Abstract
The design of efficient and cost-effective electrocatalysts to replace Pt in an oxygen reduction reaction (ORR) is crucial for advancing proton exchange membrane fuel cell (PEMFC) technologies. This study synthesized Pd-Co bimetallic alloy nanoparticles supported on reduced graphene oxide (rGO) through a simple chemical-reduction method, making it suitable for low-cost, large-scale fabrication and significantly reducing the need for Pt. The nanostructures were systematically characterized using various analytical techniques, including X-ray diffraction (XRD), high-resolution transmission electron microscopy (HR-TEM), energy-dispersive X-ray spectroscopy (EDX), and cyclic voltammetry (CV). Electrochemical investigations revealed that the Pd-Co/rGO catalyst exhibits remarkable ORR performance in an alkaline environment, with an electrode-area-normalized activity rivaling that of the commercial Pt/C catalyst. Remarkably, Pd-Co/rGO demonstrated an onset potential (Eonset) of 0.944 V (vs. RHE) and a half-wave potential (E1/2) of 0.782 V (vs. RHE), highlighting its excellent ORR activity. Furthermore, the Pd-Co/rGO catalyst displayed superior methanol-tolerant ORR activity, outperforming Pt/C and monometallic Pd/rGO and Co/rGO systems. The enhanced electrocatalytic performance is attributed to the smallest size, consistent shape, and good dispersion of the alloy structure on the RGO surface. These findings establish Pd-Co/rGO as a promising alternative to Pt-based catalysts, addressing key challenges such as methanol crossover while advancing PEMFC technology in alkaline media.
1. Introduction
To achieve highly efficient fuel cell technologies, advanced catalyst design is needed to enable effective acceleration of key energy conversion processes such as methanol oxidation, a formic acid oxidation reaction, and ORR [1,2,3]. Accelerating the electrochemical reaction rates and in particular that of the slow cathodic ORR, is necessary in order to enhance the performance of fuel cell energy systems. However, studies have proven that the energy requirement for ORR is about four times higher than that for anodic reactions like the hydrogen oxidation reaction (HOR) and methanol oxidation reaction (MOR) [4,5]. To improve the efficiency of proton exchange membrane fuel cells (PEMFCs), cathode structures are often designed by incorporating increased quantities of Pt-based precious catalysts. However, the excessive use of Pt-based materials raises internal resistance and increases the device’s overall cost, emphasizing the pressing requirement for strategies to optimize Pt utilization [6,7,8]. Several non-precious metal electrocatalysts have been explored as potential substitutes for conventional Pt-based electrocatalysts in alkaline environments [9]. These include nanocarbons doped with nonmetals [10] and composites of carbon and transition metals [11], as well as various oxides of transition metals [12], such as cobalt oxides, perovskites, and spinels [13,14,15,16,17]. While these electrocatalysts are promising because of their affordability, their ORR performance remains substantially inferior when compared to Pt-based electrocatalysts [9,10].
Of all the precious metals, Pd has attracted significant interest as an effective substitute for Pt in ORR under alkaline conditions. This is primarily due to its superior ORR activity, which is largely attributed to the similar electronic properties shared by Pd and Pt [18,19]. Over the past decade, the average cost of Pd has been considerably lower than that of Pt; however, since 2018, the price of Pd has surpassed that of Pt. To lower the expense of Pd-based electrocatalysts and enhance their ORR performance, studies have concentrated on alloying Pd with different transition metals. This approach aims to alter the electronic characteristics of the metallic phase within the electrocatalysts [20,21,22,23,24,25,26]. A major challenge in these studies is correlating the changes observed in ORR activity to the modifications in the electronic characteristics of Pd-based electrocatalysts. Recent studies have linked the ORR electrocatalytic performance in alkaline solutions to the charge-transfer interactions observed between the atoms of Pd and a secondary metal. These interactions influence the d-band properties, including its center position, bandwidth, and electron filling, which subsequently affect the electronic structure of the metal catalyst [27,28]. The d-band characteristics, in conjunction with the binding energies of surface molecules and the electron interaction between the metal surface and the surface species, play a crucial role in determining the attraction force and reactivity of substances on metallic surfaces. Notably, a shifted d-band position below the Fermi level reduces the adhesion energy of oxygen-containing adsorbates, as shown for oxygen attachment on different metals and alloys [29,30,31].
Optimizing the position of the d-band center is a critical factor in the development of highly efficient bimetallic electrocatalysts for ORR. This optimal positioning governs the balance between oxygen-binding strength and the energy barrier required for the dissociation of oxygen adsorbates [19]. Transition metals like cobalt, molybdenum, and copper exhibit strong oxygen binding with minimal or negligible energy barriers for dissociation, whereas noble metals such as gold display weaker oxygen binding coupled with higher energy barriers for dissociative adsorption [32]. Incorporating Pd with another transition metal seeks to strike a delicate balance ensuring the oxygen–metal interaction is robust enough to facilitate sufficient adsorption of oxygen species while avoiding excessively strong bonds that could impede subsequent reaction steps [32]. The commercialization of fuel cells faces significant challenges because of the elevated cost, sluggish reaction rates, limited methanol tolerance, and increased loading requirements of traditional Pt/C catalysts for ORR [6,7,8]. To overcome these limitations, extensive research has shifted towards the development of alternative catalysts. Among these, Pd-based bimetallic nanocatalysts have shown considerable potential for ORR in alkaline conditions [33,34,35]. Recent studies have investigated various Pd-based bimetallic systems, including Pd-Cu [36], Pd-Co [37], Pd-Ni [38], Pd-Ag [39], Pd-Au [21], and Pd-Fe [40], as efficient electrocatalysts in alkaline environments. The growing interest in alkaline electrolytes is driven by their ability to create a less corrosive environment for catalysts, facilitate faster ORR kinetics compared to acidic media, and promote the activation of peroxide intermediates, thereby enhancing the efficiency of the four-electron transfer pathway [41]. In addition, the ORR current density of the Pd-Co catalyst (5.1 × 10−6 mA cm−2) is the highest as compared to other palladium-based bimetal alloy catalysts, including Pd-Ni (7.9 × 10−7 mA cm−2) and Pd-Cr (2.2 × 10−7 mA cm−2). It produces a four-electron transfer and executes the one-pathway ORR rule to produce water.
