Abstract
Advanced oxidation processes (AOPs) utilizing peroxymonosulfate (PMS) have recently gained attention for effectively removing organic dyes. Biochar, a carbon-based material, can act as a catalyst carrier for PMS activation. This study developed a nitrogen-doped biochar catalyst (NCMR800–2) from waste Chinese medicine residue (CMR) through one-step pyrolysis to efficiently remove Rhodamine B (RhB) from wastewater. Results indicate that NCMR800–2 rapidly achieved complete removal of 20 mg/L Rhodamine B (RhB), the primary focus of this study, within 30 min, while maintaining high degradation efficiencies for other pollutants and significantly outperforming the unmodified material. The material demonstrates strong resistance to ionic interference and operates effectively across a wide pH range. Quenching experiments and in situ testing identified singlet oxygen (1O2) as the primary active species in RhB degradation. Electrochemical analysis showed that nitrogen doping significantly enhanced the electrical conductivity and electron transfer efficiency of the catalyst, facilitating PMS decomposition and RhB degradation. Liquid chromatography–mass spectrometry (LC-MS) identified intermediate products in the RhB degradation process. Seed germination experiments and TEST toxicity software confirmed a significant reduction in the toxicity of degradation products. In conclusion, this study presents a cost-effective, efficient catalyst with promising applications for removing persistent organic dyes.
1. Introduction
The rise in domestic sewage and industrial pollutants has led to the infiltration of micropollutants into water bodies, posing potential threats to human health and ecosystems [1,2,3]. Synthetic dyes, prime examples of these pollutants, are extensively used in industries like textiles, leather, and papermaking [4,5]. With over 10,000 varieties and an annual production surpassing 700,000 tons, synthetic organic dyes represent a major concern [6]. As the leading textile producer, China faces significant challenges relating to organic dye pollution. During dyeing, 10% to 45% of dyes are lost, resulting in nearly 2 × 105 tons of colored wastewater annually [7]. Traditional methods for treating contaminated water, namely, physical–chemical techniques like adsorption and electrochemistry, and biological approaches such as contact oxidation and membrane bioreactors face challenges including high operational costs, secondary pollution, and incomplete pollutant removal [8,9,10]. In contrast, AOPs utilizing PMS have gained attention for their efficiency and environmental compatibility. PMS oxidation in particular has emerged as a key area of research concentration due to its effectiveness in degrading common organic pollutants in water [11]. The development of AOPs leveraging PMS has received notable attention in recent years, attributed to their high efficacy in removing pollutants and alignment with green economy principles [12,13]. Among these, PMS oxidation approaches have gained prominence in research due to their high efficiency in degrading organic pollutants in water bodies [14,15]. While PMS is a potent oxidizing agent, its direct oxidation and degradation of organic pollutants under standard conditions is challenging, necessitating activation through appropriate means to generate free radicals or other reactive species. Current activation strategies include the use of alkaline conditions, organic substrates, and carbon-based materials [16,17,18]. Therefore, there is an urgent need to develop environmentally friendly, cost-effective, and highly efficient catalysts. Activation approaches leveraging multifunctional synergistic effects can effectively enhance pollutant degradation efficiency.
Human activities have led to the annual production of over 1.6 billion tons of biomass waste, often dismissed as useless [19]. However, biomass waste is a renewable resource with considerable potential as an energy carrier [20]. Recent advancements in AOPs have highlighted the role of carbon-based materials in activating PMS processes, making them a research focal point [21,22]. Studies on biochar derived from CMR have predominantly examined its physical adsorption capabilities for pollutant removal from water. For instance, Wang et al. developed a chitosan-modified magnetic biochar from ligusticum chuanxiong residues, achieving removal efficiencies exceeding 95%, 99%, and 99% for Cr(VI), As(III), and Pb(II) in contaminated water, respectively [23]. Yuan et al. found that biochar made from traditional CMR has good potential for adsorbing heavy metal lead (II) [24]. Li et al. employed a one-step carbonization method to produce biochar from atropine residue and revealed its exceptional adsorption efficacy for norfloxacin [25]. Despite these compelling findings, research endeavors exploring the application of biochar derived from CMR within AOPs remain relatively scarce. This study, therefore, explores the use of TCM residues as a precursor for biochar in AOPs. Modifying biochar is crucial for its practical application in environmental remediation. Widely recognized modification methods include acid–base treatment [26], oxidant treatment [27], and heteroatom doping [28]. The literature documents that nitrogen (N) doping confers enhancements in catalytic performance through the augmentation of surface functional group abundance, pore dimensions, and pore volume; these effects collectively contribute to the amelioration of both adsorption and degradation efficiencies toward target pollutants [29,30,31]. Urea, a cost-effective biofertilizer with high nitrogen content, has been extensively used in biochar modification research [32,33]. For instance, Ge et al. successfully synthesized N-doped biochar polymer composites using urea modification, achieving a tetracycline removal rate of 97.83% [34]. Similarly, Lin et al. utilized urea-modified porous carbon materials to attain a bisphenol A removal rate of 97.20% [35]. While significant progress has been made, the underlying removal mechanisms for various pollutants warrant further investigation. Although PMS is an efficient oxidant, it presents certain safety and environmental concerns [36,37]. PMS is corrosive and may generate halogenated by-products or residual toxicity if not properly managed [38,39]. Therefore, strict handling, quenching of residual PMS, and monitoring of by-products are necessary to ensure safe application.
In summary, this study utilized herbal medicine residue as a biomass precursor, which was modified with urea and subjected to a one-step pyrolysis method to prepare an eco-friendly, economical, and high-performance catalyst. The degradation experiment conditions were optimized, and the reaction mechanism for activating PMS to degrade pollutants was explored to address current water pollution issues. Structural characterization analyses were conducted to examine the physical structure of the obtained catalyst material. Further investigations were carried out to determine the optimal conditions for pollutant removal, including evaluating the effects of catalyst and PMS dosage, pollutant type and concentration, initial pH, and water environment. The practicality of the catalyst was assessed through ion interference experiments, cyclic stability tests, and toxicity experiments. The catalytic activation of PMS for organic pollutant removal from water was investigated through radical quenching experiments, electron paramagnetic resonance (EPR) measurements, Raman spectroscopy, electrochemical analyses, and LC-MS. This research offers an efficient and sustainable approach to water treatment, enabling the elimination of contaminants from aquatic environments under mild reaction conditions.
2. Results and Discussion
2.1. Material Characterization
Figure 1a illustrates the preparation procedures of NCMRT-X material, where T represents the activation temperature and X represents the mass ratio of urea to Chinese medicine residue. Since the catalytic and adsorption properties of materials are strongly influenced by their microstructure [40], we used scanning electron microscopy (SEM) to investigate the morphological characteristics of NCMR800–2. Figure 1b illustrates its highly porous nature, while Figure 1c reveals distinctive wrinkles on the catalyst surface, probably resulting from urea decomposition at elevated temperatures. This process generates gases that form numerous pores within the material. The presence of abundant large pores and channels in the catalyst minimizes mass transfer resistance, facilitating pollutant penetration into the catalyst interior and thereby improving removal efficiency. Further microstructural analysis of NCMR800–2 through transmission electron microscopy (TEM), as shown in Figure 1d,e, reveals a strip-like morphology with well-defined pore structures. Figure 1f–j demonstrate uniform distribution of C, N, and O elements on the material surface, indicating success as well as uniform nitrogen doping into the catalyst framework. Moreover, Figure 1i,j reveal the presence of Si and Al elements in the material, likely to be trace elements self-doped from the CMR.
