Photocatalytic Degradation of Ant Trail Pheromones by P25 TiO2
Abstract
1. Introduction
2. Results and Discussion
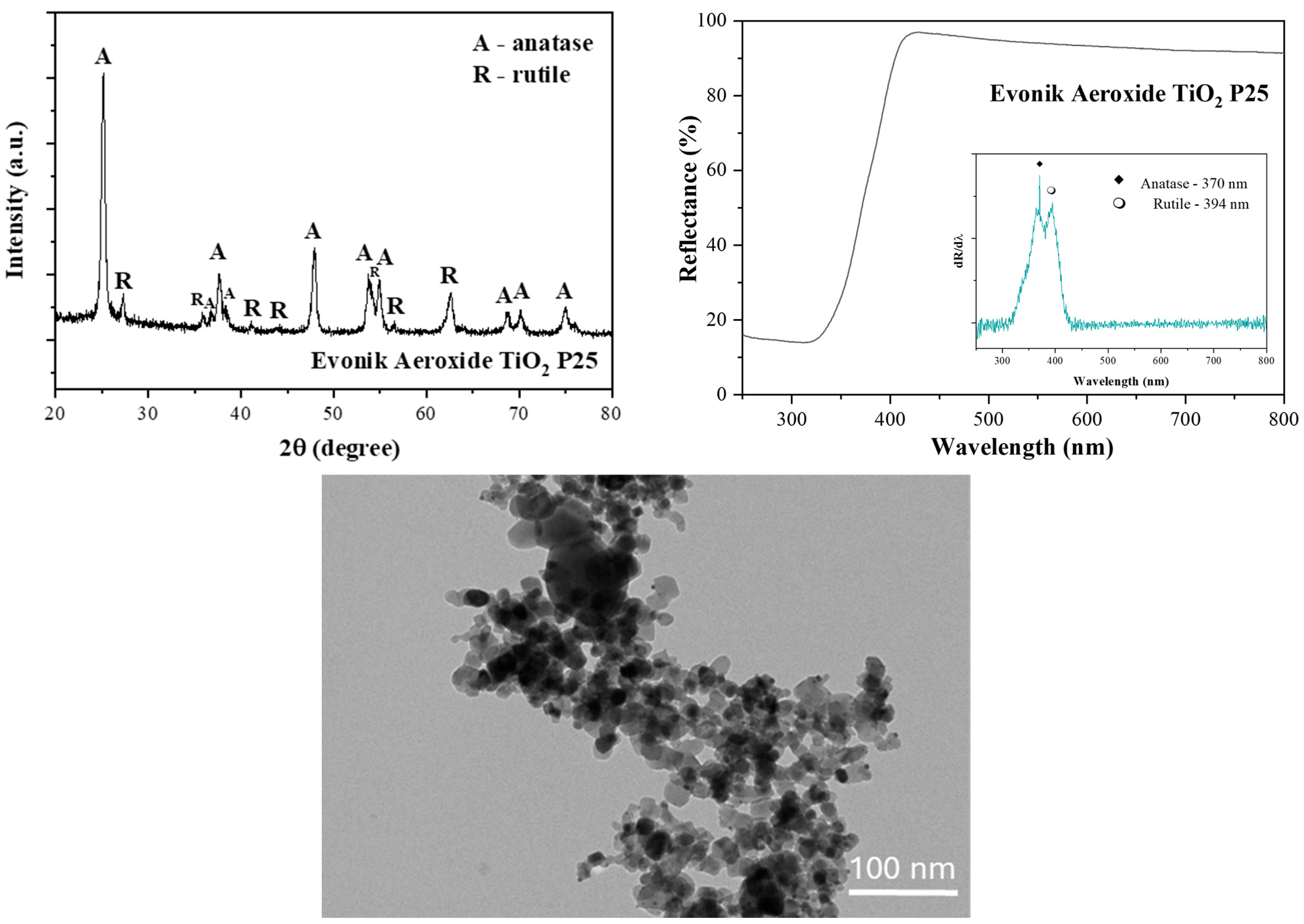
2.1. The Basic Properties of Evonik Aeroxide P25
2.2. The Interaction Between the Pheromone Trail and Evonik Aeroxide P25
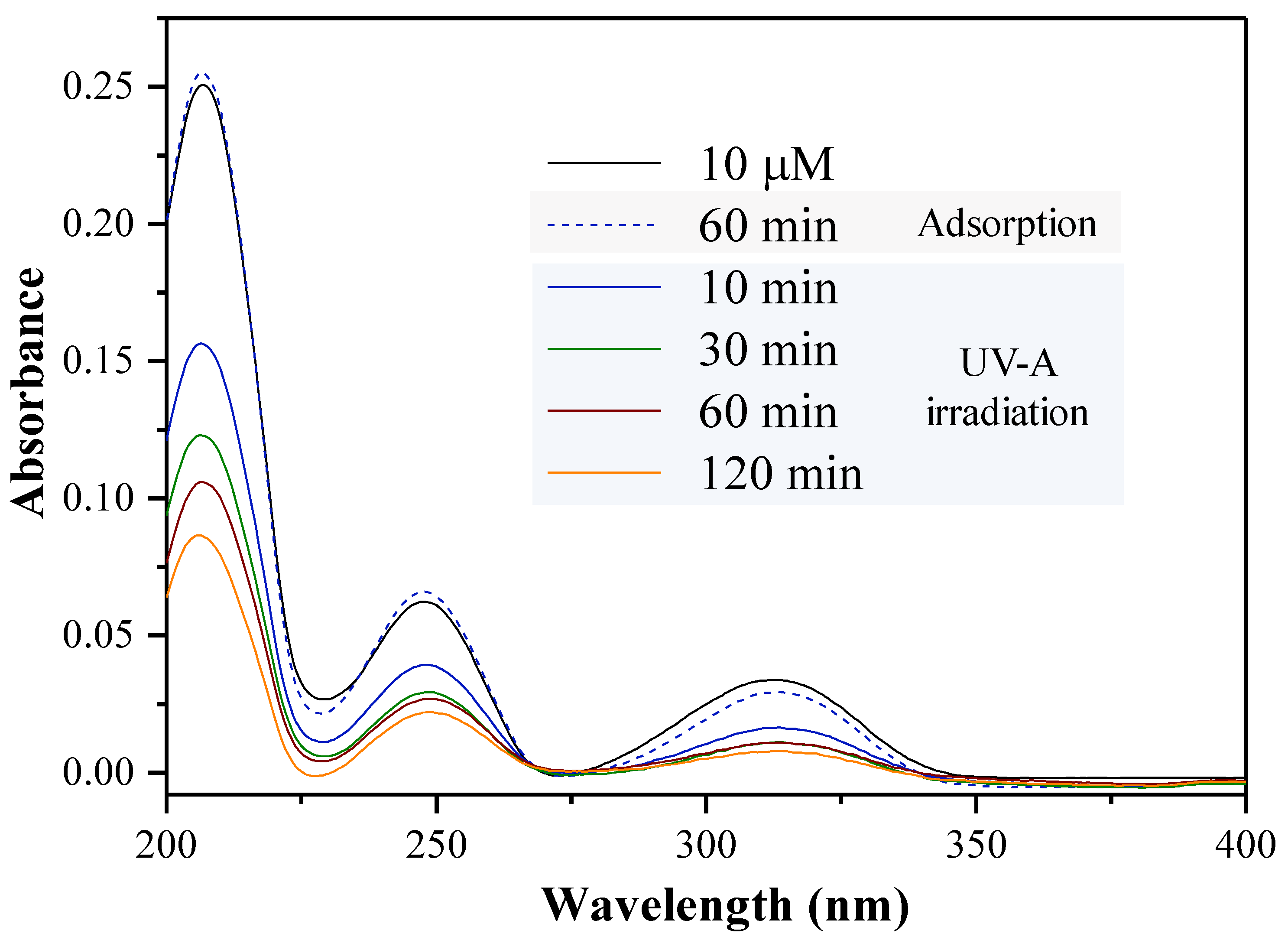
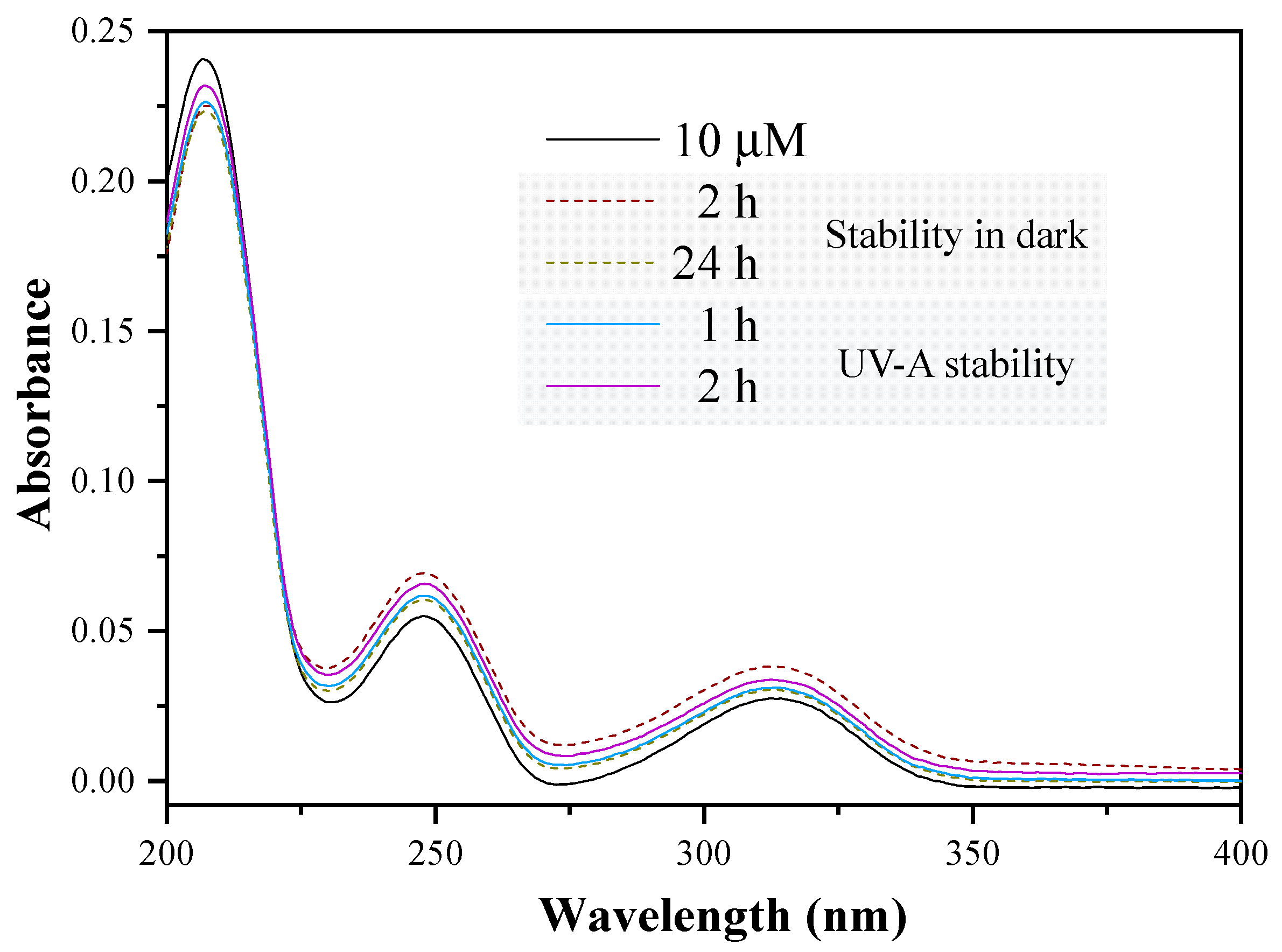
2.3. Photocatalytic Degradation Experiments of Mellein Pheromone Component
3. Materials and Methods
3.1. Instrumentation
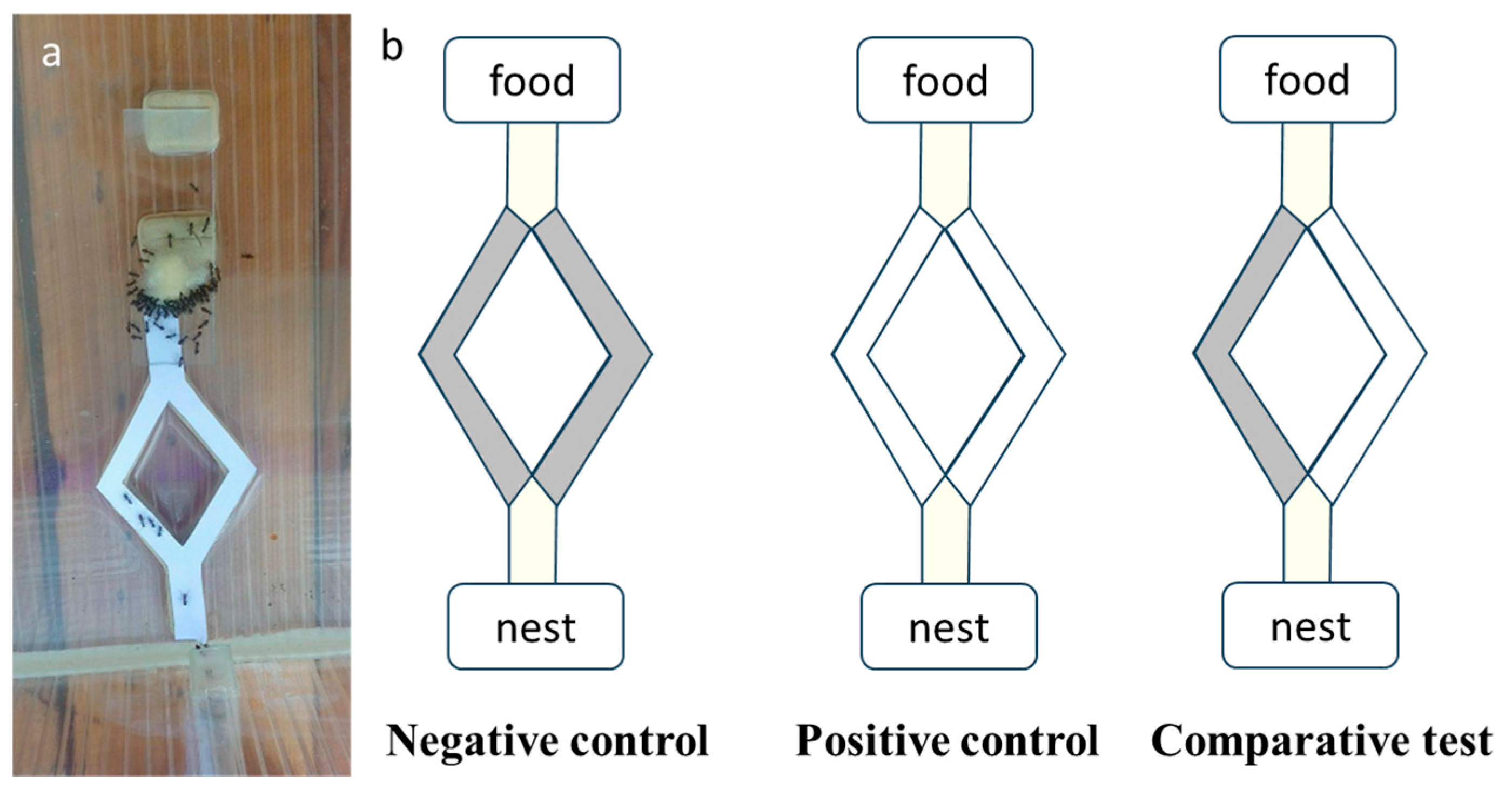
3.2. Pheromone-Based Foraging Test Method with Lasius niger Ant Species in the Presence of TiO2
3.3. Photocatalytic Degradation Method of the Mellein Pheromone Component
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- Robichaud, C.O.; Uyar, A.E.; Darby, M.R.; Zucker, L.G.; Wiesner, M.R. Estimates of Upper Bounds and Trends in Nano-TiO2 Production as a Basis for Exposure Assessment. Environ. Sci. Technol. 2009, 43, 4227–4233. [Google Scholar] [CrossRef]
- Solymos, K.; Babcsányi, I.; Ariya, B.; Gyulavári, T.; Ágoston, Á.; Kukovecz, Á.; Kónya, Z.; Pap, Z. Environmental significance of the interaction between titanium dioxides and soil solutions. Environ. Sci. Eur. 2024, 36, 85. [Google Scholar] [CrossRef]
