A Review on the Application of Catalytic Membranes Technology in Water Treatment
Abstract
1. Introduction
2. Construction and Mechanism of Catalytic Membranes
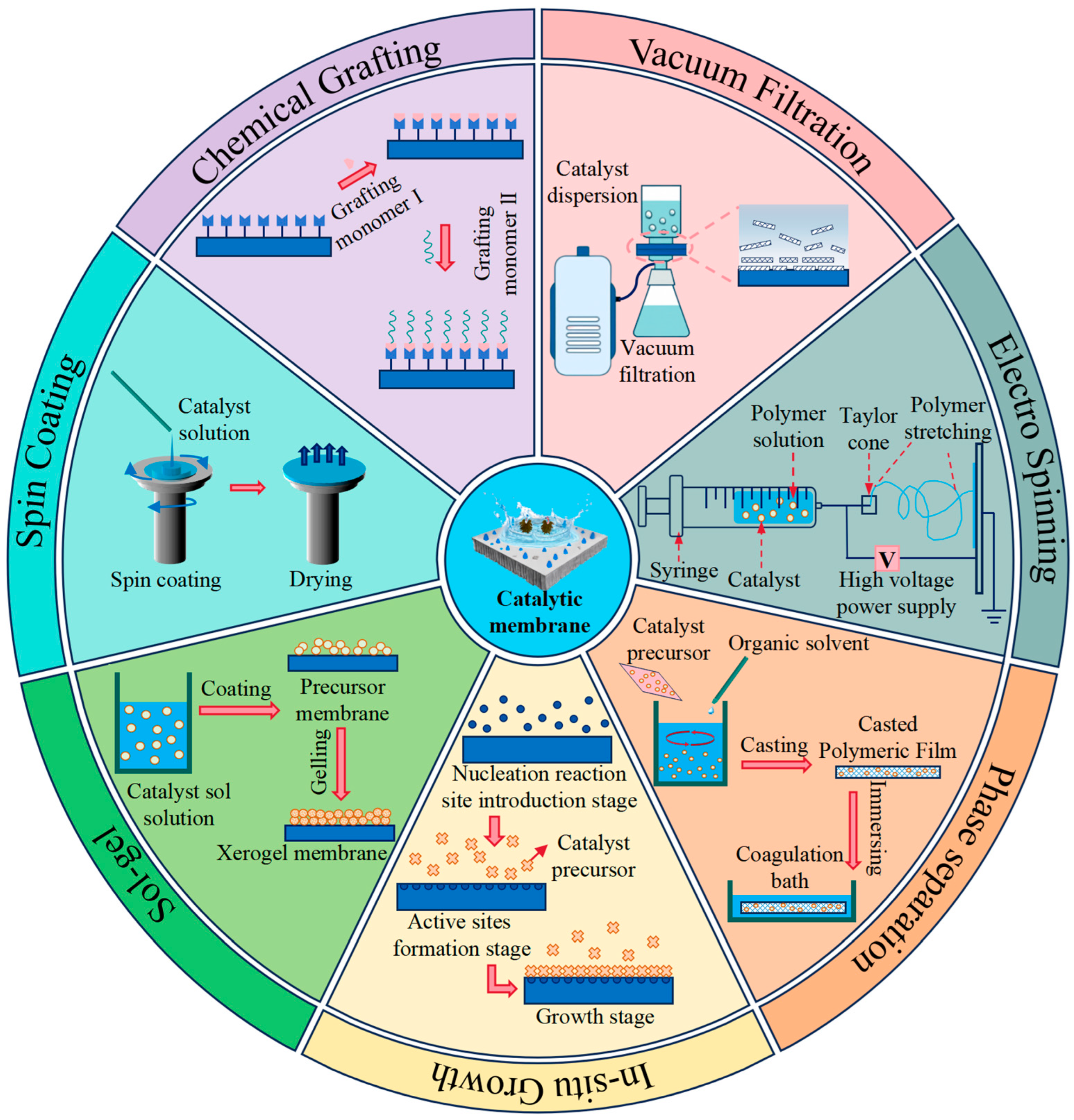
2.1. Construction of Catalytic Membranes
| Method of Preparation | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|
| Blending | Phase Inversion | Simple equipment; Simple operation | Limited capacity; time-consuming | [17] |
| Electrospinning | Controllable process; simple equipment | Requires a large amount of chemical reagents; Low product yield; Poor strength of the CM. | [18] | |
| Sol–Gel | The decorative layer is uniform. | Time-consuming and costly; poor catalytic stability due to delamination, cracking, and poor adhesion. | [19] | |
| Surface Coating | Spin Coating | Easy to operate; High density; Precise control of coating thickness | Severe paint waste; only suitable for simple flat film coatings; not suitable for mass production. | [20] |
| Immersion Deposition | Simple operation; easy control of preparation conditions | Due to the limitations of the wedge effect, thickness is uneven; the coating is unstable, and porosity is relatively low. | [21] | |
| Vacuum Filtration | Easy to prepare on a large scale; Simple operation process; Fast preparation speed. | High energy consumption; not suitable for coating dense base membranes | [22] | |
| Precision Synthesis | Chemical Grafting | Can suppress catalyst detachment; stable covalent bonds can form between the catalyst and the membrane surface. | High catalyst consumption; multiple and complex reaction steps | [23] |
| Chemical Vapor Deposition | Nanoscale catalyst coatings can be obtained; Coating thickness is easily controlled; No solvent addition is required during operation. | Uneven deposition may lead to impurities or defects on the film surface; residual gases after reaction may be toxic. | [24] | |
| Layer-by-Layer Assembly | Flexible operation with low cost; easy to control layer thickness | Requires reliance on cumbersome manual operations; Requires large quantities of polymer solutions, which may cause secondary pollution. | [25] | |
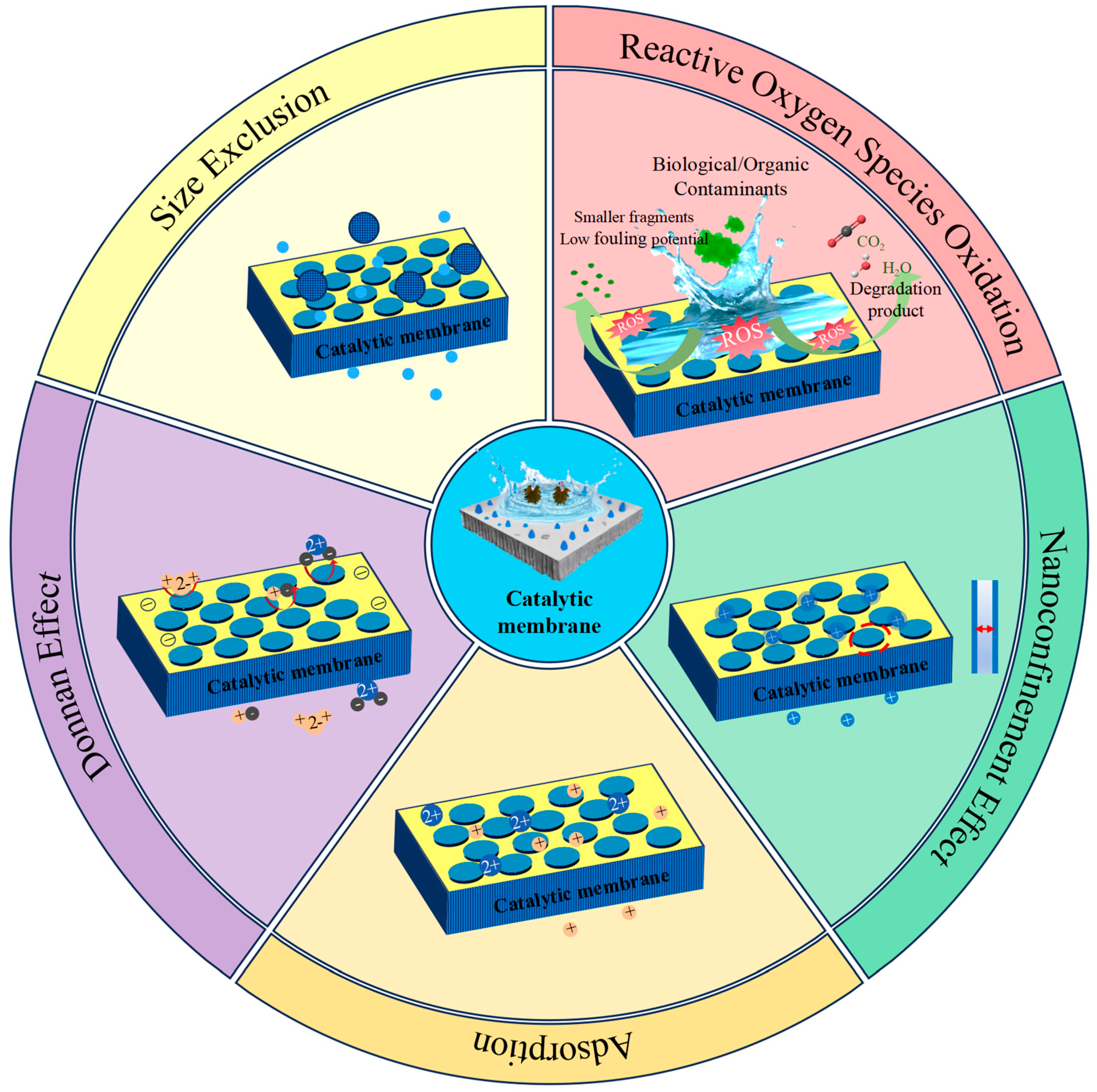
2.2. Synergistic Effects of Membrane Separation and Catalysis
3. Research Progress in Catalytic Membrane Applications
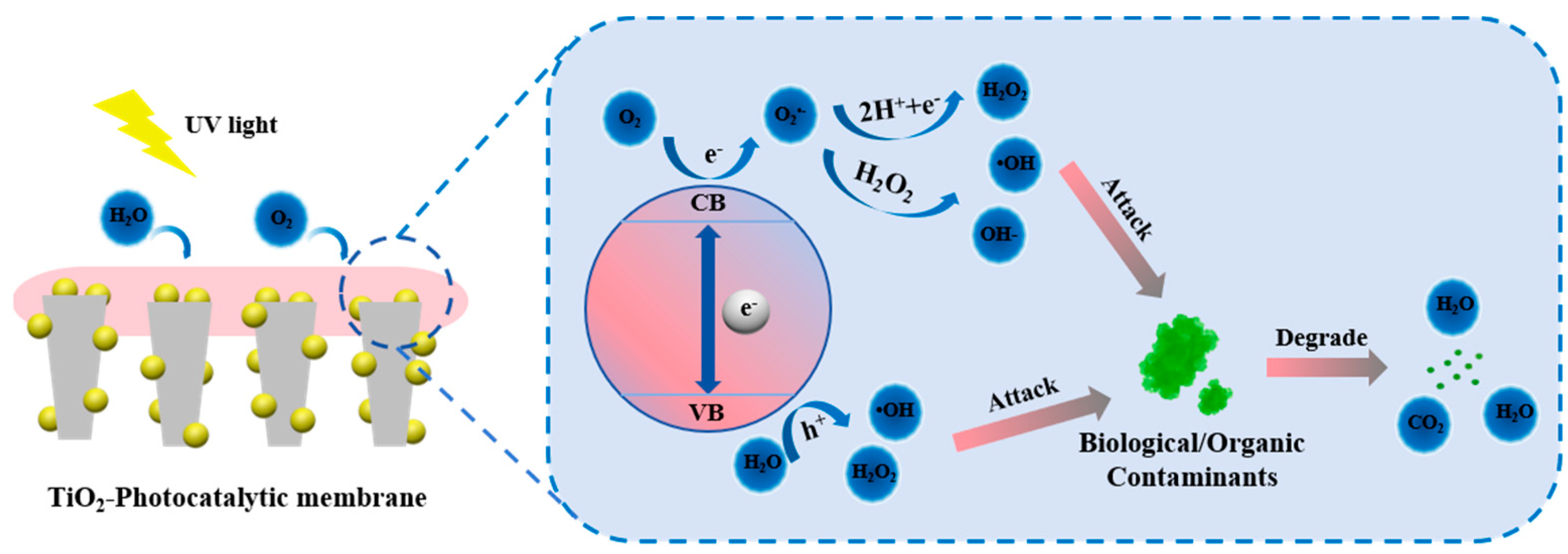
3.1. Photocatalytic Membrane
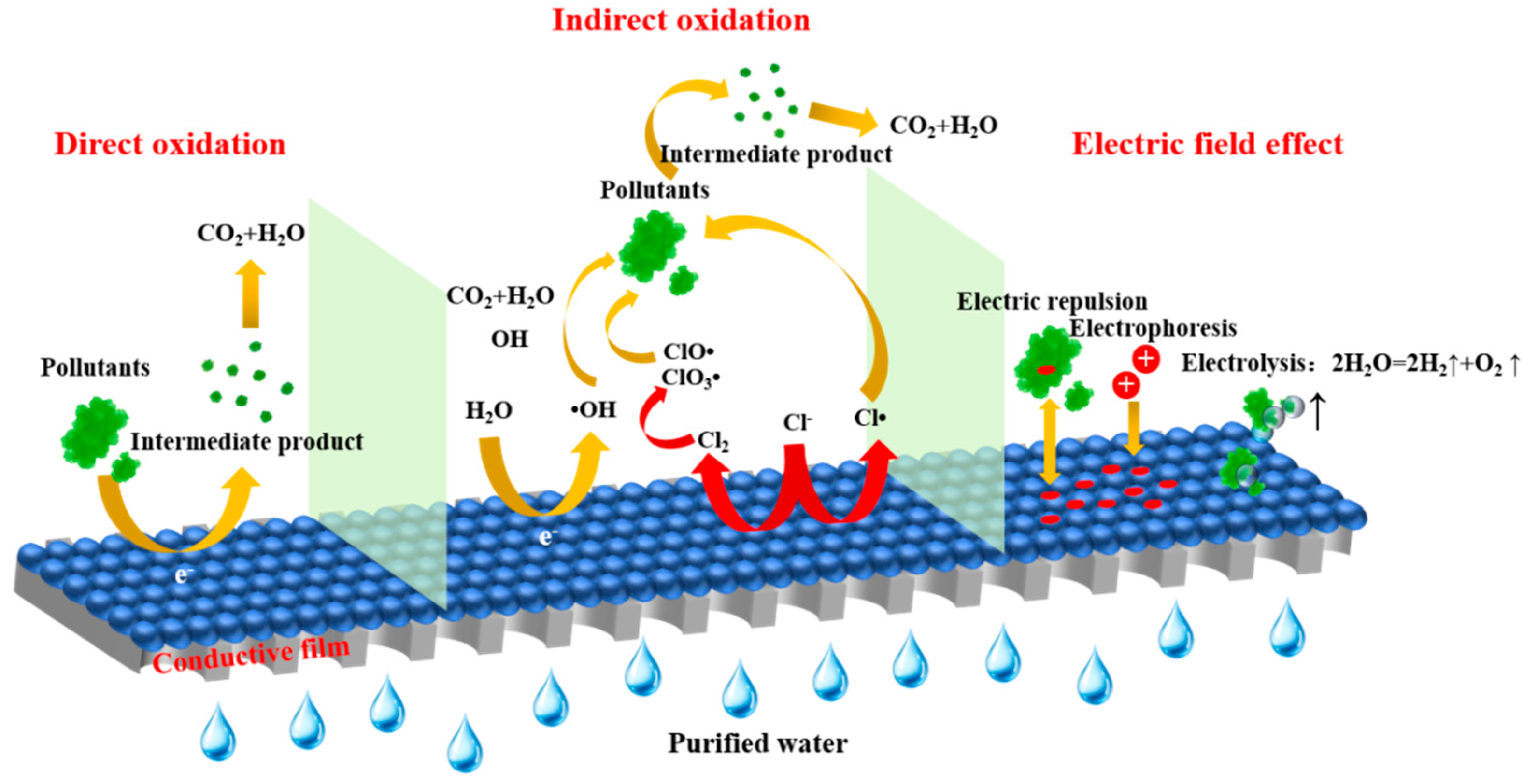
3.2. Electrocatalytic Membrane
| Materials | Pollutant Name and Concentration | Reaction Conditions | Decomposed Pollutant (%) | Energy Consumption | Ref. | ||
|---|---|---|---|---|---|---|---|
| Anode | Cathode | Electrolytes and Their Concentrations | Voltage/Current Density | ||||
| Ti/SnO2-Sb | Stainless steel mesh | RhB (50 mg/L) | Na2SO4 (0.1 M) | 10 (mA·cm−2) | 92.29 | 5.51 (kW·h·m−3) | [44] |
| TiO2-BNTs/ SnO2-Sb | Nickel mesh | Reverse osmosis concentrate (COD: 261~ 295 mg/L) | Cl− (1197~1248 mg·L−1) | 30 (mA·cm−2) | 69.1 | 10.5 [kW·h· (kg·COD)−1] | [50] |
| 1%GONs@7Ti4O7 | Ti/RuO2-IrO2 | 1,4-Dioxane (0.01 mg/L) | Cl− (28.40 ± 0.3 mg·L−1) | 20 (mA·cm−2) | 63.2 | 0.2~0.6 (kW·h·m−3) | [49] |
| Ti/Pd | Titanium mesh | Reverse osmosis concentrate (COD: 160~ 170 mg/L) | Cl− (900 mg·L−1) | 5 V | 82.3 | 45 [kW·h· (kg·COD)−1] | [51] |
| ZIF-67@ CNT/ACF | Titanium mesh | TC (10 mg/L) | Na2CO3 (10 mmol·L−1) | 3 V | 81 | - | [52] |
| PPy@CCM | Titanium plate | Phenol (50 mg/L) | Na2SO4 (2500 mg·L−1) | 2 V | 99.51 | 0.5 (kW·h·m−3) | [53] |
3.3. Fenton-Type Catalytic Membrane
3.4. Peroxymonosulfate-Catalyzed Membrane
3.5. Ozone Catalytic Membrane
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guan, Y.; Qiang, Y.; Li, B.; Zhang, N.N.; Xiao, Y.; Lu, W.T. Assessing the integrated environmental impacts and typological characteristics of water pollution and scarcity in Chinese cities. J. Hydrol. 2025, 661, 133662. [Google Scholar] [CrossRef]
