Abstract
This study compares unsupported NiO nanoflowers (NiEG) and ZrO2-supported NiO (25NiZ) for methane activation and hydrogenation, focusing on the impact of catalyst morphology. The NiEG catalyst demonstrated superior performance, achieving a high methane activation rate of 1.79 mol/(s·gNiO) and unique product selectivity. It produced ethylene and ethane at 503 K and higher hydrocarbons (C4–C6) at 593 K. Furthermore, the NiEG catalyst exhibited enhanced coke resistance, forming less-deactivating carbon nanotubes compared to the filamentous coke prevalent on the 25NiZ catalyst. We attribute this performance to the nanoflower morphology, which provides highly exposed and stable Ni sites that facilitate C-H cleavage and stabilize reaction intermediates.
1. Introduction
Methane emissions, originating from chemical industries, conventional fuels, and other anthropogenic sources, are a major driver of global warming due to their high global warming potential. For 2022, these emissions were projected at 9390 million metric tons of CO2 equivalent [1,2,3]. Despite its environmental impact, methane is a valuable raw material for numerous processes, including the production of fine chemicals [4,5,6], renewable fuels from biomass feedstocks [4,7,8], and as a precursor for hydrogen via catalytic reforming [6,9,10]. A critical and persistent challenge in utilizing methane, however, lies in activating its strong C-H bonds (439 kJ/mol). Therefore, understanding the mechanism of CH4 activation over transition metals like nickel is essential for designing effective and stable catalysts for methane reforming and valorization [11,12,13,14].
A powerful and widely studied strategy involves fabricating catalysts from nickel nanoparticles dispersed on high-surface-area supports, which maximizes the active surface area and can tune the electronic properties at the metal-support interface [9,15,16]. While acid-base catalysts and single-atom configurations have been explored for direct methane conversion [17,18], the process on nickel typically initiates with the dissociative chemisorption of methane [19,20]. Recent work on single-atom Ni1/CeO2 (111) catalysts reveals that the metal-support interaction can drastically lower the activation barrier for C-H bond. Lee et al. [21] recently published a review on nanoscience and nanotechnology as promising fields in materials science and processes. Among them, a considerable interest emerged for flower-shaped nanostructures, known as nanoflowers. These materials have great advantages due to the higher surface-to-volume ratio compared to spherical nanoparticles and environmentally friendly synthesis. Zhou et al. [22] synthesized NiO nanoflowers using various precursors in the presence/absence of surfactants/templates since the morphologies of the shaped NiO nanostructures are influenced by the reaction conditions. Godlaveeti et al. [23] reported the synthesis of flower-like NiO nanostructures with three different morphologies using a template-free hydrothermal method.
Based on these previous remarks, this work investigates methane activation through a two-stage process on two distinct catalyst morphologies: NiO supported on ZrO2 and unsupported NiO nanoflowers. The study specifically examines (i) the dissociative chemisorption of methane as a function of temperature, and (ii) the subsequent hydrogenation of the formed surface carbon fragments. The effects of reaction temperature, methane flow rate, and catalyst nature on the distribution of light hydrocarbon products will be systematically analyzed.
2. Results
2.1. Structure and Morphology of the Catalysts
Figure 1 displays the XRD diffractograms of these materials. The ZrO2 modified nanomaterials showed low crystallinity, exhibiting patterns with cubic structure [24,25,26,27,28,29,30,31].
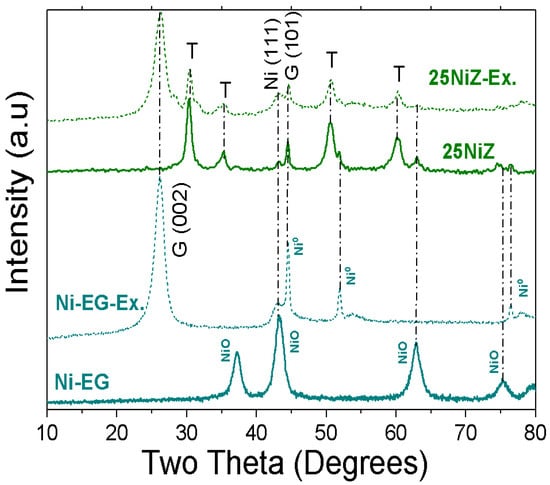
Figure 1.
X-ray Diffraction patterns for: 25NiZ and NiEG fresh and after reaction catalysts (Ex). T = Tetragonal ZrO2, G = Graphite, Ex = Exhaust catalyst.
The X-ray diffractogram in Figure 1 exhibits reflections corresponding to the (111), (200), (220), (311), and (222) planes of the tetragonal ZrO2 phase (JCPDS 50-1089), located at 2θ = 30.2°, 34.9°, 50.4°, 60.3°, and 62.9° [26,27]. Concurrently, reflections corresponding to the cubic NiO phase (JCPDS 04-0850) were observed at 2θ = 43.28°, 75.38°, and 79.75°. The calculated crystallite sizes for these phases are presented in Table 1. The NiEG catalyst exhibited smaller NiO crystallites compared to the 25NiZ catalyst. The cubic structure of the unsupported NiEG material was well-defined, with an additional peak indicative of graphitic carbon (JCPDS 00-041-1487) [28]. In contrast, the 25NiZ catalyst showed evidence of graphite formation on the (002) and (110) planes, which is attributed to the sintering of nickel particles and subsequent carbon deposition [26]. As summarized in Table 1, the particle sizes of both NiO and metallic Ni0 were significantly smaller for the fresh NiEG sample. Following the reaction, the crystallite sizes for both catalysts were approximately similar. This suggests that significant sintering did not occur; however, the presence of graphite peaks confirms carbon formation.

Table 1.
Crystalline, composition and texture parameters of the nickel catalysts used.
The NiEG material consists of unsupported NiO (JCPDS 04-0850). Consequently, its low reported “NiO content” from X-ray fluorescence (XRF) analysis reflects the stoichiometry of the pure nickel oxide phase and any associated surface species, rather than a dilution effect from a support material. The morphology of both catalysts was examined by scanning electron microscopy (SEM). Figure 2 presents SEM images of the supported (25NiZ) and unsupported (NiEG) catalysts, showing nanosphere and nanoflower arrangements, respectively, both before Figure 2a,c and after reaction Figure 2b,d at 593 K [29,30].

Figure 2.
SEM Image for samples: (a,b) 25NiZ fresh and after reaction, (c,d) NiEG fresh and after reaction.
