Recent Advances in Raman Spectroscopy for Resolving Material Surfaces/Interfaces
Abstract
1. Introduction
2. Development and Foundations of Raman Spectroscopy
2.1. Development and Principles of Raman Spectroscopy
2.2. Fundamentals of SERS
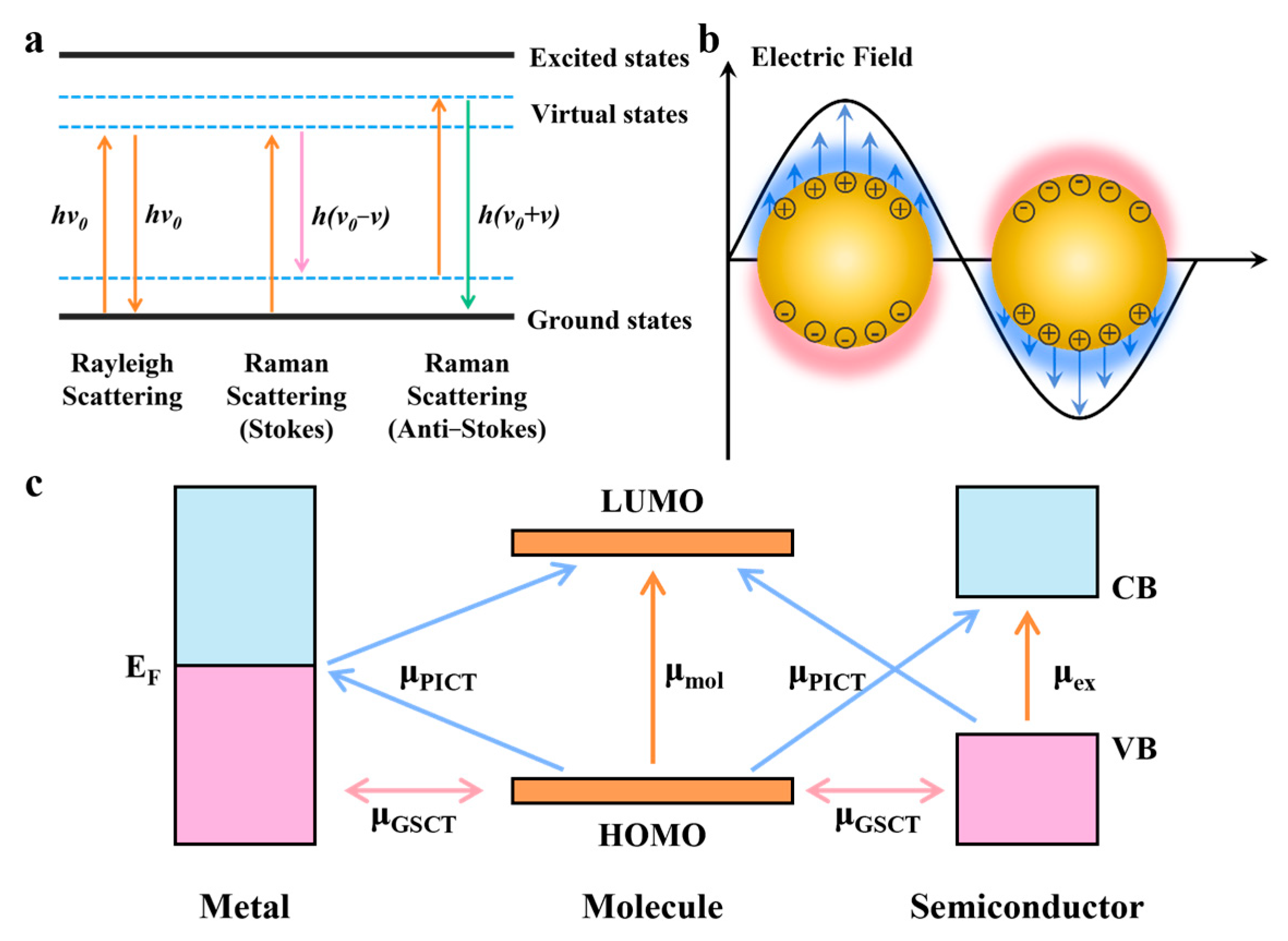
2.3. Expansion of SERS Technology
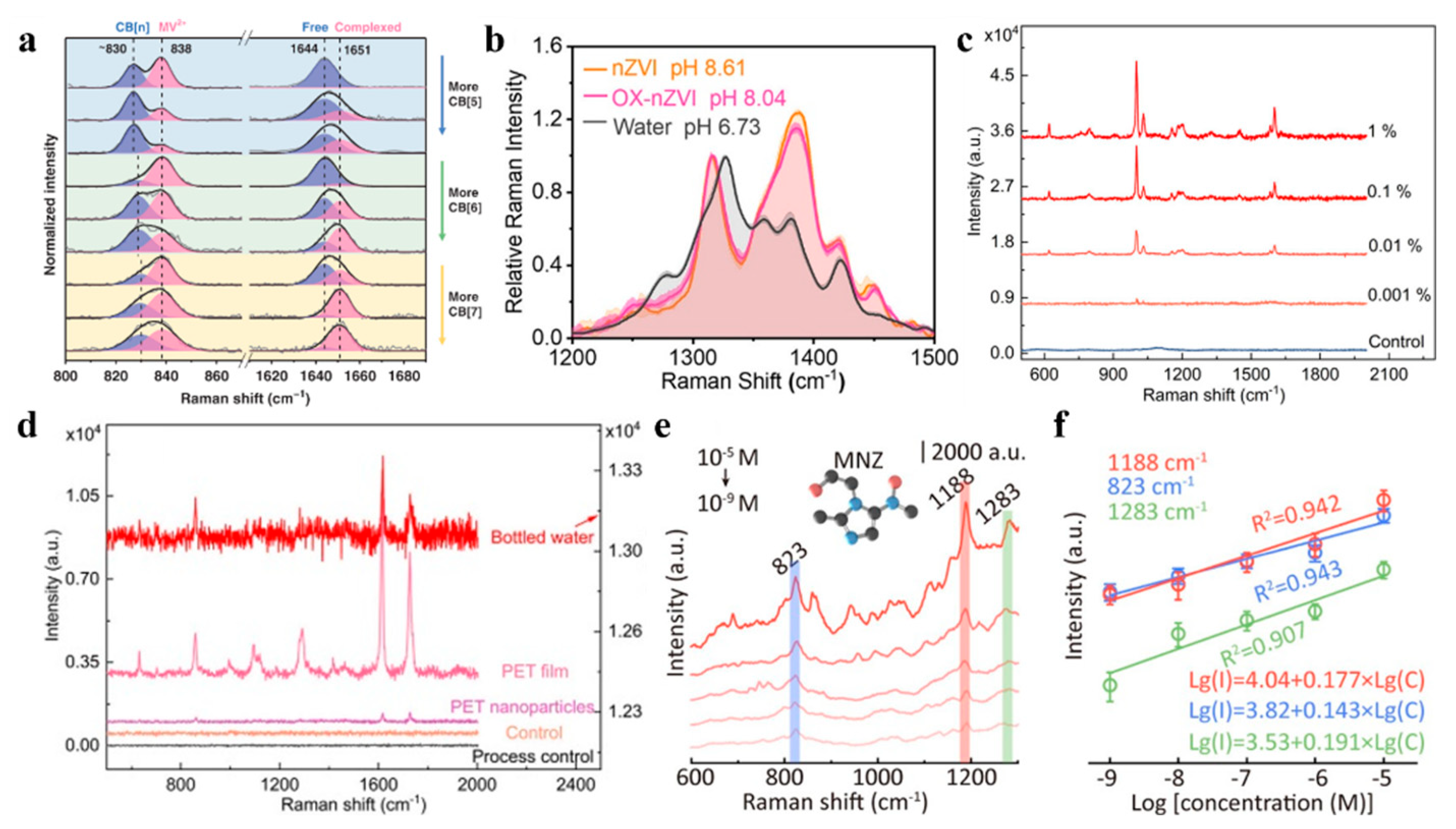
3. Application of Raman Spectroscopy in Interface Resolution
3.1. Studying the Molecular Structure and Roles of Interfaces
3.1.1. Solid–Liquid Interface
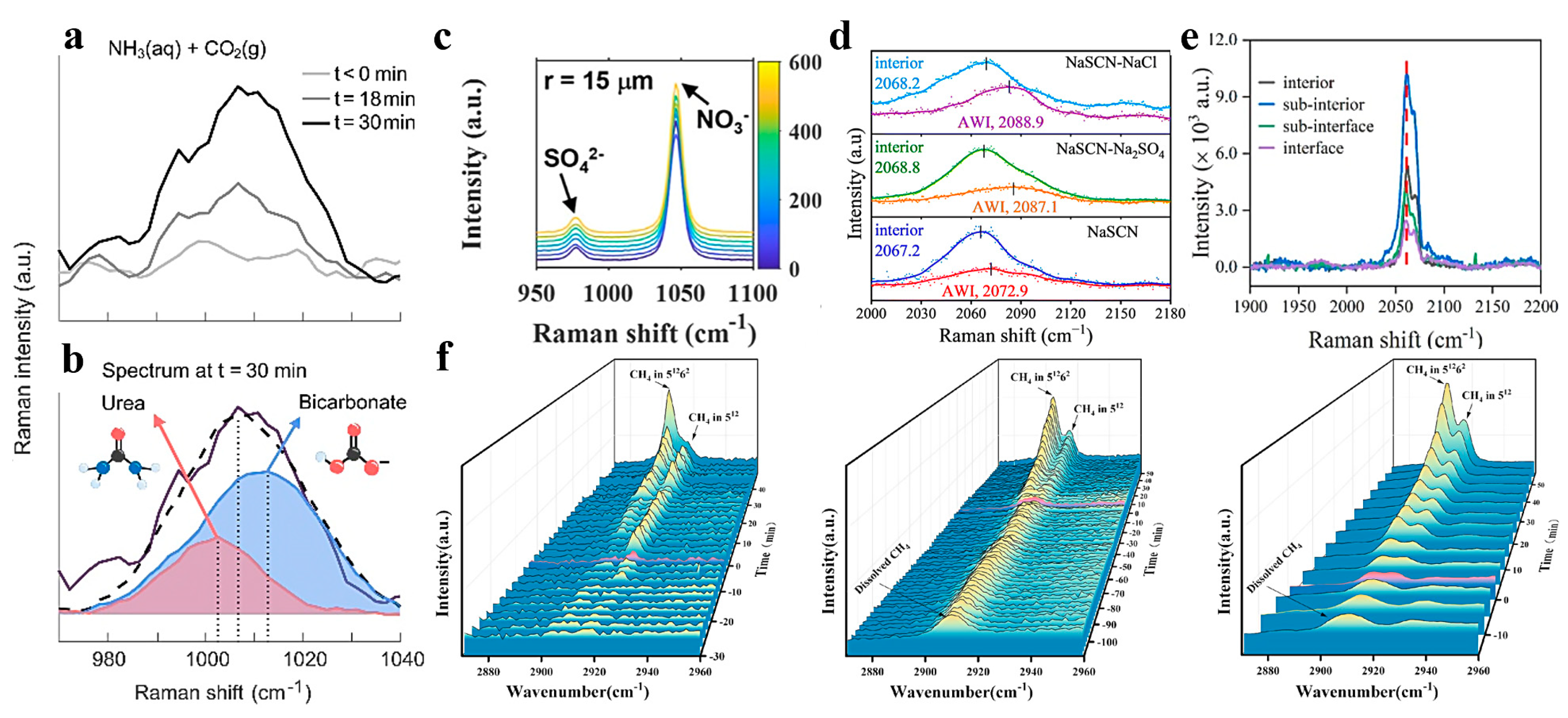
3.1.2. Gas–Liquid Interface
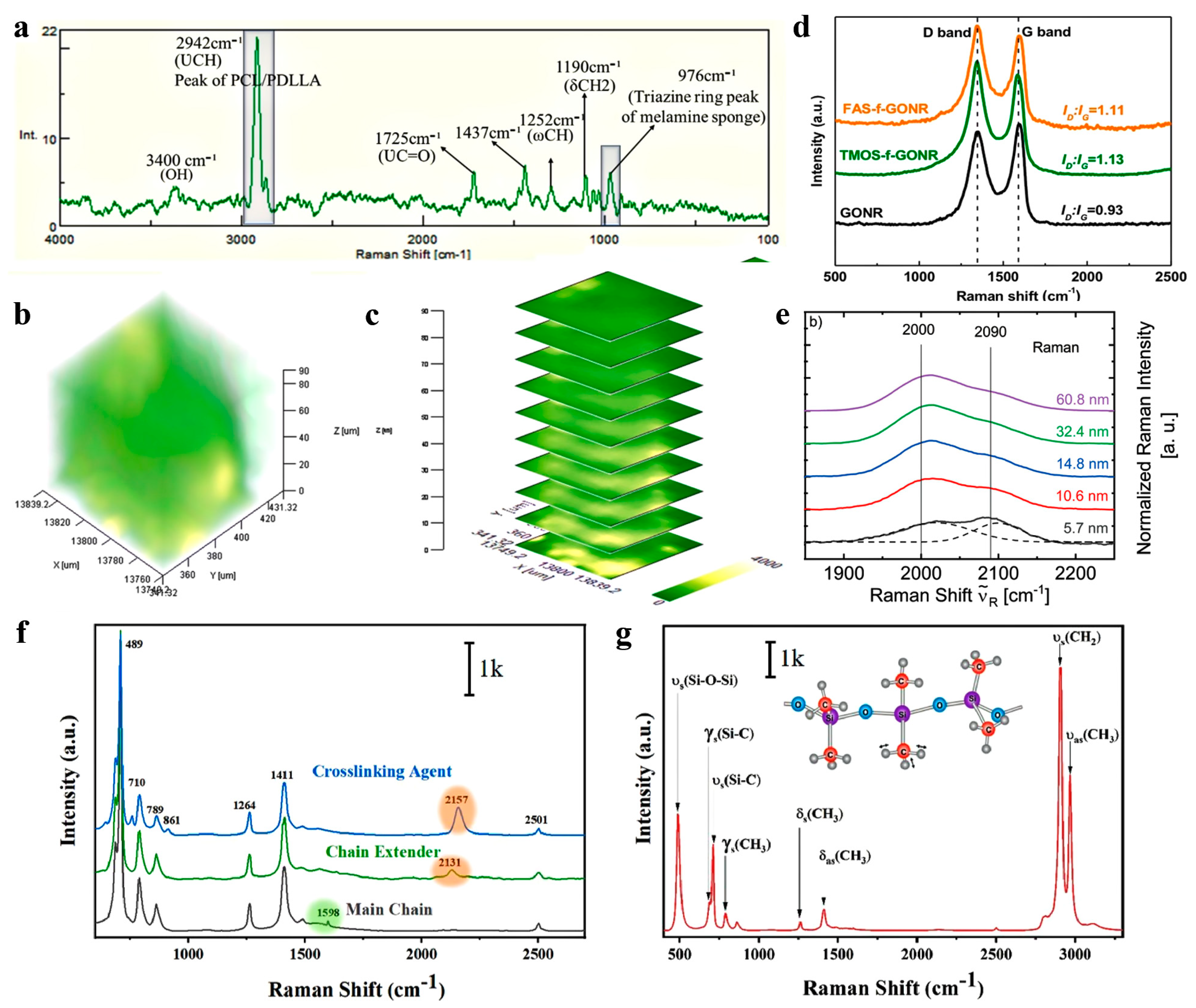
3.1.3. Solid–Solid Interface
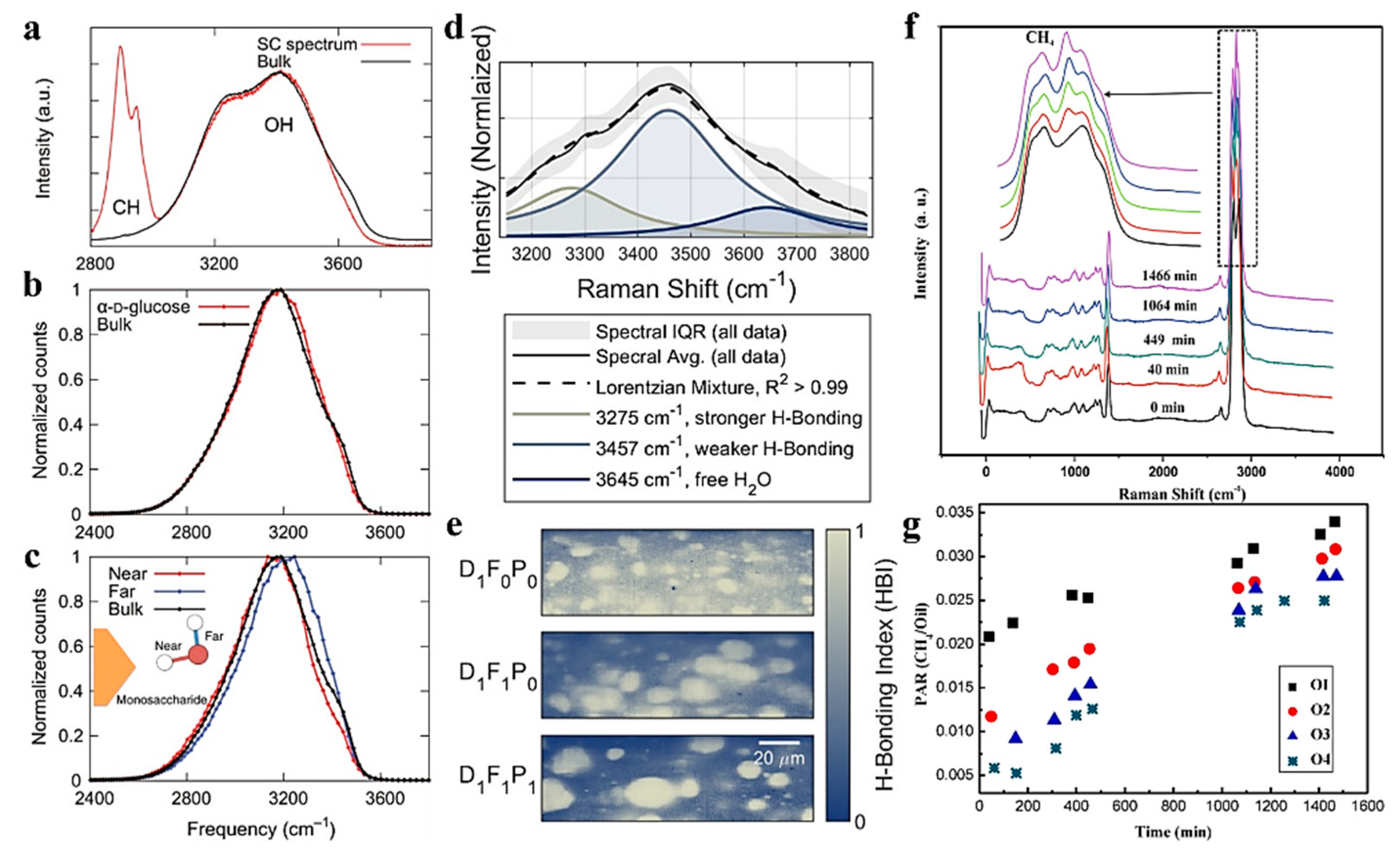
3.2. Analyzing Physical and Chemical Reactions at Water–Oil Interfaces
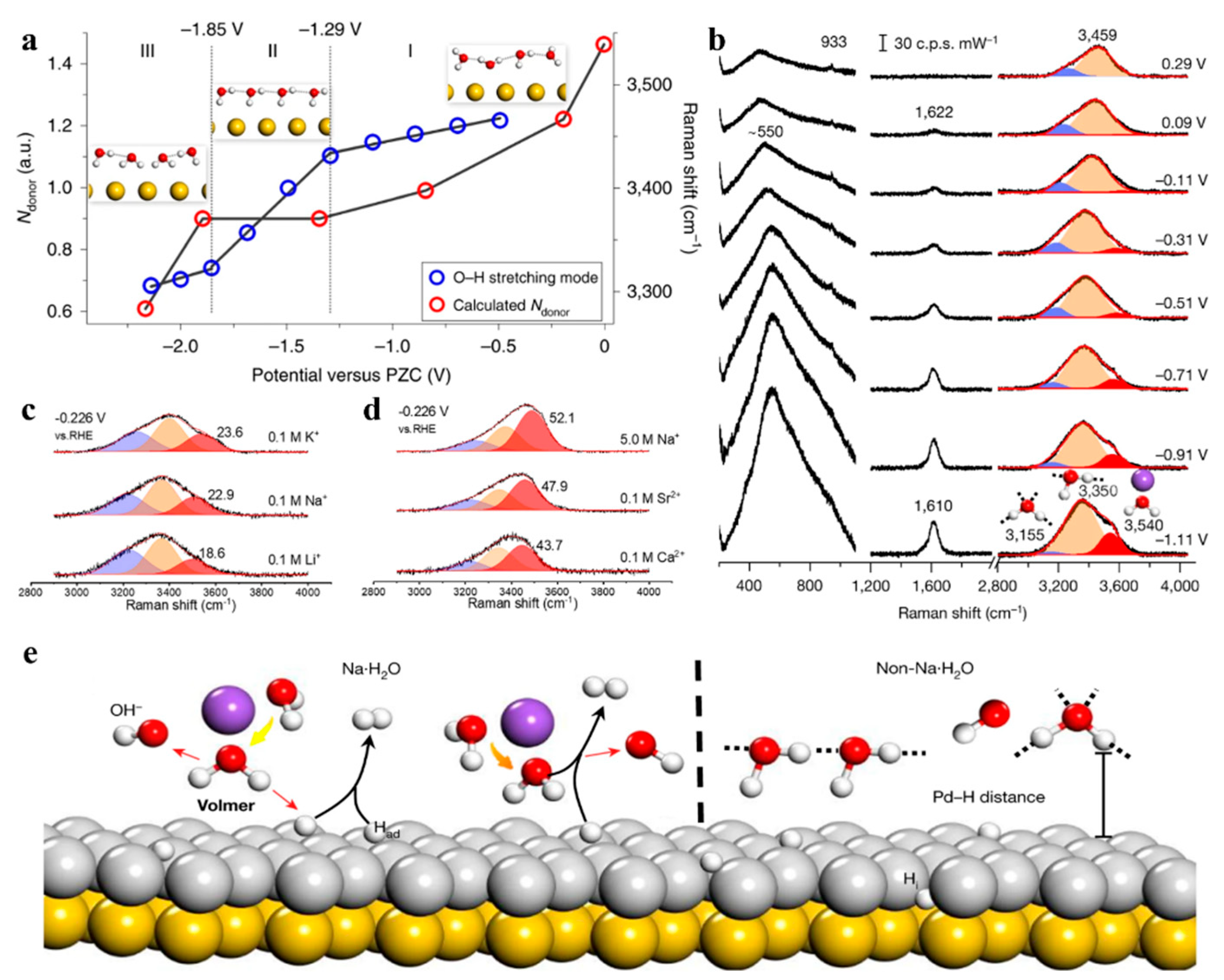
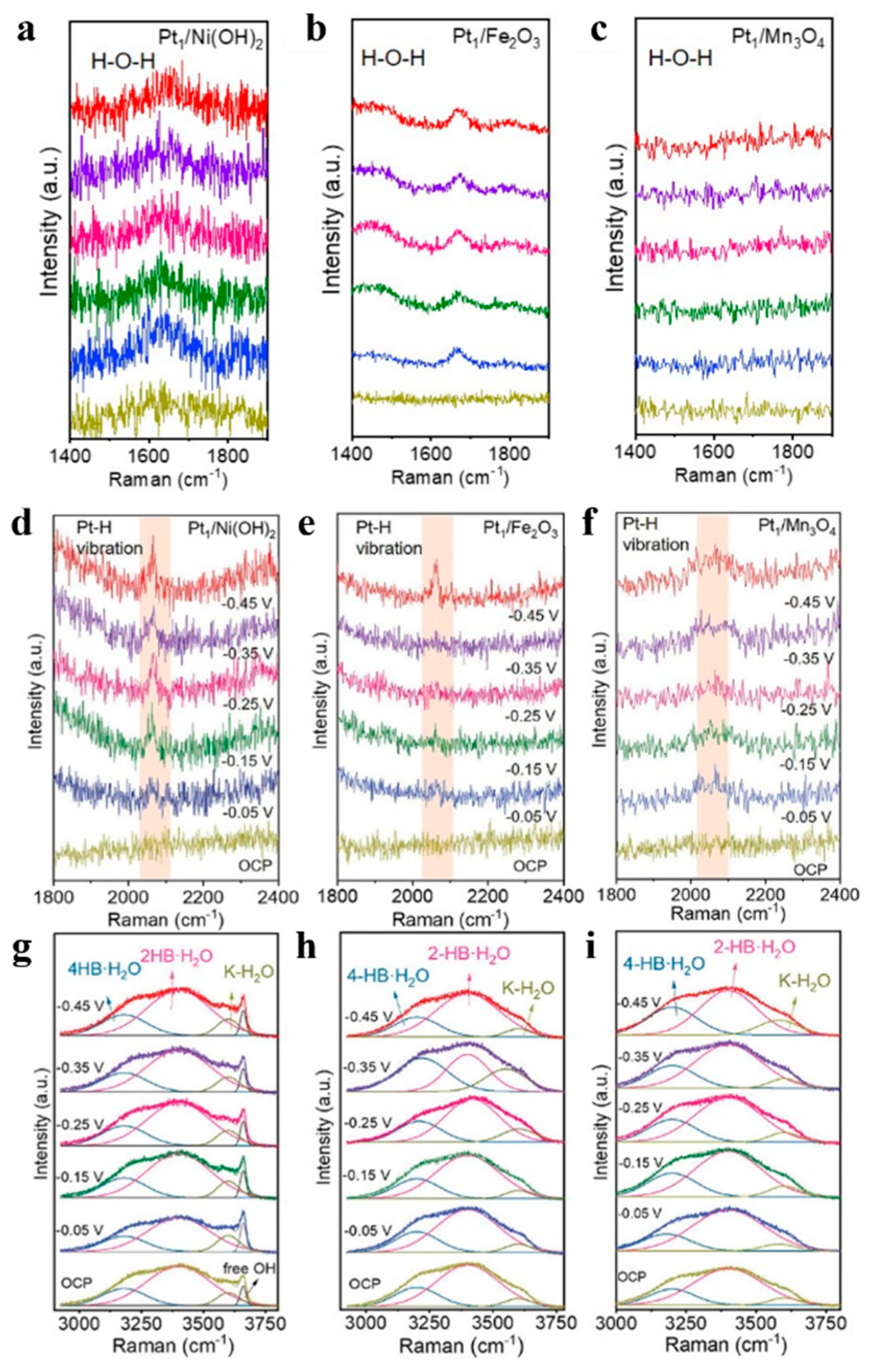
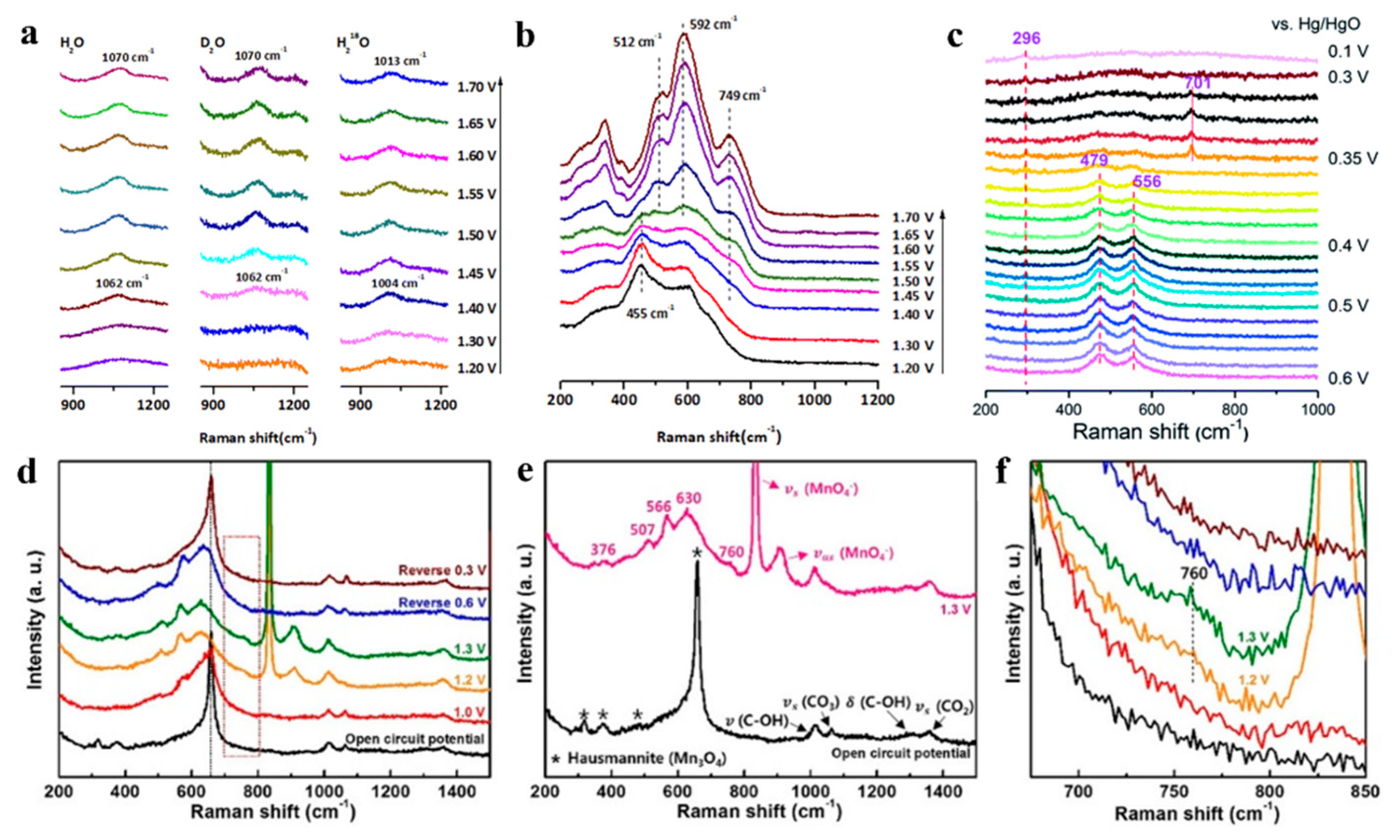
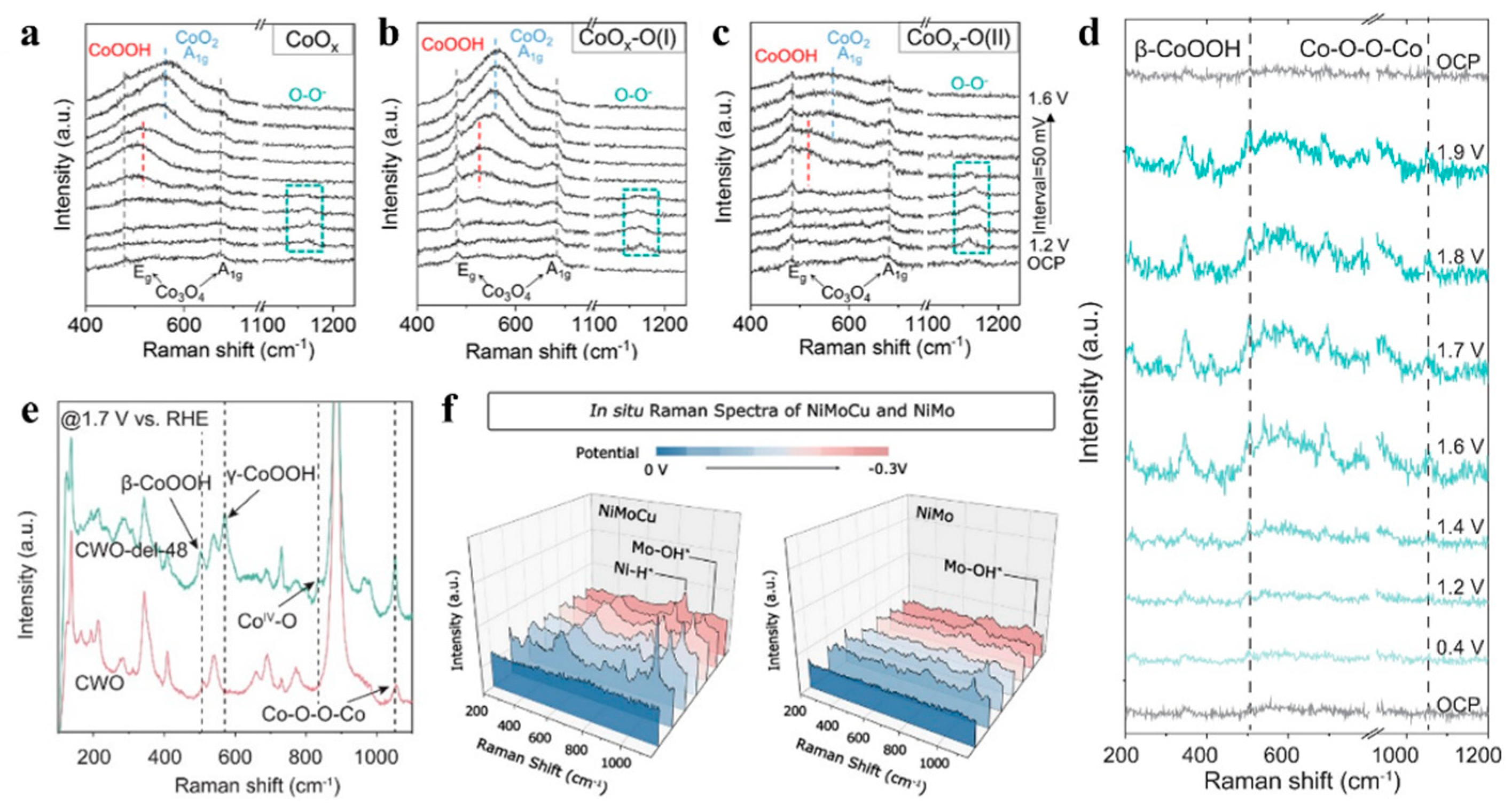
3.3. Tracking Species Evolutions at Electrochemical Interfaces
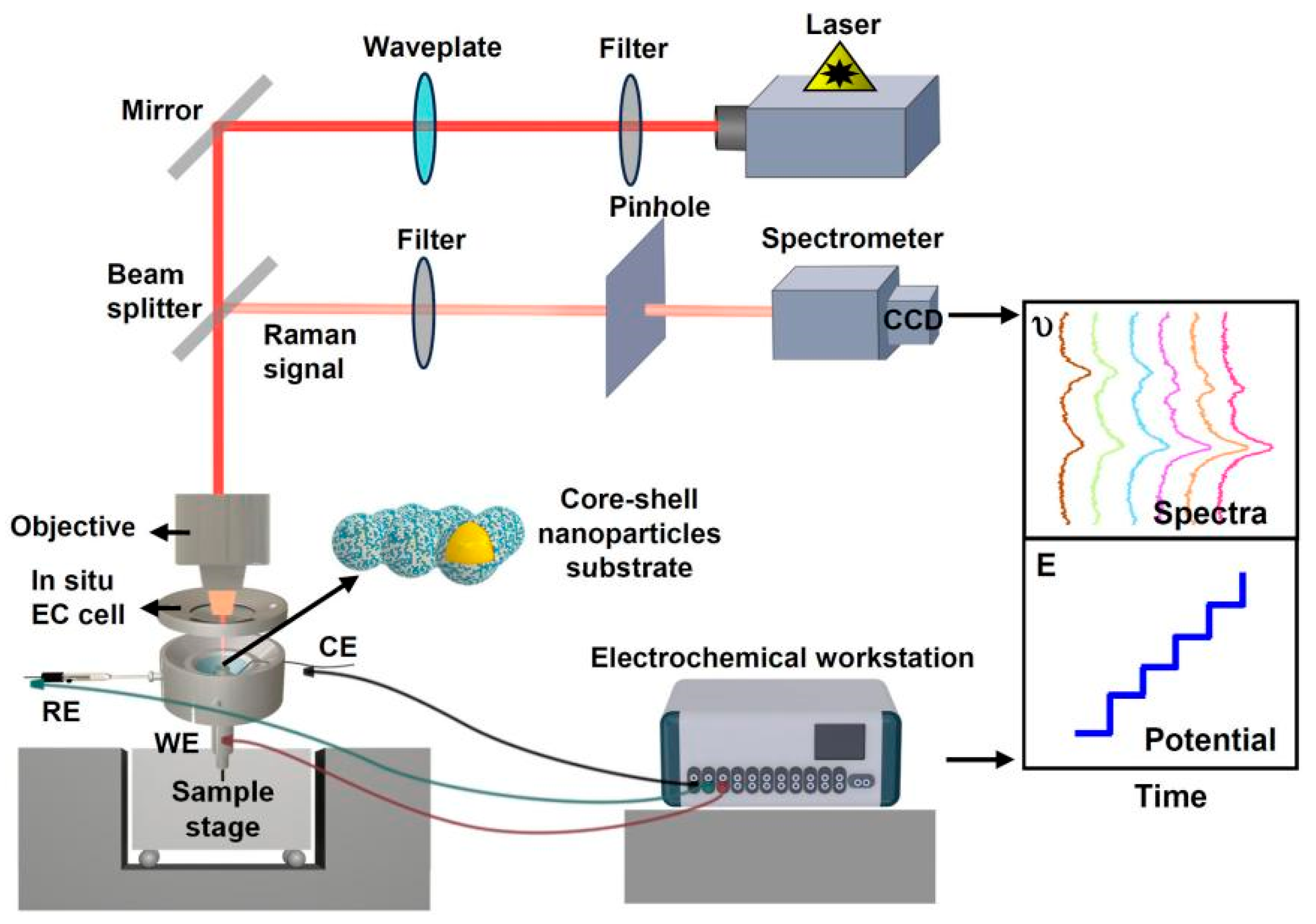
3.3.1. Coupling of SERS with In Situ Electrochemical Techniques
3.3.2. Analyzing Catalytic Reactions by Electrochemical Raman Spectroscopy
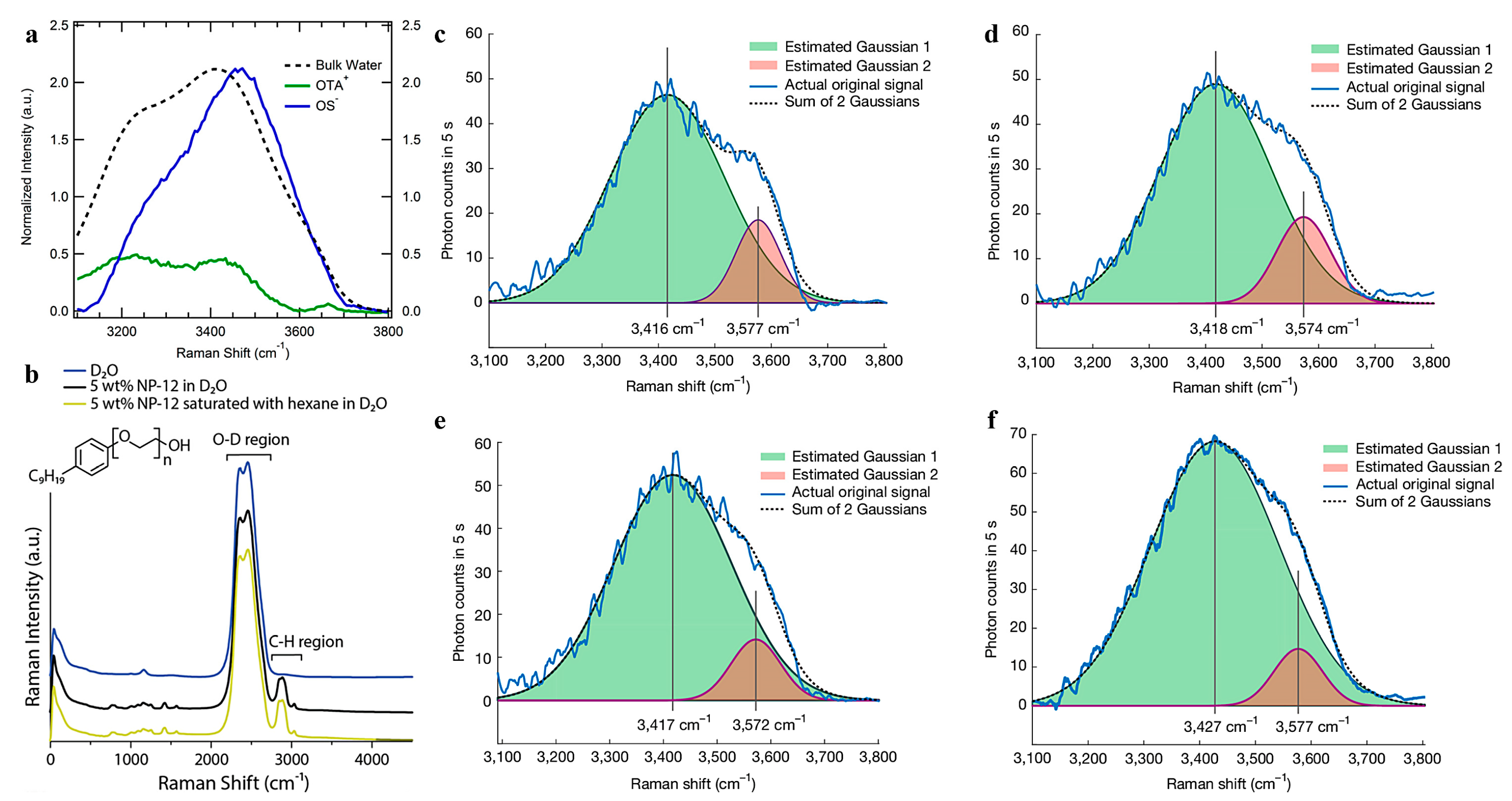
3.4. Raman Spectroscopy Combined with Data Processing to Analyze Interface Substances
4. Challenges of Raman Spectroscopy for Interface Detection
4.1. Stability and Design Complexity of SERS Substrates
4.2. Uniformity and Repeatability of SERS Signals
4.3. Interference of Test Conditions on SERS Signals
4.4. Inherent Difficulties in the Detection of Electrochemical Reaction Intermediates
5. Conclusions and Outlooks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bao, Y.-F.; Zhu, M.-Y.; Zhao, X.-J.; Chen, H.-X.; Wang, X.; Ren, B. Nanoscale Chemical Characterization of Materials and Interfaces by Tip-Enhanced Raman Spectroscopy. Chem. Soc. Rev. 2024, 53, 10044–10079. [Google Scholar] [CrossRef]
- Björneholm, O.; Hansen, M.H.; Hodgson, A.; Liu, L.-M.; Limmer, D.T.; Michaelides, A.; Pedevilla, P.; Rossmeisl, J.; Shen, H.; Tocci, G.; et al. Water at Interfaces. Chem. Rev. 2016, 116, 7698–7726. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.-C.; Huang, T.-X.; Su, H.-S.; Zhong, J.-H.; Zeng, Z.-C.; Li, M.-H.; Ren, B. Tip-Enhanced Raman Spec-troscopy for Surfaces and Interfaces. Chem. Soc. Rev. 2017, 46, 4020–4041. [Google Scholar] [CrossRef]
- Xu, S.; Wu, J.; Guo, Y.; Zhang, Q.; Zhong, X.; Li, J.; Ren, W. Applications of Machine Learning in Surfaces and Interfaces. Chem. Phys. Rev. 2025, 6, 011309. [Google Scholar] [CrossRef]
- Sun, W.; Xu, Y.; Zhou, Y.; Zeng, Z.; Wang, L.; Ouyang, J. Topographic Scanning Electronic Microscopy Reveals the 3D Surface Structure of Materials. Adv. Funct. Mater. 2025, 35, 2420372. [Google Scholar] [CrossRef]
- Kiyama, R.; Yoshida, M.; Nonoyama, T.; Sedlačík, T.; Jinnai, H.; Kurokawa, T.; Nakajima, T.; Gong, J.P. Nanoscale TEM Imaging of Hydrogel Network Architecture. Adv. Mater. 2023, 35, 2208902. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Rostamabadi, H.; Assadpour, E.; Jafari, S.M. Morphology and Microstructural Analysis of Bioactive-Loaded Micro/Nanocarriers via Microscopy Techniques; CLSM/SEM/TEM/AFM. Advances Colloid Interface Sci. 2020, 280, 102166. [Google Scholar] [CrossRef]
- Elemans, J.A.A.W. Externally Applied Manipulation of Molecular Assemblies at Solid-Liquid Interfaces Revealed by Scan-ning Tunneling Microscopy. Adv. Funct. Mater. 2016, 26, 8932–8951. [Google Scholar] [CrossRef]
- Mallick, S.; Jena, M.; Das, B.; Mohanty, B.; Patel, B.K.; Das, D.P.; Rath, A. Tuning and Understanding of Layered 2D MoS2 –MoO3 Interface for Enhanced Photocatalytic Activities. Adv. Funct. Mater. 2025, 35, 2422645. [Google Scholar] [CrossRef]
- Wang, J.; Hsu, C.-S.; Wu, T.-S.; Chan, T.-S.; Suen, N.-T.; Lee, J.-F.; Chen, H.M. In Situ X-Ray Spectroscopies beyond Con-ventional X-Ray Absorption Spectroscopy on Deciphering Dynamic Configuration of Electrocatalysts. Nat. Commun. 2023, 14, 6576. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Tao, J.; Chen, Y.; White, R.G.; Zhang, L.; Li, J.; Huang, Z.; Lin, Y. Unraveling the Evolution of Cathode–Solid Electrolyte Interface Using Operando X-Ray Photoelectron Spectroscopy. Adv. Powder Mater. 2024, 3, 100184. [Google Scholar] [CrossRef]
