Atomically Dispersed Rhodium on TiO2 for Tandem Hydrogenation–H/D Exchange of Cinnamic Acid
Abstract
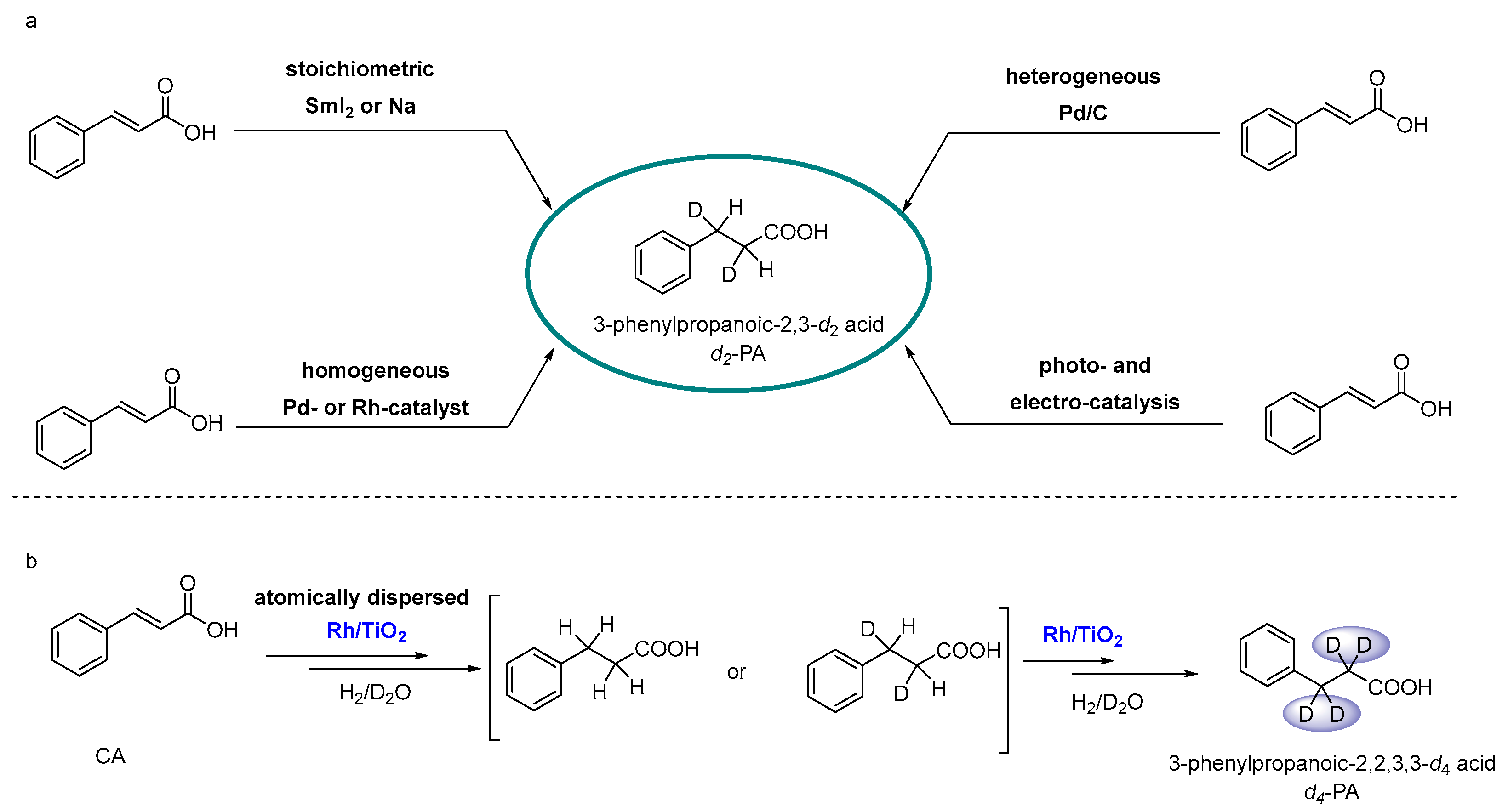
1. Introduction
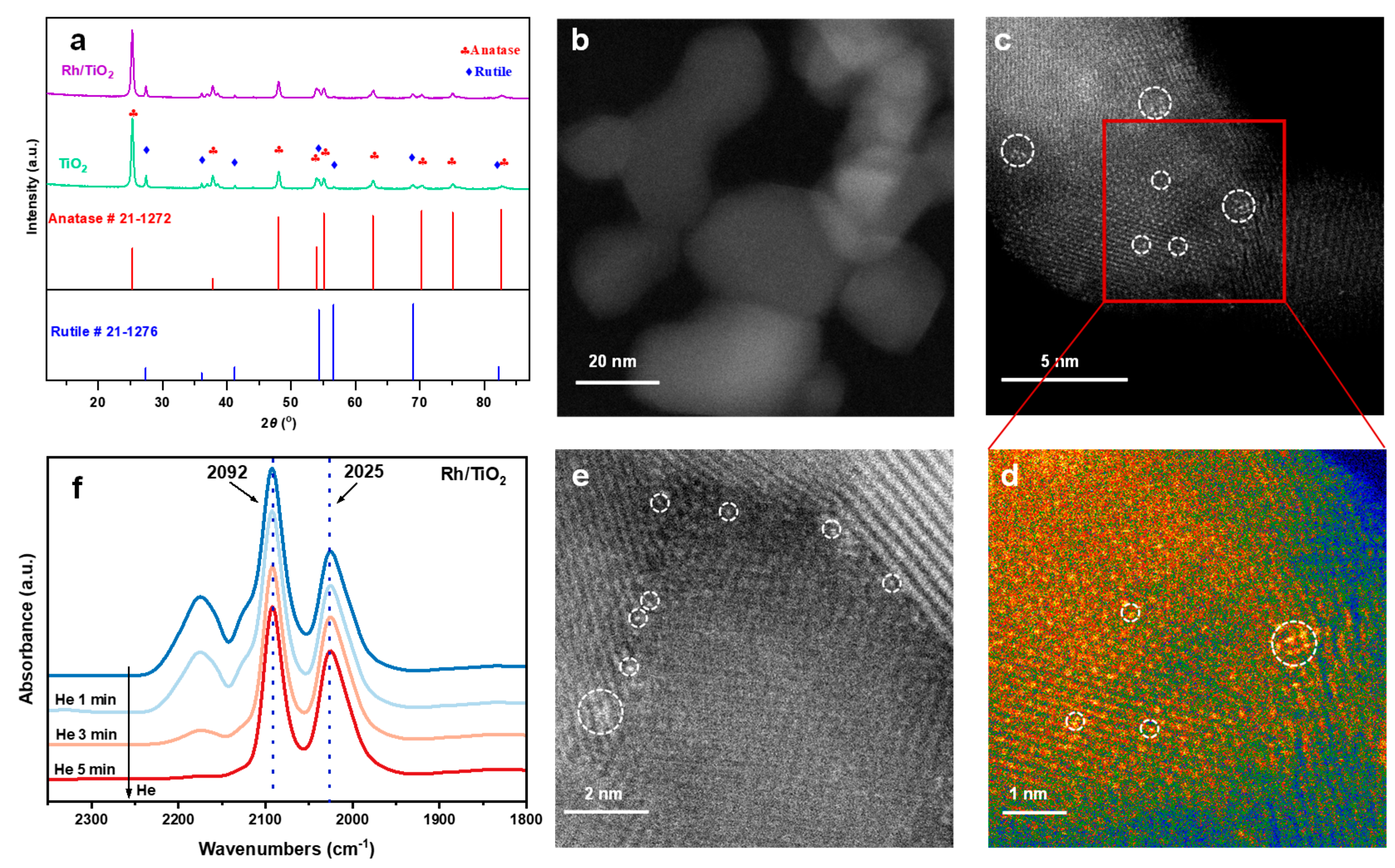
2. Results and Discussion
3. Materials and Methods
3.1. Reagents and Product Characterization
3.2. Catalyst Preparation and Characterization Techniques
3.2.1. The Preparation Procedure for Rh Catalyst
3.2.2. Details for the Catalytic Characterization Techniques
3.3. General Procedures for the Reaction
3.4. Procedures for the Recycling Reaction
3.5. Procedures for the Kinetic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CA | Cinnamic acid |
| PA | Phenyl propanoic acid |
References
- Gant, T.G. Using deuterium in drug discovery: Leaving the label in the drug. J. Med. Chem. 2013, 57, 3595–3611. [Google Scholar] [CrossRef]
- Rao, N.; Kini, R.; Kad, P. Deuterated drugs. Pharm. Chem. J. 2022, 55, 1372–1377. [Google Scholar] [CrossRef]
- Di Martino, R.M.C.; Maxwell, B.D.; Pirali, T. Deuterium in drug discovery: Progress, opportunities and challenges. Nat. Rev. Drug Discov. 2023, 22, 562–584. [Google Scholar] [CrossRef]
- Chen, Y.; Du, Y. The application of deuteration strategy in drug design. ChemMedChem 2025, 20, e202400836. [Google Scholar] [CrossRef] [PubMed]
- Atzrodt, J.; Derdau, V.; Kerr, W.J.; Reid, M. CH Functionalisation for Hydrogen Isotope Exchange. Angew. Chem. Int. Ed. Engl. 2018, 57, 3022–3047. [Google Scholar] [CrossRef]
- Kopf, S.; Bourriquen, F.; Li, W.U.; Neumann, H.; Junge, K.; Beller, M. Recent developments for the deuterium and tritium labeling of organic molecules. Chem. Rev. 2022, 122, 6634–6718. [Google Scholar] [CrossRef] [PubMed]
- Lepron, M.; Daniel-Bertrand, M.; Mencia, G.; Chaudret, B.; Feuillastre, S.; Pieters, G. Nanocatalyzed hydrogen isotope exchange. Accounts Chem. Res. 2021, 54, 1465–1480. [Google Scholar] [CrossRef]
- Yang, X.; Ben, H.; Ragauskas, A.J. Recent Advances in the Synthesis of Deuterium-Labeled Compounds. Asian J. Org. Chem. 2021, 10, 2473–2485. [Google Scholar] [CrossRef]
