Facile Preparation of High-Performance Non-Enzymatic Glucose Sensors Based on Au/CuO Nanocomposites
Abstract
1. Introduction
2. Results and Discussion
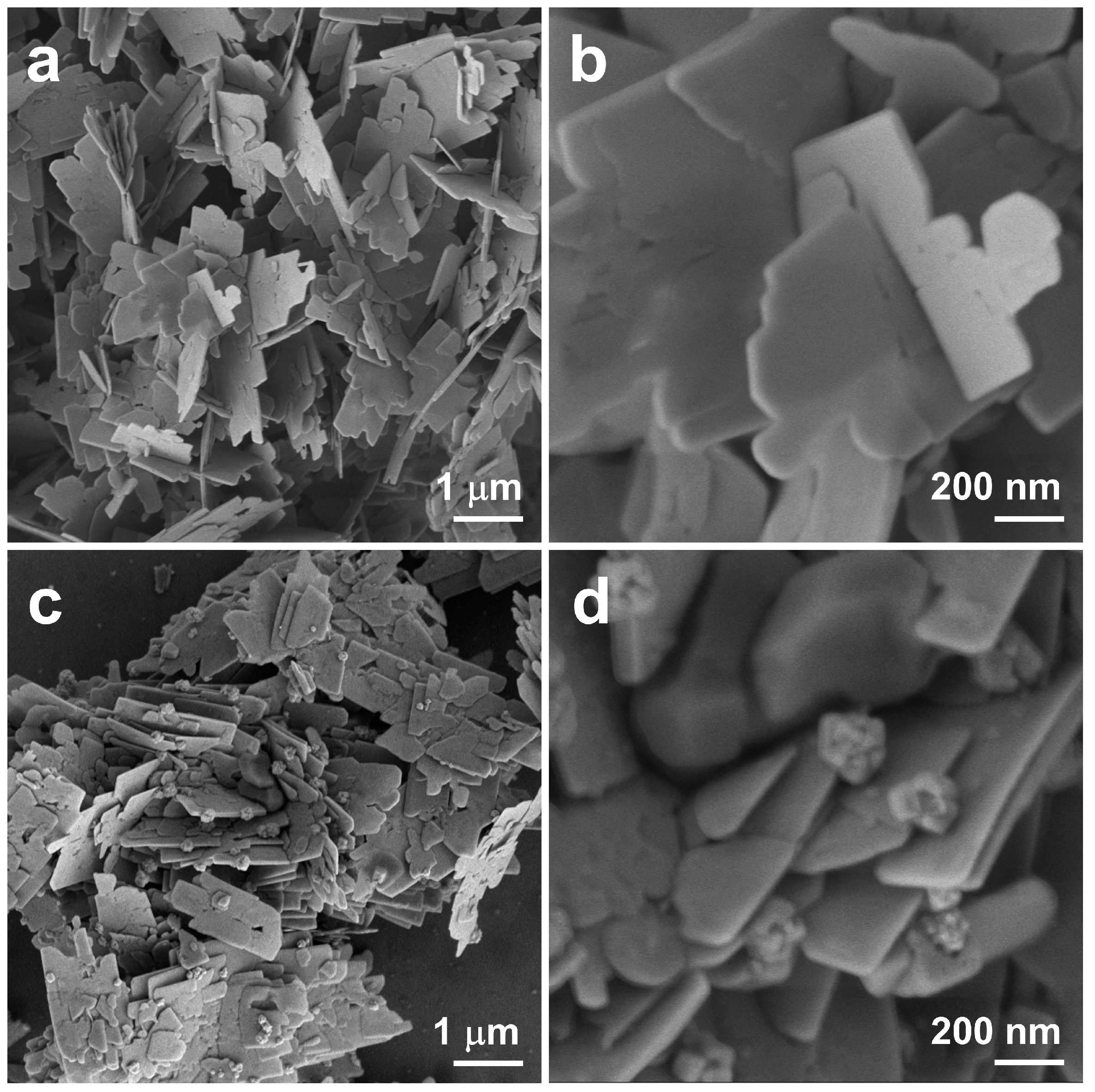
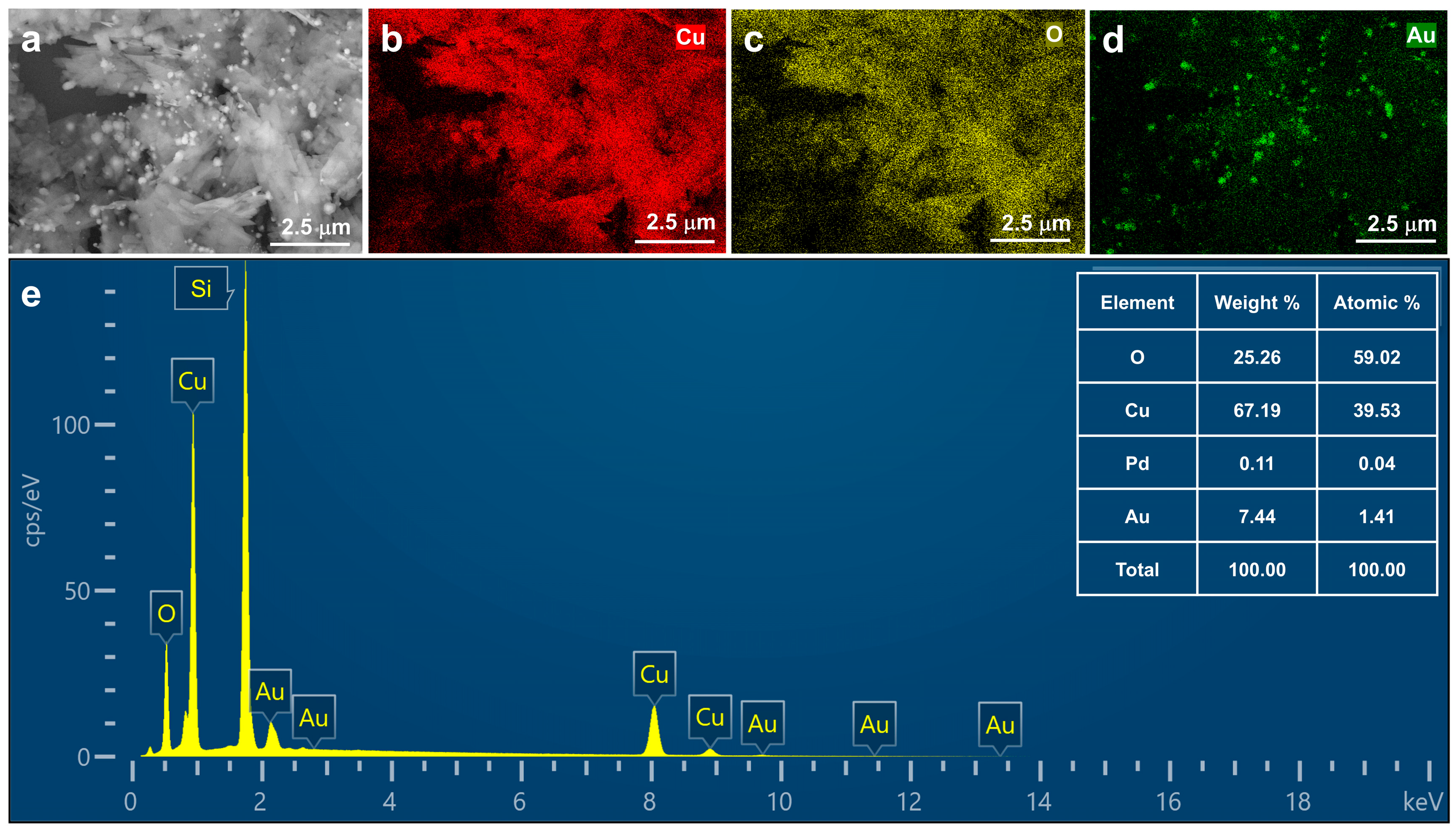
2.1. Morphological and Structural Characterization
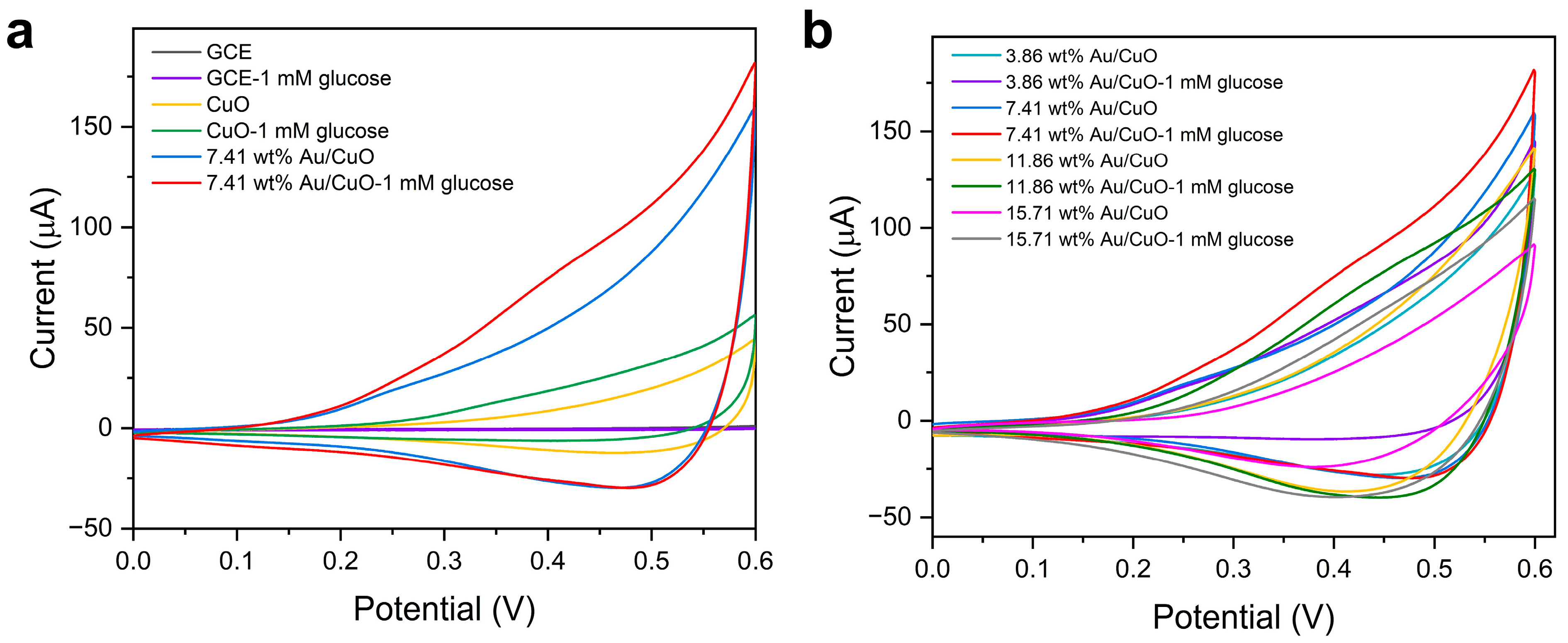
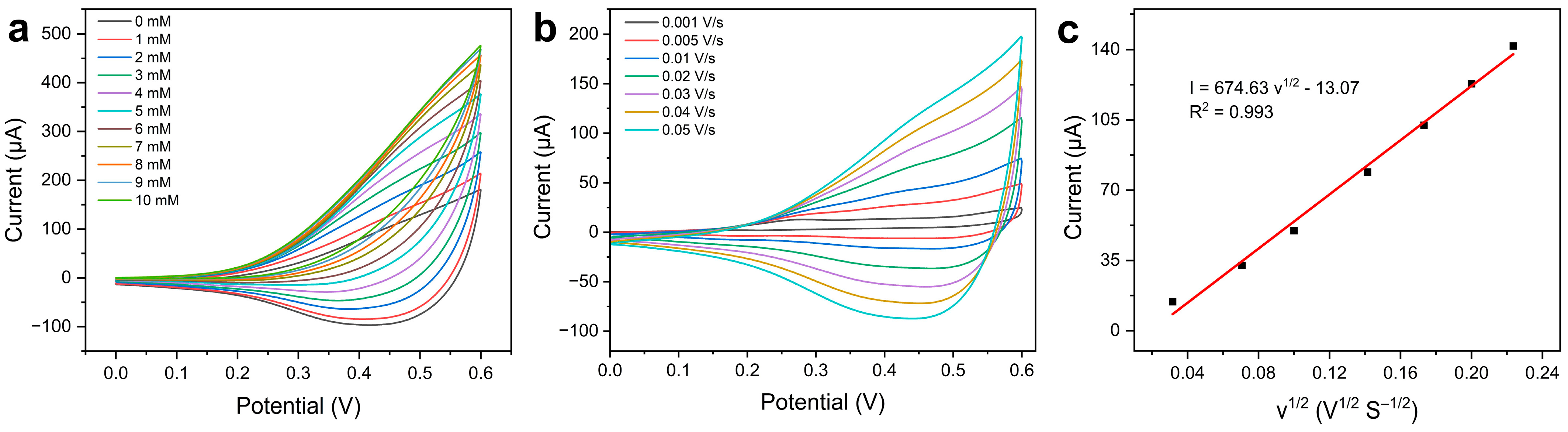
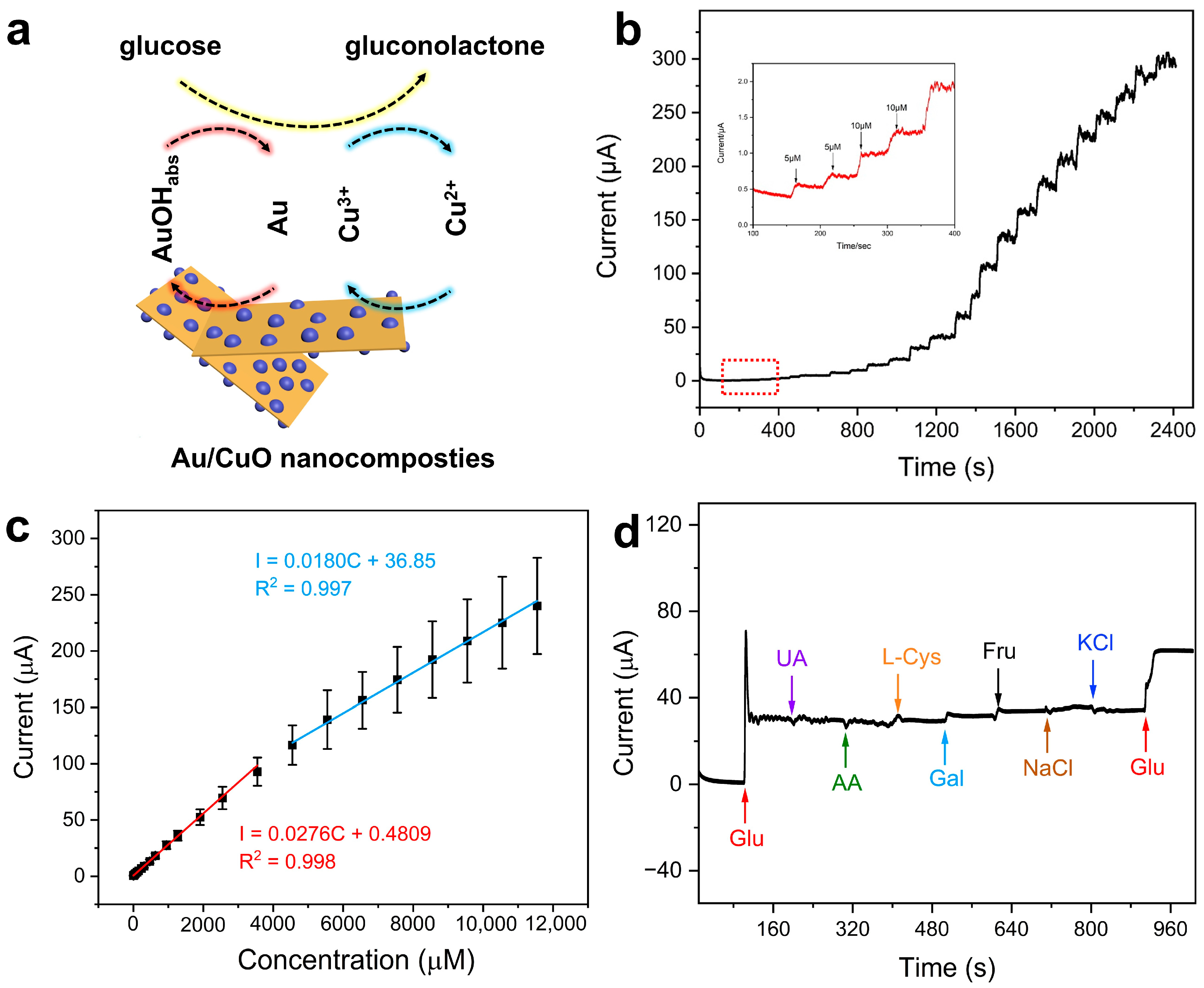
2.2. Electrochemical Behaviors of the Au/CuO Nanocomposites Modified Electrode
3. Materials and Methods
3.1. Materials
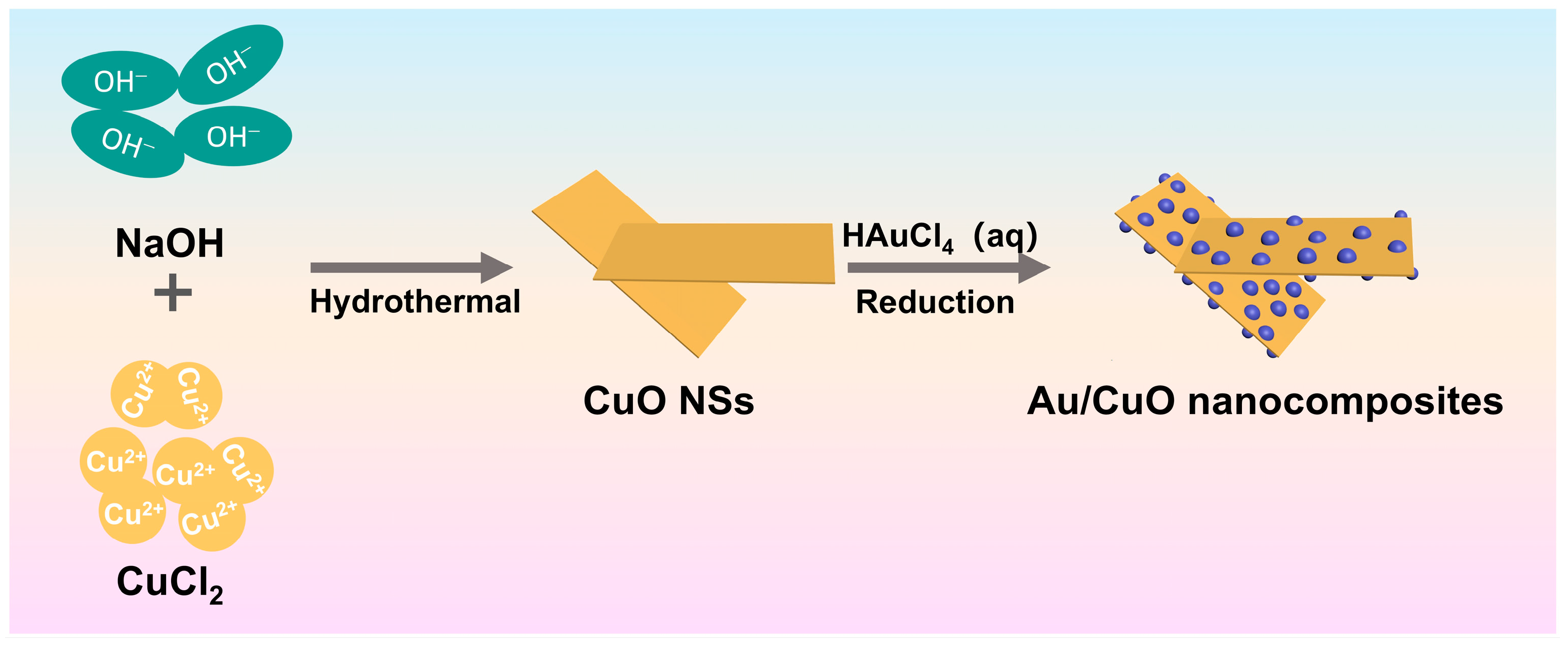
3.2. Hydrothermal Synthesis CuO Nanosheets
3.3. Synthesis Au/CuO Nanocomposites
3.4. Materials Characterization
3.5. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bolla, A.S.; Priefer, R. Blood glucose monitoring-an overview of current and future non-invasive devices. Diabetes Metab. Synd. 2020, 14, 739–751. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, M.; Cong, X.; Li, J.; Shi, Q.; Min, J.Z. Sweeteners in diabetes and blood glucose management: Advances, challenges, and trends in the food industry. Food Rev. Int. 2025, 1–26. [Google Scholar] [CrossRef]
- Wang, L.; Chang, S.J.; Chen, C.J.; Liu, J.T. Metal-organic frameworks for electrochemical glucose sensors: Progress and challenges. cardiovascular disease, and mortality in diabetes: Epidemiology, pathogenesis, and management. Coord. Chem. Rev. 2025, 543, 216907. [Google Scholar]
- Li, S.; Zhang, H.; Zhu, M.; Kuang, Z.; Li, X.; Xu, F.; Miao, S.; Zhang, Z.; Lou, X.; Li, H.; et al. Electrochemical biosensors for whole blood analysis: Recent progress, challenges, and future perspectives. Chem. Rev. 2023, 123, 7953–8039. [Google Scholar] [CrossRef]
