Fungal–Algal Co-Pellets from Coffee Effluent: A Sustainable Biorefinery Approach for Bioproducts and Waste Treatment
Abstract
1. Introduction
2. Results and Discussions
2.1. Optimization of Parameters for Cyano–Fungal Pelletizing Process
2.1.1. pH, Glucose, and Shaking Speed
2.1.2. Effect of Initial Algal Biomass on the Pelletization of Fungal-Algal Pellets
2.2. Structural and Elemental Characterization of Pellets Under Different Culture Conditions
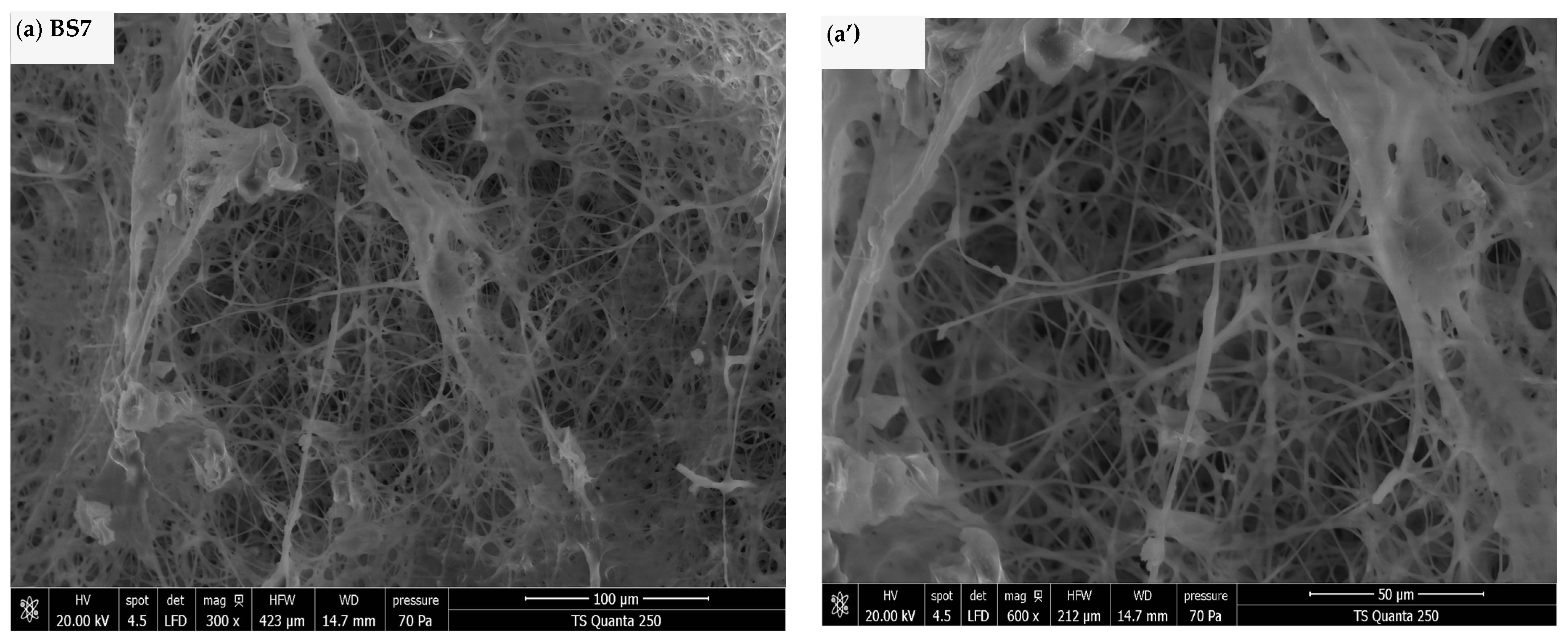
2.2.1. Pellets Morphology
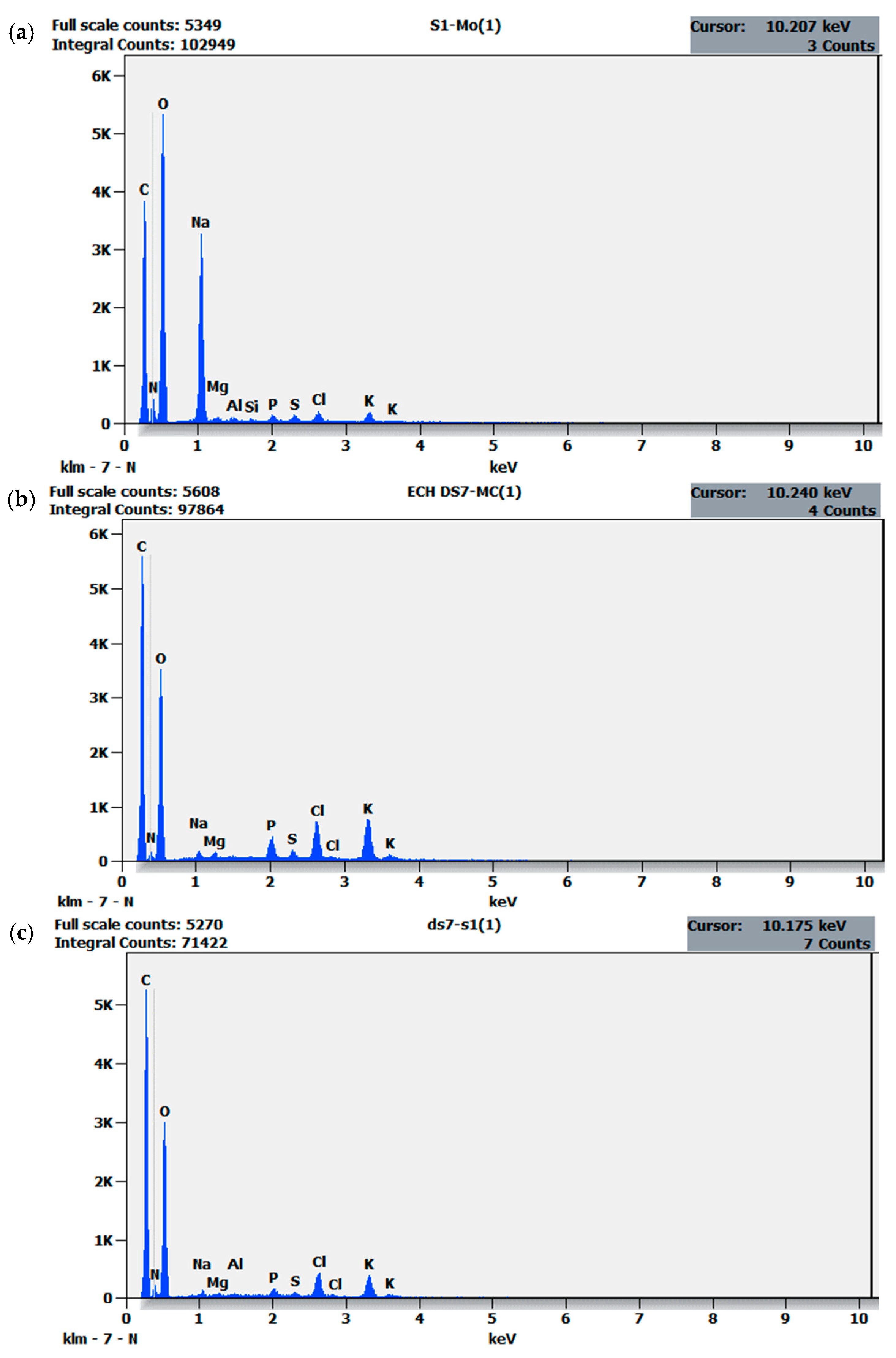
2.2.2. Elemental Composition
2.3. Performance Evaluation of Fungal, Algal, and Fungal-Algal Co-Pellets in the Treatment of Coffee Effluent
2.3.1. Fermentative Broth Analysis: Physicochemical Characterization
2.3.2. Phenolic Profile
2.3.3. Biomass, EPS, and IPS Yields
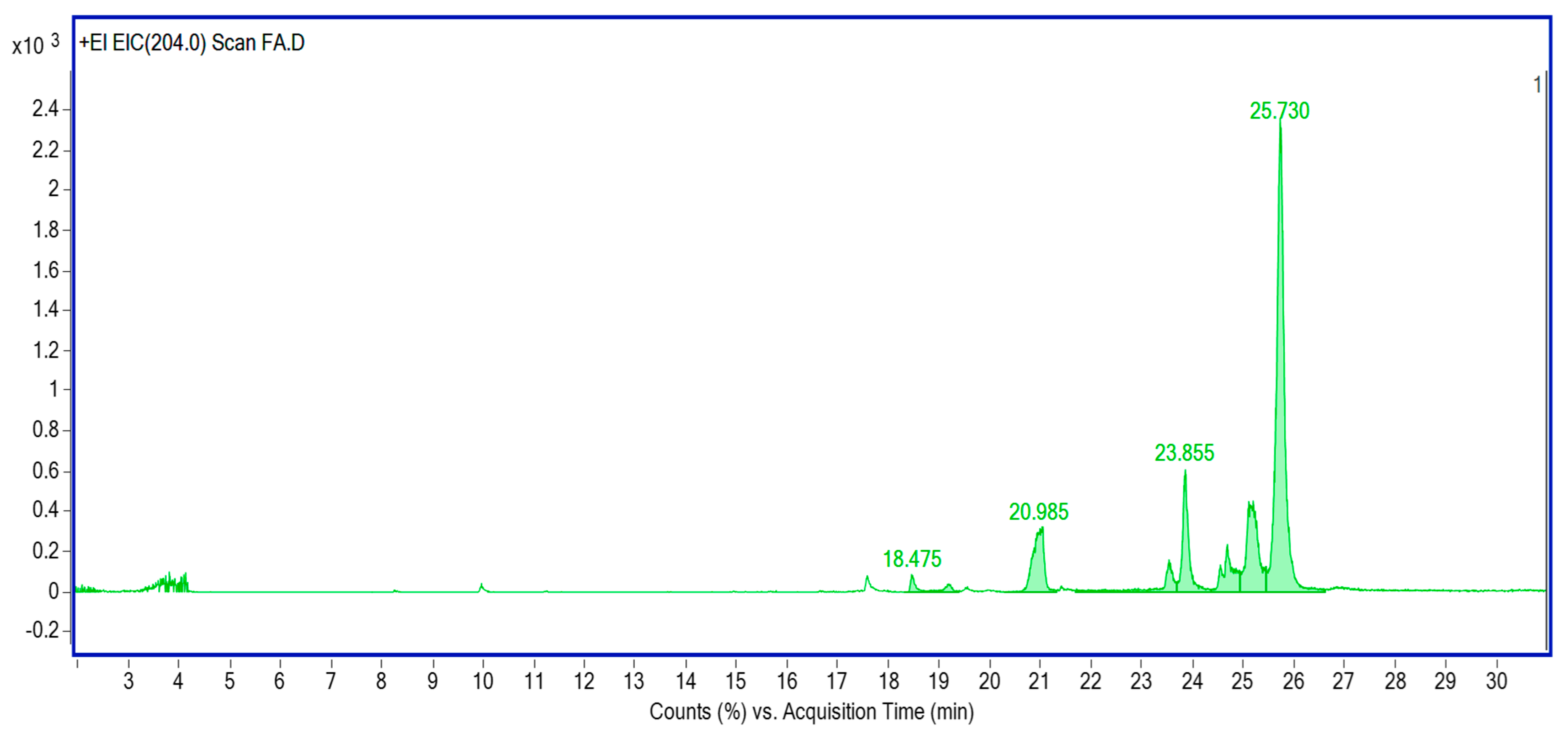
2.3.4. Lipid Profiling of Cellular Biomass and Fermentative Broth
3. Materials and Methods
3.1. Brown-Colored Coffee Effluent (CE)
3.2. Strains and Culture Conditions
3.3. Standard Cultivation
3.4. Pelletization Process: Screening of Factors
Structural and Chemical Characterization of Pellets
3.5. Performance Evaluation of Fungal, Algal, and Fungal–Algal Co-Pellets in the Treatment of Coffee Effluent (CE)
3.5.1. Analytical Methods
Biomass and Fermentative Broth Analysis
Phenolic Compounds Content and Chromatography Analysis
Polysaccharides, Lipid Content, and Chromatography Analysis
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CE | Model brown-colored coffee effluent; |
| CWW | Coffee wastewater; |
| SCG | Spend coffee ground; |
| COD | Chemical oxygen demand; |
| BOD | Biological oxygen demand; |
| BS7 | Trametes versicolor fungal strain; |
| S1 | Persinema sp. cyanobacterial strain; |
| BG-11 | Blue-Green medium; |
| EPSs | Extracellular polymeric substances; |
| SEM | Scanning electron microscopy; |
| MAGs | Monoacylglycerols; |
| DAGs | Diacylglycerols. |
References
- Chiang, P.F.; Zhang, T.; Claire, M.J.; Maurice, N.J.; Ahmed, J.; Giwa, A.S. Assessment of Solid Waste Management and Decarbonization Strategies. Processes 2024, 12, 1473. [Google Scholar] [CrossRef]
- Gil-Gómez, J.A.; Florez-Pardo, L.M.; Leguizamón-Vargas, Y.C. Valorization of coffee by-products in the industry, a vision towards circular economy. Discov. Appl. Sci. 2024, 6, 480. [Google Scholar] [CrossRef]
- Ijanu, E.M.; Kamaruddin, M.A.; Norashiddin, F.A. Coffee processing wastewater treatment: A critical review on current treatment technologies with a proposed alternative. Appl. Water Sci. 2020, 10, 11. [Google Scholar] [CrossRef]
- Gururaj, A.; Kumar, B.M.; Achyuth, K.N.; Manoj, B.R. Coffee Processing Wastewater Management: An Overview. J. Eng. Res. Rep. 2021, 20, 52–61. [Google Scholar] [CrossRef]
- Paiva Foresti, M., Jr.; Curi De Siqueira, J.; Dos Reis Souza, A.; Pimentel De Matos, M.; Fia, R. Use of by-products generated in the processing of coffee berries: A review. Coffee Sci. 2023, 18, 1–10. [Google Scholar] [CrossRef]
- Battista, F.; Barampouti, E.M.; Mai, S.; Bolzonella, D.; Malamis, D.; Moustakas, K.; Loizidou, M. Added-value molecules recovery and biofuels production from spent coffee grounds. Renew. Sustain. Energy Rev. 2020, 131, 110007. [Google Scholar] [CrossRef]
