Promotional Effect of Semiconductor-Supported Plasmonic Copper Nanoparticles in Visible-Light-Driven Photocatalytic Oxidative Homocoupling of Alkynes
Abstract
1. Introduction
2. Results and Discussion
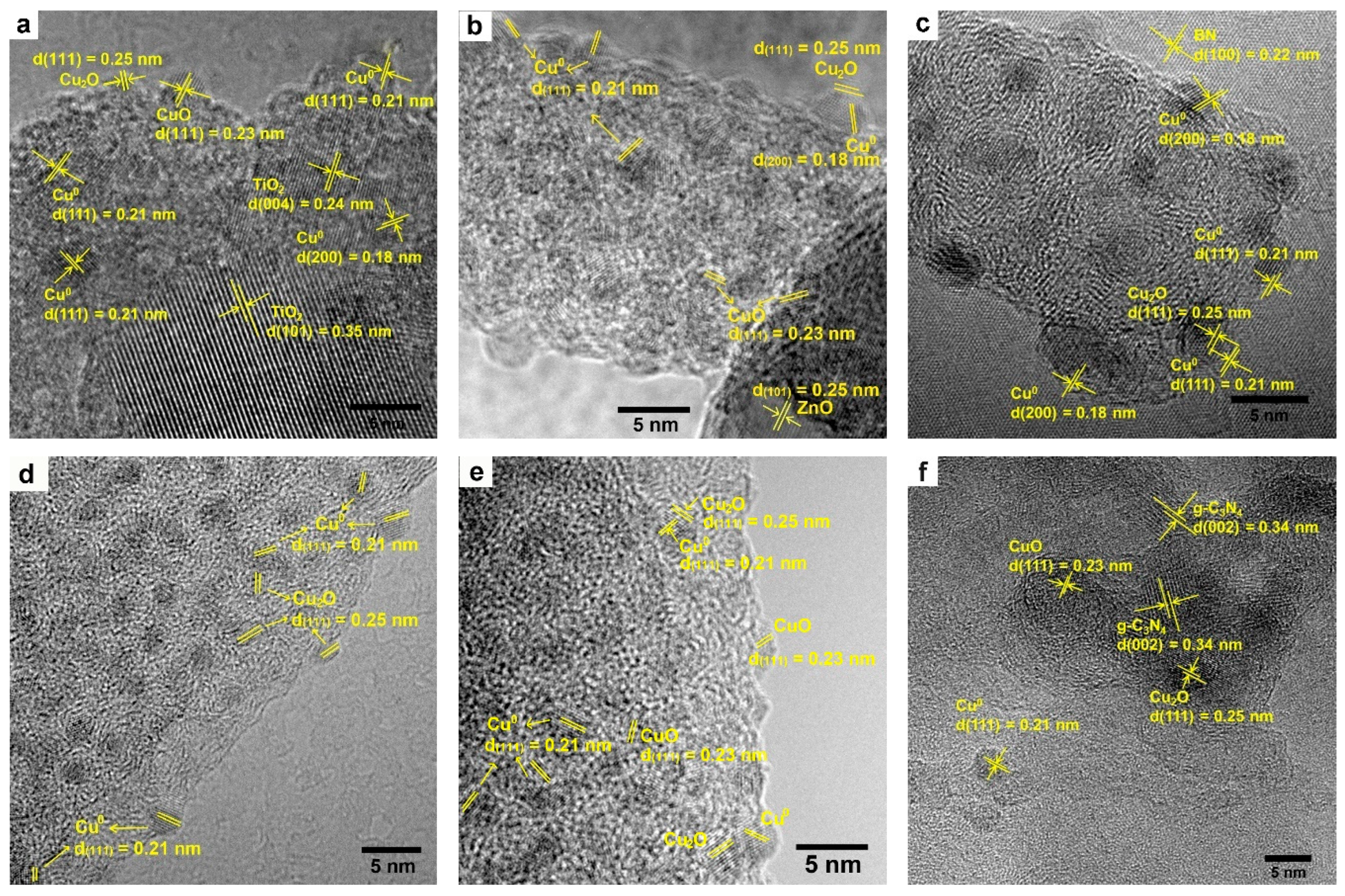
2.1. Characterization of Cu/X Composite Photocatalysts
2.2. Photocatalytic Performance of the Cu/X Catalysts
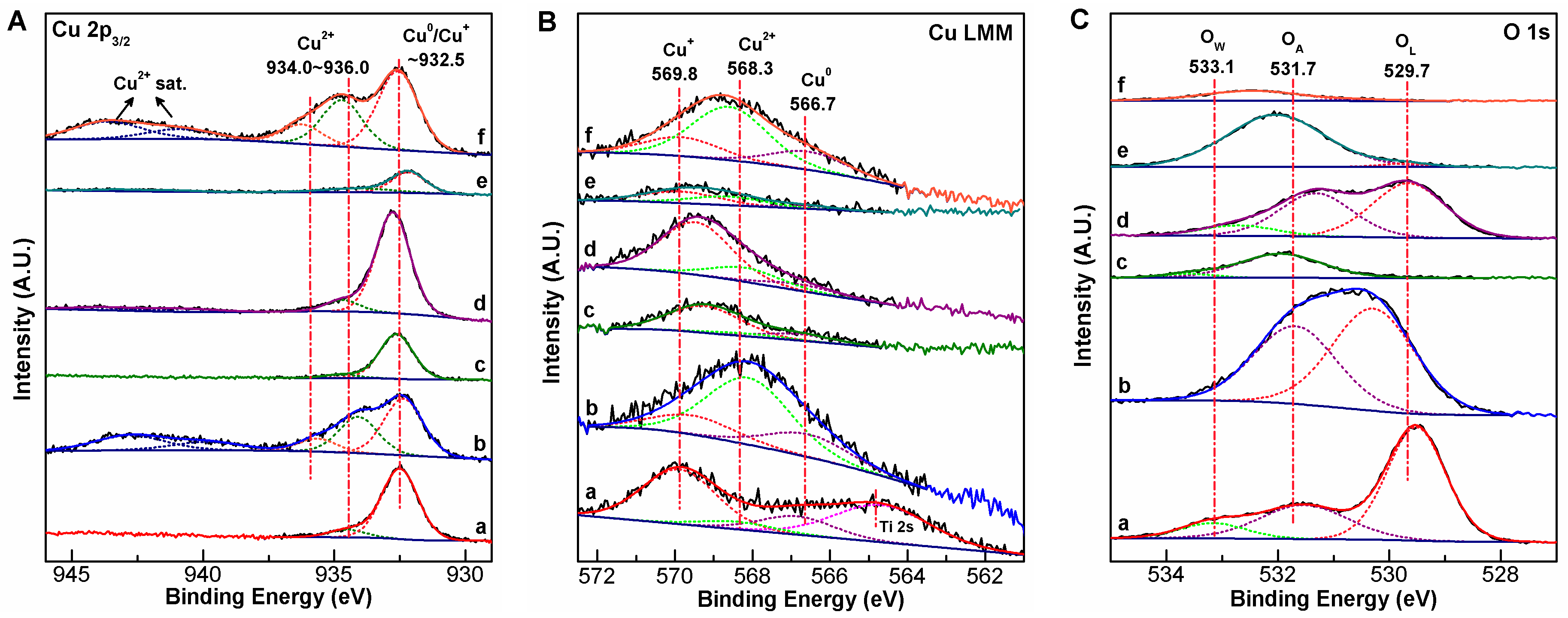
2.3. Evidence for Metal-Support Interactions
3. Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Photocatalytic Oxidative Coupling of Phenylacetylene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Tian, Y.M.; König, B. Energy- and Atom-Efficient Chemical Synthesis with Endergonic Photocatalysis. Nat. Rev. Chem. 2022, 6, 745–755. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Wang, Y.; Sui, X.; Wu, S.; Dai, W.; Zhang, Z.; Ding, Z.; Long, J. Insight into the Selectivity-Determining Step of Various Photocatalytic CO2 Reduction Products by Inorganic Semiconductors. ACS Catal. 2024, 14, 10760–10788. [Google Scholar] [CrossRef]
- Ban, L.; Zhang, Y.; Sun, D.; Zhou, Y.; Li, Y.; Xu, C.; Yang, S.; Zhang, H. Photocatalytic Hydrogen Evolution Driven by Advanced Metal Sulfides from Sustainable Multilevel Biomass and Waste Plastics. Adv. Funct. Mater. 2025, 35, 2506114. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-Metal Nanostructures for Efficient Conversion of Solar to Chemical Energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Li, H.; Gu, H.; Wang, J.; Zou, Y.; Qi, G.; Xu, J.; Deng, F.; Shen, W.; Li, J.; et al. Beyond the Thermal Equilibrium Limit of Ammonia Synthesis with Dual Temperature Zone Catalyst Powered by Solar Light. Chem 2019, 5, 2702–2717. [Google Scholar] [CrossRef]
- Xin, Y.; Yu, K.; Zhang, L.; Yang, Y.; Yuan, H.; Li, H.; Wang, L.; Zeng, J. Copper-Based Plasmonic Catalysis: Recent Advances and Future Perspectives. Adv. Mater. 2021, 33, 2008145. [Google Scholar] [CrossRef]
- Sayed, M.; Yu, J.; Liu, G.; Jaroniec, M. Non-Noble Plasmonic Metal-Based Photocatalysts. Chem. Rev. 2022, 122, 10484–10537. [Google Scholar] [CrossRef]
- Siemsen, P.; Livingston, R.C.; Diederich, F. Acetylenic Coupling: A Powerful Tool in Molecular Construction. Angew. Chem. Int. Ed. 2000, 39, 2632–2657. [Google Scholar] [CrossRef]
- Shi, W.; Lei, A. 1,3-Diyne Chemistry: Synthesis and Derivations. Tetrahedron Lett. 2014, 55, 2763–2772. [Google Scholar] [CrossRef]