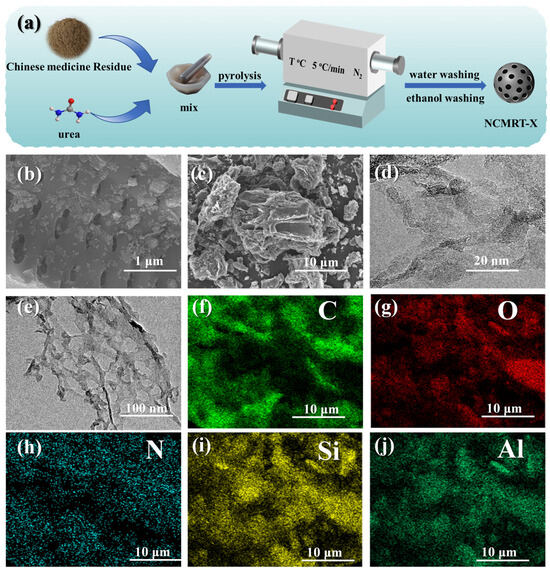
Figure 1.
(a) Preparation scheme for NCMRT-X material, (b,c) SEM, (d,e) TEM, and (f–j) TEM mapping.
The pore characteristics and specific surface area (SSA) of the catalysts with varying mass ratios were evaluated through nitrogen adsorption–desorption measurements [12]. The results depicted in Figure 2a demonstrate that the introduction of nitrogen elements led to a decrease in SSA from 59 m2/g to 55 m2/g. The nitrogen adsorption–desorption isotherm of the catalyst exhibits a distinct hysteresis loop, signifying the development of a mesoporous structure post-doping. This finding aligns with the pore size distribution curve in Figure S1 and Table S1. The mesopores enhance the availability of reactive sites for Rhodamine B (RhB) degradation. The crystalline architecture of catalysts was determined through X-ray diffraction (XRD) analysis, as depicted in Figure 2b. A diffraction peak at 2θ = 23.5° indicates the presence of an amorphous (002) diffraction plane characteristic of carbonaceous materials [41]. Furthermore, distinct diffraction peaks at 2θ = 20.8° and 2θ = 26.6° correspond to the (100) and (101) diffraction planes of silicon dioxide (SiO2) [42]. The crystallinity of the sample changes, structure, and defects were assessed using Raman spectroscopy [43]. The D peak and G peak are common features in carbon-based materials like graphite and graphene. The D peak, located at approximately 1350 cm−1, indicates structural defects or disorder, whereas the G peak, at around 1580 cm−1, represents the planar vibrational mode of sp2 hybridized carbon atoms [12]. The ratio of the D peak to the G peak (ID/IG) is a pivotal metric for assessing the crystalline integrity and defect density of carbon materials [44]. In Figure 2c, the ID/IG value decreases progressively with increasing modification ratio and temperature, indicating a relatively complete crystal structure and reduced defect level in the modified and activated carbon material. Additionally, a prominent absorption peak at 1080 cm−1 was observed, assigned to the high-temperature SiO2 phase [45].
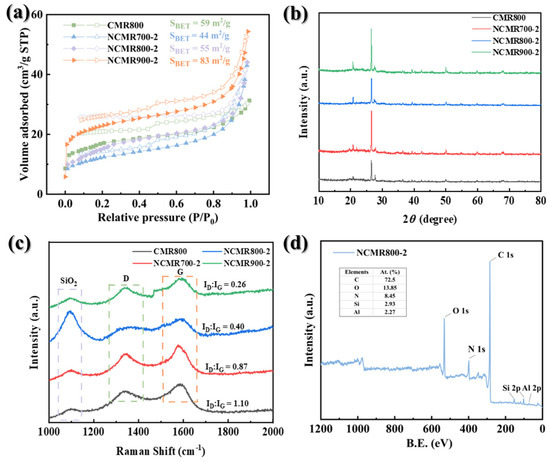
Figure 2.
(a) N2 adsorption–desorption isotherms, (b) XRD patterns of prepared samples, (c) Raman spectra, (d) full-range XPS surveys of NCMR800–2.
X-ray photoelectron spectroscopy (XPS) was employed for analyzing the elemental composition and chemical state of materials. The XPS spectrum of NCMR800–2, depicted in Figure 2d, revealed the presence of five elements: C, O, N, Si, and Al. This observation validates the effective doping of N, with incidental detection of Si and Al in minute quantities originating from the herbal residue. This deduction is supported by the XRD and energy-dispersive energy dispersive spectroscopy (EDS) spectra. Subsequent XPS analysis was performed to investigate the molecular structure and chemical surroundings of the doped impurity atoms in NCMR800–2. The C 1s peak exhibited four distinct components at 284.65 eV (graphitic C/C-C/C-H), 286.4 eV (C=N), 288.5 eV (C=O), and 291 eV (COOH) [46]. Similarly, the O 1s spectrum of NCMR800–2 displayed peaks for C=O (530.6 eV), O-Al (531.5 eV), O-H (532.8 eV), and O-Si (533.7 eV). The N 1s spectrum revealed peaks at 398.2 eV, 399.5 eV, 401.2 eV, and 402.8 eV, corresponding to pyridine-N (N−6), pyrrole-N (N−5), graphite-N (N-Q), or oxidized-N (N-O) [47]. Analysis of the Si 2p spectrum revealed peaks at 101.8 eV, 102.65 eV, and 103.5 eV, representing Si3N4, Si-O bonds, and Si-OH bonds, respectively [48]. Furthermore, high-resolution Al 2p spectroscopy identified characteristic peaks at 73.79 eV, 74.65 eV, and 75.02 eV, corresponding to Al(OH)3, AlOOH, and Al2O3, respectively [49].
2.2. Evaluation of Catalyst Activation Performance
Catalyst performance in PMS activation was evaluated by synthesizing catalysts at different temperatures and ratios, followed by an assessment of their catalytic efficiency to optimize the preparation process [50]. Figure 3a illustrates the adsorption–degradation profiles of the unmodified and modified catalysts. Incorporation of the catalyst effectively triggers the activation of PMS, thereby augmenting its adsorption and degradation capabilities towards pollutants. Furthermore, in contrast to the unmodified biochar, the nitrogen-doped catalyst demonstrates a significant improvement in catalytic efficiency, achieving full pollutant degradation within 30 min, highlighting the effectiveness of our modification strategy. The impact of temperature on catalyst performance was also scrutinized, with the degradation efficiency of the catalyst evaluated across different preparation temperatures. As depicted in Figure 3b, the adsorption efficiency of the catalyst remained largely unaffected by escalating calcination temperatures. Additionally, the degradation rate curves reveal that the NCMR800–1 catalyst degraded pollutants more rapidly. Consequently, 800 °C was chosen as the calcination temperature. To identify the optimal modification ratio, catalysts with varying ratios were calcined at this temperature. As depicted in Figure 3c, increasing the urea ratio enhances the adsorptive capacity of the catalyst and pollutant removal efficiency. Notably, NCMR800–2 achieved 100% pollutant removal in 20 min, while NCMR800–3 reached the same removal rate in just 10 min. Both catalysts demonstrated exceptional performance; however, for economic reasons, NCMR800–2 was selected to achieve effective catalytic degradation at a reduced cost.
