Ni-Based Catalysts Coupled with SERP for Efficient Power-to-X Conversion
Abstract
1. Introduction
2. Results and Discussion
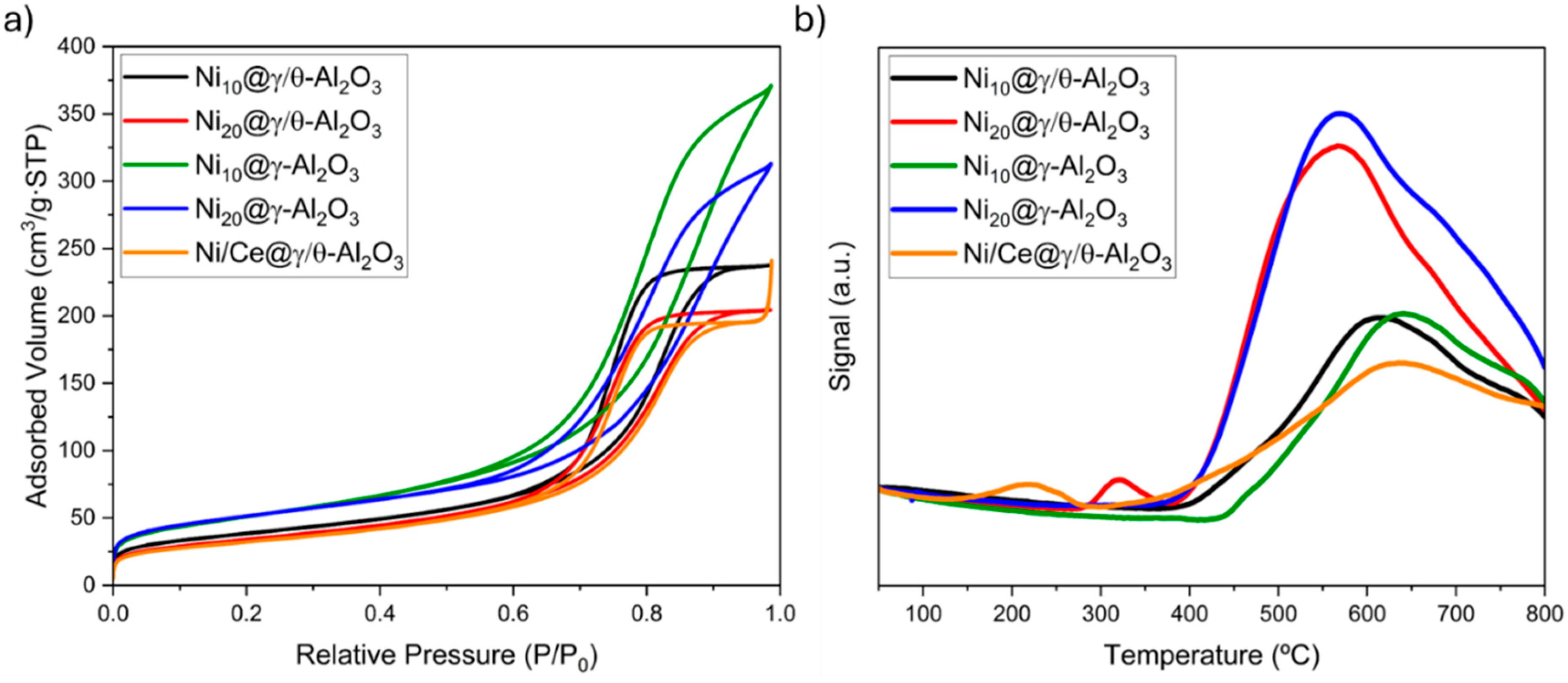
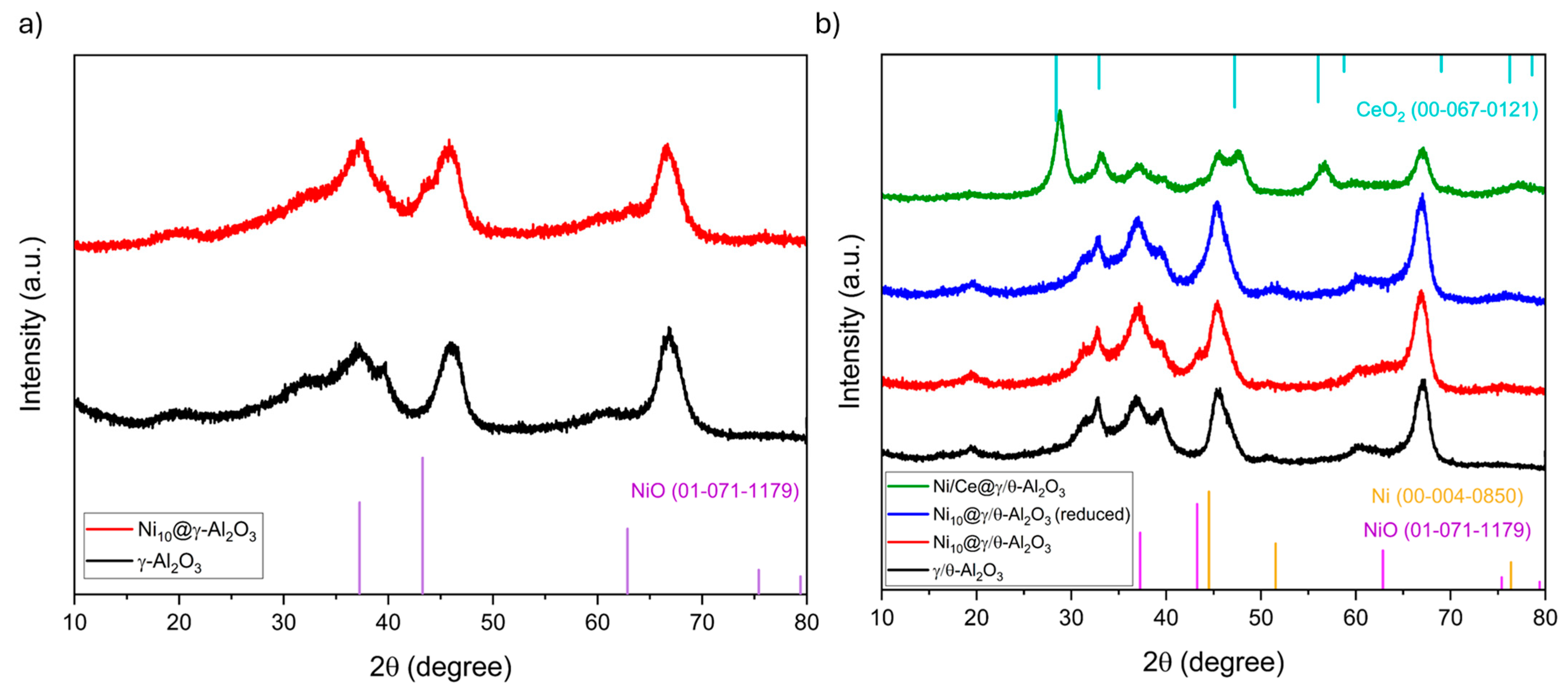
2.1. Catalysts Characterization
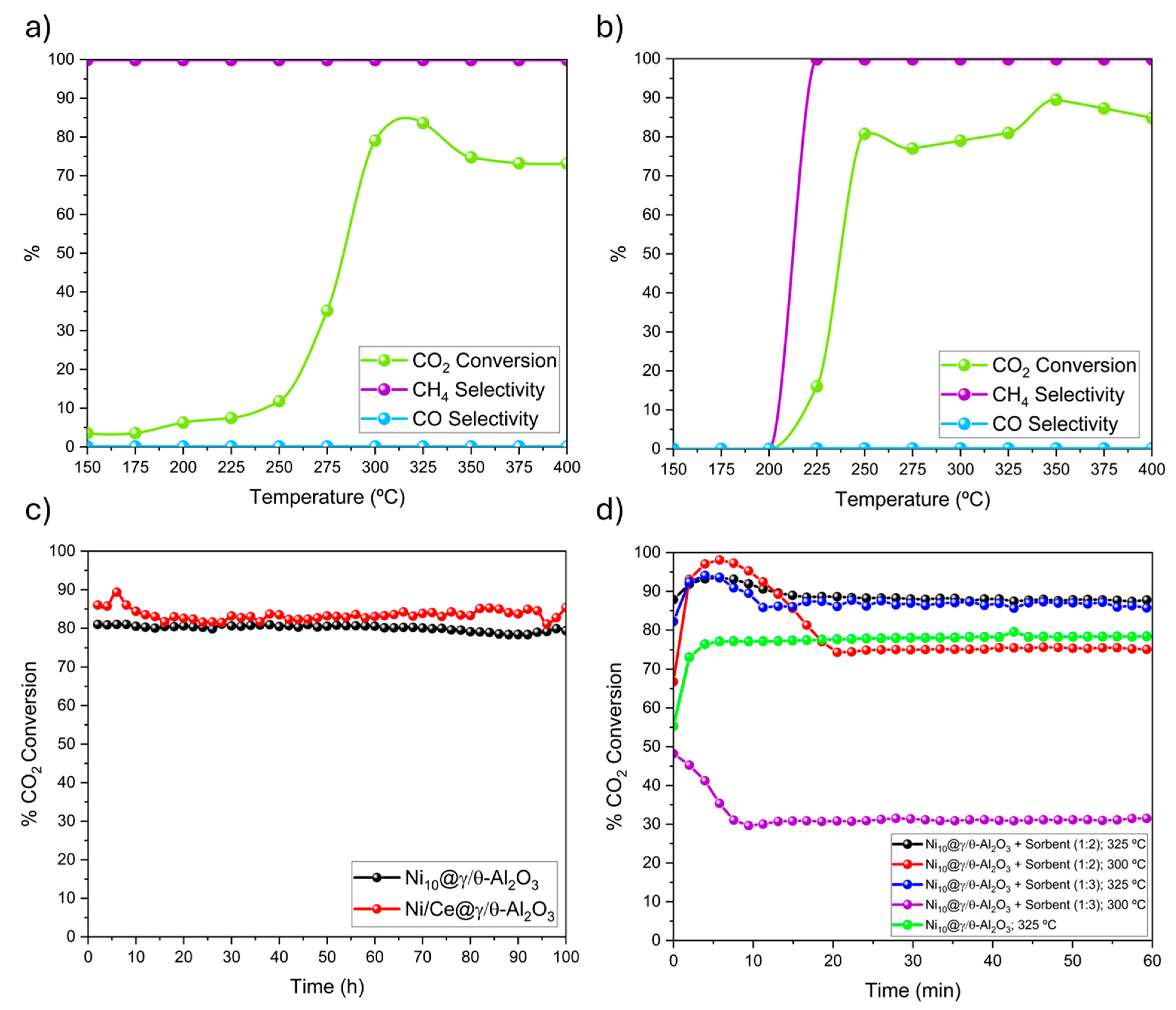
2.2. Catalytic Performance: Activity, Selectivity and Stability
3. Experimental
3.1. Catalysts Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
3.3.1. Conventional Methanation Reaction
3.3.2. SERP
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davis, S.J.; Caldeira, K.; Matthews, H.D. Future CO2 Emissions and Climate Change from Existing Energy Infrastructure. Science (1979) 2010, 329, 1330–1333. [Google Scholar] [CrossRef]
- Rogelj, J.; Den Elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement Climate Proposals Need a Boost to Keep Warming Well below 2 °C. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef]
- Zhong, J.; Zhang, X.; Zhang, D.; Wang, D.; Guo, L.; Peng, H.; Huang, X.; Wang, Z.; Lei, Y.; Lu, Y.; et al. Plausible Global Emissions Scenario for 2 °C Aligned with China’s Net-Zero Pathway. Nat Commun 2025, 16, 8102. [Google Scholar] [CrossRef]
- Ye, R.P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 Hydrogenation to High-Value Products via Heterogeneous Catalysis. Nat Commun 2019, 10, 5698. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A Thermodynamic Analysis of Methanation Reactions of Carbon Oxides for the Production of Synthetic Natural Gas. RSC Adv 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Beniwal, A.; Bagaria, A.; Chen, T.Y.; Bhalothia, D. Advancements in CO2 Conversion Technologies: A Comprehensive Review on Catalyst Design Strategies for High-Performance CO2 Methanation. Sustain Energy Fuels 2025, 9, 2261–2286. [Google Scholar] [CrossRef]