- Prakash, G.; Paul, N.; Oliver, G.A.; Werz, D.B.; Maiti, D. C–H deuteration of organic compounds and potential drug candidates. Chem. Soc. Rev. 2022, 51, 3123–3163. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Wu, X.; Zhu, C.; Xie, J. Radical deuteration. Chem. Soc. Rev. 2022, 51, 6291–6306. [Google Scholar] [CrossRef]
- Yu, Y.-J.; Kim, S.-B.; Lee, K.-H.; Back, J.-H.; Park, K.-S.; Yoon, S.-Y.; Hong, J.; You, Y.; Lee, D.G.; Park, J.; et al. Development of deuteration technology to improve lifetime of oled ex and identification of stability of deuterated materials. J. Soc. Inf. Disp. 2023, 54, 469–473. [Google Scholar] [CrossRef]
- Yao, J.; Dong, S.-C.; Tam, B.S.T.; Tang, C.W. Lifetime enhancement and degradation study of blue OLEDs using deuterated materials. Acs Appl. Mater. Interfaces 2023, 15, 7255–7262. [Google Scholar] [CrossRef] [PubMed]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Kustiana, B.A.; Widiyarti, G.; Ernawati, T. Recent advances in synthetic approaches for bioactive cinnamic acid derivatives. Beilstein J. Org. Chem. 2025, 21, 1031–1086. [Google Scholar] [CrossRef]
- Roşca, S.I.; Raluca, S.; Ungureanu, E.-M.; Gabriela, S.; Rosca, S. 13C-and D-labelled 3-phenylpropionic acids; synthesis and characterization by NMR and MS spectra. Sci. Bull. B Chem. Mater. Sci. UPB 2008, 70, 77–84. [Google Scholar]
- Concellón, J.M.; Rodríguez-Solla, H. Synthesis of different deuterated carboxylic acids from unsaturated acids promoted by samarium diiodide and D2O. Chem. A Eur. J. 2002, 8, 4493–4497. [Google Scholar] [CrossRef]
- Li, H.; Zhang, B.; Dong, Y.; Liu, T.; Zhang, Y.; Nie, H.; Yang, R.; Ma, X.; Ling, Y.; An, J. A selective and cost-effective method for the reductive deuteration of activated alkenes. Tetrahedron Lett. 2017, 58, 2757–2760. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X.; Zhao, L.; Pi, C.; Ji, J.; Cui, X.; Wu, Y. Generalized chemoselective transfer hydrogenation/hydrodeuteration. Adv. Synth. Catal. 2020, 362, 4119–4129. [Google Scholar] [CrossRef]
- Taleb, B.; Jahjah, R.; Abdel Baki, Z.; Hijazi, A.; Nehmeh, B.; El-Dakdouki, M. Rhodium-catalyzed transfer hydrogenation of cinnamic acid using formic acid as the hydrogen source. Chem. Methodol. 2025, 9, 268–276. [Google Scholar] [CrossRef]