- Lu, Y.J.; Zhou, X.Q.; Zheng, Y.; Yang, H.L.; Cao, W.B. How far do we still need to go with antibiotics in aquatic environments? Antibiotic occurrence, chemical-free or chemical-limited strategies, key challenges, and future perspectives. Water Res. 2025, 275, 123179. [Google Scholar] [CrossRef]
- Jiang, D.Y.; Nowack, B. Reconciling plastic release: Comprehensive modeling of macro- and microplastic flows to the environment. Environ. Pollut. 2025, 383, 126800. [Google Scholar] [CrossRef]
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Planet. Health 2022, 6, E535–E547. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Y.; Dai, M.; Wu, Y.N.; Peng, C.S. Emulsion system, demulsification and membrane technology in oil-water emulsion separation: A comprehensive review. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1254–1278. [Google Scholar] [CrossRef]
- Yi, M.; Xia, Q.; Tan, J.L.; Shang, J.W.; Cheng, X.W. Catalytic-separation technology for highly efficient removal of emerging pollutants, desalination, and antimicrobials: A new strategy for complex wastewater treatment. Chem. Eng. J. 2024, 493, 152568. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.Z. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants. Chem. Eng. J. 2021, 411, 128392. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, L.Z.; Wang, R.; Chou, S.R.; Dong, Z.L. A new integrated approach for dye removal from wastewater by polyoxometalates functionalized membranes. J. Hazard. Mater. 2016, 301, 462–470. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xiao, S.J.; Meng, X.G.; Yu, S.W. Research progress of MOF-based membrane reactor coupled with AOP technology for organic wastewater treatment. Environ. Sci. Pollut. Res. 2023, 30, 104958–104975. [Google Scholar] [CrossRef]
- Yao, Y.J.; Hu, H.H.; Zheng, H.D.; Wei, F.Y.; Gao, M.X.; Zhang, Y.Y.; Wang, S.B. Zn-MoS2 nanocatalysts anchored in porous membrane for accelerated catalytic conversion of water contaminants. Chem. Eng. J. 2020, 398, 125455. [Google Scholar] [CrossRef]
- Santos, E.N.; Fazekas, A.; Hodur, C.; Laszlo, Z.; Beszedes, S.; Firak, D.S.; Gyulavari, T.; Hernadi, K.; Arthanareeswaran, G.; Vereb, G. Statistical Analysis of Synthesis Parameters to Fabricate PVDF/PVP/TiO2 Membranes via Phase-Inversion with Enhanced Filtration Performance and Photocatalytic Properties. Polymers 2022, 14, 113. [Google Scholar] [CrossRef]
- Tan, H.B.; Zhang, Y.B.; Li, B.W.; Yang, H.; Hou, H.T.; Huang, Q.L. Preparation of TiO2-coated glass flat membrane and its photocatalytic degradation of methylene blue. Ceram. Int. 2023, 49, 17236–17244. [Google Scholar] [CrossRef]
- Huang, Z.H.; Zhang, X.; Wang, Y.X.; Sun, J.Y.; Zhang, H.; Liu, W.L.; Li, M.P.; Ma, X.H.; Xu, Z.L. Fe3O4/PVDF catalytic membrane treatment organic wastewater with simultaneously improved permeability, catalytic property and anti-fouling. Environ. Res. 2020, 187, 109617. [Google Scholar] [CrossRef] [PubMed]
- Muleja, A.A.; Mamba, B.B. Development of calcined catalytic membrane for potential photodegradation of Congo red in aqueous solution. J. Environ. Chem. Eng. 2018, 6, 4850–4863. [Google Scholar] [CrossRef]
- Chakraborty, S.; Loutatidou, S.; Palmisano, G.; Kujawa, J.; Mavukkandy, M.O.; Al-Gharabli, S.; Curcio, E.; Arafat, H.A. Photocatalytic hollow fiber membranes for the degradation of pharmaceutical compounds in wastewater. J. Environ. Chem. Eng. 2017, 5, 5014–5024. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Lu, D.W.; Xu, C.B.; Zhong, J.Y.; Chen, M.S.; Xu, S.; Cao, Y.; Zhao, Q.; Yang, M.; Ma, J. Synergistic oxidation—Filtration process analysis of catalytic CuFe2O4—Tailored ceramic membrane filtration via peroxymonosulfate activation for humic acid treatment. Water Res. 2020, 171, 115387. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.R.; Sanches, S.; Huertas, R.M.; Crespo, M.T.B.; Pereira, V.J. Treatment of a real water matrix inoculated with Aspergillus fumigatus using a photocatalytic membrane reactor. J. Membr. Sci. 2020, 598, 117788. [Google Scholar] [CrossRef]