In this study, we report the development of ultrafine Pd-Co nanoparticles supported on rGO as a cathodic electrocatalyst for ORR in alkaline media. The synthesis employs a straightforward chemical-reduction process, yielding 20 wt% Pd and 10 wt% Co supported on rGO, as illustrated in Scheme 1. By leveraging the synergistic interaction between Pd and Co alongside the conductive properties of rGO, this work addresses key challenges, including cost and methanol tolerance, and offers a sustainable pathway to enhancing alkaline medium fuel cell performance.
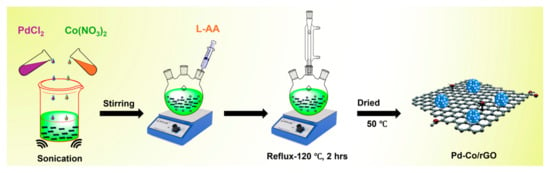
Scheme 1.
Illustrating the synthesis of Pd-Co bimetallic NPs supported on rGO.
2. Results and Discussion
The XRD analysis was performed to study the crystalline structure and phase composition of the synthesized catalysts, including Co/rGO, Pd/rGO, and Pd-Co/rGO. In Figure 1a, the XRD pattern for Co/rGO exhibits characteristic peaks at 2θ values of 44.44°, 50.66°, and 75.36°, which correspond to the (111), (200), and (220) planes of the face-centered cubic (fcc) structure of metallic Co (JCPDS #15-0806) [42]. Similarly, Pd/rGO shows diffraction peaks at 39.76°, 46.4°, and 67.28°, indexed to the (111), (200), and (220) reflections of metallic Pd in its fcc phase (JCPDS #46-1043) [43]. In the case of Pd-Co/rGO, the XRD pattern reveals peaks at 40.82°, 47.46°, and 68.72°, showing a noticeable shift toward higher Bragg angles compared to pure Pd/rGO. This shift in peak positions indicates lattice contraction due to the incorporation of Co into the Pd lattice, confirming the formation of a Pd-Co alloy. Additionally, the XRD pattern (Figure 1b) of GO displays a sharp peak at 2θ ≈ 10°, which corresponds to the (002) plane of GO. Moreover, after reduction, the rGO support shows a broad peak around 2θ ≈ 25°, indicating the partial restoration of the graphitic structure [44]. Overall, the XRD results confirm the successful synthesis of Pd-Co alloy nanoparticles on the rGO support, where the metallic phase and alloying effect are clearly observed.
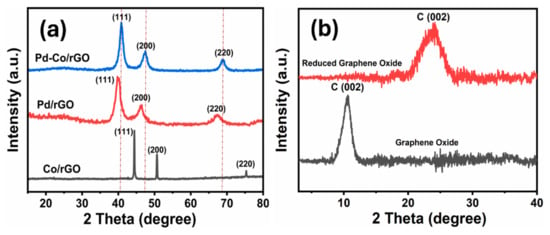
Figure 1.
(a) XRD patterns of Pd-Co/rGO, Pd/rGO, and Co/rGO catalysts, the formation of an fcc structure with characteristic metallic peaks and evidence of Pd-Co alloying. (b) Comparative XRD patterns of GO and rGO demonstrate the structural restoration and reduced interlayer spacing in rGO upon reduction, which aligns with enhanced crystallinity and decreased oxygen functionalities.
The morphology and composition of the as-synthesized Pd-Co/rGO catalyst were thoroughly characterized using transmission electron microscopy (TEM), HRTEM, and EDX, as shown in Figure 2. The wide-area TEM images reveal the structural features of the Pd-Co/rGO catalyst. The GO support exhibits a thin, wrinkled sheet-like morphology (Figure 2a), which serves as a robust support for nanoparticle dispersion and prevents agglomeration of active metal species [45]. This provides an extensive surface area for the uniform deposition of Pd-Co NPs. Figure 2b,c display the low-resolution TEM images of Pd-Co alloy nanoparticles anchored onto the rGO sheets. From the histogram analysis (inset of Figure 2b), the majority of the Pd-Co nanoparticles exhibit a uniform size distribution, with an average particle size of approximately ~1.9 nm. This small particle size enhances the electrochemical active surface area, facilitating better catalytic performance. The HRTEM image (Figure 2d) further confirms the crystalline nature of the Pd-Co NPs and their strong interaction with the rGO support. Such strong metal-support interactions are essential for maintaining stability during electrochemical processes [46]. The uniform distribution of NPs across the graphene layers minimizes agglomeration, ensuring efficient utilization of active sites. The compositional analysis was performed using EDX (Figure 2e). The EDX spectrum confirms the presence of Pd and Co in an atomic ratio of 51.35:48.65, closely aligning with the intended 1:1 nominal ratio used during synthesis. Additionally, the spectrum shows peaks corresponding to C and O elements, which originate from the partially rGO [44]. The presence of Cu in the EDX spectrum originates from the Cu grid coated with carbon used in TEM examination. Furthermore, inductively coupled plasma-optical emission spectroscopy (ICP-OES) revealed that the rGO composite contained 19.6 wt% Pd and 9.9 wt% Co, corresponding to an atomic ratio of approximately 51:49. The near-perfect agreement between the experimental atomic ratio and the designed stoichiometry suggests the successful co-reduction of Pd and Co precursors, resulting in a Pd-Co alloy with high purity and compositional control [47]. The combined analysis of TEM, HRTEM, and EDX reveals that Pd-Co NPs are evenly distributed over the rGO surface, exhibiting a narrow particle size distribution and a well-maintained alloy composition. These features are crucial for achieving superior catalytic activity in alkaline medium fuel cells.
