Mn-MOFs with Different Morphologies Derived MnOx Catalysts for Efficient CO Catalytic Oxidation
Abstract
1. Introduction
2. Results and Discussions
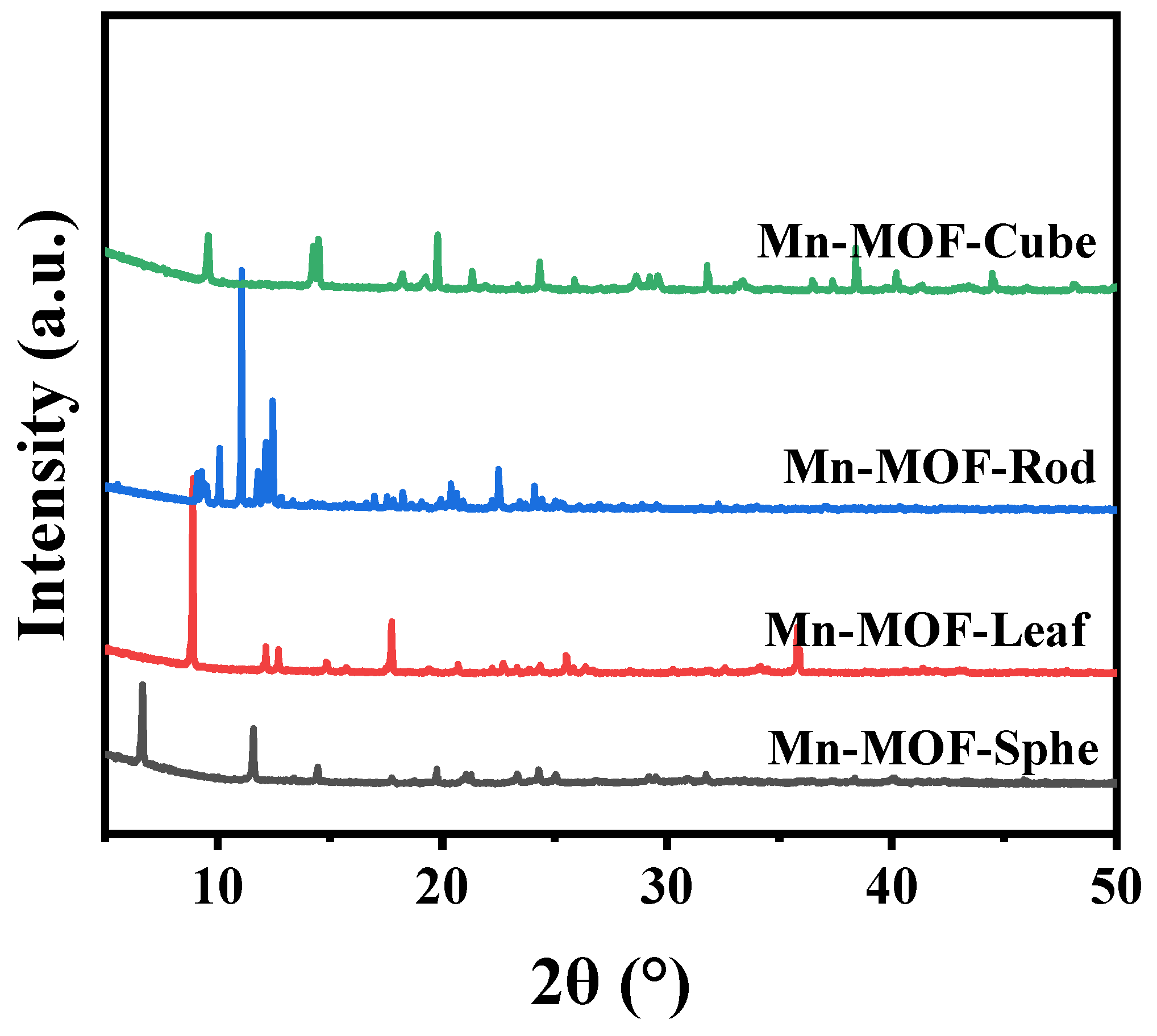
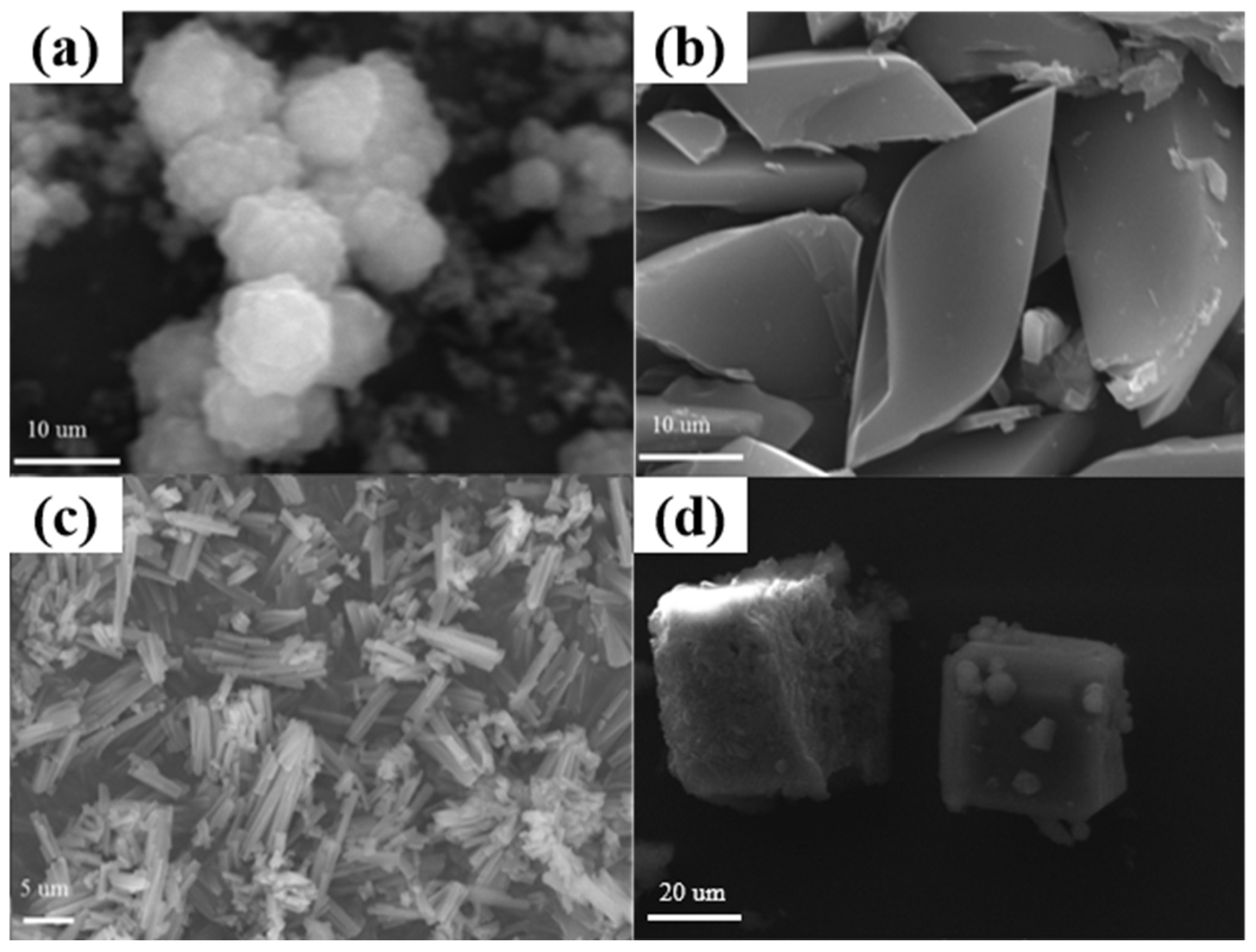
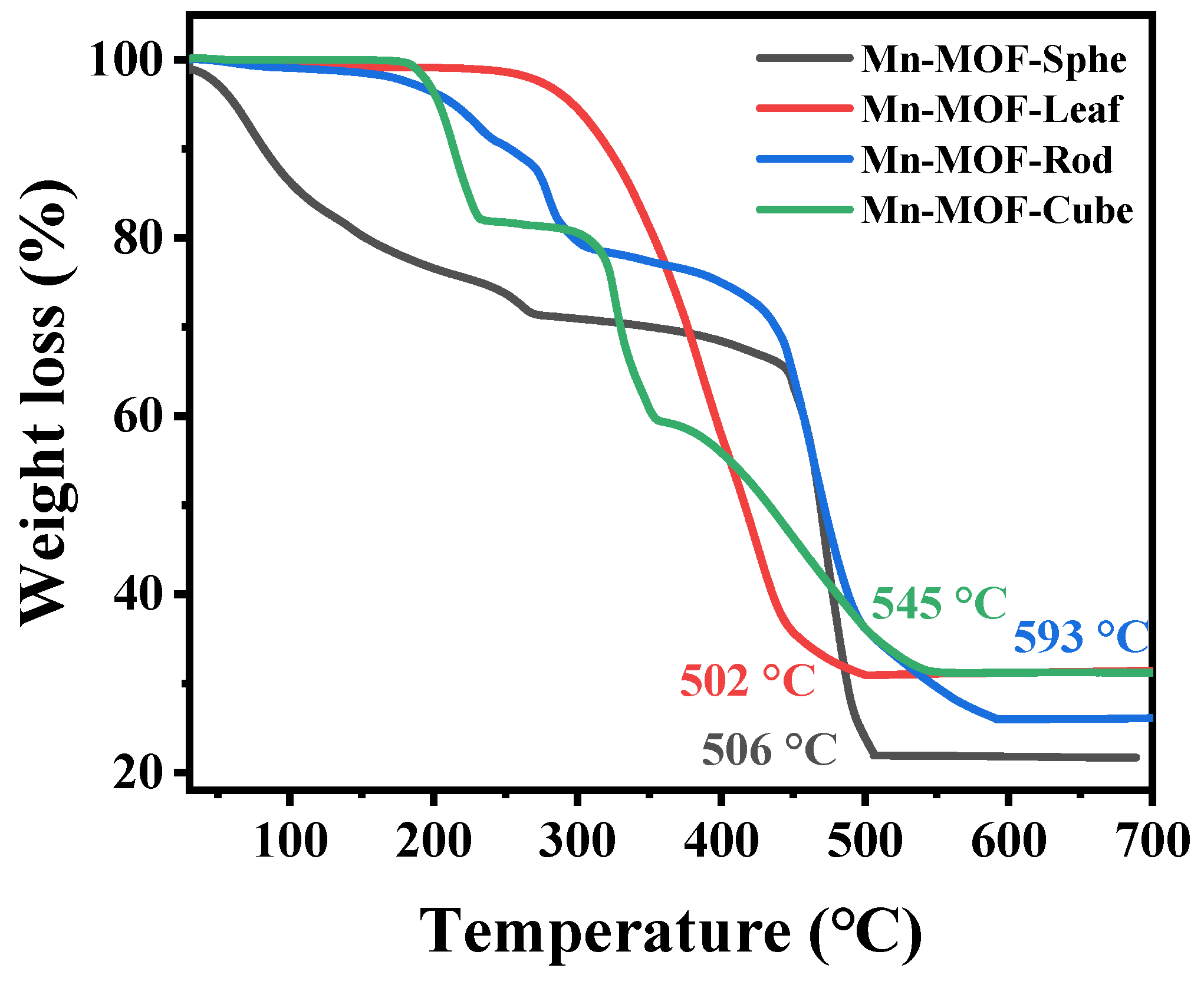
2.1. Characterization of the Mn-MOFs Precursors
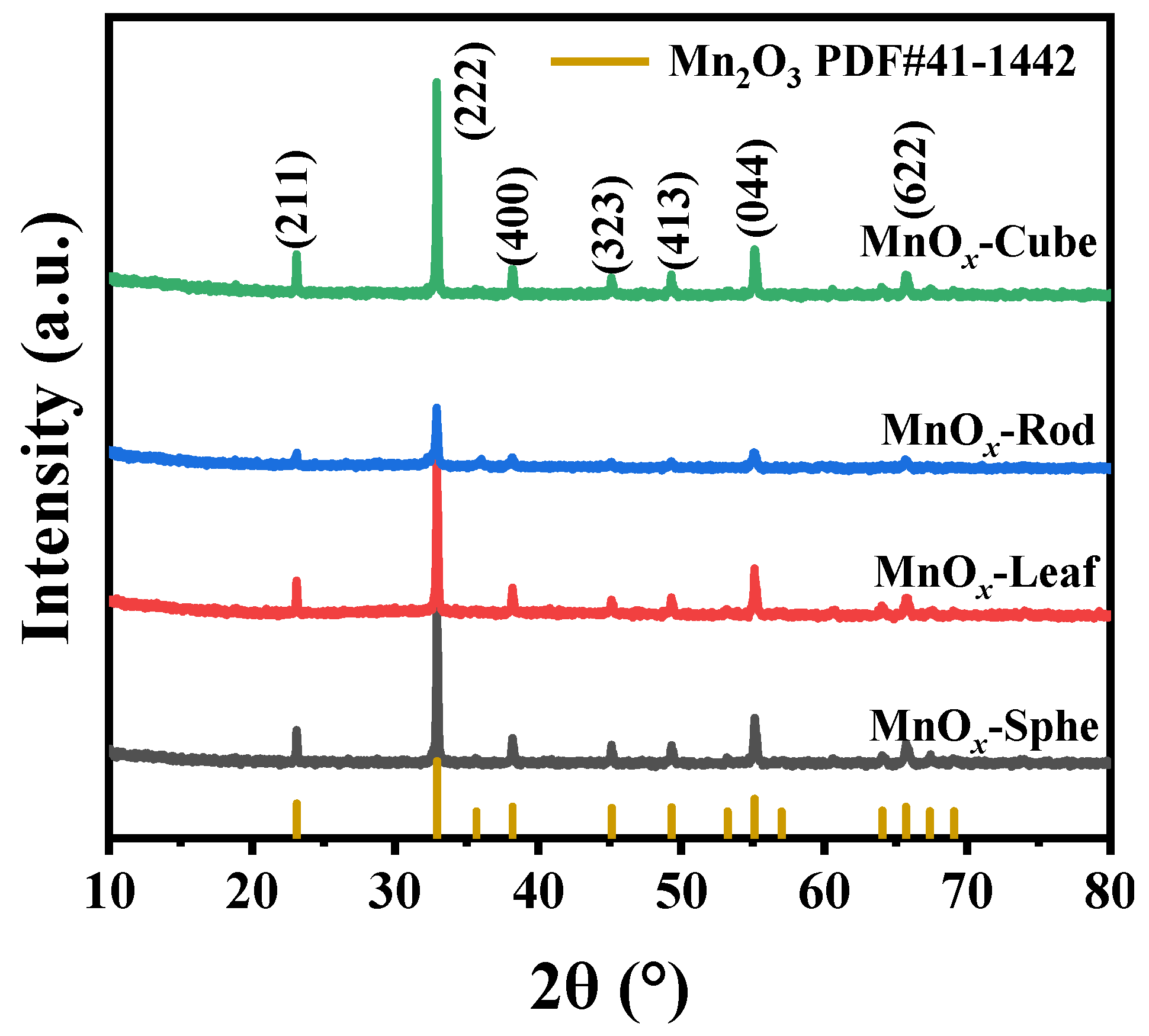
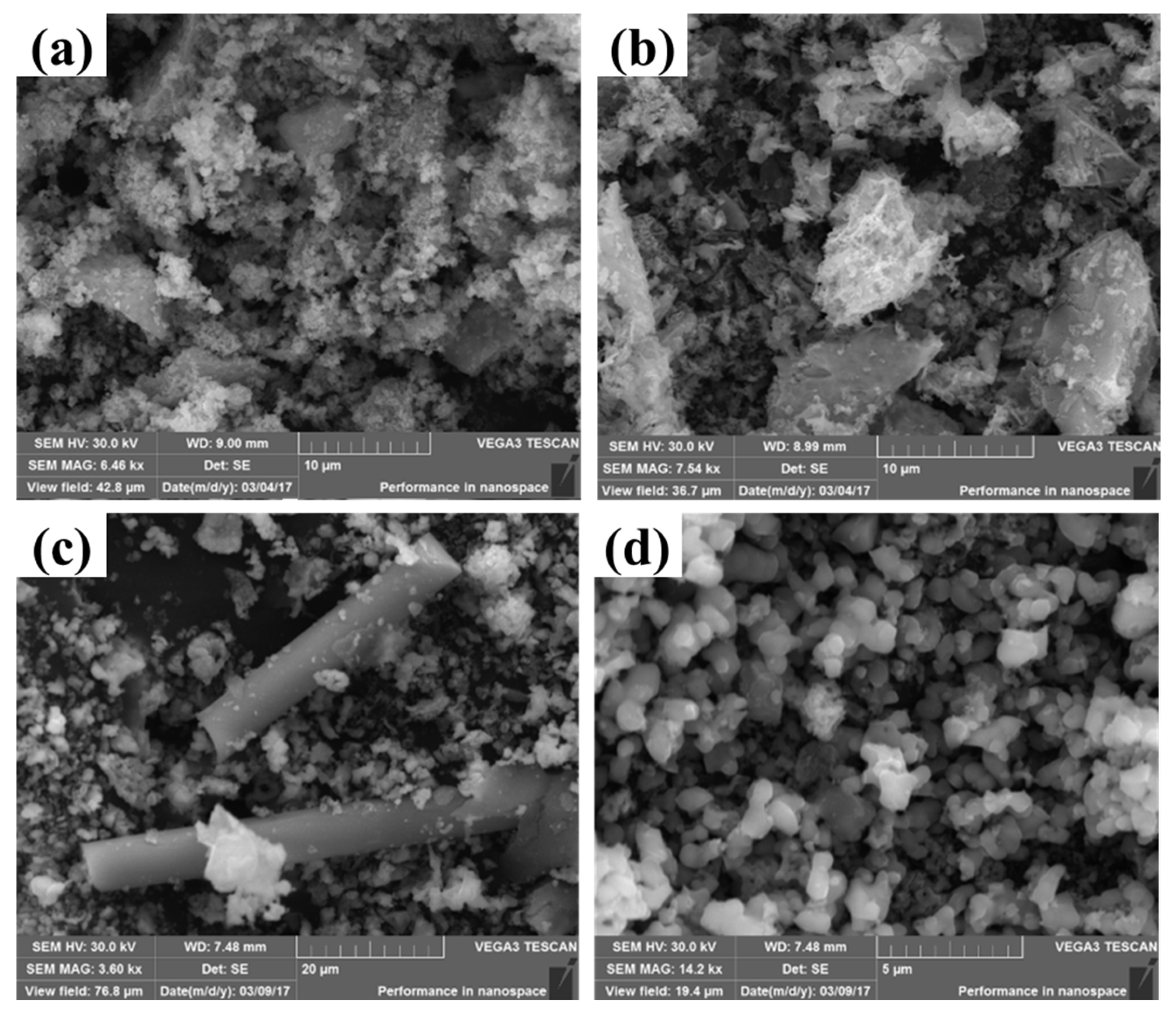
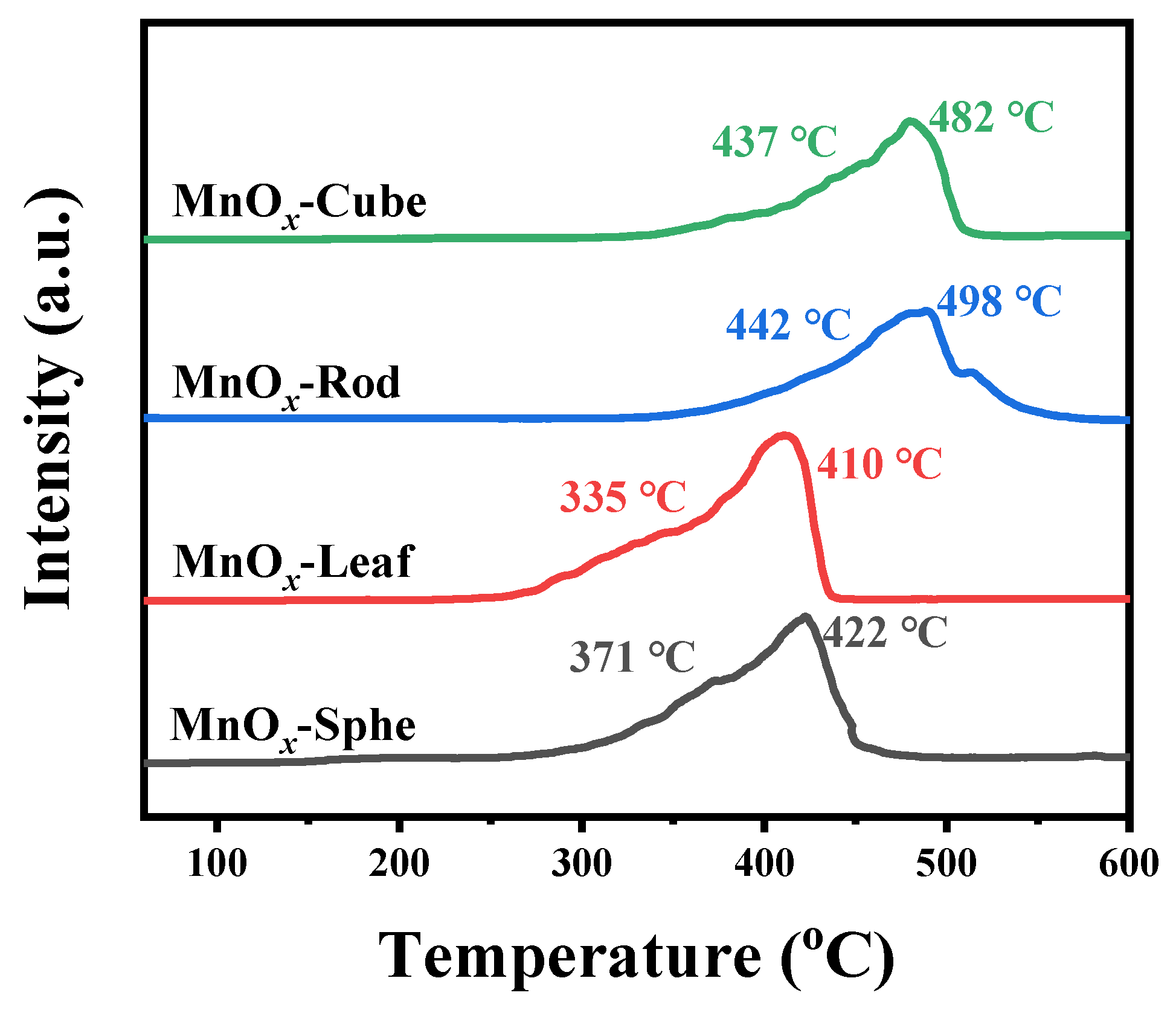
2.2. Characterizations of the Mn-MOF-Derived MnOx Catalysts
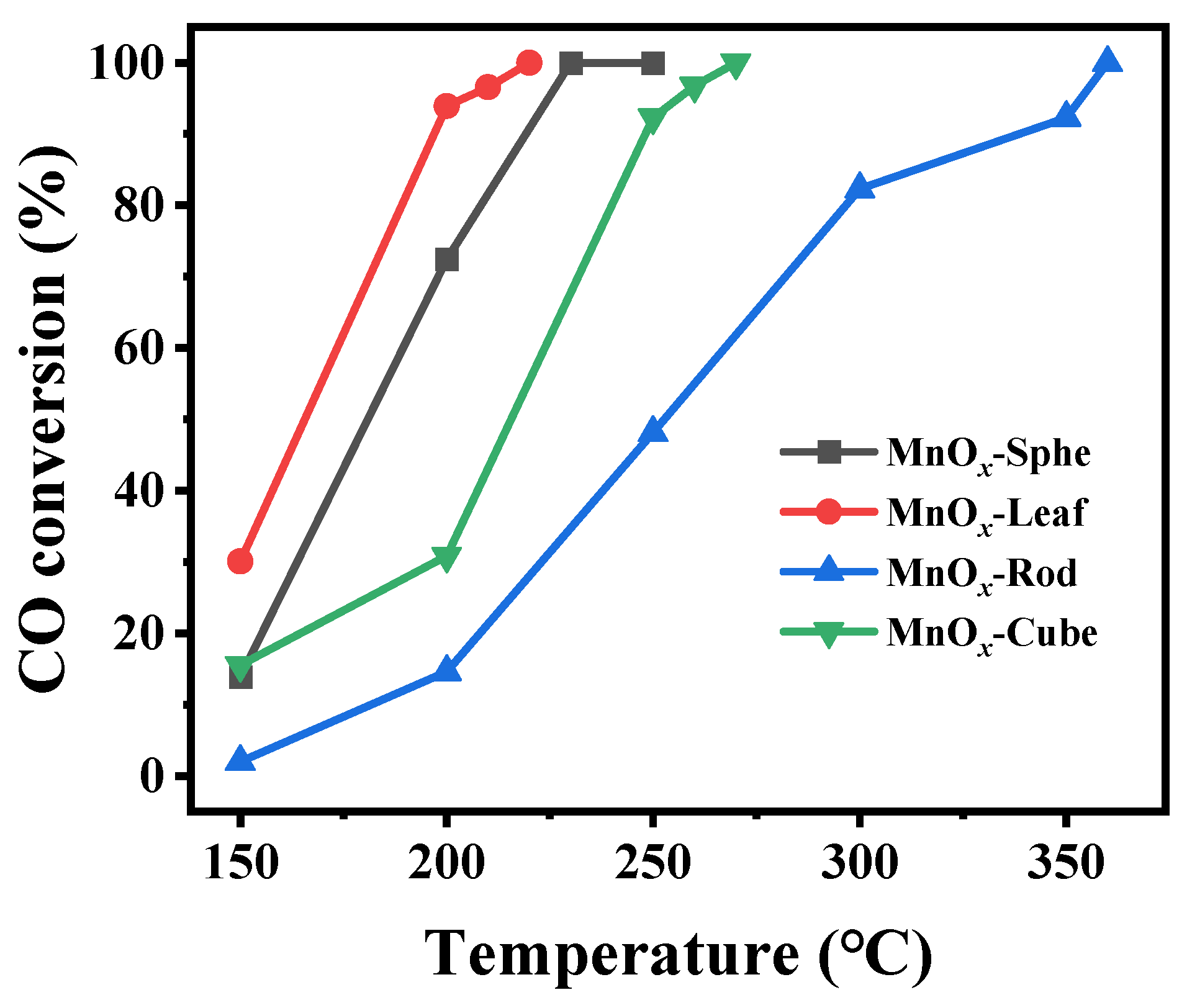
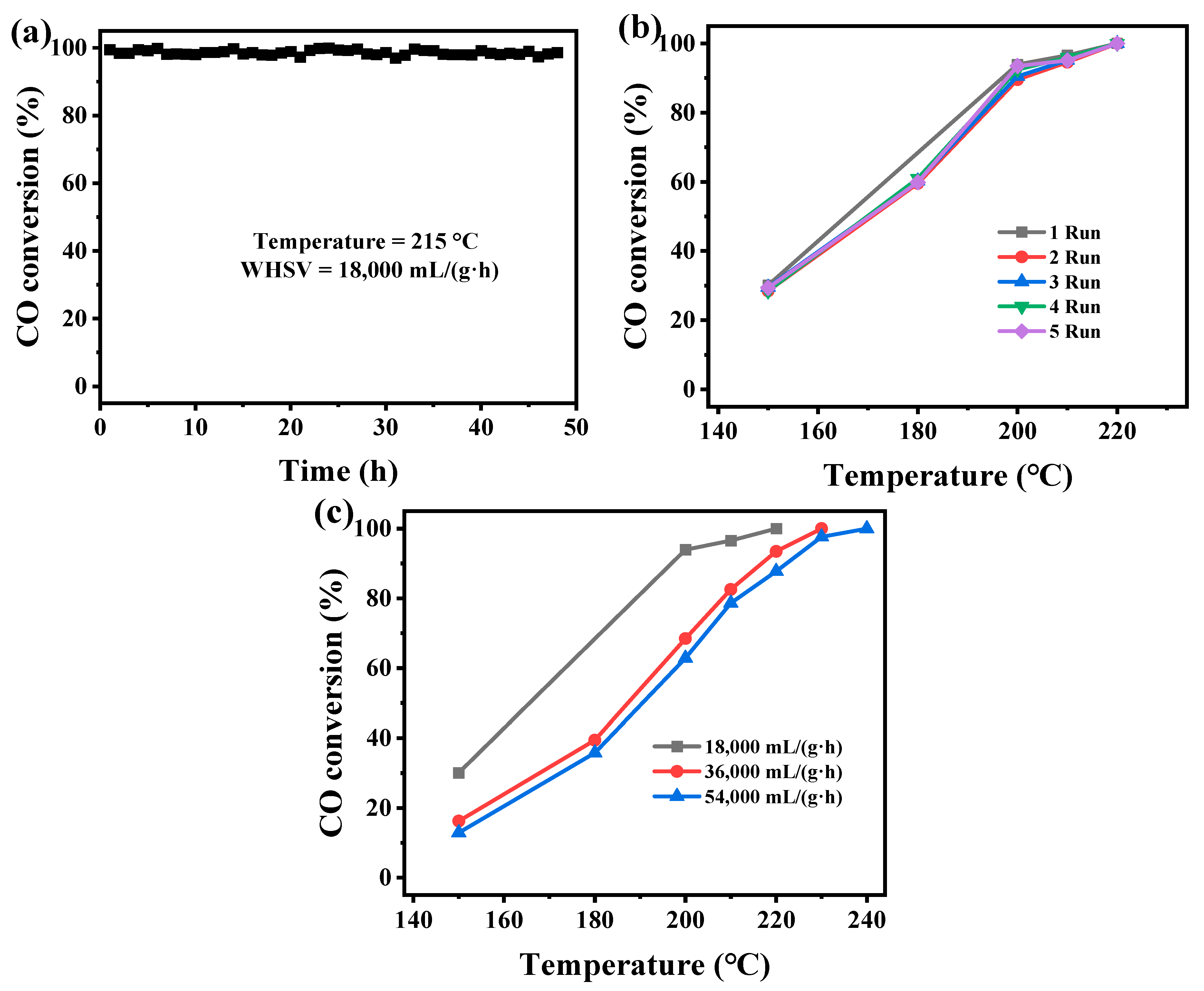
2.3. Catalytic Performance of the Mn-MOF-Derived MnOx Catalysts for CO Oxidation
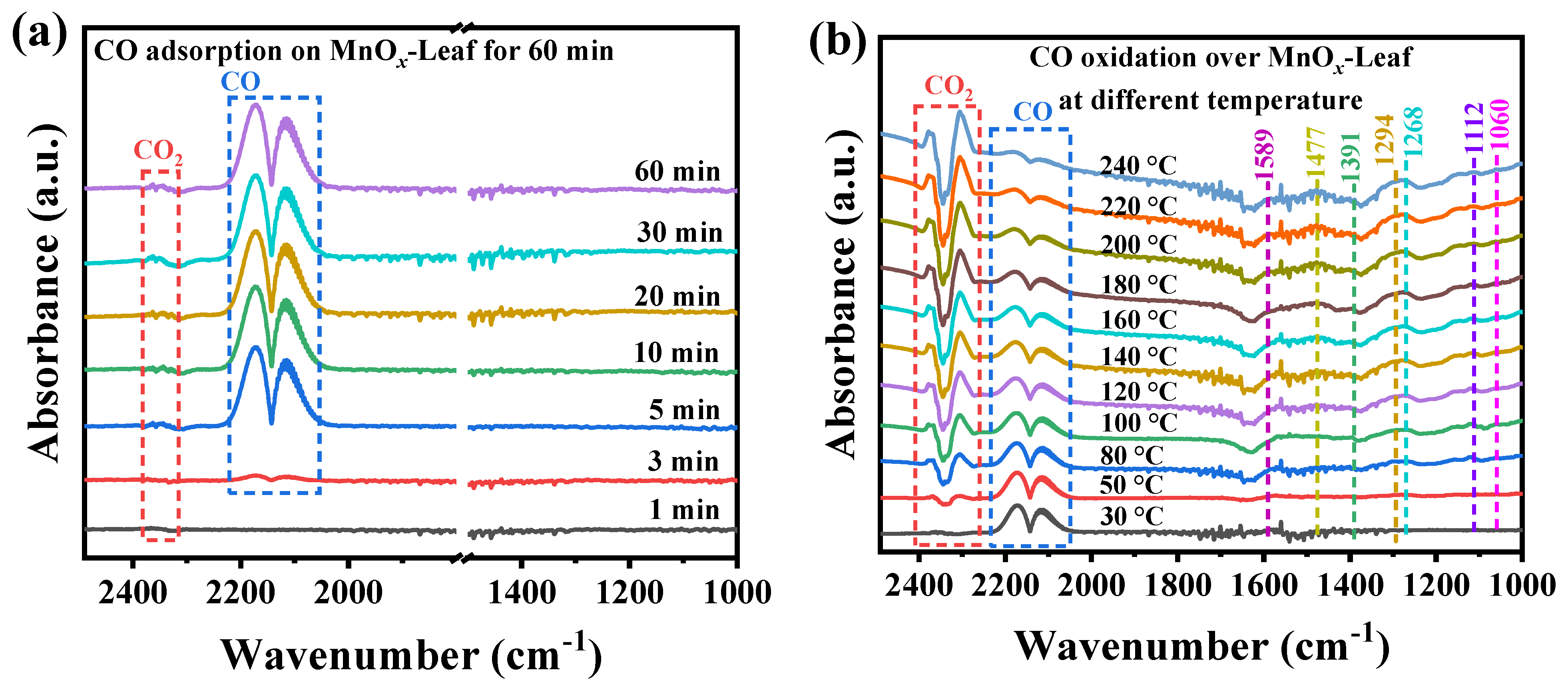
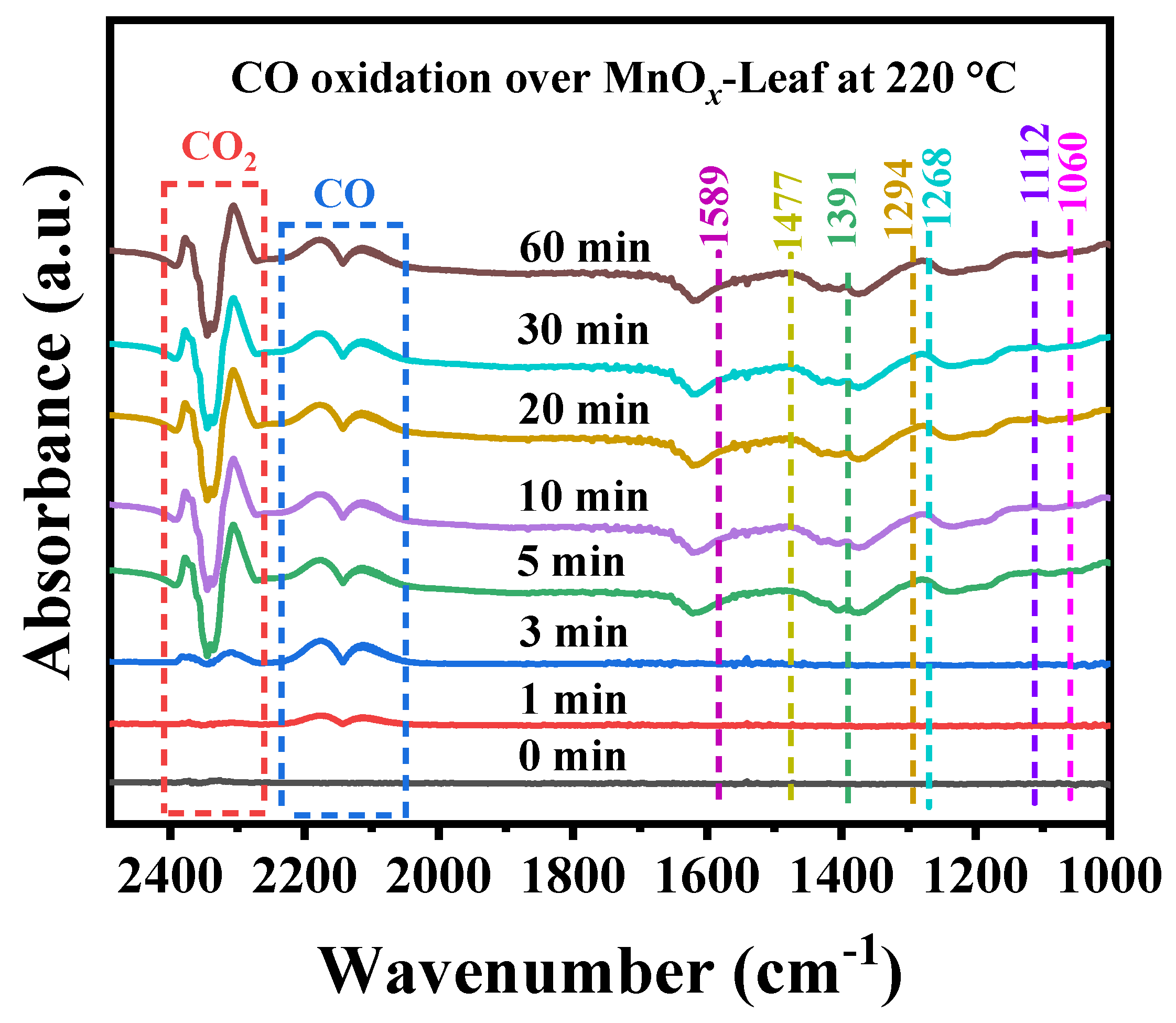
2.4. CO Oxidation Mechanism over MnOx-Leaf
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Catalysts Preparation
3.2.1. Synthesis of the Mn-MOFs Precursors with Different Morphologies
3.2.2. Synthesis of the Mn-MOF-Derived MnOx Catalysts
3.3. Catalysts Characterization
3.4. Catalytic Performance Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, H.; Chen, X.; López-Cartes, C.; Martínez-López, J.; Bu, E.; Delgado, J.J. Hydrothermal synthesis and characterization of Cu-MnOx catalysts for CO oxidation: Effect of Cu:Mn molar ratio on their structure and catalytic activity. Catal. Today 2023, 418, 114085. [Google Scholar] [CrossRef]
- Freund, H.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO Oxidation as a Prototypical Reaction for Heterogeneous Processes. Angew. Chem. Int. Ed. Engl. 2011, 50, 10064–10094. [Google Scholar] [CrossRef]
- Westberg, K.; Cohen, N.; Wilson, K.W. Carbon Monoxide: Its Role in Photochemical Smog Formation. Science 1971, 171, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Peng, B.; Zhu, X.; Guo, Y. Multi-Gas Detection System Based on Non-Dispersive Infrared (NDIR) Spectral Technology. Sensors 2022, 22, 836. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tao, H.; Chen, Z.; Zhou, M.; Jiang, N.; Chen, X.; Zhang, X. Advances in photocatalytic removal of NOx and VOCs from flue gas. Energy Environ. Prot. 2024, 38, 144–154. [Google Scholar] [CrossRef]
- Imai, K.; Fukushima, T.; Kobayashi, H.; Higashimoto, S. Visible-light responsive TiO2 for the complete photocatalytic decomposition of volatile organic compounds (VOCs) and its efficient acceleration by thermal energy. Appl. Catal. B Environ. 2024, 346, 123745. [Google Scholar] [CrossRef]
- Huang, J.; Wei, J.; Tian, F.; Bi, F.; Rao, R.; Wang, Y.; Tao, H.; Liu, N.; Zhang, X. Nitrogen-induced TiO2 electric field polarization for efficient photodegradation of high-concentration ethyl acetate: Mechanisms and reaction pathways. Mater. Today Chem. 2024, 41, 102292. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Z.; Xu, J.; Cui, H.; Tang, K.; Crawshaw, D.; Wu, J.; Zhang, X.; Tang, L. Highly Selective CO2 Conversion to CH4 by N-doped HTiNbO5/NH2-UiO-66 Photocatalyst Without Sacrificial Electron Donor. JACS Au 2025, 5, 1184–1195. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Z.; Luo, D.; Xie, T.; Zheng, W.; Hu, Z.; Yang, R. Photothermal Synergism on Pd/TiO2 Catalysts with Varied TiO2 Crystalline Phases for NOx Removal via H2-SCR: A Transient DRIFTS Study. Ind. Eng. Chem. Res. 2022, 61, 14823–14836. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, Y.; Zhou, Z.; Guo, L.; Zhu, Z.; Liu, B.; Zhang, X. Electron Beam Irradiation-Induced Defects Enhance Pt-TiO2 Photothermal Catalytic Degradation in PAEs: A Performance and Mechanism Study. Molecules 2025, 30, 697. [Google Scholar] [CrossRef]