- Kumar, A.; Castro, M.; Feller, J. Review on sensor array-based analytical technologies for quality control of food and beverages. Sensors 2023, 23, 4017. [Google Scholar] [CrossRef]
- Sun, X. Glucose detection through surface-enhanced Raman spectroscopy: A review. Anal. Chim. Acta 2022, 1206, 339226. [Google Scholar] [CrossRef] [PubMed]
- Halko, R.; Pavelek, D.; Kaykhaii, M. High performance liquid chromatography-fourier transform infrared spectroscopy coupling: A comprehensive review. Crit. Rev. Anal. Chem. 2024, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Caño, R.D.; Mahato, K.; Paz, E.D.; Chen, C.; Ding, S.; Yin, L.; Wang, J. Wearable electrochemical glucose sensors in diabetes management: A comprehensive review. Chem. Rev. 2023, 123, 7854–7889. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Reyes-De-Corcuera, J.I.; Olstad, H.E.; García-Torres, R. Stability and stabilization of enzyme biosensors: The key to successful application and commercialization. Annu. Rev. Food Sci. Technol. 2018, 9, 293–322. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.H. Enzyme-based glucose sensor: From invasive to wearable device. Adv. Healthc. Mater. 2018, 9, 293–322. [Google Scholar] [CrossRef]
- Anusha, J.R.; Kim, H.J.; Fleming, A.T.; Das, S.J.; Yu, K.H.; Kim, B.C.; Raj, C.J. Simple fabrication of ZnO/Pt/chitosan electrode for enzymatic glucose biosensor. Sens. Actuators B Chem. 2014, 202, 827–833. [Google Scholar] [CrossRef]
- Hassan, M.H.; Vyas, C.; Grieve, B.; Bartolo, P. Recent advances in enzymatic and non-enzymatic electrochemical glucose sensing. Sensors 2021, 21, 4672. [Google Scholar] [CrossRef]
- Nor, N.M.; Ridhuan, N.S.; Razak, K.A. Progress of enzymatic and non-enzymatic electrochemical glucose biosensor based on nanomaterial-modified electrode. Biosensors 2022, 12, 1136. [Google Scholar]
