Abstract
For effective water purification, the combination of membrane separation and catalytic degradation technologies not only permits continuous pollutant degradation but also successfully reduces membrane fouling. In recent years, catalytic membranes (CMs) have garnered a lot of interest in the water treatment industry. The main benefits of CMs are methodically explained in this review, emphasizing the synergistic effect of membrane separation and catalysis. These benefits include stable catalyst loading achieved through membrane structure manipulation, nanoconfinement, and effective degradation of organic pollutants. The application of catalytic membranes in water treatment is then thoroughly summarized, and they are separated into five main groups based on their unique catalytic reaction mechanisms: ozone catalytic membranes, photocatalytic membranes, electrocatalytic membranes, Fenton-type catalytic membranes, and persulfate catalytic membranes. The mechanisms and performance characteristics of each kind of CM are looked at in greater detail. Finally, research directions and future prospects for water treatment using catalytic membranes are proposed. This review provides recommendations for future research and development to ensure the effective use of catalytic membranes in water treatment, in addition to providing a thorough examination of the advancements made in their application in the treatment of various wastewaters.
1. Introduction
Industrialization has increased the complexity of wastewater composition from sectors such as textile, healthcare, and chemical manufacturing [1,2,3]. Industrial products like antibiotics, azo dyes, plastic compounds, and polycyclic aromatic hydrocarbons represent a serious threat to human health and the environment due to their high toxicity, environmental persistence, and bioaccumulation [4]. Advanced oxidation processes (AOPs), membrane separation, adsorption technology, and their combined applications are the mainstream water treatment technologies [5,6,7]. Among these, membrane separation technology stands out for its high separation efficiency, low energy consumption, and environmental sustainability [8]. This technology has been widely used in drinking water purification, wastewater treatment, and salinity wastewater desalination. However, traditional membrane technology relies on the primary physical screening methods, which restrict its capacity to simultaneously achieve deep degradation of pollutants [9]. At the same time, AOPs can also effectively oxidize non-biodegradable materials and eliminate hazardous and obstinate organic pollutants at room temperature. AOPs can produce reactive oxygen species and non-radical compounds (such as •OH, SO4•−, O2•−, and 1O2), which can purify water instead of just eliminating pollutants [10,11]. Furthermore, AOPs have surpassed the drawbacks of conventional wastewater treatment, including issues with secondary pollution and sensitivity to operational parameters like pH and temperature. However, conventional treatment technologies are no longer able to meet the current water needs due to the increase in the types and quantities of pollutants. Even the most sophisticated treatment techniques have a number of drawbacks, such as high energy consumption, the requirement for chemical additives, insufficient pollution removal, and the production of byproducts that are more dangerous than the original materials. Therefore, researchers are working to create efficient water purification systems that can effectively remove organic contaminants from water sources in order to avoid this scenario.
Catalytic membrane (CM) technology, which combines separation and degradation functions, has recently been used to overcome the drawbacks of traditional single-stage membrane processes. By combining the catalyst and membrane, in situ product separation can be achieved during the reaction process, which significantly increasing the reaction’s efficiency and selectivity. The CM technology is special because it can either disperse the catalyst in the membrane matrix or fix it to the membrane surface, creating an active layer with catalytic activity. Because of its selective permeability, this structure allows the CM to separate reactants and products while maintaining high catalytic activity in the chemical reaction. Furthermore, studies on the application of certain AOPs in conjunction with CM have advanced quickly. Membrane separation in conjunction with photocatalysis, electrocatalysis, Fenton/Fenton-like reactions, persulfate oxidation, and ozone treatment are examples of advanced oxidation processes that have demonstrated synergistic effects [12]. Higher removal rates of tolerant compounds, longer membrane stability, reduced pollution, and the elimination of toxic byproducts are some advantages of these processes [13]. However, there is still much to learn about the construction and performance of catalytic membrane reactors (CMRs) as well as the critical role membranes play in the oxidation and filtration of organic wastewater. Furthermore, the capacity of the membrane to support and separate catalysts should not be the exclusive focus of CMR research. This study requires a detailed examination of the processes of free radical generation and degradation in oxidation reactions. Significant progress in this field depends on determining how membrane separation technology and reactive oxygen species (AOPs) work in concert. The total of these effects is much less important than the sum of their individual effects. The main causes of these synergistic effects must be determined because increasing the effectiveness of wastewater treatment necessitates a careful examination of the mechanisms underlying the interactions between catalytic membranes and AOPs.
This article describes the four primary processes—size selection and adsorption, catalyst stabilization, nanoscale confinement, and reactive oxygen species (ROS) oxidation—that underpin the combined effects of membrane separation and catalysis. It also looks closely at the methods used to synthesize catalytic membranes. The effectiveness of CM in treating contaminated wastewater is improved by these mechanisms working in concert. This technology’s wide range of applications in water treatment has been demonstrated by its expansion from single-stage wastewater treatment to the control of complex pollutants. This article thoroughly examines the most recent developments in CM technology for water treatment and offers a thorough analysis of how these technologies address the drawbacks of conventional single-stage treatment, taking into account the pressing issues facing water treatment and the substantial benefits of this technology. The mechanisms, benefits, and drawbacks of photocatalysis, electro-catalysis, Fenton-like CM, persulfate catalytic membranes, and ozone catalytic membranes are examined in this article. Their potential to increase the effectiveness of pollutant removal, lessen environmental impact, and mitigate membrane fouling is also examined. Establishing a scientific foundation to support the useful implementation of catalytic membrane systems in the fight against new contaminants in aquatic environments is the goal of this review.
2. Construction and Mechanism of Catalytic Membranes
2.1. Construction of Catalytic Membranes
Three categories of CM preparation techniques can be distinguished based on how the catalyst and membrane are combined: blending, surface coating, and “bottom-up” precision synthesis. Phase inversion, vacuum filtration, and electrospinning are examples of blending techniques; spin coating, immersion deposition, and sol–gel are examples of surface coating techniques; and chemical grafting, chemical vapor deposition, and layer-by-layer assembly are examples of “bottom-up” synthesis techniques. A schematic diagram of a few CM preparation techniques is displayed in Figure 1. Significant variations exist in the pollutant removal efficiency, membrane flux variation trends, and membrane fouling characteristics of CM made using various techniques. The benefits and drawbacks of various CM preparation techniques are compiled through performance comparison and analysis, as indicated in Table 1.
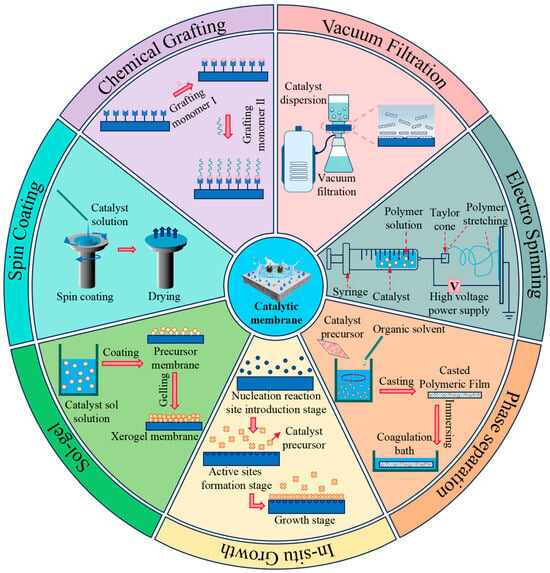
Figure 1.
Schematic diagram of different catalyst membranes preparation methods.
