Abstract
The increasing demand for sustainable chemical processes has fostered the development of advanced catalytic systems for biomass valorization. In this work, a series of mono-, bi-, and trimetallic oxides (FeO, FeCuO, FeZnO, and FeCuZnO) were successfully synthesized using MIL-101-based MOFs as sacrificial templates. The obtained materials were thoroughly characterized by N2 adsorption–desorption, XRD, FTIR, and TEM/STEM-EDX to investigate their structural, morphological, and textural properties. Their catalytic performance was evaluated in the selective oxidation of benzyl alcohol, a lignin-derived platform molecule, into benzaldehyde under microwave irradiation as a sustainable heating strategy. The results demonstrate that MOF-derived oxides exhibit superior activity compared to their parent MOFs, highlighting the beneficial effect of thermal treatment on the exposure of active sites. Among the catalysts, heterometallic oxides showed enhanced performance due to synergistic effects between metals. In particular, FeZnO reached a maximum yield of 62.1% towards benzaldehyde at 150 °C and 30 min, outperforming the monometallic oxide. Recycling tests revealed that FeZnO retained higher overall performance than FeCuO, which suffered from progressive copper leaching. These findings confirm the potential of MOF-derived multimetallic oxides as efficient and reusable heterogeneous catalysts for selective biomass-derived alcohol oxidation. The combination of microwave-assisted processes and the tuneable nature of MOF-derived oxides provides a promising pathway for designing sustainable catalytic systems with industrial relevance.
1. Introduction
The contemporary era has been characterised by significant population growth and urbanisation, which have led to a sharp increase in both energy demand and chemical production as compared to previous decades. To meet these demands, the chemical industry has traditionally relied on fossil fuels such as oil, coal and natural gas. However, these non-renewable resources are responsible for more than 75% of global greenhouse gas emissions, thus, contributing substantially to climate change and environmental degradation [1]. This has intensified the search for sustainable alternatives.
Among the different options, lignocellulosic biomass has attracted considerable attention due to its abundance, global availability, and potential to be transformed into a wide range of valuable products [2,3]. Biomass is regarded as carbon-neutral, since the CO2 released during its transformation is reabsorbed by photosynthetic organisms, enabling a short carbon cycle [4,5]. Importantly, lignocellulosic biomass does not compete with food production, making it one of the most promising feedstocks for sustainable chemical processes. It is mainly composed of cellulose (40–50%), hemicellulose (20–30%), and lignin (20–25%), with minor contributions from proteins, lipids, soluble sugars, and minerals [5,6].
While cellulose and hemicellulose are carbohydrate polymers, lignin is a complex hydrophobic polymer formed from p-coumaryl, coniferyl, and sinapyl alcohols. Its predominant linkage, the β-O-4 bond, represents 60–70% of its structure [7,8,9]. Controlled depolymerisation of lignin enables the release of simple aromatic molecules that can serve as platform compounds [8]. Among these, benzyl alcohol is especially relevant, since it can be selectively oxidised to benzaldehyde, an intermediate of broad industrial application in perfumes, dyes, pharmaceuticals, and agrochemicals [10].
Traditionally, benzaldehyde has been obtained through hydrolysis of benzyl chloride [11] or direct oxidation of toluene [12,13]. However, these processes present severe drawbacks, including toxic by-products and contamination of the final product. As a result, heterogeneous catalysis has emerged as a more sustainable strategy, enabling selective oxidations under milder conditions with reduced environmental impact [5].
MOFs are coordination polymers in which metal centres are connected by organic ligands to form ordered one-, two-, or three-dimensional structures [3,14]. Their exceptional crystallinity, porosity, and tuneable surface properties make them versatile for a wide range of applications, including gas storage, separation, sensing, drug delivery, and catalysis [3,14,15,16,17,18,19,20,21].
Nevertheless, pristine MOFs often suffer from limited chemical and thermal stability, which restrict their direct use in catalysis. To address this limitation, MOFs are increasingly employed as sacrificial templates to generate more robust functional materials, such as metal oxides, metal nanoparticles embedded in carbon matrices, or single-atom catalysts (SACs) [3,22,23]. These MOF-derived materials combine the morphological advantages of the parent frameworks with improved durability, while offering higher surface areas, more accessible active sites, and enhanced catalytic stability [24,25,26].
Therefore, MOF-derived materials lead to the formation of their respective metal oxides upon carbonisation. Historically, metal oxides have served as excellent heterogeneous catalysts in a wide variety of reactions, as summarised in Table 1.

Table 1.
Oxide catalysts in heterogeneous catalysis.
In this context, the synthesis of MOF-derived metal oxides provides a promising route to fabricate efficient catalysts for selective oxidation. During thermal treatment, the organic ligands decompose, leading to the formation of oxides with high specific surface areas and increased numbers of active centres. Such materials frequently outperform their MOF precursors due to improved stability and catalytic efficiency [23,25,26].
In this work, MOFs and their corresponding oxides were synthesised and evaluated as catalysts for the selective oxidation of benzyl alcohol into benzaldehyde under microwave-assisted conditions. Microwave irradiation was chosen as a sustainable alternative to conventional heating, since it allows achieving comparable results in significantly shorter times owing to its homogeneous and efficient energy transfer [32]. In line with the principles of green chemistry, we focused on earth-abundant and low-cost metals, avoiding the use of noble metals such as Pt, Pd, Au, Ag, Ru, or Rh, which are traditionally applied in alcohols oxidation [33,34,35]. Iron-based catalysts were selected because Fe is inexpensive, abundant, and able to generate reactive oxygen species (ROS) in the presence of oxidants, thereby promoting selective oxidation [36].
To further enhance catalytic efficiency, recent studies have emphasised the importance of synergistic interactions between multiple metals. Incorporating two or more metals into a catalyst can improve redox behaviour, surface acidity, and stability. In this study, bi- and trimetallic MOFs were synthesised and transformed into the corresponding oxides through calcination, exploiting these synergistic effects [37,38]. Notably, Fe has demonstrated cooperative behaviour when combined with Cu [39] or Zn [40], suggesting that Fe–Cu–Zn systems could offer superior catalytic performance.
To contextualise the relevance of this research, Table 2 summarises representative studies from the literature on the selective oxidation of benzyl alcohol to benzaldehyde, highlighting catalysts, reaction conditions, and yields achieved.

Table 2.
Catalysts and reactions carried out for the selective oxidation of benzyl alcohol.
Therefore, the aim of this research was to synthesise and characterise mono-(Fe), bi-(Fe–Cu, Fe–Zn), and trimetallic (Fe–Cu–Zn) MOFs and their derived oxides, and to evaluate their performance in the microwave-assisted selective oxidation of benzyl alcohol. Special emphasis was placed on verifying that oxide derivatives outperform their respective MOF precursors, in agreement with previously reported in the literature, and on elucidating the role of synergistic interactions in multimetallic systems.
2. Results and Discussion
2.1. Characterization Results
2.1.1. N2 Physisorption
Figure 1 shows the N2 adsorption–desorption isotherms (−196 °C) of the synthesised MOFs and their corresponding derived oxides. In each case, the isotherms of the parent MOF (light trace) and the calcined oxide (dark trace) are compared, grouped according to metal composition. According to the IUPAC classification, MOFs usually display type I isotherms, characteristic of microporous materials. However, in all cases studied, type IV isotherms were observed for both MOFs and their calcined derivatives, indicative of mesoporous materials’ behaviour.
