Effect of Support on Complete Hydrocarbon Oxidation over Pd-Based Catalysts
Abstract
1. Introduction
2. Results
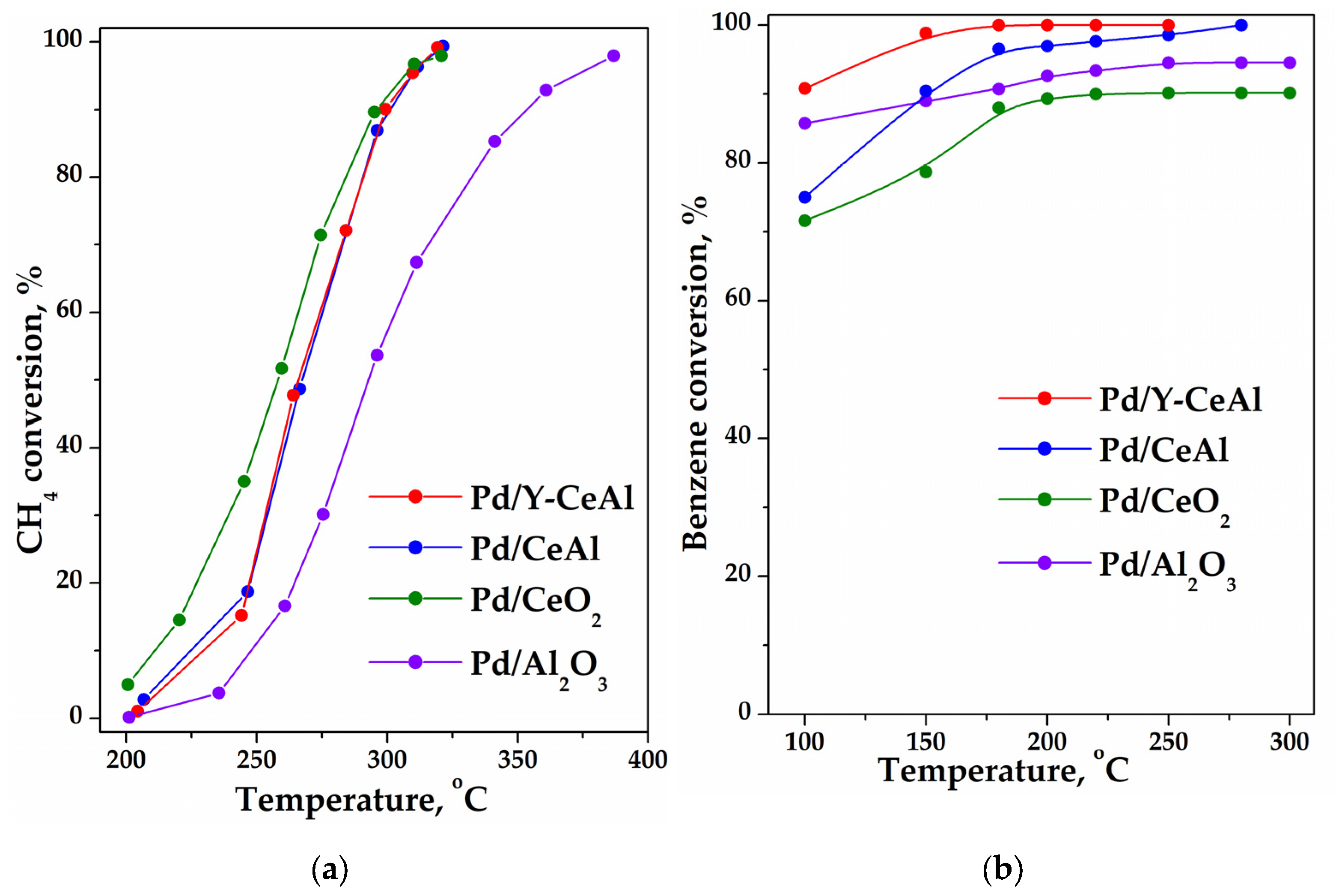
2.1. Catalytic Activity Measurements
2.2. Catalyst Characterization
2.2.1. Chemical Composition
2.2.2. Textural Characteristics
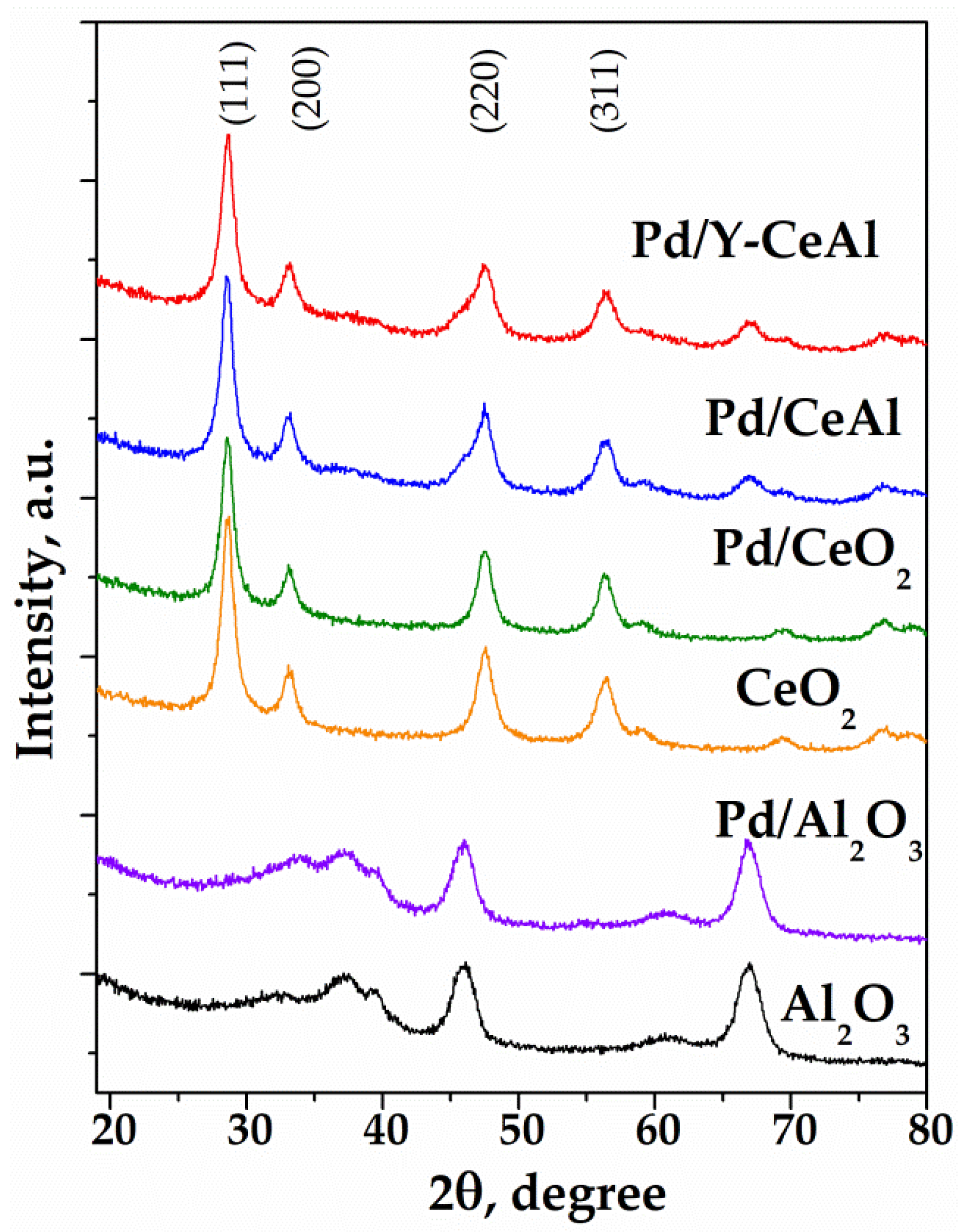
2.2.3. X-Ray Powder Diffraction (XRD) Measurements
2.2.4. X-Ray Photoelectron Spectroscopy (XPS) Measurements
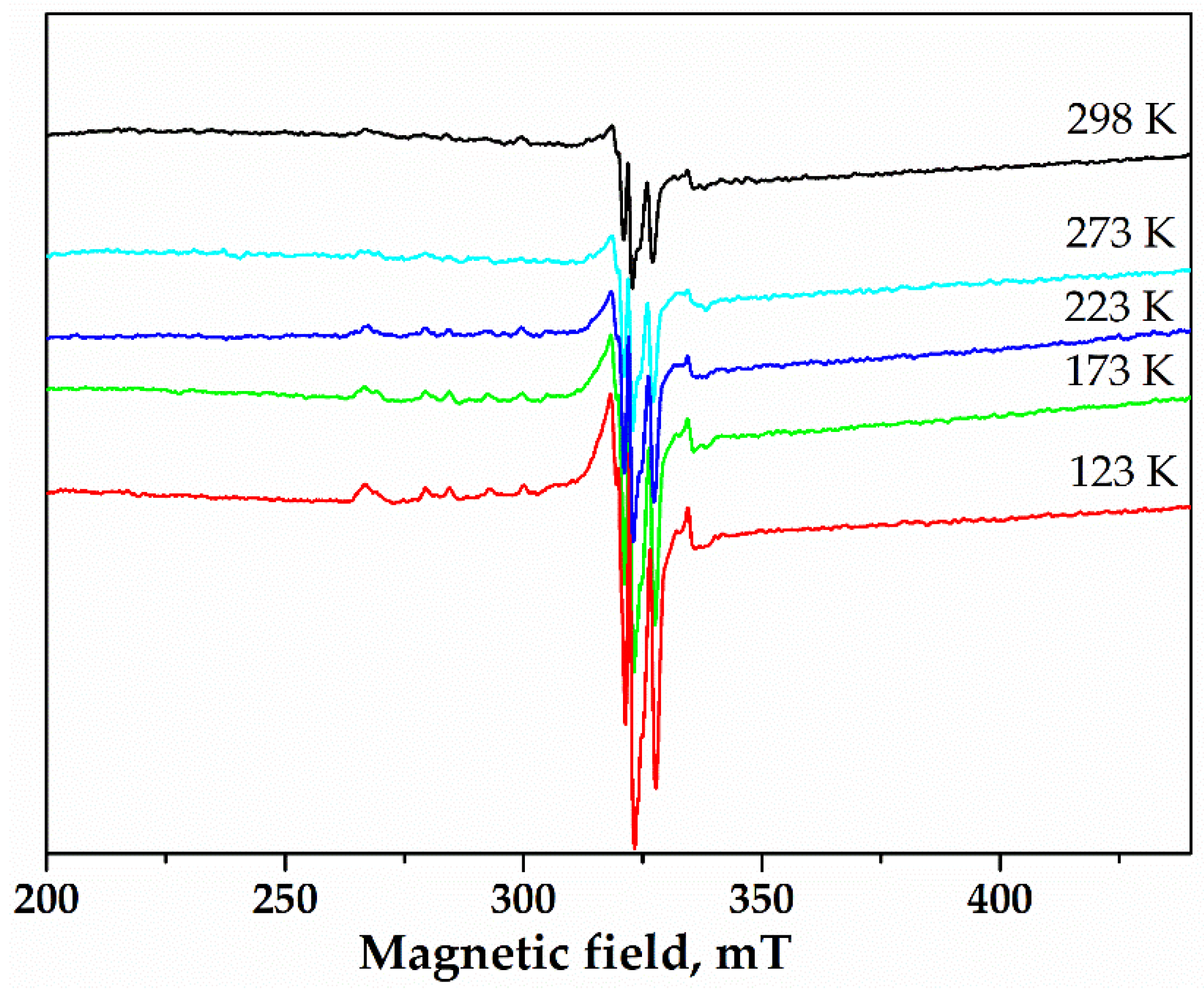
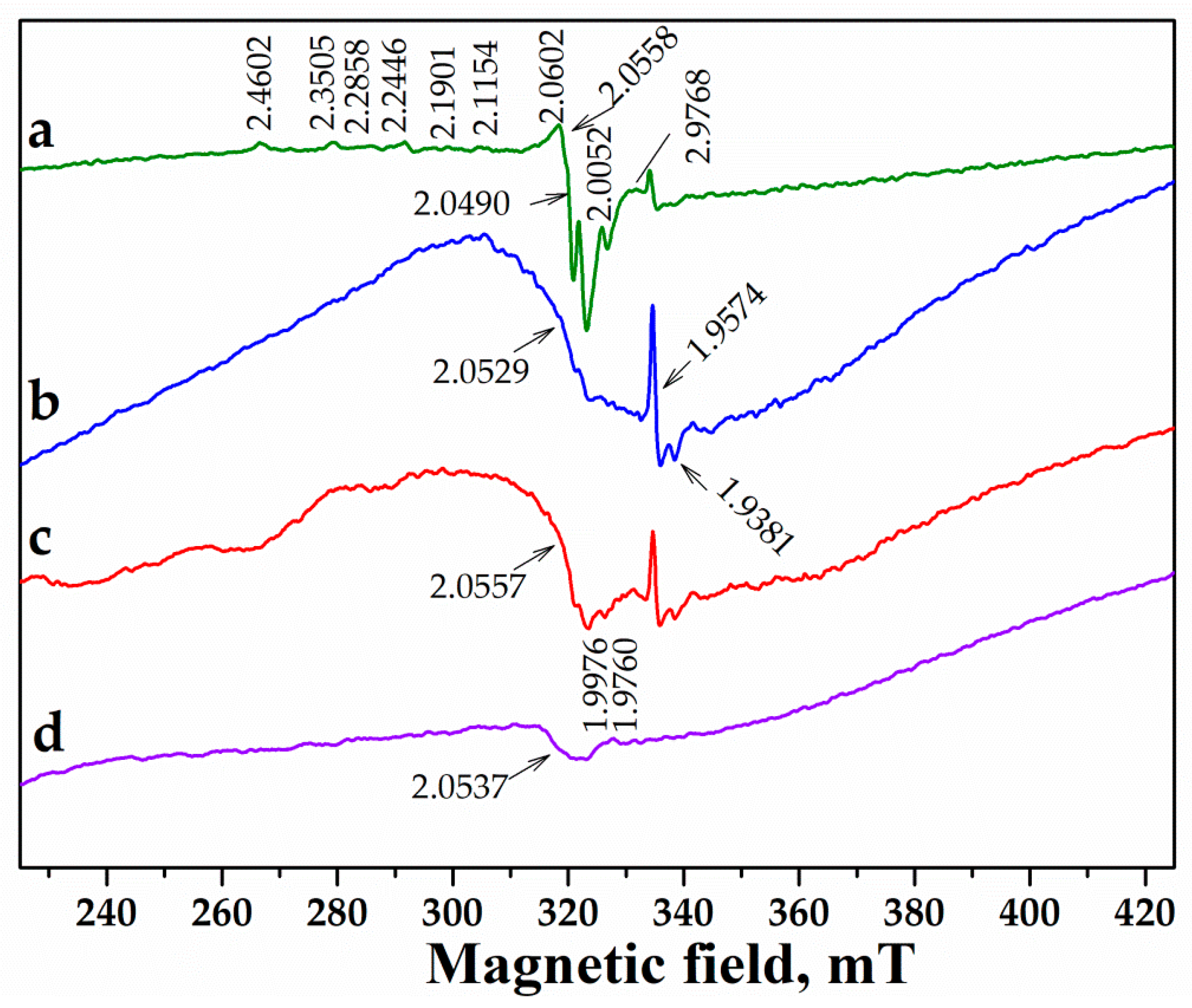
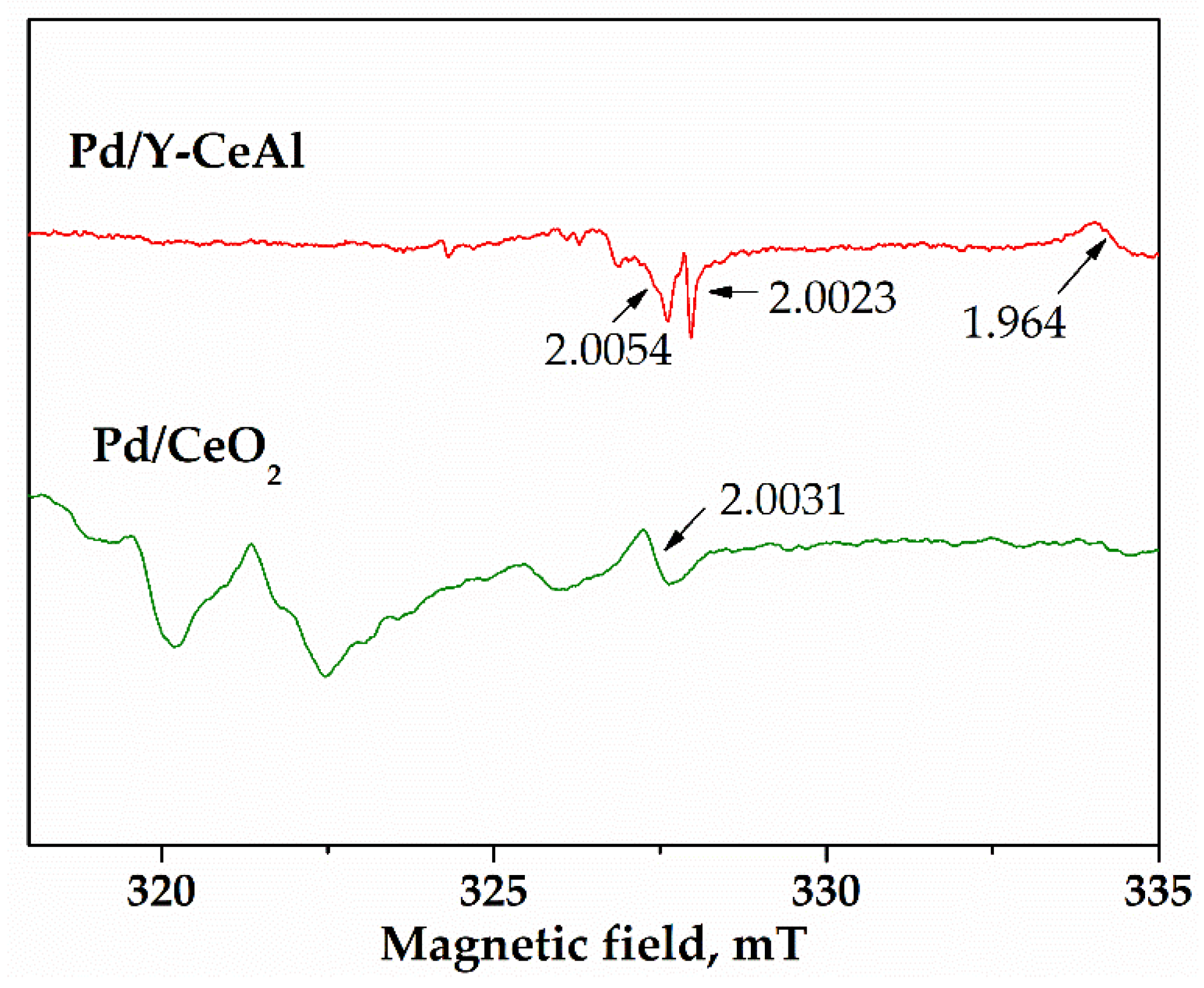
2.2.5. Electron Paramagnetic Resonance (EPR) Measurements
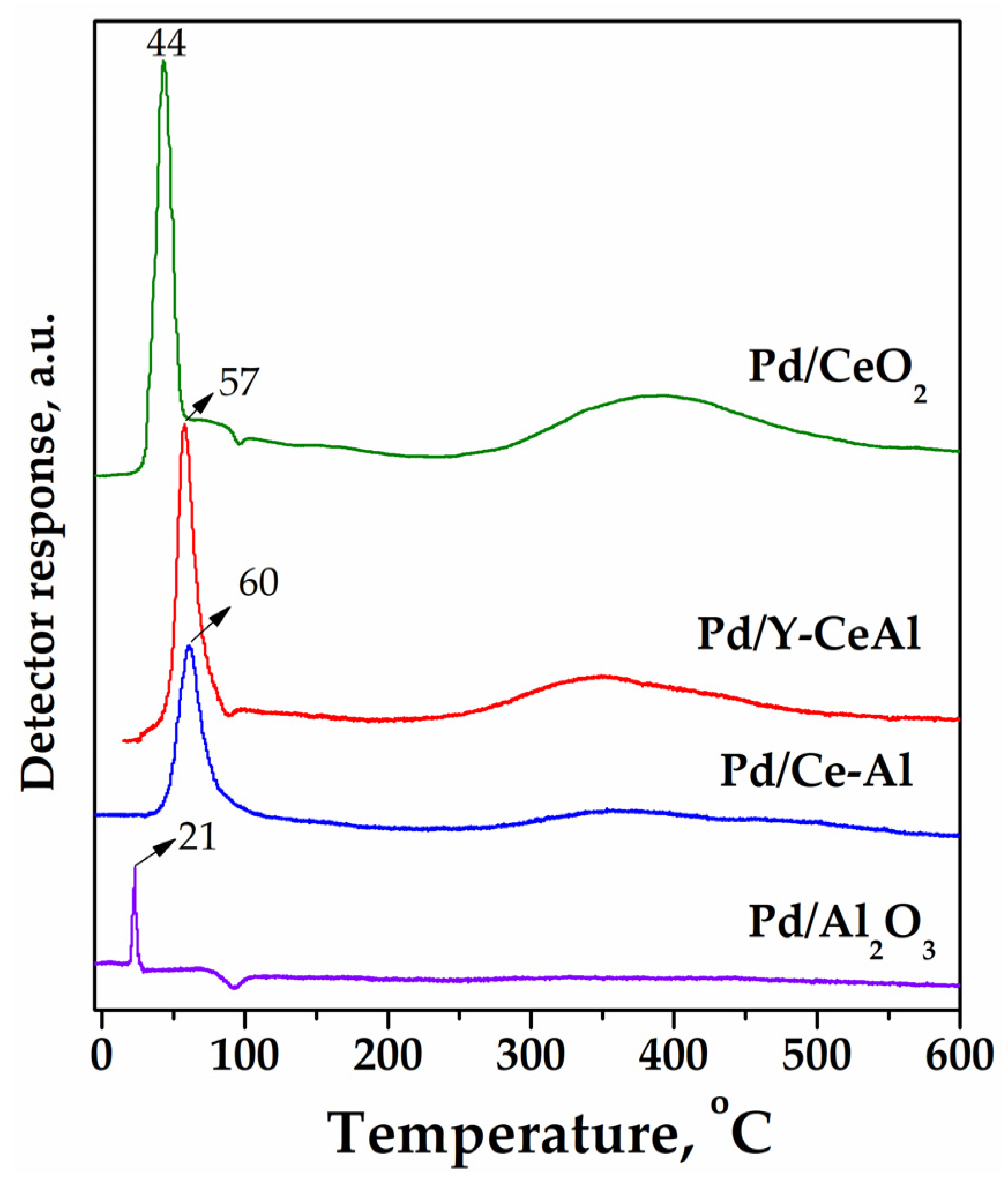
2.2.6. Temperature-Programmed Reduction (H2-TPR) Measurements
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Catalysts Preparation
4.3. Catalyst Characterization
4.4. Catalytic Activity Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moores, F.C. Climate change and air pollution: Exploring the synergies and potential for mitigation in industrializing countries. Sustainability 2009, 1, 43–54. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 505570. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Gelles, T.; Krishnamurthy, A.; Adebayo, B.; Rownaghi, A.; Rezaei, F. Abatement of gaseous volatile organic compounds: A material perspective. Catal. Today 2020, 350, 3–18. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B Environ. 2021, 281, 119447. [Google Scholar] [CrossRef]
- Todorova, S.; Naydenov, A.; Velinova, R.; Kolev, H.; Larin, A.V.; Stoyanova, D.; Shopska, M.; Tenchev, K.; Stefanov, P. Pd–MeOx/Al2O3 (Me = Co, La, Ce) catalysts for methane combustion. React. Kin. Mech. Catal. 2019, 126, 663–678. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Y.; Tang, J. Catalytic methane removal to mitigate its environmental effect. Sci. China Chem. 2023, 66, 1032–1051. [Google Scholar] [CrossRef]