The catalytic filtration membrane can be prepared by phase inversion, electrospinning, or the sol–gel process once the catalyst or precursor solution has been thoroughly combined with the membrane-forming liquid by heating, melting, or ultrasonography. The type, loading amount, catalyst performance, and properties of the membrane-forming liquid are important aspects of the blending process. This process usually results in a catalytic membrane that is evenly dispersed throughout the membrane matrix [14]. The homogeneity of the catalyst in the membrane-forming liquid has a significant impact on the derived catalytic membrane’s performance [15]. The phrase “surface coating membrane process” refers to a method of surface functionalization that uses spin coating, impregnation precipitation, and vacuum filtration to coat the surface of a commercial membrane with a catalyst solution, suspension, or colloid. The type and thickness of the catalyst, the surface roughness of the initial membrane, and the coating method all have a substantial impact on the membrane flux, anti-fouling capability, and performance of the prepared CM [16]. A new technique for preparing CM is the bottom-up membrane process. Its fundamental idea is that, under particular circumstances, various catalyst precursors react to form active components on the membrane substrate surface. Afterwards, a CM with several layers of active components is progressively produced by regulating the quantity of reactions. Numerous bottom-up preparation techniques, such as layer-by-layer assembly, chemical grafting, and chemical vapor deposition, have been developed. The CM’s capacity to self-clean and effectively treat water is greatly influenced by the characteristics of the products produced by the in situ reactions, as well as the temperature and duration of the precursor reaction.

Table 1.
Advantages and disadvantages of different catalyst membranes preparation methods.
Table 1.
Advantages and disadvantages of different catalyst membranes preparation methods.
| Method of Preparation | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|
| Blending | Phase Inversion | Simple equipment; Simple operation | Limited capacity; time-consuming | [17] |
| Electrospinning | Controllable process; simple equipment | Requires a large amount of chemical reagents; Low product yield; Poor strength of the CM. | [18] | |
| Sol–Gel | The decorative layer is uniform. | Time-consuming and costly; poor catalytic stability due to delamination, cracking, and poor adhesion. | [19] | |
| Surface Coating | Spin Coating | Easy to operate; High density; Precise control of coating thickness | Severe paint waste; only suitable for simple flat film coatings; not suitable for mass production. | [20] |
| Immersion Deposition | Simple operation; easy control of preparation conditions | Due to the limitations of the wedge effect, thickness is uneven; the coating is unstable, and porosity is relatively low. | [21] | |
| Vacuum Filtration | Easy to prepare on a large scale; Simple operation process; Fast preparation speed. | High energy consumption; not suitable for coating dense base membranes | [22] | |
| Precision Synthesis | Chemical Grafting | Can suppress catalyst detachment; stable covalent bonds can form between the catalyst and the membrane surface. | High catalyst consumption; multiple and complex reaction steps | [23] |
| Chemical Vapor Deposition | Nanoscale catalyst coatings can be obtained; Coating thickness is easily controlled; No solvent addition is required during operation. | Uneven deposition may lead to impurities or defects on the film surface; residual gases after reaction may be toxic. | [24] | |
| Layer-by-Layer Assembly | Flexible operation with low cost; easy to control layer thickness | Requires reliance on cumbersome manual operations; Requires large quantities of polymer solutions, which may cause secondary pollution. | [25] | |
2.2. Synergistic Effects of Membrane Separation and Catalysis
CMRs technology offers a promising way to eradicate water pollutants and reduce membrane fouling by selectively separating organic pollutants while also degrading them. CMRs have major advantages over conventional membrane separation technologies in terms of wastewater reduction, membrane fouling prevention, and water purification. Additionally, the catalyst is immobilized within CMRs as opposed to single activated oxygen treatment processes (AOPs), which diminishes the catalyst’s dilution effect while simultaneously improving its effective contact with the reactants through forced convection within the pores. Thus, membrane filtration in conjunction with activated oxygen treatment techniques can effectively and sustainably address water pollution problems. As shown in Figure 2, CM-AOPs work in concert to degrade water pollutants in four primary ways: size screening and adsorption, stable catalyst loading, nanoconfinement, and activated oxygen oxidation.
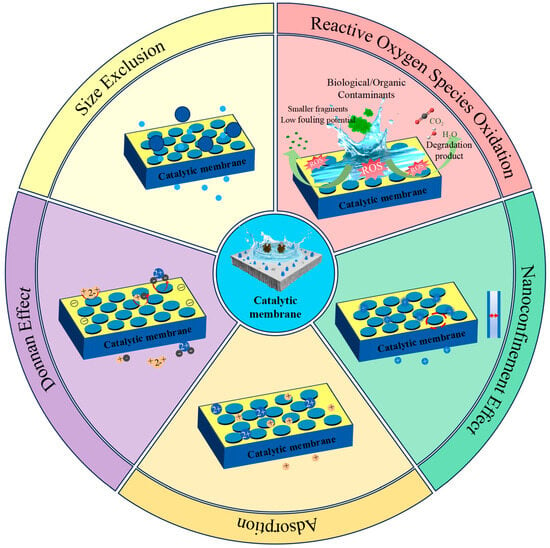
Figure 2.
Schematic diagram of synergistic effects between membrane separation and catalysis.
The ability to filter organic molecules selectively through size screening is an inherent benefit of porous membranes. One efficient solution to the selective catalytic oxidation issue is to incorporate catalysts into the membrane. Ozone-resistant organic pollutants can be eliminated more quickly thanks to catalysts’ ability to accelerate oxidation and increase ROS generation. Compared to conventional catalytic processes, CMs offer numerous benefits due to the high loading capacity of membrane materials. The intricate micro/nano channels found in the pores of the porous membrane give nano/microcatalysts a large loading area, preventing catalyst loss and producing a lot of micro/nano reactors [26]. Effectively preventing catalytic site passivation, this design can swiftly introduce target pollutants or oxidants and release degradation products right away. However, because of their brief lifetime in the aqueous phase, these ROS hinder the mass transfer process from the site of generation to the aqueous phase pollutants by reducing their availability [27]. To attain the optimal condition, embedding the catalyst into the narrow membrane pore structure and establishing a closed area for catalytic reaction within these channels can significantly boost the utilization efficiency of free radicals in the oxidation reaction [28]. The oxidation reaction’s rate of free radical utilization can be efficiently increased using this technique.
Organic contaminants on CM can be efficiently broken down by ROS, such as hydroxyl radicals (•OH) and peroxyl radical intermediates (•OOH) produced by AOPs [29]. The issue of decreased reactive oxygen scavenger efficiency in multi-pollutant water matrices is frequently disregarded, and the lifetime of ROS in the aqueous phase is severely constrained. When treating complex water matrices, membrane reactors have proven to have exceptional membrane tolerance and Fenton degradation capabilities for a range of common organic pollutants. By successfully blocking the entry of organic macromolecules with pore sizes greater than the membrane pores into the catalytic active sites, the CM design scheme can reduce the possibility of catalyst contamination and quenching of free radicals [30]. Under the influence of non-oxides (NOMs), reactive oxygen species (•OH) are produced, which degrades small pollutants that readily enter the internal pores [31]. To achieve selectivity in the catalytic process and lessen the effects of non-oxides, more research on more complex membrane design strategies is still required, despite the fact that this field has seen significant advancements.