- Zeng, H.J.; Yu, Z.X.; Shao, L.Y.; Li, X.H.; Zhu, M.; Liu, Y.C.; Feng, X.F.; Zhu, X.M. Ag2CO3@UiO-66-NH2 embedding graphene oxide sheets photocatalytic membrane for enhancing the removal performance of Cr(VI) and dyes based on filtration. Desalination 2020, 491, 114558. [Google Scholar] [CrossRef]
- Chen, W.; Ye, T.; Xu, H.; Chen, T.Y.; Geng, N.N.; Gao, X.H. An ultrafiltration membrane with enhanced photocatalytic performance from grafted N-TiO2/graphene oxide. RSC Adv. 2017, 7, 9880–9887. [Google Scholar] [CrossRef]
- Mehravar, S.; Fatemi, S.; Komiyama, M. Magnetic property and structural study of nickel supported on reduced graphene oxide prepared by chemical vapor deposition. Surf. Interface Anal. 2020, 52, 547–552. [Google Scholar] [CrossRef]
- Luo, J.; Chen, W.W.; Song, H.W.; Liu, J.R. Fabrication of hierarchical layer-by-layer membrane as the photocatalytic degradation of foulants and effective mitigation of membrane fouling for wastewater treatment. Sci. Total Environ. 2020, 699, 134398. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.B.; Fang, Q.; Wang, W.; Li, G.L.; Guan, J.M.; Shen, Y.; Ye, J.R.; Liu, F. Prussian blue/PVDF catalytic membrane with exceptional and stable Fenton oxidation performance for organic pollutants removal. Appl. Catal. B Environ. 2020, 273, 119047. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, G.; Liu, H.J.; Qu, J.H. Confining Free Radicals in Close Vicinity to Contaminants Enables Ultrafast Fenton-like Processes in the Interspacing of MoS2 Membranes. Angew. Chem. 2019, 58, 8134–8138. [Google Scholar] [CrossRef]
- Zhou, P.; Wan, J.F.; Wang, X.R.; Chen, J.; Gong, Y.G.; Xu, K. Three-Dimensional Hierarchical Porous Carbon Cathode Derived from Waste Tea Leaves for the Electrocatalytic Degradation of Phenol. Langmuir 2019, 35, 12914–12926. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, S.; Zhang, J.; Lu, J.H.; Shan, C.; Zhang, Y.Y.; Dionysiou, D.D.; Lv, L.; Pan, B.C.; Zhang, W.M. Enhancing the performance of Fenton-like oxidation by a dual-layer membrane: A sequential interception-oxidation process. J. Hazard. Mater. 2021, 402, 123766. [Google Scholar] [CrossRef]
- Xie, J.; Liao, Z.P.; Zhang, M.; Ni, L.H.; Qi, J.W.; Wang, C.H.; Sun, X.Y.; Wang, L.J.; Wang, S.B.; Li, J.S. Sequential Ultrafiltration-Catalysis Membrane for Excellent Removal of Multiple Pollutants in Water. Environ. Sci. Technol. 2021, 55, 2652–2661. [Google Scholar] [CrossRef]
- Qiu, Z.; Xiao, X.; Yu, W.T.; Zhu, X.Y.; Chu, C.H.; Chen, B.L. Selective Separation Catalysis Membrane for Highly Efficient Water and Soil Decontamination via a Persulfate-Based Advanced Oxidation Process. Environ. Sci. Technol. 2022, 56, 3234–3244. [Google Scholar] [CrossRef]
- Song, Y.F.; Li, Y.J.; Chen, X.M.; Meng, C.C.; Ma, S.F.; Li, T.M.; Jiang, K.; Hu, C. Simultaneous degradation and separation of antibiotics in sewage effluent by photocatalytic nanofiltration membrane in a continuous dynamic process. Water Res. 2023, 229, 119460. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, Z.H.; Graimed, B.H.; Ammar, S.H.; Sabit, D.A.; Najim, A.A.; Radeef, A.Y.; Taher, A.G. The latest progress in the design and application of semiconductor photocatalysis systems for degradation of environmental pollutants in wastewater: Mechanism insight and theoretical calculations. Mater. Sci. Semicond. Process. 2024, 173, 108153. [Google Scholar] [CrossRef]