- Liu, R.; Wu, H.; Shi, J.; Xu, X.; Zhao, D.; Ng, Y.H.; Zhang, M.; Liu, S.; Ding, H. Recent progress on catalysts for catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2022, 12, 6945–6991. [Google Scholar] [CrossRef]
- Zhang, K.; Ding, H.; Pan, W.; Mu, X.; Qiu, K.; Ma, J.; Zhao, Y.; Song, J.; Zhang, Z. Research progress of a composite metal oxide catalyst for VOC degradation. Environ. Sci. Technol. 2022, 56, 9220–9236. [Google Scholar] [CrossRef]
- Song, S.; Zhang, S.; Zhang, X.; Verma, P.; Wen, M. Advances in catalytic oxidation of volatile organic compounds over Pd-supported catalysts: Recent trends and challenges. Front. Mater. 2020, 7, 595667. [Google Scholar] [CrossRef]
- Monai, M.; Montini, T.; Gorte, R.J.; Fornasiero, P. Catalytic Oxidation of Methane: Pd and Beyond. Eur. J. Inorg. Chem. 2018, 2018, 2884–2893. [Google Scholar] [CrossRef]
- Tabakova, T. State of the Art and Challenges in Complete Benzene Oxidation: A Review. Molecules 2024, 29, 5484. [Google Scholar] [CrossRef]
- Padilla, J.M.; Del Angel, G.; Navarrete, J. Improved Pd/γ-Al2O3-Ce catalysts for benzene combustion. Catal. Today 2008, 133–135, 541–547. [Google Scholar] [CrossRef]
- Ramírez-López, R.; Elizalde-Martinez, I.; Balderas-Tapia, L. Complete catalytic oxidation of methane over Pd/CeO2-Al2O3: The influence of different ceria loading. Catal. Today 2010, 150, 358–362. [Google Scholar] [CrossRef]
- Sun, M.; Hu, W.; Yuan, S.; Zhang, H.; Cheng, T.; Wang, J.; Chen, Y. Effect of the loading sequence of CeO2 and Pd over Al2O3 on the catalytic performance of Pd-only close-coupled catalysts. Mol. Catal. 2020, 482, 100332. [Google Scholar] [CrossRef]