- Stefani, H.A.; Guarezemini, A.S.; Cella, R. Homocoupling Reactions of Alkynes, Alkenes and Alkyl compounds. Tetrahedron 2010, 66, 7871–7918. [Google Scholar] [CrossRef]
- Alonso, F.; Yus, M. Heterogeneous Catalytic Homocoupling of Terminal Alkynes. ACS Catal. 2012, 2, 1441–1451. [Google Scholar] [CrossRef]
- Lei, J.; Su, L.; Zeng, K.; Chen, T.; Qiu, R.; Zhou, Y.; Au, C.-T.; Yin, S.-F. Recent Advances of Catalytic Processes on the Transformation of Alkynes into Functional Compounds. Chem. Eng. Sci. 2017, 171, 404–425. [Google Scholar] [CrossRef]
- Chaabane, L.; Beyou, E.; Luneau, D.; Baouab, M.H.V. Functionalization of Graphene Oxide Sheets with Magnetite Nanoparticles for the Adsorption of Copper Ions and Investigation of its Potential Catalytic Activity toward the Homocoupling of Alkynes under Green Conditions. J. Catal. 2020, 388, 91–103. [Google Scholar] [CrossRef]
- Wan, W.; Lin, C.; Qiao, Y.; Chen, L.; Chen, J. Cu Catalyst Supported on Nitrogen and Phosphorus Co-Doped Carbon Nanosheets for Homocoupling of Terminal Alkynes Using CO2 as a Soft Oxidant. ACS Appl. Nano Mater. 2021, 4, 4839–4852. [Google Scholar] [CrossRef]
- Tang, J.; Jiao, B.; Chen, W.; Ruan, F.; Li, F.; Cui, P.; Wan, C.; Ha, M.N.; Nguyen, V.N.; Ke, Q. Revealing Efficient Catalytic Performance of N-CuOx for Aerobic Oxidative Coupling of Aliphatic alkynes: A Langmuir-Hinshelwood Reaction Mechanism. Nano Res. 2022, 15, 6076–6083. [Google Scholar] [CrossRef]
- Shen, Q.; Jin, C.; Xing, Y.; Jia, Z.; Zhang, Y.; Feng, G.; Wen, X. Oxidation Coupling of Terminal Alkynes over CuPd Bimetallic Alloy Enhanced by Optimized Charge Transfer and Alloy Structure. Chem. Eng. J. 2023, 470, 144193. [Google Scholar] [CrossRef]
- Muzalevskiy, V.M.; Shastin, A.V.; Tirkasheva, S.I.; Ziyadullaev, O.E.; Parmanov, A.B.; Nenajdenko, V.G. CCl4-TMEDA-CuCl—A Novel Convenient Catalytic System for Dimerization of Terminal Acetylenes in Mild Conditions. Catalysts 2023, 13, 1330. [Google Scholar] [CrossRef]
- Ding, R.; Xia, H.; Wu, Y.; Li, Y.; Yang, W.; Xu, H. Ultrafine Copper Oxide Nanoparticles Immobilized on Poly(4-vinylpyridine)-grafted UiO-66-NH2 as Heterogeneous Catalyst for Oxidative Homocoupling of Terminal Alkynes. Colloids Surf. A 2024, 703, 135328. [Google Scholar] [CrossRef]
- Sagadevan, A.; Charpe, V.P.; Hwang, K.C. Copper(I) Chloride Catalysed Room Temperature Csp-Csp Homocoupling of Terminal Alkynes Mediated by Visible Light. Catal. Sci. Technol. 2016, 6, 7688–7692. [Google Scholar] [CrossRef]