- Chen, M.; Liu, D.; Qiao, L.; Zhou, P.; Feng, J.; Ng, K.W.; Liu, Q.; Wang, S.; Pan, H. In-Situ/Operando Raman Techniques for in-Depth Understanding on Electrocatalysis. Chem. Eng. J. 2023, 461, 141939. [Google Scholar] [CrossRef]
- Hess, C. New Advances in Using Raman Spectroscopy for the Characterization of Catalysts and Catalytic Reactions. Chem. Soc. Rev. 2021, 50, 3519–3564. [Google Scholar] [CrossRef]
- Dodo, K.; Fujita, K.; Sodeoka, M. Raman Spectroscopy for Chemical Biology Research. J. Am. Chem. Soc. 2022, 144, 19651–19667. [Google Scholar] [CrossRef]
- Tittel, J.; Knechtel, F.; Ploetz, E. Conquering Metal–Organic Frameworks by Raman Scattering Techniques. Adv. Funct. Mate-Rials 2024, 34, 2307518. [Google Scholar] [CrossRef]
- Hu, W.; Luo, Y.; Zhu, E.; Zhang, A.; Wang, L. Revealing the Electrocatalytic Reaction Mechanism of Water Splitting by In Situ Raman Technique. Adv. Sustain. Syst. 2024, 8, 2400387. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, Q.; Zhou, J.; Wang, X.; Huang, M.; Jiang, H.; Cölfen, H. Toward Understanding the Formation Mechanism and OER Catalytic Mechanism of Hydroxides by In Situ and Operando Techniques. Angew. Chem. Int. Ed. 2023, 62, e202309293. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, N.; Xiong, Y. In Situ Raman Characterizations for Enhanced Understandings on Electrocatalysis. J. Phys. Chem. C 2024, 128, 13651–13665. [Google Scholar] [CrossRef]
- Wu, S.; Liang, Z.; Wang, T.; Liu, X.; Huang, S. In Situ Characterization Techniques: Main Tools for Revealing OER/ORR Catalytic Mechanism and Reaction Dynamics. Inorg. Chem. Front. 2025, 12, 848–875. [Google Scholar] [CrossRef]
- Ding, S.-Y.; You, E.-M.; Tian, Z.-Q.; Moskovits, M. Electromagnetic Theories of Surface-Enhanced Raman Spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Zrimsek, A.B.; Chiang, N.; Mattei, M.; Zaleski, S.; McAnally, M.O.; Chapman, C.T.; Henry, A.-I.; Schatz, G.C.; Van Duyne, R.P. Single-Molecule Chemistry with Surface- and Tip-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 7583–7613. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.-J.; Wei, T.; Ma, X.; Zheng, X.-S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioa-nalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-F.; Zhang, Y.-J.; Ding, S.-Y.; Panneerselvam, R.; Tian, Z.-Q. Core–Shell Nanoparticle-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef]
- Kurouski, D.; Dazzi, A.; Zenobi, R.; Centrone, A. Infrared and Raman Chemical Imaging and Spectroscopy at the Nanoscale. Chem. Soc. Rev. 2020, 49, 3315–3347. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously Intense Raman Spectra of Pyridine at a Silver Electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Zhu, Y.-Z.; Zhou, R.-Y.; Hu, S.; Li, J.-F.; Tian, Z.-Q. Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy: Toward High Sensitivity and Broad Applicability. Acs Nano 2024, 18, 32287–32298. [Google Scholar] [CrossRef]
- Qian, Z.-X.; Zeng, J.-S.; Zhao, S.; Zheng, Q.-N.; Tian, J.-H.; Xu, Q.-C.; Zhang, H.; Li, J.-F. In Situ Exploration of Oxygen Electrocatalysis Using Core-Shell Nanostructure-Enhanced Raman Spectroscopy. Nano Mater. Sci. 2024, in press. [Google Scholar] [CrossRef]
- Roy, S.S.; Nagappan, S.; Satheesan, A.K.; Karmakar, A.; Kundu, S. Surface-Enhanced Raman Scattering Coupled with In Situ Raman Spectroscopy for the Detection of the OER Mechanism: A Mini-Review. J. Phys. Chem. C 2024, 128, 13634–13650. [Google Scholar] [CrossRef]
- Huang, Z.; Peng, J.; Xu, L.; Liu, P. Development and Application of Surface-Enhanced Raman Scattering (SERS). Nano-Mater. 2024, 14, 1417. [Google Scholar] [CrossRef]
- Jensen, L.; Aikens, C.M.; Schatz, G.C. Electronic Structure Methods for Studying Surface-Enhanced Raman Scattering. Chem. Soc. Rev. 2008, 37, 1061. [Google Scholar] [CrossRef]