Scanning electron microscopy (SEM) reveals distinct morphologies for the two fresh catalysts. The 25NiZ catalyst shows NiO particles uniformly dispersed on the ZrO2 support, forming small nanospheroidal arrays consistent with the cubic zirconia phase (Figure 2a) [30,31,32]. In stark contrast, the unsupported NiEG catalyst exhibits a unique three-dimensional nanoflower morphology comprising interconnected nanofoils (Figure 2c). This distinctive structure is a direct consequence of the ethylene glycol-mediated synthesis, which promotes the self-assembly of highly ordered nanomaterials due to the controlled hydrophobicity of the system [33,34]. The curved nature of the NiO nanoflakes, a consequence of the cubic crystal structure, suggests a high density of exposed active sites [35]. This intricate nanoflower architecture correlates with the high surface area measured by BET (Table 1) and is a principal contributor to its enhanced catalytic activity. Scanning electron microscopy (SEM) of the spent catalysts reveals a dense network of filamentous carbon on the 25NiZ catalyst (Figure 2b), a morphology known to encapsulate active sites, induce mechanical stress, and lead to rapid deactivation [36]. In stark contrast, the spent NiEG catalyst (Figure 2d) primarily forms carbon nanotubes, with the underlying nanoflower morphology remaining largely intact. This tendency to form structured nanotubes instead of encapsulating filaments is often associated with unique morphologies or electronic properties that facilitate controlled carbon diffusion [37,38]. The preservation of the nanoflower structure and its active sites underscores the role of the initial morphology in promoting a non-deactivating coking pathway, which is key to the superior stability of the NiEG catalyst.
2.2. Temperature Programmed Reaction (TPSR) with Methane
The reducibility and methane decomposition activity of the NiEG and 25NiZ catalysts were investigated by CH4 temperature-programmed surface reaction (CH4-TPSR). The evolution profiles of the gaseous products (H2, CH4, H2O), shown in Figure 3, reveal distinct mechanistic pathways and intrinsic activities, reflecting their differing structural properties.
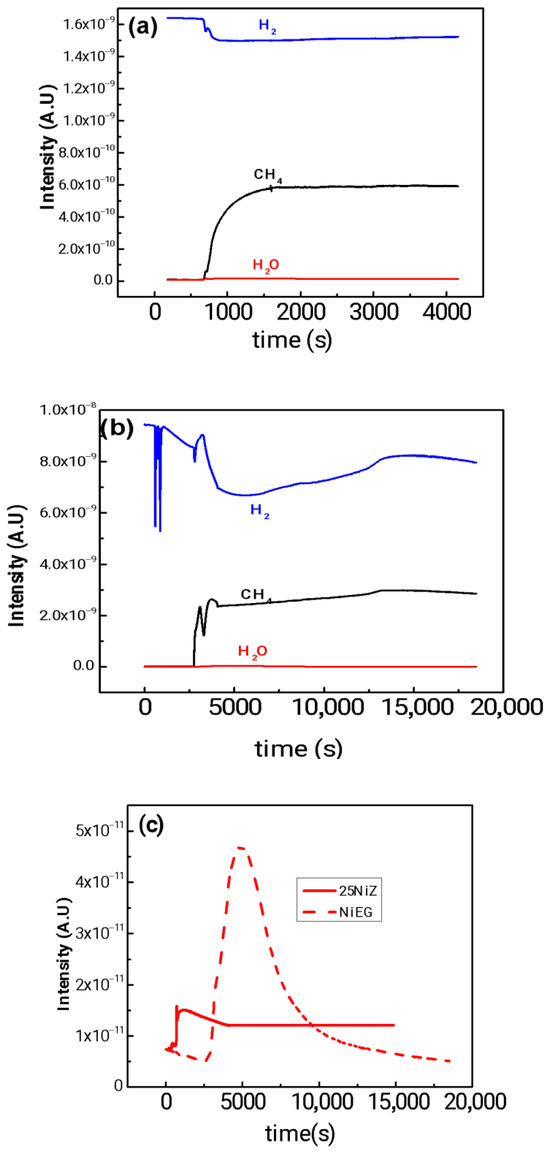
Figure 3.
TPSR spectra of (a) NiEG and (b) 25NiZ catalysts under pure CH4 flow: H2 signal (blue line) and CH4 signal (black line). (c) Corresponding H2O formation signal for NiEG and 25NiZ plotted on a separate scale. Conditions: 30 mg catalyst, 50 cm3 min−1 pure CH4, heating from ambient temperature to 773 K at 10 K min−1. Signals for H2, H2O, and CH4 were monitored by mass spectrometry.
A critical feature of the profile is the initial consumption of H2, marked by a distinct dip in the signal. Since the reacting atmosphere is pure CH4, this hydrogen must originate from the dissociative adsorption of methane on a limited number of pre-reduced metallic sites. This in situ-generated H2 is immediately consumed in the reduction of adjacent Ni2+ species, either at the support interface or within larger NiO crystallites (NiO + H2 → Ni0 + H2O). This reduction phase, evidenced by the concurrent release of H2O, is a prerequisite for generating a sufficient population of active Ni0 sites. Only once this process is complete does the catalyst achieve significant activity, marked by a sharp increase in H2 production from the methane decomposition reaction (CH4 → C(s) + 2H2).
In contrast, the NiEG nanoflower catalyst displays immediate hydrogen evolution from the onset of the CH4-TPSR experiment, absent of any induction period or H2 consumption. This indicates that the nickel in the as-synthesized NiEG catalyst is either predominantly in a pre-reduced metallic state (Ni0) or comprises highly dispersed oxide species that undergo facile reduction under the methane stream. The sustained H2 production profile points to a high and stable intrinsic activity for methane dehydrogenation. This performance underscores how the nanoflower architecture not only maximizes the density of accessible active sites but also confers remarkable stability against deactivation by coking. This resistance is attributed to structure-sensitive properties, such as enhanced carbon diffusion or a lowered activation barrier for the initial C-H bond cleavage on specific surface facets inherent to this morphology [39,40].
2.3. Catalytic Tests at 773 K
Catalyst stability was evaluated over 8 h at 773 K (See Figure 4). The NiEG catalyst demonstrated stable performance at low conversion levels, whereas the 25NiZ catalyst exhibited significant activity oscillations over time.
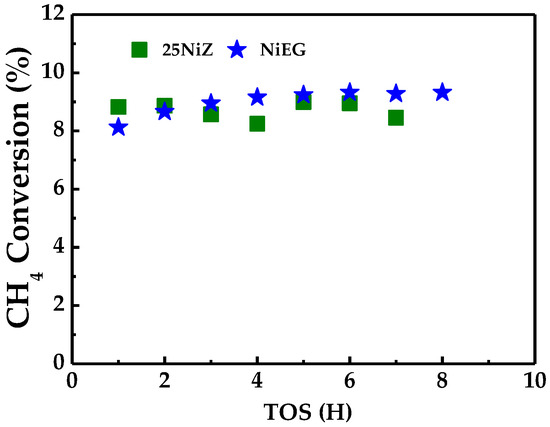
Figure 4.
Stability tests for catalysts exposed to CH4: Conditions: 30 cm3 min−1 CH4, 1 Atm, 773K.