- Zhu, Y.; Deng, N.; Feng, M.; Liu, P. On the Comparable Activity in Plasmonic Photocatalytic and Thermocatalytic Oxidative Homocoupling of Alkynes over Prereduced Copper Ferrite. Chin. J. Catal. 2019, 40, 1505–1515. [Google Scholar] [CrossRef]
- Cai, J.; Li, Y.; Zhang, M.; Li, Z. Cooperation in Cu-MOF-74-Derived Cu-Cu2O-C Nanocomposites To Enable Efficient Visible-Light-Initiated Phenylacetylene Coupling. Inorg. Chem. 2019, 58, 7997–8002. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, Y.-Q.; Zhuang, G.; Li, Q.; Yang, F.-L.; Wang, X.; Han, X. Supporting a Cu@In2O3 Core-Shell Structure on N-doped Graphitic Carbon Cuboctahedral Cages for Efficient Photocatalytic Homo-Coupling of Terminal Alkynes. J. Mater. Chem. A 2021, 9, 24909–24914. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, J.; Zhang, M. Dual Photoactive Species in CuPd for Light-switched Homo-coupling of Alkynes in Anaerobic and Base Free Condition. ChemCatChem 2024, 16, e202400715. [Google Scholar] [CrossRef]
- Yan, X.; Xu, T.; Zhan, W.; Yang, Y.; Yu, Y.; Yi, J.; He, X.; Yang, L.; Zhao, J.; Sun, L.; et al. Engineering Cu2O/Cu/N-C Interface to Induce Directional Migration of Charge for Driving Photocatalytic Homo-Coupling of Terminal alkynes. Nano Res. 2024, 17, 6895–6902. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Zhu, Y.; Wang, Y.; Wang, C. Enhanced CO2 Photoreduction Activity of Black TiO2-Coated Cu Nanoparticles under Visible Light Irradiation: Role of Metallic Cu. Appl. Catal. A Gen. 2016, 510, 34–41. [Google Scholar] [CrossRef]
- DeSario, P.A.; Gordon, W.O.; Balboa, A.; Pennington, A.M.; Pitman, C.L.; McEntee, M.; Pietron, J.J. Photoenhanced Degradation of Sarin at Cu/TiO2 Composite Aerogels: Roles of Bandgap Excitation and Surface Plasmon Excitation. ACS Appl. Mater. Interfaces 2021, 13, 12550–12561. [Google Scholar] [CrossRef]
- Liu, P.; Dörfler, A.; Tabrizi, A.A.; Skokan, L.; Rawach, D.; Wang, P.; Peng, Z.; Zhang, J.; Ruediger, A.P.; Claverie, J.P. In Operando Photoswitching of Cu Oxidation States in Cu-Based Plasmonic Heterogeneous Photocatalysis for Efficient H2 Evolution. ACS Appl. Mater. Interfaces 2023, 15, 27832–27844. [Google Scholar] [CrossRef]