- Cong, S.; Liu, X.; Jiang, Y.; Zhang, W.; Zhao, Z. Surface Enhanced Raman Scattering Revealed by Interfacial Charge-Transfer Transitions. Innovation 2020, 1, 100051. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Y.; Qiu, H.; Yang, Y.; Wu, Q.; Fan, X.; Ding, Y.; Yi, L.; Ge, K.; Shen, Y. Recent Progress on Noble-Free Substrates for Surface-Enhanced Raman Spectroscopy Analysis. Coord. Chem. Rev. 2023, 497, 215425. [Google Scholar] [CrossRef]
- Wen, B.-Y.; Chen, Q.-Q.; Radjenovic, P.M.; Dong, J.-C.; Tian, Z.-Q.; Li, J.-F. In Situ Surface-Enhanced Raman Spectroscopy Characterization of Electrocatalysis with Different Nanostructures. Annu. Rev. Phys. Chem. 2021, 72, 331–351. [Google Scholar] [CrossRef]
- Yi, J.; You, E.-M.; Hu, R.; Wu, D.-Y.; Liu, G.-K.; Yang, Z.-L.; Zhang, H.; Gu, Y.; Wang, Y.-H.; Wang, X.; et al. Sur-face-Enhanced Raman Spectroscopy: A Half-Century Historical Perspective. Chem. Soc. Rev. 2025, 54, 1453–1551. [Google Scholar] [CrossRef]
- Goel, R.; Chakraborty, S.; Awasthi, V.; Bhardwaj, V.; Dubey, S.K. Exploring the Various Aspects of Surface Enhanced Raman Spectroscopy (SERS) with Focus on the Recent Progress: SERS-Active Substrate, SERS-Instrumentation, SERS-Application. Sensors Actuators A Phys. 2024, 376, 115555. [Google Scholar] [CrossRef]
- Van Duyne, R.P.; Haushalter, J.P. Surface-Enhanced Raman Spectroscopy of Adsorbates on Semiconductor Electrode Sur-faces: Tris(Bipyridine)Ruthenium(II) Adsorbed on Silver-Modified n-Gallium Arsenide(100). J. Phys. Chem. 1983, 87, 2999–3003. [Google Scholar] [CrossRef]
- Fleischmann, M.; Tian, Z.Q.; Li, L.J. Raman Spectroscopy of Adsorbates on Thin Film Electrodes Deposited on Silver Sub-strates. J. Electroanal. Chem. Interfacial Electrochem. 1987, 217, 397–410. [Google Scholar] [CrossRef]
- Leung, L.W.H.; Weaver, M.J. Extending Surface-Enhanced Raman Spectroscopy to Transition-Metal Surfaces: Carbon Monoxide Adsorption and Electrooxidation on Platinum- and Palladium-Coated Gold Electrodes. J. Am. Chem. Soc. 1987, 109, 5113–5119. [Google Scholar] [CrossRef]
- Langer, J.; De Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. Acs Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Duan, S.; Radjenovic, P.M.; Tian, Z.-Q.; Li, J.-F. Core–Shell Nanostructure-Enhanced Raman Spectroscopy for Surface Catalysis. Acc. Chem. Res. 2020, 53, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Haryanto, A.; Lee, C.W. Shell Isolated Nanoparticle Enhanced Raman Spectroscopy for Mechanistic Investigation of Elec-trochemical Reactions. Nano Converg. 2022, 9, 9. [Google Scholar] [CrossRef]
- Huh, J.-H.; Lee, J.; Lee, S. Comparative Study of Plasmonic Resonances between the Roundest and Randomly Faceted Au Nanoparticles-on-Mirror Cavities. Acs Photonics 2018, 5, 413–421. [Google Scholar] [CrossRef]
- Feng, R.; Fu, S.; Liu, H.; Wang, Y.; Liu, S.; Wang, K.; Chen, B.; Zhang, X.; Hu, L.; Chen, Q.; et al. Single-Atom Site SERS Chip for Rapid, Ultrasensitive, and Reproducible Direct-Monitoring of RNA Binding. Adv. Healthc. Mater. 2024, 13, 2301146. [Google Scholar] [CrossRef]
- Chen, B.; Meng, S.; Liu, D.; Deng, Q.; Wang, C. In Situ SERS Monitoring of Schiff Base Reactions via Nanoparticles on a Mirror Platform. Catalysts 2024, 14, 803. [Google Scholar] [CrossRef]
- Mahapatra, S.; Li, L.; Schultz, J.F.; Jiang, N. Tip-Enhanced Raman Spectroscopy: Chemical Analysis with Nanoscale to Angstrom Scale Resolution. J. Chem. Physics 2020, 153, 010902. [Google Scholar] [CrossRef]
- Wessel, J. Surface-Enhanced Optical Microscopy. J. Opt. Soc. Am. B 1985, 2, 1538. [Google Scholar] [CrossRef]
- Stöckle, R.M.; Suh, Y.D.; Deckert, V.; Zenobi, R. Nanoscale Chemical Analysis by Tip-Enhanced Raman Spectroscopy. Chem. Phys. Lett. 2000, 318, 131–136. [Google Scholar] [CrossRef]
- Hayazawa, N.; Inouye, Y.; Sekkat, Z.; Kawata, S. Metallized Tip Amplification of Near-Field Raman Scattering. Opt. Commun. 2000, 183, 333–336. [Google Scholar] [CrossRef]