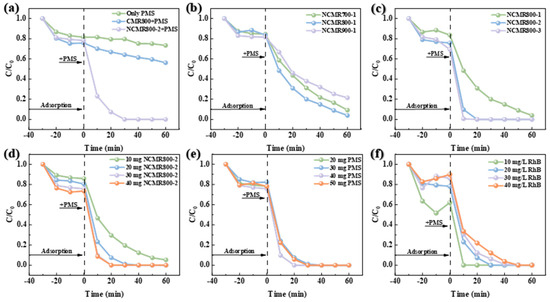
Figure 3.
Removal of RhB using (a) various processes, (b) different preparation temperatures, (c) different doping ratios, influence of (d) NCMR800–2 dose, (e) PMS dose, (f) pollution concentration. Reaction conditions: [RhB] = 20 mg/L, [Catalyst] = 0.15 g/L, [PMS] = 0.15 g/L, T = 25 °C.
Catalyst dosage critically influences pollutant removal efficiency. This study evaluated various catalyst dosages on degradation performance. As depicted in Figure 3d, increased catalyst dosage correlates with higher pollutant adsorption during the initial stage, with both 30 mg and 40 mg doses achieving complete pollutant removal within 20 min. For optimal efficiency and cost-effectiveness, a 30 mg catalyst dose was chosen for further research. Similarly, oxidant dosage impacts pollutant removal efficiency. We examined different PMS dosages under a constant catalyst dosage, as shown in Figure 3e. Even at a low PMS dosage of 20 mg, effective pollutant degradation was achieved, leading to its selection for subsequent studies. Further analysis explored the effect of varying pollutant concentrations on degradation performance, illustrated in Figure 3f. Increased pollutant concentration diminishes removal efficiency. To ensure efficient removal within a limited timeframe, a 20 mg/L RhB solution was selected for further investigation. These findings suggest that N co-doping significantly enhances PMS activation and RhB degradation.
In natural aquatic systems, anions such as chloride ions (Cl−), nitrate ions (NO3−), sulphate ions (SO42−), and phosphate ions (PO43−) coexist alongside natural organic compounds like humic acid (HA). This study investigated the system of NCMR800–2/PMS resistance to interference during pollutant degradation by introducing these common anions and organic matter at specific concentrations. As depicted in Figure 4, the presence of these ions did not notably impact pollutant adsorption by the catalyst. However, they variably influenced the catalytic activation of PMS for pollutant degradation. Figure 4a,b illustrate that Cl− and NO3− mildly inhibited PMS activation for RhB degradation. Figure 4c indicates that increasing SO42− concentration progressively heightened this inhibitory effect. Notably, Figure 4d reveals that PO43− strongly suppressds the catalytic degradation efficiency; once pollutants reached adsorption saturation, degradation virtually ceased. The addition of HA (Figure 4e) enhances the catalyst-activated PMS degradation of pollutants at low concentrations (5, 10 mg/L), significantly boosting catalytic efficiency. However, excessive HA can inhibit catalyst performance. The initial pH of the solution is critical in determining the reactive oxygen species (ROS) type and intermediate products formed. As depicted in Figure 4f, acidic conditions, with increased H+ ions, improved the catalytic efficiency of NCMR800–2. In contrast, weakly acidic, neutral, and weakly alkaline conditions showed negligible effects on catalytic degradation. Under strongly alkaline conditions, while the adsorptive capacity of the catalyst is enhanced, its degradation performance declines markedly, probably due to metal ions within the catalyst. Studies indicate that in alkaline environments, OH− ions react with metal ions to form complexes, significantly inhibiting the catalytic efficiency of many metal ions.
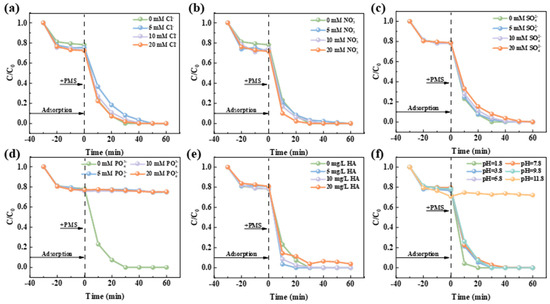
Figure 4.
Influence of inorganic anions on the NCMR800–2/PMS system: (a) Cl−, (b) NO3−, (c) SO42−, (d) PO43−, (e) HA and (f) impact of initial pH on RhB removal. Reaction conditions: [RhB] = 20 mg/L, [Catalyst] = 0.15 g/L, [PMS] = 0.15 g/L, T = 25 °C.
2.3. Practical Application of the NCMR800–2/PMS System in Water Treatment
The zeta potential test results (Figure 5a) indicate that the surface of catalyst was negatively charged. In aqueous solution, RhB molecules undergo ionization, generating positively charged species and releasing Cl−. This process significantly enhances the adsorption capacity on the catalyst surface, primarily due to the electrostatic interaction between its cationic form and the negatively charged catalyst surface. However, in strongly alkaline conditions, PMS exists as SO52−, leading to strong electrostatic repulsion with the catalyst. This repulsion hinders PMS adsorption on the catalyst surface, resulting in poor catalytic degradation efficiency under alkaline conditions. The catalytic performance of NCMR800–2 was evaluated in real water bodies to assess its applicability under more complex environmental conditions. As illustrated in Figure S3, the catalytic degradation performance of NCMR800–2 in actual water samples was limited, likely due to the presence of interfering ions such as HCO3− and PO43−, which significantly impacted the catalytic performance of the catalyst. Furthermore, the abundant microorganisms in natural water bodies may have inhibited the activation of PMS by the catalyst to some extent. Despite these challenges, NCMR800–2 exhibited exceptional catalytic degradation efficiency for various pollutants, including RhB, AO7, HTC, OTC, and MB, each at a concentration of 20 mg/L, with removal rates of 100%, 100%, 87.57%, 97.29%, and 72.63%, respectively (Figure 5b). To evaluate the stability of the catalyst, a fixed-bed flow reactor was used to determine its catalytic efficiency (Figure S4). As shown in Figure 5c, the NCMR800–2 catalyst exhibited excellent removal performance for RhB in the flow mode in the presence of PMS. After 40 h of cycling, NCMR800–2 still exhibited good stability. To test the recyclability of the catalyst, used NCMR800–2 was recovered and recycled. After four cycles, the catalyst still exhibited strong catalytic activity, with a pollutant removal rate of 88.54% (Figure 5d). This result indicates that the catalyst has strong practical applicability.
