Abstract
Lipases (EC 3.1.1.3) catalyze the hydrolysis of triacylglycerols into mono- and diacylglycerols and free fatty acids. This study investigated the production of lipase by Hypocrea pseudokoningii under solid-state fermentation (SSF), followed by its immobilization, purification, and biochemical characterization. Maximum activity was achieved using wheat fiber after 168 h of cultivation. Supplementation with oils enhanced production, particularly palm oil (315U; 1.58-fold increase) and soybean oil (Glycine max) (298U; 1.49-fold increase). The addition of micronutrients further improved yields, with Khanna (445U) and Vogel (400U) salts promoting more than a two-fold increase in activity. Immobilization on Octyl-Sepharose significantly altered the enzyme’s properties. The free lipase exhibited optimal activity at 45 °C and pH 4.5–5.5, while the immobilized enzyme showed maximum activity at 35–40 °C and pH 5.5. Thermal stability was notable enhanced: the free lipase had a half-life of 10 min at 50 °C, whereas the immobilized enzyme remained stable for 60 min and retained over 30% activity at 70 °C. Both the free and immobilized forms were stable across a broad pH range (4.0–10.0), maintaining more than 70% residual activity. The enzyme was stabilized by Tween 80 but inhibited by SDS. It was activated by Ca2+ and showed resistance to Pb2+, Zn2+, and Cu2+. Hydrolytic assays revealed murumuru (Astrocaryum murumuru), cupuaçu (Theobroma grandiflorum), and soybean oils as preferred substrates. TLC confirmed the formation of mono- and diglycerides, as well as the presence of fatty acids.
1. Introduction
Lipases (triacylglycerol acylhydrolases; EC 3.1.1.3) are members of the hydrolase family that act on ester linkages of carboxylic esters. Their primary physiological role is to catalyze the hydrolysis and sequential synthesis of triacylglycerols, releasing diacylglycerides, monoacylglycerides, glycerol, and free fatty acids [1,2]. In addition to hydrolysis, lipases can catalyze a wide range of reactions, including esterification, transesterification (such as interesterification, alcoholysis, and acidolysis), aminolysis (for amide synthesis), and lactonization, depending on the amount of water present in the reaction medium [3,4].
Due to their versatility, lipases have found rapidly expanding applications across diverse industrial sectors [1,5,6]. These include cosmetic, food emulsifiers, dicarboxylic acids, detergent industries, fat and oil processing, biodiesel synthesis, textile, dairy, egg, bakery, meat and fish industries, pulp and paper, pharmaceuticals, perfumery, tea processing, medical and biosensor applications, waste treatment, polymer synthesis, and other “green chemistry” processes [4,7,8,9,10].
Lipases are found in various biological sources, but microbial lipases are the most commercially attractive [7,9] due to their high yields, ease of manipulation, and extracellular secretion. Microbial lipases can be produced by submerged fermentation (SmF), one of the most widely used bioprocesses in industry. SmF enables precise control of enzyme production and typically yields reliable results for extracellular lipases; however, it also generates large volumes of wastewater, increasing downstream processing and disposal costs [6,10,11].
Solid-state fermentation (SSF) has emerged as an alternative to SmF and has been widely applied to produce microbial lipases due to its economic and environmental advantages [6,10]. SSF is particularly valuable in countries with abundant biomass and agro-industrial residues, which can serve as nutrient-rich substrates while reducing production costs. It can also be performed in situ, using industrial waste directly as a nutrient source for the fermentation process [6,12,13,14,15]. Moreover, studies have reported higher lipase yields in SSF compared to SmF [10,12,16]. Pereira et al. [17], for example, produced lipase from the fungus H. pseudokoningii using both fermentation methods and observed that SSF resulted in greater enzymatic activity levels. Thus, SSF contributes to environmental sustainability by reducing pollution and providing a productive destination for industrial waste, while enhancing enzyme yields. An additional advantage of this method is the high microbial growth rate on biomass and the low proteolytic activity observed during fermentation [18].
Beyond biomass, nutritionally and pharmaceutically important oils are also incorporated into SSF substrates to stimulate microbial lipase production. These lipases are capable of hydrolyzing both common and exotic oils, expanding their range of industrial applications. For instance, Bacuri (Platonia insignis) seed oil is used to make soap, in the treatment of skin diseases and wounds in animals, and in the cosmetic industry. Murumuru (Astrocaryum murumuru) is commonly utilized in the production of cosmetics, soaps, creams, shampoos, and as a drying agent in the paint industry. Fats can also be employed in butter manufacturing and biodiesel production [11,19]. Cupuaçu (Theobroma grandiflorum) oil is rich in essential nutrients, antioxidants, vitamins, and phytonutrients such as polyphenols and theobromine, showing beneficial properties for skin health and the treatment of irritations like eczema and dermatitis [20]. Similarly, butter oil enhances skin elasticity, slows the aging process, improves hydration, and offers protection against UV-A and UV-B radiation [21,22].
Following enzyme production, various strategies can be applied to improve enzyme stability and thermotolerance, such as immobilization on different chemical supports. Immobilization adds value to biocatalysts by facilitating enzyme purification [23,24,25,26], improving thermal and pH stability, and allowing recovery and reuse during industrial processes.
Considering this, the present study aimed to produce lipase from the fungus H. pseudokoningii through SSF using agro-industrial residues and different oils, followed by enzyme immobilization, purification, and biochemical characterization. The immobilized lipase obtained was successfully applied to the hydrolysis of oils relevant to the food and pharmaceutical industries.
2. Results
2.1. Improvement in Lipase Production Under SSF: Effect of the Supplementation of Diverse Carbon Sources, Oils, and Monosaccharides
Fourteen types of agro-industrial wastes, each with distinct compositions, were evaluated for their ability to support the growth of H. pseudokoningii and stimulate lipase production. The cultures were incubated under static conditions for seven days at 30 °C. H. pseudokoningii was able to grow and produce lipase in all tested media; however, the levels of enzyme production varied significantly (Table 1).

Table 1.
Lipase activity and specific activity obtained in SSF with different agro-industrial waste by H. pseudokoningii.
Fine sawdust, sawdust, oatmeal flour, jatropha cake, and wheat grain acted as weak inducers (1.8–46.5 U). Dehydrated orange flour, chopped buriti nuts, rye flour, and sorghum grain were classified as moderate inducers (97.4–223 U). In contrast, rolled oats, soybeans, and rice straw promoted lipase production of approximately 344 U. The highest yields were obtained with wheat bran (531 U) and wheat fiber (572 U). Wheat fiber, which provided the maximum activity, was therefore selected as the carbon source for subsequent experiments (Table 1).
Knowing that oils and fats induce the production of microbial lipases, different oil compositions were tested to evaluate their effect on enzyme activity. Only macaúba almond oil (29.0 ± 7.7%), palm oil (58.8 ± 4.0%), soybean oil (51.8 ± 11.2%), olive oil (37.8 ± 4.9%), and sunflower oil (30.0 ± 8.7%) increased lipase activity compared to the control. The remaining oils, mineral, sesame, pequi pulp, castor bean, nim, corn, macaúba pulp, and canola, showed the opposite trend, exhibiting only about 50% of the control activity (Figure 1A). H. pseudokoningii grew in all evaluated oils, although growth was reduced in mineral, sesame, and pequi pulp oils (Figure 1B). The highest specific activities were achieved in palm and soybean oils (Figure 1C).
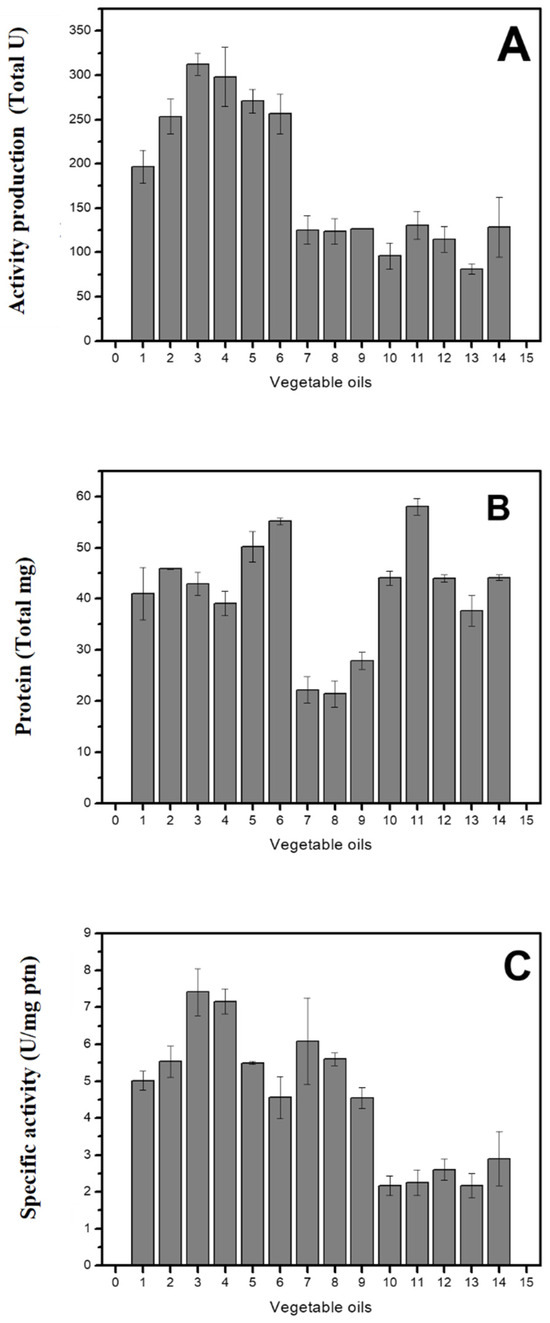
Figure 1.