- Imsong, R.; Ahmed, F.U.; Purkayastha, D.D. Tailoring MXene-based electrospun membranes with spindle-like structures for multifunctional oily wastewater treatment. Chem. Eng. J. 2025, 520, 165721. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, X.; Xu, X.F.; Wu, P.; Li, Z.Z.; Zhang, G.Y. Hierarchically structured CoAl-LDH derived hybrid membrane for synergistic solar-driven water purification and photocatalytic CO2 reduction. Desalination 2025, 613, 118992. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, X.X.; Hu, J.P.; Chen, S.J.; Hu, S.G.; Wu, Y.Q.; Liu, B.C.A.; Xiao, K.K.; Liang, S.; Yang, J.K.; et al. Efficient degradation of refractory pollutant in a microbial fuel cell with novel hybrid photocatalytic air-cathode: Intimate coupling of microbial and photocatalytic processes. Bioresour. Technol. 2021, 340, 125717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Q.; Li, X.; Gao, G.Q.; Sun, H.J.; Liu, X.F. The BiOBr/PVDF film prepared by in situ deposition and phase transformation has high piezo-photocatalytic performance for efficient degradation of Rh B. J. Mater. Sci. Mater. Med. 2025, 36, 1414. [Google Scholar] [CrossRef]
- Costa, I.G.F.; Ribeiro, S.; Nascimento, L.L.; Patrocinio, A.O.T.; Cardoso, V.L.; Batista, F.R.X.; Reis, M.H.M. Well-dispersed titanium dioxide and silver nanoparticles on external and internal surfaces of asymmetric alumina hollow fibers for enhanced chromium (VI) photoreductions. Environ. Sci. Pollut. Res. 2023, 30, 62508–62521. [Google Scholar] [CrossRef]
- Zhang, T.Q.; Guo, Z.F.; Huang, Z.T.; Zhu, L.Y.; Xie, Y.S.; Liu, B.X.; Yuan, K.K.; Jin, X.T.; Zhang, G.H.; Wang, X.Q. Performance-enhanced oxygen vacancy-rich C-TiO2 nanofiber membranes for dual photocatalytic/bactericidal water treatment. Sep. Purif. Technol. 2025, 377, 134169. [Google Scholar] [CrossRef]
- Zhou, H.D.; Jiang, S.Q.; Wang, X.; Zhi, X.H.; Zhang, Z.Y.; Li, X.Z.; Tai, Y.T.; Xu, X.X. Polydopamine-modified g-C3N4/TiO2/GO PVDF membranes for enhanced quinolone antibiotic photocatalytic removal. J. Water Process Eng. 2025, 76, 108280. [Google Scholar] [CrossRef]
- Chen, M.N.; Song, Q.Q.; Li, Z.K.; Bai, W.W.; Xu, M.Y.; Li, X.; Li, W.P.; Nan, H.Y.; Wang, J.; Zhang, Y.T.; et al. COFs functionalized self-cleaning loose nanofiltration membranes for efficient dye/salt separation. Desalination 2025, 593, 118206. [Google Scholar] [CrossRef]
- Li, S.T.; Zhuang, Y.; Wu, H.Z.; Sang, C.; Wang, L.K.; Pang, S.Y.; Yao, S.Y.; Yang, H.W.; Guo, Z.J.; Lu, L.; et al. Self-cleaning PDMS membranes via UV-triggered integration with photocatalytic MOF. J. Membr. Sci. 2025, 728, 124154. [Google Scholar] [CrossRef]
- Yi, Q.Y.; Li, Y.; Dai, R.B.; Li, X.S.; Li, Z.Y.; Wang, Z.W. Efficient removal of neonicotinoid by singlet oxygen dominated MoSx/ ceramic membrane-integrated Fenton-like process. J. Hazard. Mater. 2022, 439, 129672. [Google Scholar] [CrossRef]
- Hu, J.F.; Zhu, H.; Lin, M.; Wu, D.E.; Yao, J.G.; Sun, T.Y.; Ma, X.J.; Xia, Y.J. Highly-efficient removal of Rhodamine B using a flow-through electrocatalytic filtration system: Characteristics, efficiency and mechanism. J. Electroanal. Chem. 2023, 946, 117742. [Google Scholar] [CrossRef]
- Yu, J.F.; Wang, J.B.; Yu, H.J.; Hu, J.W.; Jiang, L.X.; Wang, W.Y. Development of Ti4O7 reactive electrochemical membrane and electrochemical oxidation of naphthols in aqueous solution. Process Saf. Environ. Prot. 2024, 182, 497–508. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Lee, S.; Tijing, L.; Shon, H.K.; Hong, S. Electrically conductive membrane for fouling control: Its mechanisms and applications. Desalination 2024, 578, 117445. [Google Scholar] [CrossRef]
