Recent Progress in Preparations and Multifunctional Applications Towards MOF/GDY Composites and Their Derivative Materials
Abstract
1. Introduction
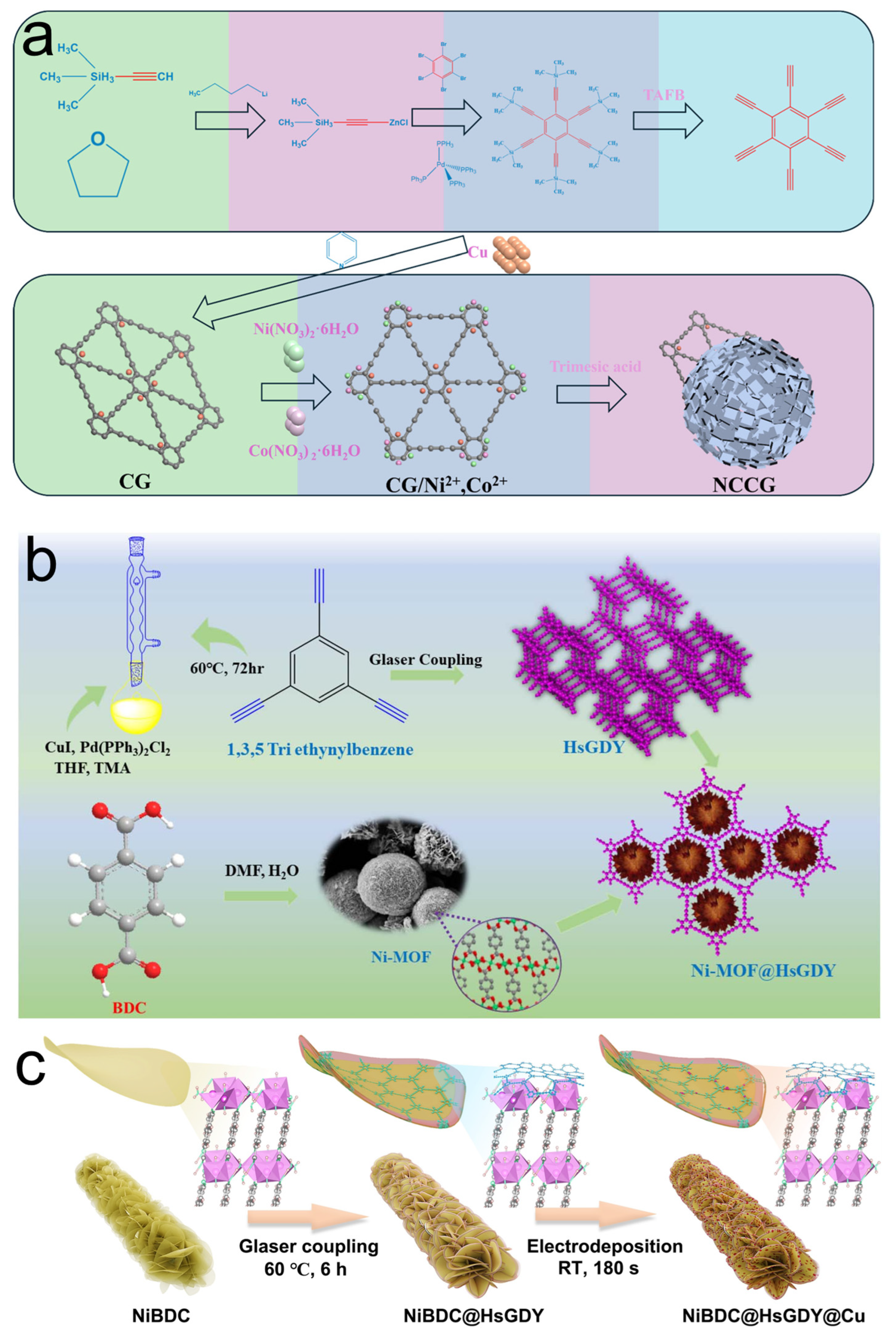
2. Preparations of MOF/GDY Composite Materials
2.1. Physical Mixing Strategy
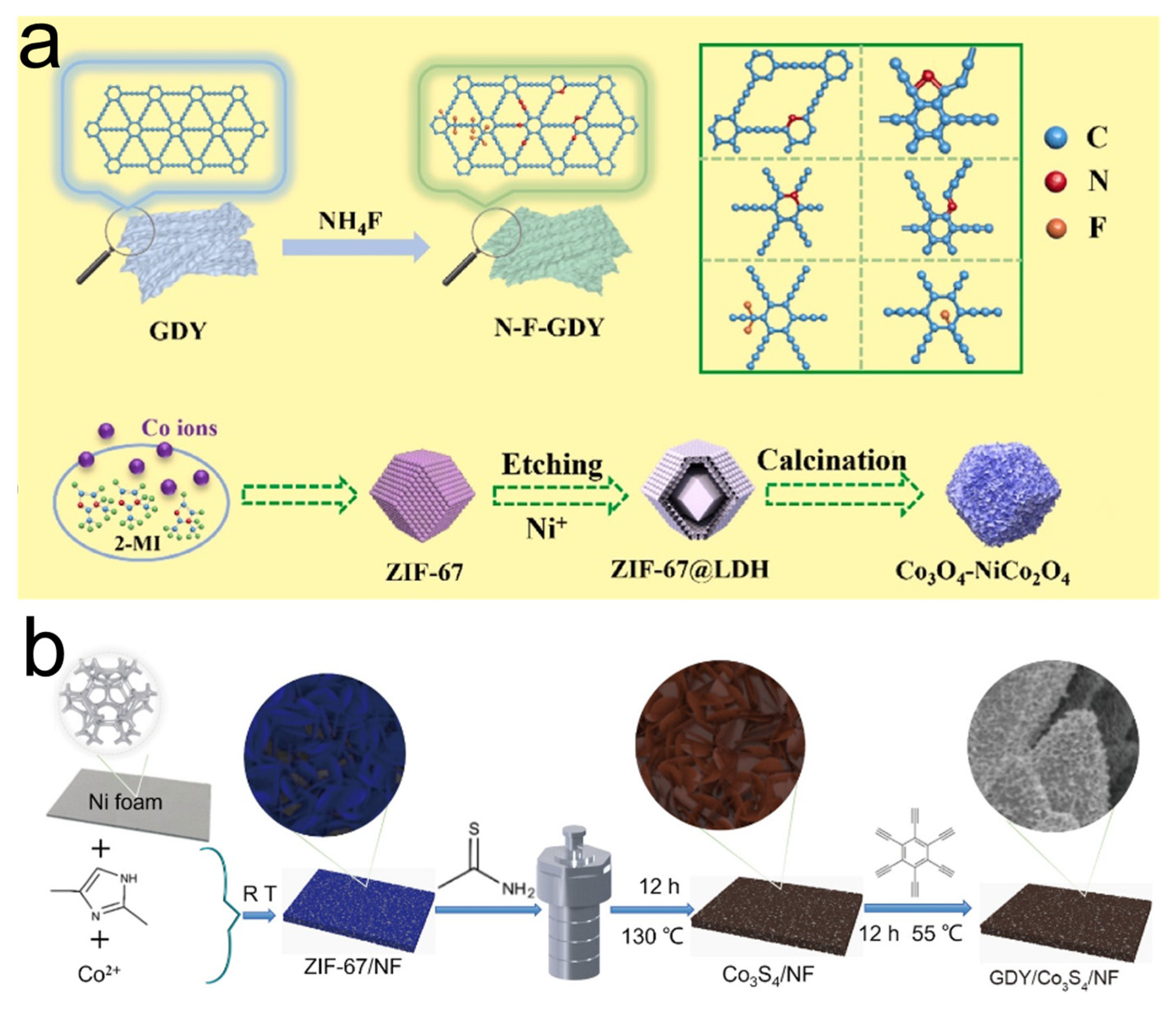
2.2. MOFs In Situ Anchored on GDY
2.3. GDY In Situ Grown on MOFs
2.4. MOF/GDY Derivative Composites
3. Applications of MOF/GDY Composites and Derivative Materials
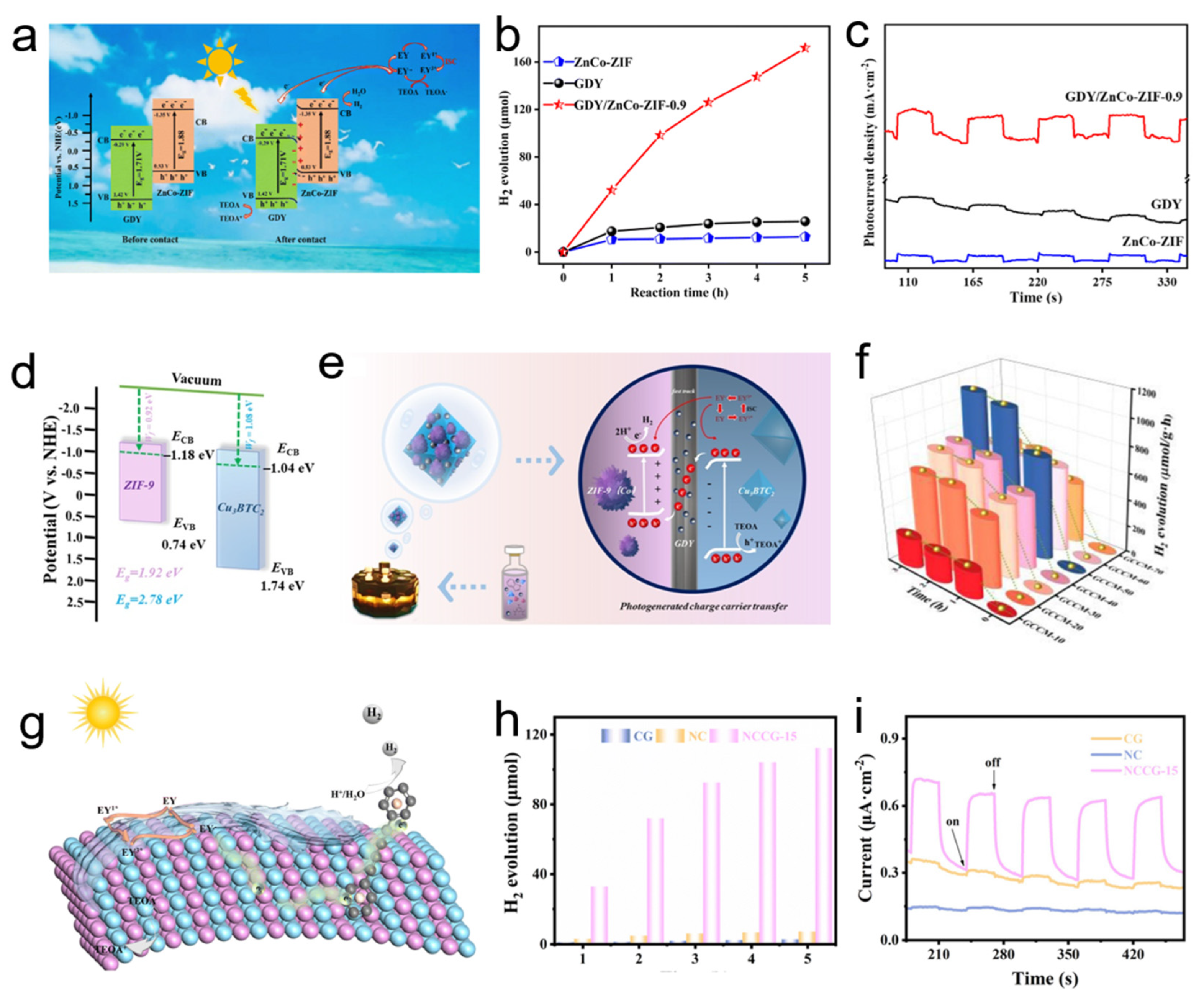
3.1. Application in Catalysis
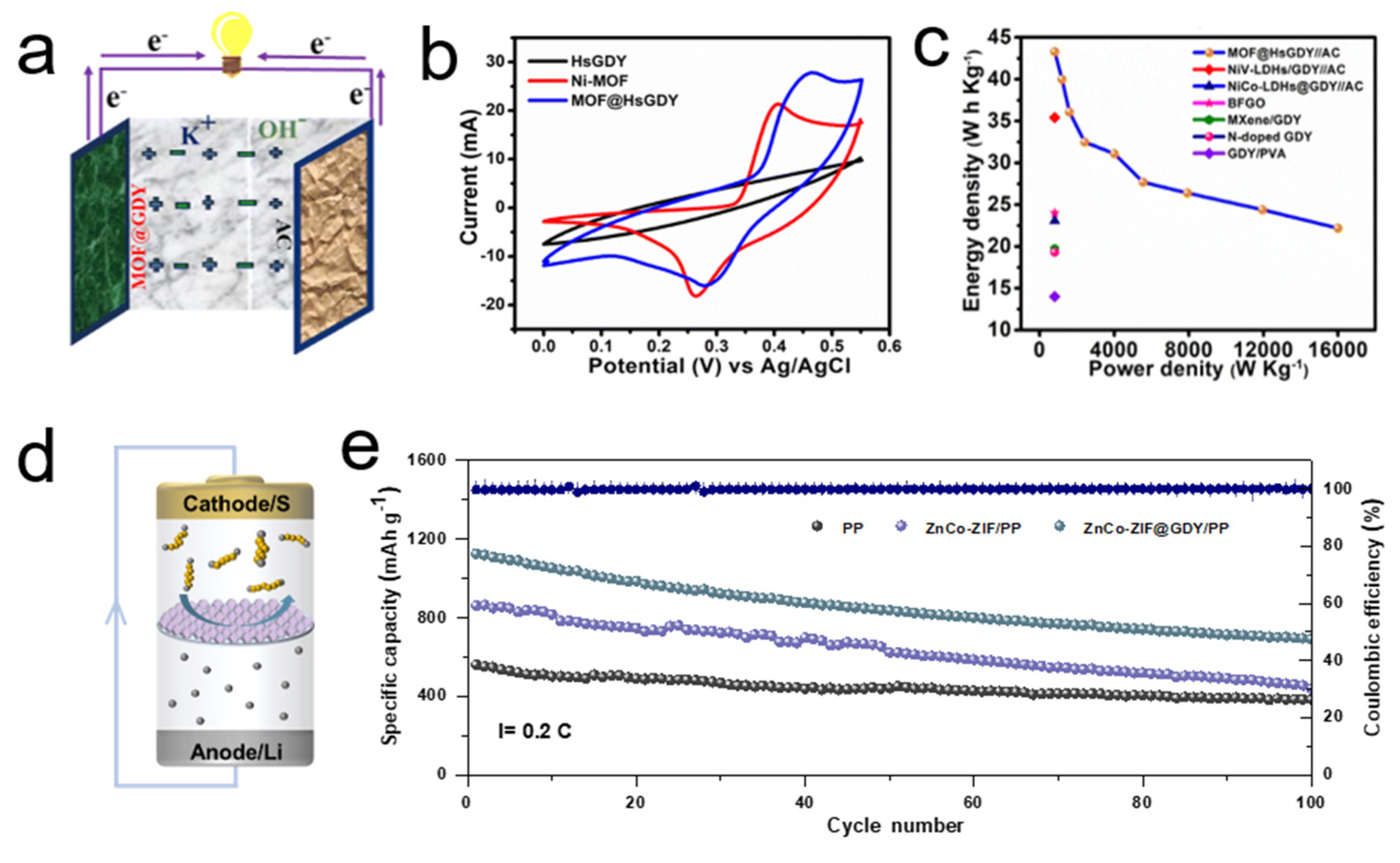
3.2. Application in Energy Storage
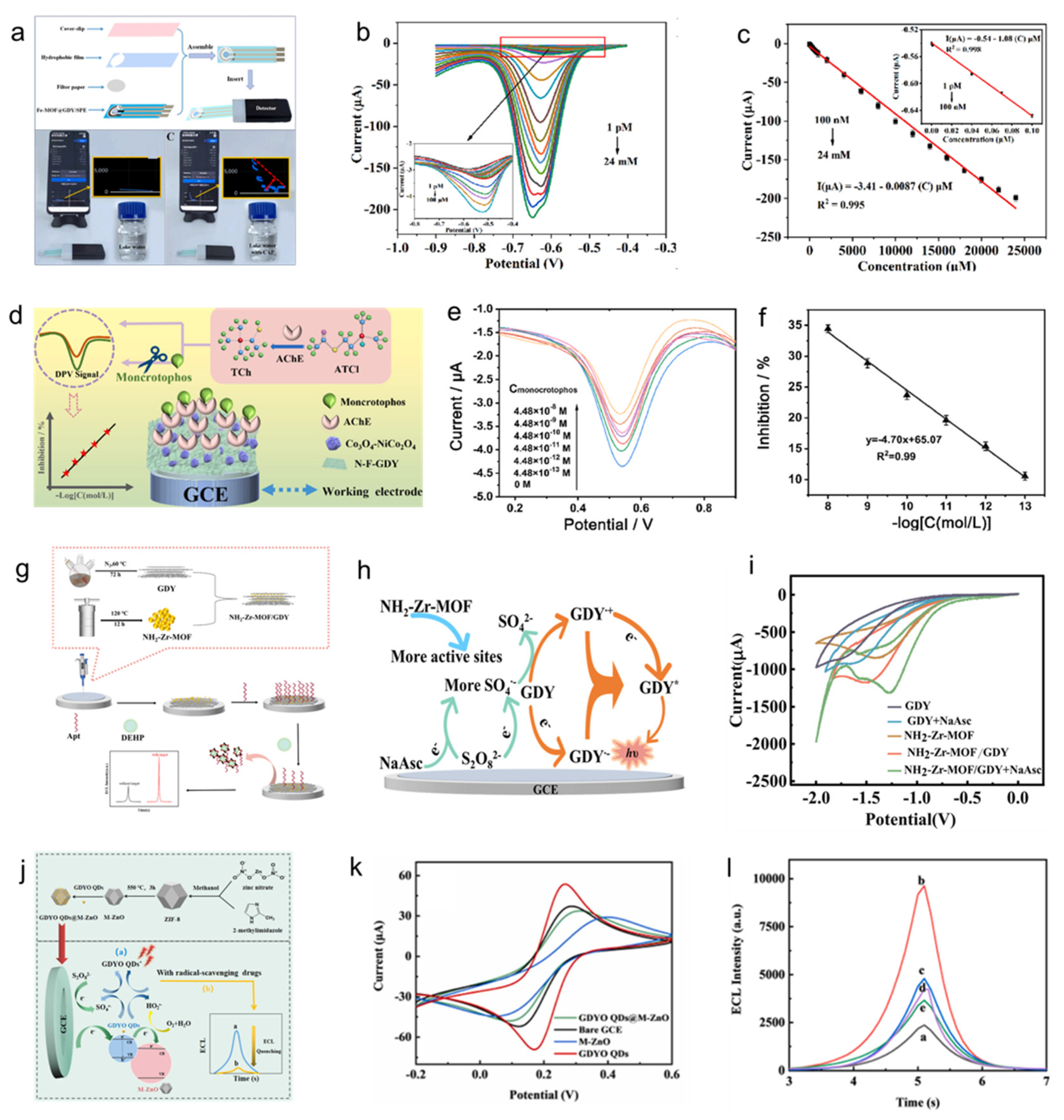
3.3. Application in Biological Sensors
| Materials | Performance | Role of GDY | Refs. | |
|---|---|---|---|---|
| GDY/ZnCo-ZIF-0.9 | Catalysis | H2 evolution: 171.79 µmol (6.67× vs. GDY) (13.5× vs. ZnCo-ZIF) | S-scheme heterojunction | [82] |
| GDY/CoMo-MOF-60 | Catalysis | H2 evolution: 300 µmol (19.61× vs. GDY) (9.03× vs. CoMo-MOF) | S-scheme heterojunction | [83] |
| NiCo-MOF | Catalysis | H2 evolution: 6.89 µmol | [86] | |
| NiCo-MOF/Cu-GDY-15 | H2 evolution: 112.22 µmol (47.0× vs. Cu-GDY) (16.3× vs. NiCo-MOF) | Strongly coupled electronic interface and ohmic contact | ||
| NiBDC | Catalysis | Onset potential: −142 mV vs. RHE | [88] | |
| HsGDY@Cu | Onset potential: 65 mV vs. RHE | |||
| Ni-MOFs@HsGDY@Cu | Onset potential: 90.5 mV vs. RHE | Conductive and reactive bridge | ||
| Co3S4/NF | Catalysis | Overpotential: 230 mV @ 10 mA cm−2 Tafel Slope: 78.9 mV dec−1 | [90] | |
| GDY/Co3S4/NF | Overpotential: 223 mV @ 10 mA cm−2 Tafel Slope: 46.5 mV dec−1 | Modulating the electronic configuration and enhancing durability | ||
| Cu3BTC2/ZIF-9(Co)/GDY | Catalysis | H2 Evolution Rate: 1126 μmol g−1 h−1 (~30× vs. Cu3BTC2) (~16× vs. ZIF-9(Co)) | Electron transport layer | [91] |
| Polymer-HKUST-1/CF | Catalysis | H2O2 conversions: ~68% | [93] | |
| HKUST-1/GDY/CF | H2O2 conversions: ~94% | Facilitate electron transfer | ||
| HsGDY | Energy storage | Specific Capacitance: 154 F g−1 @ 1 A g−1 | [87] | |
| Ni-MOF | Specific Capacitance: 426 F g−1 @ 1 A g−1 | |||
| Ni-MOF@HsGDY | Specific Capacitance: 982 F g−1 @ 1 A g−1 (~6.4× vs. HsGDY) (~2.3× vs. Ni-MOF) | Conductive substrate | ||
| ZnCo-ZIF/PP | Energy storage | Initial Specific Capacity: 862.2 mAh g−1 @ 0.2C | [95] | |
| ZnCo-ZIF@GDY | Initial Specific Capacity: 1126.1 mAh g−1 @ 0.2C | Enhance electrical conductivity; Suppress polysulfide shuttle effect | ||
| Fe-MOF | Biological sensors | Limit of detection: 0.011μM | [84] | |
| Fe-MOF@GDY | Limit of detection: 0.54 pM Detection Range: 1 pM–24 mM | Charge transfer enhancer and anchoring substrate | ||
| IL1-MWCNTs/AChE/GCE | Biological sensors | Limit of detection: 33 pM Detection Range: 0.1–5000 nM | [89] | |
| AChE-NF/NiCo2O4–Co3O4/N-F-GDY/GCE | Limit of detection: 0.0166 fM Detection Range: 0.448 pM–44.8 nM | Synergistic signal amplification | ||
| NH2-Zr-MOF/GDY/GCE | Biological sensors | ECL intensity: ~1.5 × vs. GDY | ECL Luminophore | [97] |