- Fertal, D.; Bukhovko, M.; Ding, Y.; Billor, M.; Banerjee, A. Particle size and PdO support interactions in PdO/CeO2-γAl2O3 catalysts and effect on methane combustion. Catalysts 2020, 10, 976. [Google Scholar] [CrossRef]
- Yang, X.; Du, C.; Guo, Y.; Guo, Y.; Wang, L.; Wang, Y.; Zhan, W. Al2O3 supported hybrid Pd–CeO2 colloidal spheres and its enhanced catalytic performances for methane combustion. J. Rare Earths 2019, 37, 714–719. [Google Scholar] [CrossRef]
- Ilieva, L.; Petrova, P.; Liotta, L.F.; Sobczak, J.W.; Lisowski, W.; Kaszkur, Z.; Munteanu, G.; Tabakova, T. Gold catalysts on Y-doped ceria supports for complete benzene oxidation. Catalysts 2016, 6, 99. [Google Scholar] [CrossRef]
- Ilieva, L.; Venezia, A.M.; Petrova, P.; Pantaleo, G.; Liotta, L.F.; Zanella, R.; Kaszkur, Z.; Tabakova, T. Effect of Y modified ceria support in mono and bimetallic Pd-Au catalysts for complete benzene oxidation. Catalysts 2018, 8, 283. [Google Scholar] [CrossRef]
- Venezia, A.M.; Di Carlo, G.; Liotta, L.F.; Pantaleo, G.; Kantcheva, M. Effect of Ti(IV) loading on CH4 oxidation activity and SO2 tolerance of Pd catalysts supported on silica SBA-15 and HMS. Appl. Catal. B Environ. 2011, 106, 529–539. [Google Scholar] [CrossRef]
- Tabakova, T.; Ilieva, L.; Petrova, P.; Venezia, A.M.; Avdeev, G.; Zanella, R.; Karakirova, Y. Complete benzene oxidation over mono and bimetallic Au–Pd catalysts supported on Fe-modified ceria. Chem. Eng. J. 2015, 260, 133–141. [Google Scholar] [CrossRef]
- Kalvachev, Y.; Todorova, T.; Kolev, H.; Merker, D.; Popov, C. Benzene oxidation over Pt loaded on fly ash zeolite X. Catalysts 2023, 13, 1128. [Google Scholar] [CrossRef]