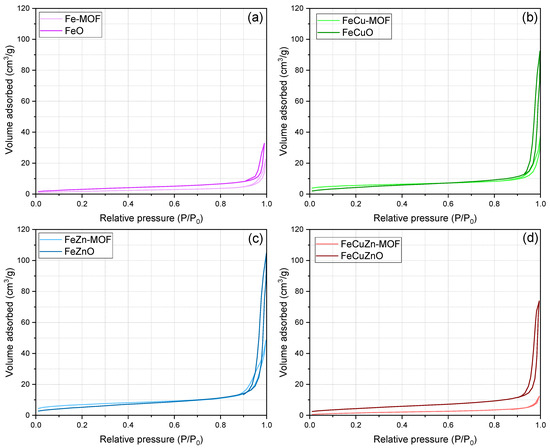
Figure 1.
Adsorption-desorption N2 isotherms of MOFs and metallic oxides. (a) Fe, (b) FeCu, (c) FeZn and (d) FeCuZn.
As shown in Figure 1, the metal oxides adsorb significantly larger N2 volumes than their MOF precursors, most likely due to enhanced pore accessibility after thermal treatment. This phenomenon can be attributed to the removal of organic linkers and the concomitant formation of interparticle porosity [46].
In addition, the bimetallic and trimetallic oxides (FeCuO, FeZnO, FeCuZnO) exhibit isotherms with similar profiles and higher N2 volume adsorbed as compared to the monometallic oxide (FeO). This suggests that the incorporation of more than one metal contributes to an increased pore volume.
Table 3 summarises the specific surface area, total pore volume, and mesopore volume for all the samples, calculated from the N2 adsorption–desorption isotherms of the synthesised materials.

Table 3.
Values obtained from adsorption isotherm range of surface area, total pore volume and mesopore volume.
As anticipated from the isotherms shown in Figure 1, the surface area values obtained for the MOFs are significantly lower than those reported in the literature for MIL-101-type structures [47]. In contrast, the derived metal oxides exhibit higher surface areas than their respective MOF precursors. Unexpectedly, this behaviour is opposite to the expected decrease in surface area typically associated with framework collapse upon calcination. Such a discrepancy can be rationalised by considering the synthesis conditions described previously: specifically, the substitution of hydrofluoric acid (HF) with hydrochloric acid (HCl) may have affected the integrity and porosity of the material. While HF promotes the formation of well-defined, highly porous structures due to its strong nucleation capacity, HCl lacks the same efficiency [48].
A general increase in both total and mesopore volume is also observed after thermal treatment and transformation of the MOFs into oxides. This effect is particularly pronounced in the bimetallic and trimetallic oxides and can be attributed to the generation of interparticle porosity upon removal of the organic linker.
2.1.2. X-Ray Diffraction
Figure 2 depicts the diffraction patterns of MOFs (Figure 2a) and oxides derived from them (Figure 2b).
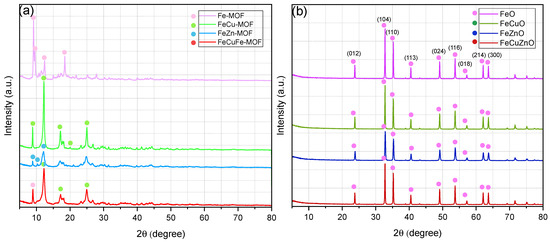
Figure 2.
X-Ray diffraction peaks of (a) Fe, FeCu, FeZn and FeCuZn MOFs and (b) derived-MOFs oxides.
Figure 2 shows the XRD patterns of the synthesised MOF-type materials. The diffraction peaks observed match the characteristic crystallographic pattern of Fe-MIL-101 reported in the literature, with intense reflections at 2θ = 9.0°, 9.5°, 12.5°, and 18.4° [49,50].
Upon incorporation of a second metal, such as copper (FeCu-MIL-101), new and more intense peaks appear at 2θ = 8.8°, 12.2°, 17.2°, 19.9°, and 25.0°. This behaviour can be attributed to competition between Cu and Fe for coordination with the organic ligand, resulting in structural modifications in which the crystalline contribution of the Cu-MOF becomes dominant [49]. A similar trend is observed for the other bimetallic MOF (FeZn-MIL-101), where the main reflections (2θ = 9.0°, 10.3°, and 12.1°) correspond to the Zn-MOF structure. However, in this case the signal intensity is lower and the peaks are broader, suggesting a more amorphous character. This loss of structural order may arise from two phenomena: (i) the “breathing effect,” in which the framework expands or contracts due to hydrogen-bonding interactions with the solvent (DMF), causing significant variations in the unit cell; and (ii) crystalline disorder associated with the simultaneous incorporation of metal ions of different ionic ratio [51,52]. Finally, the trimetallic FeCuZn-MIL-101 displays an intermediate diffraction pattern between those of the bimetallic phases, exhibiting greater disorder than FeCu-MIL-101 but more structural organisation than FeZn-MIL-101. This behaviour may be linked to distortions induced by the simultaneous incorporation of three cations; in particular, the reduced crystallinity appears to be mainly associated with Zn, consistent with the behaviour previously observed.
Overall, the results suggest that the incorporation of more than one metal does not lead to the formation of a new crystalline phase, but rather to the coexistence of phases corresponding to the individual monometallic MOFs, which appear to be well integrated within the same material as happened in other similar studies [15].
Figure 2b presents the XRD patterns of the MOF-derived oxides. All samples display a defined crystalline structure, with reflections at 2θ = 24.0°, 33.0°, 36.0°, 41.0°, 49.0°, 54.0°, 58.0°, 62.0°, and 64.0°, corresponding to the (012), (104), (110), (113), (024), (116), (018), (214), and (300) planes, respectively [53]. These reflections are characteristic of hematite (α-Fe2O3), indicating that the dominant crystalline phase in all oxides is determined by the calcination of the Fe-MOF precursor. In contrast, Cu and Zn oxides appear to be structurally amorphous, since no characteristic diffraction peaks of these compounds were detected.
2.1.3. FTIR-ATR
Attenuated total reflectance Fourier-transform infrared spectroscopy (FTIR-ATR) was employed to investigate the surface structure of the synthesised materials. The corresponding spectra are shown in Figure 3.
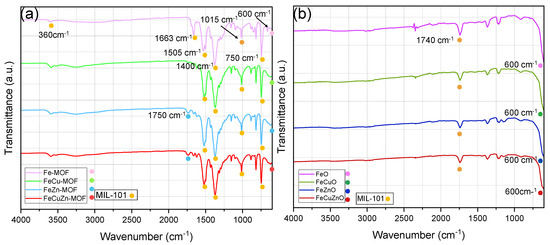
Figure 3.
FTIR-ATR spectra of (a) Fe, FeCu, FeZn and FeCuZn MOFs and (b) derived-MOFs oxides.
Figure 3a shows the FTIR-ATR spectra of the synthesised MOF-type materials. In all cases, characteristic bands associated with MIL-101-type structures are observed, consistent with the presence of the terephthalic acid linker used in the synthesis. Specifically, the bands at 750 and 1015 cm−1 correspond to C–H bending vibrations, while the band at 1505 cm−1 confirms the presence of C=C stretching modes of the aromatic ring. In addition, the bands at 1400 and 1600 cm−1 are attributed to the symmetric and asymmetric stretching vibrations, respectively, of the carboxylate group (–COO−). The band at 1663 cm−1 is related to C=O stretching of uncoordinated carboxyl groups [45]. Interestingly, for the Zn-containing materials (FeZn-MIL-101 and FeCuZn-MIL-101), an additional band at 1750 cm−1 is detected, which can be assigned to the C=O stretching of free carboxylic acid groups (COOH), possibly indicating the presence of non-coordinated groups or residual solvent interactions. Finally, weak bands at around 600 cm−1 are associated with metal–oxygen (M–O) vibrations [15,54]. Overall, these results confirm a similar organic surface structure for all MOFs, in line with the use of the same organic linker in their synthesis, and are consistent with the structural features of MIL-101-type frameworks.