- Machado, M.; Ferreira, H.; Oliveira, M.B.P.P.; Alves, R.C. Coffee by-products: An underexplored source of prebiotic ingredients. Crit. Rev. Food Sci. Nutr. 2024, 64, 7181–7200. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Iriondo-DeHond, M.; Del Castillo, M.D. Applications of Compounds from Coffee Processing By-Products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Cho, E.-J.; Maskey, S.; Nguyen, D.-T.; Bae, H.-J. Value-Added Products from Coffee Waste: A Review. Molecules 2023, 28, 3562. [Google Scholar] [CrossRef]
- Da Silva, C.N.; Da Silva, R.M.; Lemes, A.C.; Ribeiro, B.D. Recovery of Phenolic Compounds by Deep Eutectic Solvents in Orange By-Products and Spent Coffee Grounds. Sustainability 2024, 16, 7403. [Google Scholar] [CrossRef]
- Corrêa, C.L.O.; Penha, E.M.; Freitas-Silva, O.; Luna, A.S.; Gottschalk, L.M.F. Enzymatic Technology Application on Coffee Co-Products: A Review. Waste Biomass Valor. 2021, 12, 3521–3540. [Google Scholar] [CrossRef]
- Budžaki, S.; Velić, N.; Ostojčić, M.; Stjepanović, M.; Rajs, B.B.; Šereš, Z.; Maravić, N.; Stanojev, J.; Hessel, V.; Strelec, I. Waste Management in the Agri-Food Industry: The Conversion of Eggshells, Spent Coffee Grounds, and Brown Onion Skins into Carriers for Lipase Immobilization. Foods 2022, 11, 409. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.-C.; Kim, Y.Y.; Yang, J.E.; Lee, H.M.; Hwang, I.M.; Park, H.W.; Kim, H.M. Utilization of coffee waste as a sustainable feedstock for high-yield lactic acid production through microbial fermentation. Sci. Total Environ. 2024, 912, 169521. [Google Scholar] [CrossRef]
- Mirón-Mérida, V.A.; Barragán-Huerta, B.E.; Gutiérrez-Macías, P. Coffee waste: A source of valuable technologies for sustainable development. In Valorization of Agri-Food Wastes and By-Products; Elsevier: Amsterdam, The Netherlands, 2021; pp. 173–198. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Campos, R.C.; Pinto, V.R.A.; Melo, L.F.; Rocha, S.J.S.S.D.; Coimbra, J.S. New sustainable perspectives for “Coffee Wastewater” and other by-products: A critical review. Future Foods 2021, 4, 100058. [Google Scholar] [CrossRef]
- Skorupa, A.; Worwąg, M.; Kowalczyk, M. Coffee industry and ways of using By-Products as bioadsorbents for removal of pollutants. Water 2022, 15, 112. [Google Scholar] [CrossRef]
- Pires, J.F.; Viana, D.C.; Braga, R.A., Jr.; Schwan, R.F.; Silva, C.F. Protocol to select efficient microorganisms to treat coffee wastewater. J. Environ. Manag. 2020, 278, 111541. [Google Scholar] [CrossRef]
- Ashenafi Hailemariam, F.; Velmurugan, P.; Selvaraj, S.K. Treatment of wastewater from coffee (Coffea arebica) industries using mixed culture Pseudomonas florescence and Escherichia coli bacteria. Mater. Today Proc. 2021, 46, 7396–7401. [Google Scholar] [CrossRef]
- Erazo, S.; Agudelo-Escobar, L.M. Determination of Electrogenic Potential and Removal of Organic Matter from Industrial Coffee Wastewater Using a Native Community in a Non-Conventional Microbial Fuel Cell. Processes 2023, 11, 373. [Google Scholar] [CrossRef]
- Agudelo-Escobar, L.M.; Cabrera, S.E.; Avignone Rossa, C. A bioelectrochemical system for waste degradation and energy recovery from industrial coffee wastewater. Front. Chem. Eng. 2022, 4, 814987. [Google Scholar] [CrossRef]