- Faria, C.; Rocha, C.; Miguel, C.; Rodrigues, A.; Madeira, L.M. Process Intensification Concepts for CO2 Methanation − A Review. Fuel 2025, 386, 134269. [Google Scholar] [CrossRef]
- Bengaouer, A.; Bedel, L. CO2 Hydrogenation to Methane. In Carbon Dioxide Utilisation: Transformations; North, M., Styring, P., Eds.; De Gruyter: Berlin, 2019; Volume 2, pp. 385–411. ISBN 9783110665147. [Google Scholar]
- Wang, H.; Guo, S.; Qin, Z.; Li, Z.; Wang, G.; Dong, M.; Fan, W.; Wang, J. A Thermodynamic Consideration on the Synthesis of Methane from CO, CO2, and Their Mixture by Hydrogenation. JFCT 2024, 52, 1453–1461. [Google Scholar] [CrossRef]
- Daiyan, R.; Macgill, I.; Amal, R. Opportunities and Challenges for Renewable Power-to-X. ACS Energy Lett 2020, 5, 3843–3847. [Google Scholar] [CrossRef]
- Sorrenti, I.; Harild Rasmussen, T.B.; You, S.; Wu, Q. The Role of Power-to-X in Hybrid Renewable Energy Systems: A Comprehensive Review. Renew Sust Energ Rev 2022, 165, 112380. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A Technological and Economic Review. Renew Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Hussain, I.; Jalil, A.A.; Hassan, N.S.; Hamid, M.Y.S. Recent Advances in Catalytic Systems for CO2 Conversion to Substitute Natural Gas (SNG): Perspective and Challenges. J. Energy Chem. 2021, 62, 377–407. [Google Scholar] [CrossRef]
- Ren, J.; Lou, H.; Xu, N.; Zeng, F.; Pei, G.; Wang, Z. Methanation of CO/CO2 for Power to Methane Process: Fundamentals, Status, and Perspectives. J. Energy Chem. 2023, 80, 182–206. [Google Scholar] [CrossRef]
- Moioli, E.; Mutschler, R.; Züttel, A. Renewable Energy Storage via CO2 and H2 Conversion to Methane and Methanol: Assessment for Small Scale Applications. Renew Sust Energ Rev 2019, 107, 497–506. [Google Scholar] [CrossRef]