- Li, N.; Wang, W.Y.; Ma, C.; Zhu, L.Y.; Chen, X.Y.; Zhang, B.J.; Zhong, C.L. A novel conductive rGO/ZnO/PSF membrane with superior water flux for electrocatalytic degradation of organic pollutants. J. Membr. Sci. 2022, 641, 119901. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Huang, G.H.; Li, Y.P.; Chen, X.J.; Yao, Y.; Ren, S.J.; Li, M.N.; Wu, Y.W.; An, C.J. Electrically conductive inorganic membranes: A review on principles, characteristics and applications. Chem. Eng. J. 2022, 427, 131987. [Google Scholar] [CrossRef]
- Li, W.; Xiao, R.L.; Xu, J.L.; Lin, H.; Yang, K.; He, K.C.; Tang, L.X.; Chen, J.; Wu, Y.P.; Lv, S.H. Interface engineering strategy of a Ti4O7 ceramic membrane via graphene oxide nanoparticles toward efficient electrooxidation of 1,4-dioxane. Water Res. 2022, 216, 118287. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Li, W.; Zhu, H.W.; Yu, J.T.; Wei, K.J.; Gao, Z.F.; Zhang, Y.H.; Chen, H.M.; Gu, L.K.; Han, W.Q. Electrochemical treatment of municipal reverse osmosis concentrates by a TiO2-BNTs/SnO2-Sb reactive electrochemical membrane. Sep. Purif. Technol. 2024, 331, 125726. [Google Scholar] [CrossRef]
- Ren, L.H.; Li, Y.; Guo, Y.; Yang, K.; Yi, Q.Y.; Wang, X.Y.; Wu, Z.C.; Wang, Z.W. Electrochemical oxidation of reverse osmosis concentrate using a pilot-scale reactive electrochemical membrane filtration system: Performance and mechanisms. J. Hazard. Mater. 2024, 465, 133315. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Zhang, H.Y.; Zhou, Y.; Li, C.J. Enhanced electrocatalytic degradation of tetracycline by ZIF-67@CNT coupled with a self-standing aligned carbon nanofiber anodic membrane. Nanotechnology 2024, 35, 145701. [Google Scholar] [CrossRef]
- Pan, Z.L.; Xu, S.; Xin, H.; Yuan, Y.; Xu, R.S.; Wang, P.C.; Yan, X.Q.; Fan, X.F.; Song, C.W.; Wang, T.H. High performance polypyrrole coated carbon-based electrocatalytic membrane for organic contaminants removal from aqueous solution. J. Colloid Interface Sci. 2022, 626, 283–295. [Google Scholar] [CrossRef]
- Qu, S.Y.; Wang, W.H.; Pan, X.Y.; Li, C.L. Improving the Fenton catalytic performance of FeOCl using an electron mediator. J. Hazard. Mater. 2020, 384, 121494. [Google Scholar] [CrossRef]
- Xie, A.T.; Cui, J.Y.; Yang, J.; Chen, Y.Y.; Lang, J.H.; Li, C.X.; Yan, Y.S.; Dai, J.D. Graphene oxide/Fe(III)-based metal-organic framework membrane for enhanced water purification based on synergistic separation and photo-Fenton processes. Appl. Catal. B Environ. 2020, 264, 118548. [Google Scholar] [CrossRef]
- He, Z.J.; Mahmud, S.; Yang, Y.; Zhu, L.J.; Zhao, Y.B.; Zeng, Q.Y.; Xiong, Z.; Zhao, S.F. Polyvinylidene fluoride membrane functionalized with zero valent iron for highly efficient degradation of organic contaminants. Sep. Purif. Technol. 2020, 250, 117266. [Google Scholar] [CrossRef]
- Liu, H.L.; Sun, Y.M.; Xu, H.Y.; Qin, Y.; Huang, Q.L.; Chen, K.K.; Shu, W.; Xiao, C.F. Dual-functional design of tubular polyvinyl chloride hybrid nanofiber membranes for the simultaneous oil/water separation and in-situ catalytic degradation. J. Membr. Sci. 2022, 661, 120955. [Google Scholar] [CrossRef]
- Song, Q.; Li, Y.H.; Xie, W.C.; Gao, C.F.; Liu, L.F.; Liu, B.C. Catalytic degradation of carbamazepine by metal organic frameworks (MOFs) derived magnetic catalyst Fe@PC in an electro-Fenton coupled membrane filtration system: Performance, pathway, and mechanism. Sep. Purif. Technol. 2023, 309, 122988. [Google Scholar] [CrossRef]
- Piao, H.W.; Zhao, J.; Liu, M.Y.; Zhang, S.J.; Huang, Q.L.; Liu, Y.; Xiao, C.F. Ultra-low power light driven lycopodium-like nanofiber membrane reinforced by PET braid tube with robust pollutants removal and regeneration capacity based on photo-Fenton catalysis. Chem. Eng. J. 2022, 450, 138204. [Google Scholar] [CrossRef]
- Zhang, L.P.; Liu, Z.; Zhou, X.L.; Zhang, C.; Cai, Q.W.; Xie, R.; Ju, X.J.; Wang, W.; Faraj, Y.; Chu, L.Y. Novel composite membranes for simultaneous catalytic degradation of organic contaminants and adsorption of heavy metal ions. Sep. Purif. Technol. 2020, 237, 116364. [Google Scholar] [CrossRef]