- Dulta, K.; Awasthi, Y.K.; Aman, J.; Khirwar, R.; Kulwanshi, S.; Kumar, K. Dual Application of Waste Treatment and Fungal Cultivation/Metabolite Production. In Bioprospecting of Multi-Tasking Fungi for a Sustainable Environment; Uppuluri, K.B., Selvasembian, R., Eds.; Springer Nature: Singapore, 2024; pp. 361–377. [Google Scholar] [CrossRef]
- Khalatbari, S.; Sotaniemi, V.-H.; Leiviskä, T. Introducing a Dual approach to industrial wastewater reclamation and microalgae harvesting by filamentous fungus. J. Environ. Chem. Eng. 2025, 13, 116035. [Google Scholar] [CrossRef]
- Muradov, N.; Taha, M.; Miranda, A.F.; Wrede, D.; Kadali, K.; Gujar, A.; Stevenson, T.; Ball, A.S.; Mouradov, A. Fungal-assisted algal flocculation: Application in wastewater treatment and biofuel production. Biotechnol. Biofuels 2015, 8, 24. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Mathur, M.; Kumar, P.; Prajapati, S.K.; Malik, A. A rapid method for fungal assisted algal flocculation: Critical parameters & mechanism insights. Algal Res. 2017, 21, 42–51. [Google Scholar] [CrossRef]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular Bioeconomy in Action: Transforming Food Wastes into Renewable Food Resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef]
- Talukdar, S.; Barzee, T.J. Metabolically active fungus is not always required for fungal-assisted microalgae immobilization. Algal Res. 2025, 86, 103908. [Google Scholar] [CrossRef]
- Gao, Z.; Jiang, C.; Lyu, R.; Yang, Z.; Zhang, T. Optimization of the preparation of fungal-algal pellets for use in the remediation of arsenic-contaminated water. Environ. Sci. Pollut. Res. 2020, 27, 36789–36798. [Google Scholar] [CrossRef]
- Wang, S.-K.; Yang, K.-X.; Zhu, Y.-R.; Zhu, X.-Y.; Nie, D.-F.; Jiao, N.; Angelidaki, I. One-step co-cultivation and flocculation of microalgae with filamentous fungi to valorize starch wastewater into high-value biomass. Bioresour. Technol. 2022, 361, 127625. [Google Scholar] [CrossRef]
- Hamidi, M.; Okoro, O.V.; Milan, P.B.; Khalili, M.R.; Samadian, H.; Nie, L.; Shavandi, A. Fungal exopolysaccharides: Properties, sources, modifications, and biomedical applications. Carbohydr. Polym. 2022, 284, 119152. [Google Scholar] [CrossRef]
- Qamar, S.A.; Riasat, A.; Jahangeer, M.; Fatima, R.; Bilal, M.; Iqbal, H.M.N.; Mu, B. Prospects of microbial polysaccharides-based hybrid constructs for biomimicking applications. J. Basic Microbiol. 2022, 62, 1319–1336. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.; Mandari, V.; Santhosh Kumar, D. Efficient production of biomass and exopolysaccharide from P. ostreatus and physio-chemical characterization of biomass powder. Food Biosci. 2023, 55, 103073. [Google Scholar] [CrossRef]
- Umar, A.; Abid, I.; Elshikh, M.S.; Dufossé, L.; Abdel-Azeem, A.M.; Ali, I. Agitation role (Dissolved Oxygen) in production of laccase from newly identified Ganoderma multistipitatum sp. nov. and its effect on mycelium morphology. BMC Microbiol. 2023, 23, 280. [Google Scholar] [CrossRef]
- Chu, R.; Li, S.; Yin, Z.; Hu, D.; Zhang, L.; Xiang, M.; Zhu, L. A fungal immobilization technique for efficient harvesting of oleaginous microalgae: Key parameter optimization, mechanism exploration and spent medium recycling. Sci. Total Environ. 2021, 790, 148174. [Google Scholar] [CrossRef]