- Khan, A.; Pivert, M.L.; Ranjbari, A.; Dragoe, D.; Bahena-Uribe, D.; Colbeau-Justin, C.; Herrero, C.; Rutkowska-Zbik, D.; Deschamps, J.; Remita, H. Cu-Based MOF/TiO2 Composite Nanomaterials for Photocatalytic Hydrogen Generation and the Role of Copper. Adv. Funct. Mater. 2025, 2501736. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Z.; Gao, G.; Xiao, G.; Du, A.; Bottle, S.; Sarina, S.; Zhu, H. Stable Copper Nanoparticle Photocatalysts for Selective Epoxidation of Alkenes with Visible Light. ACS Catal. 2017, 7, 4975–4985. [Google Scholar] [CrossRef]
- Lou, Y.; Zhang, Y.; Cheng, L.; Chen, J.; Zhao, Y. A Stable Plasmonic Cu@Cu2O/ZnO Heterojunction for Enhanced Photocatalytic Hydrogen Generation. ChemSusChem 2018, 11, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Jiang, P.; Yuan, H.; He, R.; Zhu, W.; Wang, L. Cu-Based Nanocrystals on ZnO for Uranium Photoreduction: Plasmon-Assisted Activity and Entropy-Driven Stability. Appl. Catal. B Environ. 2021, 288, 119978. [Google Scholar] [CrossRef]
- Zhang, S.-W.; Li, S.; Zhang, B.-P.; Yu, D.; Zhang, Z.; Li, J.-F. Copper-Nanoparticle-Dispersed Amorphous BaTiO3 Thin Films as Hole-Trapping Centers: Enhanced Photocatalytic Activity and Stability. RSC Adv. 2019, 9, 5045–5052. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, G.; Zhang, W.; Zhu, L.; Cao, B.; Gao, J.; Shi, X.; Huang, Y.; Liu, P.; Hojamberdiev, M. Dual-Functional Copper (Cu0/Cu2+)-Modified SrTiO3-δ Nanosheets with Enhanced Photothermal Catalytic Performance for CO2 Reduction and H2 Evolution. Chem. Eng. J. 2023, 452, 139378. [Google Scholar] [CrossRef]
- Li, M.-Y.; Lu, W.-D.; He, L.; Schüth, F.; Lu, A.-H. Tailoring the Surface Structure of Silicon Carbide Support for Copper Catalyzed Ethanol Dehydrogenation. ChemCatChem 2019, 11, 481–487. [Google Scholar] [CrossRef]
- Zhou, G.; Yang, F.; Zhu, X.; Feng, W.; Chen, D.; Fang, J. Copper-Copper Oxide Heterostructural Nanocrystals Anchored on g-C3N4 Nanosheets for Efficient Visible-Light-Driven Photo-Fenton-like Catalysis. Molecules 2025, 30, 144. [Google Scholar] [CrossRef]
- Liu, P.; Hensen, E.J.M. Highly Efficient and Robust Au/MgCuCr2O4 Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde. J. Am. Chem. Soc. 2013, 135, 14032–14035. [Google Scholar] [CrossRef]
- Yue, L.; Wang, J.; Liu, P. Promoting Effect of Copper Doping on LaMO3 (M = Mn, Fe, Co, Ni) Perovskite-Supported Gold Catalysts for Selective Gas-Phase Ethanol Oxidation. Catalysts 2025, 15, 176. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Yue, L.; Filot, I.A.W.; Hensen, E.J.M.; Liu, P. Unveiling the Au-Mn-Cu Synergy in Au/LaMnCuO3 Catalysts for Selective Ethanol Oxidation. Chin. J. Catal. 2025, 75, 34–48. [Google Scholar] [CrossRef]