- Krishnan, P.; Zhang, M.-H.; Cheng, Y.; Riang, D.T.; Yu, L.E. Photocatalytic degradation of SO2 using TiO2-containing silicate as a building coating material. Constr. Build. Mater. 2013, 43, 197–202. [Google Scholar] [CrossRef]
- Yang, Y.; Bi, F.; Wei, J.; Han, X.; Gao, B.; Qiao, R.; Xu, J.; Liu, N.; Zhang, X. Boosting the Photothermal Oxidation of Multicomponent VOCs in Humid Conditions: Synergistic Mechanism of Mn and K in Different Oxygen Activation Pathways. Environ. Sci. Technol. 2025, 59, 11341–11352. [Google Scholar] [CrossRef]
- Kumar, M.S.; Alphin; Manigandan, S.; Vignesh, S.; Vigneshwaran, S.; Subash, T. A review of comparison between the traditional catalyst and zeolite catalyst for ammonia-selective catalytic reduction of NOx. Fuel 2023, 344, 128125. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, S.; You, M.; Yu, D.; Zhang, C.; Gao, S.; Yu, X.; Zhao, Z. Research Progress on Metal Oxides for the Selective Catalytic Reduction of NOx with Ammonia. Catalysts 2023, 13, 1086. [Google Scholar] [CrossRef]
- Bi, F.; Feng, X.; Huang, J.; Wei, J.; Wang, H.; Du, Q.; Liu, N.; Xu, J.; Liu, B.; Huang, Y.; et al. Unveiling the Influence Mechanism of Impurity Gases on Cl-Containing Byproducts Formation During VOC Catalytic Oxidation. Environ. Sci. Technol. 2025, 59, 15526–15537. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, M.; Niu, Y.; Yi, L.; Yi, H.; Zhou, Y.; Zhao, S.; Tang, X.; Gao, F. Advances in CO catalytic oxidation on typical noble metal catalysts: Mechanism, performance and optimization. Chem. Eng. J. 2024, 495, 153523. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Huang, J.; Ma, S.; Zhang, Y.; Bi, F.; Zhang, X. Electron Beam Irradiation Modified UiO-66 Supported Pt Catalysts for Low-Temperature Ethyl Acetate Catalytic Degradation. Catalysts 2025, 15, 220. [Google Scholar] [CrossRef]
- Beg, M.B.; Ali, L.; Shittu, T.; Khaleel, A.; Vermeire, F.H.; Altarawneh, M. Non-noble catalysts formulations using CuO-CeO2/Nb2O5 for low-temperature catalytic oxidation of carbon monoxide. J. Environ. Chem. Eng. 2024, 12, 113177. [Google Scholar] [CrossRef]
- Yang, W.; Huang, H.; Liu, X.; Ren, J.; Ma, K.; Pan, Z.; Ding, Z.; Ding, X.; Gao, Z. Screening the activity of single-atom catalysts for the catalytic oxidation of sulfur dioxide with a kinetic activity model. Chem. Commun. 2020, 56, 11657–11660. [Google Scholar] [CrossRef]
- Bi, F.; Wei, J.; Zhou, Z.; Zhang, Y.; Gao, B.; Liu, N.; Xu, J.; Liu, B.; Huang, Y.; Zhang, X. Insight into the Synergistic Effect of Binary Nonmetallic Codoped Co3O4 Catalysts for Efficient Ethyl Acetate Degradation Under Humid Conditions. JACS Au 2025, 5, 363–380. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Luo, Q.; Zhang, J.; Wang, C.; Ge, X.; Zhang, W.; Xiao, F.-S. Two-dimensional manganese oxide on ceria for the catalytic partial oxidation of hydrocarbons. Chem. Synth. 2022, 2, 2. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Hu, H.; Wei, J.; Qiao, R.; Bi, F.; Zhang, X. Recent progress of cerium-based catalysts for the catalytic oxidation of volatile organic compounds: A review. Fuel 2025, 399, 135603. [Google Scholar] [CrossRef]