For the thermally treated materials, shown in Figure 3b, no bands corresponding to the organic groups of H2BDC are observed, confirming their removal after high-temperature calcination. A weak band at approximately 1740 cm−1 is still present, associated with residual C=O vibrations, which may arise from incomplete decomposition of the linker. This suggests that higher calcination temperatures or longer treatment times could be required to ensure total removal of organic moieties. Finally, all derived oxides exhibit a band around 600 cm−1, corresponding to M–O stretching vibrations [15].
2.1.4. HR-TEM
To investigate the particle morphology of the synthesised materials, the bimetallic and trimetallic oxides were analysed by high-resolution transmission electron microscopy (HR-TEM). The corresponding images are shown in Figure 4.
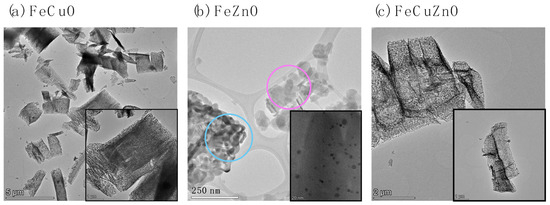
Figure 4.
HR-TEM images of (a) FeCuO, (b) FeZnO and (c) FeCuZn catalysts.
For the bimetallic FeCuO oxide (Figure 4a), well-defined cylindrical structures with large diameters, regular edges, and compact morphology can be distinguished. The magnified image (bottom right inset) reveals a rough surface, attributed to the loss of organic ligands during calcination, which results in the formation of superficial porosity [15].
In contrast, the incorporation of zinc in FeZnO (Figure 4b) leads to the development of two clearly differentiated structures. In the regions where iron is predominant (highlighted in blue), dense and irregular aggregates are observed. In the Zn-rich regions (marked in fuchsia and enlarged in the lower-right inset), smaller and more dispersed iron particles were detected, which may be related to a dispersion effect promoted by Zn during oxide formation.
Finally, the trimetallic FeCuZnO oxide (Figure 4c) again exhibits thick cylindrical structures, similar to those of FeCuO. This suggests that copper acts as the dominant structural element, favouring the formation of a more homogeneous and compact network.
2.1.5. STEM-EDX
STEM-EDX analysis was performed on the same materials studied by HR-TEM in order to evaluate the spatial distribution of metals in the catalysts. The technique was applied to the FeCuO, FeZnO, and FeCuZnO oxides to assess the dispersion of the metallic elements within each sample. The complete elemental mapping (C, O, Fe, Cu, and Zn) is provided in Figure A1 in Appendix A.
For FeCuO (Figure 5a), iron is predominantly distributed across the entire surface of the material, with copper present to a lesser extent. This observation suggests that Fe is the dominant element both structurally (as confirmed by XRD) and at the surface level in the final material.

Figure 5.
STEM images of (a) FeCuO, (b) FeZnO and (c) FeCuZn catalysts.
In contrast, for FeZnO (Figure 5b), zinc is mainly located on the outer regions of the catalyst, while iron appears more confined in the interior. This result indicates that, despite following a similar synthetic protocol, the metals distribution in the overall material depends on their intrinsic nature and structural affinity, reflecting differences in their behaviour during the oxide formation.
For the trimetallic FeCuZnO oxide (Figure 5c), a mixed distribution of the three metals is observed. Certain regions resemble the distribution found in FeZnO, whereas in the majority of the material the configuration is closer to that of FeCuO. These analyses together with those obtained by HR-TEM suggests that copper plays a dominant role in controlling the structural and morphological organisation of the system, as its presence is directly associated with the cylindrical structures and the prevailing metallic distribution when the three metals are combined.
2.2. Catalytic Results
2.2.1. Comparation Between MOFs and Derived Metallic Oxides
The evaluation of the catalytic performance of the as-synthesised MOFs materials and their corresponding metal oxides was carried out using the selective benzyl alcohol oxidation to benzaldehyde. Typically, catalytic tests were carried out under the following standard conditions: 120 °C, 30 min, 0.1 M benzyl alcohol in acetonitrile, and a substrate-to-oxidant molar ratio of 1:4. In addition, control experiments were performed in the absence of both catalyst and oxidant, as well as in the absence of either one individually. In all cases, negligible conversion was observed; therefore, these results are not included in the figures. Thus, these controls highlight the essential role of the catalyst and of TBHP as oxidant, since its absence or use in insufficient proportions (substrate/oxidant < 1:4) resulted in nearly null conversion and selectivity.
The results related to the catalytic activity of the evaluated material in the microwave-assisted benzyl alcohol oxidation reaction are summarised in Figure 6, which shows, for each catalyst, the conversion of benzyl alcohol (blue line) together with the selectivity to benzaldehyde and benzoic acid (stacked bars).
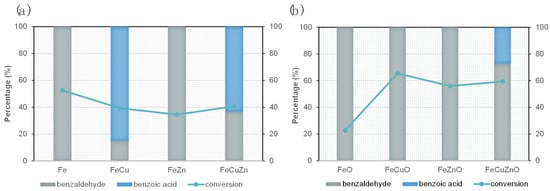
Figure 6.
Conversion and selectivity in the oxidation reaction catalyzed by (a) MOFs and (b) oxides. Reaction conditions: 30 min, 120 °C, 0.1 M benzylic alcohol in ACN (molar relation BA/TBHP 1:4).
The catalysts screening showed higher conversion values for the metal oxides (Figure 6b) as compared to their respective MOF precursors (Figure 6a), confirming that MOF-derived oxides exhibit superior catalytic activity, in agreement with previous reports [24]. This enhancement can be attributed to the removal of the organic linker during calcination, which increases the accessibility of exposed metallic active sites. The lower performance achieved by the MOF materials may also be related to their reduced crystallinity and specific surface area, as evidenced by the XRD patterns (Figure 2) and N2 adsorption–desorption measurements (Table 2). These structural and textural limitations result in reduced access to active sites and, consequently, lower catalytic activity.
It is also noteworthy that the oxides display higher selectivity towards the desired product, benzaldehyde, whereas the MOFs tend to over-oxidise the substrate to benzoic acid. This behaviour may be associated with the stronger oxidative character of the MOFs, which is detrimental to selectivity in this reaction, thereby positioning the oxides as more promising candidates for optimising the catalytic conditions.
Within the group of metal oxides (Figure 6b), the bimetallic and trimetallic catalysts (FeCuO, FeZnO, FeCuZnO) exhibit clearly higher conversions than the monometallic FeO, evidencing a positive synergistic effect between the combined metals. These results reinforce the hypothesis that the incorporation of a second or third metal enhances catalytic activity [37,39]. Based on these findings, and given the superior performance of the oxides compared to MOF materials, the rest of this study focused exclusively on the comparative analysis and optimisation of the reaction conditions for the MOF-derived oxides.
2.2.2. Study of the Effect of Temperature on Conversion and Product Selectivity
To evaluate whether the initially selected temperature (120 °C) was optimal for the catalytic process, and to analyse the response of the catalytic system to this parameter, additional experiments were performed at 90 and 150 °C while keeping the other experimental conditions constant (30 min, 0.1 M benzyl alcohol, AB/TBHP molar ratio 1:4). The results are presented in Figure 7 (a: 90 °C, b: 120 °C, c: 150 °C).
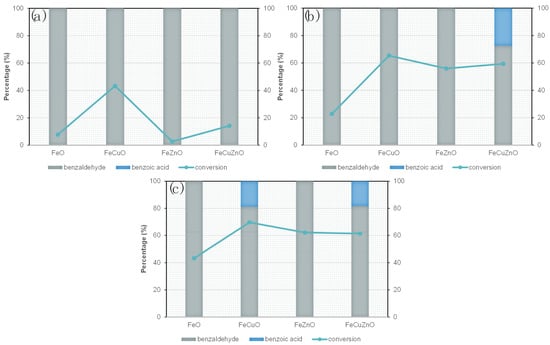
Figure 7.