- Dabera, G.D.M.R.; Walker, M.; Sanchez, A.M.; Pereira, H.J.; Beanland, R.; Hatton, R.A. Retarding Oxidation of Copper Nanoparticles Without Electrical Isolation and the Size Dependence of Work Function. Nat. Commun. 2017, 8, 1894. [Google Scholar] [CrossRef]
- Liu, P.; Li, T.; Chen, H.; Hensen, E.J.M. Optimization of Au0-Cu+ Synergy in Au/MgCuCr2O4 Catalysts for Aerobic Oxidation of Ethanol to Acetaldehyde. J. Catal. 2017, 347, 45–56. [Google Scholar] [CrossRef]
- Hu, W.; Li, D.; Yang, Y.; Li, T.; Chen, H.; Liu, P. Copper Ferrite Supported Gold Nanoparticles as Efficient and Recyclable Catalyst for Liquid-Phase Ethanol Oxidation. J. Catal. 2018, 357, 108–117. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, C.; Du, M.; Wu, Y.; Ren, C.; Ding, K.; Song, M.; Huang, C. Porous Hexagonal Boron Nitride Sheets: Effect of Hydroxyl and Secondary Amino Groups on Photocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2018, 1, 4566–4575. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Zhang, M.; Zhao, L.-B.; Feng, J.-M.; Wu, D.-Y.; Ren, B.; Tian, Z. Activation of Oxygen on Gold and Silver Nanoparticles Assisted by Surface Plasmon Resonances. Angew. Chem. Int. Ed. 2014, 53, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Aslam, U.; Boerigter, C.; Morabito, M. Photochemical Transformations on Plasmonic Metal Nanoparticles. Nat. Mater. 2015, 14, 567–576. [Google Scholar] [CrossRef]
- DeSario, P.A.; Pietron, J.J.; Brintlinger, T.H.; McEntee, M.; Parker, J.F.; Baturina, O.; Stroud, R.M.; Rolison, D.R. Oxidation-Stable Plasmonic Copper Nanoparticles in Photocatalytic TiO2 Nanoarchitectures. Nanoscale 2017, 9, 11720–11729. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, J.; Chen, S.; Bai, J.; Li, J.; Zhang, Y.; Li, L.; Xia, L.; Rahim, M.; Xu, Q.; et al. Bird-Nest Structured ZnO/TiO2 as a Direct Z-Scheme Photoanode with Enhanced Light Harvesting and Carriers Kinetics for Highly Efficient and Stable Photoelectrochemical Water Splitting. Appl. Catal. B Environ. 2020, 267, 118599. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Huang, D.; Zeng, G.; Huang, J.; Lai, C.; Zhou, C.; Wang, W.; Guo, H.; Xue, W.; et al. Boron Bitride Quantum Dots Decorated Ultrathin Porous g-C3N4: Intensified Exciton Dissociation and Charge Transfer for Promoting Visible-Light-Driven Molecular Oxygen Activation. Appl. Catal. B Environ. 2019, 245, 87–99. [Google Scholar] [CrossRef]