- Cai, D.; Chen, B.; Huang, Z.; Zeng, X.; Xiao, J.; Zhou, S.-F.; Zhan, G. Metal oxide/CeO2 nanocomposites derived from Ce-benzene tricarboxylate (Ce-BTC) adsorbing with metal acetylacetonate complexes for catalytic oxidation of carbon monoxide. RSC Adv. 2021, 11, 21057–21065. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, J.; Fang, S.; Yao, S. Revealing the differences in CO oxidation activity of Fe-CeO2, Fe2O3, and CeO2 using operando CO2-DRIFTS-MS: Carbonate species and desorption process. J. Alloy. Compd. 2025, 1010, 177414. [Google Scholar] [CrossRef]
- Pulleri, J.K.; Singh, S.K.; Yearwar, D.; Saravanan, G.; Al-Fatesh, A.S.; Labhasetwar, N.K. Morphology Dependent Catalytic Activity of Mn3O4 for Complete Oxidation of Toluene and Carbon Monoxide. Catal. Lett. 2020, 151, 172–183. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Wang, Y.; Lyu, Y.-K. Performance and mechanism comparison of manganese oxides at different valence states for catalytic oxidation of NO. Chem. Eng. J. 2019, 361, 1161–1172. [Google Scholar] [CrossRef]
- Guo, L.; Bi, F.; Liu, N.; Zhang, X. Degradation of volatile organic pollutants over manganese-based catalysts by defect engineering: A review. Sep. Purif. Technol. 2025, 362, 131934. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, H.; Song, Z.; Liu, W.; Zhao, J.; Ma, Z.; Zhao, M.; Xing, Y. Insight into the effect of oxygen species and Mn chemical valence over MnOx on the catalytic oxidation of toluene. Appl. Surf. Sci. 2019, 493, 9–17. [Google Scholar] [CrossRef]
- Pandit, B.; Goda, E.S.; Abu Elella, M.H.; Rehman, A.U.; Hong, S.E.; Rondiya, S.R.; Barkataki, P.; Shaikh, S.F.; Al-Enizi, A.M.; El-Bahy, S.M.; et al. One-pot hydrothermal preparation of hierarchical manganese oxide nanorods for high-performance symmetric supercapacitors. J. Energy Chem. 2022, 65, 116–126. [Google Scholar] [CrossRef]
- Balamurugan, M.; Sivaprakash, P.; Sivakumar, S.; Ramachandran, S.; Saravanan, S. Effect of different precursors on structural and luminescence properties of Mn3O4 nanoparticles prepared by co-precipitation method. Mater. Sci. Eng. Technol. 2022, 53, 723–731. [Google Scholar] [CrossRef]
- Yarbrough, R.; Davis, K.; Dawood, S.; Rathnayake, H. A sol–gel synthesis to prepare size and shape-controlled mesoporous nanostructures of binary (II–VI) metal oxides. RSC Adv. 2020, 10, 14134–14146. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, G.; Hu, J.; Feng, W.; Zeng, Q.; Liu, Y.; Pang, H. Recent progress in strategies for preparation of metal-organic frameworks and their hybrids with different dimensions. Chem. Synth. 2023, 3, 1. [Google Scholar] [CrossRef]
- Dou, C.; Liu, Y.; Shu, H.; Li, S.; Shi, Q.; Luo, X. Evolutionary trends and photothermal catalytic reduction performance of carbon dioxide by MOF-derived MnOx. Fuel 2025, 384, 133949. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R. Metal–Organic Framework (MOF)-Derived Metal Oxides for Selective Catalytic Reduction (SCR) of NOx. Molecules 2025, 30, 2836. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Guo, L.; Shen, Q.; Bi, F.; Li, C.; Zhang, X. The application of metal–organic frameworks and their derivatives in the catalytic oxidation of typical gaseous pollutants: Recent progress and perspective. Sep. Purif. Technol. 