- Kurita, T.; Aoki, F.; Mizumoto, T.; Maejima, T.; Esaki, H.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Facile and convenient method of deuterium gas generation using a Pd/C-catalyzed H2–D2 exchange reaction and its application to synthesis of deuterium-labeled compounds. Chem. Eur. J. 2008, 14, 3371–3379. [Google Scholar] [CrossRef]
- Mándity, I.M.; Martinek, T.A.; Darvas, F.; Fülöp, F. A simple, efficient, and selective deuteration via a flow chemistry approach. Tetrahedron Lett. 2009, 50, 4372–4374. [Google Scholar] [CrossRef]
- Modvig, A.; Andersen, T.L.; Taaning, R.H.; Lindhardt, A.T.; Skrydstrup, T. Two-chamber hydrogen generation and application: Access to pressurized deuterium gas. J. Org. Chem. 2014, 79, 5861–5868. [Google Scholar] [CrossRef]
- Xu, P.; Jiang, H.-Q.; Xu, H.; Wang, S.; Jiang, H.-X.; Zhu, S.-L.; Yin, L.; Guo, D.; Zhu, X.U. Photocatalytic deuterocarboxylation of alkynes with oxalate. Chem. Sci. 2024, 15, 13041–13048. [Google Scholar] [CrossRef]
- Liu, X.; Liu, R.; Qiu, J.; Cheng, X.; Li, G. Chemical-reductant-free electrochemical deuteration reaction using deuterium oxide. Angew. Chem. Int. Ed. 2020, 59, 13962–13967. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, X.; Lv, M.; Pan, X.; Wang, H.; Li, C.; Chen, M.; Chen, W.; Zhang, B.O.; Yu, G.; et al. “Suspended” Single Rhenium Atoms on Nickel Oxide for Efficient Electrochemical Oxidation of Glucose. J. Am. Chem. Soc. 2025, 147, 4886–4895. [Google Scholar] [CrossRef]
- Zhang, F.; Hong, F.; Qin, X.; Du, X.; Jiang, X.; Li, Y.; Guo, H.; Liu, P.; Cui, W.; Min, X.; et al. Achieving “true” selective hydrogenation by CO treatment of the Pt/TiO2 catalyst. J. Am. Chem. Soc. 2025, 147, 26319–26328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.I.; Huang, Y.; Wang, Y.; Wang, Z.; Huang, C.; Yang, X.; Qiao, B.; Wang, X.; Zhang, T. Enzyme-mimicking copper single-atom catalyst for selective oxidation of methane to liquid oxygenates. J. Am. Chem. Soc. 2025, 147, 29496–29504. [Google Scholar] [CrossRef]