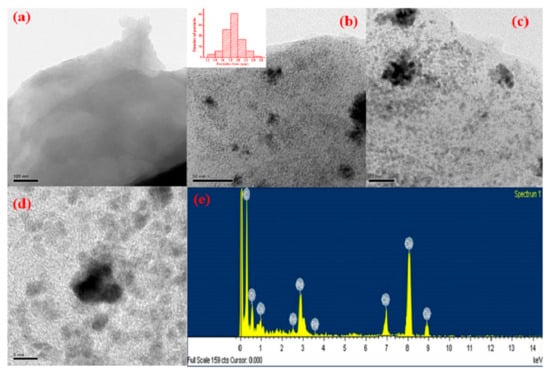
Figure 2.
TEM and compositional analysis of Pd-Co/rGO catalysts. (a) TEM image of graphene oxide (GO). (b,c) Low-resolution TEM images of Pd-Co/rGO showing uniform dispersion of nanoparticles; inset in (b) shows the particle size distribution with an average size of ~1.9 nm. (d) HRTEM image of Pd-Co/rGO nanoparticles revealing their crystalline nature. (e) EDX spectrum confirming the atomic ratio of Pd to Co as 1:1, indicating the successful formation of Pd-Co alloy.
The CV results for the synthesized catalysts in 0.1 M KOH electrolyte are presented in Figure 3. CV analysis for Pd-Co/rGO, Pd/rGO, and Pt/C was conducted in a nitrogen-rich atmosphere, covering a potential range from 0.06 to 1.36 V vs. RHE, with a scan rate of 50 mV/s. In the anodic region, the oxidation peaks observed are attributed to the formation of metal oxides, particularly facilitated by the oxophilic nature of Co, which promotes oxygen adsorption and surface oxidation. The reduction peaks in the cathodic region correspond to the reduction of preformed oxides, leading to reduced reactivity of the active metal. Due to the presence of more oxygenated surface groups on rGO, the Pd-Co/rGO catalyst exhibits a significantly larger double-layer capacitance compared to commercial Pt/C. These oxygenated groups play a crucial role in enhancing specific activity, increasing electron transfer, and improving active site accessibility [48]. Additionally, the Pd-Co/rGO catalyst demonstrates a significant improvement in current density in both oxidation and reduction regions compared to Pd/rGO and Pt/C, indicating enhanced electrochemical activity. The presence of Co in the Pd-Co alloy increases oxophilicity, contributing to better catalytic performance by promoting electron transfer and enhancing oxygen-binding capabilities [49]. Furthermore, the conductive rGO support enhances faster charge transfer and stabilizes the active site, thereby boosting the catalyst’s performance. These observations highlight the superior electrochemical properties of Pd-Co/rGO, making it a promising material for further studies related to electrocatalytic applications, such as the ORR.
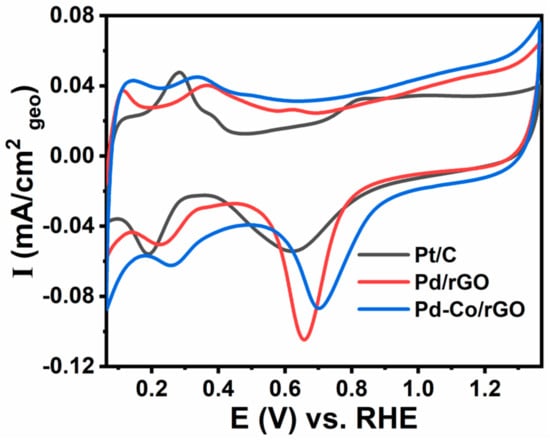
Figure 3.
CV curves of Pd-Co/rGO, Pd/rGO, and Pt/C catalysts in N2-saturated 0.1 M KOH solution at a scan rate of 50 mV/s, showing the electrochemical behavior in the potential range of 0.06 to 1.36 V vs. RHE.
The ORR activity of the synthesized catalysts was evaluated in O2-saturated 0.1 M KOH using linear sweep voltammetry (LSV) at a scan rate of 5 mV/s. Figure 4a compares the ORR polarization curves of Pd-Co/rGO, along with Pd/rGO, and Co/rGO, and the commercial Pt/C benchmark catalyst. Notably, Pd-Co/rGO exhibited superior electrocatalytic performance with an onset potential of 0.944 V and half-wave potential of 0.782 V, both of which are close to those of Pt/C (0.945 V and 0.784 V, respectively). In contrast, Pd/rGO and Co/rGO demonstrated more negative onset potentials (0.864 V and 0.714 V) and half-wave potentials (0.774 V and 0.544 V), confirming the enhanced ORR kinetics resulting from the synergistic effect of Pd and Co NPs supported on rGO. The limiting current densities (Figure 4b) further emphasize the superior catalytic activity of Pd-Co/rGO, which outperforms other materials. Specifically, the limiting current densities were recorded as −5.33 mA cm−2 corresponding to Pt/C, −5.29 mA cm−2 attributed to Pd-Co/rGO, −3.50 mA cm−2 observed for Pd/rGO, and −1.64 mA cm−2 measured for Co/rGO. The higher limiting current density of Pd-Co/rGO, when compared to Pd/rGO and Co/rGO, is primarily due to the well-optimized electronic structure and the even distribution of Pd-Co NPs on the rGO support, facilitating efficient oxygen adsorption and reduction pathways. To account for the intrinsic activity of the catalysts, the mass-normalized current densities were calculated and are presented in Figure 4c. Pd-Co/rGO displayed the highest mass activity, significantly surpassing that of Pd/rGO and Co/rGO. This observation highlights the efficient utilization of active sites and the reduced Pd loading due to the synergistic effect with Co.
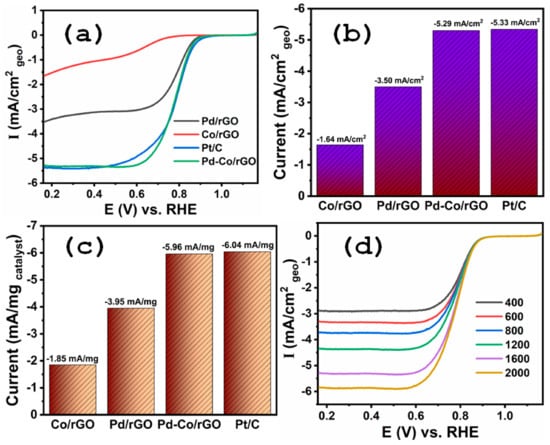
Figure 4.