2024, 340, 126772. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, X.; Zhang, J.; Mai, Y.; Chen, J. Highly efficient MnOx catalysts derived from Mn-MOFs for chlorobenzene oxidation: The influence of MOFs precursors, oxidant and doping of Ce metal. Mol. Catal. 2023, 551, 113653. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, J.; Zhang, Q.; Liu, Z.; Fu, M.; Wu, J.; Hu, Y.; Ye, D. Enhanced oxygen vacancies to improve ethyl acetate oxidation over MnOx-CeO2 catalyst derived from MOF template. Chem. Eng. J. 2019, 371, 78–87. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Song, L.; Hou, F.; Yang, Y.; Wang, Y.; Liu, N. Enhanced catalytic performance for CO oxidation and preferential CO oxidation over CuO/CeO2 catalysts synthesized from metal organic framework: Effects of preparation methods. Int. J. Hydrogen Energy 2018, 43, 18279–18288. [Google Scholar] [CrossRef]
- Feng, F.; Zeng, Y.; Yan, D.; Ren, Q.; Lan, B.; Zhong, J.; Liu, B.; Dong, T.; Huang, H. Construction of hollow sphere MnOx with abundant oxygen vacancy for accelerating VOCs degradation: Investigation through operando spectroscopycombined with on-line mass spectrometry. J. Colloid Interface Sci. 2024, 673, 746–755. [Google Scholar] [CrossRef]
- Lee, J.H.; Sa, Y.J.; Kim, T.K.; Moon, H.R.; Joo, S.H. A transformative route to nanoporous manganese oxides of controlled oxidation states with identical textural properties. J. Mater. Chem. A 2014, 2, 10435–10443. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, F.; Zhu, Z.; Yang, Y.; Zhao, S.; Chen, J.; Lv, X.; Wang, Y.; Xu, J.; Liu, N. The promoting effect of H2O on rod-like MnCeOx derived from MOFs for toluene oxidation: A combined experimental and theoretical investigation. Appl. Catal. B Environ. 2021, 297, 120393. [Google Scholar] [CrossRef]
- Fan, Z.; Ren, J.; Bai, H.; He, P.; Hao, L.; Liu, N.; Chen, B.; Niu, R.; Gong, J. Shape-controlled fabrication of MnO/C hybrid nanoparticle from waste polyester for solar evaporation and thermoelectricity generation. Chem. Eng. J. 2022, 451, 138534. [Google Scholar] [CrossRef]
- Zhuang, S.; Lei, Y.; Xue, F.; Wei, T.; Chu, J.; Cui, M.; Zhang, Z.; Wei, R.; Tang, J.; Qiao, X. Synergistic role of CuO-Cu+ sites and oxygen vacancies on MOF-derived Cu supported CeO2 for enhancing hydrogenation of cyclohexyl acetate to cyclohexanol. Appl. Surf. Sci. 2025, 709, 163840. [Google Scholar] [CrossRef]
- Liu, Q.; Ye, J.; Han, Y.; Wang, P.; Fei, Z.; Chen, X.; Zhang, Z.; Tang, J.; Cui, M.; Qiao, X. Defective UiO-67 for enhanced adsorption of dimethyl phthalate and phthalic acid. J. Mol. Liq. 2021, 321, 114477. [Google Scholar] [CrossRef]
- Abbas, M.Q.; Javeria, H.; Nazir, A.; Du, Z.; Keshta, B.E. Enhanced ibuprofen removal from wastewater using Ni-doped ZIF-67 MOF: Synthesis, characterization, and impact of doping on adsorption performance. Environ. Funct. Mater. 2025. [Google Scholar] [CrossRef]
- Wang, Q.; Li, T.; Tian, H.; Zou, D.; Zeng, J.; Chen, S.; Xie, H.; Zhou, G. Effect of pore size distribution of biomass activated carbon adsorbents on the adsorption capacity. J. Chem. Technol. Biotechnol. 2024, 99, 1148–1156. [Google Scholar] [CrossRef]