- Singh, K.; Maurya, K.K.; Malviya, M.; Cheng, S.; Zhang, M.; Cong, X.; Li, J.; Shi, Q.; Min, J.Z. Review of Electrochemical Sensors and Biosensors Based on First-Row Transition Metals, Their Oxides, and Noble Metals Nanoparticles. J. Anal. Test. 2024, 8, 143–159. [Google Scholar] [CrossRef]
- Wei, M.; Qiao, Y.; Zhao, H.; Liang, J.; Li, T.; Luo, Y.; Lu, S.; Shi, X.; Lu, W.; Sun, X. Electrochemical non-enzymatic glucose sensors: Recent progress and perspectives. Chem. Commun. 2020, 56, 14553–14569. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; He, X.; Wang, L.; Gu, A.; Huang, Y.; Fang, B.; Geng, B.; Zhang, X. Non-enzymatic electrochemical sensing of glucose. Microchim. Acta 2013, 180, 161–186. [Google Scholar] [CrossRef]
- He, C.; Asif, M.; Liu, Q.; Xiao, F.; Liu, H.; Xia, B.Y. Noble metal construction for electrochemical nonenzymatic glucose detection. Adv. Mater. Technol. 2023, 8, 2200272. [Google Scholar] [CrossRef]
- Li, D.; Yang, M.; Li, W.; Wang, A.; Wang, X.; Hong, L.; Dong, L.; Xu, H.; Wang, G. WS2/CuO-based non-enzymatic sensor for the detection of glucose in sweat. Anal. Chim. Acta 2025, 1378, 344696. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, W.; Li, Y. Recent progress in MOF-based electrochemical sensors for non-enzymatic glucose detection. Molecules 2023, 28, 4891. [Google Scholar] [CrossRef]
- Shen, L.; Liang, Z.; Chen, Z.; Wu, C.; Hu, X.; Zhang, J.; Jiang, Q.; Wang, Y. Reusable electrochemical non-enzymatic glucose sensors based on Au-inlaid nanocages. Nano Res. 2022, 15, 6490–6499. [Google Scholar]
