A Ternary Spinel Strategy for Increasing the Performances of Oxygen Reduction Reaction and Anion Exchange Membrane Fuel Cell Based on Mn-Co Spinel Oxides
Abstract
1. Introduction
2. Results
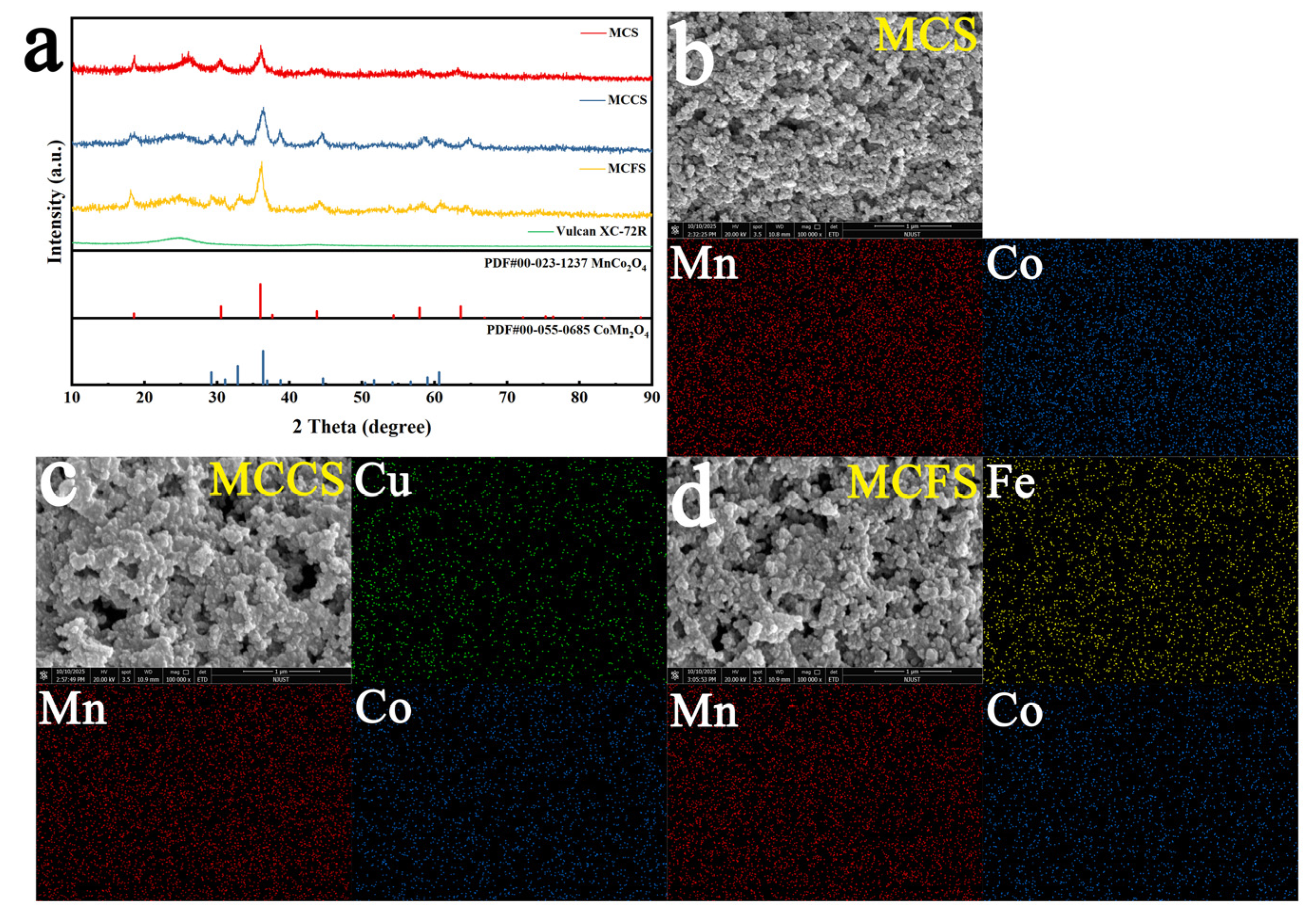
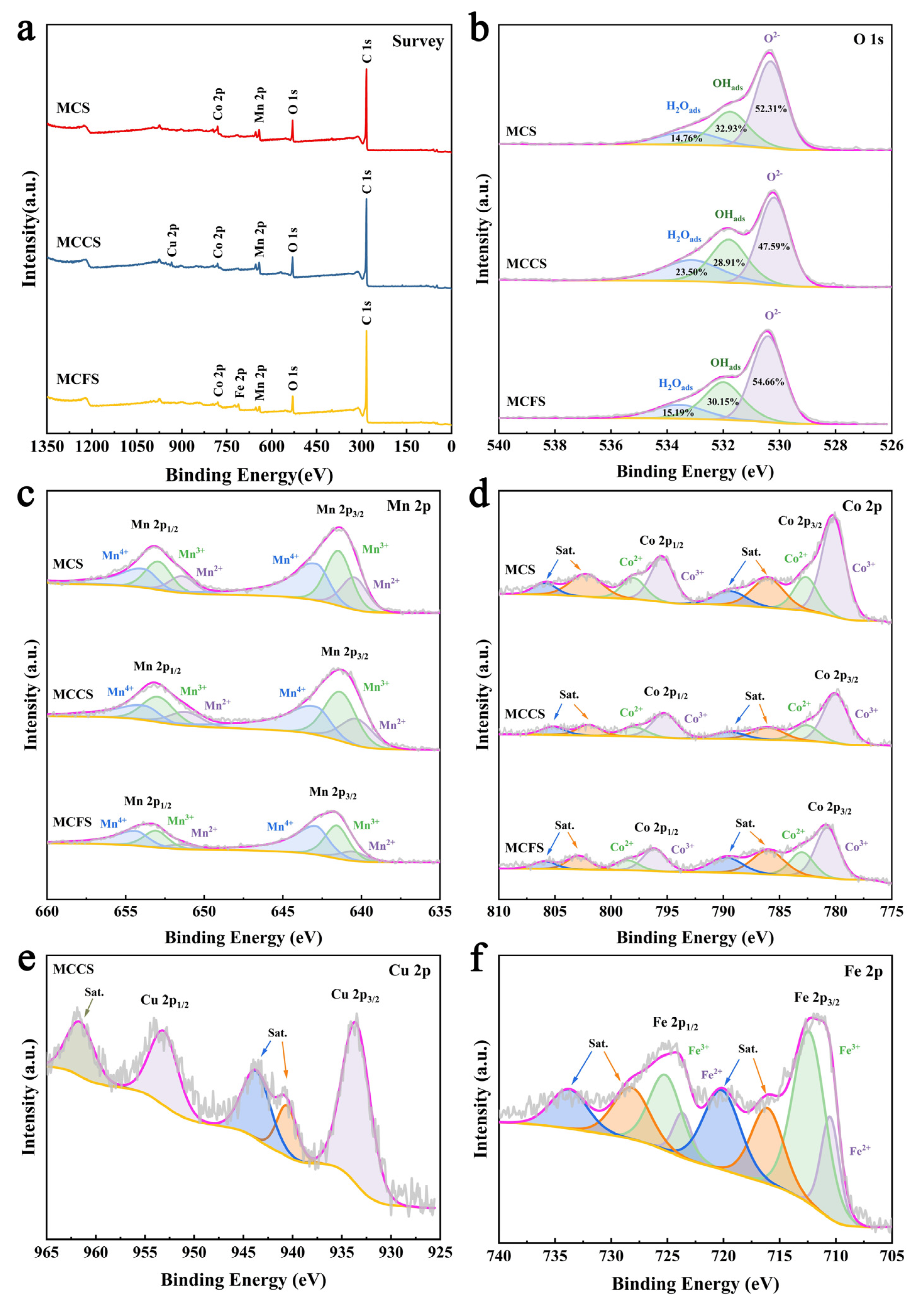
2.1. Characterization of Materials
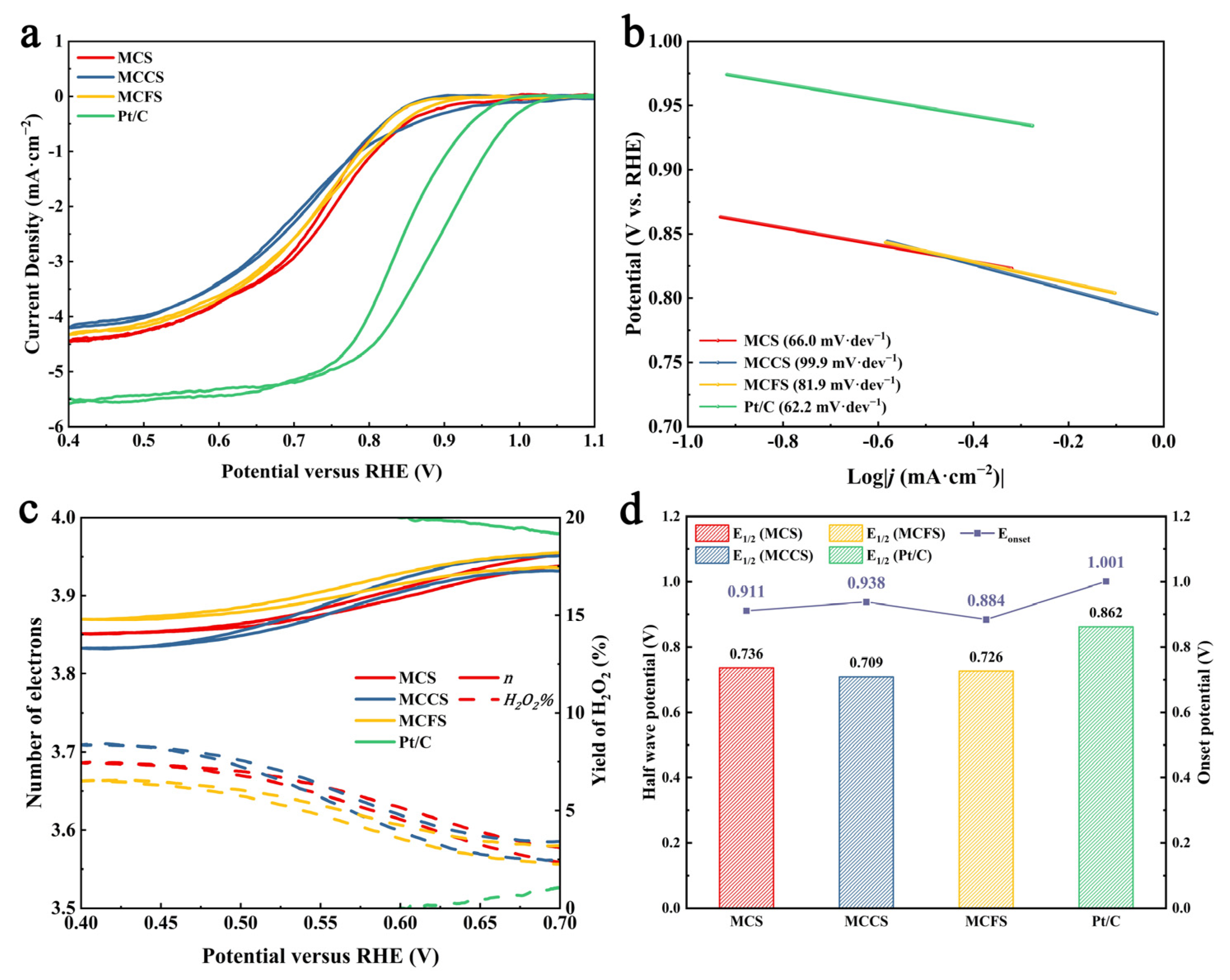
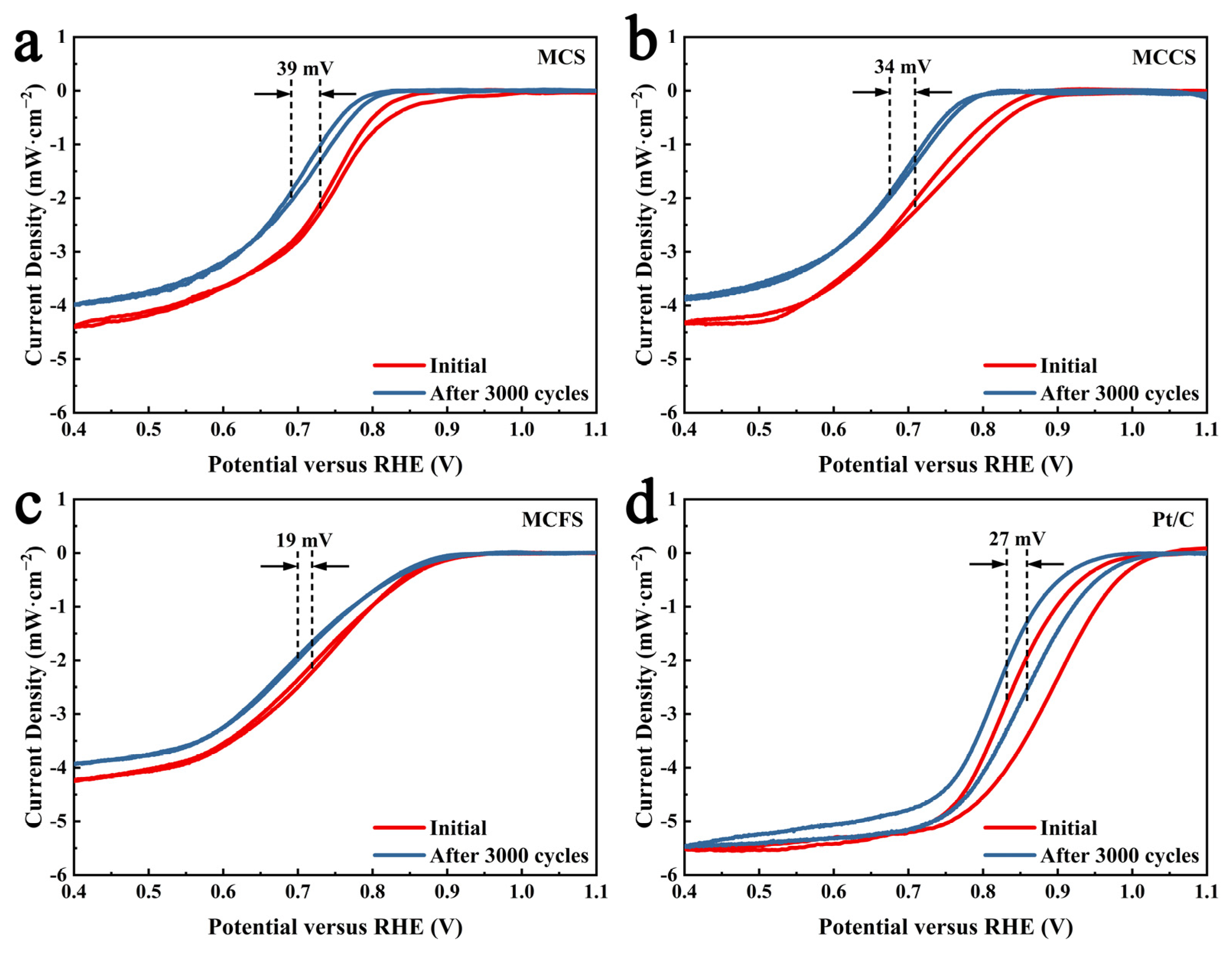
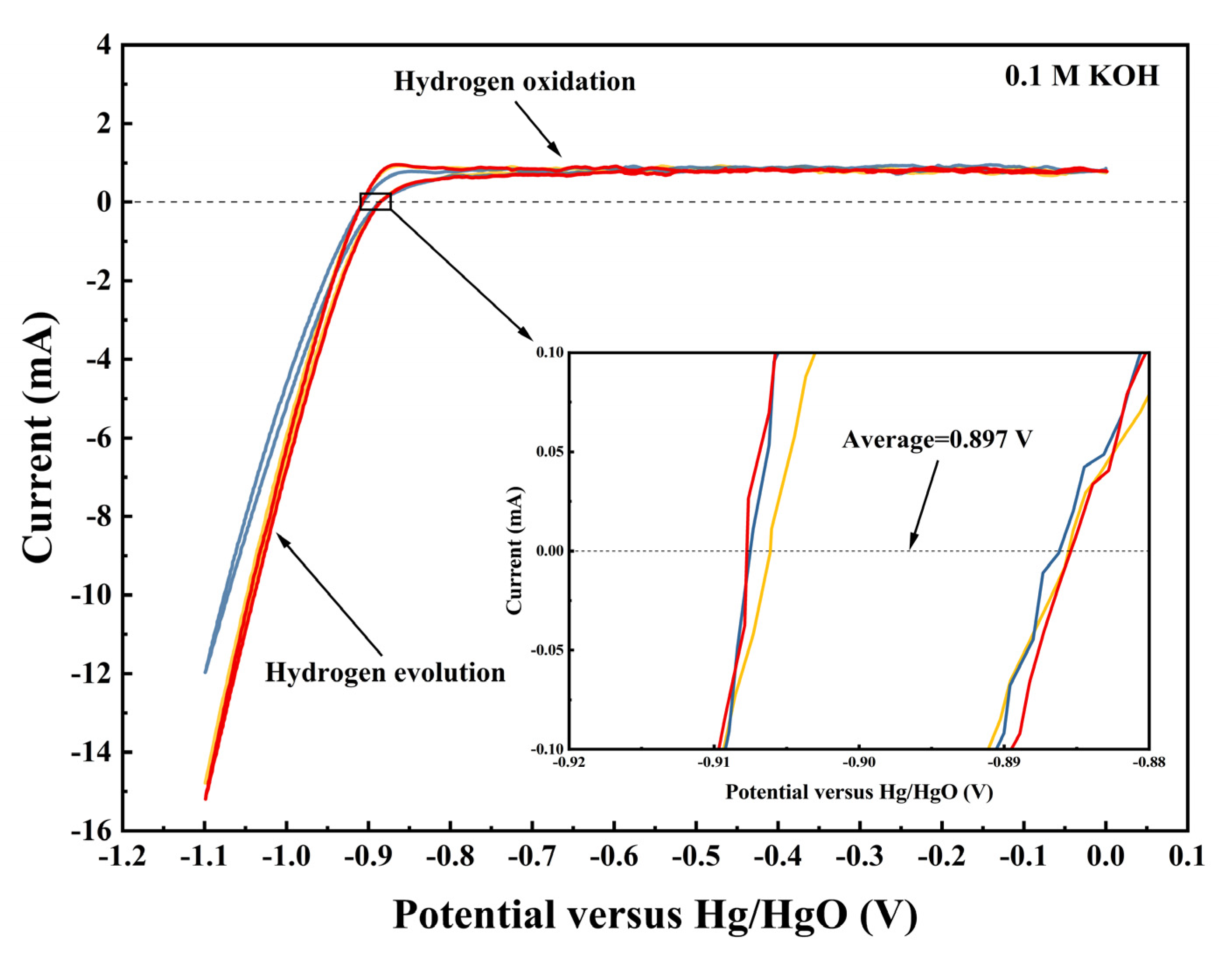
2.2. Electrochemical Performance
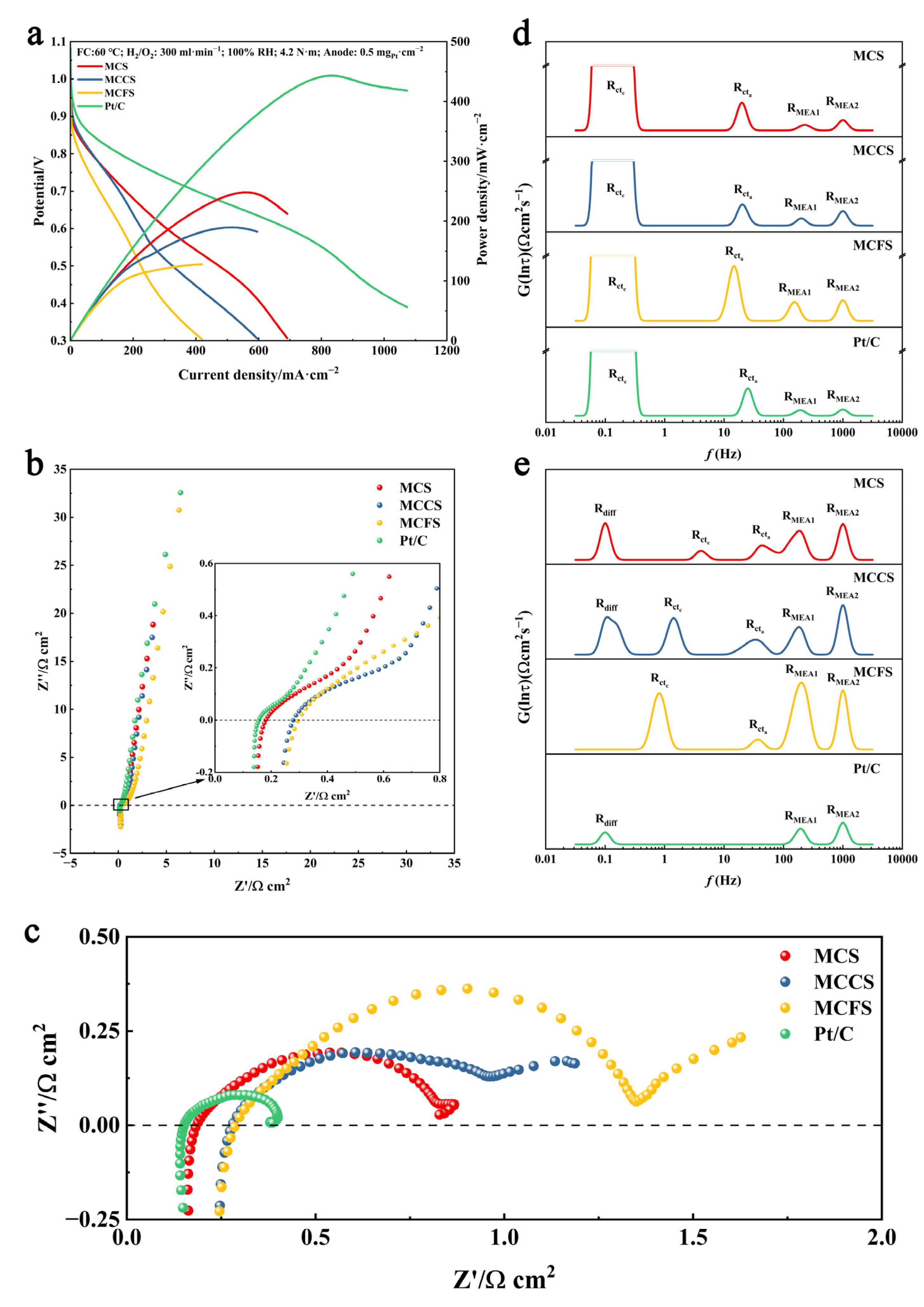
2.3. Fuel Cell Test
2.4. Mechanism Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Catalyst Preparation
4.3. Material Characterization
4.4. Electrochemical Measurements
4.5. Fuel Cell Tests
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cigolotti, V.; Genovese, M.; Fragiacomo, P. Comprehensive Review on Fuel Cell Technology for Stationary Applications as Sustainable and Efficient Poly-Generation Energy Systems. Energies 2021, 14, 4963. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Saseendran, D.P.A.; Huang, C.; Triana, C.A.; Marks, W.R.; Chen, H.; Zhao, H.; Patzke, G.R. Oxygen Evolution/Reduction Reaction Catalysts: From In Situ Monitoring and Reaction Mechanisms to Rational Design. Chem. Rev. 2023, 123, 6257–6358. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yang, Y.; Joress, H.; Padgett, E.; Gupta, U.; Yarlagadda, V.; Agyeman-Budu, D.N.; Huang, X.; Moylan, T.E.; Zeng, R.; et al. Revealing the atomic ordering of binary intermetallics using in situ heating techniques at multilength scales. Proc. Natl. Acad. Sci. USA 2019, 116, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, D.; Yang, P.X.; Du, L.; Lu, X.Y.; Li, R.P.; Liu, L.L.; Zhang, J.Q.; An, M.Z. A hierarchically porous Fe-N-C synthesized by dual melt-salt-mediated template as advanced electrocatalyst for efficient oxygen reduction in zinc-air battery. Appl. Catal. B-Environ. Energy 2022, 305, 121040. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, Y.; Holtz, M.E.; Feng, X.R.; Zeng, R.; Chen, G.; DiSalvo, F.J.; Muller, D.A.; Abruña, H.D. Octahedral spinet electrocatalysts for alkaline fuel cells. Proc. Natl. Acad. Sci. USA 2019, 116, 24425–24432. [Google Scholar] [CrossRef]
- Jin, Y.M.; Su, L.X.; Jia, H.N.; Men, Y.; Luo, W. Nickel-Based Electrocatalysts for Hydrogen Oxidation Reaction Under Alkaline Electrolytes. ChemCatChem 2023, 15, e202300056. [Google Scholar] [CrossRef]
- Yang, Y.; Peltier, C.R.; Zeng, R.; Schimmenti, R.; Li, Q.H.; Huang, X.; Yan, Z.F.; Potsi, G.; Selhorst, R.; Lu, X.Y.; et al. Electrocatalysis in Alkaline Media and Alkaline Membrane-Based Energy Technologies. Chem. Rev. 2022, 122, 6117–6321. [Google Scholar] [CrossRef]
- Adabi, H.; Shakouri, A.; Ul Hassan, N.; Varcoe, J.R.; Zulevi, B.; Serov, A.; Regalbuto, J.R.; Mustain, W.E. High-performing commercial Fe-N-C cathode electrocatalyst for anion-exchange membrane fuel cells. Nat. Energy 2021, 6, 834–843. [Google Scholar] [CrossRef]
- Lu, X.Y.; Li, Y.Q.; Yang, P.X.; Wan, Y.B.; Wang, D.; Xu, H.; Liu, L.L.; Xiao, L.H.; Li, R.P.; Wang, G.Z.; et al. Atomically dispersed Fe-N-C catalyst with densely exposed Fe-N4 active sites for enhanced oxygen reduction reaction. Chem. Eng. J. 2024, 485, 149529. [Google Scholar] [CrossRef]
- Huang, M.X.; Zhu, X.Y.; Shi, W.W.; Qin, Q.Q.; Yang, J.; Liu, S.S.; Chen, L.F.; Ding, R.M.; Gan, L.; Yin, X. Manipulating the coordination dice: Alkali metals directed synthesis of Co-N-C catalysts with CoN4 sites. Sci. Adv. 2025, 11, eads6658. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, E.; Hu, Y.C.; Jiao, L.; Kolla, P.; Liu, Y.C.; Tang, M.H.; Luo, J.; Sun, Q.; Chen, S.L.; et al. Understanding the ORR Electrocatalysis on Co-Mn Oxides. J. Phys. Chem. C 2021, 125, 25470–25477. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Jia, S.F.; Wang, X.M.; Lyu, K.J.; Peng, Y.Q.; Zheng, H.; Wei, X.; Ren, H.; Xiao, L.; et al. Synergistic Mn-Co catalyst outperforms Pt on high-rate oxygen reduction for alkaline polymer electrolyte fuel cells. Nat. Commun. 2019, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Yang, Y.; Feng, X.R.; Li, H.Q.; Gibbs, L.M.; DiSalvo, F.J.; Abruña, H.D. Nonprecious transition metal nitrides as efficient oxygen reduction electrocatalysts for alkaline fuel cells. Sci. Adv. 2022, 8, eabj1584. [Google Scholar] [CrossRef]
- Shui, Z.Y.; Tian, H.Y.; Yu, S.L.; Xiao, H.; Zhao, W.; Chen, X. La0.75Sr0.25MnO3-based perovskite oxides as efficient and durable bifunctional oxygen electrocatalysts in rechargeable Zn-air batteries. Sci. China-Mater. 2023, 66, 1002–1012. [Google Scholar] [CrossRef]
- Hughes, L.; Roy, A.; Downing, C.; Browne, M.P.; Zhussupbekova, A.; Shvets, I.V.; Nicolosi, V. Surface Reduced Manganese States as a Source of Oxygen Reduction Activity in BaMnO3. Adv. Funct. Mater. 2023, 33, 2214883. [Google Scholar] [CrossRef]
- Tegl, G.; Hanson, J.; Chen, H.M.; Kwan, D.H.; Santana, A.G.; Withers, S.G. Facile Formation of β-thioGlcNAc Linkages to Thiol-Containing Sugars, Peptides, and Proteins using a Mutant GH20 Hexosaminidase. Angew. Chem.-Int. Ed. 2019, 58, 1632–1637. [Google Scholar] [CrossRef]
- Wu, C.H.; Liu, C.; Su, D.; Xin, H.L.L.; Fang, H.T.; Erens, B.; Zhang, S.; Murray, C.B.; Salmeron, M.B. Bimetallic synergy in cobalt-palladium nanocatalysts for CO oxidation. Nat. Catal. 2019, 2, 78–85. [Google Scholar] [CrossRef]
- Huang, J.J.; Yang, Y.; Weinstock, D.; Bundschu, C.R.; Li, Q.H.; Sarker, S.; Ruff, J.P.C.; Arias, T.A.; Abruna, H.D.; Singer, A. Multimodal in situ X-ray mechanistic studies of a bimetallic oxide electrocatalyst in alkaline media. Nat. Catal. 2025, 8, 116–125. [Google Scholar] [CrossRef]