- Tabakova, T.; Ivanov, I.; Zanella, R.; Karakirova, Y.; Sobczak, J.W.; Lisowski, W.; Kaszkur, Z.; Ilieva, L. Unraveling the effect of alumina-supported Y-doped ceria composition and method of preparation on the WGS activity of gold catalysts. Int. J. Hydrog. Energy 2020, 45, 26238–26253. [Google Scholar] [CrossRef]
- Liotta, L.F.; Pantaleo, G.; Puleo, F.; Venezia, A.M. Au/CeO2-SBA-15 catalysts for CO oxidation: Effect of ceria loading on physicochemical properties and catalytic performances. Catal. Today 2012, 187, 10–19. [Google Scholar] [CrossRef]
- Filimonov, I.N.; Ikonnikov, I.A.; Loginov, A.Y. EPR investigation of paramagnetic species on palladium-promoted yttria and lanthana. J. Chem. Soc. Faraday Trans. 1994, 90, 219–226. [Google Scholar] [CrossRef]
- Il’ichev, A.N.; Shashkin, D.P.; Khomenko, T.I.; Fattakhova, Z.T.; Korchak, V.N. Structure and surface properties of ZrO2, CeO2, and Zr0.5Ce0.5O2 prepared by a microemulsion method according to X-ray diffraction, TPR, and EPR data. Kinet. Catal. 2010, 51, 743–753. [Google Scholar] [CrossRef]
- Askrabic, S.; Dohcevic-Mitrovic, Z.D.; Araujo, V.D.; Ionita, G.; de Lima, M.M., Jr.; Cantarero, A. F-centre luminescence in nanocrystalline CeO2. J. Phys. D Appl. Phys. 2013, 46, 495306. [Google Scholar] [CrossRef]
- Soria, J.; Martinez-Arias, A.; Coronado, J.M.; Conesa, J.C. Electron paramagnetic resonance spectroscopy study of the adsorption of O2 and CO on a Pt/CeO2/Al2O3 catalyst. Coll. Surf. A Physicochem. Eng. Asp. 1996, 115, 215–221. [Google Scholar] [CrossRef]
- Wang, J.B.; Tai, Y.-L.; Dow, W.-P.; Huang, T.-J. Study of ceria-supported nickel catalyst and effect of yttria doping on carbon dioxide reforming of methane. Appl. Catal. A Gen. 2001, 218, 69–79. [Google Scholar] [CrossRef]