| GDYO QDs @M-ZnO | Biological sensors | ECL intensity: ~5 × vs. GDYO QDs | ECL Luminophore | [98] |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.C.; Jiang, H.L. Metal-organic framework-based hierarchically porous materials: Synthesis and applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.L. Metal-organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable metal-organic frameworks: Design, synthesis, and applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- Zheng, H.; Yan, W.; Zhang, J. Porous organic framework-based materials (MOFs, COFs and HOFs) for lithium-/sodium-/potassium-/zinc-/aluminum-/calcium-ion batteries: A review. Electrochem. Energy Rev. 2025, 8, 3. [Google Scholar] [CrossRef]
- Gao, X.; Dong, Y.; Li, S.; Zhou, J.; Wang, L.; Wang, B. MOFs and COFs for batteries and supercapacitors. Electrochem. Energy Rev. 2019, 3, 81–126. [Google Scholar] [CrossRef]
- Ye, Z.; Jiang, Y.; Li, L.; Wu, F.; Chen, R. Rational design of MOF-based materials for next-generation rechargeable batteries. Nano-Micro Lett. 2021, 13, 203. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.L.; Xu, Q. Metal-organic frameworks as platforms for catalytic applications. Adv. Mater. 2017, 30, 1703663. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.Q.; Jiang, H.L.; Sun, L.B. Metal-organic frameworks for heterogeneous basic catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Z.; Yin, Y.; Xu, H.; Wang, Y.; Yang, K.; Zhang, Z.; Wang, J.; He, X. Efficient capture and separation of CO2-Boosted carbon neutralization enabled by tailorable metal-organic frameworks: A review. EcoEnergy 2023, 1, 217–247. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, X.; Kang, Z.; Liu, X.; Sun, D. Isoreticular chemistry within metal-organic frameworks for gas storage and separation. Coord. Chem. Rev. 2021, 443, 213968. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2011, 112, 1105–1125. [Google Scholar] [CrossRef]
- Olorunyomi, J.F.; Geh, S.T.; Caruso, R.A.; Doherty, C.M. Metal-organic frameworks for chemical sensing devices. Mater. Horiz. 2021, 8, 2387–2419. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef]
- Xu, G.; Zhu, C.; Gao, G. Recent progress of advanced conductive metal-organic frameworks: Precise synthesis, electrochemical energy storage applications, and future challenges. Small 2022, 18, 2203140. [Google Scholar] [CrossRef]
- Xie, L.S.; Skorupskii, G.; Dincă, M. Electrically conductive metal-organic frameworks. Chem. Rev. 2020, 120, 8536–8580. [Google Scholar] [CrossRef]
- Sun, L.; Campbell, M.G.; Dincă, M. Electrically conductive porous metal-organic frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Dutta, S.; Hossain, M.S.A.; Shiddiky, M.J.A.; Tung, K.L.; Shieh, F.K.; Tsung, C.K.; Wu, K.C.W.; Yamauchi, Y. Strategies for improving the functionality of zeolitic imidazolate frameworks: Tailoring nanoarchitectures for functional applications. Adv. Mater. 2017, 29, 1700213. [Google Scholar] [CrossRef]
- Bennett, T.D.; Keen, D.A.; Tan, J.C.; Barney, E.R.; Goodwin, A.L.; Cheetham, A.K. Thermal amorphization of zeolitic imidazolate frameworks. Angew. Chem. Int. Ed. 2011, 50, 3067–3071. [Google Scholar] [CrossRef]
- Hughes, J.T.; Bennett, T.D.; Cheetham, A.K.; Navrotsky, A. Thermochemistry of zeolitic imidazolate frameworks of varying porosity. J. Am. Chem. Soc. 2012, 135, 598–601. [Google Scholar] [CrossRef]