(a) ORR polarization curves of Pt/C, Pd-Co/rGO, Pd/rGO, and Co/rGO catalysts in O2-saturated 0.1 M KOH at a scan rate of 5 mV/s. (b) Limiting current densities for all synthesized catalysts under the same conditions. (c) Mass-normalized current densities of the catalysts showing intrinsic activity based on catalyst loading. (d) ORR performance of Pd-Co/rGO catalyst at varying rotation speeds, indicating diffusion-controlled behavior.
Pd-Co/rGO’s ORR performance was further evaluated at different rotation speeds (Figure 4d), showing a clear dependence of the current density on the rotating disk electrode (RDE) speed. This behavior suggests a typical diffusion-controlled process, confirming the robustness of the catalyst under alkaline conditions. A major challenge for cathode electrocatalysts in alkaline fuel cells is their resistance to methanol crossover. Figure 5 illustrates the methanol tolerance of the synthesized catalysts (Pd-Co/rGO, Pd/rGO) and the commercial Pt/C benchmark. The Pd-Co/rGO catalyst exhibited negligible current response in the presence of methanol, whereas Pt/C experienced significant methanol oxidation interference. Similarly, Pd/rGO displayed lower methanol oxidation currents compared to Pt/C, but its performance remained inferior to Pd-Co/rGO. The superior methanol tolerance of Pd-Co/rGO primarily stems from the unique electronic and compositional characteristics of the Pd-Co alloy. The distinct electronic properties of Pd and Co enable alloying, which creates a catalytic surface that modifies the electron density around Pd atoms. This modification shifts the d-band center of Pd, improving oxygen reduction efficiency by enhancing interactions with oxygen species and minimizing methanol binding [27,28]. Methanol, being larger and less reactive than oxygen, exhibits weak binding to the Pd-Co active sites. The alloying of Pd with Co induces a synergistic effect, fine tuning the d-band center of Pd to enhance oxygen intermediate adsorption while reducing methanol adsorption. Density Functional Theory (DFT) studies on Pd-based alloys support this selective adsorption mechanism, demonstrating that methanol is inhibited from competing with oxygen at the active sites [50,51,52,53]. Furthermore, Co contributes an oxophilic nature to the catalyst, enhancing the selective adsorption of oxygen species necessary for the ORR. This oxophilic property facilitates a four-electron ORR pathway while minimizing side reactions caused by methanol oxidation [54]. The high conductivity and large surface area of rGO further augment the activity by providing efficient charge-transfer pathways and abundant active sites. As a result, Pd-Co/rGO demonstrates exceptional ORR activity, surpassing the standard Pt/C catalyst in methanol tolerance, and effectively addresses methanol crossover issues. This synergistic effect between Pd and Co alloy nanoparticles, combined with the advantageous properties of rGO, positions Pd-Co/rGO as a strong candidate for practical applications in alkaline medium fuel cells.
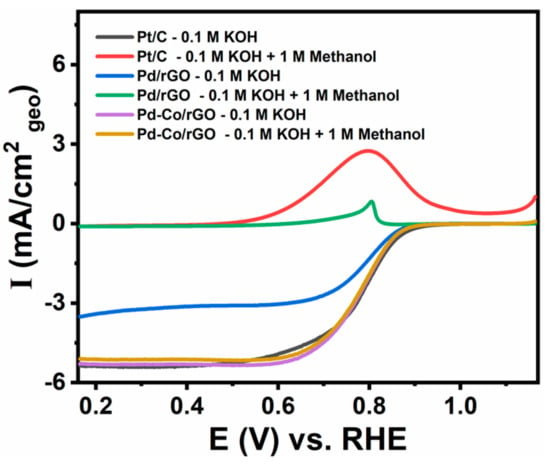
Figure 5.
Methanol crossover tests for the catalysts, highlighting methanol tolerance of Pd-Co/rGO in contrast with Pd/rGO and Pt/C.
3. Materials and Methods
3.1. Chemicals
Palladium (II) chloride (PdCl2, 99.0%, Sigma-Aldrich, St. Louis, MO, USA), cobalt nitrate hexahydrate (Co(NO3)2·6H2O, Sigma-Aldrich), L-ascorbic acid (C6H8O6, 99%, Sigma-Aldrich), and tri-sodium citrate were procured from Sigma-Aldrich. Sodium hydroxide, graphite powder, methanol, ethanol, and deionized (DI) water were used at analytical grade and not further purified. Synthesis and experimental processes of all chemicals were used directly.
3.2. Synthesis of Graphene Oxide (GO)
The precursor used for GO synthesis was a natural graphite powder, and it was synthesized using a modified version of the Hummers method [55]. Amounts total loading 3 g of graphite powder and 6 g of NaNO3 were mixed in 120 mL of H2SO4 (250 mL three-necked flask). The reaction was allowed to occur at a low temperature via cooling in an ice bath. We added 12 g of KMnO4 slowly so that the temperature did not go above 10 °C, as heating KMnO4 can generate excess heat. After stirring the reaction for 6 h under these controlled conditions, 15 mL of H2O2 was slowly added to the mixture to quench any unreacted permanganate and facilitate further oxidation. The mixture was stirred at ambient temperature for 2 additional hours, resulting in a viscous dark-brown slurry indicative of GO formation. The reaction product was carefully washed several times with DI water until the pH of the supernatant was neutral. Finally, the purified GO was collected by centrifugation and dried overnight at 80 °C in a hot-air oven, yielding a fine oxidized powder suitable for further applications.