- Shan, H.R.; Dong, X.Y.; Cheng, X.T.; Si, Y.; Yu, J.Y.; Ding, B. Highly flexible, mesoporous structured, and metallic Cu-doped C/SiO2 nanofibrous membranes for efficient catalytic oxidative elimination of antibiotic pollutants. Nanoscale 2019, 11, 14844–14856. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Yu, Y.L.; Yang, Y.; Sun, T.J.; Dong, S.J.; Yang, H.L.; Liu, Y.M.; Fan, X.F.; Song, C.W. Improved separation performance of carbon nanotube hollow fiber membrane by peroxydisulfate activation. Sep. Purif. Technol. 2021, 276, 119328. [Google Scholar] [CrossRef]
- Zhao, X.F.; Yi, X.B.; Song, J.J.; Yuan, X.Y.; Yu, S.M.; Nie, Y.H.; Zhang, J.; Cao, G.G. Mesoporous and flexible polyimide aerogel as highly active catalytic membrane for AO7 degradation by peroxymonosulfate activation. Chem. Eng. J. 2022, 431, 134286. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Zhong, L.L.; Zhang, Z.Q.; Li, H.R.; Shi, W.X.; Cui, F.Y.; Wang, W. Gravity driven ultrafast removal of organic contaminants across catalytic superwetting membranes. J. Mater. Chem. A 2017, 5, 25266–25275. [Google Scholar] [CrossRef]
- Du, X.; Zhang, K.M.; Xie, B.H.; Zhao, J.; Cheng, X.X.; Kai, L.; Nie, J.X.; Wang, Z.H.; Li, G.B.; Liang, H. Peroxymonosulfate-assisted electro-oxidation/coagulation coupled with ceramic membrane for manganese and phosphorus removal in surface water. Chem. Eng. J. 2019, 365, 334–343. [Google Scholar] [CrossRef]
- Du, X.; Yang, W.P.; Liu, Y.; Zhang, W.X.; Wang, Z.H.; Nie, J.X.; Li, G.B.; Liang, H. Removal of manganese, ferrous and antibiotics from groundwater simultaneously using peroxymonosulfate-assisted in-situ oxidation/coagulation integrated with ceramic membrane process. Sep. Purif. Technol. 2020, 252, 117492. [Google Scholar] [CrossRef]
- Ma, D.R.; Lian, Q.Y.; Zhang, Y.X.; Huang, Y.J.; Guan, X.Y.; Liang, Q.W.; He, C.; Xia, D.H.; Liu, S.W.; Yu, J.G. Catalytic ozonation mechanism over M1-N3C1 active sites. Nat. Commun. 2023, 14, 7011. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, Y.Z.; Zhao, D.R.; Liu, C.; Dong, P.H.; Gu, H.X. Research on MnFe2O4 Modified Ceramic Membrane Catalyzed Ozone Oxidation Treatment of Phenol-Containing Wastewater. Water Air Soil Pollut. 2025, 236, 568. [Google Scholar] [CrossRef]
- Qian, K.; Meng, L.Y.; Zhang, G.Y.; Li, J.; Lyu, J.Z. Neural network-like CeO2-loaded polyvinylidene fluoride membrane for tri-phase interface catalytic ozonation of antibiotics. J. Environ. Chem. Eng. 2025, 13, 117757. [Google Scholar] [CrossRef]
- Zhang, J.L.; Yu, H.T.; Quan, X.; Chen, S.; Zhang, Y.B. Ceramic membrane separation coupled with catalytic ozonation for tertiary treatment of dyestuff wastewater in a pilot-scale study. Chem. Eng. J. 2016, 301, 19–26. [Google Scholar] [CrossRef]
- Han, L.W.; Liu, J.; Li, F.; Zhao, Y.Y.; Guo, X.F.; Wang, S.Z.; Ji, Z.Y. CoMn-MOF-74 coated ceramic membranes for catalytic ozonation of azo dye in wastewater by membrane dispersion—Membrane catalysis process. J. Environ. Chem. Eng. 2025, 13, 116612. [Google Scholar] [CrossRef]
- Bai, H.K.; Liang, L.L.; Cao, P.K.; Zhang, H.G.; Chen, S.; Yu, H.T.; Quan, X. MgAl2O4 incorporated catalytic ceramic membrane for catalytic ozonation of organic pollutants. Appl. Catal. B Environ. 2024, 343, 123527. [Google Scholar] [CrossRef]
- Chen, P.; Cheng, Z.L.; Zhang, X.; Zhang, L.P.; Zhang, X.Z.; Tang, J.S.; Qiu, F.C. Efficient degradation of dye wastewater by catalytic ozonation reactive ceramic membrane with facile spraying of nano Ti-Mn oxides: A pilot scale attempt. J. Water Process Eng. 2023, 55, 104143. [Google Scholar] [CrossRef]