Post-reaction characterization of the spent catalysts provided insight into their differing stabilities. Contrary to an initial visual inspection suggesting minimal coke formation (Figure 2a), detailed analysis revealed distinct carbon deposition modes. The 25NiZ catalyst was found to be predominantly deactivated by filamentous carbon (Figure 2b). In contrast, the NiEG catalyst formed carbon nanotubes, with no evidence of the encapsulating carbon that typically leads to severe deactivation. A stark contrast in product distribution is evident at 503 K when comparing methane flow rates. Under a high flow rate of 250 cm3 min−1 (Condition 2)—a condition typically associated with shortened residence time and lower conversion—the selectivity shifts markedly. Instead of 100% ethylene (C2H4) observed at lower flows, the products comprise a mixture of C2H4 (65.4%) and ethane (C2H6) (34.6%). This unexpected result implies that a higher conversion regime is achieved under these specific conditions. We propose that the increased methane flux drives higher initial activation rates, generating a sufficient surface hydrogen concentration to promote the secondary hydrogenation of the primary ethylene product to ethane.
Selectivity of the Catalysts
These marked differences in the unsupported NiEG catalyst compared to the supported 25NiZ suggested determining the product distribution or selectivity only for the NiEG catalyst. The molar selectivity for different feed conditions is shown in Table 2. The product distribution and selectivity under varying flows and temperatures, the calculation of reaction rates for both stages.

Table 2.
Molar selectivity for the NiEG catalyst under a two-stage reaction protocol: (A) Methane Activation: 1 min pulse of CH4 (30 or 250 cm3 min−1); (B) Subsequent Carbon Hydrogenation: under H2 (60 cm3 min−1). All steps performed at 503 K or 593 K.
The pronounced differences in performance between the unsupported NiEG and supported 25NiZ catalysts, particularly the superior activity and unique product spectrum of NiEG, warranted a detailed analysis of its selectivity. The product distribution for the NiEG catalyst was therefore determined under various feed conditions. Table 2 presents the molar selectivity towards different hydrocarbons while summarizing the evolution of product distribution with varying methane flow rates and reaction temperatures. Furthermore, the corresponding reaction rates for the principal steps of methane activation and conversion are quantified in Table 3.

Table 3.
Reaction rates for the two stages of activation and hydrogenation on 25NiZ and NiEG catalysts.
3. Discussion
The catalytic behavior observed in this study can be interpreted within the established framework of methane activation on nickel-based catalysts. Arevalo et al. [41] proposed that methane activation proceeds via the dissociative adsorption of methyl and methylene groups (CHx, where x < 3) [42,43,44]. Our results align with this mechanism, as the production of light olefins, particularly ethylene, increased during the initial activation stage. This is consistent with the catalytic coupling of CHx intermediates on highly dispersed nickel clusters [45,46]. The observed enhancement in olefin formation with increasing methane flow (Table 2) further supports this pathway, suggesting that higher feedstock flux promotes the coupling reaction. For the NiEG catalyst, the selectivity toward ethylene was notably high and increased following the hydrogenation stage, indicating the stabilization and desorption of surface intermediates as olefins [47]. However, this selectivity decreased at elevated temperatures. This loss is attributed to the onset of encapsulating carbon formation, which blocks active sites and shifts the product distribution toward heavier hydrocarbons [48,49]. The general trend confirms that the NiEG nanoflower morphology preferentially promotes the formation of ethane and ethylene at 503 K, primarily through the coupling of CHx fragments generated from initial C-H bond cleavage. The decline in selectivity at higher temperatures and flows is consistent with accelerated coking rates and the subsequent progression of reactions toward higher molecular weight products [47].
The catalytic performance of the NiEG material can be rationalized by considering the intrinsic activity of its exposed crystal facets. Theoretical and experimental studies indicate that the (111) plane of cubic nickel crystals exhibits superior activity for methane dissociation and CHx fragment coupling compared to other facets [48]. This plane provides an optimal electronic environment for stabilizing C-H bond cleavage intermediates, thereby increasing the probability of initial activation and subsequent C-C coupling reactions.
The consistent formation of ethylene during the initial activation stage across all conditions suggests a complex interplay of surface processes. This may involve a hydrogen spillover effect, where hydrogen atoms generated from dissociation migrate across the surface, potentially influencing further methane activation and the nature of carbon deposits [50]. The product distribution confirms that the active sites on the NiEG catalyst are effective not only for activation but also for hydrogenation, facilitating the formation of higher hydrocarbons like butane and propane [51,52]. The stability of these products implies the involvement of stable, non-graphitic carbon intermediates during their formation [53].
The influence of temperature is clearly linked to the appearance of heavier products. Elevated temperatures lower the activation energy for successive C-C coupling steps [46], promoting the formation of longer-chain hydrocarbons. The process begins with methane chemisorption on active Ni sites, enabling temporary C-H activation and C-C coupling. This step can be quantified by the amount of chemisorbed methane. The formation of non-graphitic, reactive carbon species is critical, as it avoids encapsulating the nickel particles, thereby preserving site activity for subsequent hydrogenation steps where olefins are formed and desorbed.
Based on this mechanistic framework, the consumption rates of methane and hydrogen during each stage were calculated. The reaction rate for the initial chemisorption stage and the turnover frequency (TOF) for the hydrogenation stage [54] are summarized in Table 3.
The quantitative data presented in Table 3 provide strong support for the proposed interpretation. The intrinsic activity, measured by the turnover frequency (TOF), demonstrates that the active sites on the NiEG catalyst are approximately ten times more efficient (TOF = 2.92 × 10−2 s−1) than those on the 25NiZ catalyst (TOF = 3.09 × 10−3 s−1). This superior site efficiency is a direct consequence of the distinctive nanoflower morphology.
H2 chemisorption data confirm that the NiEG catalyst exhibits high metallic dispersion (18.7%) and a small average Ni0 crystallite size (5.39 nm). These parameters are consistent with a structure that maximizes the density of accessible, active surface sites. In contrast, the supported 25NiZ catalyst, despite its higher total nickel loading (7.82 wt% vs. 2.50 wt%), possesses low metallic dispersion (2.9%) and large Ni0 crystallites (16.9 nm). This results in a significantly smaller fraction of exposed nickel atoms, explaining its lower intrinsic activity and the prolonged reduction period observed in the CH4-TPSR experiments.
The significantly higher turnover frequency (TOF) and methane reaction rate (rCH4) observed for the NiEG catalyst indicate not only superior methane activation but also enhanced stabilization of carbonaceous intermediates on the nickel active sites. This stability is crucial, as it allows these intermediates to undergo coupling and desorption as olefins rather than progressing toward deactivating carbon deposits.
In contrast, the 25NiZ catalyst exhibits much lower TOF and reaction rates, likely a consequence of sintering and lower metallic dispersion. Interestingly, this catalyst shows a higher total H2 uptake and turnover number (TONH2). This can be attributed to the ZrO2 support facilitating hydrogen spillover, whereby hydrogen atoms migrate from the metal particles to the support surface, increasing the overall measured uptake. The NiEG catalyst, as an unsupported material, lacks this specific effect but demonstrates an exceptional ability to form stable, high-surface-area nanostructures that may exhibit resistance to deactivation.
The high activity of the NiEG catalyst stems from its unique nanoarchitecture of nickel clusters, which provides a high density of accessible active sites. This performance is notable when contextualized with literature; strategies for methane activation often involve complex catalysts, such as molybdenum supported on zeolites or mesoporous materials, frequently promoted with metals like Ga, Pt, or Ru, to achieve olefin production [55,56,57]. The unsupported NiEG nanoflowers presented here achieve high activity through morphological control alone, offering a promising and potentially simpler catalytic system. The efficacy of the NiEG catalyst is further highlighted when compared to state-of-the-art systems for methane valorization. Table 4 compiles literature data for methane activation over various catalysts, including those designed for methane cracking and CO2 methanation.