3.3. Synthesis of Pd-Co Bimetallic NPs on rGO
To synthesize 30 wt% Pd-Co bimetallic nanoparticles supported on rGO, 70 mg of as-prepared GO was dispersed in 50 mL of DI water. The suspension was ultrasonicated for 30 min to exfoliate the GO sheets. Subsequently, 33 mg of PdCl2 was added, and the sonication was continued for another 30 min to promote the dispersion of Pd2+ ions onto the GO surface. The resulting mixture was stirred overnight at room temperature to ensure uniform adsorption of Pd ions on GO. In the next step, the Pd-GO dispersion was transferred into a 250 mL three-necked flask containing a pre-refluxed solution of 400 mg of L-ascorbic acid (L-AA) and 100 mg of tri-sodium citrate dissolved in DI water. The synthesis blend was heated to 100 °C and stirred persistently for 1 h to ensure thorough mixing. Subsequently, 2 mL of 5 M NaOH solution was added to adjust the pH, and the temperature was raised to 120 °C. The refluxing was continued for 5 h, during which L-AA acted as a reducing agent, reducing both Pd2+ to metallic Pd0 and GO to rGO. This step resulted in the formation of Pd NPs supported by rGO. To achieve a final composition of 20 wt% Pd and 10 wt% Co in the Pd-Co/rGO catalyst, an aqueous solution of 53 mg of Co(NO3)2·6H2O was slowly added to the Pd/rGO solution while maintaining the temperature at 120 °C. The synthesis blend was stirred for an additional 2 h to ensure the alloying of Pd and Co. The resulting product, Pd-Co/rGO, was cooled to room temperature, washed several times with DI water and ethanol to remove impurities, and then collected via centrifugation. The purified product was dried overnight at 50 °C in a hot-air oven. For comparative analysis, 20 wt% Pd/rGO and 20 wt% Co/rGO catalysts were also synthesized following the same procedure, substituting Co(NO3)2 or PdCl2 as required.
3.4. Material Characterization
The structural and morphological characteristics of the synthesized materials, including particle size and distribution, were examined using a high-resolution FEI Technai G2 S–Twin transmission electron microscope (TEM, FEI Company, Hillsboro, OR, USA) operated at an accelerating voltage of 200 kV. For TEM imaging, a small quantity of the catalyst powder was ultrasonically dispersed in ethanol, and a drop of the suspension was carefully deposited onto a carbon-coated copper grid. The sample was then dried at room temperature prior to analysis to ensure a uniform distribution of particles on the grid. XRD measurements were performed to analyze the crystalline structure and phase composition of the GO, rGO, and metal/rGO electrocatalysts. The diffraction patterns were recorded over a 2θ range of 5–90° using a Rigaku Ultima IV diffractometer, operating at 40 kV and 30 mA. A Ni-filtered Cu-Kα radiation source (λ = 1.541 Å) was employed for the measurements, ensuring high resolution and accuracy in phase identification. Additionally, an Optima 5300 DV (PerkinElmer, Waltham, MA, USA) inductively coupled plasma-optical emission spectrometer was utilized to determine metal loadings on a glassy carbon electrode (GCE) and the atomic composition of Pd and Co on the rGO support.
3.5. Electrochemical Measurements
All electrochemical measurements were conducted at room temperature using a standard three-electrode electrochemical cell, connected to a CHI potentiostat (Model CHI 6002 E, CH Instruments, Austin, TX, USA). The reference electrode was an Ag/AgCl (saturated KCl) electrode (E° = −0.197 V vs. NHE), and a Pt wire served as the auxiliary electrode. The working electrode was constructed on a glassy carbon disk (3 mm diameter, geometric area of 0.0706 cm2). Initially, the GCE was polished meticulously using alumina powder of particle sizes 1.0, 0.5, and 0.05 µm, followed by ultrasonic cleaning in ethanol and DI water multiple times. Subsequently, 2 mg of the catalyst was dispersed in a solvent mixture composed of deionized water (800 µL), ethanol (700 µL), and a 0.5 wt% Nafion solution (100 µL). This dispersion was sonicated for 30 min to ensure uniform distribution. A 5 µL aliquot of the resulting solution was then applied onto the surface of the glassy carbon electrode using a micropipette, followed by air drying for 24 h before electrochemical testing. CV measurements were conducted in an N2-saturated 0.1 M KOH solution, with the electrode potential scanned from 0.06 to 1.36 V vs. RHE at a scan rate of 50 mV/s. All potentials reported in this study are recalculated and presented with respect to the reversible hydrogen electrode (RHE) using the equation ERHE = EAg/AgCl + 0.059 × pH + 0.197 V, where the pH of the 0.1 M KOH solution is approximately 13. Upon stabilization of the voltammograms, the electrolyte was saturated with O2, and LSV measurements for the ORR were performed using an RDE configuration. The potential was swept from 0.16 to 1.17 V vs. RHE at various rotation rates of 400, 600, 800, 1200, 1600, and 2000 rpm, at a scan rate of 5 mV/s.
4. Conclusions
In this investigation, a suite of palladium-based catalysts, Pd-Co/rGO, Pd/rGO, and Co/rGO NPs, were successfully produced through a straightforward one-step chemical-reduction process. The ORR electrocatalytic activity was evaluated using the rotating disk electrode technique in an O2-saturated 0.1 M KOH solution. A commercial Pt/C catalyst exhibited similar performance to the Pd−Co/rGO catalyst for ORR electrocatalysis. This catalyst also demonstrated greater tolerance to methanol than Pt/C and exhibited superior performance compared to Pd/rGO and Co/rGO monometallic systems. These findings underscore the potential of Pd-Co/rGO as an excellent and economical replacement for Pt-based catalysts, particularly in mitigating the methanol crossover challenge in PEMFCs. Enhanced performance was realized due to the optimized alloy structure and synergistic interactions between Pd and Co. Future research will focus on further improving the catalyst’s durability and scaling up the synthesis for practical fuel cell applications.