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The Renaissance of the Sabatier Reaction and Its Applications on Earth and in Space. Nat Catal 2019, 2, 188–197. [Google Scholar] [CrossRef]
- Du, J.; Gao, J.; Gu, F.; Zhuang, J.; Lu, B.; Jia, L.; Xu, G.; Liu, Q.; Su, F. A Strategy to Regenerate Coked and Sintered Ni/Al2O3 Catalyst for Methanation Reaction. Int J Hydrogen Energy 2018, 43, 20661–20670. [Google Scholar] [CrossRef]
- Olesen, S.E.; Andersson, K.J.; Damsgaard, C.D.; Chorkendorff, I. Deactivating Carbon Formation on a Ni/Al2O3 Catalyst under Methanation Conditions. J. Phys. Chem. C 2017, 121, 15556–15564. [Google Scholar] [CrossRef]
- Medina, O.E.; Amell, A.A.; López, D.; Santamaría, A. Comprehensive Review of Nickel-Based Catalysts Advancements for CO2 Methanation. Renew Sust Energ Rev 2025, 207, 114926. [Google Scholar] [CrossRef]
- Lv, C.; Xu, L.; Chen, M.; Cui, Y.; Wen, X.; Li, Y.; Wu, C.E.; Yang, B.; Miao, Z.; Hu, X.; et al. Recent Progresses in Constructing the Highly Efficient Ni Based Catalysts with Advanced Low-Temperature Activity toward CO2 Methanation. Front Chem 2020, 8, 269. [Google Scholar] [CrossRef]
- Li, L.; Zeng, W.; Song, M.; Wu, X.; Li, G.; Hu, C. Research Progress and Reaction Mechanism of CO2 Methanation over Ni-Based Catalysts at Low Temperature: A Review. Catalysts 2022, 12, 244. [Google Scholar] [CrossRef]
- Usman, M.; Podila, S.; Alamoudi, M.A.; Al-Zahrani, A.A. Current Research Status and Future Perspective of Ni- and Ru-Based Catalysts for CO2 Methanation. Catalysts 2025, 15, 203. [Google Scholar] [CrossRef]
- Martínez, J.; Hernández, E.; Alfaro, S.; Medina, R.L.; Aguilar, G.V.; Albiter, E.; Valenzuela, M.A. High Selectivity and Stability of Nickel Catalysts for CO2 Methanation: Support Effects. Catalysts 2019, 9, 24. [Google Scholar] [CrossRef]