Table 4.
Comparative overview of catalytic performance and properties for various methane activation, methane pyrolysis, and CO2 methanation systems.
The NiEG catalyst operates at a significantly lower temperature (593 K) compared to other methane activation catalysts, which typically require temperatures above 923 K [63,64] and often exceed 1123 K [58,59,60]. This lower operational temperature underscores a superior intrinsic activity for C-H bond cleavage, which we attribute to the highly exposed and stable active sites presented by the nanoflower morphology. While the methane conversion over NiEG (10%) is moderate compared to some high-temperature systems like 7Ni3Al (68% at 923 K) [63], it is crucial to note that conversion is a function of both temperature and gas hourly space velocity (GHSV). The NiEG catalyst achieves this conversion under a substantially higher GHSV (186 L h−1 gcat−1), indicating a much higher throughput and a more efficient contact time compared to systems like 20Ni-20Co/AC (GHSV = 1.5 L h−1 gcat−1) [59].
While many methane activation systems are geared towards the production of hydrogen and carbon nanotubes (CNTs) or filaments [61,63,64], the NiEG catalyst facilitates the direct formation of valuable C6 hydrocarbons with a selectivity of 8.2%. This demonstrates its ability to promote C-C coupling and chain growth under remarkably mild conditions. For instance, while NixPy/SiO2 produces a broader range of hydrocarbons (C2–C10) [60], it does so at a much higher temperature (1173 K) and with lower BET surface area, suggesting that the nanoflower structure of NiEG provides a more optimized environment for specific chain growth pathways.
The NiEG catalyst possesses a high BET surface area (180 m2 g−1), which is comparable to or greater than many supported catalysts [58,61,63]. This high surface area, combined with a small NiO crystallite size (5.2 nm), is consistent with its high metallic dispersion and the abundance of active sites, as discussed previously. This contrasts with catalysts like Ni/La2Ce2O7, which, despite a respectable surface area (87 m2·g−1), features larger crystallites (11.5 nm) and operates at a higher temperature for a lower conversion [63].
When compared to catalysts for related reactions, the distinct role of NiEG in methane activation becomes even clearer. Catalysts for CO2 methanation (CM), such as 1.0% Ru/Ni Nanowires [65], are highly efficient for that specific reaction but do not facilitate the C-C coupling necessary for higher hydrocarbon synthesis. Similarly, the Photocatalytic Non-Oxidative Coupling of Methane (PNOCM) over Co0.1/GaN Nanowires [66] achieves excellent selectivity for C2 species but operates through a completely different, light-driven mechanism.
The unsupported NiEG nanoflowers exhibit a remarkably high TON of 1.79 × 10−2 s−1 for methane activation. This value is comparable to those estimated for the highly optimized 1.0% Ru/Ni Nanowires (2.1 × 10−2 s−1) and RuNi/CeZr_TSI (1.5 × 10−2 s−1) catalysts in CO2 methanation [65,67]. This is a significant finding, as it demonstrates that the morphological control in the unsupported NiEG system can achieve an intrinsic site activity rivaling that of noble-metal-promoted (Ru-Ni) and carefully engineered nanostructured (Ni nanowires) catalysts, but for the more challenging C-H bond cleavage and C-C coupling of methane activation versus the hydrogenation-focused CO2 methanation.
The Ru-Ni nanowire system achieves its high activity at a very low temperature (452 K) [65], a benefit attributed to the synergistic effect where Ru enhances H2 dissociation and spillover, facilitating the hydrogenation of surface intermediates on Ni and thereby suppressing carbon polymerization. Similarly, the RuNi/CeZr_TSI catalyst leverages strong metal-support interactions to maintain high activity and stability at 623 K [67]. In the present study, the NiEG catalyst, while operating at a higher temperature than these methanation catalysts (593 K), achieves its high TON without any noble metal promoter. We attribute this to its nanoflower morphology, which provides a high density of exposed, stable Ni sites that facilitate efficient C-H activation and intermediate stabilization, mirroring the effect of Ru in preventing the formation of deactivating carbon by promoting a controlled reaction pathway towards structured carbon nanotubes rather than encapsulating coke. This is in stark contrast to the supported 25NiZ catalyst, which exhibited a much lower TON (3.09 × 10−4 s−1) and was prone to deactivation by filamentous coke.
Therefore, the unsupported NiO nanoflower (NiEG) catalyst demonstrates high suitability for the direct production of higher hydrocarbons and olefins from methane. This efficacy is directly linked to its unique morphology (Figure 3b), which maximizes the exposure of active sites, thereby facilitating the initial C-H bond cleavage and subsequent hydrogenation of the resulting surface intermediates. Based on the experimental evidence, we propose a reaction pathway for methane conversion over the NiEG catalyst, as illustrated in Figure 5.
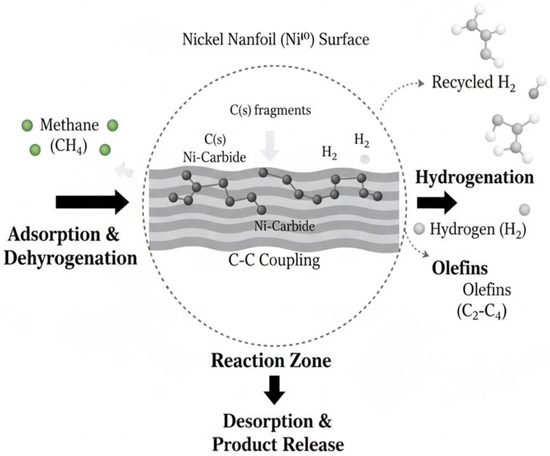
Figure 5.
Proposed reaction Path for NiEG nanofoils.
The detection of longer-chain hydrocarbons in low proportions indicates the occurrence of secondary hydrogenation pathways. These reactions involve the addition of hydrogen to surface carbonaceous species generated during the initial methane activation.
4. Materials and Methods
4.1. Catalysts Synthesis
Zirconia (ZrO2) was selected as a support due to its thermal stability, redox properties, and ability to moderate metal-support interactions, providing a contrasting system to the unsupported nanoflowers. A Ni/ZrO2 catalyst with a nominal loading of 25 wt.% NiO (denoted 25NiZ) was prepared by incipient wetness impregnation. The ZrO2 support was synthesized by calcining Zr(OH)4 (MEL Chemicals) at 923 K for 3 h. While this calcination typically yields predominantly monoclinic zirconia (m-ZrO2), the incorporation of nickel at loadings ≥ 20 wt.% is known to stabilize the metastable tetragonal phase (t-ZrO2) [10]. The support was impregnated with an aqueous solution of Ni(NO3)2·6H2O to achieve the target loading. The material was then dried at 373 K for 24 h and calcined in flowing synthetic air at 923 K for 3 h using a ramp rate of 10 °C min−1.