- Luo, S.; Wu, X.; Jiang, H.; Yu, M.; Liu, Y.; Min, A.; Li, W.; Ruan, R. Edible fungi-assisted harvesting system for efficient microalgae bio-flocculation. Bioresour. Technol. 2019, 282, 325–330. [Google Scholar] [CrossRef]
- Alrubaie, G.; Al-Shammari, R.H.H. Microalgae Chlorella vulgaris Harvesting Via Co-Pelletization with Filamentous Fungus. Baghdad Sci. J. 2018, 15, 31. [Google Scholar] [CrossRef]
- Oliveira, H.R.; Bassin, I.D.; Cammarota, M.C. Bioflocculation of cyanobacteria with pellets of Aspergillus niger: Effects of carbon supplementation, pellet diameter, and other factors in biomass densification. Bioresour. Technol. 2019, 294, 122167. [Google Scholar] [CrossRef]
- Ogawa, M.; Moreno-García, J.; Barzee, T.J. Filamentous fungal pellets as versatile platforms for cell immobilization: Developments to date and future perspectives. Microb. Cell Fact. 2024, 23, 280. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.M.; Sánchez-Elordi, E.; Santiago, R.; Vicente, C.; Legaz, M.E. Algal-Fungal Mutualism: Cell Recognition and Maintenance of the Symbiotic Status of Lichens. J. Vet. Med. Res. 2016, 3, 1052. [Google Scholar]
- Rokaya, N.; Carr, E.C.; Tiwari, S.; Wilson, R.A.; Jin, C. Design of Co-Culturing system of diazotrophic cyanobacteria and filamentous fungi for potential application in Self-Healing Concrete. Mater. Today Commun. 2025, 44, 112093. [Google Scholar] [CrossRef]
- Righini, H.; Francioso, O.; Martel Quintana, A.; Roberti, R. Cyanobacteria: A natural source for controlling agricultural plant diseases caused by fungi and oomycetes and improving plant growth. Horticulturae 2022, 8, 58. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lin, F.-Y.; Hsu, T.-H. Effects of nitrogen from different sources on mycelial biomass and polysaccharide production and pellet morphology in submerged cultures of Grifola frondosa. BioResources 2021, 16, 2937–2952. [Google Scholar] [CrossRef]
- Nawaz, T.; Joshi, N.; Nelson, D.; Saud, S.; Abdelsalam, N.R.; Abdelhamid, M.M.A.; Jaremko, M.; Rahman, T.U.; Fahad, S. Harnessing the potential of nitrogen-fixing cyanobacteria: A rich bio-resource for sustainable soil fertility and enhanced crop productivity. Environ. Technol. Innov. 2024, 36, 103886. [Google Scholar] [CrossRef]
- Daâssi, D.; Alhumairi, A.M.; Mellah, B.; Fdhil, N.; Baccar, N.; Chamkha, M. Physico-mechanical, thermal, and biodegradation performance of mycelium biocomposites derived from residual agrowastes. Polym. Bull. 2025, 82, 11295–11321. [Google Scholar] [CrossRef]
- Jiang, M.; Li, H.; Zhou, Y.; Zhang, J. The interactions of an algae–fungi symbiotic system influence nutrient removal from synthetic wastewater. J. Chem. Tech. Biotech. 2019, 94, 3993–3999. [Google Scholar] [CrossRef]
- Fathali, Z.; Rezaei, S.; Faramarzi, M.A.; Habibi-Rezaei, M. Catalytic phenol removal using entrapped cross-linked laccase aggregates. Int. J. Biol. Macromol. 2019, 122, 359–366. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Sharma, P.; Singh, V.; Sharma, S.; Singh, J. Fungal laccase-based technologies for the remediation of industrial wastewater: A comprehensive review. J. Environ. Manag. 2023, 340, 117865. [Google Scholar]