- Muroyama, H.; Tsuda, Y.; Asakoshi, T.; Masitah, H.; Okanishi, T.; Matsui, T.; Eguchi, K. Carbon Dioxide Methanation over Ni Catalysts Supported on Various Metal Oxides. J Catal 2016, 343, 178–184. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Zhu, M.; Han, Y.F. Essential Role of the Support for Nickel-Based CO2 Methanation Catalysts. ACS Catal 2020, 10, 14581–14591. [Google Scholar] [CrossRef]
- Gac, W.; Zawadzki, W.; Rotko, M.; Greluk, M.; Słowik, G.; Kolb, G. Effects of Support Composition on the Performance of Nickel Catalysts in CO2 Methanation Reaction. Catal Today 2020, 357, 468–482. [Google Scholar] [CrossRef]
- Deng, G.; Nie, G.; He, X.; Li, L.; Sun, Z.; Duan, L. La2O3-Modified Ni-Based Catalysts Supported on Ordered Mesoporous Silicas for CO2 Methanation. ACS Appl Nano Mater 2024, 7, 28436–28447. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Bibak, F.; Shakeri, M.; Meshkani, F. The Influence of Ce, La, and Y Promoters on the Catalytic Performance of Ni/Cr2O3 Catalysts for CO2 Methanation. J Mol Struct 2025, 1328, 141369. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. Bimetallic Ni-Based Catalysts for CO2 Methanation: A Review. Nanomaterials 2021, 11, 1–34. [Google Scholar] [CrossRef]
- Barreau, M.; Salusso, D.; Zhang, J.; Haevecker, M.; Teschner, D.; Efimenko, A.; Borfecchia, E.; Sobczak, K.; Zafeiratos, S. Thermal Activation and Deactivation of Ni-Doped Ceria Catalysts in CO2 Methanation. SMALL SCI 2025, 5, 2400540. [Google Scholar] [CrossRef]
- Tarifa, P.; Megías-Sayago, C.; Cazaña, F.; González-Martín, M.; Latorre, N.; Romeo, E.; Delgado, J.J.; Monzón, A. Highly Active Ce- And Mg-Promoted Ni Catalysts Supported on Cellulose-Derived Carbon for Low-Temperature CO2 Methanation. Energy Fuels 2021, 35, 17212–17224. [Google Scholar] [CrossRef]