Conversion and selectivity of MOFs derived metal oxides at different temperatures (a) 90 °C, (b) 120 °C and (c) 150 °C. Reaction conditions: 30 min, 0.1 M benzylic alcohol in ACN (molar relation BA/TBHP 1:4).
The results show that the lowest temperature evaluates, at 90 °C, the catalysts exhibited a negligible catalytic activity. At 120 °C, however, a clear improvement is observed, with significantly higher catalytic performance. A further temperature raise up to 150 °C produces only a slight additional rise in conversion, indicating that 120 °C is already close to the optimal temperature for this system.
In terms of selectivity, the copper-containing oxides (FeCuO and FeCuZnO) display a stronger oxidative character, which results in a higher proportion of benzoic acid at elevated temperatures. Although STEM analysis revealed that copper is not predominantly located at the surface, the relatively high conversion values obtained for FeCuO suggest the presence of accessible active sites, or alternatively, that metal dispersion within the oxide matrix contributes to its catalytic activity.
By contrast, zinc-containing oxides show improved selectivity towards benzaldehyde. According to STEM, Zn is preferentially located at the surface, which could enhance the reactivity of the system and favour partial oxidation. In addition, the Zn-based oxides exhibited the largest surface area and porosity (Table 2), which would promote efficient diffusion of reactants to active sites and thereby explain their catalytic behaviour. Moreover, according to the literature, ZnO not only exhibits semiconducting properties but also possesses Lewis acidity, which could be directly involved in the reaction mechanism or activate a secondary pathways [55]. In addition, the synergistic effect between Zn and other transition metals, an interaction that as has been demonstrated in previous studies may have a positive impact on the selectivity towards benzaldehyde [56]. Both hypotheses are plausible and open new perspectives for further investigation, which should consider: (i) the influence of the Zn content, (ii) the intrinsic effect of Zn, and (iii) the use of additional characterization techniques to correlate these results with the physicochemical properties of the catalysts.
Although benzoic acid was detected as a by-product under some conditions, benzaldehyde remained the major product in all cases. These results confirm the potential of the synthesised materials as promising catalysts for selective oxidation processes.
2.2.3. Study of the Effect of Reaction Time on Conversion and Product Selectivity
To investigate the kinetic evolution of the oxidation reaction and optimise the operating time, FeCuO was selected as catalyst, since it showed the best compromise between high conversion and limited by-product formation in the previous tests. The reaction was conducted at 150 °C, and different reaction times (5, 15, 30, and 45 min) were evaluated while keeping the other experimental parameters constant (0.1 M benzyl alcohol, AB/TBHP molar ratio 1:4).
As shown in Figure 8, the conversion of benzyl alcohol increases progressively with reaction time, reaching a maximum of 71.2% at 45 min. This behaviour can be attributed to the longer contact time between the substrate and the catalyst, which allows more reactant molecules to access the active sites.
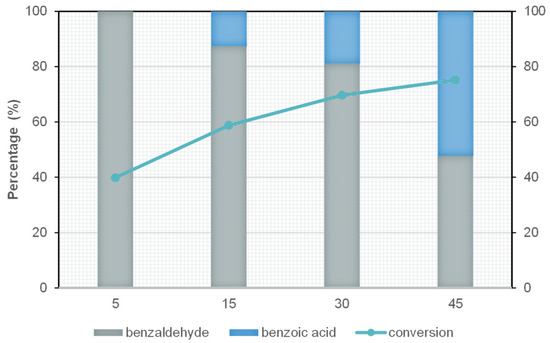
Figure 8.
Conversion and selectivity of FeCuO at different time intervales (5, 15, 30 and 45 min) at 150 °C (0.1M benzyl alcohol, molar relation BA/TBHP 1:4).
However, the increase in conversion is accompanied by a loss of selectivity towards benzaldehyde, particularly evident at 45 min, where a significant amount of benzoic acid is formed. This effect can be explained by the consecutive oxidation of benzaldehyde: as it remains longer in the system and adsorbed on the catalyst surface, it is further oxidised to benzoic acid, thereby reducing the proportion of the targeted product and the overall yield.
Based on these results, 30 min has been selected as the optimal reaction time, as it provides a suitable balance between conversion (69.7%) and selectivity to benzaldehyde (81.2%). Under these conditions, a final yield of 56.5% is achieved, compared to only 35.9% at 45 min.
2.2.4. Recyclability Study
Finally, the reusability of the bimetallic catalysts that exhibited the highest yields—FeCuO (56.5%) and FeZnO (62.1%)—was evaluated under the previously optimised reaction conditions (150 °C, 30 min, BA/TBHP molar ratio 1:4), as shown in Figure 7c. Between successive cycles, the catalysts were subjected to a cleaning protocol consisting of recovery by centrifugation, followed by two washing steps with acetonitrile and two with acetone. The solids were then dried at 80 °C to remove residual solvent before being used in the next catalytic cycle. The results corresponding to the recyclability study are presented on Figure 9.
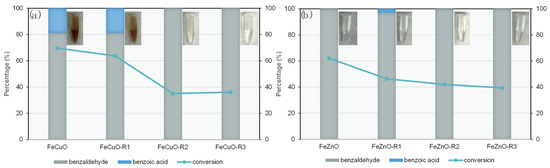
Figure 9.
Conversion and selectivity for FeCuO (a) and FeZnO (b) reuses. Reaction conditions: 150 °C, 30 min, 0.1 M benzyl alcohol, molar relation BA/TBHP 1:4.
The results show that, for both catalysts, the conversion of benzyl alcohol to benzaldehyde continuously decreases with successive reuse cycles. In the case of FeCuO (Figure 9a), the conversion drops from 69% in the first run to 63% in the second, while for FeZnO (Figure 9b) it decreases from ~62% to 46% under the same conditions. However, whereas FeZnO maintains a stable conversion around 40% in the subsequent cycles, FeCuO continues to deactivate from 63% down to values close to 40%. This indicates that, in absolute terms, FeCuO suffers a greater overall loss of activity as compared to FeZnO.
These results clearly highlight the reduced stability of both oxides under repeated reuse, which may be associated with catalyst deactivation phenomena such as leaching of active metals or accumulation of organic residues on the surface. With regard to the selectivity towards benzaldehyde, FeCuO displays an increasing preference for benzaldehyde over benzoic acid with each cycle, reflecting a progressive loss of oxidative capacity. Specifically, the selectivity to benzaldehyde rises from 81.1% to 100% after three cycles, consistent with a gradual deactivation of the catalytic system.
Evidence of metal leaching is also suggested by the colour change of the reaction mixture observed during the recycling experiments (Figure 9a), shifting from a coppery tone to colourless (see Eppendorf pictures). This behaviour is in agreement with previously reported describing the tendency of Cu to leach during heterogeneous oxidation reactions, negatively affecting both activity and durability of the catalyst [53]. To further investigate this phenomenon, an additional test was carried out under the same conditions used in the reusability study (150 °C, 30 min, BA/TBHP 1:4), employing the reaction mixture recovered from FeCuO but without the heterogeneous solid confirming that leached copper was present in solution and was partially responsible of benzyl alcohol oxidation.
In parallel, catalyst deactivation due to incomplete removal of organic by-products over the catalyst surface during the cleaning procedure after three catalytic cycles was examined by FTIR-ATR (Figure 10). For this purpose, the materials were recovered by centrifugation (5 min at 1500 rpm), washed twice with the reaction solvent and twice with acetone, and then dried at 100 °C for 30 min.