- Chang, G.; Shu, H.; Huang, Q.; Oyama, M.; Ji, K.; Liu, X.; He, Y. Synthesis of highly dispersed Pt nanoclusters anchored graphene composites and their application for non-enzymatic glucose sensing. Electrochim. Acta 2015, 157, 149–157. [Google Scholar]
- Ye, J.S.; Chen, C.W.; Lee, C.L. Pd nanocube as non-enzymatic glucose sensor. Sens. Actuators B Chem. 2015, 208, 569–574. [Google Scholar] [CrossRef]
- Tee, S.Y.; Teng, C.P.; Ye, E. Metal nanostructures for non-enzymatic glucose sensing. Biomater. Adv. 2017, 70, 1018–1030. [Google Scholar]
- Tang, J.; Tang, D. Non-enzymatic electrochemical immunoassay using noble metal nanoparticles: A review. Microchim. Acta 2015, 182, 2077–2089. [Google Scholar] [CrossRef]
- Kalhoro, K.A.; Anwar, M.; Zhang, C.; Khan, A.; Wu, D.; Rehman, A.U.; Shokouhimehr, M.; Liu, Z. Recent Trends and Prospective Developments in Metal Oxide Composites-Based Electrochemical Nonenzymatic Glucose Sensors. Talanta 2025, 295, 128366. [Google Scholar] [CrossRef]
- Yang, J.; Yin, J.; Xu, L. Electrochemical non-enzymatic glucose sensors based on CuO nanostructures. J. Alloys Compd. 2025, 1010, 177796. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Tarlochan, F. Highly efficient nonenzymatic glucose sensors based on CuO nanoparticles. Appl. Surf. Sci. 2019, 481, 712–722. [Google Scholar] [CrossRef]
- Li, K.; Fan, G.; Yang, L.; Li, F. Novel ultrasensitive non-enzymatic glucose sensors based on controlled flower-like CuO hierarchical films. Sens. Actuators B Chem. 2014, 199, 175–182. [Google Scholar]
- Haghparas, Z.; Kordrostami, Z.; Sorouri, M.; Rajabzadeh, M.; Khalifeh, R.; Cheng, S.; Zhang, M.; Cong, X.; Li, J.; Shi, Q.; et al. Fabrication of non-enzymatic electrochemical glucose sensor based on nano-copper oxide micro hollow-spheres. Biotechnol. Bioprocess Eng. 2020, 25, 528–535. [Google Scholar]