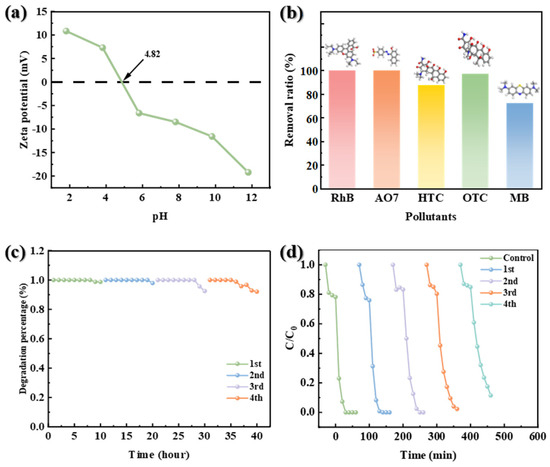
Figure 5.
(a) Zeta potential distribution at different pH, (b) experiments on the universality of the NCMR800–2/PMS system, (c) pollutant removal by catalyst over 40 h, and (d) cycling experiments.
2.4. Investigation of Active Species and Mechanistic Pathways
To explore the activation mechanism, free radical quenching experiments were conducted to identify the reactive species involved (Figure 6a) [51]. Tert-butyl alcohol (TBA) is the preferred •OH scavenger [52]. When TBA was added, it did not significantly affect the reaction, indicating that •OH was not generated during the reaction. Additionally, methanol was used to quench •OH and SO4•−, and the addition of methanol (MeOH) did not significantly inhibit the degradation of pollutants, indicating that SO4•− was not present during the reaction. p-benzoquinone (p-BQ) and L–histidine (L–His) were selected as quenchers for O2•− and 1O2, respectively. The addition of p-BQ did not inhibit the degradation of RhB, indicating that free radicals were not present in the reaction process, while the addition of L–His significantly inhibited the degradation of RhB, indicating that 1O2 participated as a potential active species in the degradation of RhB. FFA is another commonly used 1O2 quencher, but at the same concentration, its inhibitory effect was much smaller than that of L–His; so, the addition of FFA did not significantly affect the degradation of RhB in this system [53,54]. Additional research was carried out to examine the reactive species responsible for the activation of PMS by NCMR800–2 using EPR spectroscopy [55]. TEMP was employed as a scavenger for 1O2, DMPO was utilized to trap •OH and SO4•− in water-based solutions, and DMPO in methanol solution was used as a scavenger for O2•−. As shown in Figure 6b, when detecting 1O2, a weak 1:1:1 ternary signal was observed as early as 3 min into the reaction, and this signal continued to strengthen over time, indicating the presence of 1O2 in the system [56]. As shown in Figure S5, no significant signals for •OH/SO4•− or O2•− were detected in the NCMR800–2/PMS system. This outcome aligns with the results observed in the radical quenching experiment.
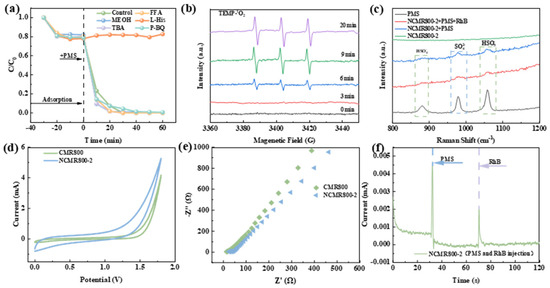
Figure 6.
(a) Effect of chemical quenchers, (b) EPR spectra of PMS activation in TEMP solutions by the NCMR800–2/PMS syste m, (c) Raman of single PMS and NMCR800–2/PMS systems, electrochemical testing (d) CV scans, (e) EIS analysis, (f) I-t curves.
Subsequently, Raman spectroscopy was employed to analyze the characteristics of the PMS and the resultant polymer on its surface. As depicted in Figure 6c, vibrational peaks corresponding to HSO5− in PMS were observed at 1060 cm−1 and 889 cm−1, while the signal for SO42− manifested as a broad peak at 963 cm−1. The attenuation of the HSO5− peak at 889 cm−1 and the SO42− peak at 963 cm−1 in the NCMR800–2/PMS system can be attributed to the degradation of PMS induced by the catalyst introduction. The shift in the HSO5− vibrational peak further validates the interaction of the O-O bond of PMS with the active sites on NCMR800–2, resulting in alterations in the stretching vibration amplitude [57]. To delve deeper into the electron transfer mechanism in the NCMR800–2/PMS system, its electrochemical characteristics were evaluated utilizing a three-electrode configuration [58]. The cyclic voltammetry curves of the catalyst material are shown in Figure 6d. NCMR800–2 exhibited a large charge potential difference and good cyclic reversibility, demonstrating that this material possessed strong conductivity and redox reaction capability at the reaction interface. Electrochemical impedance spectroscopy (EIS) indicated the charge transfer resistance characteristic of the material, with a smaller semicircular diameter in the EIS plot indicating lower resistance [59]. This suggests the material exhibited faster electron transfer kinetics and enhanced electrical conductivity. Figure 6e shows that NCMR800–2 had a smaller diameter, demonstrating its superior conductivity and electron transfer capability compared to the original carbon. Hybrid atom-doped carbon-based catalysts have garnered significant attention owing to their distinctive physicochemical characteristics, diverse active sites, and their ability to facilitate electron transfer between PMS and the catalyst [60,61]. The charge transfer between NCMR800–2, PMS, and RhB molecules was further investigated using the chronoamperometry method. As shown in Figure 6f, the addition of PMS had a significant impact on the current output, indicating the formation of a metastable complex (NCMR800–2/PMS). The current experienced a sudden increase following the addition of RhB, suggesting the formation of a current path from RhB to the complex on the NCMR800–2 surface, which may have been related to the oxidative degradation of RhB.
2.5. Possible Degradation Pathways
To further investigate the reaction mechanism of RhB degradation in the NCMR800–2/PMS system, LC-MS was used to confirm the intermediate degradation products of RhB. Based on the LC-MS analysis results for the NCMR800–2/PMS system (Figure S6) and previous research reports [62,63], three possible degradation pathways for RhB under this reaction system were proposed (Figure 7). Among these, pathways 1 and 3 are initiated by the decarbonization of carbon atoms, with 1O2 attack and electron transfer catalyzing the cleavage of RhB [64]. Pathway 2 primarily involves cleavage reactions and ring-opening reactions. Throughout degradation, large compounds were fragmented into smaller molecules, ultimately mineralizing into H2O and CO2. The total organic carbon (TOC) concentration decreased by approximately 33% after 40 min in the NCMR800–2/PMS system (Figure S7); the remaining 67% of TOC was attributed to small molecular byproducts derived from RhB degradation.