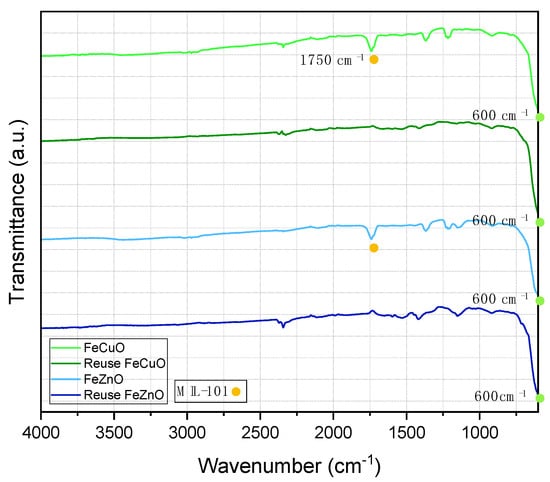
Figure 10.
FTIR-ATR spectra of FeCuO and FeZnO fresh and used catalyst.
In all spectra, bands at around 600 cm−1 are observed, corresponding to metal–oxygen vibrations as previously discussed. In both FeCuO and FeZnO, the disappearance of the band at 1750 cm−1, assigned to C=O stretching, is evident. This observation suggests that the surface structure of the catalysts undergoes modification upon reuse, which in turn impacts their catalytic performance. One possible consequence is the alteration or blocking of active sites, leading to a progressive loss of activity as the number of reuse cycles increases, mostly caused by humins on the catalytic surface due to high temperatures achieved during the process [57].
3. Materials and Methods
3.1. Materials
All reagents used for both the synthesis of the catalysts and the catalytic reactions were employed without further purification and were purchased from different suppliers depending on availability.
For the catalyst synthesis, the following chemicals were used iron (III) chloride hexahydrate (FeCl3·6H2O, Panreac, Barcelona, Spain), copper (II) chloride dihydrate (CuCl2·2H2O, Merck, Darmstadt, Germany), zinc chloride (ZnCl2, purity 96%, Probus, Badalona, Spain), terephthalic acid (H2BDC, C6H4 (CO2H)2, purity 98%, Sigma Aldrich, St. Louis, MO, USA), N,N-dimethylformamide (DMF, (CH3)2NCHO, purity 99.5%, Panreac), methanol (CH3OH, Panreac), and hydrochloric acid solution 0.1 M (HCl, prepared from 35% stock, Panreac).
For the catalytic reactions, the following were used: tert-butyl hydroperoxide (TBHP, 70% in water, Sigma Aldrich) as oxidant, benzyl alcohol (C6H5CH2OH, purity 99%, Sigma Aldrich) as substrate, and acetonitrile (CH3CN, Panreac) as solvent.
3.2. Catalyst Synthesis: MOFs Synthesis
MOF materials were obtained via the typical solvothermal route. As a reference, the synthesis protocol carried out in our research group for Fe-MIL-101 was followed [15,58]. Specifically, 1.35 g (5 mmol) of FeCl3·6H2O and 1.678 g (10 mmol) of H2BDC were dissolved in 40 mL of DMF and 0.5 mL of 0.1 M HCl, since an acidic medium promotes the formation of MOF nuclei. Subsequently, the mixture was stirred for 1 h at 300 rpm, then the homogeneous solution was transferred to a 125 mL Teflon-lined autoclave (BERGHOF, Eningen, Germany) and heated at 150 °C for 24 h. The resulting solids were recovered by filtration, washed twice with methanol, and dried overnight in an oven at 80 °C. In Figure 11 is depicted the scheme of MOFs synthesis (step 1 to 4).
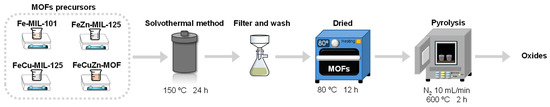
Figure 11.
Synthesis scheme of catalysts: MOFs and oxides.
An analogous procedure was applied for the synthesis of bi- and trimetallic MOFs, adjusting the amounts of metal precursors according to the desired composition. The synthesis was repeated in parallel as many times as necessary to obtain sufficient material for both characterisation and subsequent catalytic evaluation. The amount of metal precursors and organic linker used for the synthesis of MOFs and the corresponding metal oxides are summarised on Table 4.

Table 4.
Molar relation and precursors used for MOFs synthesis.
3.3. Metal Oxide Synthesis
Metal oxides were obtained from the previously synthesised mono-, bi-, and trimetallic MOFs. Once dried (step 4, Figure 1), the materials were subjected to thermal treatment in a muffle furnace under a controlled air/N2 atmosphere. The process consisted of calcination at 600 °C for 2 h, with a heating ramp of 4 °C/min. This treatment allowed the decomposition of the organic ligand and the formation of the corresponding metal oxides.
The nomenclature used throughout this work to identify both the MOFs and their derived oxides is presented in Table 5, in order to facilitate the interpretation of the results.

Table 5.
Nomenclature of catalysts obtained: MOFs and oxides.
3.4. Catalyst Characterization
The catalysts were characterised to determine their textural, structural, and physicochemical properties, which provide insight into their composition, internal organisation, and behaviour under different reaction conditions. For this purpose, several complementary analytical techniques were employed, including N2 adsorption–desorption porosimetry, X-ray diffraction (XRD), attenuated total reflectance Fourier transform infrared spectroscopy (FTIR-ATR), transmission electron microscopy (TEM), and scanning transmission electron microscopy (STEM) coupled with an energy-dispersive X-ray spectroscopy (EDX) detector from the research support service SCAI (University of Córdoba).
3.4.1. N2 Adsorption-Desorption Porosimetry
Textural properties of the porous solids were determined by nitrogen adsorption–desorption at −196 °C (liquid nitrogen). Measurements were carried out using a Micromeritics ASAP 2000 analyzer (Norcross, GA, USA). Prior to analysis, 0.1–0.2 g of each sample was degassed at 0.1 Pa for 24 h at 130 °C. Specific surface area was calculated from the linear region of the BET equation, assuming a nitrogen molecular cross-sectional area of 0.162 nm2. Total pore volume and mesopore distribution were evaluated using the BJH method.
3.4.2. X-Ray Diffraction (XRD)
XRD analysis was used to investigate the crystallinity and structure of the solid materials. Diffraction patterns were recorded on a Bruker D8 DISCOVER diffractometer (Billerica, MA, USA) using Cu Kα radiation (λ = 1.5418 Å) over the 2θ range of 5–80° with a scan rate of 1°/min.
3.4.3. FTIR-ATR Spectroscopy
FTIR-ATR measurements were performed to obtain information on the surface chemical composition of the samples. Spectra were recorded on a PerkinElmer Spectrum 3 spectrophotometer (Boston, MA, USA) equipped with a PIKE Technologies MIRacle™ single reflection accessory. Measurements were carried out in air over the range 4000–600 cm−1 with a resolution of 8 cm−1, averaging 60 scans for each spectrum.
3.4.4. Transmission Electron Microscopy (HR-TEM and STEM)
TEM analysis was used to investigate the morphology of the synthesised materials at the atomic scale. High-resolution TEM images were obtained with a Talos F200i microscope (Waltham, MA, USA) available at the Central Research Support Service (SCAI) of the University of Córdoba.
STEM imaging was performed with the same instrument equipped with an energy-dispersive X-ray spectroscopy (EDX) detector, enabling elemental mapping and distribution analysis at the nanoscale.
3.5. Catalytic Tests
The oxidation of benzyl alcohol to benzaldehyde was carried out using a CEM Discover 2.0 microwave reactor(Charlotte, NC, USA) from our research group. Reactions were performed in 10 mL Pyrex glass vials. Each reaction contained 50 mg of catalyst, 0.1 M benzyl alcohol (0.021 mL), 0.4 M tert-butyl hydroperoxide (0.111 mL), and acetonitrile (ACN) as solvent until a final volume of 2 mL. Reaction conditions were varied depending on the specific objective, with microwave power fixed at 300 W, temperature in the range of 90–150 °C (2.5 min heating ramp), and reaction time between 5 and 45 min.