Effect of the addition of oils to the culture medium. (A) Lipase activity (Total U). (B) Protein (Total mg). (C) Specific activity (U/mg). Control: SSF without adding oil. Legend: 1—control, 2—macaúba almond, 3—palm, 4—soybean, 5—olive, 6—sunflower, 7—mineral, 8—sesame, 9—pequi pulp, 10—castor beans, 11—nim, 12—corn, 13—macaúba pulp, 14—canola.
Other compounds were evaluated to assess their ability to enhance the lipase activity. The addition of glucose did not increase enzyme production; instead, it caused a marked reduction in lipase activity, suggesting an inhibitory effect on lipase synthesis and secretion (Figure 2A). In contrast, substituting distilled water with a micronutrient solution significantly increased lipase production by H. pseudokoningii. The mineral salt solutions from Vogel and Khanna media were the most effective, resulting in increases of 24 ± 1.4% and 37 ± 1.7%, respectively (Figure 2B). The time-course profile of lipase production indicated that maximum activity occurred on the seventh day of fermentation.
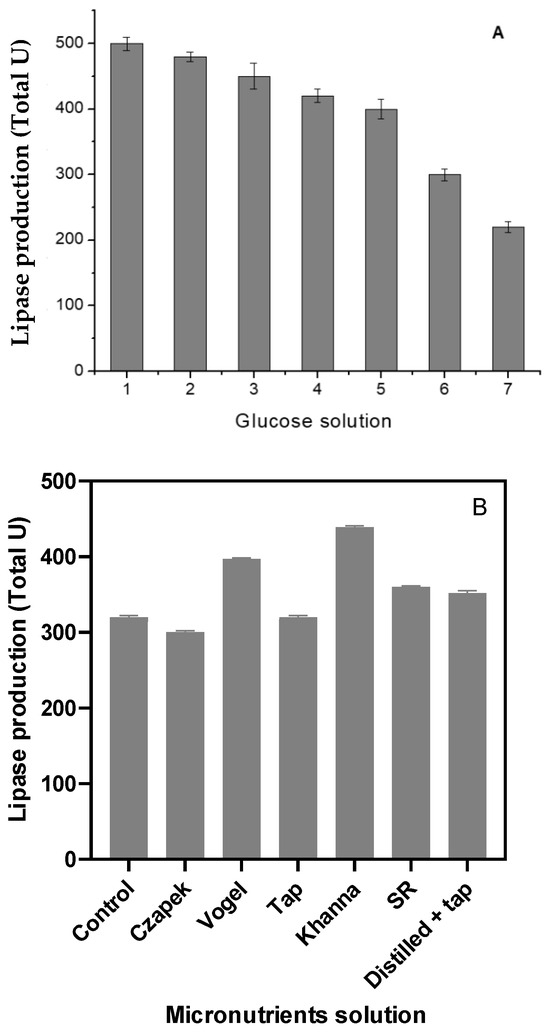
Figure 2.
Evaluation of the glucose addition (A) and micronutrient solutions (B) in lipase production. Legend: 1—without glucose; 2—0.05%; 3—0.1%; 4—0.25%; 5—0.5%; 6—1%; 7—1.5% of glucose (w/w).
H. pseudokoningii showed consistent growth and lipase production across the tested agro-industrial residues. Among the evaluated substrates, wheat fiber proved to be the most effective in promoting enzyme synthesis, and lipolytic activity increased further when Khanna salts were added to the SSF medium. Consequently, wheat fiber supplemented with Khanna salts and a cultivation period of seven days was selected for subsequent experiments.
2.2. Lipase Immobilization on Hydrophobic and on Ionic Supports
The lipase was incubated with various hydrophobic supports overnight (Table 2). 70% immobilization was observed using Purolite and Decaoctyl Sepabeads supports, achieving 68 and 90% activity recovery, respectively. On Phenyl-Sepharose and Hexyl Toyopearl supports, 63 and 68% of the protein were immobilized, and the activity recovery was 103 and 92%, respectively. On Butyl-Sepharose and Octyl-Sepharose supports, 65 and 63% of the protein was immobilized, respectively, and both showed an activity recovery of 140%, indicating an activation of 40%.

Table 2.
Immobilization on hydrophobic and ionic supports at pH 7.0.
The lipase from H. pseudokoningii was immobilized on ionic supports (Table 2). On Duolite, 65% of the total protein was immobilized, with an activity recovery of 21%. DEAE-Sepharose and DEAE-Toyopearl immobilized 50 and 61% of proteins, and the activity recovery was 42 and 59%, respectively.
2.3. Desorption of Immobilized Lipase on Hydrophobic and Ionic Supports
Desorption was carried out on all hydrophobic supports using different concentrations of Triton X-100. Complete desorption (100%) was achieved at 5% Triton X-100 for Butyl-Sepharose, Hexyl-Toyopearl, Purolite, and Decaoctyl-Sepabeads. Phenyl-Sepharose and Octyl-Sepharose showed partial desorption of 76 and 62%, respectively, under the same conditions (Table 3).

Table 3.
Desorption of lipase on hydrophobic and ionic support using Triton X-100 and NaCl.
When ionic supports were used for immobilization, lipase desorption did not exceed 31% (Table 3). Desorption levels of 20, 26, and 31% were obtained for Duolite, DEAE-Sepharose, and DEAE-Toyopearl, respectively, at 2.5 M NaCl.
2.4. Purification of Lipase
The derivatives were analyzed by electrophoresis to evaluate their degree of purity (Figure 3). Partial purification was observed for most supports, with up to three protein bands visible. Complete purification was achieved for some derivatives, notably lane 13, corresponding to the Hexyl-Toyopearl derivative, which displayed a single protein band. This derivative was therefore selected for subsequent characterization experiments.
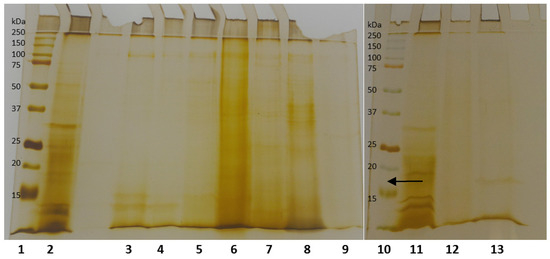
Figure 3.
SDS-PAGE of the immobilized lipase from SSF. The electrophoresis was carried out on 12% acrylamide gels. Each lane contained 20 mg of the derivative. Lanes: 1—Molecular marker, 2—Crude extract, 3—Butyl-Sepharose derivative, 4—Phenyl-Sepharose derivative, 5—Octyl-Sepharose derivative, 6—DEAE-Toyarpeal derivative, 7—Purolite derivative, 8—Sepabeads C18 derivative, 9—Duolite derivative, 10—Molecular marker, 11—Crude extract, 12—DEAE-Sepharose derivative, 13—Hexyl-Toyarpeal derivative. Stained with Silver Nitrate.
2.5. Lipase Characterization: Effect of Temperature and pH on Activity and Stability
The lipase produced in SSF exhibited an optimum temperature for lipase activity at 45 °C; interestingly, the immobilized lipase showed a shift in its optimum temperature, with the highest activity observed between 35 and 40 °C after immobilization (Figure 4A). Another interesting fact is that the derivative showed more resistance at elevated temperature, at 70 °C, the free lipase had no activity, while the Hexyl-derivative showed more than 30% activity (Figure 4A). Immobilization also improved enzyme activity at extreme pH. At pH 8.0, activity was 62.5%, and at pH 2.5, it was 74%. These values are considerably higher than those of the free enzyme, which exhibited a narrow optimum pH range of 4.5–5.5, whereas the immobilized lipase showed an optimum pH of 5.5 (Figure 4B).