- Alanazi, A.; Abid, H.R.; Usman, M.; Ali, M.; Keshavarz, A.; Vahrenkamp, V.; Iglauer, S.; Hoteit, H. Hydrogen, carbon dioxide, and methane adsorption potential on Jordanian organic-rich source rocks: Implications for underground H2 storage and retrieval. Fuel 2023, 346, 128362. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, Z.; Zhang, X.; Yan, F.; Zhao, Y.; Zhu, C.; Chen, Y. Flexible Film Constructed by Asymmetrically-Coordinated La1N4Cl1 Moieties on Interconnected Nitrogen-Doped Graphene Nanocages for High-Efficiency Electromagnetic Wave Absorption. Adv. Funct. Mater. 2024, 34, 2313483. [Google Scholar] [CrossRef]
- Wang, H.; Huang, J.; Ding, S.; Li, Z.; Zhao, Z.; Wei, J.; Zhou, D.; Chu, H.; Bi, F.; Zhang, X. Catalytic oxidation of binary VOCs over MnO2 with different crystal structures: Mechanisms of promotion and inhibition. Sep. Purif. Technol. 2026, 382, 135895. [Google Scholar] [CrossRef]
- Moreno-Román, E.; González-Cobos, J.; Guilhaume, N.; Gil, S. Toluene and 2-propanol mixture oxidation over Mn2O3 catalysts: Study of inhibition/promotion effects by in-situ DRIFTS. Chem. Eng. J. 2023, 470, 144114. [Google Scholar] [CrossRef]
- Shah, P.M.; Bailey, L.A.; Morgan, D.J.; Taylor, S.H. The Effect of Metal Ratio and Precipitation Agent on Highly Active Iron-Manganese Mixed Metal Oxide Catalysts for Propane Total Oxidation. Catalysts 2023, 13, 794. [Google Scholar] [CrossRef]
- Yu, X.; Deng, B.; Yang, L.; Zou, M.; Chen, Z.; Fan, Y.; Wei, Z.; Chen, K.; Lu, M.; Ying, T.; et al. Influence of residual anions (Cl−, SO42− and NO3−) on Mn2O3 for photothermal catalytic oxidation of toluene. J. Environ. Chem. Eng. 2022, 10, 108496. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Cao, M.; He, Z.; Qiao, R.; Bi, F.; Wang, Y.; Zhang, X. Preparation of CeXMn1−XO2 Catalysts with Strong Mn-Ce Synergistic Effect for Catalytic Oxidation of Toluene. Materials 2025, 18, 3809. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Men, Y.; Ji, F.; Shi, F.; Wang, J.; Liu, S.; Magkoev, T.T.; An, W. Boosting Catalytic Combustion of Ethanol by Tuning Morphologies and Exposed Crystal Facets of α-Mn2O3. Catalysts 2023, 13, 865. [Google Scholar] [CrossRef]
- Khder, A.E.R.S.; Altass, H.M.; Orif, M.I.; Ashour, S.S.; Almazroai, L.S. Preparation and characterization of highly active Pd nanoparticles supported Mn3O4 catalyst for low-temperature CO oxidation. Mater. Res. Bull. 2019, 113, 215–222. [Google Scholar] [CrossRef]
- Jampaiah, D.; Velisoju, V.K.; Devaiah, D.; Singh, M.; Mayes, E.L.; Coyle, V.E.; Reddy, B.M.; Bansal, V.; Bhargava, S.K. Flower-like Mn3O4/CeO2 microspheres as an efficient catalyst for diesel soot and CO oxidation: Synergistic effects for enhanced catalytic performance. Appl. Surf. Sci. 2019, 473, 209–221. [Google Scholar] [CrossRef]
- Portillo-Vélez, N.; Zanella, R. Comparative study of transition metal (Mn, Fe or Co) catalysts supported on titania: Effect of Au nanoparticles addition towards CO oxidation and soot combustion reactions. Chem. Eng. J. 2020, 385, 123848. [Google Scholar] [CrossRef]
- Zhao, G.; Guo, Y.; Huang, P.; Zhang, Z.; Zhao, C. Enhanced low-temperature CO oxidation over CuO-Co3O4 catalysts promoted with transition metal oxides. Fuel 2025, 403, 136113. [Google Scholar] [CrossRef]