Product identification was carried out by gas chromatography–mass spectrometry (GC–MS) using an Agilent 7820A GC/5977B (Santa Clara, CA, USA) instrument equipped with a 30 m × 0.25 mm × 0.25 μm HP-5ms capillary column. Analysis conditions were as follows: injector temperature 250 °C, detector temperature 300 °C, oven temperature initially 80 °C and ramped at 10 °C/min to 230 °C, giving a total analysis time of 42 min.
Quantification of the identified compounds was performed using an Agilent 5890 Series II gas chromatograph equipped with a flame ionisation detector (FID) and a non-polar Supelco Equity™-1 silica column (60 m × 0.25 mm × 0.25 μm). The injector and detector were maintained at 250 °C. The oven programme started at 60 °C and increased at 5 °C/min to 180 °C, with a total analysis time of 26 min.
Conversion, selectivity, and yield were calculated according to the following expressions:
where n0 is the initial mola amount of benzyl alcohol, nf the molar amount resting of benzyl alcohol and np is the molar amount of the desired product.
Carbon balance was assessed by GC-FID quantification, obtaining a loss-value in all cases lower than 2% of carbon loss. The carbon balance was estimated from the GC–FID analysis of the liquid phase, considering the carbon content of benzyl alcohol, benzaldehyde and benzoic acid. Peak areas were used directly to determine the relative molar amounts, assuming a similar FID response per mole of carbon for these aromatic compounds.
4. Conclusions
In this work, the catalysts FeO, FeCuO, FeZnO, and FeCuZnO were successfully synthesised from their MOF precursors: MIL-101 (Fe), MIL-101 (FeCu), MIL-101 (FeZn), and MIL-101 (FeCuZn). The structure of these materials was confirmed by a convenient selection of characterisation techniques, and their performance was evaluated in the selective oxidation of benzyl alcohol to benzaldehyde. Among the different conditions tested, the best results were obtained at 150 °C and 30 min, with FeZnO achieving the highest overall yield (62.1%).
The superior performance of FeZnO compared to the other catalysts can be explained by its physicochemical properties. STEM images revealed that Zn is mainly located at the surface, while N2 adsorption–desorption measurements showed that FeZnO has the highest surface area and pore volume among the oxides. Together, these features result in better accessibility to the active sites, enhancing catalytic activity. In contrast, the copper-containing catalysts (FeCuO and FeCuZnO) displayed lower structural stability, with an evident Cu leaching during the reaction.
Although Cu leaching could not be quantified due to temporary malfunction of the analytical equipment, its occurrence was inferred from the discoloration of the reaction medium and the progressive loss of activity over successive cycles, consistent with literature reports for Cu-based catalysts under oxidative conditions. Future studies will focus on quantifying this effect and improving Cu retention within the oxide matrix.
Reusability studies showed that both FeCuO and FeZnO gradually lose activity with increasing cycles. Although FeZnO deactivates more rapidly, it still maintains a higher overall yield as compared to FeCuO. The observed Cu leaching provides a plausible explanation for the faster deactivation of copper-based catalysts, consistent with previous literature.
Overall, these findings confirm that MOF-derived oxides are more active and selective than their respective parent MOFs, in addition to the improvement in the catalytic performance achieved by bi- and trimetallic systems. However, the catalysts stability remains a challenge, particularly in Cu-containing materials. Future work will focus on optimising the synthesis routes to stabilise copper within the oxide framework and minimise leaching, while also exploring new strategies to enhance catalyst durability and broaden their application to the selective oxidation of other biomass-derived alcohols. Additionally, the combination of MOF-derived oxides with microwave-assisted processes represents a promising and energy-efficient route for sustainable fine chemical synthesis. Nonetheless, further studies on scaling-up and long-term catalyst stability are needed to assess the system’s practical feasibility.
Author Contributions
Conceptualization, A.P.; methodology, M.R.-L.; A.Á.R. validation, A.P.; A.Á.R.; formal analysis, M.R.-L. and A.P.; investigation, C.M.-F. and M.R.-L.; resources, A.Á.R.; data curation, M.R.-L. and A.P.; writing—original draft preparation, M.R.-L. writing—review and editing A.P.; visualization A.P.; supervision, A.P.; A.Á.R.; project administration, and A.Á.R.; M.R.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Ciencia e Innovación of Spain Government under the project PID2019-109953GB-100.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
Marina Ronda-Leal gratefully acknowledges the MINECO project for the provision of an FPU contract (REF: FPU20/03875) associated with the same project in the FQM-383 Group. The authors would also like to thank the QUIEMA and technician for XRD measurements and Central Service for Research Support (SCAI) for the TEM and STEM measurements.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| BA | Benzyl alcohol |
| DMF | Dimetilformamide |
| TBHP | Tert butyl isopropoxide |
Appendix A
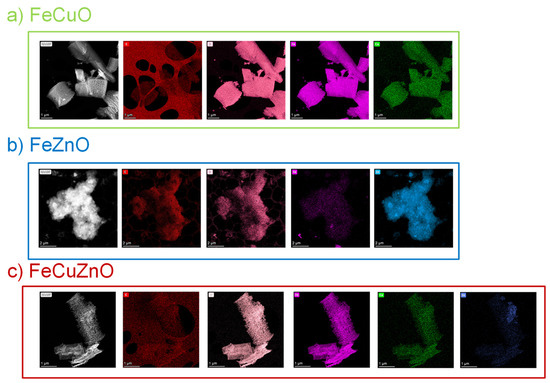
Figure A1.
HAADF-STEM images of (a) FeCuO, (b) FeZnO and (c) FeCuZnO.