- Taarit, Y.B.; Vedrine, J.C.; Dutel, J.F.; Naccache, C. EPR Investigation of the structure and reactivity of Pd(1) species generated in synthetic mordenite-type zeolite. J. Magn. Reson. 1978, 31, 251–257. [Google Scholar] [CrossRef]
- Ge, L.; Chen, T.; Liu, Z.; Chen, F. The effect of gold loading on the catalytic oxidation performance of CeO2/H2O2 system. Catal. Today 2014, 224, 209–215. [Google Scholar] [CrossRef]
- Yao, H.C.; Yao, Y.F.Y. Ceria in automotive exhaust catalysts: I. Oxygen storage. J. Catal. 1984, 86, 254–265. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Hosseini, M.; Siffert, S.; Tidahy, H.L.; Cousin, R.; Lamonier, J.-F.; Aboukais, A.; Vantomme, A.; Roussel, M.; Su, B.-L. Promotional effect of gold added to palladium supported on a new mesoporous TiO2 for total oxidation of volatile organic compounds. Catal. Today 2007, 122, 391–396. [Google Scholar] [CrossRef]
- Gil, S.; Garcia-Vargas, J.M.; Liotta, L.F.; Pantaleo, G.; Ousmane, M.; Retailleau, L.; Giroir-Fendler, A. Catalytic oxidation of propene over Pd catalysts supported on CeO2, TiO2, Al2O3 and M/Al2O3 oxides (M = Ce, Ti, Fe, Mn). Catalysts 2015, 5, 671–689. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, W.; Li, W.; Xiong, X.; Wang, Y.; Cheng, K.; Kang, J.; Zhang, Q.; Wang, Y. In-situ confinement of ultrasmall palladium nanoparticles in silicalite-1 for methane combustion with excellent activity and hydrothermal stability. Appl. Catal. B Environ. 2020, 276, 119142. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Ge, W.; Xu, Y.; Jia, H.; Li, Q. Complete oxidation of lean methane over metal oxide supported Pd catalysts: Current advancement and future perspectives. J. Environ. Chem. Eng. 2023, 11, 110712. [Google Scholar] [CrossRef]