- Zhou, K.; Mousavi, B.; Luo, Z.; Phatanasri, S.; Chaemchuen, S.; Verpoort, F. Characterization and properties of Zn/Co zeolitic imidazolate frameworks vs. ZIF-8 and ZIF-67. J. Mater. Chem. A 2017, 5, 952–957. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Zeng, G.; Cheng, M.; Huang, D.; Liu, Y.; Zhou, C.; Xiong, W.; Yang, Y.; Wang, W.; et al. Materials institute lavoisier (MIL) based materials for photocatalytic applications. Coord. Chem. Rev. 2021, 438, 213874. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, X.; Li, T.; Zhang, Y.; Xu, H.; Sun, Y.; Gu, X.; Gu, C.; Luo, J.; Gao, B. MIL series of metal organic frameworks (MOFs) as novel adsorbents for heavy metals in water: A review. J. Hazard. Mater. 2022, 429, 128271. [Google Scholar] [CrossRef]
- Zorainy, M.Y.; Gar Alalm, M.; Kaliaguine, S.; Boffito, D.C. Revisiting the MIL-101 metal-organic framework: Design, synthesis, modifications, advances, and recent applications. J. Mater. Chem. A 2021, 9, 22159–22217. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Z.; Alhassan, S.I.; Luo, Z.; Song, B.; Jin, L.; Zhao, Y.; Wang, H. Highly efficient fluoride removal from water using 2D metal-organic frameworks MIL-53(Al) with rich Al and O adsorptive centers. Environ. Sci. Ecotechnol. 2021, 8, 100123. [Google Scholar] [CrossRef]
- Adly, M.S.; El-Dafrawy, S.M.; Ibrahim, A.A.; El-Hakam, S.A.; El-Shall, M.S. Efficient removal of heavy metals from polluted water with high selectivity for Hg (I) and Pb (II) by a 2-imino-4-thiobiuret chemically modified MIL-125 metal-organic framework. RSC Adv. 2021, 11, 13940–13950. [Google Scholar] [CrossRef]
- Khosravi, M.J.; Hosseini, S.M.; Vatanpour, V. Polyamidoamine dendrimers-Mil-125(Ti) MOF embedded polyethersulfone membrane for enhanced removal of heavy metal, antibiotic and dye from water. J. Environ. Chem. Eng. 2022, 10, 108644. [Google Scholar] [CrossRef]
- Tatrari, G.; An, R.; Shah, F.U. Designed metal-organic framework composites for metal-ion batteries and metal-ion capacitors. Coord. Chem. Rev. 2024, 512, 215876. [Google Scholar] [CrossRef]
- Li, B.; Ma, J.G.; Cheng, P. Integration of metal nanoparticles into metal-organic frameworks for composite catalysts: Design and synthetic strategy. Small 2019, 15, 1804849. [Google Scholar] [CrossRef]
- Yu, J.; Mu, C.; Yan, B.; Qin, X.; Shen, C.; Xue, H.; Pang, H. Nanoparticle/MOF composites: Preparations and applications. Mater. Horiz. 2017, 4, 557–569. [Google Scholar] [CrossRef]
- Deng, X.; Yang, L.; Huang, H.; Yang, Y.; Feng, S.; Zeng, M.; Li, Q.; Xu, D. Shape-defined hollow structural Co-MOF-74 and metal nanoparticles@Co-MOF-74 composite through a transformation strategy for enhanced photocatalysis performance. Small 2019, 15, 1902287. [Google Scholar] [CrossRef]
- Mukoyoshi, M.; Kitagawa, H. Nanoparticle/metal-organic framework hybrid catalysts: Elucidating the role of the MOF. Chem. Commun. 2022, 58, 10757–10767. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.L. Metal-organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef]
- Kalaj, M.; Bentz, K.C.; Ayala, S.; Palomba, J.M.; Barcus, K.S.; Katayama, Y.; Cohen, S.M. MOF-polymer hybrid materials: From simple composites to tailored architectures. Chem. Rev. 2020, 120, 8267–8302. [Google Scholar] [CrossRef]