- Sun, J.; Xue, H.; Zhang, Y.F.; Zhang, X.L.; Guo, N.K.; Song, T.S.; Dong, H.L.; Kong, Y.; Zhang, J.W.; Wang, Q. Unraveling the Synergistic Effect of Heteroatomic Substitution andVacancy Engineering in CoFe2O4 for Superior Electrocatalysis Performance. Nano Lett. 2022, 22, 3503–3511. [Google Scholar] [CrossRef]
- Harada, M.; Kotegawa, F.; Kuwa, M. Structural Changes of Spinel MCo2O4 (M = Mn, Fe, Co, Ni, and Zn) Electrocatalysts during the Oxygen Evolution Reaction Investigated by In Situ X-ray Absorption Spectroscopy. ACS Appl. Energy Mater. 2022, 5, 278–294. [Google Scholar] [CrossRef]
- Celorrio, V.; Leach, A.S.; Huang, H.L.; Hayama, S.; Freeman, A.; Inwood, D.W.; Fermin, D.J.; Russell, A.E. Relationship between Mn Oxidation State Changes and Oxygen Reduction Activity in (La,Ca)MnO3 as Probed by In Situ XAS and XES. ACS Catal. 2021, 11, 6431–6439. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Shen, J.A.; Peng, B.; Pan, Y.D.; Tao, Z.L.; Chen, J. Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts. Nat. Chem. 2011, 3, 79–84. [Google Scholar] [CrossRef]
- Li, C.; Han, X.P.; Cheng, F.Y.; Hu, Y.X.; Chen, C.C.; Chen, J. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis. Nat. Commun. 2015, 6, 7345. [Google Scholar] [CrossRef]
- Tang, W.Q.; Tang, J.Y.; Liao, K.M.; Shao, Z.P. Self-reconstruction of highly active NiCo2O4 with triple-continuous transfer of electrons, ions, and oxygen for Zn-air batteries. Chem. Eng. J. 2023, 455, 140855. [Google Scholar] [CrossRef]