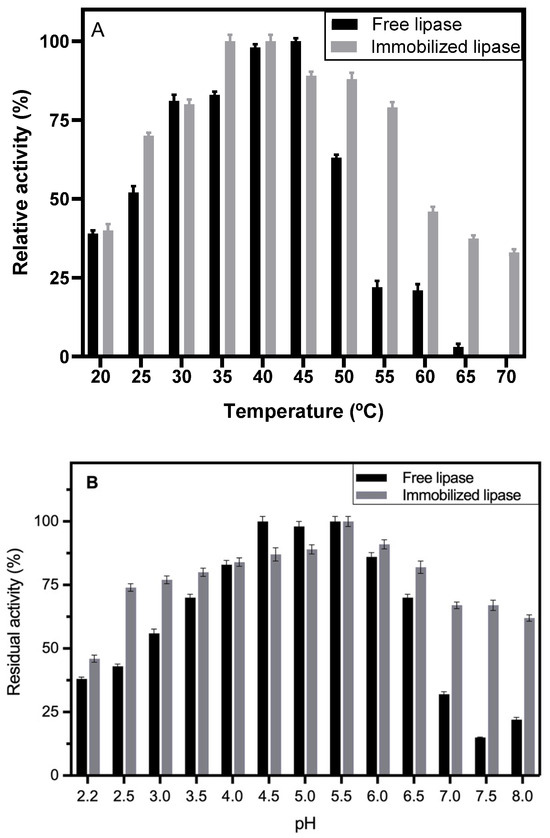
Figure 4.
Effect of temperature (A) and pH (B) on free lipase and immobilized lipase activity. Legend: black bars, free lipase; gray bars, immobilized lipase.
The immobilized lipase was more stable than the free enzyme at 40 °C. At this temperature, the derivative remained completely stable, whereas the free enzyme lost approximately 20% of its activity within 60 min (Figure 5A). The free enzyme at 50 °C had a half-life of 10 min, retaining less than 20% of its activity after 60 min. In contrast, the immobilized lipase remained fully stable for 60 min (Figure 5B).
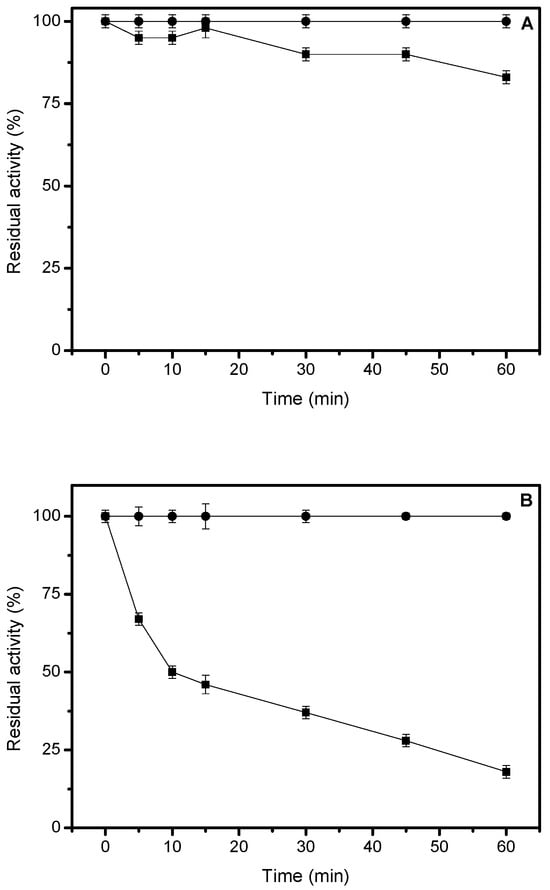
Figure 5.
Thermal stability of the H. pseudokoningii lipase. (A) 40 °C. (B) 50 °C. Symbols: Circle, immobilized lipase; square, free lipase. Inactivation was performed in 25 mM sodium acetate, pH 5, at 40 °C, and the pNPP assay was used to follow activity. Other details are described in Materials and Methods.
In general, the lipase produced in SSF exhibited good stability over a two-hour incubation period in a wide pH range (2.2 to 10). At the end of the assay, residual activity was above 80% at all pH values, except at pH 5 (68.5 ± 4.3%) and pH 7.5 (72.1 ± 1.8%). In contrast, the immobilized enzyme remained completely stable at all tested pH values (Figure 6A).
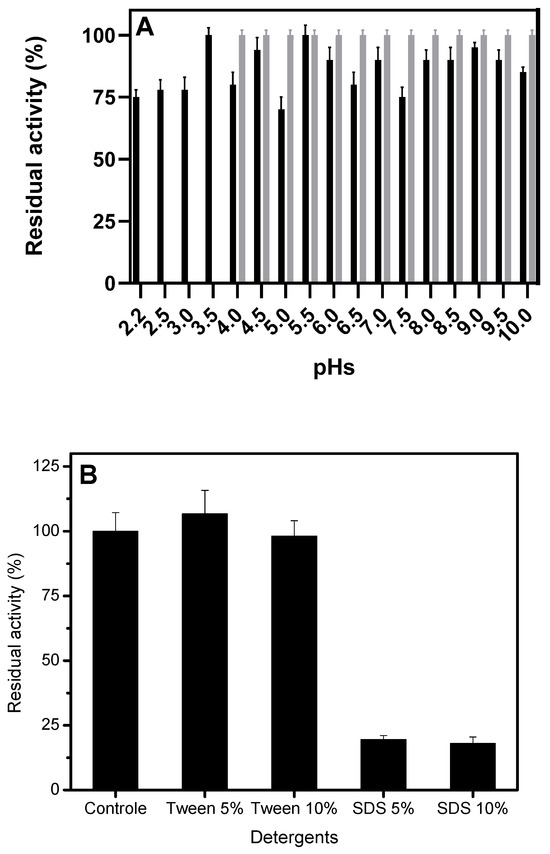
Figure 6.
Stability of H. pseudokoningii lipase at varying pH levels (A) and detergents (B). Inactivation was performed in 100 mM sodium acetate, pH 5, at 40 °C for 120 min, and activity was monitored using the pNPP assay. For pH 2.2 to 8.0, McIlvaine buffer was used; for pH 8.5 to 10, Glycine buffer was used. Other details are described in Materials and Methods. Legend: black bar, free lipase; gray bar, immobilized lipase.
Anionic detergents, such as SDS, reduced lipase activity more than non-ionic detergents, such as Tween 80. Lipases lost approximately 80% of their activity after exposure to 5 and 10% SDS. In contrast, exposure to 5 and 10% Tween 80 had little effect, with residual activity remaining above 80% during the incubation period (Figure 6B). The Hexyl derivative was not tested because the detergents could induce desorption from the support, which would prevent the desired assessment.
2.6. Effects of Ions on Immobilized Lipase Activity
The effect of ions on lipase activity was evaluated. Among the salts assessed, an increased lipolytic activity was observed using Pb2+, Mg2+, Ag2+, Na+, Al3+, K+, Co2+, and Ca2+. Interestingly, the lipase was resistant to all the heavy metals tested, including Hg2+, Cu2+, Co2+, Mn2+, Zn2+, and Pb2+ (Table 4).

Table 4.
Influence of salts and EDTA on lipase activity (%).
2.7. Hydrolysis of Oils
H. pseudokoningii derivative lipase was tested for the hydrolysis of 13 oils, and all oils were hydrolyzed (Table 5). Different levels of activity were observed among the oils. The highest activity was observed with murumuru oil (50 U/g), followed by cupuaçu (45 U/g), soybean (39 U/g), sesame (38 U/g), and coconut (38 U/g) oils. Lower activity was observed with andiroba and ucuuba nut oils, both showing 10 U/g. TLC analysis confirmed the formation of hydrolysis products, corroborating the observed enzyme activity (Figure 7).

Table 5.
Hydrolysis of oils.
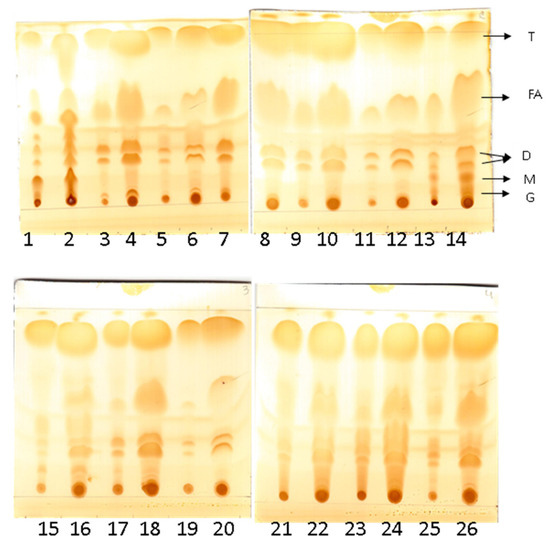
Figure 7.