- Brahmi, C.; Benltifa, M.; Vaulot, C.; Michelin, L.; Dumur, F.; Millange, F.; Frigoli, M.; Airoudj, A.; Morlet-Savary, F.; Bousselmi, L.; et al. New hybrid MOF/polymer composites for the photodegradation of organic dyes. Eur. Polym. J. 2021, 154, 110560. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, F.; Feng, W.; Zou, X.; Zhao, C.; Na, H.; Liu, C.; Sun, F.; Zhu, G. From metal-organic framework (MOF) to MOF-polymer composite membrane: Enhancement of low-humidity proton conductivity. Chem. Sci. 2013, 4, 983–992. [Google Scholar] [CrossRef]
- Wang, D.; Li, T. Toward MOF@polymer core-shell particles: Design principles and potential applications. Acc. Chem. Res. 2023, 56, 462–474. [Google Scholar] [CrossRef]
- Parsapour, F.; Moradi, M.; Safarifard, V.; Sojdeh, S. Polymer/MOF composites for metal-ion batteries: A mini review. J. Energy Storage 2024, 82, 110487. [Google Scholar] [CrossRef]
- Liu, X.W.; Sun, T.J.; Hu, J.L.; Wang, S.D. Composites of metal-organic frameworks and carbon-based materials: Preparations, functionalities and applications. J. Mater. Chem. A 2016, 4, 3584–3616. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Xue, H.; Pang, H. Metal-Organic Frameworks/Graphene-Based Materials: Preparations and Applications. Adv. Funct. Mater. 2018, 28, 1804950. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Engineering the surface of a new class of adsorbents: Metal-organic framework/graphite oxide composites. J. Colloid Interface Sci. 2015, 447, 139–151. [Google Scholar] [CrossRef]
- Wang, K.; Hui, K.N.; Hui, K.S.; Peng, S.; Xu, Y. Recent progress in metal-organic framework/graphene-derived materials for energy storage and conversion: Design, preparation, and application. Chem. Sci. 2021, 12, 5737–5766. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, J.; Mao, J.; Guo, Q.; Chen, Z.; Lai, Y. Metal-organic frameworks and their derivatives with graphene composites: Preparation and applications in electrocatalysis and photocatalysis. J. Mater. Chem. A 2020, 8, 2934–2961. [Google Scholar] [CrossRef]
- Kayal, S.; Chakraborty, A. Activated carbon (type Maxsorb-III) and MIL-101(Cr) metal organic framework based composite adsorbent for higher CH4 storage and CO2 capture. Chem. Eng. J. 2018, 334, 780–788. [Google Scholar] [CrossRef]
- Govindaraju, S.; Arumugasamy, S.K.; Chellasamy, G.; Yun, K. Zn-MOF decorated bio activated carbon for photocatalytic degradation, oxygen evolution and reduction catalysis. J. Hazard. Mater. 2022, 421, 126720. [Google Scholar] [CrossRef]
- Wang, X.; Kukkar, D.; Younis, S.A.; Vikrant, K.; Ahmadi, Y.; Boukhvalov, D.; Kim, K.H. The co-adsorption potential of metal-organic framework/activated carbon composites against both polar and non-polar volatile organic compounds in air. Sep. Purif. Technol. 2023, 306, 122594. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Mukherjee, S.; Morales, D.M.; Dubal, D.P.; Nanjundan, A.K.; Schneemann, A.; Masa, J.; Kment, S.; Schuhmann, W.; Otyepka, M.; et al. Graphene-based metal-organic framework hybrids for applications in catalysis, environmental, and energy technologies. Chem. Rev. 2022, 122, 17241–17338. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Tang, Y.; Huang, X.; Pang, H. Recent advances and challenges of metal-organic framework/graphene-based composites. Compos. Part B Eng. 2022, 230, 109532. [Google Scholar] [CrossRef]