- Liu, Y.; Ying, Y.R.; Fei, L.F.; Liu, Y.; Hu, Q.Z.; Zhang, G.G.; Pang, S.Y.; Lu, W.; Mak, C.L.; Luo, X.; et al. Valence Engineering via Selective Atomic Substitution on Tetrahedral Sites in Spinel Oxide for Highly Enhanced Oxygen Evolution Catalysis. J. Am. Chem. Soc. 2019, 141, 8136–8145. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.S.; Zhang, L.H.; Li, C.; Kumbhar, A.; Abruña, H.D.; Fang, J.Y. Facet Impact of CuMn2O4 Spinel Nanocatalysts on Enhancement of the Oxygen Reduction Reaction in Alkaline Media. ACS Catal. 2022, 12, 13663–13670. [Google Scholar] [CrossRef]
- Wang, Z.P.; Wu, P.Y.; Zou, X.B.; Wang, S.; Du, L.; Ouyang, T.; Liu, Z.Q. Optimizing the Oxygen-Catalytic Performance of Zn-Mn-Co Spinel by Regulating the Bond Competition at Octahedral Sites. Adv. Funct. Mater. 2023, 33, 2214275. [Google Scholar] [CrossRef]
- Chen, B.; Miao, H.; Yin, M.M.; Hu, R.G.; Xia, L.; Zhang, C.F.; Yuan, J.L. Mn-based spinels evolved from layered manganese dioxides at mild temperature for the robust flexible quasi-solid-state zinc-air batteries. Chem. Eng. J. 2021, 417, 129179. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, Y.; Feng, X.R.; DiSalvo, F.J.; Abruña, H.D. A Strategy for Increasing the Efficiency of the Oxygen Reduction Reaction in Mn-Doped Cobalt Ferrites. J. Am. Chem. Soc. 2019, 141, 4412–4421. [Google Scholar] [CrossRef]
- Cermenek, B.; Genorio, B.; Winter, T.; Wolf, S.; Connell, J.G.; Roschger, M.; Letofsky-Papst, I.; Kienzl, N.; Bitschnau, B.; Hacker, V. Alkaline Ethanol Oxidation Reaction on Carbon Supported Ternary PdNiBi Nanocatalyst using Modified Instant Reduction Synthesis Method. Electrocatalysis 2020, 11, 203–214. [Google Scholar] [CrossRef]
- Gillot, B.; El Guendouzi, M.; Kharroubi, M.; Tailhades, P.; Metz, R.; Rousset, A. Phase transformation-related kinetics in the oxidation of a manganese mixed oxide with a spinel structure. Mater. Chem. Phys. 1989, 24, 199–208. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Zhou, W.; Yu, J.; Chen, Y.B.; Liu, M.L.; Shao, Z.P. Enhancing Electrocatalytic Activity of Perovskite Oxides by Tuning Cation Deficiency for Oxygen Reduction and Evolution Reactions. Chem. Mater. 2016, 28, 1691–1697. [Google Scholar] [CrossRef]