- Zhang, Z.; Sheng, S.; Wang, R.; Sun, M. Tip-Enhanced Raman Spectroscopy. Anal. Chem. 2016, 88, 9328–9346. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Yuan, Y.; Yao, J.; Lu, F.; Gu, R. Structure of Water at Ionic Liquid/Ag Interface Probed by Surface Enhanced Raman Spectroscopy. Sci. China Chem. 2011, 54, 200–204. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Sun, Y.-B.; Shi, P.-C.; Liu, T.; Li, Z.-H.; Luo, S.-H.; Wang, X.-C.; Cao, X.-Y.; Ren, B.; Liu, G.-K.; et al. Revealing Unconventional Host–Guest Complexation at Nanostructured Interface by Surface-Enhanced Raman Spectroscopy. Light. Sci. Appl. 2021, 10, 85. [Google Scholar] [CrossRef]
- Ma, W.; Wang, Y.; Wang, R.; Fan, X.; Ma, S.; Tang, Y.; Ai, Z.; Yao, Y.; Zhang, L.; Gao, T. Azo-Enhanced Raman Scattering Probing Proton Transfer between Water and Nanoscale Zero-Valent Iron. J. Am. Chem. Soc. 2024, 146, 32785–32794. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, M.; Lian, E.; Xia, L.; Asimakopoulos, A.G.; Luo, S.; Wang, L. Identification of Poly(Ethylene Terephthalate) Nanoplastics in Commercially Bottled Drinking Water Using Surface-Enhanced Raman Spectroscopy. Environ. Sci. Technol. 2023, 57, 8365–8372. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lei, F.; Gong, M.; Zhou, X.; Zhao, X.; Li, Z.; Zhang, C.; Man, B.; Yu, J. In Situ Raman Monitoring of Trace Antibiotics in Different Harsh Water Environments. Energy Environ. Mater. 2024, 7, e12517. [Google Scholar] [CrossRef]
- Dou, X.; Zhao, L.; Li, X.; Qin, L.; Han, S.; Kang, S.-Z. Ag Nanoparticles Decorated Mesh-like MoS2 Hierarchical Nanostructure Fabricated on Ti Foil: A Highly Sensitive SERS Substrate for Detection of Trace Malachite Green in Flowing Water. Appl. Surf. Sci. 2020, 509, 145331. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, K.; Wang, W.; Wei, L.; Lai, Y. Quantitative and Sensitive Analysis of Polystyrene Nanoplastics down to 50 nm by Surface-Enhanced Raman Spectroscopy in Water. J. Hazard. Mater. 2022, 429, 128388. [Google Scholar] [CrossRef]
- Ruan, X.; Xie, L.; Liu, J.; Ge, Q.; Liu, Y.; Li, K.; You, W.; Huang, T.; Zhang, L. Rapid Detection of Nanoplastics down to 20 nm in Water by Surface-Enhanced Raman Spectroscopy. J. Hazard. Mater. 2024, 462, 132702. [Google Scholar] [CrossRef]
- Carreón, R.V.; Cortázar-Martínez, O.; Rodríguez-Hernández, A.G.; De La Rosa, L.E.S.; Gervacio-Arciniega, J.J.; Krishnan, S.K. Ionic Liquid-Assisted Thermal Evaporation of Bimetallic Ag–Au Nanoparticle Films as a Highly Reproducible SERS Substrate for Sensitive Nanoplastic Detection in Complex Environments. Anal. Chem. 2024, 96, 5790–5797. [Google Scholar] [CrossRef]
- Carreón, R.V.; Rodríguez-Hernández, A.G.; De La Rosa, L.E.S.; Gervacio-Arciniega, J.J.; Krishnan, S.K. Mechanically Flexible, Large-Area Fabrication of Three-Dimensional Dendritic Au Films for Reproducible Surface-Enhanced Raman Scat-tering Detection of Nanoplastics. Acs Sens. 2025, 10, 1747–1755. [Google Scholar] [CrossRef]
- Xing, F.; Duan, W.; Tang, J.; Zhou, Y.; Guo, Z.; Zhang, H.; Xiong, J.; Fan, M. Superhydrophobic Surface-Enhanced Raman Spectroscopy (SERS) Substrates for Sensitive Detection of Trace Nanoplastics in Water. Anal. Chem. 2025, 97, 2293–2299. [Google Scholar] [CrossRef]
- Li, G.; Yang, Z.; Pei, Z.; Li, Y.; Yang, R.; Liang, Y.; Zhang, Q.; Jiang, G. Single-Particle Analysis of Micro/Nanoplastics by SEM-Raman Technique. Talanta 2022, 249, 123701. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, J.; Peng, L.; Sun, X.; Wang, G.; Wang, Y.; Chen, L. A Sustainable Emulsion for Separation and Raman Identification of Microplastics and Nanoplastics. Chem. Eng. J. 2023, 469, 143992. [Google Scholar] [CrossRef]
- Shao, Q.; Zhang, X.; Liang, P.; Chen, Q.; Qi, X.; Zou, M. Fabrication of Magnetic Au/Fe3O4/MIL-101(Cr) (AF-MIL) as Sensitive Surface-Enhanced Raman Spectroscopy (SERS) Platform for Trace Detection of Antibiotics Residue. Appl. Surf. Sci. 2022, 596, 153550. [Google Scholar] [CrossRef]