- Wang, Q.; Cui, X.; Chen, J.; Zheng, X.; Liu, C.; Xue, T.; Wang, H.; Jin, Z.; Qiao, L.; Zheng, W. Well-dispersed palladium nanoparticles on graphene oxide as a non-enzymatic glucose sensor. RSC Adv. 2012, 2, 6245–6249. [Google Scholar]
- Gopal, T.S.; Jeong, S.K.; Alrebdi, T.A.; Pandiaraj, S.; Alodhayb, A.; Muthuramamoorthy, M.; Grace, A.N. MXene-based composite electrodes for efficient electrochemical sensing of glucose by non-enzymatic method. Mater. Today Chem. 2022, 24, 100891. [Google Scholar] [CrossRef]
- Dai, S.; Wang, Y.; Xiao, L.; Hao, G.; Hu, Y.; Zhang, G.; Jiang, W. 2D/2D/2D CuO-MXene-OCN heterojunction with enhanced photocatalytic removal of pharmaceuticals and personal care products: Characterization, efficiency and mechanism. J. Alloys Compd. 2022, 919, 165873. [Google Scholar] [CrossRef]
- Wang, S.Z.; Zheng, M.; Zhang, X.; Zhuo, M.; Zhou, M.; Su, Y.; Zheng, M.; Yuan, G.; Wang, Z. Flowerlike CuO/Au nanoparticle heterostructures for nonenzymatic glucose detection. ACS Appl. Nano Mater. 2021, 4, 5808–5815. [Google Scholar] [CrossRef]
- Felix, S.; Grace, A.N.; Jayavel, R. Sensitive electrochemical detection of glucose based on Au-CuO nanocomposites. J. Phys. Chem. Solids 2018, 122, 255–260. [Google Scholar] [CrossRef]
- Chakraborty, P.; Dhar, S.; Debnath, K.; Majumder, T.; Mondal, S.P. Non-enzymatic and non-invasive glucose detection using Au nanoparticle decorated CuO nanorods. Sens. Actuators B Chem. 2019, 283, 776–785. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Q.; Yang, X.; Wei, M.; Zhang, M.; Mishra, A.K.; Jarwal, D.K.; Mukherjee, B.; Kumar, A.; Ratan, S.; et al. Copper oxide coated gold Nanorods like a film: A facile route to nanocomposites for electrochemical application. J. Electroanal. Chem. 2017, 806, 8–14. [Google Scholar] [CrossRef]