- Colussi, S.; Fornasiero, P.; Trovarelli, A. Structure-activity relationship in Pd/CeO2 methane oxidation catalysts. Chin. J. Catal. 2020, 41, 938–950. [Google Scholar] [CrossRef]
- Chen, S.; Li, S.; You, R.; Guo, Z.; Wang, F.; Li, G.; Yuan, W.; Zhu, B.; Gao, Y.; Zhang, Z.; et al. Elucidation of active sites for CH4 catalytic oxidation over Pd/CeO2 via tailoring metal-support interactions. ACS Catal. 2021, 11, 5666–5677. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Properties and performance of Pd based catalysts for catalytic oxidation of volatile organic compounds. Appl. Catal. B Environ. 2009, 92, 429–436. [Google Scholar] [CrossRef]
- He, C.; Li, J.; Li, P.; Cheng, J.; Hao, Z.; Xu, Z.-P. Comprehensive investigation of Pd/ZSM-5/MCM-48 composite catalysts with enhanced activity and stability for benzene oxidation. Appl. Catal. B Environ. 2010, 96, 466–475. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Chen, Y.; Yang, M.; Wu, Y. Effects of pretreatment atmospheres on the catalytic performance of Pd/γ-Al2O3 catalyst in benzene degradation. Catal. Commun. 2014, 46, 11–16. [Google Scholar] [CrossRef]
- Kang, S.; Wang, M.; Zhu, N.; Wang, C.; Deng, H.; He, H. Significant enhancement in water resistance of Pd/Al2O3 catalyst for benzene oxidation by Na addition. Chin. Chem. Lett. 2019, 30, 1450–1454. [Google Scholar] [CrossRef]
- He, Z.; He, Z.; Wang, D.; Bo, Q.; Fan, T.; Jiang, Y. Mo-modified Pd/Al2O3 catalysts for benzene catalytic combustion. J. Environ. Sci. 2014, 26, 1481–1487. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, Y.; Li, X.; Zhuang, G.; Wang, K.; Zheng, Y.; Sun, D.; Huang, J.; Li, Q. Catalytic benzene oxidation by biogenic Pd nanoparticles over 3D-ordered mesoporous CeO2. Chem. Eng. J. 2019, 362, 41–52. [Google Scholar] [CrossRef]
