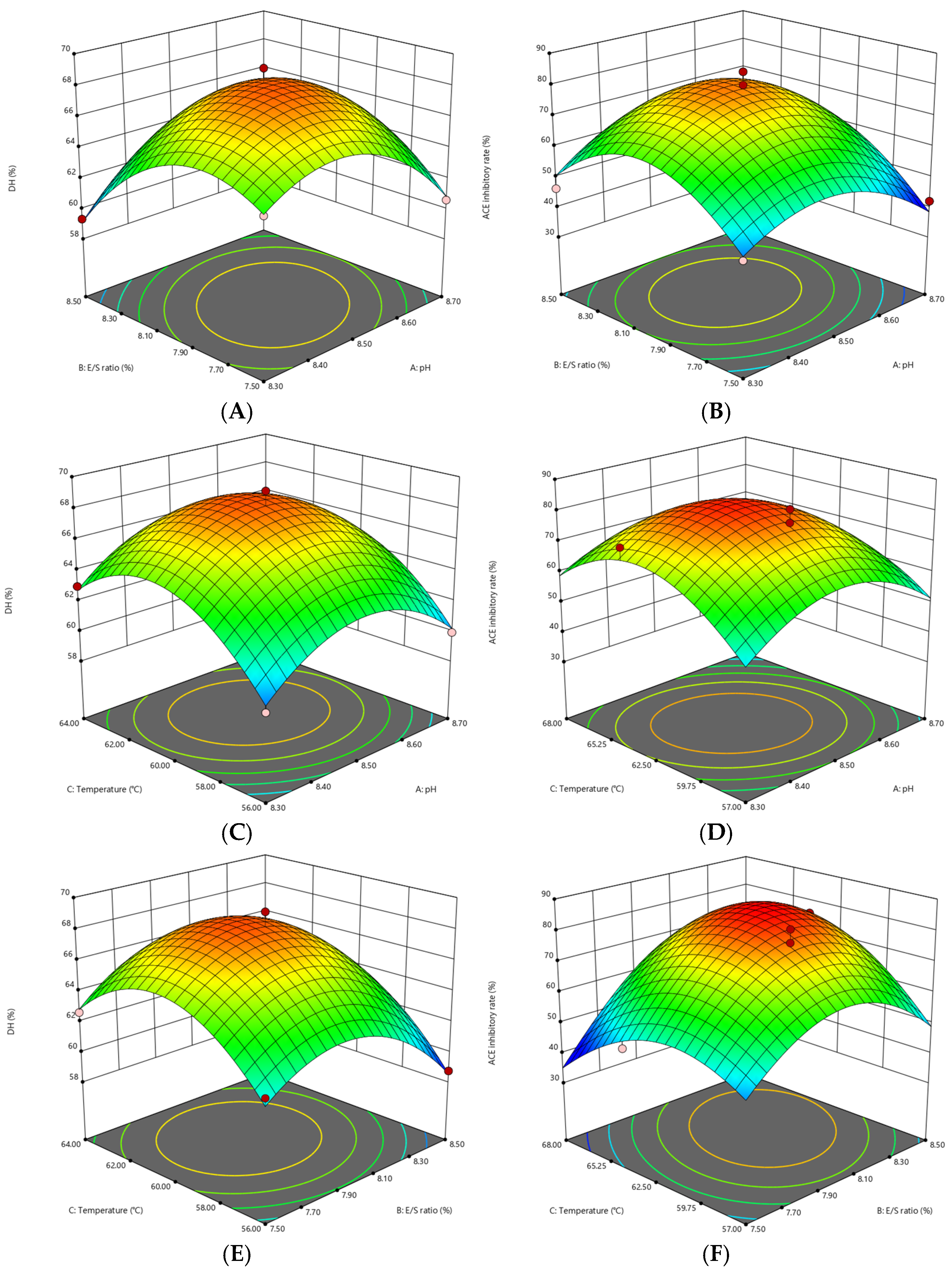



| Run | A pH | B E/S Ratio (%) | C Temperature (℃) | Y1 DH (%) | Y2 ACE Inhibitory Rate (%) |
|---|---|---|---|---|---|
| 1 | 8.5 (0) | 8 (0) | 60 (0) | 69.11 | 72.11 |
| 2 | 8.5 | 8.5 (+1) | 64 (+1) | 61.88 | 75.79 |
| 3 | 8.7 (+1) | 8 | 64 | 63.33 | 60 |
| 4 | 8.3 (−1) | 7.5 (−1) | 60 | 64.01 | 46.32 |
| 5 | 8.5 | 8 | 60 | 67.32 | 84.21 |
| 6 | 8.7 | 8 | 56 (−1) | 59.93 | 43.16 |
| 7 | 8.5 | 7.5 | 64 | 62.65 | 49.47 |
| 8 | 8.7 | 8.5 | 60 | 62.73 | 54.74 |
| 9 | 8.3 | 8 | 56 | 59.59 | 47.37 |
| 10 | 8.3 | 8 | 64 | 62.99 | 74.74 |
| 11 | 8.7 | 7.5 | 60 | 60.61 | 42.11 |
| 12 | 8.3 | 8.5 | 60 | 59.33 | 46.32 |
| 13 | 8.5 | 8 | 60 | 68.09 | 80 |
| 14 | 8.5 | 8.5 | 56 | 58.74 | 44.21 |
| 15 | 8.5 | 7.5 | 56 | 61.71 | 43.37 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.55 | 9 | 0.061 | 29.12 | 0.0009 | ** |
| A—pH | 2.6 × 10−4 | 1 | 2.6 × 10−4 | 0.12 | 0.7399 | |
| B—E/S ratio | 0.02 | 1 | 0.02 | 9.62 | 0.0268 | * |
| C—Temperature | 0.06 | 1 | 0.06 | 28.63 | 0.0031 | ** |
| AB | 0.047 | 1 | 0.047 | 22.2 | 0.0053 | ** |
| AC | 9 × 10−8 | 1 | 9 × 10−8 | 4.26 × 10−5 | 0.995 | |
| BC | 5.08 × 10−3 | 1 | 5.08 × 10−3 | 2.41 | 0.1815 | |
| A2 | 0.14 | 1 | 0.14 | 66.78 | 0.0004 | ** |
| B2 | 0.16 | 1 | 0.16 | 76.37 | 0.0003 | ** |
| C2 | 0.18 | 1 | 0.18 | 86.38 | 0.0002 | ** |
| Residual | 0.011 | 5 | 2.11 × 10−3 | |||
| Lack of Fit | 4.65 × 10−3 | 3 | 1.55 × 10−3 | 0.52 | 0.7077 | not significant |
| Pure Error | 5.91 × 10−3 | 2 | 2.95 × 10−3 | |||
| Cor Total | 0.56 | 14 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 13.09 | 9 | 1.45 | 11.57 | 0.0075 | ** |
| A-pH | 0.11 | 1 | 0.11 | 0.87 | 0.3934 | |
| B-E/S ratio | 0.87 | 1 | 0.87 | 6.96 | 0.0461 | * |