3. Research Progress in Catalytic Membrane Applications
3.1. Photocatalytic Membrane
The issues of catalyst particle agglomeration, photocorrosion, and recycling challenges in conventional photocatalysis can be effectively addressed by incorporating photocatalysts into membrane materials. Specifically, pollutants can be eliminated concurrently using the dual processes of physical screening and in situ degradation when photocatalytic activity and membrane separation are combined. Organic pollutants can be efficiently broken down by a variety of highly oxidizing free radicals, including O2•− and •OH, which are produced in light [32]. The photocatalytic membrane’s selective barrier function can enhance photocatalytic efficiency by efficiently regulating reactant and product transport. For membrane materials to survive the oxidation of active free radicals in photocatalytic reactions, they typically need to possess strong thermal and chemical stability [33]. Physical and chemical stability, availability, affordability, oxidation capacity, and non-toxic qualities are important considerations when choosing photocatalysts for photocatalytic reactions. Ti3C2Tx and other two-dimensional MXene can be employed as a conductive layer to decrease the recombination of photogenerated carriers and increase the efficiency of electron transport. A novel multifunctional MXene-TiO2/CuO@PVDF nanomembrane for the treatment of complex oily wastewater was successfully developed by Imsong et al. [34]. The membrane has outstanding anti-fouling and oil-water separation properties. Additionally, the membrane’s degradation efficiency for organic pollutants surpasses 95% when exposed to visible light. Thus, combining several uses into one membrane material not only promotes the creation of next-generation water treatment technologies but also creates new opportunities for environmentally friendly wastewater treatment in practical settings.
The mechanisms of ROS generation and anti-pollution during photocatalysis are described using TiO2 as an example. The mechanism of light-induced antibacterial and self-cleaning on TiO2 surfaces is depicted in Figure 3. Although this process also produces electrons and holes, the reaction mechanism is not the same as the superhydrophilic mechanism. In particular, electrons react with adsorbed molecular oxygen to form O2•−, which then reacts with protons to form H2O2, whereas valence band holes react with water to form •OH. •OH has the ability to disinfect some bacteria and viruses and oxidize pollutants that are adhered to the membrane surface into non-toxic compounds like CO2 and water. Additionally, new fabrication techniques like 3D printing, atomic layer deposition, and electrospinning are frequently employed to distribute and load photocatalysts precisely within the membrane matrix, guaranteeing consistent catalytic activity and lowering the possibility of leaching. Table 2 lists the uses of photocatalytic membrane technology for the breakdown of organic contaminants in water. Even though photocatalytic membranes have demonstrated remarkable promise in eliminating obstinate contaminants like dyes, antibiotics, and persistent perfluorinated compounds (PFAS), there are still many obstacles in the way of their widespread use. The first concern is long-term stability. Degradation of membrane structure, surface deactivation, and photocatalyst leaching can result from complex wastewater environments and continuous illumination. Second is the problem of energy consumption. The economic viability of most photocatalytic processes is limited because they still rely on UV lamps or intense concentrated sunlight. In order to achieve this, recent studies have suggested combining photocatalytic membranes with renewable energy sources, including microbial fuel cells, solar light collection systems, and even piezoelectrically driven catalytic systems [35,36,37].
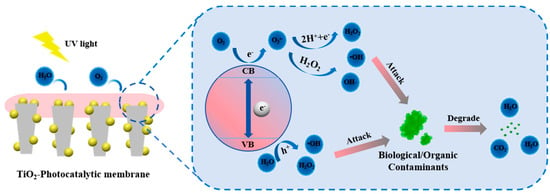
Figure 3.
Mechanism of self-cleaning and antibacterial properties in TiO2-Photocatalytic membrane.

Table 2.
Application of photocatalytic film technology for degrading organic pollutants in water.
3.2. Electrocatalytic Membrane
Electrocatalytic membrane technology has shown significant advantages and a broad range of potential uses in the removal of organic pollutants and the optimization of energy consumption. Studies have shown that electrocatalytic membranes—in particular, electrocatalytic microfiltration and ultrafiltration membranes—perform remarkably well in a variety of wastewater treatment scenarios. For instance, electrocatalytic membranes efficiently break down dye molecules in the treatment of wastewater containing dyes by producing active oxygen species (like •OH and Cl•) and accomplishing decolorization and mineralization [43]. Hu et al. [44] created a successful electrocatalytic filtration system to eliminate rhodamine B (RhB) from wastewater by employing a Ti/SnO2-Sb membrane as an anode. The membrane’s electrocatalytic active surface area is larger, its charge transfer resistance is lower, its oxygen evolution potential is higher, and its particle size is smaller. After 90 min of electrolysis, the electrocatalytic filtration system’s RhB removal rate was 90.29%. Using direct oxidation and indirect oxidation mechanisms, electrocatalytic membranes can quickly break down complex organic molecules into harmless small molecules in wastewater treatment of phenolic compounds, significantly increasing the removal rate of pollutants [45]. The rate of pollutant removal and energy consumption are two crucial parameters for the practical application of electrocatalytic membrane technology. As indicated in Table 3, the wastewater characteristics and the electrocatalytic membrane material have a major impact on efficiency and energy consumption. When treating complex wastewaters, the performance and energy consumption of various electrocatalytic membrane materials vary significantly. Future research should optimize electrocatalytic membrane materials based on wastewater characteristics to further improve pollution removal efficiency while reducing energy consumption. This will promote electrocatalytic membrane technology’s practical use.
Electrocatalytic membrane materials’ anti-pollution performance and service life are important metrics that impact their practical applications. The service life of the material is mostly determined by its electrochemical stability. By employing electrolysis, electrophoresis, and charge attraction and repulsion [46]. An external electric field can decrease membrane fouling and extend the electrocatalytic membrane’s service life, as shown in Figure 4. Membrane fouling can be reduced by changing the electrical characteristics of the electrocatalytic membrane’s surface with positive and negative potentials. This is because the electrical repulsion effect reduces the migration of charged pollutants from wastewater to the membrane surface [47]. However, if an excessively strong electrophoretic effect leads to excessively high ion concentrations at the counter electrode, which may result in concentration polarization and mixing effects, there is a higher risk of inorganic pollution [48]. In order to improve the anti-pollution performance, the electrocatalytic membrane can produce active oxygen species (like •OH) in situ and electrolyze bubbles to self-clean the membrane surface. This increases the membrane’s stability and service life. For instance, metal oxide membranes (like Ti4O7) have a strong anti-pollution ability and directly mineralize pollutants through high oxygen evolution potential; however, it is necessary to optimize the membrane surface structure to minimize pollutant accumulation under high pollution loads. Ti4O7 electrocatalytic membranes doped with graphene oxide nanoparticles showed a 2.5 to 2.8 increase in OH generation rate, which can effectively reduce membrane fouling [49]. Studies have shown that TiO2-BNTs/SnO2-Sb electrocatalytic membranes have strong anti-fouling ability; after 20 cycles, the transmembrane pressure difference only increased by 3 kPa when treating complex wastewater (reverse osmosis concentrate) [50]. Future complex wastewater treatment requirements will require surface modification, nanocatalyst loading, and multifunctional composite design to effectively balance the material’s long life and anti-fouling performance.
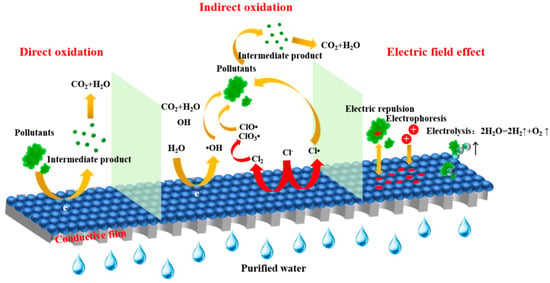
Figure 4.