- Ullah, S.; Huang, T.; Pan, Y.; Xue, Q.; Yu, Z.; Hu, Y.; Ahmed, S.M.; Ye, R.; Wang, Y.; Luo, G. Ceria-Modified High Pore Volume Ni/Al2O3 Spheres for Enhanced Low-Temperature CO2 Methanation. Fuel 2025, 390, 134736. [Google Scholar] [CrossRef]
- Alkhoori, A.A.; Elmutasim, O.; Dabbawala, A.A.; Vasiliades, M.A.; Petallidou, K.C.; Emwas, A.H.; Anjum, D.H.; Singh, N.; Baker, M.A.; Charisiou, N.D.; et al. Mechanistic Features of the CeO2-Modified Ni/Al2O3 Catalysts for the CO2 Methanation Reaction: Experimental and Ab Initio Studies. ACS Appl Energy Mater 2023, 6, 8550–8571. [Google Scholar] [CrossRef]
- Ye, R.; Ma, L.; Hong, X.; Reina, T.R.; Luo, W.; Kang, L.; Feng, G.; Zhang, R.; Fan, M.; Zhang, R.; et al. Boosting Low-Temperature CO2 Hydrogenation over Ni-Based Catalysts by Tuning Strong Metal-Support Interactions. Angew. Chem. Int. Ed. 2024, 63, e202317669. [Google Scholar] [CrossRef] [PubMed]
- De Piano, G.; Andrade Gamboa, J.J.; Condó, A.M.; Gennari, F.C. CO2 Methanation over Nickel-CeO2 Catalyst Supported on Al2O3: Different Impregnation Strategies and Ni-Ce Ratios. Int J Hydrogen Energy 2024, 56, 1007–1019. [Google Scholar] [CrossRef]
- Lei, J.; Wu, Z.; Ye, D.; Feng, Y.; Tian, Y.; Niu, J.; Cheng, C.; Wang, Y.; Li, S.; Zhao, C. Amorphous Silica Induced Loose CeO2 Clusters with Isolated Pt Atoms for Efficient Reverse-Water Gas Shift Reaction. Angew. Chem. Int. Ed. 2025, 64, e202511913. [Google Scholar] [CrossRef]
- Muravev, V.; Parastaev, A.; Van Den Bosch, Y.; Ligt, B.; Claes, N.; Bals, S.; Kosinov, N.; Hensen, E.J.M. Size of Cerium Dioxide Support Nanocrystals Dictates Reactivity of Highly Dispersed Palladium Catalysts. Science 2023, 380, 1174–1179. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, S.; Li, Y.; Shen, J.; Tian, X.; Ding, M. Highly Dispersed Cu on Hollow Spherical CeO2: An Efficient and Stable Catalyst for the RWGS Reaction. Appl Catal B 2025, 366, 125003. [Google Scholar] [CrossRef]