- Feng, T.T.; Wang, B.; Li, J.; Wang, T.; Huang, P.; Xu, X.Y. Metal-organic framework based photocatalytic membrane for organic wastewater treatment: Preparation, optimization, and applications. Sep. Purif. Technol. 2025, 355, 129540. [Google Scholar] [CrossRef]
| Catalytic Membrane (CM) | Preparation Method | Pollutant Name and Concentration | Catalytic Conditions (Light Source, Time) | Decomposed Pollutant (%) | Ref. |
|---|---|---|---|---|---|
| AgNP30-TiO2@Alumina Hollow Fiber Membrane | Mutual Conversion Method | Cr(VI) (10 mg/L) | tungsten halogen lamp (100 W, 220 V, FLC, Brazil), 3 h | 55.00 ± 0.83% | [38] |
| C-TiO2 Flexible Nanofiber Membrane | Electrospinning | Tetracycline (40 mg/L) | Xe lamp (300 W, 250–800 nm, 100 mW/cm2, AM 1.5G), 1 h | 90.8% | [39] |
| GO/g-C3N4/TiO2@PDA Composite Membrane | Immersion Precipitation Phase Transformation Technology | Ciprofloxacin (10 mg/L), Enrofloxacin (10 mg/L) and Ofloxacin (10 mg/L) | short-arc xenon lamp (800 W), 2.5 h | 94.1%, 85.8% and 85.1% | [40] |
| Porphyrin-Based COF-366 Low-Permeability Nanofiltration Membrane | Hybrid Matrix Method | Methyl Blue | short-arc xenon lamp (800 W), 1 h | 95.7% | [41] |
| MXene-TiO2/CuO@PVDF Composite Membrane | Electrospinning and Hydrothermal Method | RhB and Tetracycline | visible light irradiation (50 W, LED bulb, 5530 Lux), 0.5 h | 97% and 96% | [34] |
| MOF/PDMS Composite Membrane | Simplified Light-Curing Method | Chlorinated naphthalene (50 mg/L) | Xe lamp, 1 h | 99.08% | [42] |
| Catalytic Membrane Type | Pollutant Name and Concentration | Reaction Conditions | Response Time | Decomposed Pollutant (%) | Ref. |
|---|---|---|---|---|---|
| FeOCl/MoS | MB (50 mg/L) | H2O2 concentration: 0.6 mM, Flow velocity: 0.1 mL s−1, pH: 3~9 | 2 min | 97.3% | [54] |
| GO/M88A | MB (10 mg/L) | H2O2 concentration: 10 mM, Under visible light irradiation | 40 min | 98.81% | [55] |
| Magnetic Fe@PC | CBZ (10 mg/L) | Flow-through aeration: 50 mL/min, Catalyst dosage:50 mg, pH = 3, Potential: 7.1 V, Current density:0.67 mA cm−2 | 70 min | 98.34% | [58] |
| Fe-HP | 4-NP (10 ppm) and 2-CP (10 ppm) | 30 wt% H2O2, Membrane area: 7.1 cm2, pH = 5 | 60 min | 100% | [56] |
| PMIA/β-FeOOH Nanofiber Membrane | MB, RhB and TC (10 mg/L) | Under visible light irradiation, 20 μL 30 wt% H2O2 | 360 min | 99.99%, 99.75% and 95.28% | [59] |
| Fe3O4@PDA/PES Composite Membrane | Methylene blue (10 ppm), Pb2+ (1 ppm), Cd2+ (100 ppb) and Ag+ (500 ppb) | 30 wt% H2O2, T = 25 °C, pH = 4.0–5.4 | 80 min | 98.8%, ~ 90%, ~100% and ~100% | [60] |
| Tubular PVC/SiO2/SiO2@Ag Nanofiber Membrane | Methylene blue (10 mg/L) and kerosene (mixed with MB aqueous solution in a 1:1 volume ratio) | Negative pressure suction filtration, Operating pressure: 1.8 kPa | 8 min | 95% and 96% | [57] |
| Catalytic Membrane Type | Preparation Method | Pollutant Name and Concentration | Reaction Conditions | Response Time | Decomposed Pollutant (%) | Ref. |
|---|---|---|---|---|---|---|
| Cu@C/SiO2 NFMs | Sol–gel electrospinning and in situ carbonization reduction method | TCH | - | 40 min | 95% | [61] |
| CNT hollow fiber membrane | Wet spinning combined with pyrolysis process | Phenol (10 mg/L) | pH = 6, 0.5 mM PDS and HRT = 39.6 s | 120 min | 97% | [62] |
| PI aerogel film | - | AO7 (20.0 mg/L) | PMS: 100 g/L, T = 35 °C | 5 min | 99.8% | [63] |
| MnO/Co@SiO2-CNFMs | Electrospinning technology and Self-Reducing pyrolysis method | Methylene blue | PMS | - | 99.5% | [64] |
| Flat-sheet ceramic ultrafiltration membrane | - | Mn2+ (0–1.0 mg/L) and P (0–0.8 mg/L) | 100µM PMS, I = 0.2 A, Electrolysis time: 60 s, pH = 7.5 | 60 min | 75% and 85% | [65] |
| Ceramic ultrafiltration membrane | - | Fe2+ (2.0–4.2 mg/L), Mn2+ (0.99–4.12 mg/L) and Sulfamethoxazole (400–800 µg/L) | 100 µM PMS, T = 25 °C | - | 100% 100% and 93.1% | [66] |