- Zhao, B.; Cheng, Z.; Zheng, J.; Wang, Z.; Zuo, S. Synthesis of C21H38ClN assisted Si pillared clays and the effects of CeO2 addition on its supported palladium catalyst for benzene oxidation. Catal. Lett. 2021, 151, 3287–3297. [Google Scholar] [CrossRef]
- Zuo, S.; Du, Y.; Liu, F.; Han, D.; Qi, C. Influence of ceria promoter on shell-powder-supported Pd catalyst for the complete oxidation of benzene. Appl. Catal. A Gen. 2013, 451, 65–70. [Google Scholar] [CrossRef]


| Sample | Chemical Composition (wt.%) | |||
|---|---|---|---|---|
| Pd | CeO2 | Y2O3 | Al2O3 | |
| Pd/Al2O3 | 1.03 | - | - | 98.97 |
| Pd/CeO2 | 1.10 | 98.90 | - | - |
| Pd/CeAl | 1.06 | 29.68 | - | 69.26 |
| Pd/Y-CeAl | 1.01 | 29.40 | 0.29 | 69.29 |
| Sample | SBET (m2 g−1) | Vpore (cm3 g−1) | Dpore (nm) | Dceria (nm) | αceria (nm) |
|---|---|---|---|---|---|
| γ-Al2O3 | 200.0 | 0.53 | 10.6 | - | - |
| Pd/Al2O3 | 195.8 | 0.52 | 9.7 | - | - |
| CeO2 | 63.0 | 0.29 | 20.3 | 6.3 | 0.5424 (2) |
| Pd/CeO2 | 67.6 | 0.28 | 16.5 | 6.2 | 0.5424 (4) |
| Ce/Al | 165.0 | 0.39 | 9.5 | 6.1 | 0.5422 (1) |
| Pd/CeAl | 161.2 | 0.45 | 9.5 | 5.8 | 0.5424 (1) |
| Y-Ce/Al | 168.0 | 0.43 | 10.0 | 6.1 | 0.5421 (2) |
| Pd/Y-CeAl | 166.4 | 0.42 | 9.6 | 5.8 | 0.5420 (1) |
| Catalyst | Ce 3d5/2 (eV) | Pd 3d5/2 (eV) | Y 3d5/2 (eV) | Y/Pd | Pd/(Ce + Al) | Ce3+/(Ce3+ + Ce4+) |
|---|---|---|---|---|---|---|
| Pd/Al2O3 | - | 336.7 | - | - | 0.017 0.048 * | - |
| Pd/CeO2 | 881.7 | 337.1 | - | - | 0.140 0.0163 * | 0.32 |
| Pd/CeAl | 881.8 | 336.6 | - | - | 0.044 0.0062 * | 0.33 |
| Pd/Y-CeAl | 881.8 | 336.7 | 157.8 | 0.21 0.28 * | 0.041 0.0063 * | 0.40 |