- Wu, K.C.-W.; Yamauchi, Y.; Hong, C.-Y.; Yang, Y.-H.; Liang, Y.-H.; Funatsu, T.; Tsunoda, M. Biocompatible, surface functionalized mesoporous titania nanoparticles for intracellular imaging and anticancer drug delivery. Chem. Commun. 2011, 47, 5232. [Google Scholar] [CrossRef]
- Eshaghi, M.M.; Pourmadadi, M.; Rahdar, A.; Díez-Pascual, A.M. Improving quercetin anticancer activity through a novel polyvinylpyrrolidone/polyvinyl alcohol/TiO2 nanocomposite. J. Drug Deliv. Sci. Technol. 2023, 81, 104304. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.; Fowler, P.; Fernandez, M.J.F.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; Husøy, T.; et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.-J.; Huang, S.-C.; Chen, Y.-P.; Chiueh, L.-C.; Shih, D.Y.-C. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J. Food Drug Anal. 2015, 23, 587–594. [Google Scholar] [CrossRef]
- Kokkonen, M.; Talebi, P.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; Hashmi, S.G. Advanced research trends in dye-sensitized solar cells. J. Mater. Chem. A 2021, 9, 10527–10545. [Google Scholar] [CrossRef]
- Chen, M.C.; Koh, P.W.; Ponnusamy, V.K.; Lee, S.L. Titanium dioxide and other nanomaterials based antimicrobial additives in functional paints and coatings: Review. Prog. Org. Coat. 2022, 163, 106660. [Google Scholar] [CrossRef]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H.; Rehan, M. Biodiesel production from used cooking oil using a novel surface functionalised TiO2 nano-catalyst. Appl. Catal. B 2017, 207, 297–310. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled Environmental Concentrations of Engineered Nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for Different Regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef]
- Heilgeist, S.; Sekine, R.; Sahin, O.; Stewart, R.A. Finding Nano: Challenges Involved in Monitoring the Presence and Fate of Engineered Titanium Dioxide Nanoparticles in Aquatic Environments. Water 2021, 13, 734. [Google Scholar] [CrossRef]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef]
- Knaden, M.; Graham, P. The Sensory Ecology of Ant Navigation: From Natural Environments to Neural Mechanisms. Annu. Rev. Entomol. 2016, 61, 63–76. [Google Scholar] [CrossRef]
- Czaczkes, T.J.; Grüter, C.; Jones, S.M.; Ratnieks, F.L.W. Synergy between social and private information increases foraging efficiency in ants. Biol. Lett. 2011, 7, 521–524. [Google Scholar] [CrossRef]
- Czaczkes, T.J. Communication: Recruitment to Resources. In Encyclopedia of Social Insects; Springer International Publishing: Cham, Switzerland, 2021; pp. 267–274. [Google Scholar] [CrossRef]
- Morgan, E.D. Trail pheromones of ants. Physiol. Entomol. 2009, 34, 1–17. [Google Scholar] [CrossRef]
- Székely, I.; Baia, M.; Magyari, K.; Boga, B.; Pap, Z. The effect of the pH adjustment upon the WO3-WO3·0.33H2O-TiO2 ternary composite systems’ photocatalytic activity. Appl. Surf. Sci. 2019, 490, 469–480. [Google Scholar] [CrossRef]
- Ferrara, G.; Salvaggio, A.; Pecoraro, R.; Scalisi, E.M.; Presti, A.M.; Impellizzeri, G.; Brundo, M.V. Toxicity assessment of nano-TiO2 in Apis mellifera L. 1758: Histological and immunohistochemical assays. Microsc. Res. Tech. 2020, 83, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Papa, G.; Di Prisco, G.; Spini, G.; Puglisi, E.; Negri, I. Acute and chronic effects of Titanium dioxide (TiO2) PM1 on honey bee gut microbiota under laboratory conditions. Sci. Rep. 2021, 11, 5946. [Google Scholar] [CrossRef]
- Czekes, Z.; Bai, D.; Vincze, J.; Gál, E.; Réthi-Nagy, Z.; Baia, L.; Pap, Z. Commercial photocatalyst changes the behavior of Formica pratensis and Formica polyctena. Environ. Sci. Nano 2023, 10, 72–79. [Google Scholar] [CrossRef]
- Gutiérrez-Ramírez, J.A.; Betancourt-Galindo, R.; Aguirre-Uribe, L.A.; Cerna-Chávez, E.; Sandoval-Rangel, A.; Del Ángel, E.C.; Chacón-Hernández, J.C.; García-López, J.I.; Hernández-Juárez, A. Insecticidal Effect of Zinc Oxide and Titanium Dioxide Nanoparticles against Bactericera cockerelli Sulc. (Hemiptera: Triozidae) on Tomato Solanum lycopersicum. Agronomy 2021, 11, 1460. [Google Scholar] [CrossRef]