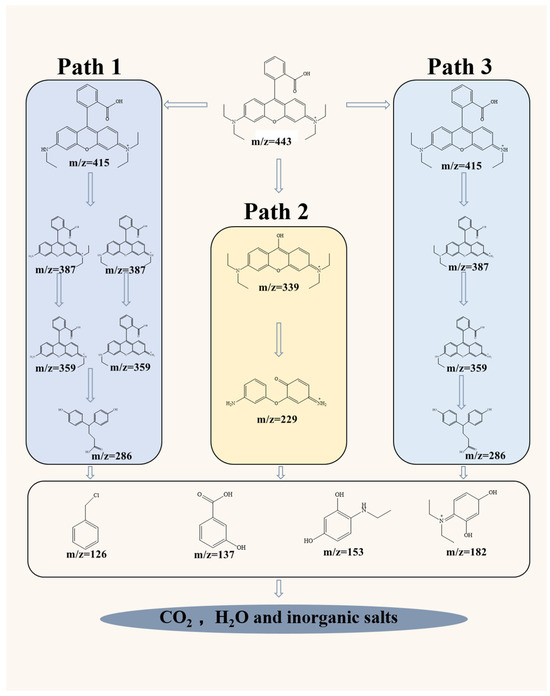
Figure 7.
Proposed degradation pathways of RhB degradation by NCMR800–2/PMS system.
2.6. Evaluation of the Toxicity of RhB Degradation Products
Toxicity assessment is a very important aspect of the NCMR800–2/PMS system. Although the RhB solution appeared visually degraded and decolorized after treatment, complete mineralization into CO2 and H2O remains challenging, and the degradation intermediates may still pose environmental risks. Therefore, it is essential to evaluate the toxicity of the byproducts formed during the RhB degradation process. Previous studies have commonly used seed germination experiments to study the toxic effect of pollutant solutions [12,65]. In this study, Shanghai greens, wheat, and pea seeds were employed as model crops. The toxicity of both treated and untreated RhB solutions was assessed by examining the average root and shoot growth of the three model crop seeds. Lower root and stem growth inhibition indicates lower solution toxicity. As shown in Figure 8a–c, for peas, those grown in distilled water exhibited longer roots and stems, with an average length longer than those grown in the degraded culture medium and the pollutant culture medium, while the treated culture medium also performed better than the pollutant solution. Similarly, the same growth trend was observed in wheat and Shanghai greens. Additionally, it was found that the average root length after treatment was closer to that in distilled water but significantly better than that in the untreated RhB solution; this indicates that the ecological toxicity of the RhB solution treated with the NCMR800–2/PMS system was significantly reduced, and NCMR800–2 demonstrated satisfactory catalytic performance.
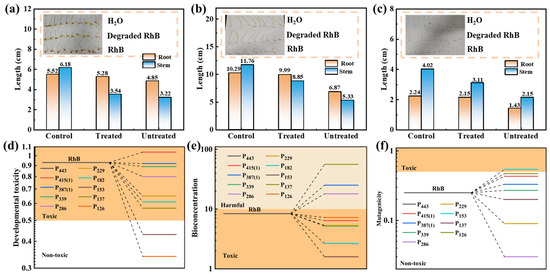
Figure 8.
Seed germination experiments: (a) pea, (b) wheat, (c) Shanghai greens (d–f) ecotoxicity analysis of RhB and its intermediates.
The evaluation of several toxicity parameters using TEST software (Toxicity Estimation Software Tool, version 5.1) provides a comprehensive understanding of the potential environmental risks of this catalyst system during degradation. As shown in Figure 8d–f, developmental toxicity, bioaccumulation factors and mutagenicity were evaluated. In these tests, the degradation products P11 and P12 showed low or negligible toxicity. Test results showed that the biological toxicity and developmental toxicity of the main intermediate products generated during degradation exhibited a significant decreasing trend, with the toxicity of the main end products significantly reduced or non-toxic; although some intermediate products still exhibited toxicity during degradation, their concentrations were reduced by extending the reaction time, thereby lowering their toxicity to aquatic ecosystems. In summary, the present study introduces an economical and straightforward way to prepare modified carbon-based catalysts for the green and efficient removal of pollutants from water.
3. Experiments
3.1. Chemicals
Urea (CH4N2O), peroxymonosulfate (PMS), L–histidine(L–His), tert-butanol (C4H10O), furfuryl alcohol (FFA), methylene blue (MB), tetracycline hydrochloride (HTC), Acid Orange 7(AO7), Oxytetracycline(OTC), sodium chloride (NaCl), sodium hydrogen carbonate (NaHCO3), sodium nitrate (NaNO3), sodium sulfate (Na2SO4), sodium hydroxide (NaOH), trisodium phosphate (Na3PO4), and methanol (MeOH) were purchased from Aladdin Reagent Co., Ltd., Shanghai, China. Naphthol, humic acid (HA), and Rhodamine B (RhB) were obtained from Macklin Biochemical Co., Ltd. (Shanghai, China). 5,5-dimethyl−1-pyrroline-N-oxide (DMPO) and 2,2,6,6-tetramethyl−4-piperidinol (TEMP) were purchased from J&K Scientific Ltd., Shanghai, China. Peas, wheat, shanghai cabbage, and Chinese medicine residue (CMR) were collected from Hanzhong Tianyang Biotechnology Co., Ltd., Hanzhong, Shaanxi, China. All chemicals are highly purified reagents without further purification.
3.2. Preparation and Characterization of Catalysts
The preparation procedure of NCMRT-X material is illustrated in Figure 1a, with comprehensive experimental details available in the Supporting Information.
4. Conclusions
This work reports the development of a highly efficient catalyst based on nitrogen-doped biochar (NCMR800–2) derived from waste CMR using a one-step pyrolysis method, specifically designed for the removal of synthetic dyes (RhB) from water. Experimental results demonstrate that NCMR800–2 achieved 100% removal of RhB—a primary target dye in this study—within 30 min, significantly outperforming untreated traditional Chinese medicine residues and other comparison samples; its high catalytic performance is attributed to the significant increase in specific surface area due to nitrogen doping, which introduces abundant mesoporous structures and active sites, thereby enhancing adsorption capacity and PMS activation capacity for RhB. Further characterization techniques confirmed the successful nitrogen doping and its regulatory effect on material structure and chemical state. In practical applications, the NCMR800–2/PMS system demonstrated excellent RhB removal efficiency under various pH conditions and ionic interference, exhibiting strong interference resistance and broad applicability. Cycling experiments showed that the catalyst maintained an 88.54% removal rate after four cycles, demonstrating good cycling stability. Investigations into the reaction mechanism indicate that 1O2 serves as the predominant reactive species in the NCMR800–2/PMS system for RhB degradation. Electrochemical analysis further revealed that nitrogen doping improved conductivity of the catalyst and enhanced its electron transfer efficiency, thereby facilitating the decomposition of PMS and the degradation of RhB. Additionally, toxicity assessment experiments confirmed the active sites and reaction mechanism of NCMR800–2 and demonstrated that the treated RhB solution exhibited significantly reduced toxicity in simulated crop growth conditions. In summary, NCMR800–2, as a low-cost, high-efficiency catalyst, holds promising application prospects in the field of water treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15100926/s1, Figure S1: Pore size distributions of catalysts; Figure S2: The XPS gross mapping of NCMR800–2 (a) C 1s; (b) O 1s; (c) N 1s; (d) Si 2p; (e) Al 2p; Figure S3. RhB removal experiments in various water matrixes; Figure S4: Flow cycle apparatus diagram; Figure S5: EPR spectra of (a) TEMP-O2•−, (b) DMPO-•OH and DMPO-SO4•− adduct; Figure S6: m/z of intermediates obtained by LC-MS analysis; Figure S7: TOC removal efficiency for RhB in the PMS process ([RhB] = 20 mg/L, [PMS] = 20 mg/L, catalyst dosage = 0.15 g/L, T = 298 K); Table S1: Pore characteristics of carbon materials.