- Gao, C.; Wang, P.; Wang, Z.; Kær, S.K.; Zhang, Y.; Yue, Y. The disordering-enhanced performances of the Al-MOF/graphene composite anodes for lithium ion batteries. Nano Energy 2019, 65, 104032. [Google Scholar] [CrossRef]
- Jahan, M.; Bao, Q.; Loh, K.P. Electrocatalytically active graphene-porphyrin MOF composite for oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 6707–6713. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Dubal, D.P.; Schneemann, A.; Ranc, V.; Perez-Reyes, C.; Stráská, J.; Kment, Š.; Otyepka, M.; Fischer, R.A.; Zbořil, R. Shape-assisted 2D MOF/graphene derived hybrids as exceptional lithium-ion battery electrodes. Adv. Funct. Mater. 2019, 29, 1902539. [Google Scholar] [CrossRef]
- Yang, C.; You, X.; Cheng, J.; Zheng, H.; Chen, Y. A novel visible-light-driven in-based MOF/graphene oxide composite photocatalyst with enhanced photocatalytic activity toward the degradation of amoxicillin. Appl. Catal. B Environ. 2017, 200, 673–680. [Google Scholar] [CrossRef]
- Huang, H.; Chen, Y.; Chen, Z.; Chen, J.; Hu, Y.; Zhu, J.J. Electrochemical sensor based on Ce-MOF/carbon nanotube composite for the simultaneous discrimination of hydroquinone and catechol. J. Hazard. Mater. 2021, 416, 125895. [Google Scholar] [CrossRef]
- Jabbari, V.; Veleta, J.M.; Zarei-Chaleshtori, M.; Gardea-Torresdey, J.; Villagrán, D. Green synthesis of magnetic MOF@GO and MOF@CNT hybrid nanocomposites with high adsorption capacity towards organic pollutants. Chem. Eng. J. 2016, 304, 774–783. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Z.; Chai, L.; Xu, L.; Zhu, Z.; Hu, Y.; Qian, J.; Huang, S. MOF derived N-doped carbon coated CoP particle/carbon nanotube composite for efficient oxygen evolution reaction. Carbon 2019, 141, 643–651. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Shrestha, L.K.; Ma, R.; Hill, J.P.; Yamauchi, Y.; Ariga, K. Metal-organic framework on fullerene (MOFOF) as a hierarchical composite by the integration of coordination chemistry and supramolecular chemistry. ACS Appl. Mater. Interfaces 2024, 16, 41363–41370. [Google Scholar] [CrossRef]
- Han, J.; Wu, H.; Fan, H.; Ding, L.; Hai, G.; Caro, J.; Wang, H. Tuning the phase composition of metal-organic framework membranes for helium separation through incorporation of fullerenes. J. Am. Chem. Soc. 2023, 145, 14793–14801. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Liu, H.; Guo, Y.; Li, Y.; Zhu, D. Architecture of graphdiyne nanoscale films. Chem. Commun. 2010, 46, 3256–3258. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Y.; Qi, L.; Xue, Y.; Li, Y. 2D graphdiyne: An emerging carbon material. Chem. Soc. Rev. 2022, 51, 2681–2709. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Wang, D.; Zhang, J. Graphdiyne: Synthesis, properties, and applications. Chem. Soc. Rev. 2019, 48, 908–936. [Google Scholar] [CrossRef]
- Jia, Z.; Li, Y.; Zuo, Z.; Liu, H.; Huang, C.; Li, Y. Synthesis and properties of 2D carbon-graphdiyne. Acc. Chem. Res. 2017, 50, 2470–2478. [Google Scholar] [CrossRef]
- Zuo, Z.; Wang, D.; Zhang, J.; Lu, F.; Li, Y. Synthesis and applications of graphdiyne-based metal-free catalysts. Adv. Mater. 2018, 31, 1803762. [Google Scholar] [CrossRef]