- Gutiérrez-Ramírez, J.A.; Betancourt-Galindo, R.; Aguirre-Uribe, L.A.; Cerna-Chávez, E.; Sandoval-Rangel, A.; Ángel, E.C.; Chacón-Hernández, J.C.; García-López, J.I.; Hernández-Juárez, A. Investigating the Insecticidal Properties of Titanium Oxide Nanoparticles against Periplaneta americana. Entomol. Lett. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- De Almeida, T.; Mesléard, F.; Santonja, M.; Gros, R.; Dutoit, T.; Blight, O. Above- and below-ground effects of an ecosystem engineer ant in Mediterranean dry grasslands. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201840. [Google Scholar] [CrossRef]
- Folgarait, P.J. Ant biodiversity and its relationship to ecosystem functioning: A review. Biodivers. Conserv. 1998, 7, 1221–1244. [Google Scholar] [CrossRef]
- Sanders, D.; van Veen, F.J.F. Ecosystem engineering and predation: The multi-trophic impact of two ant species. J. Anim. Ecol. 2011, 80, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Kehres, J.; Andreasen, J.W.; Krebs, F.C.; Molenbroek, A.M.; Chorkendorff, I.; Vegge, T. Combined in situ small- and wide-angle X-ray scattering studies of TiO2 nanoparticle annealing to 1023 K. J. Appl. Crystallogr. 2010, 43, 1400–1408. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ahmed, S. Easy and green synthesis of TiO2 (Anatase and Rutile): Estimation of crystallite size using Scherrer equation, Williamson-Hall plot, Monshi-Scherrer Model, size-strain plot, Halder- Wagner Model. Results Mater. 2023, 20, 100492. [Google Scholar] [CrossRef]
- Beckers, R.; Deneubourg, J.L.; Goss, S. Trail laying behaviour during food recruitment in the ant Lasius niger (L.). Insectes Soc. 1992, 39, 59–72. [Google Scholar] [CrossRef]
- Czaczkes, T.J.; Grüter, C.; Ellis, L.; Wood, E.; Ratnieks, F.L.W. Ant foraging on complex trails: Route learning and the role of trail pheromones in Lasius niger. J. Exp. Biol. 2012, 216, 188–197. [Google Scholar] [CrossRef]
- Bestmann, H.J.; Kern, F.; Schafer, D.; Witschel, M.C. 3,4-Dihydroisocoumarins, a New Class of Ant Trail Pheromones. Angew. Chem. Int. Ed. Engl. 1992, 31, 795–796. [Google Scholar] [CrossRef]
- Klowden, M.J. Communication Systems. In Physiological Systems in Insects; Elsevier: Amsterdam, The Netherlands, 2008; pp. 597–642. [Google Scholar] [CrossRef]
- Proutsos, N.; Alexandris, S.; Liakatas, A.; Nastos, P.; Tsiros, I.X. PAR and UVA composition of global solar radiation at a high altitude Mediterranean forest site. Atmos. Res. 2022, 269, 106039. [Google Scholar] [CrossRef]