Author Contributions
Conceptualization, X.L. (Xiao Liu) and D.L.; methodology, W.Z.; software, X.G.; validation, W.Z. and X.G.; formal analysis, X.S.; investigation, D.L.; resources, X.S.; data curation, D.L. and W.Z.; writing—original draft preparation, X.L. (Xiaoyun Lei); writing—review and editing, X.L. (Xiaoyun Lei); visualization, D.L. and X.G.; supervision, T.W.; project administration, X.S.; funding acquisition, T.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Shaanxi Province Science and Technology Innovation Team Project, grant number 2025RS-CXTD-040 and the Shaanxi University of Technology Doctoral Research Start Foundation, grant number SLGRCQD004.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- He, Y.; Wang, Z.; Wang, H.; Almatrafi, E.; Qin, H.; Huang, D.; Zhu, Y.; Zhou, C.; Tian, Q.; Xu, P.; et al. Confinement of ZIF-derived copper-cobalt-zinc oxides in carbon framework for degradation of organic pollutants. J. Hazard. Mater. 2022, 440, 129811. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, X.; Song, W.; Pan, Y.; Lambropoulou, D.; Zhong, Y.; Du, Y.; Nie, J.; Yang, X. Photochemical oxidation of PPCPs using a combination of solar irradiation and free available chlorine. Sci. Total Environ. 2019, 682, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; He, H.; Lei, L.; Zhu, K.; He, D.; Huang, J.; Ai, Y. Green synthesis of novel Fe nanoparticles embedded in N-doped biochar composites derived from bagasse for sulfadiazine degradation via peroxymonosulfate activator: Mechanism insight and performance assessment. J. Water Process Eng. 2022, 49, 103131. [Google Scholar] [CrossRef]
- Nghia, N.T.; Nguyen, V.D. Application of Response Surface Methodology for the Optimization of Basic Red 46 Dye Degradation in an Electrocoagulation–Ozonation Hybrid System. Molecules 2025, 30, 2627. [Google Scholar] [CrossRef] [PubMed]
- Faraos, A.; Maroulas, K.N.; Nikoloudakis, E.; Drivas, C.; Isaacs, M.A.; Kyzas, G.Z.; Ladomenou, K. Synergistic effect of porphyrin and NiO in chitosan/graphene oxide aerogels for enhanced photocatalytic degradation of cationic and anionic dyes. RSC Adv. 2025, 15, 27685–27699. [Google Scholar] [CrossRef]
- Yener, J.; Kopac, T.; Dogu, G.; Dogu, T. Dynamic analysis of sorption of Methylene Blue dye on granular and powdered activated carbon. Chem. Eng. J. 2008, 144, 400–406. [Google Scholar] [CrossRef]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of textile dyes on health and ecosystem: A review of structure, causes, and potential solutions. Environ. Sci. Pollut. Res. 2023, 30, 9207–9242. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Kurniawan, S.B.; Iwuozor, K.O.; Aniagor, C.O.; Ajala, O.J.; Oba, S.N.; Iwuchukwu, F.U.; Ahmadi, S.; Igwegbe, C.A. A review of treatment technologies for the mitigation of the toxic environmental effects of acid mine drainage (AMD). Process Saf. Environ. Prot. 2022, 157, 37–58. [Google Scholar] [CrossRef]
- Nure, J.F.; Nkambule, T.T.I. The recent advances in adsorption and membrane separation and their hybrid technologies for micropollutants removal from wastewater. J. Ind. Eng. Chem. 2023, 126, 92–114. [Google Scholar] [CrossRef]
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Emerging contaminants of high concern and their enzyme-assisted biodegradation–A review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef]
- Song, X.; Shi, Y.; Wu, Z.; Huang, B.; Wang, X.; Zhang, H.; Zhou, P.; Liu, W.; Pan, Z.; Xiong, Z.; et al. Unraveling the discriminative mechanisms for peroxy activation via atomically dispersed Fe-N5 sites for tunable water decontamination. Appl. Catal. B Environ. 2024, 340, 123240. [Google Scholar] [CrossRef]
- Di, X.; Zeng, X.; Zhang, X.; Tang, T.; Zhao, Z.; Wang, W.; Liu, Z.; Jin, L.; Ji, X.; Shao, X. Nitrogen-phosphorus codoped biochar prepared from tannic acid for degradation of trace antibiotics in wastewater. Environ. Res. 2025, 266, 120589. [Google Scholar] [CrossRef]
- Gao, X.; Liu, C.; Di, X.; Shi, Y.; Liu, D.; Yang, J.; Zhao, Z.; Wang, W.; Ji, X.; Shao, X. Non-radical pathway dominated mechanism of nitrogen-phosphorus co-doped porous carbon activated peroxymonosulfate: Synergistic regulation of N-P and ecological toxicity reduction. J. Environ. Chem. Eng. 2025, 13, 119005. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, Q.; Yuan, X.; Wang, N.; Wu, J.; Lian, X.; Miao, K.; Feng, G.; Luo, X. Achieving a balance between catalytic activity and stability of MnCo2O4 for sustainable flow-through wastewater treatment in peroxymonosulfate-advanced oxidation process. Chem. Eng. J. 2025, 505, 159580. [Google Scholar] [CrossRef]
- Manickavasagam, G.; Saaid, M.; Oh, W.-D. Hydrochar as sustainable redox catalyst for advanced oxidation processes-based wastewater treatment. Pure Appl. Chem. 2025. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Zhao, H.; Qi, W.; Tan, X.; Xu, X.; Song, F.; Shao, X. Metal single-atom catalysts derived from silicon-based materials for advanced oxidation applications. Chin. Chem. Lett. 2025, 36, 110898. [Google Scholar] [CrossRef]
- Lei, X.; Zhao, H.; Bai, C.; Geng, L.; Xu, X. Wood-derived catalysts for green and stable Fenton-like chemistry: From basic mechanisms to catalytic modules and future inspiration. Chin. Chem. Lett. 2025, 36, 111550. [Google Scholar] [CrossRef]
- Oluyinka, O.A.; Oke, E.A.; Oyelude, E.O.; Abugri, J.; Raheem, S.A. Recapitulating potential environmental and industrial applications of biomass wastes. J. Mater. Cycles Waste Manag. 2022, 24, 2089–2107. [Google Scholar] [CrossRef]
- Cavali, M.; Libardi Junior, N.; de Sena, J.D.; Woiciechowski, A.L.; Soccol, C.R.; Belli Filho, P.; Bayard, R.; Benbelkacem, H.; de Castilhos Junior, A.B. A review on hydrothermal carbonization of potential biomass wastes, characterization and environmental applications of hydrochar, and biorefinery perspectives of the process. Sci. Total Environ. 2023, 857, 159627. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Z.; Kang, R.; Wang, J.; Lu, Q.; Wang, T.; Tian, D.; Xu, Y.; Wang, Z.; Ding, H. N-Rich Algal Sludge Biochar for Peroxymonosulfate Activation toward Sulfadiazine Removal. Coatings 2023, 13, 431. [Google Scholar] [CrossRef]