- Yu, W.; Difa Xu, D.; Peng, T. Enhanced Photocatalytic Activity of g-C3N4 for Selective CO2 Reduction to CH3OH via Facile Coupling of ZnO: A Direct Z-Scheme Mechanism. J. Mater. Chem. A 2015, 3, 19936–19947. [Google Scholar] [CrossRef]
- Hao, C.-H.; Guo, X.-N.; Pan, Y.-T.; Chen, S.; Jiao, Z.-F.; Yang, H.; Guo, X.-Y. Visible-Light-Driven Selective Photocatalytic Hydrogenation of Cinnamaldehyde over Au/SiC Catalysts. J. Am. Chem. Soc. 2016, 138, 9361–9364. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, M.; Gao, K.; Fu, R.; Zhang, S.; Xiao, Y.; Guo, J.; Wang, Z.; Wang, H.; Zhao, Y.; et al. Boosting Photocatalytic Degradation of Levofloxacin over Plasmonic TiO2-x/TiN Heterostructure. Appl. Surf. Sci. 2024, 655, 159516. [Google Scholar] [CrossRef]
- Madasu, M.; Huang, M.H. Cu2O Polyhedra for Aryl Alkyne Homocoupling Reactions. Catal. Sci. Technol. 2020, 10, 6948–6952. [Google Scholar] [CrossRef]
- Pary, F.F.; Tirumala, R.T.A.; Andiappan, M.; Nelson, T.L. Copper(I) Oxide Nanoparticle-Mediated C–C Couplings for Synthesis of Polyphenylenediethynylenes: Evidence for a Homogeneous Catalytic Pathway. Catal. Sci. Technol. 2021, 11, 2414–2421. [Google Scholar] [CrossRef]
- Shanmugam, M.; Sagadevan, A.; Charpe, V.P.; Pampana, V.K.K.; Hwang, K.C. Cu2O Nanocrystals-Catalyzed Photoredox Sonogashira Coupling of Terminal Alkynes and Arylhalides Enhanced by CO2. ChemSusChem 2020, 13, 287–292. [Google Scholar] [CrossRef]
- Lin, Z.; Xiao, Z.; Liu, Y.; Wang, Y.; Chen, S.; Zhang, J.; Chen, Y.; Zhang, X.; Zhang, G.; Li, D.; et al. Insights into Copper(I) Phenylacetylide with in-situ Transformation of Oxygen and Enhanced Visible-Light Response for Water Decontamination: Cu–O Bond Promotes Exciton Dissociation and Charge Transfer. J. Colloid Interf. Sci. 2024, 671, 1–14. [Google Scholar] [CrossRef]
- Wang, K.; Bielan, Z.; Endo-Kimura, M.; Janczarek, M.; Zhang, D.; Kowalski, D.; Zielinska-Jurek, A.; Markowska-Szczupak, A.; Bunsho Ohtani, B.; Kowalska, E. On the Mechanism of Photocatalytic Reactions on CuxO@TiO2 Core-Shell Photocatalysts. J. Mater. Chem. A 2021, 9, 10135–10145. [Google Scholar] [CrossRef]