- Yang, H.; Wang, S.; Wang, X.; Zhang, P.; Yan, C.; Luo, Y.; Chen, L.; Li, M.; Fan, F.; Zhou, Z.; et al. Grain boundary enriched CuO nanobundle for efficient non-invasive glucose sensors/fuel cells. J. Colloid Interface Sci. 2022, 609, 139–148. [Google Scholar] [CrossRef]
- Pu, F.; Miao, H.; Lu, W.; Zhang, X.; Yang, Z.; Kong, C. High-performance non-enzymatic glucose sensor based on flower-like Cu2O-Cu-Au ternary nanocomposites. Appl. Surf. Sci. 2022, 581, 152389. [Google Scholar] [CrossRef]
- Chang, H.W.; Chen, S.C.; Chen, P.W.; Liu, F.J.; Tsai, Y.C. Constructing morphologically tunable copper oxide-based nanomaterials on Cu wire with/without the deposition of manganese oxide as bifunctional materials for glucose sensing and supercapacitors. Int. J. Mol. Sci. 2022, 23, 3299. [Google Scholar] [CrossRef]
- Mamleyev, E.R.; Weidler, P.G.; Nefedov, A.; Szabó, D.V.; Islam, M.; Mager, D.; Korvink, J.G. Nano-and microstructured copper/copper oxide composites on laser-induced carbon for enzyme-free glucose sensors. ACS Appl. Nano Mater. 2021, 4, 13747–13760. [Google Scholar] [CrossRef]
- Li, S.; Xia, H.; Liu, Y.; Cao, C.; Li, S.; Wang, X.; Tian, N.; Liu, L.; Lu, P.; Quan, C.; et al. Room-temperature and gram-scale constructed Cu@CuO with promoted kinetics for glucose electrooxidation in the Faraday process. Sci. China Mater. 2023, 66, 4396–4402. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, Z.; Cui, F.; Shao, J.; Zhou, H.S. CuO/Cu composite nanospheres on a TiO2 nanotube array for amperometric sensing of glucose. Microchim. Acta 2020, 187, 123. [Google Scholar] [CrossRef]