- Xu, S.; Wang, P.; Mi, X.; Bao, Y.; Zhang, H.; Mo, F.; Zhou, Q.; Zhan, S. N, S, and Cl tri-doped carbon boost the switching of radical to non-radical pathway in Fenton-like reactions: Synergism of N species and defects. J. Hazard. Mater. 2024, 466, 133321. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qiao, J.; Yuan, J.; Tang, Z.; Chu, T.; Lin, R.; Wen, H.; Zheng, C.; Chen, H.; Xie, H.; et al. Novel chitosan-modified biochar prepared from a Chinese herb residue for multiple heavy metals removal: Characterization, performance and mechanism. Bioresour. Technol. 2024, 402, 130830. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wang, C.; Tang, Z.; Chu, T.; Zheng, C.; Han, Q.; Chen, H.; Tan, Y. Biochar derived from traditional Chinese medicine residues: An efficient adsorbent for heavy metal Pb(II). Arab. J. Chem. 2024, 17, 105606. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Z.; Zhang, M.; Su, H.; Zhao, T.; Feng, W.; Zhang, Z. Exploring the Potential of Biochar Derived from Chinese Herbal Medicine Residue for Efficient Removal of Norfloxacin. Molecules 2024, 29, 2063. [Google Scholar] [CrossRef]
- Wang, Z.; Du, C.; Yang, S.; Li, X.; Chen, R.; Ding, D. Active surface area determines the activity of biochar in Fenton-like oxidation processes. J. Hazard. Mater. 2025, 487, 137272. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, X.; Zhao, H.; Yang, C. Synergistic advanced oxidation process for enhanced degradation of organic pollutants in spent sulfuric acid over recoverable apricot shell-derived biochar catalyst. RSC Adv. 2022, 12, 1904–1913. [Google Scholar] [CrossRef]
- Ramirez, L.A.; Alvarez, M.; Gutierrez, V.S. From agro-alimentary residue to catalyst: Transforming sunflower seed husk waste into modified biochar for efficient ibuprofen degradation in water. J. Water Process Eng. 2025, 72, 107458. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.; Jing, J.; Song, G.; Zhang, X.; Zhou, M.; Dewil, R. Synergistically S/N self-doped biochar as a green bifunctional cathode catalyst in electrochemical degradation of organic pollutant. Green Energy Environ. 2025, 10, 214–230. [Google Scholar] [CrossRef]
- Wu, J.; Wen, Y.; Leng, Z.; Zhang, S.; Li, S.; Zhou, Z.; Zhou, Z.; Zhou, N. Enhanced atrazine degradation in water by N, P co-doped biochar based on peroxymonosulfate: Performance, mechanism and phytotoxicity reduction. J. Environ. Chem. Eng. 2025, 13, 116002. [Google Scholar] [CrossRef]
- Wen, Q.; Wang, J. Degradation of sulfamethoxazole using peroxymonosulfate activated by nitrogen-doped biochar: Insights into the active sites and activation mechanism. Chem. Eng. J. 2025, 508, 161020. [Google Scholar] [CrossRef]
- Meng, Z.; Mo, R.; Wang, Q.; Zheng, K.; Li, W.; Qin, C. Nitrogen-doped porous carbon derived from graphite of solid waste for activating peroxymonosulfate to degradation tetracycline. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 130984. [Google Scholar] [CrossRef]
- Kifle, G.A.; Huang, Y.; Xiang, M.; Tesfamichael, T.; Wang, W.; Wei, Y.; Wang, C.; Li, C.; Li, H. Synergistic enhancement of peroxymonosulfate activation by bimetallic (Bi, Fe) supported NaHCO3 activated and urea-modified biochar for sulfamethoxazole degradation: DFT calculations, toxicity assessments, and mechanistic studies. J. Environ. Chem. Eng. 2024, 12, 111675. [Google Scholar] [CrossRef]
- Huang, J.; Wang, M.; Luo, S.; Li, Z.; Ge, Y. In situ preparation of highly graphitized N-doped biochar geopolymer composites for efficient catalytic degradation of tetracycline in water by H2O. Environ. Res. 2023, 219, 115166. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Wu, F.; Yin, Z.; He, C.; Qian, J.; Mao, Y.; You, X.; Lin, G.; Yang, X.; Huang, B. Activation of peroxymonosulfate by tannin-derived porous carbon for enhanced bisphenol A degradation: Synergistic effect of surface nitrogen and oxygen functional groups. Appl. Surf. Sci. 2023, 640, 158308. [Google Scholar] [CrossRef]
- Yan, B.; Li, Q.; Chen, X.; Deng, H.; Feng, W.; Lu, H. Application of O3/PMS Advanced Oxidation Technology in the Treatment of Organic Pollutants in Highly Concentrated Organic Wastewater: A Review. Separations 2022, 9, 444. [Google Scholar] [CrossRef]
- Deepika, R.; Sethuraman, M.G. Unleashing the potential of carbon dots bound Pd/CeO2@zeolite 13X as an oxone activator for the degradation of methylene blue dye: Insights into the catalytic mechanism and toxicity assessment. J. Water Process Eng. 2025, 74, 107817. [Google Scholar] [CrossRef]
- Du, Y.; Wang, W.-L.; Wang, Z.-W.; Yuan, C.-J.; Ye, M.-Q.; Wu, Q.-Y. Overlooked Cytotoxicity and Genotoxicity to Mammalian Cells Caused by the Oxidant Peroxymonosulfate during Wastewater Treatment Compared with the Sulfate Radical-Based Ultraviolet/Peroxymonosulfate Process. Environ. Sci. Technol. 2023, 57, 3311–3322. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Hu, J. Performance comparison of O3 and O3/Peroxymonosulfate (PMS) advanced oxidation processes for the antibiotics removal in wastewater treatment. J. Hazard. Mater. 2025, 496, 139248. [Google Scholar] [CrossRef]
- Hwang, S.; Chen, X.; Zhou, G.; Su, D. In Situ Transmission Electron Microscopy on Energy-Related Catalysis. Adv. Energy Mater. 2020, 10, 1902105. [Google Scholar] [CrossRef]
- Abdel-Salam, M.O.; Farghal, H.H.; El Sawy, E.; Yoon, T.; El-Sayed, M.M.H. Activation of peroxymonosulfate for rhodamine-B removal from water: Enhanced efficiency with cobalt-enriched, magnetically recoverable CNTs. RSC Adv. 2025, 15, 6371–6383. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; Salem, J.K.; Kuhn, S.; Hammad, T.; Hempelmann, R.; Al Bhaisi, S. Synthesis and characterization of silica-, meso-silica- and their functionalized silica-coated copper oxide nanomaterials. J. Sol.-Gel. Sci. Technol. 2016, 79, 573–583. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, H.; Yuan, L.; Ding, X.; Zhang, S.; Li, K.; Sun, J.; Liu, Q. Temperature-Dependent Structural Evolution of Coal-Based Graphite During Synthetic Graphitization Process. Energy Fuels 2024, 38, 17370–17379. [Google Scholar] [CrossRef]