- Quan, X.; Guo, F.; Li, R.; Zhang, H.; Shen, Z.; Zhang, J. Palladium−Copper Alloy Nanoparticles Supported on Carbon Nitride Nanosheets as a Photocatalyst for Degradation of Ciprofloxacin. ACS Appl. Nano Mater. 2024, 7, 23228–23239. [Google Scholar] [CrossRef]
- Hu, T.; Yue, Z.; Wang, Y.; Yu, Y.; Chang, Y.; Pei, L.; Chen, W.; Han, P.; Martens, W.; Waclawik, E.R.; et al. Cu@CuOx/WO3 with Photo-Regulated Singlet Oxygen and Oxygen Adatoms Generation for Selective Photocatalytic Aromatic Amines to Imines. J. Colloid Interf. Sci. 2024, 663, 632–643. [Google Scholar] [CrossRef]
- Tirumala, R.T.A.; Gyawali, S.; Le, T.; Kumar, S.; Tan, S.; Wang, B.; Bristow, A.D.; Andiappan, M. Visible-Light-Driven Photocatalytic Carbon-Carbon Coupling Reaction under Atmospheric Temperature and Pressure Conditions Using Hybrid Cu2O-Pd Nanostructures. ACS Appl. Mater. Interfaces 2025, 17, 26651–26660. [Google Scholar] [CrossRef]
- Tirumala, R.T.A.; Ramakrishnan, S.B.; Mohammadparast, F.; Khatri, N.; Arumugam, S.M.; Tan, S.; Kalkan, A.K.; Andiappan, M. Structure-Property-Performance Relationships of Dielectric Cu2O Nanoparticles for Mie Resonance-Enhanced Dye Sensitization. ACS Appl. Nano Mater. 2022, 5, 6699–6707. [Google Scholar] [CrossRef]
- Tirumala, R.T.A.; Khatri, N.; Ramakrishnan, S.B.; Mohammadparast, F.; Khan, M.T.; Tan, S.; Wagle, P.; Puri, S.; McIlroy, D.N.; Kalkan, A.K.; et al. Tuning Catalytic Activity and Selectivity in Photocatalysis on MieResonant Cuprous Oxide Particles: Distinguishing Electromagnetic Field Enhancement Effect from the Heating Effect. ACS Sustainable Chem. Eng. 2023, 11, 15931–15940. [Google Scholar] [CrossRef]
- Simukaitis, M.; Stewart, S.; Sun, Y. Visible-Light-Driven Desulfurization Reaction Using Partially Oxidized TiN Nanoparticles as Photocatalysts. J. Phys. Chem. C 2025, 129, 7780–7786. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Wu, Z.; Liang, J.; Chen, X.; Leng, L.; Wang, H.; Wang, H. Metal-Free Efficient Photocatalyst for Stable Visible-Light Photocatalytic Degradation of Refractory Pollutant. Appl. Catal. B Environ. 2018, 221, 715–725. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Li, J.; Guo, X.; Bai, G.; Tong, X.; Jin, G.; Guo, X. Photocatalytic Sonogashira Reaction over Silicon Carbide Supported Pd–Cu Alloy Nanoparticles under Visible Light Irradiation. Catal. Sci. Technol. 2018, 8, 3357–3362. [Google Scholar] [CrossRef]
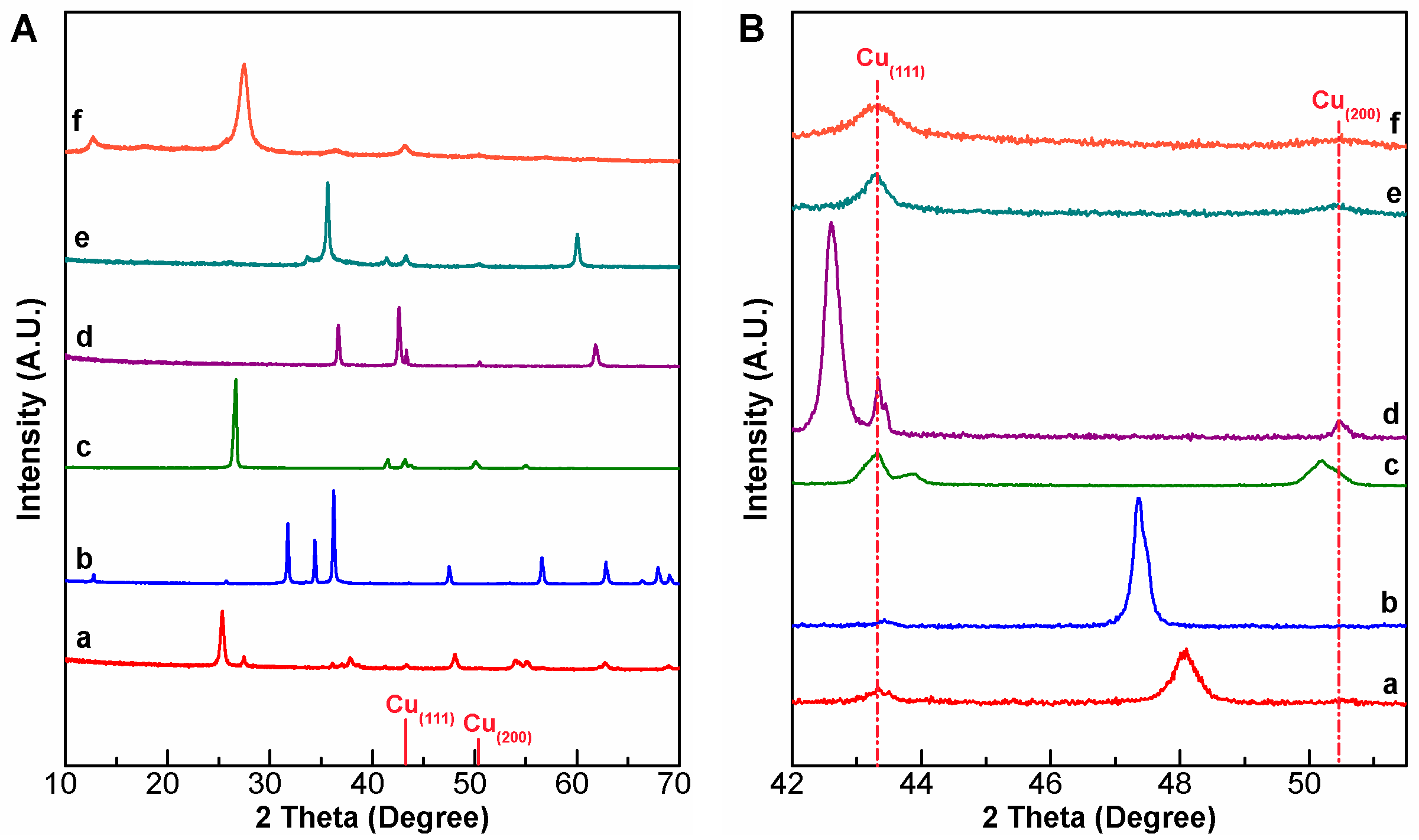