Author Contributions
Conceptualization, C.S.Y.; methodology, C.S.Y.; software, V.V.; validation, T.H.K., J.O. and P.K.R.K.; formal analysis, V.V. and P.K.R.K.; investigation, C.S.Y.; resources, C.S.Y.; data curation, C.S.Y.; writing—original draft preparation, C.S.Y.; writing—review and editing, C.S.Y. and V.V.; visualization, C.S.Y.; supervision, T.H.K. and J.O.; funding acquisition, T.H.K. and J.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Research Foundation of Korea (NRF) under grant numbers NRF-2020R1A2C1014918 and NRF-2021R1A6A1A03039503. Additional support was provided by the Korea Innovation Foundation under grant number 2023-DD-UP-0007, funded by the Ministry of Science and ICT (MSIT). This work was also supported by the Korea Basic Science Institute (National Research Facilities and Equipment Center) under grant number 2022R1A6C101B794 funded by the Ministry of Education (MOE). Further support was received from the Soonchunhyang University research fund.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors would like to thank Loka Subramanyam Sarma in the Department of Chemistry at Yogi Vemana University, India for providing invaluable support with electrochemical analysis during this research.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kaur, J.; Sharma, V.; Kumar Das, D.; Pandit, B.; Shahzad Samdani, M.; Shkir, M.; Aslam Manthrammel, M.; Nangan, S.; Jagadeesha Angadi, V.; Ubaidullah, M. Single-Atom Catalysts for Oxygen Reduction Reaction and Methanol Oxidation Reaction. Fuel 2024, 358, 130241. [Google Scholar] [CrossRef]
- Shen, T.; Zhang, J.; Chen, K.; Deng, S.; Wang, D. Recent Progress of Palladium-Based Electrocatalysts for the Formic Acid Oxidation Reaction. Energy Fuels 2020, 34, 9137–9153. [Google Scholar] [CrossRef]
- Vo, T.; Gao, J.; Liu, Y. Recent Development and Future Frontiers of Oxygen Reduction Reaction in Neutral Media and Seawater. Adv. Funct. Mater. 2024, 34, 2314282. [Google Scholar] [CrossRef]
- Wang, S.; Sheng, T.; Yuan, Q. Low-Pt Octahedral PtCuCo Nanoalloys: “One Stone, Four Birds” for Oxygen Reduction and Methanol Oxidation Reactions. Inorg. Chem. 2023, 62, 11581–11588. [Google Scholar] [CrossRef]
- Yang, X.; Lin, L.; Guo, X.; Zhang, S. Design of Multifunctional Electrocatalysts for ORR/OER/HER/HOR: Janus Makes Difference. Small 2024, 20, 2404000. [Google Scholar] [CrossRef]
- Martinaiou, I.; Daletou, M.K. Enhancing Electrode Efficiency in Proton Exchange Membrane Fuel Cells with PGM-Free Catalysts: A Mini Review. Energies 2024, 17, 3443. [Google Scholar] [CrossRef]
- Saeidfar, A.; Yesilyurt, S. Durability Investigation of Low Pt-Loaded PEM Fuel Cells with Different Catalyst Layer Morphologies. ECS Trans. 2024, 113, 3–10. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, S.Y.; Lee, H.J.; In Lee, H.; Lim, D.-H.; Lee, Y.S.; Kim, H.S.; Woo, S.H. Strategies for the Design and Synthesis of Pt-Based Nanostructured Electrocatalysts in Proton Exchange Membrane Fuel Cells (PEMFCs). ACS Eng. Au 2024. [Google Scholar] [CrossRef]
- Charalampopoulos, G.; Maniatis, I.; Daletou, M. Non-PGM Cathode Electrocatalysts for PEM Fuel Cells. ECS Trans. 2023, 112, 335–341. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Brouzgou, A.; Song, S.; Liang, Z.-X.; Tsiakaras, P. Non-Precious Electrocatalysts for Oxygen Reduction Reaction in Alkaline Media: Latest Achievements on Novel Carbon Materials. Catalysts 2016, 6, 159. [Google Scholar] [CrossRef]
- Goswami, C.; Hazarika, K.K.; Bharali, P. Transition Metal Oxide Nanocatalysts for Oxygen Reduction Reaction. Mater. Sci. Energy Technol. 2018, 1, 117–128. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, L.; Zhang, B.; Jin, J.; Su, D.S.; Wang, S.; Sun, G. Controllable Synthesis of Cobalt Monoxide Nanoparticles and the Size-Dependent Activity for Oxygen Reduction Reaction. ACS Catal. 2014, 4, 2998–3001. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled Preparation, Oxygen Reduction/Evolution Reaction Application, and Beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef]
- Wei, C.; Feng, Z.; Scherer, G.G.; Barber, J.; Shao-Horn, Y.; Xu, Z.J. Cations in Octahedral Sites: A Descriptor for Oxygen Electrocatalysis on Transition-Metal Spinels. Adv. Mater. 2017, 29, 1606800. [Google Scholar] [CrossRef]
- Safakas, A.; Bampos, G.; Bebelis, S. Oxygen Reduction Reaction on La0.8Sr0.2CoxFe1-XO3-δ Perovskite/Carbon Black Electrocatalysts in Alkaline Medium. Appl. Catal. B 2019, 244, 225–232. [Google Scholar] [CrossRef]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the Rational Design of Non-Precious Transition Metal Oxides for Oxygen Electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, Q.; Chen, H.; Shao, M. Recent Advances in Palladium-Based Electrocatalysts for Fuel Cell Reactions and Hydrogen Evolution Reaction. Nano Energy 2016, 29, 198–219. [Google Scholar] [CrossRef]