- Cañada, J.; Esteve, A.R.; Marín, M.J.; Utrillas, M.P.; Tena, F.; Martínez-Lozano, J.A. Study of erythemal, UV (A + B) and global solar radiation in Valencia (Spain). Int. J. Climatol. 2008, 28, 693–702. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Das, S.; Balan, R.; Lekshmi, I.C. TiO2-ZrO2 nanocomposite with tetragonal zirconia phase and photocatalytic degradation of Alizarin Yellow GG azo dye under natural sunlight. Mater. Today Proc. 2021, 47, 4641–4646. [Google Scholar] [CrossRef]
- Landi, S.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]
- Bhatkar, A.; Whitcomb, W.H. Artificial Diet for Rearing Various Species of Ants. Fla. Entomol. 1970, 53, 229. [Google Scholar] [CrossRef]
- Grüter, C.; Czaczkes, T.J.; Ratnieks, F.L.W. Decision making in ant foragers (Lasius niger) facing conflicting private and social information. Behav. Ecol. Sociobiol. 2011, 65, 141–148. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
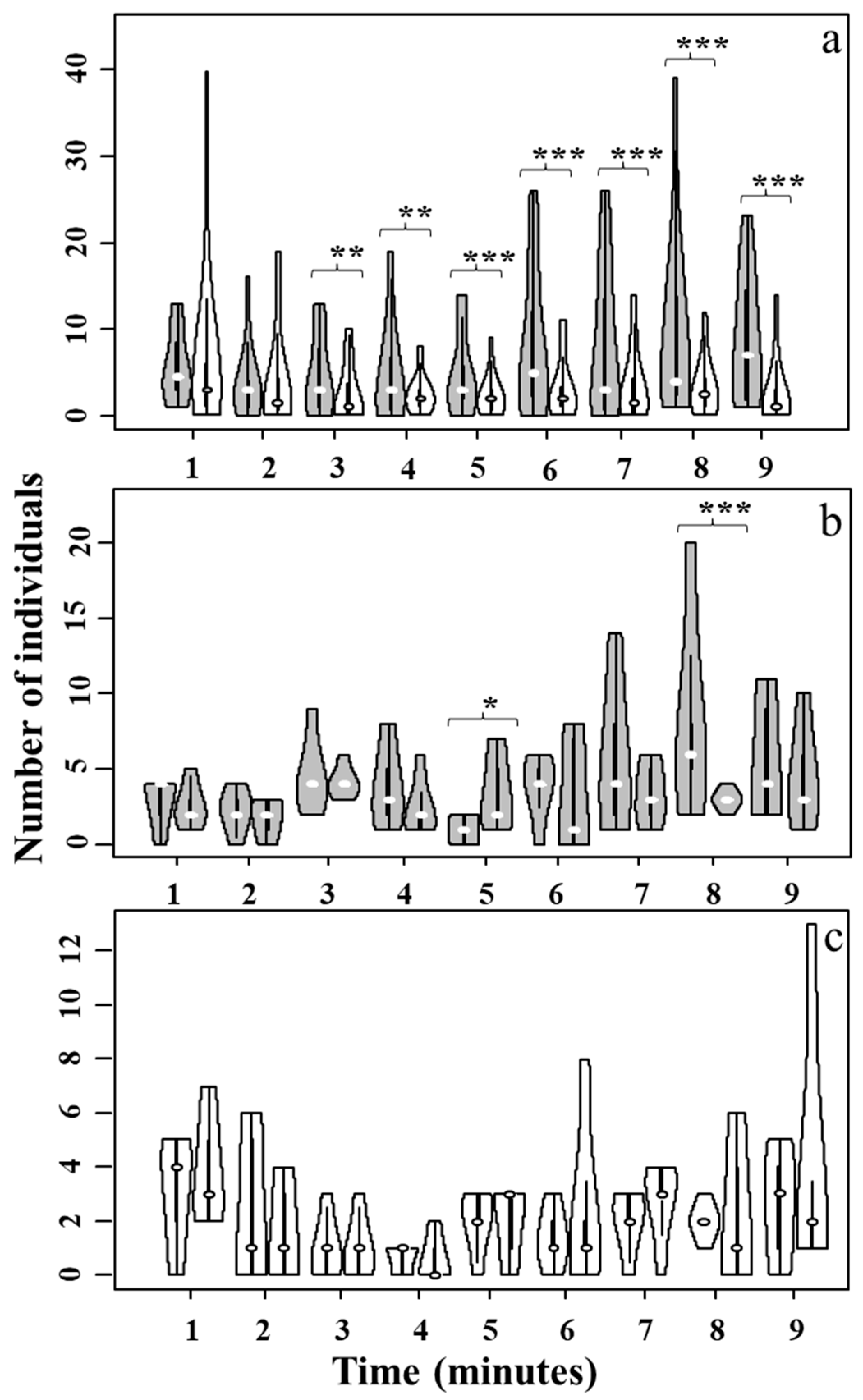


| Experimental Setup | z | p |
|---|---|---|
| positive control | ||
| 1. min. | −0.50 | 0.61 |
| 2. min. | 0.88 | 0.37 |
| 3. min. | - | - |
| 4. min. | - | - |
| 5. min. | - | - |
| 6. min. | −1.13 | 0.25 |
| 7. min. | −0.81 | 0.41 |
| 8. min. | −0.71 | 0.47 |
| 9. min. | −1.05 | 0.29 |
| negative control | ||
| 1. min. | −0.19 | 0.84 |
| 2. min. | −0.44 | 0.65 |
| 3. min. | −0.30 | 0.76 |
| 4. min. | −1.24 | 0.21 |
| 5. min. | 2.405 * | 0.016 |
| 6. min. | −0.50 | 0.61 |
| 7. min. | −1.76 | 0.07 |