- Yu, Z.K.; Jiang, M.; Dai, S.; Zhan, W.; Wang, Z.Q.; Gong, X.Q. Valence Restrictive Metal-Support Interaction for Boosting Catalytic Activity of Rh/CeO2 in CO2 Hydrogenation. Nat Commun 2025, 16, 9072. [Google Scholar] [CrossRef]
- Wei, L.; Azad, H.; Haije, W.; Grenman, H.; de Jong, W. Pure Methane from CO2 Hydrogenation Using a Sorption Enhanced Process with Catalyst/Zeolite Bifunctional Materials. Appl Catal B 2021, 297, 120399. [Google Scholar] [CrossRef]
- Gómez, L.; Martínez, I.; Navarro, M.V.; García, T.; Murillo, R. Sorption-Enhanced CO and CO2 Methanation (SEM) for the Production of High Purity Methane. CEJ 2022, 440, 135842. [Google Scholar] [CrossRef]
- Gómez, L.; Martínez, I.; Navarro, M.V.; Murillo, R. Selection and Optimisation of a Zeolite/Catalyst Mixture for Sorption-Enhanced CO2 Methanation (SEM) Process. J CO2 UTIL 2023, 77, 102611. [Google Scholar] [CrossRef]
- Gómez, L.; Martínez, I.; Grasa, G.; Murillo, R. Experimental Demonstration of a Sorption-Enhanced Methanation (SEM) Cyclic Process on a Lab-Scale TRL-3 Fixed Bed Reactor. CEJ 2024, 491, 151744. [Google Scholar] [CrossRef]
- Cañada-Barcala, A.; Larriba, M.; Águeda Maté, V.I.; Delgado Dobladez, J.A. CO2 Methanation Enhanced with a Cyclic SERP Process Using a Commercial Ni-Based Catalyst Mixed with 3A Zeolite as Adsorbent. CEJ 2023, 461, 141897. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Piertotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure & Applied Chemistry 1985, 57, 603–619. [Google Scholar]
- Kim, M.J.; Youn, J.R.; Kim, H.J.; Seo, M.W.; Lee, D.; Go, K.S.; Lee, K.B.; Jeon, S.G. Effect of Surface Properties Controlled by Ce Addition on CO2 Methanation over Ni/Ce/Al2O3 Catalyst. Int J Hydrogen Energy 2020, 45, 24595–24603. [Google Scholar] [CrossRef]