- Lima, F.H.B.; Zhang, J.; Shao, M.H.; Sasaki, K.; Vukmirovic, M.B.; Ticianelli, E.A.; Adzic, R.R. Catalytic Activity−d-Band Center Correlation for the O 2 Reduction Reaction on Platinum in Alkaline Solutions. J. Phys. Chem. C 2007, 111, 404–410. [Google Scholar] [CrossRef]
- Raghavendra, P.; Sekhar, Y.C.; Reddy, G.V.; Chandana, P.S.; Sarma, L.S. Hetero-Epitaxial Grown Pt@Au Core-Shell Bimetallic Nanoparticles on Reduced Graphene Oxide (RGO) as Electrocatalyst for Oxygen Reduction Reaction in Alkaline Media. Adv. Nat. Sci. Nanosci. Nanotechnol. 2024, 15, 015005. [Google Scholar] [CrossRef]
- Raghavendra, P.; Vishwakshan Reddy, G.; Sivasubramanian, R.; Sri Chandana, P.; Subramanyam Sarma, L. Reduced Graphene Oxide-Supported Pd@Au Bimetallic Nano Electrocatalyst for Enhanced Oxygen Reduction Reaction in Alkaline Media. Int. J. Hydrogen Energy 2018, 43, 4125–4135. [Google Scholar] [CrossRef]
- Gunji, T.; Wakabayashi, R.H.; Noh, S.H.; Han, B.; Matsumoto, F.; DiSalvo, F.J.; Abruña, H.D. The Effect of Alloying of Transition Metals (M = Fe, Co, Ni) with Palladium Catalysts on the Electrocatalytic Activity for the Oxygen Reduction Reaction in Alkaline Media. Electrochim. Acta 2018, 283, 1045–1052. [Google Scholar] [CrossRef]
- Cui, N.; Li, W.; Guo, Z.; Xu, X.; Zhao, H. Electrocatalytic Performance of Carbon Supported WO3-Containing Pd–W Nanoalloys for Oxygen Reduction Reaction in Alkaline Media. Catalysts 2018, 8, 225. [Google Scholar] [CrossRef]
- Yang, T.; Ma, Y.; Huang, Q.; Cao, G. Palladium–Iridium Nanocrystals for Enhancement of Electrocatalytic Activity toward Oxygen Reduction Reaction. Nano Energy 2016, 19, 257–268. [Google Scholar] [CrossRef]
- Wang, G.; Guan, J.; Xiao, L.; Huang, B.; Wu, N.; Lu, J.; Zhuang, L. Pd Skin on AuCu Intermetallic Nanoparticles: A Highly Active Electrocatalyst for Oxygen Reduction Reaction in Alkaline Media. Nano Energy 2016, 29, 268–274. [Google Scholar] [CrossRef]
- Yin, H.; Liu, S.; Zhang, C.; Bao, J.; Zheng, Y.; Han, M.; Dai, Z. Well-Coupled Graphene and Pd-Based Bimetallic Nanocrystals Nanocomposites for Electrocatalytic Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2014, 6, 2086–2094. [Google Scholar] [CrossRef]
- Fan, J.; Du, H.; Zhao, Y.; Wang, Q.; Liu, Y.; Li, D.; Feng, J. Recent Progress on Rational Design of Bimetallic Pd Based Catalysts and Their Advanced Catalysis. ACS Catal. 2020, 10, 13560–13583. [Google Scholar] [CrossRef]
- Chasanah, U.; Trisunaryanti, W.; Triyono; Santoso, I.; Fatmawati, D.A.; Purbonegoro, J. Effect of Stabilizer Agent Type on the Characteristics of Pd–Ni Nanoparticles Deposited on Reduced Graphene Oxide as Electrocatalysts for the Oxygen Reduction Reaction. J. Mater. Sci. 2024, 59, 20593–20605. [Google Scholar] [CrossRef]
- Hammer, B.; Nørskov, J.K. Electronic Factors Determining the Reactivity of Metal Surfaces. Surf. Sci. 1995, 343, 211–220. [Google Scholar] [CrossRef]
- Xu, Y.; Ruban, A.V.; Mavrikakis, M. Adsorption and Dissociation of O 2 on Pt-Co and Pt-Fe Alloys. J. Am. Chem. Soc. 2004, 126, 4717–4725. [Google Scholar] [CrossRef]
- Mavrikakis, M.; Hammer, B.; Nørskov, J.K. Effect of Strain on the Reactivity of Metal Surfaces. Phys. Rev. Lett. 1998, 81, 2819–2822. [Google Scholar] [CrossRef]
- Castegnaro, M.V.; Paschoalino, W.J.; Fernandes, M.R.; Balke, B.; Alves, M.C.M.; Ticianelli, E.A.; Morais, J. Pd–M/C (M = Pd, Cu, Pt) Electrocatalysts for Oxygen Reduction Reaction in Alkaline Medium: Correlating the Electronic Structure with Activity. Langmuir 2017, 33, 2734–2743. [Google Scholar] [CrossRef] [PubMed]
- Lüsi, M.; Erikson, H.; Käärik, M.; Piirsoo, H.-M.; Aruväli, J.; Kikas, A.; Kisand, V.; Leis, J.; Kukli, K.; Tammeveski, K. One-Pot Synthesis of Pd Nanoparticles Supported on Carbide-Derived Carbon for Oxygen Reduction Reaction. Nanomaterials 2024, 14, 994. [Google Scholar] [CrossRef] [PubMed]
- Golubović, J.; Rakočević, L.; Vasiljević Radović, D.; Štrbac, S. Improved Oxygen Reduction on GC-Supported Large-Sized Pt Nanoparticles by the Addition of Pd. Catalysts 2022, 12, 968. [Google Scholar] [CrossRef]
- Golubović, J.; Rakočević, L.; Štrbac, S. The Effect of Sulphate and Chloride Palladium Salt Anions on the Morphology of Electrodeposited Pd Nanoparticles and Their Catalytic Activity for Oxygen Reduction in Acid and Alkaline Media. Int. J. Electrochem. Sci. 2022, 17, 220943. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, P.; Zhang, H.; Deng, K.; Yu, H.; Xu, Y.; Li, X.; Wang, H.; Wang, L. PdCu Bimetallene for Enhanced Oxygen Reduction Electrocatalysis. Inorg. Chem. 2023, 62, 5622–5629. [Google Scholar] [CrossRef]