References
- Perea-Moreno, M.-A.; Samerón-Manzano, E.; Perea-Moreno, A.-J. Biomass as Renewable Energy: Worldwide Research Trends. Sustainability 2019, 11, 863. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, J.; Shan, R.; Yuan, H.; Chen, Y. Advances in Thermochemical Valorization of Biomass towards Carbon Neutrality. Resour. Conserv. Recycl. 2025, 212, 107905. [Google Scholar] [CrossRef]
- Ronda-Leal, M.; Lázaro, N.; Pineda, A.; Romero, A.A.; Luque, R. Selective Catalytic Conversion of Gamma-Valerolactone to Isopropyl Valerate Using MOF-Derived Pd-ZrO2@C Materials. Sustain. Chem. Pharm. 2024, 38, 101467. [Google Scholar] [CrossRef]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-Industrial Lignocellulosic Biomass a Key to Unlock the Future Bio-Energy: A Brief Review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Lorenci Woiciechowski, A.; Dalmas Neto, C.J.; Porto de Souza Vandenberghe, L.; de Carvalho Neto, D.P.; Novak Sydney, A.C.; Letti, L.A.J.; Karp, S.G.; Zevallos Torres, L.A.; Soccol, C.R. Lignocellulosic Biomass: Acid and Alkaline Pretreatments and Their Effects on Biomass Recalcitrance—Conventional Processing and Recent Advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Katahira, R.; Elder, T.J.; Beckham, G.T. Chapter 1. A Brief Introduction to Lignin Structure. In Lignin Valorization: Emerging Approaches; Royal Society of Chemistry: Cambridge, UK, 2018; pp. 1–20. [Google Scholar]
- Mukhtar, A.; Zaheer, M.; Saeed, M.; Voelter, W. Synthesis of Lignin Model Compound Containing a β-O-4 Linkage. Z Für Naturforschung B 2017, 72, 119–124. [Google Scholar] [CrossRef]
- Shinde, S.H.; Hengne, A.; Rode, C.V. Lignocellulose-Derived Platform Molecules. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–31. [Google Scholar]
- Chen, M.; Zhong, W.; Wu, K.; Wei, G.; Hu, Z.; Zheng, W.; Ruan, H.; Zhang, H.; Xiao, R. Catalytic Pyrolysis Mechanism of β-O-4 Type of Lignin Dimer: The Role of H Proton. Energy Fuels 2021, 35, 575–582. [Google Scholar] [CrossRef]
- Gao, G.; Rong, R.; Zhang, Z.; Pan, B.; Sun, X.; Zhang, Q.; Zheng, G.; Xu, K.; Gao, L. Selective Oxidation of Benzyl Alcohol to Benzaldehyde over CoFe2O4 Nanocatalyst. Catal. Commun. 2023, 183, 106757. [Google Scholar] [CrossRef]
- Luong, G.K.T.; Ku, Y. Selective Oxidation of Benzyl Alcohol in the Aqueous Phase by TiO2-Based Photocatalysts: A Review. Chem. Eng. Technol. 2021, 44, 2178–2190. [Google Scholar] [CrossRef]
- Lapa, H.M.; Martins, L.M.D.R.S. Toluene Oxidation: CO2 vs Benzaldehyde: Current Status and Future Perspectives. ACS Omega 2024, 9, 26780–26804. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Chen, W.; Hu, J.; Zhang, F.; Hu, X. Highly Selective Oxidation of Toluene to Benzaldehyde in Alkaline Systems. Ind. Eng. Chem. Res. 2023, 62, 10051–10056. [Google Scholar] [CrossRef]
- Claudio-Rizo, J.A.; Cano Salazar, L.F.; Flores-Guia, T.E.; Cabrera-Munguia, D.A. Estructuras Metal-Orgánicas (MOFs) Nanoestructuradas Para La Liberación Controlada de Fármacos. Mundo Nano. Rev. Interdiscip. Nanociencias Nanotecnología 2020, 14, 1e–29e. [Google Scholar] [CrossRef]
- Ronda-Leal, M.; Osman, S.M.; Won Jang, H.; Shokouhimehr, M.; Romero, A.A.; Luque, R. Selective Hydrogenation of Furfural Using TiO2-Fe2O3/C from Ti-Fe-MOFs as Sacrificial Template: Microwave vs Continuous Flow Experiments. Fuel 2023, 333, 126221. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, W.; Liu, Q.; Xia, Z. CH3 NH3 PbBr3 Perovskite Nanocrystals Encapsulated in Lanthanide Metal–Organic Frameworks as a Photoluminescence Converter for Anti-Counterfeiting. ACS Appl. Mater. Interfaces 2018, 10, 27875–27884. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Ayyoubzadeh, S.M.; Ghorbani-Bidkorbeh, F.; Shahhosseini, S.; Dadashzadeh, S.; Asadian, E.; Mosayebnia, M.; Siavashy, S. An Investigation of Affecting Factors on MOF Characteristics for Biomedical Applications: A Systematic Review. Heliyon 2021, 7, e06914. [Google Scholar] [CrossRef]
- Giménez-Marqués, M.; Hidalgo, T.; Serre, C.; Horcajada, P. Nanostructured Metal–Organic Frameworks and Their Bio-Related Applications. Coord. Chem. Rev. 2016, 307, 342–360. [Google Scholar] [CrossRef]
- Gedanken, A. Using Sonochemistry for the Fabrication of Nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Nabipour, H.; Pahlevani, F.; Zhao, Y.; Hussain, Z.; Hojjati-Najafabadi, A.; Hoang, H.Y.; Pei, R. Recent Progress on Adsorption of Cadmium Ions from Water Systems Using Metal-Organic Frameworks (MOFs) as an Efficient Class of Porous Materials. Environ. Res. 2022, 214, 114113. [Google Scholar] [CrossRef]
- Othong, J.; Boonmak, J.; Promarak, V.; Kielar, F.; Youngme, S. Sonochemical Synthesis of Carbon Dots/Lanthanoid MOFs Hybrids for White Light-Emitting Diodes with High Color Rendering. ACS Appl. Mater. Interfaces 2019, 11, 44421–44429. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cao, X.; Jiao, L. MOFs-Derived Carbon-Based Metal Catalysts for Energy-Related Electrocatalysis. Small 2021, 17, e2004398. [Google Scholar] [CrossRef]
- Dong, X.; Yan, E.; Lv, Y.; Zhou, Y.; Chu, X. Engineering MOF-Derived Hollow Metal Oxides toward Enhanced Electrocatalytic Oxygen Evolution Reaction. Appl. Catal. A Gen. 2024, 681, 119772. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, S.; Tariq, S.; Luque, R.; Verpoort, F. Self-Sacrifice MOFs for Heterogeneous Catalysis: Synthesis Mechanisms and Future Perspectives. Mater. Today 2022, 55, 137–169. [Google Scholar] [CrossRef]
- Almazán, F.; Lafuente, M.; Echarte, A.; Imizcoz, M.; Pellejero, I.; Gandía, L.M. UiO-66 MOF-Derived Ru@ZrO2 Catalysts for Photo-Thermal CO2 Hydrogenation. Chemistry 2023, 5, 720–729. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zhou, J.; Wang, L. Metal-Organic Framework-Derived Multifunctional Photocatalysts. Chin. J. Catal. 2022, 43, 971–1000. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Bhonsle, A.K.; Trivedi, J.; Bangwal, D.P.; Singh, L.P.; Atray, N. Synthesis and characterization of coal fly ash supported zinc oxide catalyst for biodiesel production using used cooking oil as feed. Renew. Energy 2021, 170, 302–314. [Google Scholar] [CrossRef]
- Mansir, N.; Teo, S.H.; Mijan, N.-A.; Taufiq-Yap, Y.H. Efficient reaction for biodiesel manufacturing using bi-functional oxide catalyst. Catal. Commun. 2021, 149, 106201. [Google Scholar] [CrossRef]
- Al-Saadi, A.; Mathan, B.; He, Y. Esterification and transesterification over SrO–ZnO/Al2O3 as a novel bifunctional catalyst for biodiesel production. Renew. Energy 2020, 158, 388–399. [Google Scholar] [CrossRef]
- Alhassan, F.H.; Rashid, U.; Taufiq-Yap, Y.H. Synthesis of waste cooking oil-based biodiesel via effectual recyclable bi-functional Fe2O3MnOSO42−/ZrO2 nanoparticle solid catalyst. Fuel 2015, 142, 38–45. [Google Scholar] [CrossRef]