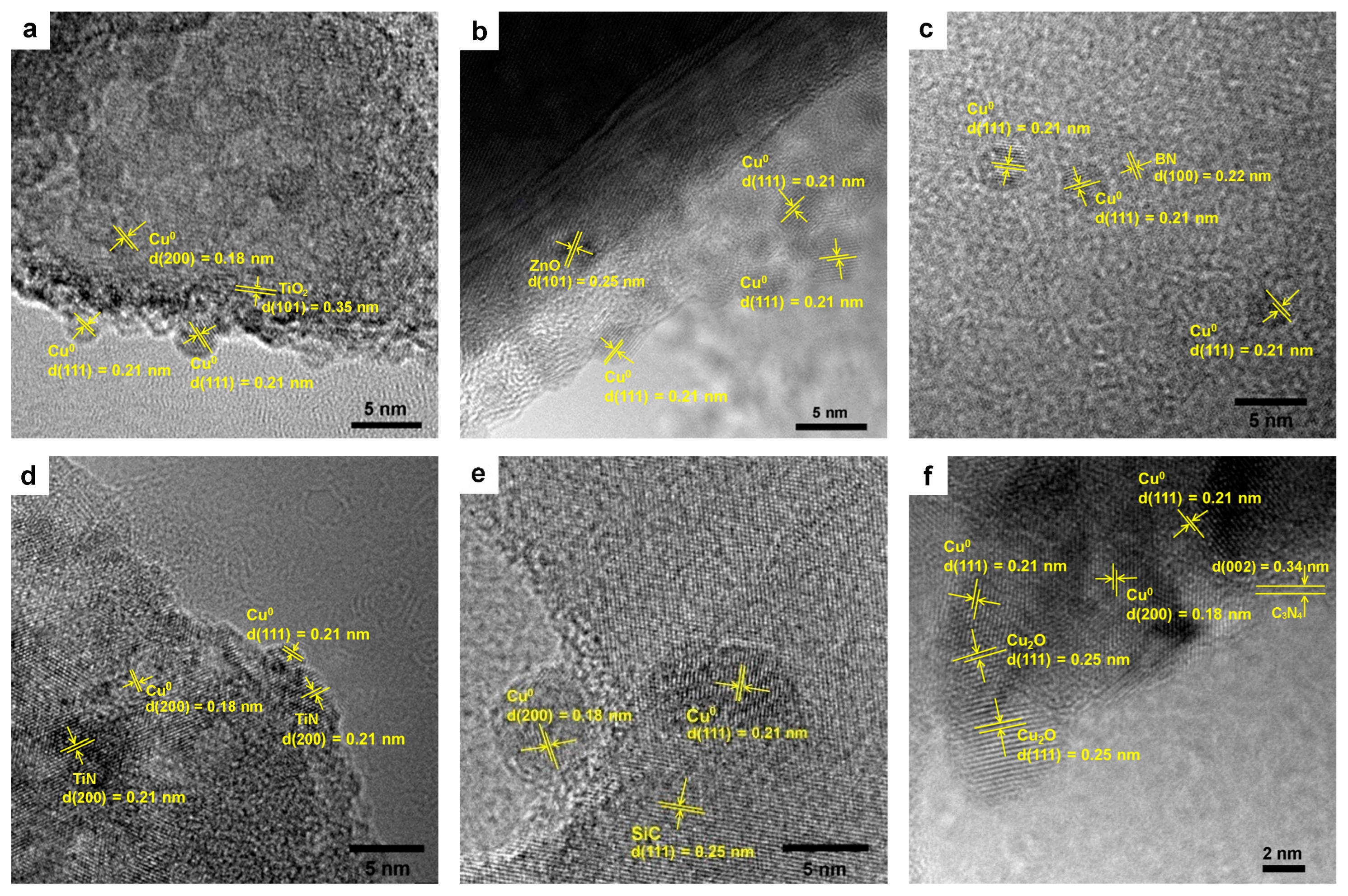

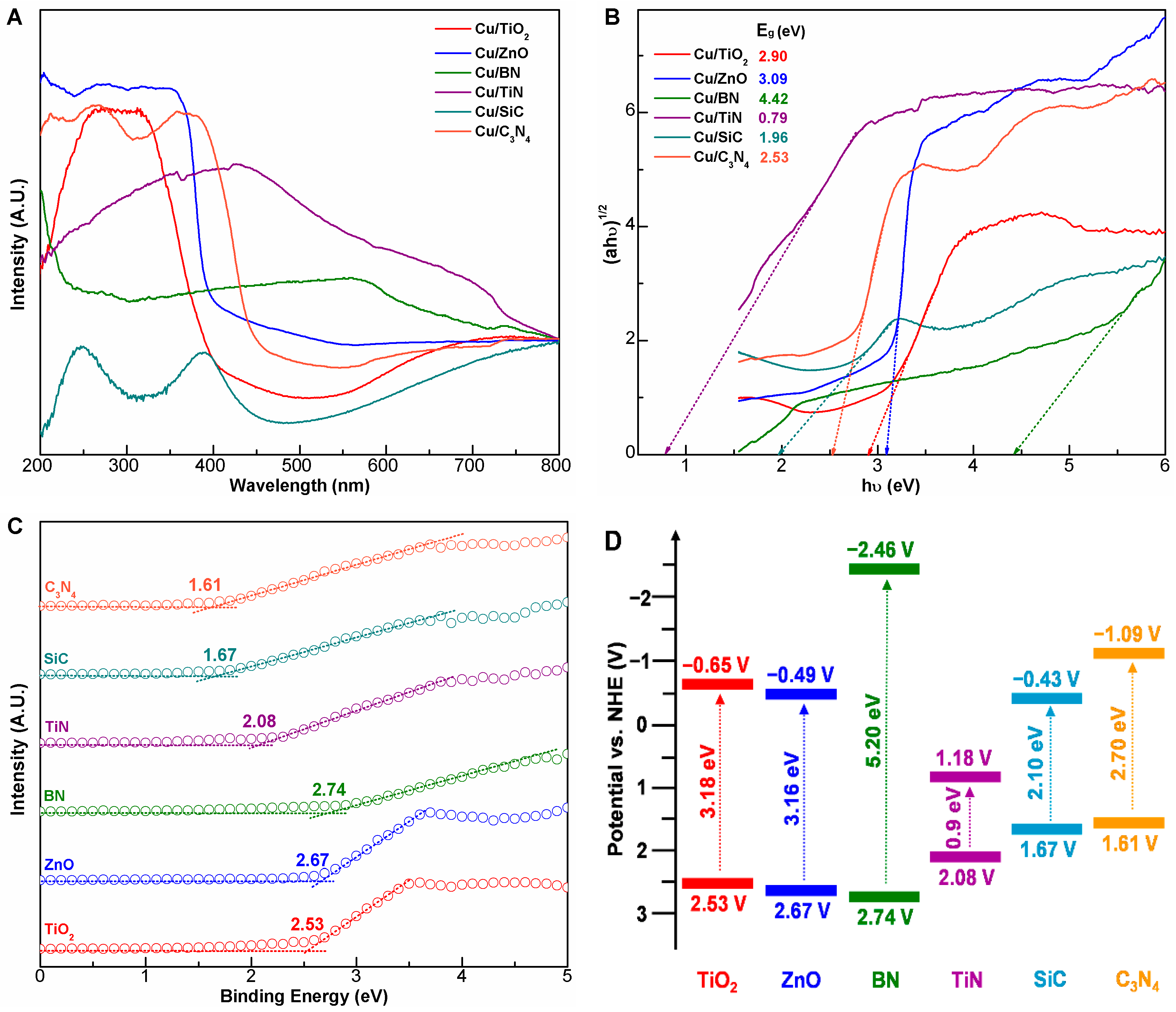
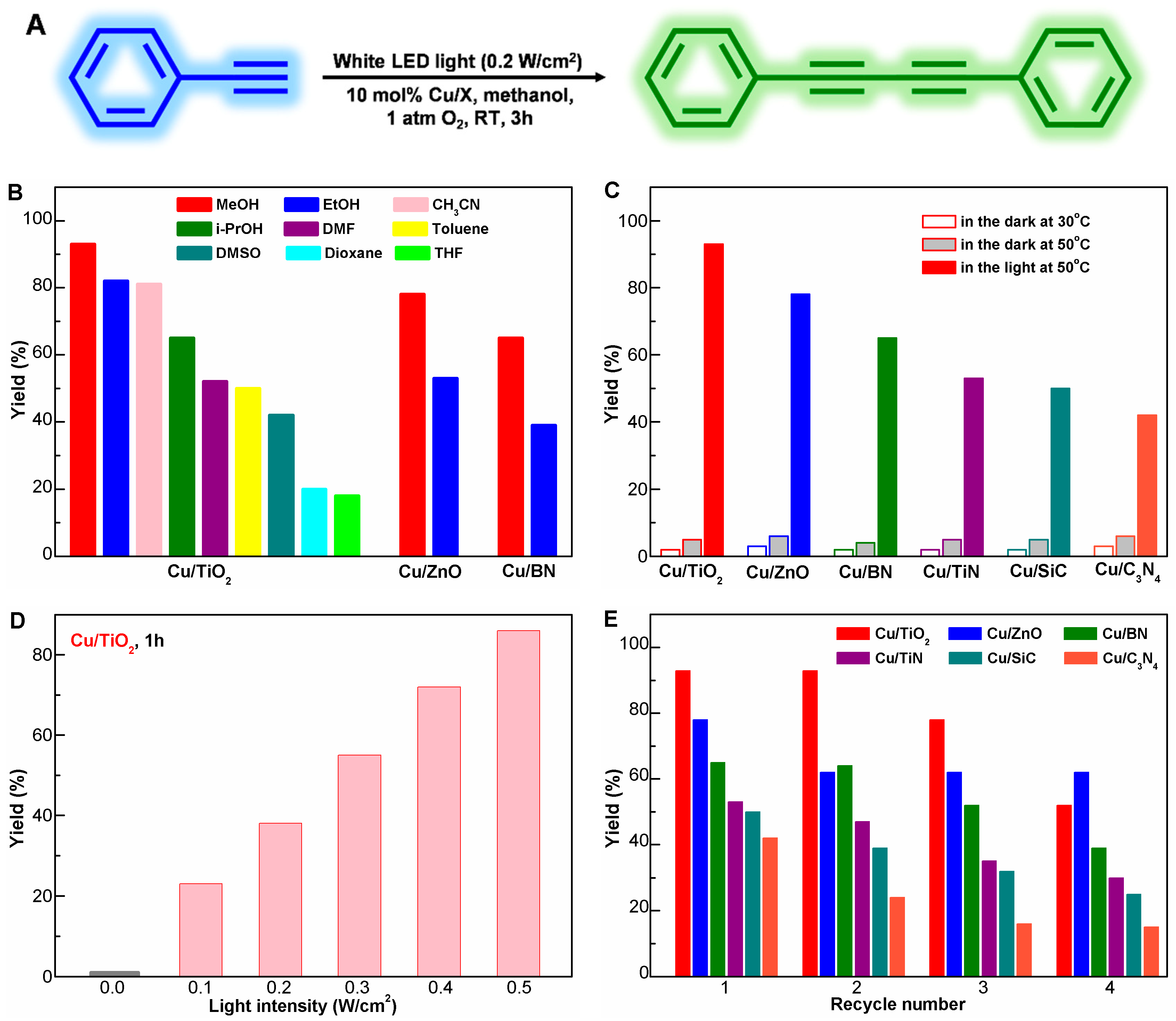
| Catalyst | dCu a (nm) | [Cu] b (wt%) | SBET (m2/g) | XPS Peak Area Ratio (%) | Eg c (eV) | |||
|---|---|---|---|---|---|---|---|---|
| Cu0/Cu | Cu+/Cu | OL/O | OA/O | |||||
| Cu/TiO2 | 2.3 | 4.9 | 46.8 | 38 | 62 | 88 | 12 | 2.90 (3.18) |
| Cu/ZnO | 2.2 | 4.3 | 16.8 | 14 | 38 | 60 | 40 | 3.09 (3.16) |
| Cu/BN | 2.5 | 5.1 | 6.8 | 17 | 83 | 0 | 74 | 4.42 (5.20) |
| Cu/TiN | 3.3 | 5.0 | 2.3 | 12 | 88 | 49 | 37 | 0.79 (0.90) |
| Cu/SiC | 4.7 | 5.2 | 43.0 | 15 | 85 | 0 | 100 | 1.96 (2.10) |
| Cu/C3N4 | 5.5 | 3.7 | 20.4 | 24 | 45 | 14 | 86 | 2.53 (2.70) |
| Catalyst | dCu a (nm) | Cu Content (wt%) b | XPS Peak Area Ratio (%) c | Yield d (%) | TOF e (h−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | 1st | 4th | Cu0/Cu | Cu+/Cu | OL/O | OA/O | ||||
| Cu/TiO2 | 2.8 | 4.9 | 4.3 | 3.2 | 23 | 68 | 63 | 28 | 52 | 3.4 |
| Cu/ZnO | 2.7 | 4.3 | 3.7 | 3.3 | 21 | 18 | 57 | 43 | 62 | 4.0 |
| Cu/BN | 3.0 | 5.1 | 4.4 | 3.1 | 18 | 75 | 0 | 91 | 39 | 2.7 |
| Cu/TiN | 2.6 | 5.0 | 3.8 | 2.8 | 9 | 82 | 50 | 40 | 30 | 2.3 |
| Cu/SiC | 2.8 | 5.2 | 3.5 | 2.1 | 10 | 52 | 3 | 97 | 25 | 2.5 |
| Cu/C3N4 | 4.3 | 3.7 | 2.7 | 1.1 | 20 | 21 | 7 | 93 | 15 | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, N.; Wu, Y.; Sun, Y.; Liu, P. Promotional Effect of Semiconductor-Supported Plasmonic Copper Nanoparticles in Visible-Light-Driven Photocatalytic Oxidative Homocoupling of Alkynes. Catalysts 2025, 15, 1045. https://doi.org/10.3390/catal15111045
Deng N, Wu Y, Sun Y, Liu P. Promotional Effect of Semiconductor-Supported Plasmonic Copper Nanoparticles in Visible-Light-Driven Photocatalytic Oxidative Homocoupling of Alkynes. Catalysts. 2025; 15(11):1045. https://doi.org/10.3390/catal15111045
Chicago/Turabian StyleDeng, Nan, Yaqi Wu, Yi Sun, and Peng Liu. 2025. "Promotional Effect of Semiconductor-Supported Plasmonic Copper Nanoparticles in Visible-Light-Driven Photocatalytic Oxidative Homocoupling of Alkynes" Catalysts 15, no. 11: 1045. https://doi.org/10.3390/catal15111045
APA StyleDeng, N., Wu, Y., Sun, Y., & Liu, P. (2025). Promotional Effect of Semiconductor-Supported Plasmonic Copper Nanoparticles in Visible-Light-Driven Photocatalytic Oxidative Homocoupling of Alkynes. Catalysts, 15(11), 1045. https://doi.org/10.3390/catal15111045