- Qi, L.; Gao, Y.; Gao, Y.; Zheng, Z.; Luan, X.; Zhao, S.; Chen, Z.; Liu, H.; Xue, Y.; Li, Y. Controlled growth of metal atom arrays on graphdiyne for seawater oxidation. J. Am. Chem. Soc. 2024, 146, 5669–5677. [Google Scholar] [CrossRef]
- Zheng, X.; Xue, Y.; Chen, S.; Li, Y. Advances in hydrogen energy conversion of graphdiyne-based materials. EcoEnergy 2023, 1, 45–59. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, L.; Ma, J.; Fang, Y.; Yang, J.; Chen, X.; Zheng, J.; Zhang, S.; Chen, W.; Pan, C.; et al. Rapid ozone decomposition over water-activated monolithic MoO3/graphdiyne nanowalls under high humidity. Angew. Chem. Int. Ed. 2023, 62, e202309158. [Google Scholar] [CrossRef]
- Ma, K.; Wu, J.; Wang, X.; Sun, Y.; Xiong, Z.; Dai, F.; Bai, H.; Xie, Y.; Kang, Z.; Zhang, Y. Periodically interrupting bonding behavior to reformat delocalized electronic states of graphdiyne for improved electrocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2022, 61, e202211094. [Google Scholar] [CrossRef]
- Wang, N.; He, J.; Wang, K.; Zhao, Y.; Jiu, T.; Huang, C.; Li, Y. Graphdiyne-based materials: Preparation and application for electrochemical energy storage. Adv. Mater. 2019, 31, 1803202. [Google Scholar] [CrossRef]
- Du, Y.; Zhou, W.; Gao, J.; Pan, X.; Li, Y. Fundament and application of graphdiyne in electrochemical energy. Acc. Chem. Res. 2020, 53, 459–469. [Google Scholar] [CrossRef]
- An, J.; Zhang, H.; Wang, Y.; Kong, Z.; Li, W.; Gao, X.; Song, J.; Yao, Y. Application and prospects of interface engineering in energy storage and conversion of graphdiyne-based materials. EcoEnergy 2025, 3, 77–104. [Google Scholar] [CrossRef]
- Li, H.; Lim, J.H.; Lv, Y.; Li, N.; Kang, B.; Lee, J.Y. Graphynes and graphdiynes for energy storage and catalytic utilization: Theoretical insights into recent advances. Chem. Rev. 2023, 123, 4795–4854. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, D.; Yang, Z.; Xu, Y.; Wang, X.; Huang, H.; Qiu, F.; Huang, C. Doped-graphdiyne: Synthesis, theoretical prediction and application for electrochemical energy storage. Nanoscale Horiz. 2025, 10, 2239–2261. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Li, X.; Wang, N.; Huang, C. Graphdiyne ink for ionic liquid gated printed transistor. Adv. Electron. Mater. 2020, 6, 2000157. [Google Scholar] [CrossRef]
- Niu, G.; Wang, Y.; Yang, Z.; Cao, S.; Liu, H.; Wang, J. Graphdiyne and its derivatives as efficient charge reservoirs and transporters in semiconductor devices. Adv. Mater. 2023, 35, 2212159. [Google Scholar] [CrossRef]
- Do, D.P.; Hong, C.; Bui, V.Q.; Pham, T.H.; Seo, S.; Do, V.D.; Phan, T.L.; Tran, K.M.; Haldar, S.; Ahn, B.W.; et al. Highly efficient van der waals heterojunction on graphdiyne toward the high-performance photodetector. Adv. Sci. 2023, 10, 2300925. [Google Scholar] [CrossRef]
- Rana, S.; Kumar, A.; Dhiman, P.; Sharma, G.; Amirian, J.; Stadler, F.J. Progress in graphdiyne and phosphorene based composites and heterostructures as new age materials for photocatalytic hydrogen evolution. Fuel 2024, 356, 129630. [Google Scholar] [CrossRef]