- Bai, J.; Zhao, T.; Xu, M.; Mei, B.; Yang, L.; Shi, Z.; Zhu, S.; Wang, Y.; Jiang, Z.; Zhao, J.; et al. Monosymmetric Fe-N4 sites enabling durable proton exchange membrane fuel cell cathode by chemical vapor modification. Nat. Commun. 2024, 15, 4219. [Google Scholar] [CrossRef]
- Yuan, X.-Z.; Song, C.; Wang, H.; Zhang, J. Electrochemical Impedance Spectroscopy in PEM Fuel Cells: Fundamentals and Applications; Springer: London, UK, 2010. [Google Scholar]
- Ahn, S.; Tatarchuk, B.J. Air electrode: Identification of intraelectrode rate phenomena via ac impedance. J. Electrochem. Soc. 1995, 142, 4169. [Google Scholar] [CrossRef]
- Romero-Castañón, T.; Arriaga, L.G.; Cano-Castillo, U. Impedance spectroscopy as a tool in the evaluation of MEA’s. J. Power Sources 2003, 118, 179–182. [Google Scholar] [CrossRef]
- León, M.I.; Romero-Castañón, T.; Flores-Hernández, J.R.; Nava, J.L. Experimental determination of HOR and ORR kinetic parameters in an AEMFC by the distribution of relaxation times method. J. Power Sources 2025, 629, 236000. [Google Scholar] [CrossRef]
- Douglin, J.C.; Vijaya Sankar, K.; Biancolli, A.L.G.; Santiago, E.I.; Tsur, Y.; Dekel, D.R. Quantifying the resistive losses of the catalytic layers in anion-exchange membrane fuel cells. ChemSusChem 2023, 16, e202301080. [Google Scholar] [CrossRef]
- Risch, M. Perovskite electrocatalysts for the oxygen reduction reaction in alkaline media. Catalysts 2017, 7, 154. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Z.Y.; Xu, L.F.; Li, J.Q.; Gan, Q.Q.; Du, X.L.; Ouyang, M.G. A comparative study of equivalent circuit model and distribution of relaxation times for fuel cell impedance diagnosis. Int. J. Energy Res. 2021, 45, 15948–15961. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Dubal, D.P.; Nagar, B.; Ranc, V.; Tomanec, O.; Petr, M.; Datta, K.K.R.; Zboril, R.; Gómez-Romero, P.; Fischer, R.A. Ultrathin Hierarchical Porous Carbon Nanosheets for High-Performance Supercapacitors and Redox Electrolyte Energy Storage. Adv. Mater. 2018, 30, 1705789. [Google Scholar] [CrossRef]
- Baldovino-Medrano, V.G.; Niño-Celis, V.; Giraldo, R.I. Systematic Analysis of the Nitrogen Adsorption-Desorption Isotherms Recorded for a Series of Materials Based on Microporous-Mesoporous Amorphous Aluminosilicates Using Classical Methods. J. Chem. Eng. Data 2023, 68, 2512–2528. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.H.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Bundschu, C.R.; Ahmadi, M.; Méndez-Valderrama, J.F.; Yang, Y.; Abruña, H.D.; Arias, T.A. Oxygen Reduction Pathway for Spinel Metal Oxides in Alkaline Media: An Experimentally Supported Ab Initio Study. J. Am. Chem. Soc. 2024, 146, 4680–4686. [Google Scholar] [CrossRef]
- Ji, Q.Q.; Bi, L.; Zhang, J.T.; Cao, H.J.; Zhao, X.S. The role of oxygen vacancies of ABO3 perovskite oxides in the oxygen reduction reaction. Energy Environ. Sci. 2020, 13, 1408–1428. [Google Scholar] [CrossRef]
- Cui, X.; Gao, L.K.; Lu, C.H.; Ma, R.; Yang, Y.K.; Lin, Z.Q. Rational coordination regulation in carbon-based single-metal-atom catalysts for electrocatalytic oxygen reduction reaction. Nano Converg. 2022, 9, 34. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, S.H.; Tang, Y.; Wang, G.; Pang, H.; Yu, F. Inhibiting demetalation of Zn-N-C via bimetallic CoZn alloy for an efficient and durable oxygen reduction reaction. J. Colloid Interface Sci. 2025, 689, 137276. [Google Scholar] [CrossRef]