- Feng, Y.; Dai, J.; Wang, C.; Zhou, H.; Li, J.; Ni, G.; Zhang, M.; Huang, Y. Ag Nanoparticle/Au@Ag Nanorod Sandwich Structures for SERS-Based Detection of Perfluoroalkyl Substances. Acs Appl. Nano Mater. 2023, 6, 13974–13983. [Google Scholar] [CrossRef]
- Kukralova, K.; Miliutina, E.; Guselnikova, O.; Burtsev, V.; Hrbek, T.; Svorcik, V.; Lyutakov, O. Dual-Mode Electrochemical and SERS Detection of PFAS Using Functional Porous Substrate. Chemosphere 2024, 364, 143149. [Google Scholar] [CrossRef]
- Lada, Z.G.; Mathioudakis, G.N.; Beobide, A.S.; Andrikopoulos, K.S.; Voyiatzis, G.A. Generic Method for the Detection of Short & Long Chain PFAS Extended to the Lowest Concentration Levels of SERS Capability. Chemosphere 2024, 363, 142916. [Google Scholar] [CrossRef] [PubMed]
- Mohajer, M.A.; Basuri, P.; Evdokimov, A.; David, G.; Zindel, D.; Miliordos, E.; Signorell, R. Spontaneous formation of urea from carbon dioxide and ammonia in aqueous droplets. Science 2025, 388, 1426–1430. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Q.; Liu, Y.-X.; Yin, J.; Pang, S.-F.; Liu, P.; Zhang, Y.-H.; Ge, M. Microdroplet Surface Drives and Accel-erates Proton-Controlled, Size-Dependent Nitrate Photolysis. J. Am. Chem. Soc. 2025, 147, 19595–19601. [Google Scholar] [CrossRef]
- Li, K.; Ge, Q.; Liu, Y.; Wang, L.; Gong, K.; Liu, J.; Xie, L.; Wang, W.; Ruan, X.; Zhang, L. Highly Efficient Photocatalytic H2 O2 Production in Microdroplets: Accelerated Charge Separation and Transfer at Interfaces. Energy Environ. Sci. 2023, 16, 1135–1145. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Wang, T.; Ge, Q.; Li, K.; Liu, J.; You, W.; Wang, L.; Xie, L.; Fu, H.; et al. Significantly Accelerated Pho-tosensitized Formation of Atmospheric Sulfate at the Air–Water Interface of Microdroplets. J. Am. Chem. Soc. 2024, 146, 6580–6590. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Ding, X.-L.; Liu, A.-X.; Cui, H.; Zhong, J.-R.; Dai, Y.-M. Biodegradable MAM-Based Amphiphilic Block Copolymers: Toward Efficient and Eco-Friendly Kinetic Inhibitors for Methane Hydrate Formation. Chem. Eng. J. 2024, 500, 157347. [Google Scholar] [CrossRef]
- Li, K.; You, W.; Wang, W.; Gong, K.; Liu, Y.; Wang, L.; Ge, Q.; Ruan, X.; Ao, J.; Ji, M.; et al. Significantly Accelerated Pho-tochemical Perfluorooctanoic Acid Decomposition at the Air–Water Interface of Microdroplets. Environ. Sci. Technol. 2023, 57, 21448–21458. [Google Scholar] [CrossRef]
- Hao, G.; Shu-Xi, D.; Cheng-Feng, S.; Chao, W.; Ya-Bin, H.; Zu-Liang, D. In Situ Raman Spectroscopy of Langmuir Mono-layers at Air-Water Interface. Acta Phys.-Chim. Sin. 2006, 22, 1061–1064. [Google Scholar] [CrossRef]
- Arunagiri, V.; Prasannan, A.; Udomsin, J.; Lai, J.-Y.; Wang, C.-F.; Hong, P.-D.; Tsai, H.C. Facile Fabrication of Eco-Friendly Polycaprolactone (PCL)/Poly-D, L-Lactic Acid (PDLLA) Modified Melamine Sorbent for Oil-Spill Cleaning and Water/Oil (W/O) Emulsion Separation. Sep. Purif. Technol. 2021, 259, 118081. [Google Scholar] [CrossRef]
- Qiang, F.; Hu, L.-L.; Gong, L.-X.; Zhao, L.; Li, S.-N.; Tang, L.-C. Facile Synthesis of Super-Hydrophobic, Electrically Con-ductive and Mechanically Flexible Functionalized Graphene Nanoribbon/Polyurethane Sponge for Efficient Oil/Water Sep-aration at Static and Dynamic States. Chem. Eng. J. 2018, 334, 2154–2166. [Google Scholar] [CrossRef]
- Fischer, B.; Lambertz, A.; Nuys, M.; Beyer, W.; Duan, W.; Bittkau, K.; Ding, K.; Rau, U. Insights into the Si─H Bonding Configuration at the Amorphous/Crystalline Silicon Interface of Silicon Heterojunction Solar Cells by Raman and FTIR Spectroscopy. Adv. Mater. 2023, 35, 2306351. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Y.; Feng, H.; Liang, H.; Shen, J.; Diao, Y.; Liu, Y.; Zeng, X.; Yu, Z.; Sun, R.; et al. In Situ Raman Spectroscopy Monitoring of Interface Aging in Aluminum-Filled Polydimethylsiloxane Composites. Nano Mater. Sci. 2025, S2589965125000558. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, B.G.; Yun, S.J.; Lee, Y.H.; Lee, S.M.; Jeong, M.S. Unveiling Defect-Related Raman Mode of Monolayer WS2 via Tip-Enhanced Resonance Raman Scattering. Acs Nano 2018, 12, 9982–9990. [Google Scholar] [CrossRef]