- Kuivanen, J.; Penttilä, M.; Richard, P. Metabolic engineering of the fungal D-galacturonate pathway for L-ascorbic acid production. Microb. Cell Fact. 2015, 14, 2. [Google Scholar] [CrossRef]
- Martins, L.C.; Monteiro, C.C.; Semedo, P.M.; Sá-Correia, I. Valorisation of pectin-rich agro-industrial residues by yeasts: Potential and challenges. Appl. Microbiol. Biotechnol. 2020, 104, 6527–6547. [Google Scholar] [CrossRef]
- Magengelele, M.; Malgas, S.; Pletschke, B.I. Bioconversion of spent coffee grounds to prebiotic mannooligosaccharides—An example of biocatalysis in biorefinery. RSC Adv. 2023, 13, 3773–3780. [Google Scholar] [CrossRef]
- Gultom, S.; Zamalloa, C.; Hu, B. Microalgae Harvest through Fungal Pelletization—Co-Culture of Chlorella vulgaris and Aspergillus niger. Energies 2014, 7, 4417–4429. [Google Scholar] [CrossRef]
- Phyu, K.K.; Zhi, S.; Liang, J.; Yang, Z.; Zhao, R.; Liu, J.; Cao, Y.; Wang, H.; Zhang, K. Biomass growth, nutrient removal, and microbial community dynamics in mono-, Co-, and sequential culture of screened cyanobacteria with microalgae for dairy wastewater treatment. Bioresour. Technol. 2026, 439, 133329. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Pap, B.; Maróti, G.; Van Staden, J.; Ördög, V. Cyanobacteria-Fungi Co-Cultures: Which partner contributes to antifungal activity? Curr. Microbiol. 2024, 81, 401. [Google Scholar] [CrossRef]
- Pyrzynska, K. Useful Extracts from Coffee By-Products: A Brief Review. Separations 2024, 11, 334. [Google Scholar] [CrossRef]
- Alebooye, P.; Falah, F.; Vasiee, A.; Yazdi, F.T.; Mortazavi, S.A. Spent coffee grounds as a potential culture medium for γ-aminobutyric acid (GABA) production by Levilactobacillus brevis PML1. LWT 2023, 189, 115553. [Google Scholar] [CrossRef]
- Wainaina, S.; Kisworini, A.D.; Fanani, M.; Wikandari, R.; Millati, R.; Niklasson, C.; Taherzadeh, M.J. Utilization of food waste-derived volatile fatty acids for production of edible Rhizopus oligosporus fungal biomass. Bioresour. Technol. 2020, 310, 123444. [Google Scholar] [CrossRef]
- Dahmen-Ben Moussa, I.; Chtourou, H.; Karray, F.; Sayadi, S.; Dhouib, A. Nitrogen or phosphorus repletion strategies for enhancing lipid or carotenoid production from Tetraselmis marina. Bioresour. Technol. 2017, 238, 325–332. [Google Scholar] [CrossRef]
- Annal, U.N.; Vaithiyanathan, R.; Natarajan, A.; Rajadurai, V.; Kumar, P.S.M.; Li, Y.-Y. Electrolytic biodiesel production from spent coffee grounds: Optimization through response surface methodology and artificial neural network. J. Taiwan Inst. Chem. Eng. 2024, 165, 105697. [Google Scholar] [CrossRef]
- Kaiser, B.K.; Carleton, M.; Hickman, J.W.; Miller, C.; Lawson, D.; Budde, M.; Warrener, P.; Paredes, A.; Mullapudi, S.; Navarro, P.; et al. Fatty aldehydes in cyanobacteria are a metabolically flexible precursor for a diversity of biofuel products. PLoS ONE 2013, 8, e58307. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.F.; Pessoa, J.G.B.; Ríos Pinto, L.F.; Maciel Filho, R.; Fregolente, L.V. Mono- and diglyceride production from microalgae: Challenges and prospects of high-value emulsifiers. Trends Food Sci. Technol. 2021, 118, 589–600. [Google Scholar] [CrossRef]