- Wang, R.; Shi, M.; Xu, F.; Qiu, Y.; Zhang, P.; Shen, K.; Zhao, Q.; Yu, J.; Zhang, Y. Graphdiyne-modified TiO2 nanofibers with osteoinductive and enhanced photocatalytic antibacterial activities to prevent implant infection. Nat. Commun. 2020, 11, 4465. [Google Scholar] [CrossRef]
- Gao, N.; Ren, G.; Zhang, M.; Mao, L. Electroless deposition of palladium nanoparticles on graphdiyne boosts electrochemiluminescence. J. Am. Chem. Soc. 2024, 146, 3836–3843. [Google Scholar] [CrossRef]
- He, T.; Matta, S.K.; Will, G.; Du, A. Transition-metal single atoms anchored on graphdiyne as high-efficiency electrocatalysts for water splitting and oxygen reduction. Small Methods 2019, 3, 1800419. [Google Scholar] [CrossRef]
- Pei, Y.; Wu, X.; Ren, Z.; Lv, Y.; Xue, R.; Guo, J.; Jia, D. Reinforcement of C-NFO@GDY membranes via the synergistic effect of the graphdiyne honeycomb nanostructure and electronegativity for high-efficiency oil-in-water emulsion separation. Adv. Fiber Mater. 2025, 7, 1195–1207. [Google Scholar] [CrossRef]
- Wang, K.; Kong, X.; Xie, H.; Li, S.; Wang, M.; Jin, Z. High performance of visible-light driven hydrogen production over graphdiyne (g-CnH2n−-2)/MOF S-scheme heterojunction. Dalton Trans. 2023, 52, 8716–8727. [Google Scholar] [CrossRef]
- Ding, L.; Lei, M.; Wang, T.; Wang, J.; Jin, Z. Graphdiyne coordinated CoMo-MOF formed S-scheme heterojunction boosting photocatalytic hydrogen production. Carbon Lett. 2024, 34, 2099–2112. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Z.; Li, Y.; Wu, X.; Ma, Z.; Sun, W.; Ming Li, C.; Guo, C. Graphdiyne chelated iron-based metal–organic frameworks for electrochemical sensing of antibiotic chloramphenicol with ultralow detection limit. Microchem. J. 2024, 201, 110526. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Li, Y.; Jin, Z. Graphdiyne (CnH2n−2) coupled with ZnCo-MOF double S-scheme heterojunction forms an efficient electron transport layer and its characterization via in situ XPS. Catal. Sci. Technol. 2023, 13, 3667–3681. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Liu, G.; Jin, Z.; Tsubaki, N. NiCo-MOFs in situ anchored on graphdiyne with metal-like properties form a strongly coupled electron transport interface and construct an ohmic contact to achieve efficient charge-hole spatial separation. Nanoscale 2024, 16, 19322–19334. [Google Scholar] [CrossRef]
- Abbas, Z.; Hussain, N.; Mobin, S.M. Crumpled fibrous graphdiyne network decorated metal-organic framework: A promising heterostructure for improved energy storage performance. J. Mater. Chem. A 2024, 12, 20170–20178. [Google Scholar] [CrossRef]
- Wang, B.; Ma, J.; Yang, R.; Meng, B.; Yang, X.; Zhang, Q.; Zhang, B.; Zhuo, S. Bridging nickel-MOF and copper single atoms/clusters with H-substituted graphdiyne for the tandem catalysis of nitrate to ammonia. Angew. Chem. Int. Ed. 2024, 63, e202404819. [Google Scholar] [CrossRef]
- Song, D.; Xu, X.; Huang, X.; Li, G.; Wang, X.; Zhao, Y.; Gao, F. Nitrogen and fluoride co-doped graphdiyne with metal-organic framework (MOF)-derived NiCo2O4-Co3O4 nanocages as sensing layers for ultra-sensitive pesticide detection. Anal. Chim. Acta 2023, 1252, 341012. [Google Scholar] [CrossRef]
- Lu, M.; Zhao, X.; Zhang, S.; Jian, H.; Wang, M.; Lu, T. Stabilization of MOF-derived Co3S4 nanoparticles via graphdiyne coating for efficient oxygen evolution. Sci. China Mater. 2024, 67, 1882–1890. [Google Scholar] [CrossRef]