- Sun, S.; Shi, N.; Liao, X.; Zhang, B.; Yin, G.; Huang, Z.; Chen, X.; Ximing Pu, X. Facile synthesis of CuO/Ni(OH)2 on carbon cloth for non-enzymatic glucose sensing. Appl. Surf. Sci. 2020, 529, 147067. [Google Scholar] [CrossRef]
- Chang, G.; Shu, H.; Ji, K.; Oyama, M.; Liu, X.; He, Y.; Cheng, S.; Zhang, M.; Cong, X.; Li, J.; et al. Gold nanoparticles directly modified glassy carbon electrode for non-enzymatic detection of glucose. Appl. Surf. Sci. 2014, 288, 524–529. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mukherjee, B.; Kumar, A.; Jarwal, D.K.; Ratan, S.; Kumar, C.; Satyabrata Jit, S.; Cheng, S.; Zhang, M.; Cong, X.; et al. Superficial fabrication of gold nanoparticles modified CuO nanowires electrode for non-enzymatic glucose detection. RSC Adv. 2019, 9, 1772–1781. [Google Scholar] [CrossRef]
- Fang, Q.; Wang, H.; Wei, X.; Tang, Y.; Luo, X.; Xu, W.; Hu, L.; Gu, W.; Zhu, C. Cu aerogels with sustainable Cu (I)/Cu (II) redox cycles for sensitive nonenzymatic glucose sensing. Adv. Healthc. Mater. 2023, 12, 2301073. [Google Scholar] [CrossRef] [PubMed]
- Dayakar, T.; Rao, K.V.; Bikshalu, K.K.; Malapati, V.; Sadasivuni, K.K. Non-enzymatic sensing of glucose using screen-printed electrode modified with novel synthesized CeO2@CuO core shell nanostructure. Biosens. Bioelectron. 2018, 111, 166–173. [Google Scholar]



| Sample | Added (μM) | Detected (μM) | Recovery (%) | RSD (%) (n = 3) |
|---|---|---|---|---|
| Urine 1 | 40.00 | 43.40 | 108.5 | 4.2 |
| Urine 2 | 40.00 | 42.36 | 105.9 | 8.5 |
| Urine 3 | 40.00 | 43.12 | 107.8 | 6.5 |
| Electrode | Detection Potential (V) | Sensitivity (μA·mM−1·cm−2) | Linear Range (μM) | LOD (μM) | Reference |
|---|---|---|---|---|---|
| CuO nanosheets | 0.45 | 2331 | 20–3000 | 20 | [40] |
| CuO urchins | 0.5 | 320 | 1–3300 | 7.56 | [41] |