- Choe, U. Valorization of spent coffee grounds and their applications in food science. Curr. Res. Food Sci. 2025, 10, 101010. [Google Scholar] [CrossRef]
- Satori, H.; Kawase, Y. Decolorization of dark brown colored coffee effluent using zinc oxide particles: The role of dissolved oxygen in degradation of colored compounds. J. Environ. Manag. 2014, 139, 172–179. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Chang, C.; Chen, J.; Cao, F.; Zhao, J.; Zheng, Y.; Zhu, J. Physicochemical and functional properties of proteins extracted from three microalgal species. Food Hydrocoll. 2019, 96, 510–517. [Google Scholar] [CrossRef]
- Relucenti, M.; Familiari, G.; Donfrancesco, O.; Taurino, M.; Li, X.; Chen, R.; Artini, M.; Papa, R.; Selan, L. Microscopy Methods for Biofilm Imaging: Focus on SEM and VP-SEM pros and cons. Biology 2021, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Veríssimo, L.; Finimundy, T.; Rodrigues, J.; Oliveira, I.; Gonçalves, J.; Fernandes, I.P.; Barros, L.; Heleno, S.A.; Calhelha, R.C. Chemical and Bioactive Screening of Green Polyphenol-Rich Extracts from Chestnut By-Products: An Approach to Guide the Sustainable Production of High-Added Value Ingredients. Foods 2023, 12, 2596. [Google Scholar] [CrossRef] [PubMed]
- Aroua, N.; Boukhris, M.; Choura, S.; Chamkha, M.; Salem, R.B.; Rigane, G. Characterization of Phenolic and Flavonoid Compounds in Casuarina glauca Organs From Djerba (Tunisia): A Comparative HPLC-DAD Study of Extraction Techniques. Chem. Biodivers. 2025, 22, e00618. [Google Scholar] [CrossRef]
- Shen, K.; Liu, Y.; Liu, L.; Khan, A.W.; Normakhamatov, N.; Wang, Z. Characterization, Optimization, and Scaling-up of Submerged Inonotus hispidus Mycelial Fermentation for Enhanced Biomass and Polysaccharide Production. Appl. Biochem. Biotechnol. 2025, 197, 1534–1555. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Verma, A.; Vyas, M.; Sahu, S.K. Unveiling Anticancer phytoconstituents: A Comprehensive review of GC–MS applications in Natural products. ChemistrySelect 2025, 10, e202405724. [Google Scholar] [CrossRef]




| Time (h) | Initial Algal Biomass (cells/mL) | ||||
|---|---|---|---|---|---|
| 2.5 × 106 | 1.5 × 107 | 3.5 × 107 | 4.6 × 108 | 1.6 × 109 | |
| 1 | 80.2 ± 3.0 | 66.4 ± 0.5 | 54.6 ± 1.5 | 48.2 ± 2.5 | 36.5 ± 1.8 |
| 2 | 95.3 ± 1.0 | 72.5 ± 4.0 | 67.3 ± 4.2 | 56.4 ± 1.8 | 49.1 ± 0.9 |
| 3 | 99.1 ± 2.0 | 92.4 ± 1.0 | 96.5 ± 3.5 | 66.2 ± 0.5 | 56.7 ± 1.5 |
| 4 | 99.4 ± 2.5 | 99.4 ± 0.9 | 99.2 ± 1.1 | 77.6 ± 1.5 | 69.7 ± 0.2 |
| 5 | 99.5 ± 1.0 | 99.6 ± 1.1 | 99.7 ± 0.9 | 95.8 ± 0.5 | 72.3 ± 0.5 |
| Elemental Composition (%) | |||
|---|---|---|---|
| Compounds (%) | Weight (%) | ||
| Elements | (a) S1 | (b) BS7 | (c) S1+BS7 |
| C | 24.88 | 27.88 | 27.89 |
| N | 5.27 | 13.63 | 15.24 |
| O | 51.26 | 48.83 | 50.50 |
| Na | 15.97 | 0.66 | 0.50 |
| Mg | 0.13 | 0.32 | 0.21 |
| Al | 0.20 | - | 0.14 |
| Si | 0.10 | - | - |
| P | 0.30 | 1.22 | 0.56 |
| S | 0.34 | 0.47 | 0.32 |
| Cl | 0.66 | 2.80 | 2.23 |
| K | 0.89 | 4.18 | 2.41 |
| Parameters | Culture Conditions and Removal Efficiency (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Raw CE | Fungal Monoculture [BS7] | Cyanobacterial Monoculture [S1] | Cyano–Fungal Co-Culture [BS7+S1] | |||||