- Rudolph, M.; Motylenko, M.; Rafaja, D. Structure Model of γ-Al2O3 Based on Planar Defects. IUCrJ 2019, 6, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Qin, C.; Xu, Y.; Xu, D.; Bai, J.; Ma, G.; Ding, M. Ni Nanoparticles Dispersed on Oxygen Vacancies-Rich CeO2 Nanoplates for Enhanced Low-Temperature CO2 Methanation Performance. CEJ 2021, 418, 129402. [Google Scholar] [CrossRef]
- Graça, I.; González, L.V.; Bacariza, M.C.; Fernandes, A.; Henriques, C.; Lopes, J.M.; Ribeiro, M.F. CO2 Hydrogenation into CH4 on NiHNaUSY Zeolites. Appl Catal B 2014, 147, 101–110. [Google Scholar] [CrossRef]
- Wang, D.; Kang, Y.; Doan-Nguyen, V.; Chen, J.; Küngas, R.; Wieder, N.L.; Bakhmutsky, K.; Gorte, R.J.; Murray, C.B. Synthesis and Oxygen Storage Capacity of Two-Dimensional Ceria Nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 4378–4381. [Google Scholar] [CrossRef]
- Dobladez, J.A.D.; Maté, V.I.Á.; Torrellas, S.Á.; Larriba, M.; Pascual Muñoz, G.; Alberola Sánchez, R. Comparative Simulation Study of Methanol Production by CO2 Hydrogenation with 3A, 4A and 5A Zeolites as Adsorbents in a PSA Reactor. Sep Purif Technol 2021, 262, 118292. [Google Scholar] [CrossRef]
- Cañada-Barcala, A.; Larriba, M.; Águeda Maté, V.I.; Delgado Dobladez, J.A. Synthetic Natural Gas Production through Biogas Methanation Using a Sorption-Enhanced Reaction Process. Sep. Purif. Technol. 2024, 331, 125714. [Google Scholar] [CrossRef]
- EURECAT CIUDEN y Eurecat Desarrollan Una Planta Pionera Para Producir Metanol Más Eficiente a Partir de Hidrógeno Verde y Dióxido de Carbono. Available online: https://eurecat.org/es/ciuden-y-eurecat-desarrollan-una-planta-pionera-para-producir-metanol-mas-eficiente-a-partir-de-hidrogeno-verde-y-dioxido-de-carbono/ (accessed on 10 November 2025).