| CuO/Au | 0.54 | 2455 | 10–12,000 | 0.53 | [34] |
| Cu@CuO | 0.55 | 0.03 | 1.3–2500 | 1.3 | [42] |
| CuO/Cu | 0.65 | 234 | 200–90,000 | 19 | [43] |
| CuO/Ni(OH)2 | 0.55 | 598.6 | 50–8500 | 0.31 | [44] |
| Au nanoparticles | - | 87.5 | 100–25,000 | 50 | [45] |
| Au decorated CuO NR | 0.55 | 2009 | 5–1325 | 0.17 | [36] |
| Au NP modified CuO NWs | 0.6 | 4398.8 | 0.5–5900 | 0.5 | [46] |
| Cu aerogels | 0.5 | 714.3 | 1–1000 | 0.48 | [47] |
| Au@CuO/nafion | 0.6 | 715 | 20–2000 | 18 | [48] |
| Nafion/Au-CuO | 0.6 | 3126.76 | 5–650 | 1.4 | [35] |
| Au/CuO | 0.5 | 393.92 | 5–2250 | 5 | This work |
| 269.87 | 3550–11,550 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Wang, T.; Mei, H.; You, Y.; Lin, Z.; Li, W.; Li, B.; Kang, S.; Zhu, L. Facile Preparation of High-Performance Non-Enzymatic Glucose Sensors Based on Au/CuO Nanocomposites. Catalysts 2025, 15, 1020. https://doi.org/10.3390/catal15111020
Ma L, Wang T, Mei H, You Y, Lin Z, Li W, Li B, Kang S, Zhu L. Facile Preparation of High-Performance Non-Enzymatic Glucose Sensors Based on Au/CuO Nanocomposites. Catalysts. 2025; 15(11):1020. https://doi.org/10.3390/catal15111020
Chicago/Turabian StyleMa, Lian, Tao Wang, Hao Mei, Yuhao You, Zhandong Lin, Weishuang Li, Bojie Li, Silin Kang, and Lei Zhu. 2025. "Facile Preparation of High-Performance Non-Enzymatic Glucose Sensors Based on Au/CuO Nanocomposites" Catalysts 15, no. 11: 1020. https://doi.org/10.3390/catal15111020
APA StyleMa, L., Wang, T., Mei, H., You, Y., Lin, Z., Li, W., Li, B., Kang, S., & Zhu, L. (2025). Facile Preparation of High-Performance Non-Enzymatic Glucose Sensors Based on Au/CuO Nanocomposites. Catalysts, 15(11), 1020. https://doi.org/10.3390/catal15111020