| Units | Raw CE | Values | RE (a) (%) | Values | RE (%) | Values | RE (%) | |
| pH | 6.2 ± 0.2 | 4.8 ± 0.1 | - | 6.0 ± 0.1 | - | 5.5 ± 0.2 | - | |
| Conductivity | (us/cm) | 486 ± 2.3 | 967 ± 1.5 | - | 525 ± 5.2 | - | 997 ± 1.7 | - |
| Color | (m−1) | 420 ± 4.5 | 85 ± 0.9 | 79.7 ± 1.2 | 320 ± 2.3 | 23.81 ± 1.3 | 67 ± 0.2 | 84 ± 0.05 |
| OM | (%) | 33.8 ±1.86 | 24 ± 1.95 | 28.9 ± 2.4 | 27 ± 2.25 | 20.12 ± 2.4 | 19 ± 1.1 | 43.7 ± 2.3 |
| BOD5 | (g/L) | 5.25 ± 0.1 | 2.25 ± 0.28 | 57.1 ± 2.4 | 4.2 ± 0.4 | 20.00 ± 0.5 | 1.25 ± 0.2 | 76.1 ± 0.3 |
| COD | (g/L) | 10.8 ± 0.8 | 5.49 ± 0.5 | 49.2 ± 3.1 | 8.3 ± 0.8 | 23.2 ± 1.4 | 3.5 ± 0.9 | 67.6 ± 3.5 |
| P | (%) | 2.9 ± 0.02 | 2.37 ± 0.5 | 18.2 ± 0.8 | 2.7 ± 0.7 | 6.90 ± 0.7 | 2± 0.02 | 29.3 ± 0.9 |
| TKN | (%) | 1.03 ± 0.02 | 1.0 ± 0.01 | - | 1.03 ± 0.01 | - | 1.4 ± 0.07 | - |
| FA | (%) | 1.38 ± 0.5 | 0.52 ± 0.05 | 62.31 ± 0.5 | 1.02 ± 0.02 | 26 ± 1.08 | 0.7 ± 0.02 | 49.2 ± 1.2 |
| TPC | (%) | 64 ± 0.4 | 17.04 ± 0.2 | 73.38 ± 0.4 | 45 ± 0.4 | 29.69 ± 0.4 | 16.3 ± 0.2 | 74.5 ± 0.3 |
| Compounds | Retention Time (min) | Raw CE_NT | CE-T (BS7+S1) | CE-T (BS7) | CE_T (S1) |
|---|---|---|---|---|---|
| Gallic acid | 7.161 | 0.0013 | 0.0297 | - | 0.02 |
| 3,4-Dihydroxybenzoic acid | 10.345 | 0.0032 | 0.0050 | 0.0025 | 0.0018 |
| Hydroxytyrosol | 10.347 | 0.0802 | 0.0001 | 0.0130 | 0.0672 |
| Chlorogenic acid | 11.291 | 0.0241 | 0.0010 | 0.0142 | 0.0099 |
| Catechin | 12.160 | 0.0029 | 0.0091 | 0.0026 | 0.0003 |
| Tyrosol | 12.536 | 5.8849 | 0.8276 | 1.9555 | 3.9294 |
| Caffeic acid | 13.124 | 0.0678 | 0.0005 | ND | 0.0673 |
| Epicatechin | 13.925 | 1.6549 | 0.0215 | 0.0304 | 1.5 |
| Vanillic acid | 13.974 | 0.0018 | 0.0010 | 0.0032 | ND |
| Rutin | 15.156 | 0.0123 | 0.0081 | 0.0046 | 0.011 |
| Verbascoside | 15.950 | 0.0588 | 0.0021 | 0.0012 | 0.041 |
| Luteolin glucoside | 16.568 | 0.0115 | ND | ND | 0.004 |
| p-Coumaric acid | 17.135 | 0.0114 | ND | ND | 0.01 |
| Apigenin-7-glucoside | 17.949 | 0.0454 | 0.0047 | ND | 0.033 |
| Ferulic acid | 18.282 | 0.0016 | 0.0034 | ND | 0.001 |
| Oleuropein | 18.702 | 0.0334 | ND | 0.0127 | 0.03 |
| Naringenin | 21.153 | 0.0015 | 0.0010 | 0.0003 | 0.001 |
| Luteolin | 22.241 | 0.0193 | ND | ND | 0.0182 |
| Quercetin | 22.717 | 0.0118 | 0.0141 | 0.0009 | 0.013 |
| Apigenin | 25.717 | 0.0031 | 0.0020 | 0.0009 | 0.001 |
| Ascorbic acid | 4.819 | ND | 0.0136 | 0.0001 | ND |
| Total Concentration | 7.9315 | 0.9443 | 2.0447 | 5.7391 |
| Sample | Name | Saturated/Unsaturated | Carbon: Position | Formula | (% of FAME) |
|---|---|---|---|---|---|
| CE_NT extract | Palmitic acid, methyl ester | Saturated | C16:0 | C17H34O2 | 6.72 |
| linolenic acid, methyl ester | Unsaturated | C18:3 | C19H34O2 | 7.69 | |
| Oleic acid, methyl ester | Unsaturated | C18:1 | C19H36O2 | 7.09 | |
| Stearic acid, methyl ester | Saturated | C18:0 | C19H38O2 | 2.16 | |
| Eicosanoic acid, methyl ester | Saturated | C20:0 | C21H42O2 | 1.32 | |
| Lipid extraction yield (% of the dry weight) | 25% | ||||
| BS7 pellets | Palmitic acid, methyl ester | Saturated | C16:0 | C19H40O2 | 4.14 |
| Dodecanoic acid, 2,3-bis(acetyloxy)propyl ester | Saturated | C12:0 | C19H34O6 | 1.16 | |
| α-Linolenic acid, methyl ester | Unsaturated | C18:3 | C21H38O2 | 1.36 | |