Mechanism of electrocatalytic membrane processes.

Table 3.
Summary table of removal rates and energy consumption for electrocatalytic membranes on different pollutants.
Table 3.
Summary table of removal rates and energy consumption for electrocatalytic membranes on different pollutants.
| Materials | Pollutant Name and Concentration | Reaction Conditions | Decomposed Pollutant (%) | Energy Consumption | Ref. | ||
|---|---|---|---|---|---|---|---|
| Anode | Cathode | Electrolytes and Their Concentrations | Voltage/Current Density | ||||
| Ti/SnO2-Sb | Stainless steel mesh | RhB (50 mg/L) | Na2SO4 (0.1 M) | 10 (mA·cm−2) | 92.29 | 5.51 (kW·h·m−3) | [44] |
| TiO2-BNTs/ SnO2-Sb | Nickel mesh | Reverse osmosis concentrate (COD: 261~ 295 mg/L) | Cl− (1197~1248 mg·L−1) | 30 (mA·cm−2) | 69.1 | 10.5 [kW·h· (kg·COD)−1] | [50] |
| 1%GONs@7Ti4O7 | Ti/RuO2-IrO2 | 1,4-Dioxane (0.01 mg/L) | Cl− (28.40 ± 0.3 mg·L−1) | 20 (mA·cm−2) | 63.2 | 0.2~0.6 (kW·h·m−3) | [49] |
| Ti/Pd | Titanium mesh | Reverse osmosis concentrate (COD: 160~ 170 mg/L) | Cl− (900 mg·L−1) | 5 V | 82.3 | 45 [kW·h· (kg·COD)−1] | [51] |
| ZIF-67@ CNT/ACF | Titanium mesh | TC (10 mg/L) | Na2CO3 (10 mmol·L−1) | 3 V | 81 | - | [52] |
| PPy@CCM | Titanium plate | Phenol (50 mg/L) | Na2SO4 (2500 mg·L−1) | 2 V | 99.51 | 0.5 (kW·h·m−3) | [53] |
3.3. Fenton-Type Catalytic Membrane
The Fenton catalytic membrane’s capacity to break down organic pollutants, mainly wastewater from dyes, medications, and pesticides, has been extensively studied recently. Although it usually only treats synthetic wastewater containing a single dye, the Fenton method, also known as the Fenton-like oxidation process, is currently the most widely used membrane treatment technique for dye wastewater. The FeOCl-MoS2-coated layered membrane (F/M membrane) was created by Qu et al. [54], where they prepared F/M membrane can effectively and continuously degrade MB with a degradation efficiency of up to 100%. In some comparative studies, the photo-Fenton GO/M88 membrane developed by Xie et al. degrades two or more dyes, possesses photo-Fenton performance and can break down several dyes at a removal rate of more than 98%. [55]. According to the results, this new GO/M88A composite membrane can be used as a new membrane material for water purification and environmental protection applications because of its strong photo-Fenton activity, good stability, and excellent separation performance. Additionally, the ability of Fenton-type catalytic membranes to effectively treat wastewater with high pesticide concentrations has attracted a lot of attention. He et al. [56] synthesized a layered PVDF membrane containing zero-valent iron nanoparticles (Fe-HP) degraded 4-nitrophenol (4-NP) and 2,4-dichlorophenol (2-CP) at a rate of almost 100%, in less than 30 min. Furthermore, inside the membrane, the obstinate 4-NP and 2-CP molecules partially mineralized into CO2 and H2O. Neonicotinoid pesticides can be efficiently removed by molybdenum sulfide-modified ceramic membrane systems, and a degradation rate of over 85% of clothianidin can be attained by improving Fe2+ regeneration and O2 generation capabilities [43].
An essential part of removing heavy metals from water bodies is Fenton oxidation and related oxidation processes. Heavy metals can be effectively removed from wastewater using surface Fenton-type CMs. Oily wastewater discharge that does not adhere to national regulations will also degrade water quality, damage aquatic resources, and possibly endanger human health. Fenton-like CMs can effectively address the issue of membrane fouling and separate water and oil. Liu et al. [57] synthesized a bifunctional tubular polyvinyl chloride/SiO2/SiO2@Ag nanofiber membrane that combines dye catalytic degradation with oil. In addition to its exceptional oil-water separation capabilities, the CM exhibits effective catalytic degradation capabilities. There is a new concept for the creation of multifunctional oil-water separation membranes because the oil removal rate in the oil-water mixture is 96% and the catalytic degradation rate of MB in water is 95%. According to Table 4, Fenton-type CM technology is used in water treatment. Fenton-type CM’ operational performance is generally a crucial aspect that must be taken into account when using them in practice. However, additional testing is still required to confirm this type of membrane material’s antibacterial, heavy metal removal, and self-cleaning capabilities. As of right now, there is a dearth of research on the best Fenton reaction method and membrane separation solution for various industrial wastewater treatment scenarios, as well as no widely accepted guidelines or conclusions.

Table 4.
Application of Fenton-type catalytic membrane technology in water treatment.
3.4. Peroxymonosulfate-Catalyzed Membrane
Persulfate based CMs have shown great promise in the field of wastewater treatment and have been the subject of much recent research. This technology uses CMs to activate various ROS produced by peroxymonosulfate (PMS) or peroxydisulfate (PDS) in order to degrade organic pollutants such as dyes, phenols, and antibiotics. It makes effective use of membrane materials’ filtration capabilities in addition to catalytic technology’s degradation function. With good treatment efficiency, the catalyst loaded on the CM can efficiently maintain a high membrane flux, drastically lower the buildup of membrane fouling, and create a synergistic effect. Shan et al. [61] fabricated Cu-doped carbon SiO2 nanofiber membranes with large and medium pores, high mechanical strength, and good flexibility using electrospinning. Within 40 min, up to 95% of the tetracycline hydrochloride (TCH) was removed. Sun et al. Peroxidase (PDS) was activated using carbon nanotube hollow fiber membranes to generate free radicals for phenol degradation (97%), whereas phenol degradation efficiencies of 33% and 0% were attained by using carbon nanotube hollow fiber membrane filtration and PDS alone, respectively. This phenomenon demonstrated that phenol degradation was facilitated by the complementary action of membrane filtration and PDS activation.
Persulfate-catalyzed membrane technology’s uses in water treatment are listed in Table 5. Active free radicals produced by PACMS technology can also oxidize low-valent metal ions (like Mn2+ and Fe2+) into high-valent forms (like Mn4+ and Fe3+), which precipitate. As coagulants, these precipitates efficiently adsorb organic pollutants and inorganic anions, resulting in effective wastewater treatment. Furthermore, filtration performance can be enhanced and membrane fouling can be decreased by the large flocs formed. In actual use, persulfate-catalyzed membrane technology still has a number of obstacles to overcome. First, system performance is greatly influenced by the choice of catalysts and membrane material design. The creation of CMs is currently being extensively researched using materials like MOFs, LDHs, and transition metal oxides (like NiFe2O4). Though their scalability and preparation costs need to be further optimized, these materials show good stability and high catalytic activity. The activation mechanism of persulfate is complicated and involves a number of pathways, such as mixed, non-radical, and free radical mechanisms. For the purpose of improving treatment efficiency and CM design, a deeper comprehension of these mechanisms is essential. The stability, regeneration potential, and economic viability of the CM are additional important aspects that influence its broad use. Thus, to encourage the practical use of persulfate CM technology in water treatment, future research should concentrate on effective catalyst activation, optimized membrane material design, and methodical engineering applications.