- Min, X.; Mei, Y.; Chen, B.; He, L.-B.; Song, T.; Ji, D.; Hu, Y.; Wan, B.; Chen, Q. Rhodium–Catalyzed Deuterated Tsuji–Wilkinson Decarbonylation of Aldehydes with Deuterium Oxide. J. Am. Chem. Soc. 2022, 144, 11081–11087. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Min, X.; Su, J.; Yu, B.; Cui, W.; Tang, J.; Qiao, B. Catalytic α-Site-Selective Hydrogen-Deuterium Exchange of Benzylic Alcohols by Palladium Single-Atom Catalyst. Angew. Chem. Int. Ed. 2025, 64, e202507338. [Google Scholar] [CrossRef]
- Lang, R.; Li, T.; Matsumura, D.; Miao, S.; Ren, Y.; Cui, Y.-T.; Tan, Y.; Qiao, B.; Li, L.; Wang, A.; et al. Hydroformylation of olefins by a rhodium single-atom catalyst with activity comparable to RhCl(PPh3)3. Angew. Chem. Int. Ed. 2016, 55, 16054–16058. [Google Scholar] [CrossRef]
- Gu, F.; Qin, X.; Li, M.; Xu, Y.; Hong, S.; Ouyang, M.; Giannakakis, G.; Cao, S.; Peng, M.I.; Xie, J.; et al. Selective catalytic oxidation of methane to methanol in aqueous medium over copper cations promoted by atomically dispersed rhodium on TiO2. Angew. Chem. Int. Ed. 2022, 134, e202201540. [Google Scholar] [CrossRef]
- Li, T.; Chen, F.; Lang, R.; Wang, H.; Su, Y.; Qiao, B.; Wang, A.; Zhang, T. Styrene Hydroformylation with In Situ Hydrogen: Regioselectivity Control by Coupling with the Low–Temperature Water–Gas Shift Reaction. Angew. Chem. Int. Ed. Engl. 2020, 59, 7430–7434. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asif, F.; Li, S.-X.; Min, X.-T.; Zhang, W.-T.; Qiao, B. Atomically Dispersed Rhodium on TiO2 for Tandem Hydrogenation–H/D Exchange of Cinnamic Acid. Catalysts 2025, 15, 1014. https://doi.org/10.3390/catal15111014
Asif F, Li S-X, Min X-T, Zhang W-T, Qiao B. Atomically Dispersed Rhodium on TiO2 for Tandem Hydrogenation–H/D Exchange of Cinnamic Acid. Catalysts. 2025; 15(11):1014. https://doi.org/10.3390/catal15111014
Chicago/Turabian StyleAsif, Fatima, Shu-Xian Li, Xiang-Ting Min, Wen-Ting Zhang, and Botao Qiao. 2025. "Atomically Dispersed Rhodium on TiO2 for Tandem Hydrogenation–H/D Exchange of Cinnamic Acid" Catalysts 15, no. 11: 1014. https://doi.org/10.3390/catal15111014
APA StyleAsif, F., Li, S.-X., Min, X.-T., Zhang, W.-T., & Qiao, B. (2025). Atomically Dispersed Rhodium on TiO2 for Tandem Hydrogenation–H/D Exchange of Cinnamic Acid. Catalysts, 15(11), 1014. https://doi.org/10.3390/catal15111014