| Oleic Acid, (Z)-, methyl ester | Unsaturated | C18:1 | C21H42O2 | 9.15 | |
| Lipid extraction yield (% of the dry weight) | 17.9% | ||||
| S1 pellets | Palmitic acid, methyl ester | Saturated | C16:0 | C19H40O2 | 5.57 |
| α-Linolenic acid, methyl ester | Unsaturated | C18:3 | C21H38O2 | 1.86 | |
| Oleic Acid, (Z)-, methyl ester | Unsaturated | C18:1 | C21H42O2 | 2.73 | |
| cis-8,11,14-eicosatrienoic acid methyl ester | Unsaturated | C20:3 | C21H36O2 | 1.5 | |
| Lipid extraction yield (% of the dry weight) | 12% | ||||
| BS7+S1 co-pellets | 9-Octadecenoic acid, (E), methyl ester | Unsaturated | C18:1 | C21H42O2 | 4.11 |
| Palmitic acid, methyl ester | Saturated | C16:0 | C19H40O2 | 9.99 | |
| Oleic Acid, (Z), methyl ester | Unsaturated | C18:1 | C21H42O2 | 8.91 | |
| Lipid extraction yield (% of the dry weight) | 23% |
| Name | Retention Time | Formula | % Area |
|---|---|---|---|
| 9-Octadecenoic acid, (E), TMS derivative | 18.475 | C21H42O2Si | 1.11 |
| Palmitic acid, TMS derivative | 19.185 | C19H40O2Si | 0.99 |
| Oleic acid, (Z), TMS derivative | 20.985 | C21H42O2Si | 9.91 |
| 2-Palmitoylglycerol. 2TMS derivative | 23.540 | C25H54O4Si2 | 4.66 |
| 1-Monopalmitin. 2TMS derivative | 23.855 | C25H54O4Si2 | 10.37 |
| 1.3-Dipalmitin. TMS derivative | 24.693 | C25H54O4Si2 | 6.44 |
| 2-Monooleoylglycerol trimethylsilyl ether | 25.159 | C27H56O4Si2 | 14.92 |
| 1-Monooleoylglycerol. 2TMS derivative | 25.730 | C27H56O4Si2 | 21.6 |
| Parameters | Units | Value/100 g SCG |
|---|---|---|
| pH | 6.2 ± 0.22 | |
| Conductivity | (us/cm) | 486 ± 0.8 |
| Dry matter | (%) | 34 ± 1.53 |
| Organic matter | (%) | 33.86 ± 2.86 |
| Ash | (%) | 0.67 ± 0.07 |
| BOD5 * | (g/L) | 5.25 ± 0.5 |
| COD ** | (g/L) | 10.82 ± 1.09 |
| Phosphorus | (%) | 2.9 ± 0.22 |
| Total Kjeldahl nitrogen | (%) | 1.03 ± 0.03 |
| Humidity | (%) | 65 ± 0.4 |
| Fatty acids (hexane/Soxhlet) | (%) | 1.38 ± 0.2 |
| Total phenolic compounds (TPCs) | (%) | 64 ± 0.4 |
| Total phenolic compounds (TPCs) (HPLC) | (%) | 16 ± 1.5 |
| Caffeic acid (HPLC) | (%) | 0.134 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daâssi, D.; Ghorraf, N.; Ben Ismail, I.; Maalej, A.; Ben Amor, F.; Choura, S.; Chamkha, M. Fungal–Algal Co-Pellets from Coffee Effluent: A Sustainable Biorefinery Approach for Bioproducts and Waste Treatment. Catalysts 2025, 15, 1102. https://doi.org/10.3390/catal15121102
Daâssi D, Ghorraf N, Ben Ismail I, Maalej A, Ben Amor F, Choura S, Chamkha M. Fungal–Algal Co-Pellets from Coffee Effluent: A Sustainable Biorefinery Approach for Bioproducts and Waste Treatment. Catalysts. 2025; 15(12):1102. https://doi.org/10.3390/catal15121102
Chicago/Turabian StyleDaâssi, Dalel, Nesrine Ghorraf, Ikram Ben Ismail, Amina Maalej, Fatma Ben Amor, Sirine Choura, and Mohamed Chamkha. 2025. "Fungal–Algal Co-Pellets from Coffee Effluent: A Sustainable Biorefinery Approach for Bioproducts and Waste Treatment" Catalysts 15, no. 12: 1102. https://doi.org/10.3390/catal15121102
APA StyleDaâssi, D., Ghorraf, N., Ben Ismail, I., Maalej, A., Ben Amor, F., Choura, S., & Chamkha, M. (2025). Fungal–Algal Co-Pellets from Coffee Effluent: A Sustainable Biorefinery Approach for Bioproducts and Waste Treatment. Catalysts, 15(12), 1102. https://doi.org/10.3390/catal15121102