Thin-layer chromatography of the hydrolysis product of oils catalyzed by lipase. Legend: (T) Triglycerides; (FA) fatty acids; (D) Diacylglycerol; (M) Monoacylglycerol; (G) glycerol. Lines: (1) Seed Bacuri oil without lipase; (2) Seed Bacuri oil with lipase; (3) Cupuaçu oil without lipase; (4) Cupuaçu oil with lipase; (5) Murumuru oil without lipase; (6) Murumuru oil with lipase; (7) Andiroba oil without oil; (8) Andiroba oil with lipase; (9) Nuts of Brazil oil without lipase; (10) Nuts of Brazil oil with lipase; (11) Nuts Babaçu oil without lipase; (12) Nuts Babaçu oil with lipase; (13) Nuts Ucuuba oil without lipase; (14) Nuts Ucuuba oil with lipase; (15) Sunflower oil without lipase; (16) Sunflower oil with lipase; (17) Sessame oil without lipase; (18) Sessame oil with lipase; (19) Coconut oil without lipase; (20) Coconut oil with lipase; (21) Palm oil without lipase; (22) Palm oil with lipase; (23) Soybean oil without lipase; (24) Soybean oil with lipase; (25) Olive oil without lipase; (26) Olive oil with lipase.
3. Discussion
The production of lipase using agricultural residues has been reported previously [27,28,29,30]. The use of farming residues minimizes production costs by utilizing materials that would otherwise be discarded, thus avoiding additional expenses. Furthermore, the use of waste helps reduce environmental impacts. Here, wheat bran residue was the best for lipase production of H. pseudokoningii. According to the USDA database [31], 100 g of wheat fiber contains 4.25 g of lipids, whereas the same portion of soybeans contains 6.8 g. This indicates that the total lipid content of a residue alone is not a reliable predictor of its potential to induce lipase production. Wheat bran has also been used to produce lipases and proteases via SSF by Aspergillus versicolor [32]. Aspergillus niger was cultivated in SSF using different agro-industrial residues, with wheat bran supplemented with 2% (w/w) olive oil emerging as the most effective substrate for lipase production [30]. Interestingly, the best inducers were not those typically used in the agricultural industry to produce oil or biodiesel, such as soybeans, buriti nuts, and jatropha cake, despite their high lipid content. According to Salihu et al. [33], an effective substrate for lipase production should not only induce lipolytic activity but also provide the necessary nutrients for fungal growth and a suitable surface for cell attachment.
The addition of different oils to the SSF medium influenced lipase production, total protein synthesis, and consequently, the specific activity of the enzyme (Figure 1). Supplementation with vegetable oils such as macaúba almonds, palm, soybean, and olive oils resulted in a marked increase in total lipase activity compared to the control without oil. This suggests that these oils acted as metabolic inducers, possibly providing hydrophobic substrates that stimulate lipid catabolism and the secretion of extracellular enzymes.
In contrast, a sharp decrease in total activity was observed with other oils, particularly pequi, castor bean, and neem, indicating that their composition or potential toxicity may inhibit microbial growth or enzyme secretion.
Regarding total protein production, no clear trend was observed compared with enzymatic activity. Variations were less pronounced, and some oils, such as macaúba and corn, showed relatively high protein contents without a corresponding increase in activity. This demonstrates that not all secreted proteins are active lipases, reinforcing the importance of specific activity as a key performance parameter. Some of these proteins may primarily contribute to fungal growth or metabolism rather than catalytic activity.
Consequently, the difference between lipase activity and total protein production directly affects specific activity, with higher values observed for macaúba almond and palm oils. These results indicate that, under these conditions, there was a greater proportion of active lipase relative to other secreted proteins. In contrast, pequi, neem, and castor bean oils resulted in lower specific activities, possibly due to the presence of inhibitory compounds or alterations in the medium’s physicochemical properties that interfered with enzyme expression or stability.
Considering potential downstream applications such as enzyme purification and immobilization, it is crucial to evaluate both lipase activity and total protein content, since an excess of non-enzymatic proteins may compete for immobilization sites or hinder purification, ultimately reducing process yields.
Overall, the results demonstrate that adding appropriate vegetable oils can effectively stimulate lipase production by H. pseudokoningii, with macaúba almond, palm, and soybean oils being the most promising inducers. The positive correlation between total and specific activity in these treatments indicates not only an increase in enzyme production but also an enhancement in the catalytic efficiency of the enzyme produced.
The addition of vegetable oils in SSF can enhance lipase production, indicating that they act as inducers of this enzyme. Rodriguez et al. [34] studied the lipase production by Rhizopus homothallicus and obtained an increase in the lipolytic activity using corn oil, almond, olive, peanut, grape, and sunflower oils. In addition to vegetable oils, vegetable oil cakes are also being utilized. These cakes, generated during oil extraction, have attracted considerable interest as solid substrates in SSF processes [35].
Carbon and nitrogen sources play a central role in regulating fungal growth and enzyme production. Greco-Duarte et al. [12] showed that Penicillium simplicissimum changes its physiology and morphology depending on the available carbon sources (glucose, olive oil, or a combination of both) under both SmF and SSF conditions. Similarly, glucose, maltose, and starch stimulated growth and increased lipase activity in A. versicolor [28]. In most fungi, glucose accelerates early growth and promotes higher biomass accumulation [36], which can, in turn, enhance lipase synthesis. However, H. pseudokoningii behaves differently: its lipase production increases in the absence of glucose, suggesting that carbon catabolite repression controls enzyme expression in this species. In fact, Fickers et al. [37] confirmed the role of catabolite inhibition by glucose in regulating lipase production in studies with the yeast Yarrowia lipolytica mutant LgX64.81. Although the genetic mechanism is not yet clearly understood, this finding supports the notion that catabolite inhibition plays a significant role in regulating lipase production.
Salt solutions, such as Vogel and Khanna salts, increased the yields of H. pseudokoningii lipase. A similar result was found in the work of Rodriguez et al. [34], which reported a 27% increase in lipolytic activity of R. homothallicus with the addition of a micronutrient solution (g/L) containing MnCl2, CoSO4, CuSO4, ZnSO4, and EDTA.
The time-course of lipase production by H. pseudokoningii reached its maximum on the 7th day. Veerabhadrappa et al. [28] reported that the time to output A. versicolor CJS-98 lipase was on the 5th day of incubation. Similarly, the lipases from A. niger and P. aeruginosa also reached maximum production on the 5th day [38,39].
The immobilization process can be beneficial for purifying a specific enzyme, such as lipase. In this study, hydrophobic supports were used, allowing immobilization via adsorption. Lipases possess a large hydrophobic region, facilitating their immobilization on hydrophobic supports. When immobilized on such supports, lipases often become hyperactive because the lid remains open, exposing the active site and facilitating substrate access. Lipase from H. pseudokoningii, previously produced in SmF, was immobilized on both hydrophobic and ionic supports, with increased enzyme activity observed on all hydrophobic supports [23]. The high affinity of lipases for hydrophobic supports makes these materials widely used in purification processes.
The optimum temperature for SSF H. pseudokoningii lipase activity was 45 °C, similarly, as observed for several lipases from mesophilic and thermophilic fungi [2,4,17,23,24]. The optimum temperature for lipase production by Bacillus amyloliquefaciens in SSF is 40 °C [40]. The immobilization process changed the characteristics of lipase, causing a displacement of the optimum temperature (now 35–40 °C) and improved enzyme activity at higher temperatures. This occurred because the immobilization process stabilized the protein structure.
The decrease in the apparent optimum temperature from 45 °C to 35–40 °C after immobilization may be related to structural constraints imposed by the support, which reduce the enzyme’s conformational flexibility. Additionally, diffusion limitations or microenvironmental effects, such as changes in local hydration or polarity, could influence substrate accessibility and enzyme dynamics, thereby lowering the apparent optimum temperature [41,42,43].
The biochemical characterization of H. pseudokoningii lipase produced under SSF revealed an activity range between pH 4.5 and 5.5. In contrast, the enzyme produced under SmF displayed three distinct activity peaks across different pH values, suggesting the presence of multiple lipase isoforms [17]. Similarly, Pernas et al. [44] reported more than one lipase form in Candida rugosa. The results of this study support the existence of two distinct H. pseudokoningii lipase isoforms in the crude extract, as indicated by its broad pH activity profile. However, upon immobilization, only a single form was detected (Figure 3), indicating a single distinct pH optimum.