The unsupported NiO sample (denoted NiEG) was synthesized via an ethylene glycol-mediated precipitation method adapted from the literature [68]. Briefly, Ni(NO3)2·6H2O (0.05 mol) was dissolved in ethylene glycol (150 mL). The solution was heated to 393 K under stirring and held for 30 min. An aqueous Na2CO3 solution (0.2 mol L−1, 500 mL) was added dropwise. The resulting mixture was aged at 393 K for 1 h, then filtered and washed extensively with deionized water. The solid was dried at 373 K for 16 h and calcined at 673 K for 4 h under a flow of synthetic air (50 mL min−1).
4.2. Catalysis Characterization
4.2.1. X-Ray Diffraction
X-ray diffraction patterns were acquired on a Rigaku Miniflex diffractometer operated at 30 kV and 15 mA using Cu Kα radiation (λ = 1.5406 Å). Prior to analysis, the samples were degassed under a helium flow (150 cm3 min−1) in a microreactor and subsequently stored in a vacuum desiccator.
4.2.2. Scanning Electron Microscopy (SEM)
Morphological analysis was performed by Field Emission Scanning Electron Microscopy (FE-SEM) using a Quanta 400 model (ThermoFisher, Waltham, MA, USA), operating at an accelerating voltage between 10 and 20 kV. The instrument was equipped with an energy-dispersive X-ray spectroscopy (EDS) system for microanalysis. Samples were prepared by dispersion onto a conductive carbon tape adhered to an aluminum stub.
4.2.3. X-Ray Fluorescence (XRF)
The bulk chemical composition of the catalysts was determined by X-ray fluorescence (XRF) using a Rigaku Rix 3100 spectrometer (Rigaku Americas Corporation, The Woodlands, TX, USA) with a rhodium tube as the X-ray source.
4.2.4. Temperature-Programmed Surface Reaction (TPSR) with CH4
CH4-TPSR experiments were conducted in a Micromeritics 2900 TPD/TPR system. Approximately 30 mg of catalyst was used for each experiment. The sample was exposed to a pure methane flow (50 cm3 min−1) while the temperature was ramped from room temperature to 773 K at a rate of 10 K min−1 and held for 6 h. The effluent gases were monitored using a coupled Balzers quadrupole mass spectrometer. Metal dispersion and metallic surface area were calculated from the resulting data based on established correlations [54], using Equation (1):
where XH2 is the H2 uptake (mol/gcat), NAV is the Avogadro number, and σMe shows the concentration of surface atoms, typically 1.54 × 1019 at m−2 for Nickel.
4.2.5. Catalytic Tests
Methane activation and hydrogenation were performed in a fixed-bed microreactor operating at atmospheric pressure [29]. The reactor effluent was analyzed using an online VARIAN gas chromatograph equipped with a Restek Q-PLOT capillary column.
Typically, 100 mg of the 25NiZ catalyst or 25 mg of the unsupported NiEG catalyst was loaded. The catalysts were pre-treated in situ under a H2/He mixture (60 cm3 min−1, 20% H2), heating from room temperature to 773 K at 5 °C min−1, and holding for 2 h. The system was then purged with He at 773 K for 1 h before cooling to the initial reaction temperature of 503 K.
The reaction sequence involved exposing the catalyst to a methane flow for 1 min at either 503 K or 593 K, followed by the introduction of H2 (60 cm3 min−1) at the same temperature. Two methane flow rates were tested: V1 = 30 cm3 min−1 and V2 = 250 cm3 min−1.
Methane conversion (XCH4) was calculated using Equation (2):
XCH4 = [[(FCH4)inlet − (FCH4)Outlet]/[(FCH4)inlet]] * 100
The turnover frequency (TOF) was calculated based on the number of surface nickel atoms, as shown in Equation (3):
TOFi = (Xi * Fi inlet)/((CNi) * mCat/WM Ni) * 100
Molar selectivity (S_i) for product i was calculated using Equation (4):
Si = ni/nT * 100
Where F is the molar flow of the i species (i = CH4), expressed in mol/min, mcat is the mass used in each test in g, Xi is the CH4 conversion, CNi is the weight % of Nickel, WMNi is the molecular weight of Ni (g/mol)
5. Conclusions
The NiEG catalyst showed a significantly higher methane activation rate of 1.79 mol/(s·g_NiO), far exceeding that of the 25NiZ catalyst (0.003 mol/(s·g_NiO)). This is attributed to the highly exposed and stable active sites provided by the nanoflower morphology.
NiEG produced ethylene and ethane at 503 K and higher hydrocarbons (C4–C6) at 593 K, indicating its ability to promote C-C coupling and chain growth under mild conditions. This versatility is not observed in the supported catalyst.
While the 25NiZ catalyst suffered from deactivating filamentous coke, NiEG formed less detrimental carbon nanofibers, which did not encapsulate active sites. This resulted in stable performance over time, even at high temperatures.
The nanoflower structure enhances metallic dispersion, stabilizes intermediate carbon species, and facilitates rapid adsorption/desorption cycles, thereby promoting continuous methane activation and reducing deactivation.
The exposed (111) lattice planes of NiEG favor methane dissociation and intermediate stability, leading to higher turnover frequencies (TOF = 2.92 × 10−2 s−1) compared to the supported catalyst (TOF = 3.09 × 10−3 s−1).
The unsupported NiEG nanoflowers enable methane activation at a significantly lower temperature (593 K) compared to conventional systems (>923 K) while directly producing valuable C6 hydrocarbons, an outcome distinct from typical hydrogen/CNT-producing systems.
NiEG achieves a turnover frequency (TON) comparable to noble-metal-promoted (Ru-Ni) catalysts, demonstrating that its nanoflower morphology provides an intrinsic site activity for C-H cleavage that rivals advanced, promoted systems without requiring expensive additives.
Author Contributions
Conceptualization, A.R.G.C. and M.S.; methodology, A.R.G.C. and M.S.; software, A.R.G.C.; validation, A.R.G.C.; formal analysis, A.R.G.C.; investigation, A.R.G.C.; resources, A.R.G.C. and M.S.; data curation, A.R.G.C. and M.S.; writing—original draft preparation, A.R.G.C. and M.S.; writing—review and editing, A.R.G.C. and M.S.; visualization, A.R.G.C.; supervision, A.R.G.C. and M.S.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Brazilian National Council for Scientific and Technological Development (CNPq) through a Doctoral Fellowship [Process 142194/2014-0].