- Joya, K.S.; de Groot, H.J.M. Controlled Surface-Assembly of Nanoscale Leaf-Type Cu-Oxide Electrocatalyst for High Activity Water Oxidation. ACS Catal. 2016, 6, 1768–1771. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Zhang, T.R.; Zhang, Y.; Du, J.; Han, X.P.; Chen, J. Enhancing Electrocatalytic Oxygen Reduction on MnO2 with Vacancies. Angew. Chem.-Int. Ed. 2013, 52, 2474–2477. [Google Scholar] [CrossRef]
- Masa, J.; Weide, P.; Peeters, D.; Sinev, I.; Xia, W.; Sun, Z.Y.; Somsen, C.; Muhler, M.; Schuhmann, W. Amorphous Cobalt Boride (Co2B) as a Highly Efficient Nonprecious Catalyst for Electrochemical Water Splitting: Oxygen and Hydrogen Evolution. Adv. Energy Mater. 2016, 6, 1502313. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, W.; Song, R.; Yuan, J.; Pang, H.; Zong, W.; Zhang, X.; Zhou, J. A Ternary Spinel Strategy for Increasing the Performances of Oxygen Reduction Reaction and Anion Exchange Membrane Fuel Cell Based on Mn-Co Spinel Oxides. Catalysts 2025, 15, 1031. https://doi.org/10.3390/catal15111031
Jin W, Song R, Yuan J, Pang H, Zong W, Zhang X, Zhou J. A Ternary Spinel Strategy for Increasing the Performances of Oxygen Reduction Reaction and Anion Exchange Membrane Fuel Cell Based on Mn-Co Spinel Oxides. Catalysts. 2025; 15(11):1031. https://doi.org/10.3390/catal15111031
Chicago/Turabian StyleJin, Weitao, Ruiqing Song, Jiansong Yuan, Hengxi Pang, Wen Zong, Xiao Zhang, and Juan Zhou. 2025. "A Ternary Spinel Strategy for Increasing the Performances of Oxygen Reduction Reaction and Anion Exchange Membrane Fuel Cell Based on Mn-Co Spinel Oxides" Catalysts 15, no. 11: 1031. https://doi.org/10.3390/catal15111031
APA StyleJin, W., Song, R., Yuan, J., Pang, H., Zong, W., Zhang, X., & Zhou, J. (2025). A Ternary Spinel Strategy for Increasing the Performances of Oxygen Reduction Reaction and Anion Exchange Membrane Fuel Cell Based on Mn-Co Spinel Oxides. Catalysts, 15(11), 1031. https://doi.org/10.3390/catal15111031