The lipase from H. pseudokoningii showed thermostability at 40 °C, retaining more than 80% of its residual activity and maintaining its half-life at this temperature. At 50 °C, the enzyme’s half-life was reduced to 10 min. Facchini et al. [2] reported a lipase from Fusarium verticillioides with comparable thermal properties. In contrast, lipases produced by Hypocrea under SmF conditions [17] exhibited greater thermostability than those produced under SSF conditions. Immobilization further enhanced thermal stability, as the immobilized derivative preserved 100% of its activity at both tested temperatures.
Interestingly, the H. pseudokoningii lipase, both free and immobilized, showed good stability over a broad pH range, suggesting its potential applicability across various industrial sectors. Basheer et al. [45] reported a similar finding: the lipase from A. awamori exhibited pH stability comparable to that of the lipase in this study. The A. awamori lipase has been successfully applied in bioremediation processes. Another example of an enzyme with high alkaline pH stability is the lipase from Talaromyces thermophilus, which is used in detergents [46].
Lipase activity was greatly influenced by the anionic detergent immediately after exposure (Figure 6B). In contrast, non-ionic detergents such as Tween 80 did not significantly affect the lipase activity. Similarly, the activity of lipase purified from A. terreus was inhibited by ionic detergents, whereas non-ionic detergents enhanced the enzyme activity [47]. In a previous study, the lipase produced by H. pseudokoningii under submerged fermentation exhibited an approximately 80% increase in catalytic activity when detergents (Tween 80, Triton X-100, and SDS) were added to the enzymatic assay [17]. This contrasting behavior may be attributed to several factors, including differences in fermentation conditions (solid-state versus submerged), the nature of the inducers used during enzyme production, and the enzyme purification level. Additionally, the presence of other components in the crude extract of the previous study could have stabilized or protected the enzyme’s tertiary structure, reducing the denaturing effect of SDS and enhancing its apparent activity. In the present study, the purified enzyme, free from stabilizing agents, may have been more susceptible to the disruptive effects of ionic detergents.
The observed increase in lipase activity in the presence of Pb2+, Mg2+, Ag+, Na+, Al3+, K+, Co2+, and Ca2+ can be attributed to metal–enzyme interactions through different mechanisms. Lima et al. [48] reported that lipases from Penicillium aurantiogriseum produced by SSF were activated by Mg2+, Zn2+, Co2+, and Mn2+, while Hg2+ strongly inhibited the enzyme. Similarly, Ca2+ enhanced the catalytic activity of H. pseudokoningii lipase, possibly by providing structural stabilization. As demonstrated by Tanaka et al. [49], calcium ions can stabilize lipase structure by bridging the active-site region to the second subdomain of the protein, thereby maintaining enzyme integrity and acting as a cofactor for catalytic activity [50].
Metal ions may therefore function as cofactors or structural activators, inducing conformational changes that stabilize the active form of the enzyme [51,52]. In lipases, these effects are frequently associated with the lid domain, which regulates substrate access to the active site. Divalent cations such as Ca2+ and Mg2+ are known to stabilize negatively charged residues near the catalytic triad, facilitating substrate binding and enhancing catalytic efficiency. Calcium ions are classical lipase activators, promoting enzyme rigidity and interfacial activation, while magnesium ions strengthen salt bridges and electrostatic interactions, thereby maintaining the optimal geometry of the active site [53,54,55,56].
Monovalent ions, including Na+ and K+, may modulate enzyme activity primarily by altering the ionic strength of the medium, which influences enzyme solvation, flexibility, and substrate accessibility [57].
Transition and heavy metal ions, such as Co2+ and Pb2+, although potentially toxic at high concentrations, can coordinate with histidine or aspartate residues at low concentrations, stabilizing the catalytic region [58]. Similarly, Ag+ may interact with thiol groups (-SH) from cysteine residues, promoting beneficial conformational rearrangements depending on the enzyme’s tertiary structure [55,59].
Overall, these results indicate that H. pseudokoningii lipase exhibits high tolerance and structural adaptability to various metal ions, properties that are advantageous for industrial applications under chemically complex conditions.
The oils analyzed in this study possess important nutritional and medicinal properties, with distinct compositions that provide access to a variety of fatty acids. Lipase exhibited good hydrolytic activity on all tested oils (Table 5). TLC analysis corroborated these results, revealing increasing bands corresponding to monoacylglycerol, diacylglycerol, and free fatty acids. Lipase derivatives produced by Hypocrea in SmF efficiently hydrolyzed fish oil, as well as Cupuaçu, Bacuri, and Murumuru oils [23,24]. Similarly, purified lipase from H. pseudokoningii in SmF showed high hydrolytic activity toward Bacuri, Cupuaçu, Murumuru, andiroba nut, Brazil nut, ucuuba nut, sunflower, sesame, coconut, palm, soybean, and olive oils [23,24].
4. Material and Methods
4.1. Microorganism, Culture Conditions, and Lipase Production
Conidia of H. pseudokoningii were maintained on 4% PDA slants at 30 °C. To induce lipase production from H. pseudokoningii in SSF, 1 mL of conidial suspension from a 7-day-old culture (105 conidia/mL) was inoculated into 125 mL Erlenmeyer flasks containing 5 g of agro-industrial or waste material plus 1% oil, and 10 mL of distilled water.
The following agro-industrial wastes were used: dehydrated orange flour, fine sawdust, chopped buriti nuts, rye flour, oatmeal flour, soybeans, sawdust, rolled oats, jatropha cake, sorghum grain, wheat fiber, wheat grain, rice straw, and wheat bran. The oils evaluated were macaúba almond, palm, soybean, olive, sunflower, mineral, sesame, pequi pulp, castor beans, nim, corn, macaúba pulp, or canola. A control medium without adding oil was used. Media with glucose addition (0–1.5%) were tested. Additionally, various micronutrient solutions (Vogel [60], Czapek [61,62], Khanna [63], SR [64], tap and distilled water) were added to the SSF to enhance lipase production.
The cultures were incubated in static conditions for seven days in a microbiological incubator maintained at 70% relative humidity. After this time, 25 mL of distilled water was added to each Erlenmeyer. The flask was shaken for 25 min at 4 °C, and the crude extracts were recovered by filtering through filter paper. The mixture was then centrifuged at 1000 rpm for 15 min and filtered again.
4.2. Measurement of Lipase Activity and Protein
Extracellular lipase activity was determined discontinuously using p-nitrophenyl palmitate (p-NPP) as substrate [65,66]. Standard assay conditions were 25 µL of enzymatic sample, 25 µL of distilled water, 450 µL of 100 mM McIlvaine buffer [67,68], pH 5.0, containing 0.77 mM p-nitrophenyl palmitate, 10% isopropanol, 0.5 mg/mL gum Arabic, and 0.25% Triton X-100. The mixture was incubated at 40 °C for varying periods. The assay was stopped with 0.5 mL saturated sodium tetraborate solution, and the liberated p-nitrophenolate was measured at 410 nm. One enzyme unit (U) was defined as the amount of enzyme that produced 1 µmol of p-nitrophenol per minute under the assay conditions. Total U was defined as the product of enzyme activity (U/mL) by the total volume of enzyme extract (mL). Proteins were measured using the Bradford method [69], with bovine serum albumin as a standard, and expressed in mg/L. Specific activity was defined as the relationship between the number of units and the mass of protein present in the reaction medium (U/mg) or U/g when using agro-industrial waste.
4.3. Hydrophobic Immobilization and Ionic Immobilization of H. pseudokoningii Lipase
One gram of hydrophobic supports (Butyl-Sepharose, Phenyl-Sepharose, Octyl-Sepharose, Hexyl Toyopearl, Purolite, and Decaoctyl Sepabeads), and ionic supports (DEAE-Toyopearl, DEAE-Sepharose, and Duolite) were added to 10 mL of the crude extract (30 mg of protein) in 25 mM sodium phosphate buffer, pH 7, at 4 °C. Before use, supports were washed with abundant distilled water and 10 mM sodium phosphate buffer, pH 7. The activities of the suspension and supernatant were periodically assayed as described. After immobilization, the adsorbed lipase derivatives were extensively washed with distilled water. A blank suspension was prepared by adding 1 g of Sepharose 4BCL.
The parameters of the immobilization procedures were defined as follows: the yield of immobilization (YI) was determined based on the difference in total protein concentration between the initial enzyme solution and the supernatant after immobilization, measured using the Bradford assay, and expressed as the percentage (%) of enzyme bound to the support relative to the total protein offered for immobilization. The activity recovery (AR) was calculated by dividing the total activity of the immobilized derivative by the total activity of the initial free enzyme and multiplying by 100.