| Catalytic Membrane Type | Pollutant Name and Concentration | Reaction Conditions | Response Time | Decomposed Pollutant (%) | Ref. |
|---|---|---|---|---|---|
| 15%CeO2-R@PVDF-NWF | SME (10 mg/L) | O3 concentration: 2.5 mg/L, O3 flow velocity: 40 mL/min | 60 min | 98.5% | [69] |
| Ti-Mn/CM | Dye wastewater (CODCr: 100 mg/L) | O3 concentration: 2.5 mg/L Operating pressure: 0.2 MPa O3 flow velocity: 400 mL/min | 30 d | >90% | [70] |
| 9%MnFe2O4@CM | Phenol (30 mg/L) | O3 concentration: 100 mg/L, Stirring speed: 1000 r/min, pH = 9 | 60 min | 92.38% | [68] |
| Co0.5Mn0.5-MOF-74@CM | CR | O3 concentration: 12 mg/L, O3 flow velocity: 60 mL/min | 20 min | 93.06% | [71] |
| 15 wt% MgAl2O4@CM | Ibuprofen (5 mg/L) | O3 concentration: 2 mg/L H2O flow velocity: 19.1 LMH | 25 h | 74.0% | [72] |
| TiO2/γ-MnO2-CM | RhB (20 mg/L) | O3 concentration: 2.5 g/m3, T = 25 °C, pH = 4.85 | 40 min | 100% | [73] |
| Catalytic Membrane Technology | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| photocatalytic membrane | Enhance surface membrane hydrophilicity; Increase permeation flux of catalytic membranes; Mild reaction conditions; Wide range of applications; Can be combined with other oxidation technologies. | Requires substantial light irradiation; suspended solids reduce wastewater treatment efficiency; catalytic membranes exhibit weak visible light response; ultraviolet radiation may degrade polymer membranes, posing secondary risks. | [38,39,40,41,74] |
| Electrocatalytic membrane | The process is environmentally friendly and low in pollution; Operating conditions and reaction processes are controllable; It exhibits a certain degree of corrosion resistance. | Research on high-performance anode membranes remains in its early stages; current efficiency is low; and their application in engineering has limitations. | [43,44,45,53] |
| Fenton-type catalytic membrane | Mild reaction conditions; Simple reaction equipment with convenient operation; Relatively broad application scope. | Relatively weak oxidation capacity; Operating costs are high due to chemical consumption during the oxidation process; Generates large amounts of iron sludge; Relatively narrow pH operating range. | [54,55,56,58] |
| Peroxymonosulfate-catalyzed membrane | Due to its higher oxidation potential (2.5–3.1 V), it exhibits relatively high pollutant removal efficiency; it also operates within a relatively wide pH range (2–10). | Requires consumption of additional oxidizing agents; SO produced during the reaction requires post-treatment to meet emission standards. | [61,62,63,64] |
| Ozone catalytic membrane | Rapid oxidation rate; mild reaction conditions; relatively broader operating pH range; environmentally friendly and free from secondary pollution. | Treatment of halogenated hydrocarbon and pesticide-contaminated wastewater is suboptimal; Ozone utilization efficiency is low; Energy consumption and operational costs are relatively high. | [68,69,71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Zhuang, Y.; Shah, K.J.; Sun, Y. A Review on the Application of Catalytic Membranes Technology in Water Treatment. Catalysts 2025, 15, 1081. https://doi.org/10.3390/catal15111081
Dai J, Zhuang Y, Shah KJ, Sun Y. A Review on the Application of Catalytic Membranes Technology in Water Treatment. Catalysts. 2025; 15(11):1081. https://doi.org/10.3390/catal15111081
Chicago/Turabian StyleDai, Jun, Yan Zhuang, Kinjal J. Shah, and Yongjun Sun. 2025. "A Review on the Application of Catalytic Membranes Technology in Water Treatment" Catalysts 15, no. 11: 1081. https://doi.org/10.3390/catal15111081
APA StyleDai, J., Zhuang, Y., Shah, K. J., & Sun, Y. (2025). A Review on the Application of Catalytic Membranes Technology in Water Treatment. Catalysts, 15(11), 1081. https://doi.org/10.3390/catal15111081