- Jang, W.; Yoon, S.; Song, J.; Kim, J.; An, K.; Cho, S. Selective phase transformation of layered double hydroxides into mixed metal oxides for catalytic CO oxidation. Cell Rep. Phys. Sci. 2021, 2, 100628. [Google Scholar] [CrossRef]
- Gao, Q.; Dong, C.; Hu, X.; Zhang, J.; Xue, J.; Zhao, Y.; Wang, X. Effect of the Fe2O3@TiO2 core-shell structure on CO catalytic oxidation and SO2 poisoning resistance. Mol. Catal. 2023, 547, 113308. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Sun, Y.; Fang, S.; Wu, Z.; Gao, E.; Zhu, J.; Wang, W.; Yao, S.; Li, J. Insight into the Metal-Support Interaction of Pt and β-MnO2 in CO Oxidation. Molecules 2023, 28, 6879. [Google Scholar] [CrossRef]
- Kucharczyk, B.; Tylus, W.; Okal, J.; Chęcmanowski, J.; Szczygieł, B. The Pt-NiO catalysts over the metallic monolithic support for oxidation of carbon monoxide and hexane. Chem. Eng. J. 2017, 309, 288–297. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Z.; Liu, J.; Do-Thanh, C.-L.; Chen, H.; Xu, S.; Lin, Q.; Jiao, Y.; Wang, J.; Wang, Y.; et al. Entropy-stabilized single-atom Pd catalysts via high-entropy fluorite oxide supports. Nat. Commun. 2020, 11, 3908. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.K.; Hu, H.T.; Zheng, Y.; Wang, Y.X.; Wang, Y.X.; Liu, B.L.; Dong, H.; Zhang, X.D. Regulation of Ag1Cux/SBA-15 Catalyst for Efficient CO Catalytic Degradation at Room Temperature. Catalysts 2025, 15, 676. [Google Scholar] [CrossRef]
- Zedan, A.F.; Mohamed, A.T.; El-Shall, M.S.; AlQaradawi, S.Y.; AlJaber, A.S. Tailoring the reducibility and catalytic activity of CuO nanoparticles for low temperature CO oxidation. RSC Adv. 2018, 8, 19499–19511. [Google Scholar] [CrossRef]
- Soliman, N. Factors affecting CO oxidation reaction over nanosized materials: A review. J. Mater. Res. Technol. 2019, 8, 2395–2407. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Jia, Y.; Sun, Q.; Feng, Y.; Hou, Z.; Wang, Z.; Wei, L.; Wei, Z.; Jing, L.; et al. A novel strategy for enhancing resistance to chlorine, water, and sulfur oxide of the Pt/Co-ZSM-5 catalyst by synergistic coupling of acidity and redox sites for the oxidation of multicomponent VOCs. Appl. Catal. B Environ. 2025, 378, 125557. [Google Scholar] [CrossRef]
- Luo, M.-F.; Ma, J.-M.; Lu, J.-Q.; Song, Y.-P.; Wang, Y.-J. High-surface area CuO–CeO2 catalysts prepared by a surfactant-templated method for low-temperature CO oxidation. J. Catal. 2007, 246, 52–59. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, F.; Li, H.; Wang, Y.; Liu, N.; Yang, Y. A strawsheave-like metal organic framework Ce-BTC derivative containing high specific surface area for improving the catalytic activity of CO oxidation reaction. Microporous Mesoporous Mater. 2018, 259, 211–219. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Han, Y.; Gao, Y.; Xiang, G.; Chu, G.; Luo, Y. K+-Modified Redox Properties of the CuOx/CeO2 Catalyst for Highly Efficient CO Oxidation. ACS Eng. Au 2022, 2, 486–495. [Google Scholar] [CrossRef]
- Lu, Z.; Guo, L.; Bi, F.; Ma, S.; Shen, Q.; Qiao, R.; Zhang, X. Insight into the degradation mechanism of mixed VOCs oxidation over Pd/UiO-66(Ce) catalysts: Combination of operando spectroscopy and theoretical calculation. Sep. Purif. Technol. 2025, 354, 129443. [Google Scholar] [CrossRef]