Table 5.
Application of persulfate-catalyzed membrane technology in water treatment.
3.5. Ozone Catalytic Membrane
Ozone catalysis is the most promising technology for treating organic wastewater in a practical setting because of its rapid oxidation, mild conditions, and environmental friendliness. Over the past few years, research findings on combined filtration and ozone catalytic treatment technology have increased. Among the membrane materials used are polymer membranes and ceramic membranes (CM), whose main goals are to reduce organic pollution and achieve pollutant degradation. Due to the limited ozone resistance of polymer materials and the challenges associated with the deposition of metal oxides on their surfaces (such as affinity, etc.), there are comparatively few studies on the use of polymer membranes. Since PVDF membranes are the most widely used type of polymer membrane and can have a catalyst layer deposited on their surface, research on these membranes is currently concentrated on PVDF membranes. An increasingly robust research trend on the combined use of ceramic and ozone membranes has been observed in the scientific community. Through physical separation and ozone-driven chemical oxidation, ozone catalytic membrane technology has emerged as a novel approach to wastewater treatment [67]. The utilization rate of ozone can be greatly increased by applying ceramic membranes, which have been demonstrated to effectively use shear force to reduce the size of ozone bubbles, improve the gas–liquid mass transfer efficiency in the two-phase system, and encourage full contact between ozone and pollutants. The ceramic membrane surface can also be coated with catalysts like mixed oxides, Al2O3, TiO2, Fe2O3, MgO, and ZrO2. Because the catalyst anchors to the membrane, using ceramic membranes as carriers to load catalysts onto the membrane surface or within its pores not only increases catalytic activity sites, improves pollutant removal efficiency, and effectively prevents catalyst aggregation, but it also removes the need for post-reaction recovery. Additionally, by increasing the rate at which organic pollutants degrade, ozone treatment can greatly lower ceramic membrane contamination and prolong its useful life.
Traditional wastewater treatment techniques for phenol contain issues like membrane fouling, high catalyst loss, and poor treatment efficiency. Wang et al. [68] created a MnFe2O4/CM composite material by impregnating and calcining a ceramic membrane loaded with MnFe2O4. The membrane was operated with a pH of 9.0 and an ozone concentration of 100 mg/L. After five cycles, the COD removal could still maintain 88.03%, and the membrane flux stayed at 93.29%, demonstrating that the removal rate of phenol pollutants could reach 92.38%. This study demonstrated that the system’s anti-fouling capability was greatly increased in addition to its degradation efficiency. Additional investigation also revealed that •OH’s primary benefits are its strong anti-fouling properties and its dominant role in the degradation process. Table 6 illustrates the use of ozone catalytic membrane technology in water treatment, while Table 7 compares the benefits and drawbacks of various CM technologies. But even with the impressive research findings, there are still a lot of obstacles impeding the technology’s continued adoption and promotion. On the one hand, insufficient ozone gas–liquid mass transfer results in inadequate utilization, which lowers treatment efficiency overall. On the other hand, water containing bromine may produce dangerous byproducts like bromate, which could compromise the safety of the water quality. Moreover, ceramic membranes are expensive, whereas polymer membranes are more likely to age with prolonged use. Additionally, the cost of engineering applications is increased by the high energy consumption of ozone generators. Future research must progress in four areas to address these limitations: creating effective catalysts, refining membrane structures, combining various technologies, and bolstering economic and environmental evaluations to offer a viable foundation for the widespread use of ozone catalytic membranes.

Table 6.
Application of ozone catalytic membrane technology in water treatment.

Table 7.
Comparative analysis of performance advantages and disadvantages among different catalytic membrane technologies.
4. Conclusions
The primary advantages of CMs and the importance of their synergistic effects in water treatment are thoroughly explained in this review because CMs have attracted a lot of attention in the water treatment sector. Following a synthesis of the membrane separation and catalytic processes, design considerations for CMs involving the substrate membrane, catalytic materials, and fabrication techniques are discussed. The article then goes over recent advancements in membranes based on various catalytic types, including photocatalytic membranes, ozone-catalytic membranes, persulfate-catalytic membranes, Fenton-type membranes, and electrocatalytic membranes. Even though the creation of high-performance CMs has advanced significantly, more study is required in the following areas. In addition to catalytic performance, separation performance is an important metric for evaluating the performance of CMs. Although catalysts can increase the degrading capacity of traditional separation membranes, they can also clog pores or alter the physicochemical properties of the membrane surface (such as wettability, surface charge, and pore size), which can partially reduce the membrane’s ability to separate materials.
Additionally, organic membranes are more easily broken down by oxidizing species than inorganic materials, which weakens the membrane’s structure and reduces the effectiveness of the separation. Further development of chemically oxidatively stable membrane materials is therefore required because research on the stability of polymer membranes and other novel membrane materials under catalytic conditions is still lacking. It is also crucial to acknowledge the inconsistency of the current standards used to assess CM performance. The intrinsic flexibility of these membrane materials and the variations in model pollutants could be the cause of this disparity. Consequently, developing a comprehensive framework for CM evaluation is crucial.
Author Contributions