4.4. Desorption of Lipase from Hydrophobic and Ionic Supports
Hydrophobic derivatives (Octyl-, Phenyl-, Butyl-Sepharose, Hexyl Toyopearl, Purolite, and Decaoctyl Sepabeads) were suspended in 25 mL of 5 mM sodium phosphate buffer, pH 7.0. Triton X-100 was added at increasing concentrations (0–5%), and supernatant samples were collected after 45 min of incubation at room temperature. Soluble enzyme was used as a reference. Ionic derivatives (DEAE-Sepharose, DEAE-Toyopearl, and Duolite) were suspended in 25 mL of 5 mM sodium phosphate buffer, pH 7.0. NaCl was added at concentrations ranging from 0 to 2.5 M, and supernatant samples were collected after 45 min of incubation. Soluble enzyme was used as a reference. Lipase desorption was evaluated by determining the protein content and lipase activity in the supernatant.
4.5. Electrophoresis Analysis
SDS-PAGE assessed the purity of the derivatives on 12% (w/v) polyacrylamide gels according to Laemmli [70]. Molecular weight markers ranged from 15 to 250 kDa (BioRad, Hercules, CA, USA).
4.6. Effect of pH and Temperature on Immobilized Derivatives
The optimum pH of the enzyme was determined by performing activity assays over a pH range of 2.2 to 8.0 using 50 mM McIlvaine buffer [67]. Each reaction was incubated for 1 h, after which enzymatic activity was measured as described in Section 4.2. The effect of temperature on enzyme activity was evaluated over a range of 20–80 °C using 100 mM McIlvaine buffer [67] at pH 5.0.
4.7. Thermal and pH Stability Studies
The thermal stability of the enzyme was evaluated using 20 U/g of immobilized derivatives. Stability assays were conducted at 40 °C and 50 °C, at pH 7.0. At predetermined time intervals, enzyme activity was measured as described in Section 4.2, and the remaining activity was expressed as the ratio of activity at a given time to the initial activity at the start of incubation.
For pH stability studies, 20 U/g of immobilized lipase and 20 U/mL of free enzyme were used. The derivatives and free enzyme were incubated across a pH range of 2.0 to 10.0. After the incubation period, enzymatic activity was determined as previously described, and remaining activity was calculated as the ratio of activity at each time point to that at time zero.
4.8. Stabilities of the Lipase in Detergents and Salts
Lipase stability in the presence of detergents was evaluated using 20 U/g of immobilized derivatives. The derivatives were incubated with 5 and 10% Tween 80 or sodium dodecyl sulfate (SDS) at pH 5.0 and room temperature. Remaining enzyme activity was calculated as the ratio of activity at each time point to the activity at time zero. Additionally, the effect of various salts on enzyme activity was assessed at a concentration of 1 mM. The salts tested included CuSO4, CuCl, NH4Cl, Ag2SO4, HgCl2, NaBr, EDTA, BaCl2, ZnCl2, NaF, MnCl2, FeCl3, Zn(NO3)2, Pb(C2H3O8), MgSO4·7H2O, AgNO3, NaCl, AlCl3, AgC2H3O2, KCl, CoCl2, and CaCl2.
4.9. Hydrolysis of Oils
The hydrolysis of different oils by free lipase was carried out in a 25 mM sodium acetate buffer, pH 5, using an automatic titrator. The reaction mixture consisted of 6 mL of oil, 8 mL of buffer, and 0.5 mL of Triton X-100. The reaction was initiated by adding 3 mL of lipase, and the mixture was mechanically stirred at 250 rpm for 16 h. The reaction was stopped by the addition of a solution of ethanol: acetone (1:1).
4.10. Thin-Layer Chromatography of the Hydrolysis Product of Oils Catalyzed by Lipase
The hydrolysis products generated by lipase were analyzed using ascending thin-layer chromatography (TLC) on silica plates (DC-Alufolien Kieselgel 60, without fluorescence indicator, Merck®, Rahway, NJ, USA). The reaction mixture consisted of 2 mL of oil, 3 mL of crude lipase extract, 0.3 mL of Triton X-100, and 0.7 mL of 500 mM MES buffer, pH 6.0. The mixture was incubated at 40 °C under constant agitation. Aliquots (5 µL) were removed and applied to the silica plate. The chromatographic run was performed twice using a solvent system of hexane: ethyl acetate: acetic acid (90:10:1). Hydrolysis products were visualized by iodine staining until bands corresponding to triglyceride degradation products appeared.
4.11. Reproducibility of Experiments
All experiments were performed in triplicate, and the standard deviation was calculated to confirm the results obtained. Statistical analyses were performed using OriginPro 2021 (OriginLab Corporation, Northampton, MA, USA). Data are presented as mean ± standard deviation (SD) from at least three independent experiments.
5. Conclusions
H. pseudokoningii is a high lipase producer in SSF, and simple modifications or additions of various compounds to the culture medium can regulate the amount of lipase produced by this microorganism. Based on the biochemical characterization of the immobilized lipase in this study, we conclude that it possesses key features indicating its potential for biotechnological applications, including high stability across a wide pH range, thermostability, and compatibility with mesophilic conditions. Another notable property of the lipase is its resistance to heavy metals, which enables its potential use in bioremediation processes.
Author Contributions
M.G.P.: Conceptualization, Formal analysis, Investigation, Methodology, Writing original draft, Writing—review and editing. T.M.P.: Formal analysis, Writing—review and editing. M.d.L.T.M.P.: Supervision, Project administration, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, process n° 574002/2008-1 and 2023/01547-5) and Conselho de Desenvolvimento Científico e Tecnológico (CNPq process n° 406838/2013-5). Ciência sem Fronteira (n° 242775/2012-8). M.L.T.M.P. is a Research Fellow of CNPq (process n° 310340/2021-7). M.G.P. is supported by the UEMG Research Productivity Grant Program (PQ).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Mauricio de Oliveira for the technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SSF | Solid-State Fermentation |
| U | Unit |
| TLC | Thin-Layer Chromatography |
| SmF | Submerged fermentation |
| pNPP | p-nitrophenyl palmitate |
References
- Ribeiro, E.S.; Machado, B.R.; de Farias, B.S.; Han, L.H.; dos Santos, L.O.; Duarte, S.H.; Cadaval Junior, T.R.S.; de Almeida Pinto, L.A.; Diaz, P.S. Bi-layer nanocapsules based on chitosan and xanthan gum for lipase immobilization. J. Mol. Liq. 2025, 434, 128031. [Google Scholar] [CrossRef]
- Facchini, F.D.A.; Vici, A.C.; Pereira, M.G.; Jorge, J.A.; Polizeli, M.L.T.M. Enhanced lipase production of Fusarium verticillioides by using response surface methodology and wastewater pretreatment application. J. Biochem. Technol. 2016, 6, 996–1002. [Google Scholar]
- Facchini, F.; Pereira, M.; Vici, A. Lipases: Imperative Fat-degrading Enzymes. In Fungal Enzymes; Rai, M., Ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-4665-9454-8. [Google Scholar]
- Vici, A.C.; da Cruz, A.F.; Facchini, F.D.A.; de Carvalho, C.C.; Pereira, M.G.; Fonseca-Maldonado, R.; Ward, R.J.; Pessela, B.C.; Fernandez-Lorente, G.; Torres, F.A.G.; et al. Beauveria bassiana Lipase A expressed in Komagataella (Pichia) pastoris with potential for biodiesel catalysis. Front. Microbiol. 2015, 6, 1083. [Google Scholar] [CrossRef] [PubMed]
- Chandra, K.; Dong, C.-D.; Chauhan, A.S.; Chen, C.-W.; Patel, A.K.; Singhania, R.R. Advancements in lipase immobilization: Enhancing enzyme efficiency with nanomaterials for industrial applications. Int. J. Biol. Macromol. 2025, 311, 143754. [Google Scholar] [CrossRef] [PubMed]