| C- Temperature | 3.7 | 1 | 3.7 | 29.47 | 0.0029 | ** |
| AB | 0.21 | 1 | 0.21 | 1.65 | 0.2559 | |
| AC | 0.086 | 1 | 0.086 | 0.68 | 0.4459 | |
| BC | 0.65 | 1 | 0.65 | 5.15 | 0.0725 | |
| A2 | 2.92 | 1 | 2.92 | 23.23 | 0.0048 | ** |
| B2 | 4.52 | 1 | 4.52 | 35.98 | 0.0018 | ** |
| C2 | 1 | 1 | 1 | 7.97 | 0.037 | * |
| Residual | 0.63 | 5 | 0.13 | |||
| Lack of Fit | 0.39 | 3 | 0.13 | 1.06 | 0.5189 | not significant |
| Pure Error | 0.24 | 2 | 0.12 | |||
| Cor Total | 13.71 | 14 |
| Amino Acid | Molecular Weight | F2 (mol/100 mol Amino Acid) | |
|---|---|---|---|
| F-2-1 | F-2-2 | ||
| Asp | 133.1 | 6.64 ± 0.27 | 2.62 ± 0.11 |
| Thr | 119.1 | 4.92 ± 0.22 | 1.90 ± 0.06 |
| Ser | 105.1 | 5.26 ± 0.17 | 4.59 ± 0.18 |
| Glu | 147.1 | 19.96 ± 0.63 | 8.69 ± 0.09 |
| Gly | 75.1 | 1.52 ± 0.04 | 1.58 ± 0.04 |
| Ala | 89.1 | 15.26 ± 0.33 | 17.49 ± 0.28 |
| Val | 117.1 | 9.77 ± 0.21 | 5.93 ± 0.18 |
| Met | 149.2 | 4.15 ± 0.18 | 4.31 ± 0.11 |
| Ile | 131.2 | 2.93 ± 0.06 | 1.11 ± 0.01 |
| Leu | 131.2 | 7.43 ± 0.21 | 5.02 ± 0.13 |
| Tyr | 181.2 | 4.22 ± 0.15 | 17.69 ± 0.45 |
| Phe | 165.2 | 3.88 ± 0.12 | 16.03 ± 0.39 |
| Lys | 146.2 | 4.24 ± 0.09 | 3.78 ± 0.14 |
| His | 155.2 | 1.69 ± 0.05 | 3.31 ± 0.12 |
| Arg | 174.2 | 0.00 | 0.36 ± 0.01 |
| Pro | 115.1 | 8.13 ± 0.24 | 5.60 ± 0.22 |
| Fatty amino acid | — | 39.55% | 33.86% |
| Aromatic amino acid | — | 8.10% | 33.72% |
| Basic amino acid | — | 5.93% | 7.45% |
| Cyclic amino acid | — | 8.13% | 5.60% |
| Hydrophobic amino acid | — | 55.78% | 73.17% |
| Source | Molecular Weight | Location | Sequence | Name |
|---|---|---|---|---|
| SGM G15-1 | 886.44174 | ɑS2 casein f (123–129) | NPWDQVK | P1 |
| 904.41592 | ɑS2 casein f (89–95) | VDDKHYQ | P2 | |
| 638.31442 | α whey protein f (35–40) | KDYGGV | P3 | |