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors gratefully acknowledge the financial support and scholarships provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil). The technical assistance provided by Núcleo de Catálise (NUCAT) and the Núcleo de Microscopia Eletrônica (COPPE/UFRJ) for the TEM analysis is also sincerely appreciated. This article is dedicated to the memory of Ruth L. Martins.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chu, Y.; Zhou, K.; Hu, R.; Yang, Z. Diversified hydrogen production methods can reduce carbon dioxide emissions and energy consumption across Chinese cities. Commun. Earth Environ. 2025, 6, 471. [Google Scholar] [CrossRef]
- Gao, D.; Gao, W.; Ma, Z.; Zhu, L.; Tian, J.; Liu, S.; Yu, Y.; Zhang, G.; Gao, Q. Trends and characteristics of global CH4 emissions: Insights from UNFCCC greenhouse gas inventories. Atmos. Ocean. Sci. Lett. 2025, 18, 100637. [Google Scholar] [CrossRef]
- Hureau, G.; Lecarpentier, A.; Serbutoviez, S.; Kaniewicz, J.; Madden, M.; Brooks, C.; Robertson, A.; Langston, C.; Harrison, C.; Le Ravalec, M. Global methane emissions from natural gas transmission and distribution networks. Sci. Technol. Energ. Transit. 2025, 80, 28. [Google Scholar] [CrossRef]
- Madavi, T.B.; Chauhan, S.; Madathil, V.; Sankaranarayanan, M.; Navina, B.; Velmurugan, N.K.; Choi, K.-Y.; Ankamareddy, H.; Alavilli, H.; Pamidimarri, S.D. Microbial methanotrophy: Methane capture to biomanufacturing of platform chemicals and fuels. Next Energy 2025, 8, 100251. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Huang, H.; Sui, X.; Zeng, H.; Lu, H.; Wang, Y.; Jia, Y.; Steele, J.A.; Ao, Y.; et al. Platinum Single-Atom Nests Boost Solar-Driven Photocatalytic Non-Oxidative Coupling of Methane to Ethane. J. Am. Chem. Soc. 2024, 146, 24150–24157. [Google Scholar] [CrossRef] [PubMed]
- Nesterenko, N.; Medeiros-Costa, I.C.; Clatworthy, E.B.; Cruchade, H.; Konnov, S.V.; Dath, J.-P.; Gilson, J.-P.; Mintova, S. Methane-to-chemicals: A pathway to decarbonization. Natl. Sci. Rev. 2023, 10, nwad116. [Google Scholar] [CrossRef] [PubMed]
- Baniam, M.; Gholamian, E.; Yari, M.; Mehr, A.S. Innovative integration of DMFC in polygeneration energy systems for enhanced renewable fuel and power outputs. Process Saf. Environ. Prot. 2025, 199, 107263. [Google Scholar] [CrossRef]
- Al Zakwani, S.; Ouadi, M.; Mohammed, K.; Steinberger-Wilckens, R. Simulation of Biomass Gasification and Syngas Methanation for Methane Production with H2/CO Ratio Adjustment in Aspen Plus. Energies 2025, 18, 4319. [Google Scholar] [CrossRef]
- Bampos, G.; Panagiotopoulou, P.; Kyriakidou, E.A. Catalytic Reforming and Hydrogen Production: From the Past to the Future. Catalysts 2025, 15, 332. [Google Scholar] [CrossRef]
- Szablowski, L.; Wojcik, M.; Dybinski, O. Review of steam methane reforming as a method of hydrogen production. Energy 2025, 316, 134540. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, C.; Liu, H.; Jin, Y.; Zhang, R.; Ran, J. Unraveling the effects of Ni particle size and facet on CH4 activation: From cluster to nanoparticle. Int. J. Hydrogen Energy 2023, 48, 19486–19493. [Google Scholar] [CrossRef]
- Osti, A.; Rizzato, L.; Costa, S.; Cavazzani, J.; Glisenti, A. Substoichiometric La0.8MnO3-based nanocomposites for PGM-free activation of CH4: Ni or Cu? Surface or bulk? Fuel 2025, 381, 133368. [Google Scholar] [CrossRef]
- Scheiblehner, D.; Neuschitzer, D.; Wibner, S.; Sprung, A.; Tunes, M.A.; Leuchtenmüller, M.; Scherr, C.; Antrekowitsch, H.; Luidold, S. The catalytic effect of Ni in methane pyrolysis using molten SnNi alloys for hydrogen production. Int. J. Hydrogen Energy 2025, 102, 1045–1054. [Google Scholar] [CrossRef]
- González, J.M.; Sabadell-Rendón, A.; Kaźmierczak, K.; Euzenat, F.; Montroussier, N.; Curulla-Ferré, D.; López, N. Nickel Dynamics Switches the Selectivity of CO2 Hydrogenation. Angew. Chem. Int. Ed. 2025, 64, e202417392. [Google Scholar] [CrossRef]
- Sun, X.; Tang, M.; Yu, M.; Fan, Y.; Qin, C.; Cao, J.; Wang, Y. UV-activated CH4 gas sensor based on Pd@Ni/ZnO microspheres. Mater. Today Commun. 2024, 40, 109551. [Google Scholar] [CrossRef]
- Fite, M.C.; Karse, S.D.; Gode, L.M. Optical and photocatalytic properties of nickel oxide nanoparticles. J. Cryst. Growth 2025, 660, 128163. [Google Scholar] [CrossRef]
- Xu, W.; Liu, H.; Hu, Y.; Wang, Z.; Huang, Z.; Huang, C.; Lin, J.; Chang, C.; Wang, A.; Wang, X.; et al. Metal-Oxo Electronic Tuning via In Situ CO Decoration for Promoting Methane Conversion to Oxygenates over Single-Atom Catalysts. Angew. Chem. Int. Ed. 2024, 63, e202315343. [Google Scholar] [CrossRef]
- He, C.; Gong, Y.; Li, S.; Wu, J.; Lu, Z.; Li, Q.; Wang, L.; Wu, S.; Zhang, J. Single-Atom Alloys Materials for CO2 and CH4 Catalytic Conversion. Adv. Mater. 2024, 36, 2311628. [Google Scholar] [CrossRef]
- Sanwal, P.; Gu, X.; Zhang, Y.; Li, G. The Tiara Nickel Cluster Story from Theory to Catalytic Applications. Precis. Chem. 2025, 3, 157–171. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, Y.; Sánchez, J.S.G.; Pérez-Lemus, G.R.; Rico, P.F.Z.; Delferro, M.; de Pablo, J.J. Free-Energy Landscapes and Surface Dynamics in Methane Activation on Ni(511) via Machine Learning and Enhanced Sampling. ACS Catal. 2025, 15, 8931–8942. [Google Scholar] [CrossRef]
- Lee, S.J.; Jang, H.; Lee, D.N. Recent advances in nanoflowers: Compositional and structural diversification for potential applications. Nanoscale Adv. 2023, 5, 5165–5213. [Google Scholar] [CrossRef]
- Zhou, M.; Xiong, W.; Li, H.; Zhang, D.; Lv, Y. Emulsion-template synthesis of mesoporous nickel oxide nanoflowers composed of crossed nanosheets for effective nitrogen reduction. Dalton Trans. 2021, 50, 5835–5844. [Google Scholar] [CrossRef]