- Lv, D.; Liu, J.; Zhang, G.; Wang, Y.; Zhao, Y.; Li, G. Novel insights for simultaneous NOx and CO Removal: Cu+-Sm3+-Ov-Ti4+ asymmetric active site promoting NH3-SCR coupled with CO oxidation reaction. Chem. Eng. J. 2024, 481, 148534. [Google Scholar] [CrossRef]
- Yu, Z.H.; Cai, J.Y.; Meng, Y.D.; Li, J.; Liang, W.J.; Fan, X. Loading Eu2O3 Enhances the CO Oxidation Activity and SO2 Resistance of the Pt/TO2 Catalyst. Catalysts 2025, 15, 783. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, Z.; Dong, H.; Liu, Y.; Hu, B.; Huang, S.; Zhang, X.; Lei, J.; Liu, N. In situ electronic modulation of g-C3N4/UiO66 composites via N species functionalized ligands for enhanced photocatalytic CO2 reduction. Sep. Purif. Technol. 2025, 379, 134964. [Google Scholar] [CrossRef]
- Yadav, P.K.; Kumari, S.; Naveena, U.; Deshpande, P.A.; Sharma, S. Insights into the substitutional chemistry of La1−xSrxCo1−yMyO3 (M = Pd, Ru, Rh, and Pt) probed by in situ DRIFTS and DFT analysis of CO oxidation. Appl. Catal. A Gen. 2022, 643, 118768. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Deng, Y.-Q.; Tian, P.; Shang, H.-H.; Xu, J.; Han, Y.-F. Dynamic active sites over binary oxide catalysts: In situ/operando spectroscopic study of low-temperature CO oxidation over MnOx-CeO2 catalysts. Appl. Catal. B Environ. 2016, 191, 179–191. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Y.; Li, P.; Zhang, S.; Sun, X.; Cheng, S.; Jiang, Y. High-performance MnOX-CuO catalysts for low-temperature CO oxidation: Metal interaction and reaction mechanism. Mol. Catal. 2025, 576, 114945. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Z.; Gao, E.; Zhu, J.; Yao, S.; Li, J. Revealing the role of oxygen vacancies on α-MnO2 of different morphologies in CO oxidation using operando DRIFTS-MS. Appl. Surf. Sci. 2023, 618, 156643. [Google Scholar] [CrossRef]
- Zhang, Q.; Mo, S.; Li, J.; Sun, Y.; Zhang, M.; Chen, P.; Fu, M.; Wu, J.; Chen, L.; Ye, D. In situ DRIFT spectroscopy insights into the reaction mechanism of CO and toluene co-oxidation over Pt-based catalysts. Catal. Sci. Technol. 2019, 9, 4538–4551. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, K.; Wang, H.; Wang, Y.; Tian, D.; Wei, Y.; Zhu, X.; Zeng, C.; Luo, Y. Structure dependence and reaction mechanism of CO oxidation: A model study on macroporous CeO2 and CeO2-ZrO2 catalysts. J. Catal. 2016, 344, 365–377. [Google Scholar] [CrossRef]
- El-Moemen, A.A.; Abdel-Mageed, A.M.; Bansmann, J.; Parlinska-Wojtan, M.; Behm, R.J.; Kučerová, G. Deactivation of Au/CeO2 catalysts during CO oxidation: Influence of pretreatment and reaction conditions. J. Catal. 2016, 341, 160–179. [Google Scholar] [CrossRef]
- Li, K.; Liu, K.; Ni, H.; Guan, B.; Zhan, R.; Huang, Z.; Lin, H. Electric field promoted ultra-lean methane oxidation over Pd-Ce-Zr catalysts at low temperature. Mol. Catal. 2018, 459, 78–88. [Google Scholar] [CrossRef]
- Chen, S.; Luo, L.; Jiang, Z.; Huang, W. Size-Dependent Reaction Pathways of Low-Temperature CO Oxidation on Au/CeO2 Catalysts. ACS Catal. 2015, 5, 1653–1662. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Ning, P.; Liu, X.; Wang, H.; Wei, G.; Zhang, T. In situ DRIFTS investigation of low temperature CO oxidation over manganese oxides supported Pd catalysts. J. Taiwan Inst. Chem. Eng. 2019, 97, 280–287. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, H.; Yildirim, T. Enhanced H2 Adsorption in Isostructural Metal−Organic Frameworks with Open Metal Sites: Strong Dependence of the Binding Strength on Metal Ions. J. Am. Chem. Soc. 2008, 130, 15268–15269. [Google Scholar] [CrossRef]