| 657.36784 | κ casein f (88–93) | VRSPAQ | P4 | |
| 775.47125 | β lactoglobulin f (94–100) | TKIPAVF | P5 | |
| 641.27769 | β lactoglobulin f (114–118) | DTDYK | P6 | |
| 758.40832 | ɑS1 casein | VVAPFPE | P7 | |
| 1028.48947 | ɑS1 casein | SDIPNPIGSE | P8 | |
| 686.39842 | β casein f (217–222) | RGPFPI | P9 | |
| 747.35191 | β casein f (58–63) | DELQDK | P10 | |
| 575.32214 | β casein f (116–120) | TMVPK | P11 |
| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A—pH | 8.3 | 8.5 | 8.7 |
| B—E/S ratio/(%) | 7.5 | 8 | 8.5 |
| C—Temperature/(℃) | 56 | 60 | 64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Shu, G.; Lei, H.; Du, G.; Liu, Z.; Chen, L. Preparation of Novel ACE Inhibitory Peptides from Skimmed Goat Milk Hydrolyzed by Multi-Enzymes: Process Optimization, Purification, and Identification. Catalysts 2025, 15, 140. https://doi.org/10.3390/catal15020140
Hu W, Shu G, Lei H, Du G, Liu Z, Chen L. Preparation of Novel ACE Inhibitory Peptides from Skimmed Goat Milk Hydrolyzed by Multi-Enzymes: Process Optimization, Purification, and Identification. Catalysts. 2025; 15(2):140. https://doi.org/10.3390/catal15020140
Chicago/Turabian StyleHu, Wenjing, Guowei Shu, Huan Lei, Guanli Du, Zhengxin Liu, and Li Chen. 2025. "Preparation of Novel ACE Inhibitory Peptides from Skimmed Goat Milk Hydrolyzed by Multi-Enzymes: Process Optimization, Purification, and Identification" Catalysts 15, no. 2: 140. https://doi.org/10.3390/catal15020140
APA StyleHu, W., Shu, G., Lei, H., Du, G., Liu, Z., & Chen, L. (2025). Preparation of Novel ACE Inhibitory Peptides from Skimmed Goat Milk Hydrolyzed by Multi-Enzymes: Process Optimization, Purification, and Identification. Catalysts, 15(2), 140. https://doi.org/10.3390/catal15020140