- Shao, J.; Chen, F.; Su, W.; Zeng, Y.; Lu, H.-W. Multimodal Nanoscopic Study of Atomic Diffusion and Related Localized Optoelectronic Response of WS2 /MoS2 Lateral Heterojunctions. Acs Appl. Mater. Interfaces 2021, 13, 20361–20370. [Google Scholar] [CrossRef]
- Garg, S.; Fix, J.P.; Krayev, A.V.; Flanery, C.; Colgrove, M.; Sulkanen, A.R.; Wang, M.; Liu, G.-Y.; Borys, N.J.; Kung, P. Na-noscale Raman Characterization of a 2D Semiconductor Lateral Heterostructure Interface. Acs Nano 2022, 16, 340–350. [Google Scholar] [CrossRef]
- Rahaman, M.; Marino, E.; Joly, A.G.; Stevens, C.E.; Song, S.; Alfieri, A.; Jiang, Z.; O’Callahan, B.T.; Rosen, D.J.; Jo, K.; et al. Tunable Localized Charge Transfer Excitons in Nanoplatelet–2D Chalcogenide van Der Waals Heterostructures. Acs Nano 2024, 18, 15185–15193. [Google Scholar] [CrossRef]
- Milekhin, I.A.; Rahaman, M.; Anikin, K.V.; Rodyakina, E.E.; Duda, T.A.; Saidzhonov, B.M.; Vasiliev, R.B.; Dzhagan, V.M.; Milekhin, A.G.; Latyshev, A.V.; et al. Resonant Tip-Enhanced Raman Scattering by CdSe Nanocrystals on Plasmonic Sub-strates. Nanoscale Adv. 2020, 2, 5441–5449. [Google Scholar] [CrossRef]
- Meng, G.; Chan, J.C.K.; Rousseau, D.; Li-Chan, E.C.Y. Study of Protein−Lipid Interactions at the Bovine Serum Albumin/Oil Interface by Raman Microspectroscopy. J. Agric. Food Chem. 2005, 53, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Niu, D.; Zhang, H.; Chang, C.; Gu, L.; Su, Y.; Yang, Y. Ovalbumin/Gum Arabic-Stabilized Emulsion: Rheology, Emulsion Characteristics, and Raman Spectroscopic Study. Food Hydrocoll. 2016, 52, 607–614. [Google Scholar] [CrossRef]
- Han, Z.; Xu, S.; Sun, J.; Yue, X.; Wu, Z.; Shao, J.-H. Effects of Fatty Acid Saturation Degree on Salt-Soluble Pork Protein Conformation and Interfacial Adsorption Characteristics at the Oil/Water Interface. Food Hydrocoll. 2021, 113, 106472. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Zhao, G.; Li, Y.; Yang, L.; Zhu, L.; Liu, H. Protease-Induced Soy Protein Isolate (SPI) Characteristics and Structure Evolution on the Oil–Water Interface of Emulsion. J. Food Eng. 2022, 317, 110849. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Gupta, P.; Singamaneni, S.; Lee, B.; Jun, Y.-S. The Roles of Oil–Water Interfaces in Forming Ultrasmall CaSO4 Nanoparticles. Acs Appl. Mater. Interfaces 2024, 16, 29390–29401. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Jin, X.; Wang, D.; Hao, D.; Li, Y.; Zhu, Z.; Tian, Y.; Jiang, L. Interfacial Water-Dictated Oil Adhesion Based on Ion Modulation. J. Am. Chem. Soc. 2023, 145, 24145–24152. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, Y.; Xia, Q.; Du, L.; He, J.; Xu, J.; Zhou, C.; Pan, D. Hydrophobic Interaction at the O/W Interface: Impacts on the Interfacial Stability, Encapsulation and Bioaccessibility of Polyphenols. Food Hydrocoll. 2023, 140, 108622. [Google Scholar] [CrossRef]
- Tomobe, K.; Yamamoto, E.; Kojić, D.; Sato, Y.; Yasui, M.; Yasuoka, K. Origin of the Blueshift of Water Molecules at Interfaces of Hydrophilic Cyclic Compounds. Sci. Adv. 2017, 3, e1701400. [Google Scholar] [CrossRef]
- Taylor, J.N.; Bando, K.; Tsukagoshi, S.; Tanaka, L.; Fujita, K.; Fujita, S. Microscopic Water Dispersion and Hydrogen-Bonding Structures in Margarine Spreads with Raman Hyperspectral Imaging and Machine Learning. Food Chem. 2025, 465, 142035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, W.; Hao, J.; Li, X.; Xu, D.; Cao, Y. In Vitro Digestion of Solid-in-Oil-in-Water Emulsions for Delivery of CaCO3. Food Hydrocoll. 2022, 129, 107605. [Google Scholar] [CrossRef]
- Guo, D.; Ou, W.; Ning, F.; Fang, B.; Liu, Z.; Fang, X.; Lu, W.; Zhang, L.; Din, S.U.; He, Z. The Effects of Hydrate Formation and Dissociation on the Water-Oil Interface: Insight into the Stability of an Emulsion. Fuel 2020, 266, 116980. [Google Scholar] [CrossRef]