- Jin, F.; Yang, B.; Wang, X.; Li, T.; Tsubaki, N.; Jin, Z. Facilitating efficient photocatalytic hydrogen evolution via enhanced carrier migration at MOF-on-MOF S-scheme heterojunction interfaces through a graphdiyne (CnH2n−–2) electron transport layer. Chin. J. Struct. Chem. 2023, 42, 100198. [Google Scholar] [CrossRef]
- Cui, J.; Liu, J.; Wang, C.; Rong, F.; He, L.; Song, Y.; Zhang, Z.; Fang, S. Efficient electrocatalytic water oxidation by using the hierarchical 1D/2D structural nanohybrid of CoCu-based zeolitic imidazolate framework nanosheets and graphdiyne nanowires. Electrochim. Acta 2020, 334, 135577. [Google Scholar] [CrossRef]
- Sun, H.P.; Hye, M.K.; Mariana, L.D.; Sunggi, L.; Nak, C.J. Hydroquinone-treated Cu3(BTC)2: A mixed-valence Cu(I/II) MOF catalyst for efficient cycloadditions. Chem. Commun. 2024, 60, 14577. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Ke, Z. Metal-organic frameworks/graphdiyne/copper foam composite membranes for catalytic applications. ACS Appl. Mater. Interfaces 2023, 15, 40933–40941. [Google Scholar] [CrossRef]
- Guo, L.; Shang, H.; Peng, J.; Li, H.; Pan, H. In-situ growth of graphdiyne on ZnCo-ZIF for enhanced lithium-sulfur battery performance. J. Mater. Chem. A 2025, 13, 36436–36443. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Mansouri Majd, S.; Salimi, A. Polarity-switchable dual-mode photoelectrochemical cancer marker immunoassay based on a metal-organic framework@nitrogen-doped graphdiyne heterojunction. ACS Appl. Mater. Interfaces 2025, 17, 27759–27771. [Google Scholar] [CrossRef]
- Dong, M.; Jiang, D.; Cao, Q.; Wang, W.; Shiigi, H.; Chen, Z. A metal-organic framework regulated graphdiyne-based electrochemiluminescence sensor with a electrocatalytic self-acceleration effect for the detection of di-(2-ethylhexyl) phthalate. Analyst 2023, 148, 4470–4478. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, J.; Tan, X.; Huang, K. Electrochemiluminescence sensor based on graphdiyne quantum dots functionalized MOF-derived ZnO with self-enhancing effect for the detection of radical scavenging drugs. Sensors Actuat. B-Chem. 2023, 382, 133541. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Tian, Z.; Zhao, T.; Shang, H.; Wu, J. Recent Progress in Preparations and Multifunctional Applications Towards MOF/GDY Composites and Their Derivative Materials. Catalysts 2025, 15, 1041. https://doi.org/10.3390/catal15111041
Peng J, Tian Z, Zhao T, Shang H, Wu J. Recent Progress in Preparations and Multifunctional Applications Towards MOF/GDY Composites and Their Derivative Materials. Catalysts. 2025; 15(11):1041. https://doi.org/10.3390/catal15111041
Chicago/Turabian StylePeng, Jia, Zhiwei Tian, Tonghe Zhao, Hong Shang, and Jing Wu. 2025. "Recent Progress in Preparations and Multifunctional Applications Towards MOF/GDY Composites and Their Derivative Materials" Catalysts 15, no. 11: 1041. https://doi.org/10.3390/catal15111041
APA StylePeng, J., Tian, Z., Zhao, T., Shang, H., & Wu, J. (2025). Recent Progress in Preparations and Multifunctional Applications Towards MOF/GDY Composites and Their Derivative Materials. Catalysts, 15(11), 1041. https://doi.org/10.3390/catal15111041