- Mutz, B.; Carvalho, H.W.P.; Mangold, S.; Kleist, W.; Grunwaldt, J.D. Methanation of CO2: Structural Response of a Ni-Based Catalyst under Fluctuating Reaction Conditions Unraveled by Operando Spectroscopy. J Catal 2015, 327, 48–53. [Google Scholar] [CrossRef]


| Sample | LM (wt. %) a | SABET (m2/g) b | Dp (nm) c | Vp (cm3/g) d | DNi (%) e | SNi (m2/g) f | dNi (nm) g |
|---|---|---|---|---|---|---|---|
| g/q-Al2O3 | - | 154 | 8.8 | 0.4 | - | - | - |
| g-Al2O3 | - | 218 | 9.9 | 0.7 | - | - | - |
| Ni10@g/q-Al2O3 | 11 | 136 | 7.9 | 0.4 | 5.6 | 4.3 | 18 |
| Ni20@g/q-Al2O3 | 20 | 121 | 8.1 | 0.3 | 2.5 | 3.3 | 41 |
| Ni10@g-Al2O3 | 12 | 182 | 10.3 | 0.6 | 1.8 | 2.4 | 56 |
| Ni20@g-Al2O3 | 20 | 180 | 10.1 | 0.5 | 1.1 | 0.9 | 90 |
| Ni/Ce@g/q-Al2O3 | Ni: 10 Ce: 8 | 115 | 8.1 | 0.3 | 4.0 | 2.6 | 26 |
| Exp. | Ni (w%) | Support | Flow (mLN/min) | T (°C) | Conv. (%) | CH4 sel. (%) |
|---|---|---|---|---|---|---|
| 1 | 10 | g-Al2O3 | 100 | 300 | 41 | >99 |
| 2 | 20 | g-Al2O3 | 100 | 300 | 72 | >99 |
| 3 | 10 | g/q-Al2O3 | 100 | 300 | 41 | >99 |
| 4 | 20 | g/q-Al2O3 | 100 | 300 | 76 | >99 |
| 5 | 10 | g-Al2O3 | 300 | 300 | 70 | >99 |
| 6 | 20 | g-Al2O3 | 300 | 300 | 46 | >99 |
| 7 | 10 | g/q-Al2O3 | 300 | 300 | 81 | >99 |
| 8 | 20 | g/q-Al2O3 | 300 | 300 | 80 | >99 |
| 9 | 10 | g-Al2O3 | 100 | 400 | 56 | >99 |
| 10 | 20 | g-Al2O3 | 100 | 400 | 77 | >99 |
| 11 | 10 | g/q-Al2O3 | 100 | 400 | 67 | >99 |
| 12 | 20 | g/q-Al2O3 | 100 | 400 | 56 | >99 |
| 13 | 10 | g-Al2O3 | 300 | 400 | 64 | >99 |
| 14 | 20 | g-Al2O3 | 300 | 400 | 76 | >99 |
| 15 | 10 | g/q-Al2O3 | 300 | 400 | 72 | >99 |
| 16 | 20 | g/q-Al2O3 | 300 | 400 | 79 | >99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrola, M.; Miró, R.; Vicente, I.; Gual, A. Ni-Based Catalysts Coupled with SERP for Efficient Power-to-X Conversion. Catalysts 2025, 15, 1082. https://doi.org/10.3390/catal15111082
Pedrola M, Miró R, Vicente I, Gual A. Ni-Based Catalysts Coupled with SERP for Efficient Power-to-X Conversion. Catalysts. 2025; 15(11):1082. https://doi.org/10.3390/catal15111082
Chicago/Turabian StylePedrola, Marina, Roger Miró, Isabel Vicente, and Aitor Gual. 2025. "Ni-Based Catalysts Coupled with SERP for Efficient Power-to-X Conversion" Catalysts 15, no. 11: 1082. https://doi.org/10.3390/catal15111082
APA StylePedrola, M., Miró, R., Vicente, I., & Gual, A. (2025). Ni-Based Catalysts Coupled with SERP for Efficient Power-to-X Conversion. Catalysts, 15(11), 1082. https://doi.org/10.3390/catal15111082