- Phan-Quang, G.C.; Lee, H.K.; Ling, X.Y. Isolating Reactions at the Picoliter Scale: Parallel Control of Reaction Kinetics at the Liquid–Liquid Interface. Angew. Chem. Int. Ed. 2016, 55, 8304–8308. [Google Scholar] [CrossRef]
- Xiong, H.; Lee, J.K.; Zare, R.N.; Min, W. Strong Electric Field Observed at the Interface of Aqueous Microdroplets. J. Phys. Chem. Lett. 2020, 11, 7423–7428. [Google Scholar] [CrossRef]
- Argyri, S.-M.; Stark, A.; Eriksson, V.; Evenäs, L.; Martinelli, A.; Bordes, R. Crystallization at the Hexadecane/Water Interface Observed under Acoustic Levitation. J. Environ. Sci. 2025, 158, 197–206. [Google Scholar] [CrossRef]
- Lin, X.-M.; Sun, Y.-L.; Chen, Y.-X.; Li, S.-X.; Li, J.-F. Insights into Electrocatalysis through in Situ Electrochemical Sur-face-Enhanced Raman Spectroscopy. EScience 2024, 100352. [Google Scholar] [CrossRef]
- Liu, S.; D’Amario, L.; Jiang, S.; Dau, H. Selected Applications of Operando Raman Spectroscopy in Electrocatalysis Research. Curr. Opin. Electrochem. 2022, 35, 101042. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman Spectra from Electrode Surfaces. J. Chem. Soc., Chem. Commun. 1973, 80. [Google Scholar] [CrossRef]
- Wu, D.-Y.; Li, J.-F.; Ren, B.; Tian, Z.-Q. Electrochemical Surface-Enhanced Raman Spectroscopy of Nanostructures. Chem. Soc. Rev. 2008, 37, 1025. [Google Scholar] [CrossRef]
- Shi, L.; LaCour, R.A.; Qian, N.; Heindel, J.P.; Lang, X.; Zhao, R.; Head-Gordon, T.; Min, W. Water Structure and Electric Fields at the Interface of Oil Droplets. Nature 2025, 640, 87–93. [Google Scholar] [CrossRef] [PubMed]
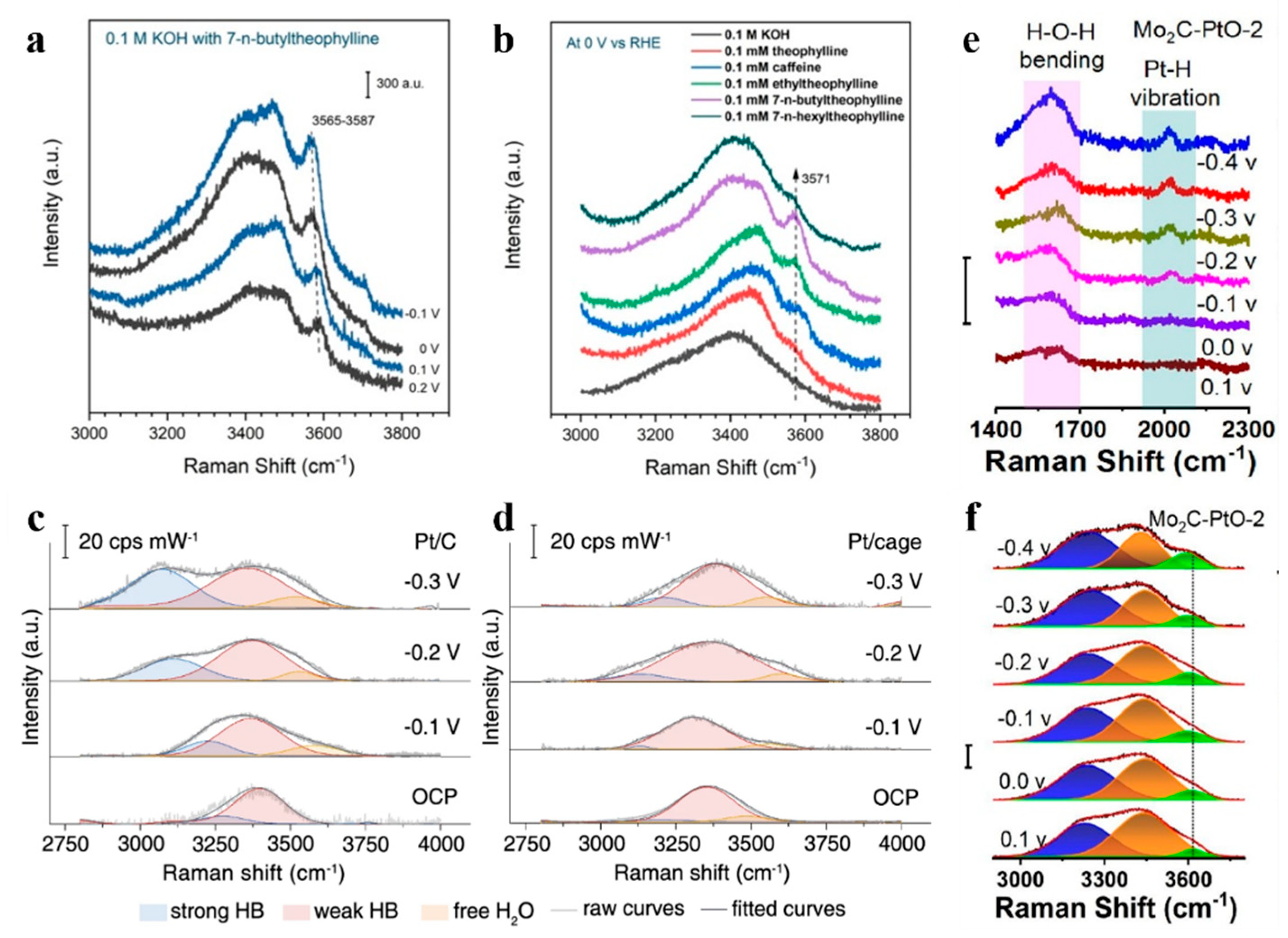
- Wen, J.; Tang, S.; Wu, X.; Xu, L.; Xie, Y.; Yin, Y.; Song, F. Unraveling the Mechanism of Hydrogen Evolution Reactions in Alkaline Media: Recent Advances in in Situ Raman Spectroscopy. Chem. Commun. 2025, 61, 8778–8789. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Le, J.-B.; Wang, Y.-H.; Chen, S.; Yang, Z.-L.; Li, J.-F.; Cheng, J.; Tian, Z.-Q. In Situ Probing Electrified Interfacial Water Structures at Atomically Flat Surfaces. Nat. Mater. 2019, 18, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Zheng, S.; Yang, W.-M.; Zhou, R.-Y.; He, Q.-F.; Radjenovic, P.; Dong, J.-C.; Li, S.; Zheng, J.; Yang, Z.-L.; et al. In Situ Raman Spectroscopy Reveals the Structure and Dissociation of Interfacial Water. Nature 2021, 600, 81–85. [Google Scholar] [CrossRef]
- You, X.; Zhang, D.; Zhang, X.-G.; Li, X.; Tian, J.-H.; Wang, Y.-H.; Li, J.-F. Exploring the Cation Regulation Mechanism for Interfacial Water Involved in the Hydrogen Evolution Reaction by In Situ Raman Spectroscopy. Nano-Micro Lett. 2024, 16, 53. [Google Scholar] [CrossRef]
- Shen, L.; Lu, B.; Li, Y.; Liu, J.; Huang-fu, Z.; Peng, H.; Ye, J.; Qu, X.; Zhang, J.; Li, G.; et al. Interfacial Structure of Water as a New Descriptor of the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2020, 59, 22397–22402. [Google Scholar] [CrossRef]
- Zhao, K.; Chang, X.; Su, H.; Nie, Y.; Lu, Q.; Xu, B. Enhancing Hydrogen Oxidation and Evolution Kinetics by Tuning the Interfacial Hydrogen-Bonding Environment on Functionalized Platinum Surfaces. Angew. Chem. Int. Ed. 2022, 61, e202207197. [Google Scholar] [CrossRef]
- Zhou, S.; Cao, W.; Shang, L.; Zhao, Y.; Xiong, X.; Sun, J.; Zhang, T.; Yuan, J. Facilitating Alkaline Hydrogen Evolution Ki-netics via Interfacial Modulation of Hydrogen-Bond Networks by Porous Amine Cages. Nat. Commun. 2025, 16, 1849. [Google Scholar] [CrossRef]
- Xiang, L.; Leng, D.; Zhang, X.; Li, H.; Wang, H.; Pi, C.; Wu, S.; Huang, L.; Li, Y.; Huo, K.; et al. PtO Nanoclusters on Ul-tra-Thin 2D Mo2C Enhance Hydrated Cation Interaction for Superior Alkaline Hydrogen Evolution Reaction. J. Colloid. Interface Sci. 2025, 688, 22–31. [Google Scholar] [CrossRef]
- Cao, D.; Gao, P.; Shen, Y.; Qiao, L.; Ma, M.; Guo, X.; Cheng, D. Fabricating Lattice-Confined Pt Single Atoms with High Electron-Deficient State for Alkali Hydrogen Evolution Under Industrial-Current Density. Adv. Mater. 2025, 37, 2414138. [Google Scholar] [CrossRef]