Conceptualization, K.J.S., J.D. and Y.S.; methodology, J.D., Y.Z. and Y.S.; resources, Y.S. and J.D.; data curation, J.D. and Y.S.; writing—original draft preparation, K.J.S. and Y.S.; writing—review and editing, J.D. and Y.S.; supervision, K.J.S. and Y.S.; project administration, J.D. and Y.S.; funding acquisition, J.D. and Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by National Key R&D Program of China (2024YFB4105500) and National Natural Science Foundation of China (51508268).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Jun Dai was employed by China Energy Investment Corporation Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Guan, Y.; Qiang, Y.; Li, B.; Zhang, N.N.; Xiao, Y.; Lu, W.T. Assessing the integrated environmental impacts and typological characteristics of water pollution and scarcity in Chinese cities. J. Hydrol. 2025, 661, 133662. [Google Scholar] [CrossRef]
- Lu, Y.J.; Zhou, X.Q.; Zheng, Y.; Yang, H.L.; Cao, W.B. How far do we still need to go with antibiotics in aquatic environments? Antibiotic occurrence, chemical-free or chemical-limited strategies, key challenges, and future perspectives. Water Res. 2025, 275, 123179. [Google Scholar] [CrossRef]
- Jiang, D.Y.; Nowack, B. Reconciling plastic release: Comprehensive modeling of macro- and microplastic flows to the environment. Environ. Pollut. 2025, 383, 126800. [Google Scholar] [CrossRef]
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Planet. Health 2022, 6, E535–E547. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Y.; Dai, M.; Wu, Y.N.; Peng, C.S. Emulsion system, demulsification and membrane technology in oil-water emulsion separation: A comprehensive review. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1254–1278. [Google Scholar] [CrossRef]
- Yi, M.; Xia, Q.; Tan, J.L.; Shang, J.W.; Cheng, X.W. Catalytic-separation technology for highly efficient removal of emerging pollutants, desalination, and antimicrobials: A new strategy for complex wastewater treatment. Chem. Eng. J. 2024, 493, 152568. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.Z. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants. Chem. Eng. J. 2021, 411, 128392. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, L.Z.; Wang, R.; Chou, S.R.; Dong, Z.L. A new integrated approach for dye removal from wastewater by polyoxometalates functionalized membranes. J. Hazard. Mater. 2016, 301, 462–470. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xiao, S.J.; Meng, X.G.; Yu, S.W. Research progress of MOF-based membrane reactor coupled with AOP technology for organic wastewater treatment. Environ. Sci. Pollut. Res. 2023, 30, 104958–104975. [Google Scholar] [CrossRef]
- Yao, Y.J.; Hu, H.H.; Zheng, H.D.; Wei, F.Y.; Gao, M.X.; Zhang, Y.Y.; Wang, S.B. Zn-MoS2 nanocatalysts anchored in porous membrane for accelerated catalytic conversion of water contaminants. Chem. Eng. J. 2020, 398, 125455. [Google Scholar] [CrossRef]
- Santos, E.N.; Fazekas, A.; Hodur, C.; Laszlo, Z.; Beszedes, S.; Firak, D.S.; Gyulavari, T.; Hernadi, K.; Arthanareeswaran, G.; Vereb, G. Statistical Analysis of Synthesis Parameters to Fabricate PVDF/PVP/TiO2 Membranes via Phase-Inversion with Enhanced Filtration Performance and Photocatalytic Properties. Polymers 2022, 14, 113. [Google Scholar] [CrossRef]
- Tan, H.B.; Zhang, Y.B.; Li, B.W.; Yang, H.; Hou, H.T.; Huang, Q.L. Preparation of TiO2-coated glass flat membrane and its photocatalytic degradation of methylene blue. Ceram. Int. 2023, 49, 17236–17244. [Google Scholar] [CrossRef]
- Huang, Z.H.; Zhang, X.; Wang, Y.X.; Sun, J.Y.; Zhang, H.; Liu, W.L.; Li, M.P.; Ma, X.H.; Xu, Z.L. Fe3O4/PVDF catalytic membrane treatment organic wastewater with simultaneously improved permeability, catalytic property and anti-fouling. Environ. Res. 2020, 187, 109617. [Google Scholar] [CrossRef] [PubMed]
- Muleja, A.A.; Mamba, B.B. Development of calcined catalytic membrane for potential photodegradation of Congo red in aqueous solution. J. Environ. Chem. Eng. 2018, 6, 4850–4863. [Google Scholar] [CrossRef]
- Chakraborty, S.; Loutatidou, S.; Palmisano, G.; Kujawa, J.; Mavukkandy, M.O.; Al-Gharabli, S.; Curcio, E.; Arafat, H.A. Photocatalytic hollow fiber membranes for the degradation of pharmaceutical compounds in wastewater. J. Environ. Chem. Eng. 2017, 5, 5014–5024. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Lu, D.W.; Xu, C.B.; Zhong, J.Y.; Chen, M.S.; Xu, S.; Cao, Y.; Zhao, Q.; Yang, M.; Ma, J. Synergistic oxidation—Filtration process analysis of catalytic CuFe2O4—Tailored ceramic membrane filtration via peroxymonosulfate activation for humic acid treatment. Water Res. 2020, 171, 115387. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.R.; Sanches, S.; Huertas, R.M.; Crespo, M.T.B.; Pereira, V.J. Treatment of a real water matrix inoculated with Aspergillus fumigatus using a photocatalytic membrane reactor. J. Membr. Sci. 2020, 598, 117788. [Google Scholar] [CrossRef]
- Zeng, H.J.; Yu, Z.X.; Shao, L.Y.; Li, X.H.; Zhu, M.; Liu, Y.C.; Feng, X.F.; Zhu, X.M. Ag2CO3@UiO-66-NH2 embedding graphene oxide sheets photocatalytic membrane for enhancing the removal performance of Cr(VI) and dyes based on filtration. Desalination 2020, 491, 114558. [Google Scholar] [CrossRef]
- Chen, W.; Ye, T.; Xu, H.; Chen, T.Y.; Geng, N.N.; Gao, X.H. An ultrafiltration membrane with enhanced photocatalytic performance from grafted N-TiO2/graphene oxide. RSC Adv. 2017, 7, 9880–9887. [Google Scholar] [CrossRef]
- Mehravar, S.; Fatemi, S.; Komiyama, M. Magnetic property and structural study of nickel supported on reduced graphene oxide prepared by chemical vapor deposition. Surf. Interface Anal. 2020, 52, 547–552. [Google Scholar] [CrossRef]
- Luo, J.; Chen, W.W.; Song, H.W.; Liu, J.R. Fabrication of hierarchical layer-by-layer membrane as the photocatalytic degradation of foulants and effective mitigation of membrane fouling for wastewater treatment. Sci. Total Environ. 2020, 699, 134398. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.B.; Fang, Q.; Wang, W.; Li, G.L.; Guan, J.M.; Shen, Y.; Ye, J.R.; Liu, F. Prussian blue/PVDF catalytic membrane with exceptional and stable Fenton oxidation performance for organic pollutants removal. Appl. Catal. B Environ. 2020, 273, 119047. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, G.; Liu, H.J.; Qu, J.H. Confining Free Radicals in Close Vicinity to Contaminants Enables Ultrafast Fenton-like Processes in the Interspacing of MoS2 Membranes. Angew. Chem. 2019, 58, 8134–8138. [Google Scholar] [CrossRef]