- Xu, W.; Yoon, D.; Yang, Y.; Xiong, Y.; Li, H.; Zeng, R.; Muller, D.A.; Abruña, H.D. MOF-Derived Bimetallic Pd-Co Alkaline ORR Electrocatalysts. ACS Appl. Mater. Interfaces 2022, 14, 44735–44744. [Google Scholar] [CrossRef]
- Schneider-Coppolino, M.; Taylor, A.; Kilham, A.M.; Gautam, S.; Tahmasebi, S.; Gates, B.D. Cathodic Pd 1-X Ni X Nanocatalyst Development for Alkaline Fuel Cell Applications. ECS Meet. Abstr. 2022, 241, 2295. [Google Scholar] [CrossRef]
- Zamora Zeledón, J.A.; Stevens, M.B.; Gunasooriya, G.T.K.K.; Gallo, A.; Landers, A.T.; Kreider, M.E.; Hahn, C.; Nørskov, J.K.; Jaramillo, T.F. Tuning the Electronic Structure of Ag-Pd Alloys to Enhance Performance for Alkaline Oxygen Reduction. Nat. Commun. 2021, 12, 620. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, Y.; Zhao, X.; Shen, T.; Zhao, T.; Gong, M.; Chen, K.; Lai, C.; Zhang, J.; Xin, H.L.; et al. Highly Active N-Doped Carbon Encapsulated Pd-Fe Intermetallic Nanoparticles for the Oxygen Reduction Reaction. Nano Res. 2020, 13, 2365–2370. [Google Scholar] [CrossRef]
- St. John, S.; Atkinson, R.W.; Dyck, O.; Sun, C.-J.; Zawodzinski, T.A.; Papandrew, A.B. Segregated Pt on Pd Nanotubes for Enhanced Oxygen Reduction Activity in Alkaline Electrolyte. Chem. Commun. 2015, 51, 16633–16636. [Google Scholar] [CrossRef] [PubMed]
- Archana, S.; Elumalai, P. Solvent-Engineered ZIF-67-Derived Cobalt-Embedded Carbon as Polysulfide Trapping Host for High-Stability Li–S Battery. Ionics 2024. [Google Scholar] [CrossRef]
- Chandra Sekhar, Y.; Raghavendra, P.; Sri Chandana, P.; Maiyalagan, T.; Subramanyam Sarma, L. Graphene Supported Pd–Cu Bimetallic Nanoparticles as Efficient Catalyst for Electrooxidation of Methanol in Alkaline Media. J. Phys. Chem. Solids 2023, 174, 111133. [Google Scholar] [CrossRef]
- Chandra Sekhar, Y.; Raghavendra, P.; Thulasiramaiah, G.; Sravani, B.; Sri Chandana, P.; Maiyalagan, T.; Sarma, L.S. Reduced Graphene Oxide (RGO)-Supported Pd–CeO2 Nanocomposites as Highly Active Electrocatalysts for Facile Formic Acid Oxidation. New J. Chem. 2022, 46, 2478–2486. [Google Scholar] [CrossRef]
- Sekhar, Y.C.; Vinothkumar, V.; Rao, H.S.; Sarma, L.S.; Oh, J.; Kim, T.H. Synergistic Effects of Platinum-Bismuth Nanoalloys on Reduced Graphene Oxide for Superior Methanol and Ethanol Oxidation in Acidic Medium. Int. J. Hydrogen Energy 2024, 81, 471–480. [Google Scholar] [CrossRef]
- Hossain, S.S.; Alwi, M.M.; Saleem, J.; Al-Hashem, H.T.; McKay, G.; Mansour, S.; Ali, S.S. Bimetallic Pd-Co Nanoparticles Supported on Nitrogen-Doped Reduced Graphene Oxide as Efficient Electrocatalysts for Formic Acid Electrooxidation. Catalysts 2021, 11, 910. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Hosseinzadeh, F.; Zardari, P.; Darbandi, M. Pd-Co Nanoparticles Decorated on Different Carbon Based Substrates as Electrocatalyst for O2 Reduction Reaction. Int. J. Hydrogen Energy 2021, 46, 28513–28526. [Google Scholar] [CrossRef]
- He, W.; Jiang, H.; Zhou, Y.; Yang, S.; Xue, X.; Zou, Z.; Zhang, X.; Akins, D.L.; Yang, H. An Efficient Reduction Route for the Production of Pd–Pt Nanoparticles Anchored on Graphene Nanosheets for Use as Durable Oxygen Reduction Electrocatalysts. Carbon 2012, 50, 265–274. [Google Scholar] [CrossRef]
- Shen, C.; Chen, H.; Qiu, M.; Shi, Y.; Yan, W.; Jiang, Q.; Jiang, Y.; Xie, Z. Introducing Oxophilic Metal and Interstitial Hydrogen into the Pd Lattice to Boost Electrochemical Performance for Alkaline Ethanol Oxidation. J. Mater. Chem. A Mater. 2022, 10, 1735–1741. [Google Scholar] [CrossRef]
- Son, D.N.; Le, O.K.; Chihaia, V.; Takahashi, K. Effects of Co Content in Pd-Skin/PdCo Alloys for Oxygen Reduction Reaction: Density Functional Theory Predictions. J. Phys. Chem. C 2015, 119, 24364–24372. [Google Scholar] [CrossRef]
- Sankarasubramanian, S.; Singh, N.; Mizuno, F.; Prakash, J. Ab Initio Investigation of the Oxygen Reduction Reaction Activity on Noble Metal (Pt, Au, Pd), Pt3M (M = Fe, Co, Ni, Cu) and Pd3M (M = Fe, Co, Ni, Cu) Alloy Surfaces, for Li O2 Cells. J. Power Sources 2016, 319, 202–209. [Google Scholar] [CrossRef]
- Maheswari, S.; Karthikeyan, S.; Murugan, P.; Sridhar, P.; Pitchumani, S. Carbon-Supported Pd-Co as Cathode Catalyst for APEMFCs and Validation by DFT. Phys. Chem. Chem. Phys. 2012, 14, 9683. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Chandra Sahu, S.; Ghosh, A.; Basu, S.; Chakraborty, B.; Jena, B.K. The Experimental and Theoretical Insights towards the CO Induced Pd-Graphene and Their Multifunctional Energy Conversion Applications. Carbon 2019, 149, 307–317. [Google Scholar] [CrossRef]
- Nasim, F.; Nadeem, M.A. Understanding the Mechanism and Synergistic Interaction of Cobalt-Based Electrocatalysts Containing Nitrogen-Doped Carbon for 4 e—ORR. J. Mater. Chem. A Mater. 2023, 11, 10095–10124. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).