| Sample | Chemical Composition (wt.%) | |||||
|---|---|---|---|---|---|---|
| Pd3+ | Pd+ | Pdx+−O2 | O2− | Ce3+ | Ce4+−O2− | |
| Pd/Al2O3 | 2.0538 1.9974 1.9609 | 2.0812 2.0661 2.0270 | - | - | ||
| Pd/CeO2 | 2.4558, 2.113, 2.0621, 1.9759 | 2.3485, 2.3050, 2.2405, 2.1872 | 2.0575 | 1.9575, 1.9395 | 2.0482, 2.0437, 2.0330, 2.0048 | |
| Pd/CeAl | 1.9759 | 1.9576, 1.9383 | 2.0638, 2.0529, 2.0332, 2.0227, 2.0064 | |||
| Pd/Y-Ce/Al | 1.9770 | 2.0557 | 1.9573, 1.9379 | 2.0640, 2.0343, 2.0067 | ||
| Catalyst | HC (mmol·g−1) |
|---|---|
| Pd/Al2O3 | 0.09 |
| Pd/CeO2 | 0.43 |
| Pd/CeAl | 0.23 |
| Pd/Y-CeAl | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabakova, T.; Grahovski, B.; Karakirova, Y.; Petrova, P.; Venezia, A.M.; Liotta, L.F.; Todorova, S. Effect of Support on Complete Hydrocarbon Oxidation over Pd-Based Catalysts. Catalysts 2025, 15, 110. https://doi.org/10.3390/catal15020110
Tabakova T, Grahovski B, Karakirova Y, Petrova P, Venezia AM, Liotta LF, Todorova S. Effect of Support on Complete Hydrocarbon Oxidation over Pd-Based Catalysts. Catalysts. 2025; 15(2):110. https://doi.org/10.3390/catal15020110
Chicago/Turabian StyleTabakova, Tatyana, Bozhidar Grahovski, Yordanka Karakirova, Petya Petrova, Anna Maria Venezia, Leonarda Francesca Liotta, and Silviya Todorova. 2025. "Effect of Support on Complete Hydrocarbon Oxidation over Pd-Based Catalysts" Catalysts 15, no. 2: 110. https://doi.org/10.3390/catal15020110
APA StyleTabakova, T., Grahovski, B., Karakirova, Y., Petrova, P., Venezia, A. M., Liotta, L. F., & Todorova, S. (2025). Effect of Support on Complete Hydrocarbon Oxidation over Pd-Based Catalysts. Catalysts, 15(2), 110. https://doi.org/10.3390/catal15020110