- Godlaveeti, S.K.; El-Marghany, A.; Parandamaiah, M.; Nasina, M.R.; Gedi, S.; Nagireddy, R.R.; Subbaiah, G.C.V.; Chintaparty, R. Low-Temperature Synthesis of NiO Structures: Tailoring Morphology for Enhanced Dielectric Performance. ECS J. Solid State Sci. Technol. 2025, 14, 23008. [Google Scholar] [CrossRef]
- Silveira, E.B.; Rabelo-Neto, R.C.; Noronha, F.B. Steam reforming of toluene, methane and mixtures over Ni/ZrO2 catalysts. Catal. Today 2017, 289, 289–301. [Google Scholar] [CrossRef]
- Fajardo, H.V.; Longo, E.; Mezalira, D.Z.; Nuernberg, G.B.; Almerindo, G.I.; Collasiol, A.; Probst, L.F.D.; Garcia, I.T.S.; Carreño, N.L.V. Influence of support on catalytic behavior of nickel catalysts in the steam reforming of ethanol for hydrogen production. Environ. Chem. Lett. 2010, 8, 79–84. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Sophiphun, O.; Bernardi, J.; Wittayakun, J.; Föttinger, K.; Rupprechter, G. Methane dry reforming over ceria-zirconia supported Ni catalysts. Catal. Today 2016, 277, 234–245. [Google Scholar] [CrossRef]
- Martínez, J.; Hernández, E.; Alfaro, S.; Medina, R.L.; Aguilar, G.V.; Albiter, E.; Valenzuela, M.A. High selectivity and stability of nickel catalysts for CO2 Methanation: Support effects. Catalysts 2019, 9, 24. [Google Scholar] [CrossRef]
- Martins, R.L.; Schmal, M. Activation of methane on NiO nanoparticles with different morphologies. J. Braz. Chem. Soc. 2014, 25, 2399–2409. [Google Scholar] [CrossRef]
- Si, J.; Liu, G.; Liu, J.; Zhao, L.; Li, S.; Guan, Y.; Liu, Y. Ni nanoparticles highly dispersed on ZrO2 and modified with La2O3 for CO methanation. RSC Adv. 2016, 6, 17836–17844. [Google Scholar] [CrossRef]
- Song, L.X.; Yang, Z.K.; Teng, Y.; Xia, J.; Du, P. Nickel oxide nanoflowers: Formation, structure, magnetic property and adsorptive performance towards organic dyes and heavy metal ions. J. Mater. Chem. A 2013, 1, 11246–11259. [Google Scholar] [CrossRef]
- Horti, N.C.; Kamatagi, M.D.; Nataraj, S.K.; Wari, M.N.; Inamdar, S.R. Structural and optical properties of zirconium oxide (ZrO2) nanoparticles: Effect of calcination temperature. Nano Express 2020, 1, 010028. [Google Scholar] [CrossRef]
- Gonzalez Caranton, A.R.; da Silva Pinto, J.C.C.; Stavale, F.; Barreto, J.; Schmal, M. Statistical analysis of the catalytic synthesis of Vinyl acetate over Pd-Cu/ZrO2 nanostructured based catalysts. Catal. Today 2020, 344, 190–200. [Google Scholar] [CrossRef]
- Duraisamy, N.; Numan, A.; Fatin, S.O.; Ramesh, K.; Ramesh, S. Facile sonochemical synthesis of nanostructured NiO with different particle sizes and its electrochemical properties for supercapacitor application. J. Colloid Interface Sci. 2016, 471, 136–144. [Google Scholar] [CrossRef]
- Bai, G.; Dai, H.; Deng, J.; Liu, Y.; Ji, K. Porous NiO nanoflowers and nanourchins: Highly active catalysts for toluene combustion. Catal. Commun. 2012, 27, 148–153. [Google Scholar] [CrossRef]
- Cárdenas-Arenas, A.; Quindimil, A.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castelló, D.; De-La-Torre, U.; Pereda-Ayo, B.; González-Marcos, J.A.; González-Velasco, J.R.; Bueno-López, A. Design of active sites in Ni/CeO2 catalysts for the methanation of CO2: Tailoring the Ni-CeO2 contact. Appl. Mater. Today 2020, 19, 100591. [Google Scholar] [CrossRef]
- Boakye, O.Y.; Hashemi, S.M.; Mahinpey, N. Investigation of Al2O3, ZrO2, SiO2, and CeO2 supported nickel catalysts for tri-reforming of methane. Int. J. Hydrogen Energy 2025, 109, 802–812. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Takriff, M.S. Methane decomposition into COx free hydrogen and multiwalled carbon nanotubes over ceria, zirconia and lanthana supported nickel catalysts prepared via a facile solid state citrate fusion method. Energy Convers. Manag. 2016, 126, 302–315. [Google Scholar] [CrossRef]
- Yang, E.; Nam, E.; Jo, Y.; An, K. Coke resistant NiCo/CeO2 catalysts for dry reforming of methane derived from core@shell Ni@Co nanoparticles. Appl. Catal. B Environ. 2023, 339, 123152. [Google Scholar] [CrossRef]
- Das, S.; Sengupta, M.; Bag, A.; Shah, M.; Bordoloi, A. Facile synthesis of highly disperse Ni-Co nanoparticles over mesoporous silica for enhanced methane dry reforming. Nanoscale 2018, 10, 6409–6425. [Google Scholar] [CrossRef]
- Sukonket, T.; Khan, A.; Saha, B.; Ibrahim, H.; Tantayanon, S.; Kumar, P.; Idem, R. Influence of the Catalyst Preparation Method, Surfactant Amount, and Steam on CO2 Reforming of CH4 over 5Ni/Ce0.6Zr0.4O2 Catalysts. Energy Fuels 2011, 25, 864–877. [Google Scholar] [CrossRef]
- Arevalo, R.L.; Aspera, S.M.; Escaño, M.C.S.; Nakanishi, H.; Kasai, H. Tuning methane decomposition on stepped Ni surface: The role of subsurface atoms in catalyst design. Sci. Rep. 2017, 7, 13963. [Google Scholar] [CrossRef]
- Chen, X.; Bella, B.; Yue, Y.; Kosari, M.; Liu, L.; Hu, F.; Cao, K.; Xiong, Y.; Mandal, A.; Chang, J.; et al. Plasma induced methane conversion: A review on COx-free production of hydrogen, valuable chemicals, and functional carbon materials. EES Catal. 2025. [Google Scholar] [CrossRef]
- Tang, P.; Zhu, Q.; Wu, Z.; Ma, D. Methane activation: The past and future. Energy Environ. Sci. 2014, 7, 2580–2591. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Han, J.; Zhu, X.; Mei, D.; Ge, Q. Simultaneous Activation of CH4 and CO2 for Concerted C-C Coupling at Oxide-Oxide Interfaces. ACS Catal. 2019, 9, 3187–3197. [Google Scholar] [CrossRef]