- Huang, A.; Tu, Y.; Yu, Q. Preparation and electrochemical properties of nitrogen-doped starch hard carbon anode materials for lithium-ion battery. Int. J. Electrochem. Sci. 2024, 19, 100774. [Google Scholar] [CrossRef]
- Liu, H.; Kaya, H.; Lin, Y.-T.; Ogrinc, A.; Kim, S.H. Vibrational spectroscopy analysis of silica and silicate glass networks. J. Am. Ceram. Soc. 2022, 105, 2355–2384. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, H.; Shahab, A.; Zhang, K.; Zeng, H.; Bacha, A.-U.-R.; Nabi, I.; Ullah, H. Comparative study on characterization and adsorption properties of phosphoric acid activated biochar and nitrogen-containing modified biochar employing Eucalyptus as a precursor. J. Clean. Prod. 2021, 303, 127046. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, L.; Li, H.; Xu, X.; Shang, Y.; Xu, Y.; Cao, X.; Liu, H.; Zhang, J. Unraveling the metal size-dependent origin in activating peracetic acid with cobalt single atoms: Collaboration of radical and non-radical mechanisms. Appl. Catal. B Environ. Energy 2025, 371, 125252. [Google Scholar] [CrossRef]
- Gross, T.; Ramm, M.; Sonntag, H.; Unger, W.; Weijers, H.M.; Adem, E.H. An XPS analysis of different SiO2 modifications employing a C 1s as well as an Au 4f7/2 static charge reference. Surf. Interface Anal. 1992, 18, 59–64. [Google Scholar] [CrossRef]
- Alaizeri, Z.M.; Alhadlaq, H.A.; Aldawood, S.; Ahamed, M. Synthesis and Characterization of MgO-Fe2O3/γ-Al2O3 Nanocomposites: Enhanced Photocatalytic Efficiency and Selective Anticancer Properties. Catalysts 2024, 14, 923. [Google Scholar] [CrossRef]
- Shi, Y.; Yin, M.; Liu, D.; Gao, X.; Liu, X.; Yang, T.; Zhao, Z.; Ji, X.; Zhao, C.; Shao, X. Single-step synthesis of nitrogen and phosphorus co-doped biochar and its application in dye removal: Synergistic effects of adsorption and peroxymonosulfate activation. Environ. Res. 2025, 279, 121866. [Google Scholar] [CrossRef]
- Feng, G.; Huang, H.; Chen, Y. Effects of emerging pollutants on the occurrence and transfer of antibiotic resistance genes: A review. J. Hazard. Mater. 2021, 420, 126602. [Google Scholar] [CrossRef]
- Hu, P.; Su, H.; Chen, Z.; Yu, C.; Li, Q.; Zhou, B.; Alvarez, P.J.J.; Long, M. Selective Degradation of Organic Pollutants Using an Efficient Metal-Free Catalyst Derived from Carbonized Polypyrrole via Peroxymonosulfate Activation. Environ. Sci. Technol. 2017, 51, 11288–11296. [Google Scholar] [CrossRef]
- Chen, Z.; Bi, S.; Zhao, G.; Chen, Y.; Hu, Y. Enhanced degradation of triclosan by cobalt manganese spinel-type oxide activated peroxymonosulfate oxidation process via sulfate radicals and singlet oxygen: Mechanisms and intermediates identification. Sci. Total Environ. 2020, 711, 134715. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Liu, Y.; Cheng, X.; Tang, W.; Zhao, C.; Guo, H. Kinetic performance of peroxymonosulfate activated by Co/Bi25FeO40: Radical and non-radical mechanism. J. Taiwan Inst. Chem. Eng. 2019, 100, 56–64. [Google Scholar] [CrossRef]
- Hassani, A.; Eghbali, P.; Mahdipour, F.; Wacławek, S.; Lin, K.-Y.A.; Ghanbari, F. Insights into the synergistic role of photocatalytic activation of peroxymonosulfate by UVA-LED irradiation over CoFe2O4-rGO nanocomposite towards effective Bisphenol A degradation: Performance, mineralization, and activation mechanism. Chem. Eng. J. 2023, 453, 139556. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, E.; Xu, D.; Guo, Q. Activation of peroxymonosulfate by bimetallic CoMn oxides loaded on coal fly ash-derived SBA−15 for efficient degradation of Rhodamine, B. Sep. Purif. Technol. 2021, 274, 119081. [Google Scholar] [CrossRef]
- Pei, J.; Liu, J.; Fu, K.; Fu, Y.; Yin, K.; Luo, S.; Yu, D.; Xing, M.; Luo, J. Non-metallic iodine single-atom catalysts with optimized electronic structures for efficient Fenton-like reactions. Nat. Commun. 2025, 16, 800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, Z.; Wang, X. A review of electrochemical oxidation technology for advanced treatment of medical wastewater. Front Chem 2022, 10, 1002038. [Google Scholar] [CrossRef] [PubMed]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef]
- Choong, Z.-Y.; Lin, K.-Y.A.; Lisak, G.; Lim, T.-T.; Oh, W.-D. Multi-heteroatom-doped carbocatalyst as peroxymonosulfate and peroxydisulfate activator for water purification: A critical review. J. Hazard. Mater. 2022, 426, 128077. [Google Scholar] [CrossRef]
- Dou, J.; Cheng, J.; Lu, Z.; Tian, Z.; Xu, J.; He, Y. Biochar co-doped with nitrogen and boron switching the free radical based peroxydisulfate activation into the electron-transfer dominated nonradical process. Appl. Catal. B Environ. 2022, 301, 120832. [Google Scholar] [CrossRef]
- Shi, X.; Hong, P.; Huang, H.; Yang, D.; Zhang, K.; He, J.; Li, Y.; Wu, Z.; Xie, C.; Liu, J.; et al. Enhanced peroxymonosulfate activation by hierarchical porous Fe3O4/Co3S4 nanosheets for efficient elimination of rhodamine B: Mechanisms, degradation pathways and toxicological analysis. J. Colloid Interface Sci. 2022, 610, 751–765. [Google Scholar] [CrossRef]
- Song, T.; Li, G.; Yu, X.; Xia, J.; Deng, Q.; Liu, X.; Gao, Y. Bi-piezoelectric and plasmonic enhanced photocatalysis using Au/Bi2WO6/PVDF flexible films for efficient dye wastewater treatment: Heterogeneous interfacial engineering, degradation pathways and mechanism insight. Appl. Surf. Sci. 2025, 679, 161163. [Google Scholar] [CrossRef]
- Xiao, G.; Xu, T.; Faheem, M.; Xi, Y.; Zhou, T.; Moryani, H.T.; Bao, J.; Du, J. Evolution of Singlet Oxygen by Activating Peroxydisulfate and Peroxymonosulfate: A Review. Int. J. Environ. Res. Public Health 2021, 18, 3344. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, E.; Treccani, S.; Ferruti, P.; Alongi, J. The Seed Germination Test as a Valuable Tool for the Short-Term Phytotoxicity Screening of Water-Soluble Polyamidoamines. Polymers 2024, 16, 1744. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).