- Zhou, P.; Wan, J.F.; Wang, X.R.; Chen, J.; Gong, Y.G.; Xu, K. Three-Dimensional Hierarchical Porous Carbon Cathode Derived from Waste Tea Leaves for the Electrocatalytic Degradation of Phenol. Langmuir 2019, 35, 12914–12926. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, S.; Zhang, J.; Lu, J.H.; Shan, C.; Zhang, Y.Y.; Dionysiou, D.D.; Lv, L.; Pan, B.C.; Zhang, W.M. Enhancing the performance of Fenton-like oxidation by a dual-layer membrane: A sequential interception-oxidation process. J. Hazard. Mater. 2021, 402, 123766. [Google Scholar] [CrossRef]
- Xie, J.; Liao, Z.P.; Zhang, M.; Ni, L.H.; Qi, J.W.; Wang, C.H.; Sun, X.Y.; Wang, L.J.; Wang, S.B.; Li, J.S. Sequential Ultrafiltration-Catalysis Membrane for Excellent Removal of Multiple Pollutants in Water. Environ. Sci. Technol. 2021, 55, 2652–2661. [Google Scholar] [CrossRef]
- Qiu, Z.; Xiao, X.; Yu, W.T.; Zhu, X.Y.; Chu, C.H.; Chen, B.L. Selective Separation Catalysis Membrane for Highly Efficient Water and Soil Decontamination via a Persulfate-Based Advanced Oxidation Process. Environ. Sci. Technol. 2022, 56, 3234–3244. [Google Scholar] [CrossRef]
- Song, Y.F.; Li, Y.J.; Chen, X.M.; Meng, C.C.; Ma, S.F.; Li, T.M.; Jiang, K.; Hu, C. Simultaneous degradation and separation of antibiotics in sewage effluent by photocatalytic nanofiltration membrane in a continuous dynamic process. Water Res. 2023, 229, 119460. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, Z.H.; Graimed, B.H.; Ammar, S.H.; Sabit, D.A.; Najim, A.A.; Radeef, A.Y.; Taher, A.G. The latest progress in the design and application of semiconductor photocatalysis systems for degradation of environmental pollutants in wastewater: Mechanism insight and theoretical calculations. Mater. Sci. Semicond. Process. 2024, 173, 108153. [Google Scholar] [CrossRef]
- Imsong, R.; Ahmed, F.U.; Purkayastha, D.D. Tailoring MXene-based electrospun membranes with spindle-like structures for multifunctional oily wastewater treatment. Chem. Eng. J. 2025, 520, 165721. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, X.; Xu, X.F.; Wu, P.; Li, Z.Z.; Zhang, G.Y. Hierarchically structured CoAl-LDH derived hybrid membrane for synergistic solar-driven water purification and photocatalytic CO2 reduction. Desalination 2025, 613, 118992. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, X.X.; Hu, J.P.; Chen, S.J.; Hu, S.G.; Wu, Y.Q.; Liu, B.C.A.; Xiao, K.K.; Liang, S.; Yang, J.K.; et al. Efficient degradation of refractory pollutant in a microbial fuel cell with novel hybrid photocatalytic air-cathode: Intimate coupling of microbial and photocatalytic processes. Bioresour. Technol. 2021, 340, 125717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Q.; Li, X.; Gao, G.Q.; Sun, H.J.; Liu, X.F. The BiOBr/PVDF film prepared by in situ deposition and phase transformation has high piezo-photocatalytic performance for efficient degradation of Rh B. J. Mater. Sci. Mater. Med. 2025, 36, 1414. [Google Scholar] [CrossRef]
- Costa, I.G.F.; Ribeiro, S.; Nascimento, L.L.; Patrocinio, A.O.T.; Cardoso, V.L.; Batista, F.R.X.; Reis, M.H.M. Well-dispersed titanium dioxide and silver nanoparticles on external and internal surfaces of asymmetric alumina hollow fibers for enhanced chromium (VI) photoreductions. Environ. Sci. Pollut. Res. 2023, 30, 62508–62521. [Google Scholar] [CrossRef]
- Zhang, T.Q.; Guo, Z.F.; Huang, Z.T.; Zhu, L.Y.; Xie, Y.S.; Liu, B.X.; Yuan, K.K.; Jin, X.T.; Zhang, G.H.; Wang, X.Q. Performance-enhanced oxygen vacancy-rich C-TiO2 nanofiber membranes for dual photocatalytic/bactericidal water treatment. Sep. Purif. Technol. 2025, 377, 134169. [Google Scholar] [CrossRef]
- Zhou, H.D.; Jiang, S.Q.; Wang, X.; Zhi, X.H.; Zhang, Z.Y.; Li, X.Z.; Tai, Y.T.; Xu, X.X. Polydopamine-modified g-C3N4/TiO2/GO PVDF membranes for enhanced quinolone antibiotic photocatalytic removal. J. Water Process Eng. 2025, 76, 108280. [Google Scholar] [CrossRef]
- Chen, M.N.; Song, Q.Q.; Li, Z.K.; Bai, W.W.; Xu, M.Y.; Li, X.; Li, W.P.; Nan, H.Y.; Wang, J.; Zhang, Y.T.; et al. COFs functionalized self-cleaning loose nanofiltration membranes for efficient dye/salt separation. Desalination 2025, 593, 118206. [Google Scholar] [CrossRef]
- Li, S.T.; Zhuang, Y.; Wu, H.Z.; Sang, C.; Wang, L.K.; Pang, S.Y.; Yao, S.Y.; Yang, H.W.; Guo, Z.J.; Lu, L.; et al. Self-cleaning PDMS membranes via UV-triggered integration with photocatalytic MOF. J. Membr. Sci. 2025, 728, 124154. [Google Scholar] [CrossRef]
- Yi, Q.Y.; Li, Y.; Dai, R.B.; Li, X.S.; Li, Z.Y.; Wang, Z.W. Efficient removal of neonicotinoid by singlet oxygen dominated MoSx/ ceramic membrane-integrated Fenton-like process. J. Hazard. Mater. 2022, 439, 129672. [Google Scholar] [CrossRef]
- Hu, J.F.; Zhu, H.; Lin, M.; Wu, D.E.; Yao, J.G.; Sun, T.Y.; Ma, X.J.; Xia, Y.J. Highly-efficient removal of Rhodamine B using a flow-through electrocatalytic filtration system: Characteristics, efficiency and mechanism. J. Electroanal. Chem. 2023, 946, 117742. [Google Scholar] [CrossRef]
- Yu, J.F.; Wang, J.B.; Yu, H.J.; Hu, J.W.; Jiang, L.X.; Wang, W.Y. Development of Ti4O7 reactive electrochemical membrane and electrochemical oxidation of naphthols in aqueous solution. Process Saf. Environ. Prot. 2024, 182, 497–508. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Lee, S.; Tijing, L.; Shon, H.K.; Hong, S. Electrically conductive membrane for fouling control: Its mechanisms and applications. Desalination 2024, 578, 117445. [Google Scholar] [CrossRef]
- Li, N.; Wang, W.Y.; Ma, C.; Zhu, L.Y.; Chen, X.Y.; Zhang, B.J.; Zhong, C.L. A novel conductive rGO/ZnO/PSF membrane with superior water flux for electrocatalytic degradation of organic pollutants. J. Membr. Sci. 2022, 641, 119901. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Huang, G.H.; Li, Y.P.; Chen, X.J.; Yao, Y.; Ren, S.J.; Li, M.N.; Wu, Y.W.; An, C.J. Electrically conductive inorganic membranes: A review on principles, characteristics and applications. Chem. Eng. J. 2022, 427, 131987. [Google Scholar] [CrossRef]
- Li, W.; Xiao, R.L.; Xu, J.L.; Lin, H.; Yang, K.; He, K.C.; Tang, L.X.; Chen, J.; Wu, Y.P.; Lv, S.H. Interface engineering strategy of a Ti4O7 ceramic membrane via graphene oxide nanoparticles toward efficient electrooxidation of 1,4-dioxane. Water Res. 2022, 216, 118287. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Li, W.; Zhu, H.W.; Yu, J.T.; Wei, K.J.; Gao, Z.F.; Zhang, Y.H.; Chen, H.M.; Gu, L.K.; Han, W.Q. Electrochemical treatment of municipal reverse osmosis concentrates by a TiO2-BNTs/SnO2-Sb reactive electrochemical membrane. Sep. Purif. Technol. 2024, 331, 125726. [Google Scholar] [CrossRef]