- Khodagholi, M.A.; Irani, M. Catalytic and noncatalytic conversion of methane to olefins and synthesis gas in an AC parallel plate discharge reactor. J. Chem. 2013, 2013, 676901. [Google Scholar] [CrossRef]
- Seenivasan, H.; Tiwari, A.K. Enhancing methane dissociation with nickel nanoclusters. Comput. Theor. Chem. 2015, 1066, 94–99. [Google Scholar] [CrossRef]
- Okolie, C.; Lyu, Y.; Kovarik, L.; Stavitski, E.; Sievers, C. Coupling of Methane to Ethane, Ethylene, and Aromatics over Nickel on Ceria–Zirconia at Low Temperatures. ChemCatChem 2018, 10, 4653–4662. [Google Scholar] [CrossRef]
- Dutta, K.; Li, L.; Gupta, P.; Gutierrez, D.P.; Kopyscinski, J. Direct non-oxidative methane aromatization over gallium nitride catalyst in a continuous flow reactor. Catal. Commun. 2018, 104, 106–110. [Google Scholar] [CrossRef]
- Hasnan, N.S.N.; Timmiati, S.N.; Lim, K.L.; Yaakob, Z.; Kamaruddin, N.H.N.; Teh, L.P. Recent developments in methane decomposition over heterogeneous catalysts: An overview. Mater. Renew. Sustain. Energy 2020, 9, 7. [Google Scholar] [CrossRef]
- Bengaard, H.S.; Nørskov, J.K.; Sehested, J.; Clausen, B.S.; Nielsen, L.P.; Molenbroek, A.M.; Rostrup-Nielsen, J.R. Steam reforming and graphite formation on Ni catalysts. J. Catal. 2002, 209, 365–384. [Google Scholar] [CrossRef]
- Lee, J.S.; Oyama, S.T. Oxidative Coupling of Methane to Higher Hydrocarbons. Catal. Rev. 1988, 30, 249–280. [Google Scholar] [CrossRef]
- Spivey, J.J.; Hutchings, G. Catalytic aromatization of methane. Chem. Soc. Rev. 2014, 43, 792–803. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Douvartzides, S.L.; Siakavelas, G.I.; Tzounis, L.; Sebastian, V.; Stolojan, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. The relationship between reaction temperature and carbon deposition on nickel catalysts based on Al2O3, ZrO2 or SiO2 supports during the biogas dry reforming reaction. Catalysts 2019, 9, 676. [Google Scholar] [CrossRef]
- Lee, K.-M.; Kim, B.; Lee, J.; Kwon, G.; Yoon, K.; Song, H.; Min, K.H.; Shim, S.E.; Hwang, S.; Kim, T. The NO reduction by CO over NiOx/CeO2 catalysts with a fixed Ni surface density: Pretreatment effects on the catalyst structure and catalytic activity. Catal. Sci. Technol. 2024, 14, 279–292. [Google Scholar] [CrossRef]
- Martins, R.L.; Baldanza, M.A.; Souza, M.M.V.M.; Schmal, M. Methane activation on alumina supported platinum, palladium, ruthenium and rhodium catalysts. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2004; Volume 147, pp. 187–192. [Google Scholar] [CrossRef]
- Jarvis, J.; He, P.; Wang, A.; Song, H. Pt-Zn/HZSM-5 as a highly selective catalyst for the Co-aromatization of methane and light straight run naphtha. Fuel 2019, 236, 1301–1310. [Google Scholar] [CrossRef]
- Guisnet, M.; Gnep, N.S.; Alario, F. Aromatization of short chain alkanes on zeolite catalysts. Appl. Catal. A Gen. 1992, 89, 1–30. [Google Scholar] [CrossRef]
- Ellison, C.; Lauterbach, J.; Smith, M.W. Activated Carbon Supported Ni, Fe, and Bimetallic NiFe Catalysts for COx-Free H2 Production by Microwave Methane Pyrolysis. Int. J. Hydrogen Energy 2024, 55, 1062–1070. [Google Scholar] [CrossRef]
- Gubanov, M.A.; Ivantsov, M.I.; Kulikova, M.V.; Kryuchkov, V.A.; Nikitchenko, N.V.; Knyazeva, M.I.; Kulikov, A.B.; Pimenov, A.A.; Maksimov, A.L. Methane Decomposition Nickel Catalysts Based on Structured Supports. Pet. Chem. 2020, 60, 1043–1051. [Google Scholar] [CrossRef]
- Dipu, A.L.; Nishikawa, Y.; Inami, Y.; Iguchi, S.; Yamanaka, I. Development of Highly Active Silica-Supported Nickel Phosphide Catalysts for Direct Dehydrogenative Conversion of Methane to Higher Hydrocarbons. Catal. Lett. 2022, 152, 199–212. [Google Scholar] [CrossRef]
- Gomez, L.A.; Bavlnka, C.Q.; Nguyen, P.T.; Alalq, I.; Sabisch, J.E.; Boscoboinik, J.A.; Resasco, D.E.; Crossley, S.P. Evolution of Ni-Mo/MgO during Catalytic Methane Pyrolysis to Produce Base-Growth Nanotubes. Cell Rep. Phys. Sci. 2025, 6, 102519. [Google Scholar] [CrossRef]
- Yang, S.Y.; Yun, J.S.; Park, H.W.; Kim, J.H.; Saidova, N.U.K.; Lee, H.; An, K.; Im, J.S.; Lee, S.H. Unveiling the Role of Metal–Support Interactions in Ni Catalysts for CO2-Free Hydrogen and Carbon Nanotube Production via Methane Pyrolysis. Int. J. Hydrogen Energy 2025, 152, 150168. [Google Scholar] [CrossRef]
- Choi, S.-B.; Kang, D.-B.; Kim, S.-J.; Park, G.-J.; Kim, Y.; Kim, W.; Ko, C.H. Tailoring Ni Particle Size to Improve Catalytic Methane Decomposition on La2Ce2O7 Supports. Catal. Today 2026, 462, 115550. [Google Scholar] [CrossRef]
- Ibrahimov, H.; Malikli, S.; Ibrahimova, Z.; Babali, R.; Aleskerova, S. Ni-γ-Al2O3 Catalysts for Obtaining Nanocarbon by Decomposition of Natural Gas. Appl. Petrochem. Res. 2021, 11, 123–128. [Google Scholar] [CrossRef]
- Siudyga, T.; Kapkowski, M.; Janas, D.; Wasiak, T.; Sitko, R.; Zubko, M.; Szade, J.; Balin, K.; Klimontko, J.; Lach, D.; et al. Nano-Ru Supported on Ni Nanowires for Low-Temperature Carbon Dioxide Methanation. Catalysts 2020, 10, 513. [Google Scholar] [CrossRef]
- Ye, Z.; Long, Z.; Zhang, B.; Navid, I.A.; Menzel, J.P.; Shen, Y.; Mondal, S.; Guo, F.; Norris, T.B.; Batista, V.S.; et al. Photocatalytic Conversion of Methane to Ethane and Propane Using Cobalt-Cluster-Activated GaN Nanowires. Angew. Chem. Int. Ed. 2025, 64, e202500158. [Google Scholar] [CrossRef]
- Ricca, A.; Renda, S.; Di Stasi, C.; Truda, L.; Palma, V. Effective H2 Conversion to Substitute Natural Gas on Ni-Based Catalysts: Role of Promoters and Synthesis Method. Renew. Energy 2026, 256, 124179. [Google Scholar] [CrossRef]
- Kattel, S.; Ramírez, P.J.; Chen, J.G.; Rodriguez, J.A.; Liu, P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 2017, 355, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).