- Xu, G.-Y.; Yue, M.-F.; Qian, Z.-X.; Du, Z.-Y.; Xie, X.-Q.; Chen, W.-P.; Zhang, Y.-J.; Li, J.-F. Metal-Support Interactions Alter the Active Species on IrO x for Electrocatalytic Water Oxidation. J. Mater. Chem. A 2023, 11, 15204–15210. [Google Scholar] [CrossRef]
- Dong, J.; Qian, Z.; Xu, P.; Yue, M.-F.; Zhou, R.-Y.; Wang, Y.; Nan, Z.-A.; Huang, S.; Dong, Q.; Li, J.-F.; et al. In Situ Raman Spectroscopy Reveals the Structure Evolution and Lattice Oxygen Reaction Pathway Induced by the Crystalline–Amorphous Heterojunction for Water Oxidation. Chem. Sci. 2022, 13, 5639–5649. [Google Scholar] [CrossRef]
- Cho, K.H.; Park, S.; Seo, H.; Choi, S.; Lee, M.Y.; Ko, C.; Nam, K.T. Capturing Manganese Oxide Intermediates in Electro-chemical Water Oxidation at Neutral pH by In Situ Raman Spectroscopy. Angew. Chem. Int. Ed. 2021, 60, 4673–4681. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, C.; Du, A.; Xiao, T.; Yu, L.; Yang, C.; Xie, W. Interfacial Evolution on Co-Based Oxygen Evolution Reaction Electrocatalysts Probed by Using In Situ Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2022, 95, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Ram, R.; Xia, L.; Benzidi, H.; Guha, A.; Golovanova, V.; Manjón, A.G.; Rauret, D.L.; Berman, P.S.; Dimi-tropoulos, M.; Mundet, B.; et al. Water-Hydroxide Trapping in Cobalt Tungstate for Proton Exchange Membrane Water Electrolysis. Science 2024, 384, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, L.; Xu, G.; Wang, N.; Chen, X.; Wang, Z.; Kong, D.; Yang, X.; Meng, C. H* Site-Blocking Alleviated Through Collaborative Copper Alloying for Large-Current Hydrogen Production. Adv. Energy Mater. 2025, 2501852. [Google Scholar] [CrossRef]
- Scheu, R.; Chen, Y.; De Aguiar, H.B.; Rankin, B.M.; Ben-Amotz, D.; Roke, S. Specific Ion Effects in Amphiphile Hydration and Interface Stabilization. J. Am. Chem. Soc. 2014, 136, 2040–2047. [Google Scholar] [CrossRef] [PubMed]
- Judd, K.D.; De Oliveira, D.M.; Urbina, A.S.; Ben-Amotz, D. Influence of H+, OH− and Salts on Hydrophobic Self-Assembly. Chem. Sci. 2024, 15, 6378–6384. [Google Scholar] [CrossRef]
- Wentworth, C.M.; Myers, R.L.; Cremer, P.S.; Zarzar, L.D. Investigating Oil Solubilization into Nonionic Micelles by Raman Multivariate Curve Resolution: Special Collection: Aggregation-Induced Processes and Functions. Aggregate 2023, 4, e385. [Google Scholar] [CrossRef]
- Davis, J.G.; Gierszal, K.P.; Wang, P.; Ben-Amotz, D. Water Structural Transformation at Molecular Hydrophobic Interfaces. Nature 2012, 491, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Hossain, I.M.; Pooja, N.; Kondeti, S.S.C.; Yamamoto, T.; Mazumder, N.; Noothalapati, H. Direct Estimation of Amylose and Amylopectin in Single Starch Granules by Machine Learning Assisted Raman Spectroscopy. Carbohydr. Polym. 2025, 366, 123929. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Zareef, M.; Chen, M.; Xu, Y.; Wang, J.; Chen, Q. Surface-Enhanced Raman Scattering Detection of Antibiotics. TrAC Trends Anal. Chem. 2025, 191, 118352. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Jiang, Y.; Feng, H.; Liu, L.; Deng, Q.; Liu, D.; Wang, C. Recent Advances in Raman Spectroscopy for Resolving Material Surfaces/Interfaces. Catalysts 2025, 15, 1131. https://doi.org/10.3390/catal15121131
Wang T, Jiang Y, Feng H, Liu L, Deng Q, Liu D, Wang C. Recent Advances in Raman Spectroscopy for Resolving Material Surfaces/Interfaces. Catalysts. 2025; 15(12):1131. https://doi.org/10.3390/catal15121131
Chicago/Turabian StyleWang, Tianyu, Yingnan Jiang, Hongyu Feng, Linlin Liu, Qingsong Deng, Danmin Liu, and Cong Wang. 2025. "Recent Advances in Raman Spectroscopy for Resolving Material Surfaces/Interfaces" Catalysts 15, no. 12: 1131. https://doi.org/10.3390/catal15121131
APA StyleWang, T., Jiang, Y., Feng, H., Liu, L., Deng, Q., Liu, D., & Wang, C. (2025). Recent Advances in Raman Spectroscopy for Resolving Material Surfaces/Interfaces. Catalysts, 15(12), 1131. https://doi.org/10.3390/catal15121131