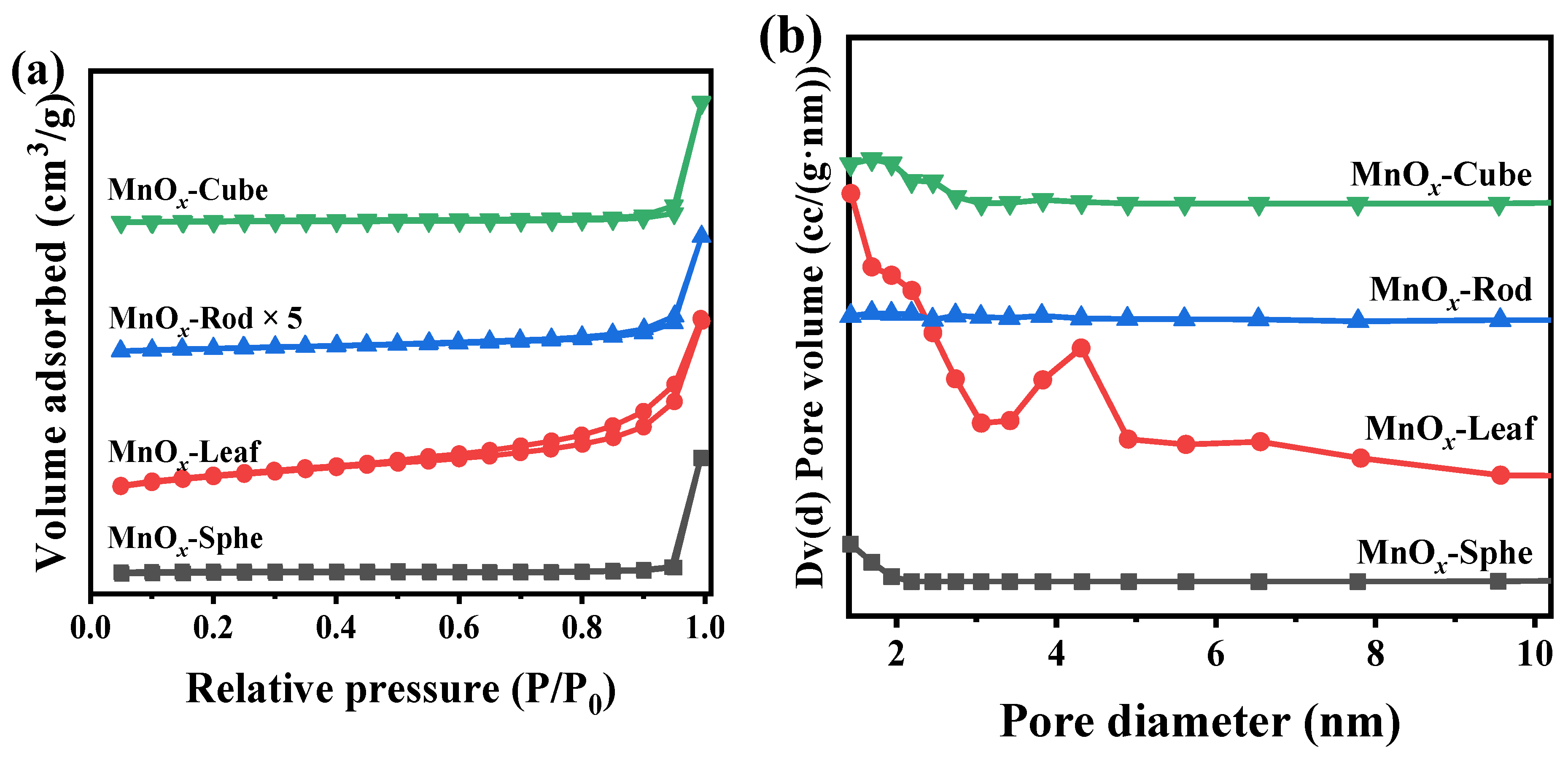
| Catalysts | SBET (m2/g) a | V (cm3/g) b | D (nm) c | H2 Consumption (μmol/g) d | Catalytic Activity (°C) | |
|---|---|---|---|---|---|---|
| T50 | T98 | |||||
| MnOx-Sphe | 4.8 | 0.032 | 1.4–2.2 | 339.9 | 180 | 228 |
| MnOx-Leaf | 32.1 | 0.091 | 1.4–5.6 | 398.3 | 165 | 214 |
| MnOx-Rod | 1.7 | 0.017 | 1.4–3.1 | 251.5 | 253 | 357 |
| MnOx-Cube | 13.5 | 0.062 | 1.4–2.4 | 280.7 | 216 | 262 |
| Catalysts | Preparation Method | CO Concentration | WHSV (mL/(g·h)) | Performance | Ref. |
|---|---|---|---|---|---|
| Mn3O4 H | Precipitation method | 4.0 vol.% | 60,000 | T100 = 347 °C | [55] |
| Mn3O4 NPs | Precipitation method | 1.0 vol.% | 60,000 | T90 = 524 °C | [56] |
| Mn/ZrTiO4 | Deposition–precipitation method | 1.0 vol.% | 120,000 | T100 = 235 °C | [57] |
| CuO-Co3O4-NiO | Sol–gel method | 0.4 vol.% | 24,000 | T100 = 250 °C | [58] |
| CuGa-S | Co-precipitation method | 2.0 vol.% | 60,000 | T50 = 235 °C | [59] |
| Fe2O3@TiO2 | Hydrothermal method | 0.1 vol.% | 15,000 | T96 = 300 °C | [60] |
| β-MnO2 | Commercial | 1.0 vol.% | 60,000 | T90 = 310 °C | [61] |
| Pt/β-MnO2 | Deposition–precipitation method | 1.0 vol.% | 60,000 | T90 = 222 °C | [61] |
| Pt(N)/Al2O3 | Impregnation method | 1.0 vol.% | 10,000 | T90 = 220 °C | [62] |
| Pd@CeO2 | - | 1.0 vol.% | 40,000 | T100 = 253 °C | [63] |
| MnOx-Leaf | MOF-derived method | 1.0 vol.% | 18,000 | T98 = 214 °C | This work |
| Position (cm−1) | Assignment | Characteristic of | Ref. |
|---|---|---|---|
| 2114, 2175 | Gaseous CO | CO | [72,73] |
| 2309, 2372 | - | CO2 | [74,75] |
| 1060 | Vibration of COO- | Carboxyl salt species | [76,77] |
| 1112 | Adsorption of O2 on M+-O2− | O2 | [78] |
| 1268 | Vibration of bicarbonate | HCO3− | [79,80] |
| 1294 | νs (OCO) stretching of bidentate carbonate species | b-CO32− | [79,81] |
| 1391 | νs (OCO) stretching of monodentate carbonate species | m-CO32− | [81,82] |
| 1477 | Polydentate carbonate | m-CO32− | [83,84] |
| 1589 | νas (OCO) stretching of bidentate carbonate species | b-CO32− | [79,82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, F.; Wang, Y.; He, J.; Qu, H.; Li, H.; Liu, B.; Wang, Y.; Zhang, X. Mn-MOFs with Different Morphologies Derived MnOx Catalysts for Efficient CO Catalytic Oxidation. Catalysts 2025, 15, 1145. https://doi.org/10.3390/catal15121145
Bi F, Wang Y, He J, Qu H, Li H, Liu B, Wang Y, Zhang X. Mn-MOFs with Different Morphologies Derived MnOx Catalysts for Efficient CO Catalytic Oxidation. Catalysts. 2025; 15(12):1145. https://doi.org/10.3390/catal15121145
Chicago/Turabian StyleBi, Fukun, Yanxuan Wang, Jingyi He, Haoyu Qu, Hongxin Li, Baolin Liu, Yuxin Wang, and Xiaodong Zhang. 2025. "Mn-MOFs with Different Morphologies Derived MnOx Catalysts for Efficient CO Catalytic Oxidation" Catalysts 15, no. 12: 1145. https://doi.org/10.3390/catal15121145
APA StyleBi, F., Wang, Y., He, J., Qu, H., Li, H., Liu, B., Wang, Y., & Zhang, X. (2025). Mn-MOFs with Different Morphologies Derived MnOx Catalysts for Efficient CO Catalytic Oxidation. Catalysts, 15(12), 1145. https://doi.org/10.3390/catal15121145