- Ren, L.H.; Li, Y.; Guo, Y.; Yang, K.; Yi, Q.Y.; Wang, X.Y.; Wu, Z.C.; Wang, Z.W. Electrochemical oxidation of reverse osmosis concentrate using a pilot-scale reactive electrochemical membrane filtration system: Performance and mechanisms. J. Hazard. Mater. 2024, 465, 133315. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Zhang, H.Y.; Zhou, Y.; Li, C.J. Enhanced electrocatalytic degradation of tetracycline by ZIF-67@CNT coupled with a self-standing aligned carbon nanofiber anodic membrane. Nanotechnology 2024, 35, 145701. [Google Scholar] [CrossRef]
- Pan, Z.L.; Xu, S.; Xin, H.; Yuan, Y.; Xu, R.S.; Wang, P.C.; Yan, X.Q.; Fan, X.F.; Song, C.W.; Wang, T.H. High performance polypyrrole coated carbon-based electrocatalytic membrane for organic contaminants removal from aqueous solution. J. Colloid Interface Sci. 2022, 626, 283–295. [Google Scholar] [CrossRef]
- Qu, S.Y.; Wang, W.H.; Pan, X.Y.; Li, C.L. Improving the Fenton catalytic performance of FeOCl using an electron mediator. J. Hazard. Mater. 2020, 384, 121494. [Google Scholar] [CrossRef]
- Xie, A.T.; Cui, J.Y.; Yang, J.; Chen, Y.Y.; Lang, J.H.; Li, C.X.; Yan, Y.S.; Dai, J.D. Graphene oxide/Fe(III)-based metal-organic framework membrane for enhanced water purification based on synergistic separation and photo-Fenton processes. Appl. Catal. B Environ. 2020, 264, 118548. [Google Scholar] [CrossRef]
- He, Z.J.; Mahmud, S.; Yang, Y.; Zhu, L.J.; Zhao, Y.B.; Zeng, Q.Y.; Xiong, Z.; Zhao, S.F. Polyvinylidene fluoride membrane functionalized with zero valent iron for highly efficient degradation of organic contaminants. Sep. Purif. Technol. 2020, 250, 117266. [Google Scholar] [CrossRef]
- Liu, H.L.; Sun, Y.M.; Xu, H.Y.; Qin, Y.; Huang, Q.L.; Chen, K.K.; Shu, W.; Xiao, C.F. Dual-functional design of tubular polyvinyl chloride hybrid nanofiber membranes for the simultaneous oil/water separation and in-situ catalytic degradation. J. Membr. Sci. 2022, 661, 120955. [Google Scholar] [CrossRef]
- Song, Q.; Li, Y.H.; Xie, W.C.; Gao, C.F.; Liu, L.F.; Liu, B.C. Catalytic degradation of carbamazepine by metal organic frameworks (MOFs) derived magnetic catalyst Fe@PC in an electro-Fenton coupled membrane filtration system: Performance, pathway, and mechanism. Sep. Purif. Technol. 2023, 309, 122988. [Google Scholar] [CrossRef]
- Piao, H.W.; Zhao, J.; Liu, M.Y.; Zhang, S.J.; Huang, Q.L.; Liu, Y.; Xiao, C.F. Ultra-low power light driven lycopodium-like nanofiber membrane reinforced by PET braid tube with robust pollutants removal and regeneration capacity based on photo-Fenton catalysis. Chem. Eng. J. 2022, 450, 138204. [Google Scholar] [CrossRef]
- Zhang, L.P.; Liu, Z.; Zhou, X.L.; Zhang, C.; Cai, Q.W.; Xie, R.; Ju, X.J.; Wang, W.; Faraj, Y.; Chu, L.Y. Novel composite membranes for simultaneous catalytic degradation of organic contaminants and adsorption of heavy metal ions. Sep. Purif. Technol. 2020, 237, 116364. [Google Scholar] [CrossRef]
- Shan, H.R.; Dong, X.Y.; Cheng, X.T.; Si, Y.; Yu, J.Y.; Ding, B. Highly flexible, mesoporous structured, and metallic Cu-doped C/SiO2 nanofibrous membranes for efficient catalytic oxidative elimination of antibiotic pollutants. Nanoscale 2019, 11, 14844–14856. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Yu, Y.L.; Yang, Y.; Sun, T.J.; Dong, S.J.; Yang, H.L.; Liu, Y.M.; Fan, X.F.; Song, C.W. Improved separation performance of carbon nanotube hollow fiber membrane by peroxydisulfate activation. Sep. Purif. Technol. 2021, 276, 119328. [Google Scholar] [CrossRef]
- Zhao, X.F.; Yi, X.B.; Song, J.J.; Yuan, X.Y.; Yu, S.M.; Nie, Y.H.; Zhang, J.; Cao, G.G. Mesoporous and flexible polyimide aerogel as highly active catalytic membrane for AO7 degradation by peroxymonosulfate activation. Chem. Eng. J. 2022, 431, 134286. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Zhong, L.L.; Zhang, Z.Q.; Li, H.R.; Shi, W.X.; Cui, F.Y.; Wang, W. Gravity driven ultrafast removal of organic contaminants across catalytic superwetting membranes. J. Mater. Chem. A 2017, 5, 25266–25275. [Google Scholar] [CrossRef]
- Du, X.; Zhang, K.M.; Xie, B.H.; Zhao, J.; Cheng, X.X.; Kai, L.; Nie, J.X.; Wang, Z.H.; Li, G.B.; Liang, H. Peroxymonosulfate-assisted electro-oxidation/coagulation coupled with ceramic membrane for manganese and phosphorus removal in surface water. Chem. Eng. J. 2019, 365, 334–343. [Google Scholar] [CrossRef]
- Du, X.; Yang, W.P.; Liu, Y.; Zhang, W.X.; Wang, Z.H.; Nie, J.X.; Li, G.B.; Liang, H. Removal of manganese, ferrous and antibiotics from groundwater simultaneously using peroxymonosulfate-assisted in-situ oxidation/coagulation integrated with ceramic membrane process. Sep. Purif. Technol. 2020, 252, 117492. [Google Scholar] [CrossRef]
- Ma, D.R.; Lian, Q.Y.; Zhang, Y.X.; Huang, Y.J.; Guan, X.Y.; Liang, Q.W.; He, C.; Xia, D.H.; Liu, S.W.; Yu, J.G. Catalytic ozonation mechanism over M1-N3C1 active sites. Nat. Commun. 2023, 14, 7011. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, Y.Z.; Zhao, D.R.; Liu, C.; Dong, P.H.; Gu, H.X. Research on MnFe2O4 Modified Ceramic Membrane Catalyzed Ozone Oxidation Treatment of Phenol-Containing Wastewater. Water Air Soil Pollut. 2025, 236, 568. [Google Scholar] [CrossRef]
- Qian, K.; Meng, L.Y.; Zhang, G.Y.; Li, J.; Lyu, J.Z. Neural network-like CeO2-loaded polyvinylidene fluoride membrane for tri-phase interface catalytic ozonation of antibiotics. J. Environ. Chem. Eng. 2025, 13, 117757. [Google Scholar] [CrossRef]
- Zhang, J.L.; Yu, H.T.; Quan, X.; Chen, S.; Zhang, Y.B. Ceramic membrane separation coupled with catalytic ozonation for tertiary treatment of dyestuff wastewater in a pilot-scale study. Chem. Eng. J. 2016, 301, 19–26. [Google Scholar] [CrossRef]
- Han, L.W.; Liu, J.; Li, F.; Zhao, Y.Y.; Guo, X.F.; Wang, S.Z.; Ji, Z.Y. CoMn-MOF-74 coated ceramic membranes for catalytic ozonation of azo dye in wastewater by membrane dispersion—Membrane catalysis process. J. Environ. Chem. Eng. 2025, 13, 116612. [Google Scholar] [CrossRef]
- Bai, H.K.; Liang, L.L.; Cao, P.K.; Zhang, H.G.; Chen, S.; Yu, H.T.; Quan, X. MgAl2O4 incorporated catalytic ceramic membrane for catalytic ozonation of organic pollutants. Appl. Catal. B Environ. 2024, 343, 123527. [Google Scholar] [CrossRef]
- Chen, P.; Cheng, Z.L.; Zhang, X.; Zhang, L.P.; Zhang, X.Z.; Tang, J.S.; Qiu, F.C. Efficient degradation of dye wastewater by catalytic ozonation reactive ceramic membrane with facile spraying of nano Ti-Mn oxides: A pilot scale attempt. J. Water Process Eng. 2023, 55, 104143. [Google Scholar] [CrossRef]
- Feng, T.T.; Wang, B.; Li, J.; Wang, T.; Huang, P.; Xu, X.Y. Metal-organic framework based photocatalytic membrane for organic wastewater treatment: Preparation, optimization, and applications. Sep. Purif. Technol. 2025, 355, 129540. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).