- Pei, U.; Ruan, L.; Zeng, P.; Fu, H.; Zeng, L.; Liu, J.; Zhang, H.; Yang, K.; Zhu, L.; Hui, B. Controlled Synthesis of RuNi-CNTs Nano-Composites and Their Catalytic Performance in Benzene Hydrogenation. Catal. Lett. 2021, 151, 773–786. [Google Scholar] [CrossRef]
- Gómez Bernal, H.; Benito, P.; Rodríguez-Castellón, E.; Raspolli Galletti, A.M.; Funaioli, T. Synthesis of Isopropyl Levulinate from Furfural: Insights on a Cascade Production Perspective. Appl. Catal. A Gen. 2019, 575, 111–119. [Google Scholar] [CrossRef]
- Hosokawa, S.; Hayashi, Y.; Imamura, S.; Wada, K.; Inoue, M. Effect of the Preparation Conditions of Ru/CeO2 Catalysts for the Liquid Phase Oxidation of Benzyl Alcohol. Catal. Lett. 2009, 129, 394–399. [Google Scholar] [CrossRef]
- Ferri, D.; Mondelli, C.; Krumeich, F.; Baiker, A. Discrimination of Active Palladium Sites in Catalytic Liquid-Phase Oxidation of Benzyl Alcohol. J. Phys. Chem. B 2006, 110, 22982–22986. [Google Scholar] [CrossRef] [PubMed]
- Mallat, T.; Baiker, A. Oxidation of Alcohols with Molecular Oxygen on Solid Catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef]
- Zhang, K.; Yan, Y. Preparation of Fe3O4 @CSAC Catalyst and Its Degradation Performance and Heat Release Mechanisms in Sewage Degradation. RSC Adv. 2024, 14, 5132–5141. [Google Scholar] [CrossRef]
- Mesa, S.; Arboleda, J.; Amaya, S.; Echavarría, A. Hidrotalcitas de NiZnFe y NiMgFe Modificadas Con V Y Cr Como Precursores de Catalizadores Para Deshidrogenación Oxidativa de Propano. Ing. Compet. 1969, 14, 169–178. [Google Scholar] [CrossRef]
- Hu, L.; Zhong, P.; Sang, X.; Zeb, A.; Lin, X.; Tong, Y.; Yang, H. Integrating Dual Active Sites on Heterogeneous Bimetallic Selenide to Facilitate Hydrogen Evolution and Ethanol Oxidation. Appl. Surf. Sci. 2023, 637, 157912. [Google Scholar] [CrossRef]
- Gómez-López, P.; Espro, C.; Rodríguez-Padrón, D.; Balu, A.M.; Ivars-Barceló, F.; Moreda, O.I.; Alvarado-Beltrán, C.G.; Luque, R. Mechanochemical Preparation of Magnetically Separable Fe and Cu-Based Bimetallic Nanocatalysts for Vanillin Production. Nanomaterials 2021, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, Z.; Kang, W.; Li, R.; Qu, K.; Wang, L.; Meng, F.; Li, H. Atomically Dispersed Fe/Zn Synergy in Sulfur-Modified Nitrogen-Doped Carbon for Boosting Oxygen Reduction Activity. J. Mater. Chem. A Mater. 2025, 13, 25423–25434. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, C.; Qiu, X.; Zhang, H.; Feng, H.; Tang, Y.; Shang, Z.; Song, H. Selective Oxidation of Benzyl Alcohol to Benzaldehyde Using Palladium Nanoparticle-Loaded Poly(Ionic Liquid)s Catalysts. Mol. Catal. 2025, 584, 115273. [Google Scholar] [CrossRef]
- Aldosari, O.F. Iron-Palladium Supported Graphene as an Efficient Bimetallic Catalyst for the Selective Oxidation of Benzyl Alcohol to Benzaldehyde. J. Saudi Chem. Soc. 2023, 27, 101671. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Chaudhari, P.A.; Narkhede, V.S. Solvent-Free Liquid Phase Oxidation of Benzyl Alcohol to Benzaldehyde by Molecular Oxygen Using Non-Noble Transition Metal Containing Hydrotalcite-like Solid Catalysts. Catal. Commun. 2003, 4, 171–175. [Google Scholar] [CrossRef]
- Mangesh, V.L.; Govindarajan, M.; Raju Chekuri, R.B.; Perumal, T.; Rajendran, K.; Chandrasekaran, K.; Siva Kumar, N.; Basivi, P.K.; Alreshaidan, S.B.; Al-Fatesh, A.S. Ni–Fe Bimetallic Catalysts with High Dispersion Supported by SBA-15 Evaluated for the Selective Oxidation of Benzyl Alcohol to Benzaldehyde. RSC Adv. 2024, 14, 2300–2310. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, M.; Kanai, M.; Matsunaga, S.; Kumagai, N. Recent Progress in Asymmetric Bifunctional Catalysis Using Multimetallic Systems. Acc. Chem. Res. 2009, 42, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Ronda-Leal, M.; Balu, A.M.; Luque, R.; Mauriello, F.; Ricchebuono, A.; Len, C.; Romero, A.A.; Paone, E. Continuous flow production of γ-valerolactone from methyl-levulinate promoted by MOF-derived Al2O3–ZrO2/C catalysts. RSC Sustain. 2025, 3, 2273–2285. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Q.; Wang, Z.; Wu, T.; Zhang, M. Synthesis of MIL-101(Cr) and Its Water Adsorption Performance. Microporous Mesoporous Mater. 2020, 297, 110044. [Google Scholar] [CrossRef]
- Afolabi, M.A.; Xiao, D.; Chen, Y. The Impact of Surface Chemistry and Synthesis Conditions on the Adsorption of Antibiotics onto MXene Membranes. Molecules 2023, 29, 148. [Google Scholar] [CrossRef]
- Ma, L.; Xu, J.; Liu, Y.; An, Y.; Pan, Z.; Yang, B.; Li, L.; Hu, T.; Lai, B. Improved Degradation of Tetracycline by Cu-Doped MIL-101(Fe) in a Coupled Photocatalytic and Persulfate Oxidation System: Efficiency, Mechanism, and Degradation Pathway. Sep. Purif. Technol. 2023, 305, 122450. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Z.; Bakhtari, M.F.; Luo, J. Preparation of GO/MIL-101(Fe,Cu) Composite and Its Adsorption Mechanisms for Phosphate in Aqueous Solution. Environ. Sci. Pollut. Res. 2021, 28, 51391–51403. [Google Scholar] [CrossRef]
- Dong, W.; Liu, X.; Shi, W.; Huang, Y. Metal–Organic Framework MIL-53(Fe): Facile Microwave-Assisted Synthesis and Use as a Highly Active Peroxidase Mimetic for Glucose Biosensing. RSC Adv. 2015, 5, 17451–17457. [Google Scholar] [CrossRef]
- Elsherbiny, A.S.; Rady, A.; Abdelhameed, R.M.; Gemeay, A.H. Efficiency and Selectivity of Cost-Effective Zn-MOF for Dye Removal, Kinetic and Thermodynamic Approach. Environ. Sci. Pollut. Res. 2023, 30, 106860–106875. [Google Scholar] [CrossRef]
- Zheng, H.; Mo, Q.; Zhang, X.; Huang, J.; Sheng, G. Efficacy and Mechanism of Activation Peroxymonosulfate for Tetracycline Degradation by AC-MIL-101(Fe)-Derived Magnetic CuO/Fe2O3/CuFe2O4. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134867. [Google Scholar] [CrossRef]
- Parikh, S.J.; Goyne, K.W.; Margenot, A.J.; Mukome, F.N.D.; Calderón, F.J. Soil Chemical Insights Provided through Vibrational Spectroscopy. In Advances in Agronomy; Academic Press: San Diego, CA, USA, 2014; pp. 1–148. [Google Scholar]
- Shojaei, A.F.; Tabatabaeian, K.; Zanjanchi, M.A. Synthesis, characterization and study of catalytic activity of Silver doped ZnO nanocomposite as an efficient catalyst for selective oxidation of benzyl alcohol. J. Chem. Sci. 2025, 127, 481–491. [Google Scholar] [CrossRef]
- Duarte, H.A.; Luggren, P.J.; Zelin, J.; Sad, M.E.; Díez, V.K.; Di Cosimo, J.I. Selective aerobic oxidation of benzyl alcohol on inexpensive and reusable ZnO/MnCO3 catalyst. Catal. Today 2022, 394, 178–189. [Google Scholar] [CrossRef]
- Ferri, D.; Baiker, A. Advances in Infrared Spectroscopy of Catalytic Solid–Liquid Interfaces: The Case of Selective Alcohol Oxidation. Top. Catal. 2009, 52, 1323–1333. [Google Scholar] [CrossRef]
- Jiang, S.; Huang, J.; Wang, Y.; Lu, S.; Li, P.; Li, C.; Li, F. Metal–Organic Frameworks Derived Magnetic Fe3O4/C for Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol. J. Chem. Technol. Biotechnol. 2021, 96, 639–649. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).