- Tanyol, M.; Uslu, G.; Yönten, V. Optimization of lipase production on agro-industrial residue medium by Pseudomonas fluorescens (NRLL B-2641) using response surface methodology. Biotechnol. Biotechnol. Equip. 2015, 29, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, J.; Zhang, H.; Zhang, M.; Qi, C.; Wang, C. Biological modification and industrial applications of microbial lipases: A general review. Int. J. Biol. Macromol. 2025, 302, 140486. [Google Scholar] [CrossRef]
- Singh, A.K.; Mukhopadhyay, M. Overview of fungal lipase: A review. Appl. Biochem. Biotechnol. 2012, 166, 486–520. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, Z.; Decatur, J.; Xie, W.; Gross, R.A. Chain Growth and Branch Structure Formation during Lipase-Catalyzed Synthesis of Aliphatic Polycarbonate Polyols. Macromolecules 2011, 44, 1471–1479. [Google Scholar] [CrossRef]
- Scheibel, D.M.; Gitsov, I.P.I.; Gitsov, I. Enzymes in “green” synthetic chemistry: Laccase and lipase. Molecules 2024, 29, 989. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, F.; Yuan, C.; Du, W.; Liu, D. Lipase-catalyzed process for biodiesel production: Enzyme immobilization, process simulation and optimization. Renew. Sustain. Energy Rev. 2015, 44, 182–197. [Google Scholar] [CrossRef]
- Greco-Duarte, J.; de Almeida, F.P.; de Godoy, M.G.; Lins, U.; Freire, D.M.G.; Gutarra, M.L.E. Simultaneous lipase production and immobilization: Morphology and physiology study of Penicillium simplicissimum in submerged and solid-state fermentation with polypropylene as an inert support. Enzym. Microb. Technol. 2023, 164, 110173. [Google Scholar] [CrossRef] [PubMed]
- Nagy, V.; Tőke, E.R.; Keong, L.C.; Szatzker, G.; Ibrahim, D.; Omar, I.C.; Szakács, G.; Poppe, L. Kinetic resolutions with novel, highly enantioselective fungal lipases produced by solid state fermentation. J. Mol. Catal. B Enzym. 2006, 39, 141–148. [Google Scholar] [CrossRef]
- Gutarra, M.L.E.; Godoy, M.G.; Maugeri, F.; Rodrigues, M.I.; Freire, D.M.G.; Castilho, L.R. Production of an acidic and thermostable lipase of the mesophilic fungus Penicillium simplicissimum by solid-state fermentation. Bioresour. Technol. 2009, 100, 5249–5254. [Google Scholar] [CrossRef]
- Rigo, E.; Ninow, J.L.; Di Luccio, M.; Oliveira, J.V.; Polloni, A.E.; Remonatto, D.; Arbter, F.; Vardanega, R.; de Oliveira, D.; Treichel, H. Lipase production by solid fermentation of soybean meal with different supplements. LWT—Food Sci. Technol. 2010, 43, 1132–1137. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Sugai-Guérios, M.H.; Krieger, N. Solid-State Fermentation. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780124095472. [Google Scholar]
- Pereira, M.G.; Vici, A.C.; Facchini, F.D.A.; Tristão, A.P.; Cursino-Santos, J.R.; Sanches, P.R.; Jorge, J.A.; Polizeli, M.L.T.d.M. Screening of filamentous fungi for lipase production: A new producer with a high biotechnological potential. Biocatal. Biotransform. 2014, 32, 74–83. [Google Scholar] [CrossRef]
- Martins, V.G.; Kalil, S.J.; Costa, J.A.V. Co-produção de lipase e biossurfactante em estado sólido para utilização em biorremediação de óleos vegetais e hidrocarbonetos. Quím. Nova 2008, 31, 1942–1947. [Google Scholar] [CrossRef][Green Version]
- Pereira Lima, R.; Souza da Luz, P.T.; Braga, M.; dos Santos Batista, P.R.; Ferreira da Costa, C.E.; Zamian, J.R.; Santos do Nascimento, L.A.; da Rocha Filho, G.N. Murumuru (Astrocaryum murumuru Mart.) butter and oils of buriti (Mauritia flexuosa Mart.) and pracaxi (Pentaclethra macroloba (Willd.) Kuntze) can be used for biodiesel production: Physico-chemical properties and thermal and kinetic studies. Ind. Crops Prod. 2017, 97, 536–544. [Google Scholar] [CrossRef]
- Barbalho, G.N.; Matos, B.N.; da Silva Brito, G.F.; da Cunha Miranda, T.; Alencar-Silva, T.; Sodré, F.F.; Gelfuso, G.M.; Cunha-Filho, M.; Carvalho, J.L.; da Silva, J.K.d.R.; et al. Skin Regenerative Potential of Cupuaçu Seed Extract (Theobroma grandiflorum), a Native Fruit from the Amazon: Development of a Topical Formulation Based on Chitosan-Coated Nanocapsules. Pharmaceutics 2022, 14, 207. [Google Scholar] [CrossRef]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef]
- Verma, K.; Kaushik, P.; Chugh, R.; Kaur, G.; Kathuria, D. Cosmeceutical applications of natural oils and fats. In Specialized Plant Metabolites as Cosmeceuticals; Elsevier: Amsterdam, The Netherlands, 2024; pp. 239–256. ISBN 9780443191480. [Google Scholar]
- Pereira, M.G.; Facchini, F.D.A.; Filó, L.E.C.; Polizeli, A.M.; Vici, A.C.; Jorge, J.A.; Fernandez-Lorente, G.; Pessela, B.C.; Guisan, J.M.; Polizeli, M.L.T.d.M. Immobilized lipase from Hypocrea pseudokoningii on hydrophobic and ionic supports: Determination of thermal and organic solvent stabilities for applications in the oleochemical industry. Process Biochem. 2015, 50, 561–570. [Google Scholar] [CrossRef]
- Pereira, M.G.; Facchini, F.D.A.; Polizeli, A.M.; Vici, A.C.; Jorge, J.A.; Pessela, B.C.; Férnandez-Lorente, G.; Guisán, J.M.; Polizeli, M.L.T.M. Stabilization of the lipase of Hypocrea pseudokoningii by multipoint covalent immobilization after chemical modification and application of the biocatalyst in oil hydrolysis. J. Mol. Catal. B Enzym. 2015, 121, 82–89. [Google Scholar] [CrossRef]
- Manoel, E.A.; Dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzym. Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Abro, A.A.; Qureshi, A.S.; Naqvi, M.; Khushk, I.; Jatt, A.N.; Ali, C.H.; Makhdoom, F.; Shafaq, U. Lipase Production from alkalophilic-thermophilic-Ionic liquid tolerant Bacillus cereus using agricultural residues for its applications in biodiesel and detergents. Ind. Crops Prod. 2024, 220, 119208. [Google Scholar] [CrossRef]
- Veerabhadrappa, M.B.; Shivakumar, S.B.; Devappa, S. Solid-state fermentation of Jatropha seed cake for optimization of lipase, protease and detoxification of anti-nutrients in Jatropha seed cake using Aspergillus versicolor CJS-98. J. Biosci. Bioeng. 2014, 117, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Martin del Campo, M.; Camacho, R.M.; Mateos-Díaz, J.C.; Müller-Santos, M.; Córdova, J.; Rodríguez, J.A. Solid-state fermentation as a potential technique for esterase/lipase production by halophilic archaea. Extremophiles 2015, 19, 1121–1132. [Google Scholar] [CrossRef]
- Damaso, M.C.T.; Passianoto, M.A.; de Freitas, S.C.; Freire, D.M.G.; Lago, R.C.A.; Couri, S. Utilization of agroindustrial residues for lipase production by solid-state fermentation. Braz. J. Microbiol. 2008, 39, 676–681. [Google Scholar] [CrossRef]
- Lyons-Wall, P. Food analysis, food composition tables, and databases. In Essentials of Human Nutrition; Oxford University Press: Oxford, UK, 2023; ISBN 9780191992902. [Google Scholar]
- Takó, M.; Kotogán, A.; Krisch, J.; Vágvölgyi, C.; Mondal, K.C.; Papp, T. Enhanced production of industrial enzymes in Mucoromycotina fungi during solid-state fermentation of agricultural wastes/by-products. Acta Biol. Hung. 2015, 66, 348–360. [Google Scholar] [CrossRef]
- Salihu, A.; Alam, M.d.Z.; AbdulKarim, M.I.; Salleh, H.M. Lipase production: An insight in the utilization of renewable agricultural residues. Resour. Conserv. Recycl. 2012, 58, 36–44. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Mateos, J.C.; Nungaray, J.; González, V.; Bhagnagar, T.; Roussos, S.; Cordova, J.; Baratti, J. Improving lipase production by nutrient source modification using Rhizopus homothallicus cultured in solid state fermentation. Process Biochem. 2006, 41, 2264–2269. [Google Scholar] [CrossRef]