| 8. min. | −3.369 *** | <0.001 |
| 9. min. | −0.70 | 0.48 |
| comparative test | ||
| 1. min. | 1.06 | 0.28 |
| 2. min. | 0.20 | 0.83 |
| 3. min. | −2.91 ** | 0.003 |
| 4. min. | −3.080 ** | 0.002 |
| 5. min. | −3.390 *** | <0.001 |
| 6. min. | −5.101 *** | <0.001 |
| 7. min. | −4.599 *** | <0.001 |
| 8. min. | −5.911 *** | <0.001 |
| 9. min. | −5.869 *** | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saszet, K.; Mátyás, E.; Almási, E.E.; Lakatos, L.V.; Czekes, Z.; Pap, Z.; Baia, L. Photocatalytic Degradation of Ant Trail Pheromones by P25 TiO2. Catalysts 2025, 15, 1040. https://doi.org/10.3390/catal15111040
Saszet K, Mátyás E, Almási EE, Lakatos LV, Czekes Z, Pap Z, Baia L. Photocatalytic Degradation of Ant Trail Pheromones by P25 TiO2. Catalysts. 2025; 15(11):1040. https://doi.org/10.3390/catal15111040
Chicago/Turabian StyleSaszet, Kata, Eszter Mátyás, Eszter Enikő Almási, Laura Vivien Lakatos, Zsolt Czekes, Zsolt Pap, and Lucian Baia. 2025. "Photocatalytic Degradation of Ant Trail Pheromones by P25 TiO2" Catalysts 15, no. 11: 1040. https://doi.org/10.3390/catal15111040
APA StyleSaszet, K., Mátyás, E., Almási, E. E., Lakatos, L. V., Czekes, Z., Pap, Z., & Baia, L. (2025). Photocatalytic Degradation of Ant Trail Pheromones by P25 TiO2. Catalysts, 15(11), 1040. https://doi.org/10.3390/catal15111040