- Costa, A.R.; Salgado, J.M.; Belo, I. Olive and sunflower cakes as suitable substrates for lipase production by Yarrowia spp.: From flasks to bioreactor. Biocatal. Agric. Biotechnol. 2023, 51, 102783. [Google Scholar] [CrossRef]
- Fickers, P.; Marty, A.; Nicaud, J.M. The lipases from Yarrowia lipolytica: Genetics, production, regulation, biochemical characterization and biotechnological applications. Biotechnol. Adv. 2011, 29, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Fickers, P.; Nicaud, J.M.; Destain, J.; Thonart, P. Involvement of hexokinase Hxk1 in glucose catabolite repression of LIP2 encoding extracellular lipase in the yeast Yarrowia lipolytica. Curr. Microbiol. 2005, 50, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, N.; Gupta, A.; Khare, S.K. Production of protease and lipase by solvent tolerant Pseudomonas aeruginosa PseA in solid-state fermentation using Jatropha curcas seed cake as substrate. Bioresour. Technol. 2008, 99, 1729–1735. [Google Scholar] [CrossRef]
- Mahadik, N.D.; Puntambekar, U.S.; Bastawde, K.B.; Khire, J.M.; Gokhale, D.V. Production of acidic lipase by Aspergillus niger in solid state fermentation. Process Biochem. 2002, 38, 715–721. [Google Scholar] [CrossRef]
- Mazhar, H.; Ullah, I.; Ali, U.; Abbas, N.; Hussain, Z.; Ali, S.S.; Zhu, H. Optimization of low-cost solid-state fermentation media for the production of thermostable lipases using agro-industrial residues as substrate in culture of Bacillus amyloliquefaciens. Biocatal. Agric. Biotechnol. 2023, 47, 102559. [Google Scholar] [CrossRef]
- Cao, L. Carrier-Bound Immobilized Enzymes: Principles, Application and Design; Wiley: Hoboken, NJ, USA, 2005; ISBN 9783527312320. [Google Scholar]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Pernas, M.A.; López, C.; Pastrana, L.; Rúa, M.L. Purification and characterization of Lip2 and Lip3 isoenzymes from a Candida rugosa pilot-plant scale fed-batch fermentation. J. Biotechnol. 2001, 84, 163–174. [Google Scholar] [CrossRef]
- Basheer, S.M.; Chellappan, S.; Beena, P.S.; Sukumaran, R.K.; Elyas, K.K.; Chandrasekaran, M. Lipase from marine Aspergillus awamori BTMFW032: Production, partial purification and application in oil effluent treatment. N. Biotechnol. 2011, 28, 627–638. [Google Scholar] [CrossRef]
- Romdhane, I.B.-B.; Fendri, A.; Gargouri, Y.; Gargouri, A.; Belghith, H. A novel thermoactive and alkaline lipase from Talaromyces thermophilus fungus for use in laundry detergents. Biochem. Eng. J. 2010, 53, 112–120. [Google Scholar] [CrossRef]
- Hasan, F.; Shah, A.A.; Hameed, A. Methods for detection and characterization of lipases: A comprehensive review. Biotechnol. Adv. 2009, 27, 782–798. [Google Scholar] [CrossRef]
- Lima, V.M.G.; Krieger, N.; Mitchell, D.A.; Fontana, J.D. Activity and stability of a crude lipase from Penicillium aurantiogriseum in aqueous media and organic solvents. Biochem. Eng. J. 2004, 18, 65–71. [Google Scholar] [CrossRef]
- Tanaka, A.; Sugimoto, H.; Muta, Y.; Mizuno, T.; Senoo, K.; Obata, H.; Inouye, K. Differential scanning calorimetry of the effects of Ca2+ on the thermal unfolding of Pseudomonas cepacia lipase. Biosci. Biotechnol. Biochem. 2003, 67, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Sabri, S.; Rahman, R.N.Z.R.A.; Leow, T.C.; Basri, M.; Salleh, A.B. Secretory expression and characterization of a highly Ca2+-activated thermostable L2 lipase. Protein Expr. Purif. 2009, 68, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Pasin, T.M.; Benassi, V.M.; Heinen, P.R.; Damasio, A.R.d.L.; Cereia, M.; Jorge, J.A.; Polizeli, M.L.T.M. Purification and functional properties of a novel glucoamylase activated by manganese and lead produced by Aspergillus japonicus. Int. J. Biol. Macromol. 2017, 102, 779–788. [Google Scholar] [CrossRef]
- Pasin, T.M.; Betini, J.H.A.; de Lucas, R.C.; Polizeli, M.L.T.M. Biochemical characterization of an acid-thermostable glucoamylase from Aspergillus japonicus with potential application in the paper bio-deinking. Biotechnol. Prog. 2024, 40, e3384. [Google Scholar] [CrossRef]
- Sharma, N.; Ahlawat, Y.K.; Stalin, N.; Mehmood, S.; Morya, S.; Malik, A.; Malathi, H.; Nellore, J.; Bhanot, D. Microbial enzymes in industrial biotechnology: Sources, production, and significant applications of lipases. J. Ind. Microbiol. Biotechnol. 2024, 52, kuaf010. [Google Scholar] [CrossRef]
- Benassi, V.M.; Pasin, T.M.; Facchini, F.D.A.; Jorge, J.A.; Polizeli, M.L.T.M. A novel glucoamylase activated by manganese and calcium produced in submerged fermentation by Aspergillus phoenicis. J. Basic Microbiol. 2014, 54, 333–339. [Google Scholar] [CrossRef]
- Foster, A.; Barnes, N.; Speight, R.; Keane, M.A. Identification, functional expression and kinetic analysis of two primary amine oxidases from Rhodococcus opacus. J. Mol. Catal. B Enzym. 2012, 74, 73–82. [Google Scholar] [CrossRef]
- Sharma, R.; Chisti, Y.; Banerjee, U.C. Production, purification, characterization, and applications of lipases. Biotechnol. Adv. 2001, 19, 627–662. [Google Scholar] [CrossRef]
- Gohara, D.W.; Di Cera, E. Molecular mechanisms of enzyme activation by monovalent cations. J. Biol. Chem. 2016, 291, 20840–20848. [Google Scholar] [CrossRef]
- Holliday, G.L.; Mitchell, J.B.O.; Thornton, J.M. Understanding the functional roles of amino acid residues in enzyme catalysis. J. Mol. Biol. 2009, 390, 560–577. [Google Scholar] [CrossRef]
- Siriwardana, K.; Wang, A.; Gadogbe, M.; Collier, W.E.; Fitzkee, N.C.; Zhang, D. Studying the Effects of Cysteine Residues on Protein Interactions with Silver Nanoparticles. J. Phys. Chem. C Nanomater. Interfaces 2015, 119, 2910–2916. [Google Scholar] [CrossRef]
- Vogel, H.J. Distribution of lysine pathways among fungi: Evolutionary implications. Am. Nat. 1964, 98, 435–446. [Google Scholar] [CrossRef]
- Pasin, T.M.; Dos Anjos Moreira, E.; de Lucas, R.C.; Benassi, V.M.; Ziotti, L.S.; Cereia, M.; Polizeli, M.L.T.M. Novel amylase-producing fungus hydrolyzing wheat and brewing residues, Aspergillus carbonarius, discovered in tropical forest remnant. Folia Microbiol. 2020, 65, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Street, G. Handbook of enzyme biotechnology. Biochem. Educ. 1977, 5, 64. [Google Scholar] [CrossRef]
- Khanna, P.; Sundari, S.S.; Kumar, N.J. Production, isolation and partial purification of xylanases from an Aspergillus sp. World J. Microbiol. Biotechnol. 1995, 11, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Rizzatti, A.C.; Jorge, J.A.; Terenzi, H.F.; Rechia, C.G.; Polizeli, M.L.T.M. Purification and properties of a thermostable extracellular beta-D-xylosidase produced by a thermotolerant Aspergillus phoenicis. J. Ind. Microbiol. Biotechnol. 2001, 26, 156–160. [Google Scholar] [CrossRef]
- Pencreac’h, G.; Baratti, J.C. Hydrolysis of p-nitrophenyl palmitate in n-heptane by the Pseudomonas cepacia lipase: A simple test for the determination of lipase activity in organic media. Enzym. Microb. Technol. 1996, 18, 417–422. [Google Scholar] [CrossRef]
- Vo, C.-V.T.; Luu, N.V.H.; Nguyen, T.T.H.; Nguyen, T.T.; Ho, B.Q.; Nguyen, T.H.; Tran, T.-D.; Nguyen, Q.-T. Screening for pancreatic lipase inhibitors: Evaluating assay conditions using p -nitrophenyl palmitate as substrate. All Life 2022, 15, 13–22. [Google Scholar] [CrossRef]
- McIlvaine, T.C. A buffer solution for colorimetric comparison. J. Biol. Chem. 1921, 49, 183–186. [Google Scholar] [CrossRef]
- Pooja; Sinha, S.K.; Datta, S. Identification of a thermostable GH6 family cellulase from Chaetomium thermophilum exhibiting high cellobiose and ionic liquid tolerance. Enzym. Microb. Technol. 2025, 192, 110755. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).